Identification of Breast-Cancer-Associated Properties of Drinking Water under a Composite-Toxicity Perspective of Mixed Contaminants: A Case Study in a High-Prevalence Area of China
Abstract
1. Introduction
2. Materials and Methods
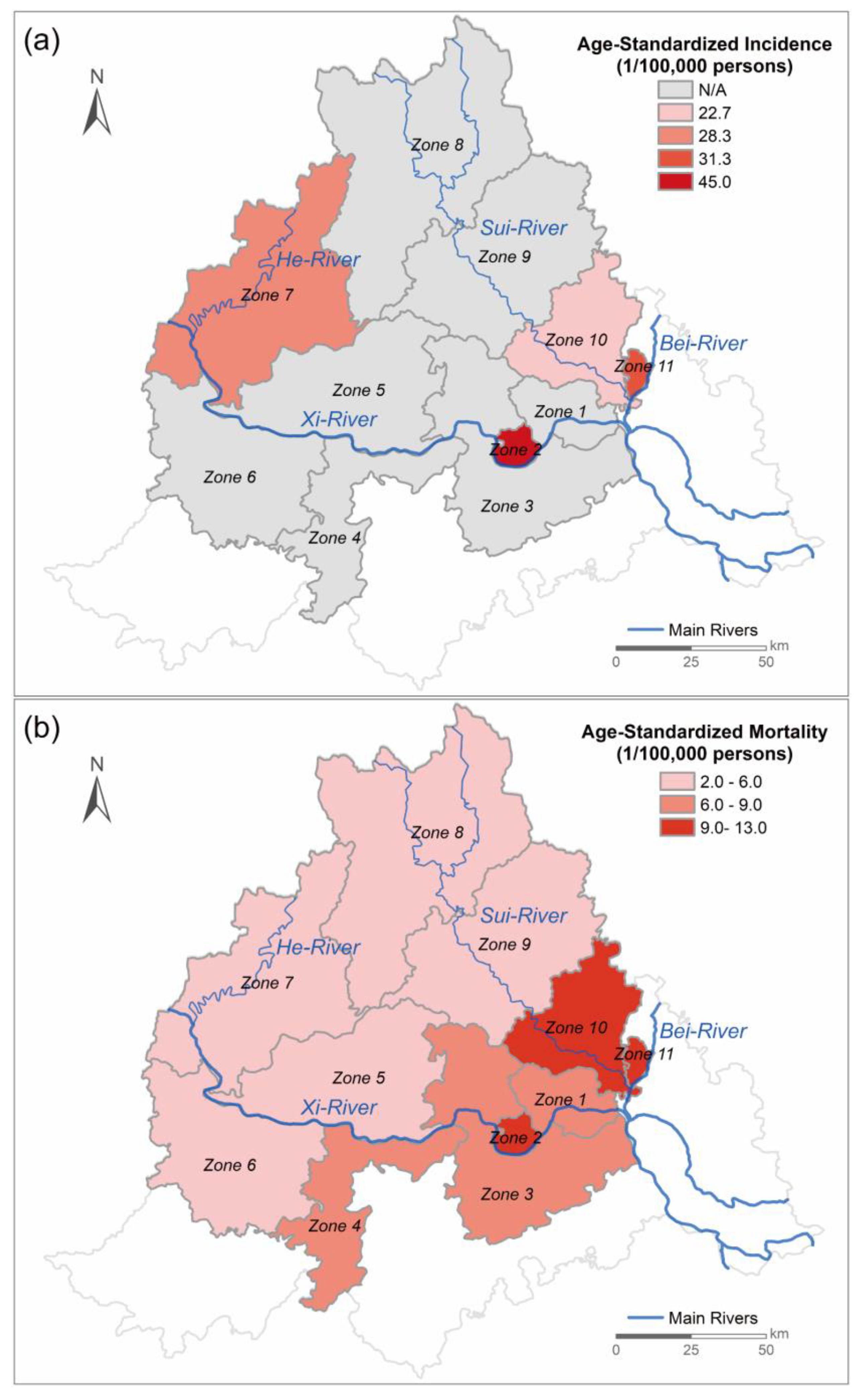
2.1. Breast Cancer Prevalence in the Study Area
2.2. Water Sample Collection
2.3. Toxicological Analysis of Water Samples
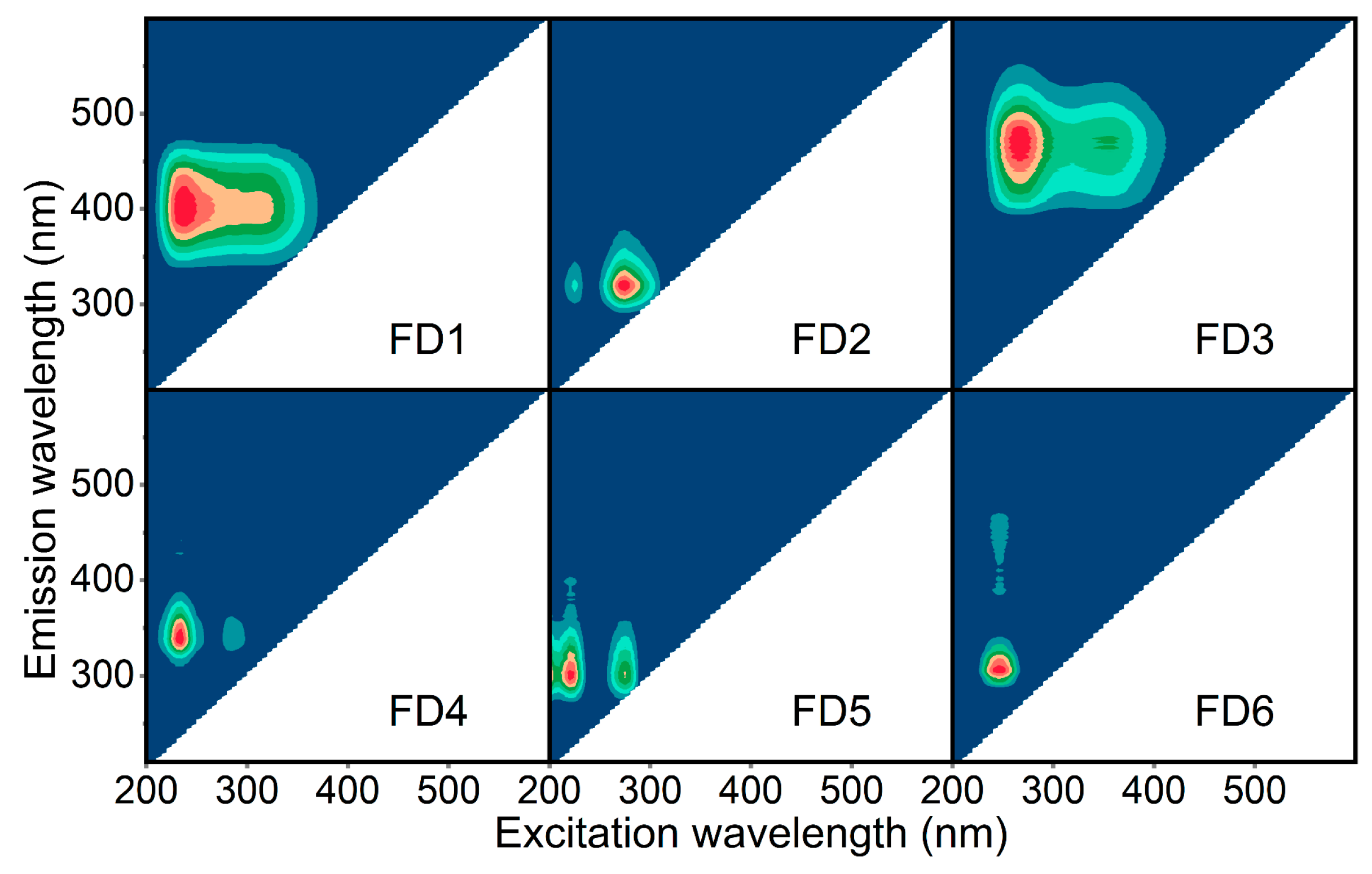
2.4. Characterization of DOM
2.5. Measurement of EDCs
2.6. Screening Process of Key Water Contaminants
2.7. PLS-PM Analysis
3. Results and Discussion
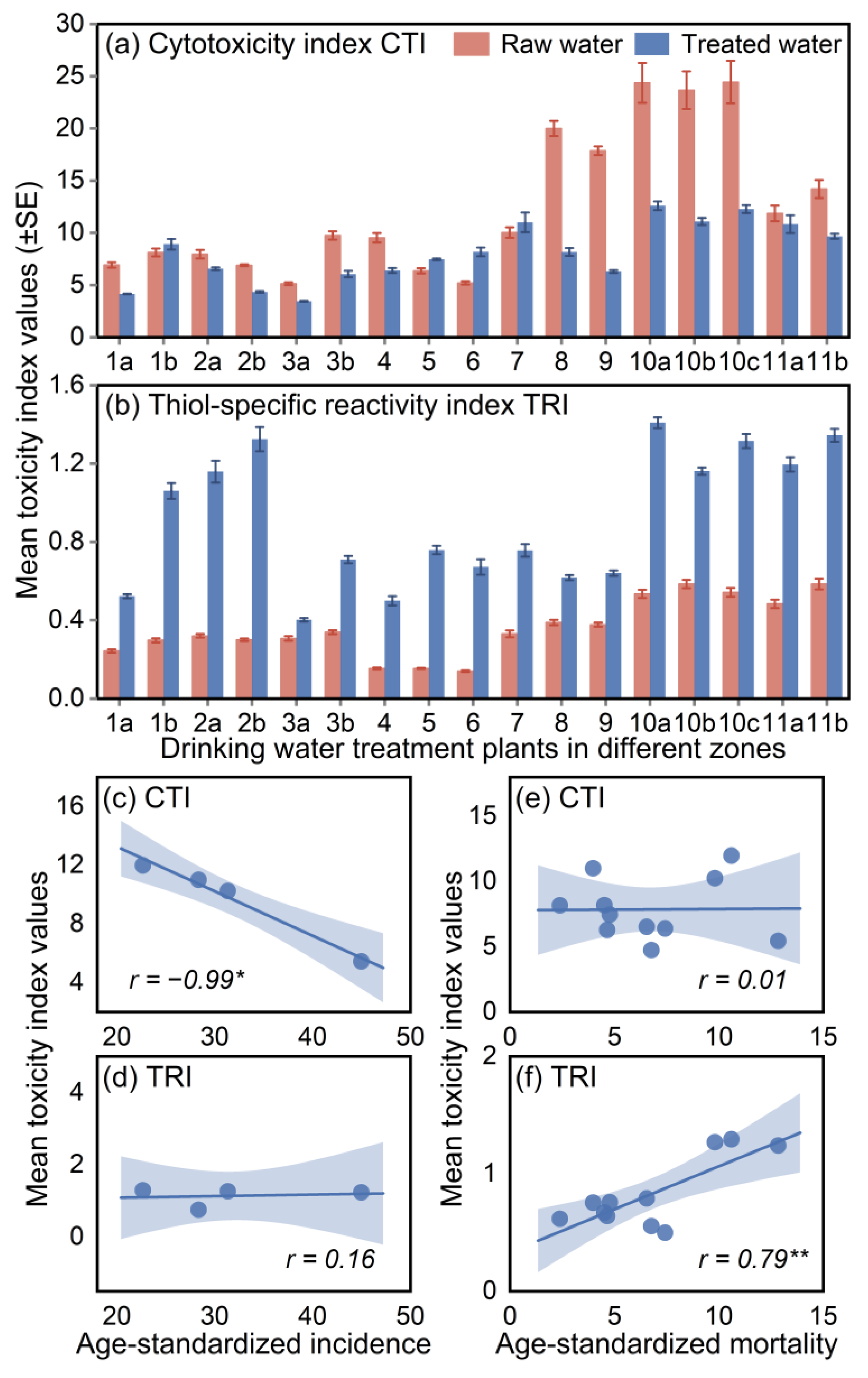
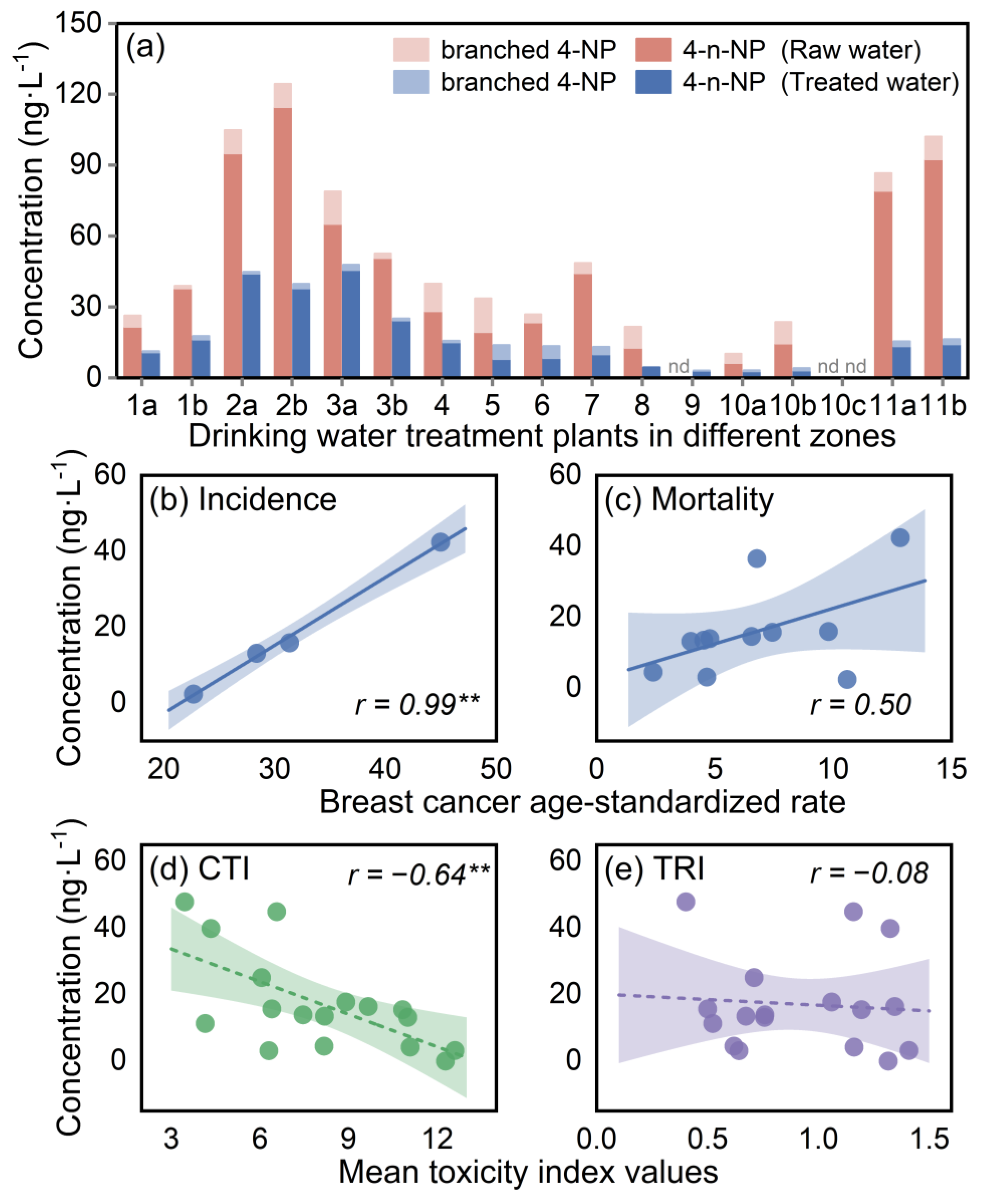
3.1. Overall Levels of Toxicity with Breast-Cancer-Associated Properties in Drinking Water and Source Water
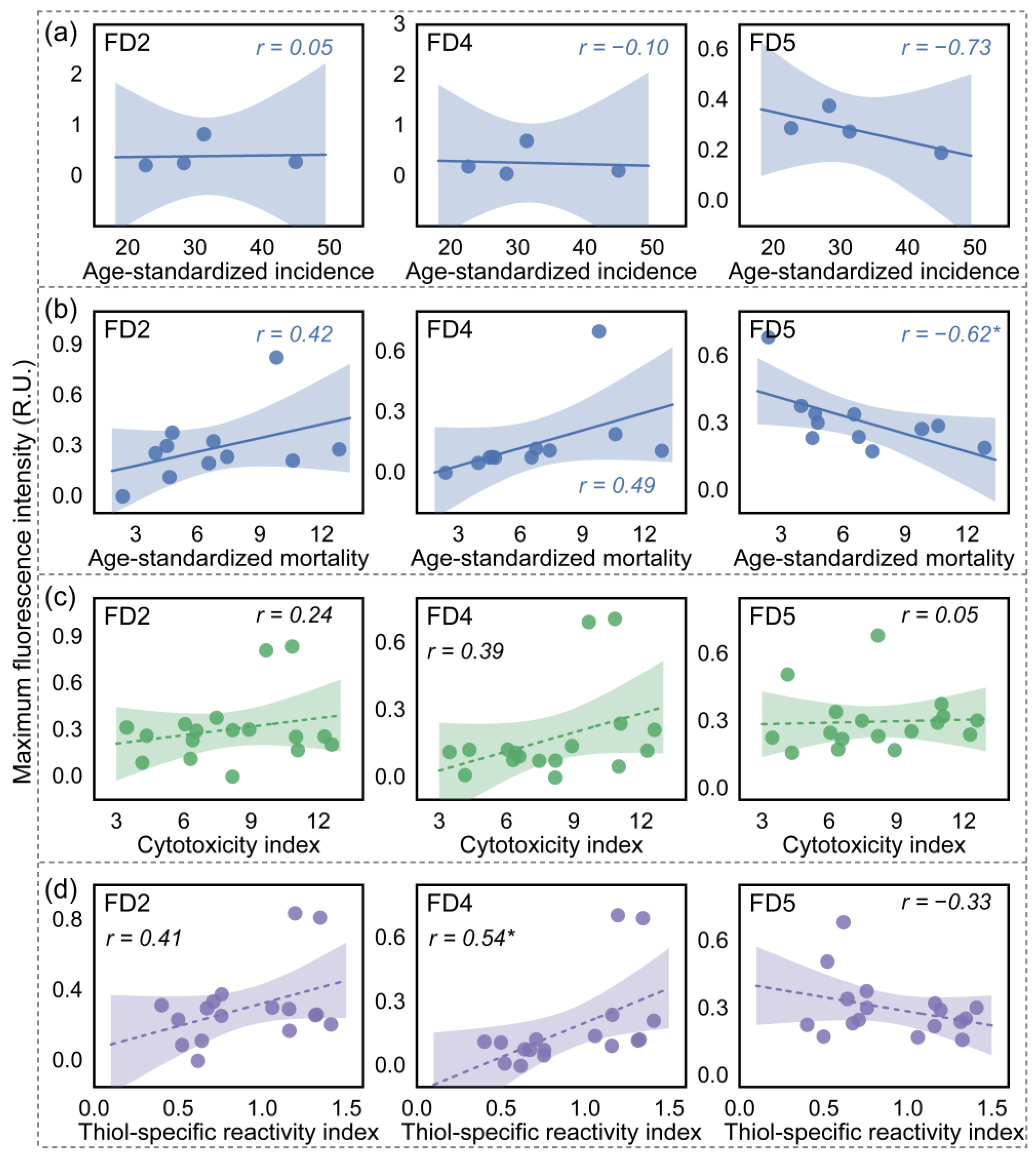
3.2. Identification of Key DOM with Breast-Cancer-Associated Properties in Water Mixture Contaminants
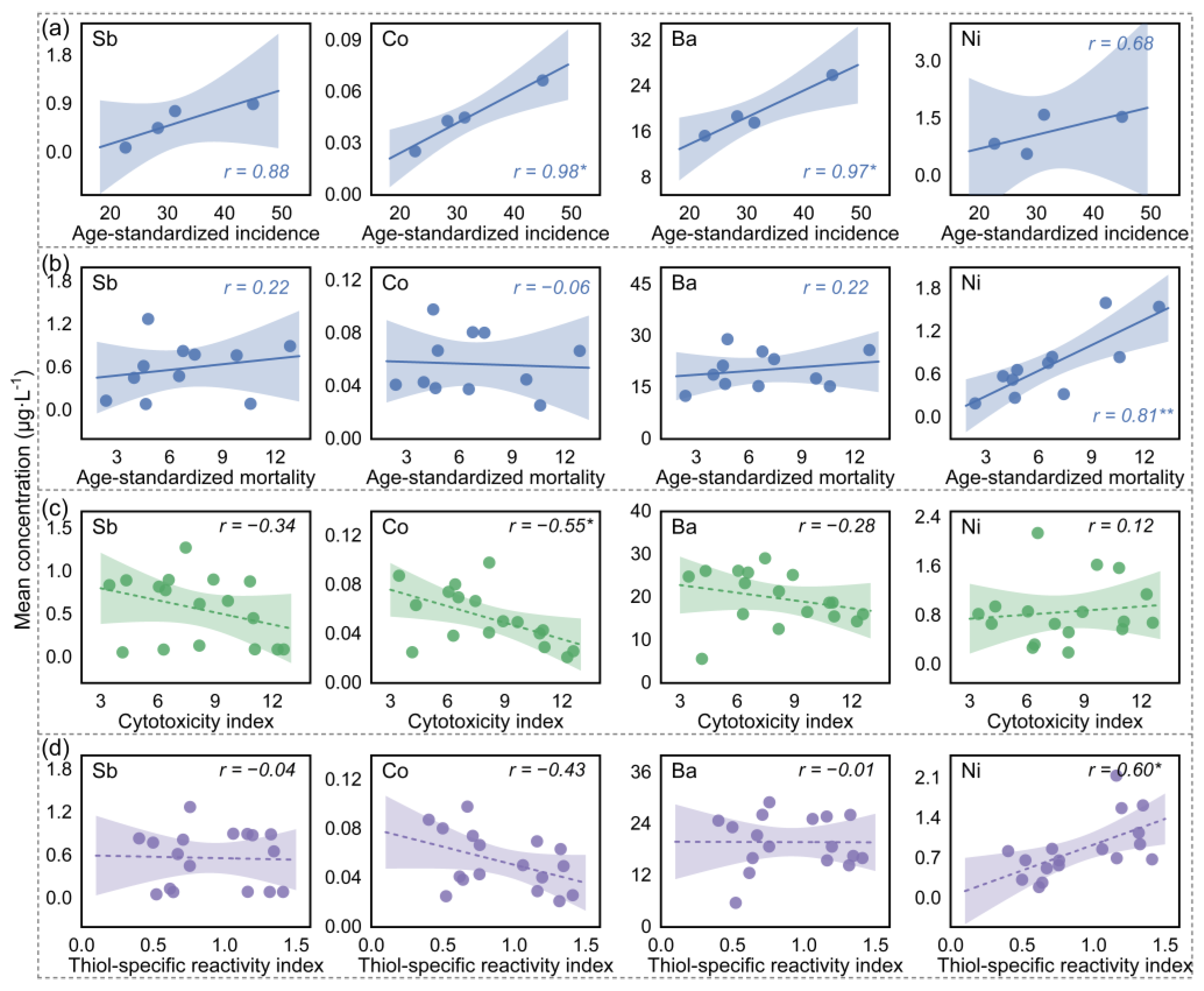
3.3. Identification of Key EDCs with Breast-Cancer-Associated Properties in Water Mixture Contaminants
3.4. Breast-Cancer-Risk-Inducing Paths Based on Water Toxicological Commonalities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Parkin, D.M. Cancers of the breast, endometrium and ovary: Geographic correlations. Eur. J. Cancer Clin. Oncol. 1989, 25, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Feuer, E.J.; Miller, B.A.; Freedma, L.S. Breast cancer clusters in the northeast United States: A geographic analysis. Am. J. Epidemiol. 1997, 146, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.G.; Rudel, R.A. Environmental pollutants and breast cancer. Environ. Health Perspect. 2003, 111, 1007–1019. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, X.-W.; Wang, T.; Tao, K.; Bao, L.-J.; Zeng, E.Y. Water quality and organic pollution with health risk assessment in China: A short review. ACS EST Water 2022, 2, 1279–1288. [Google Scholar] [CrossRef]
- Wu, C.; Maurer, C.; Wang, Y.; Xue, S.; Davis, D.L. Water pollution and human health in China. Environ. Health Perspect. 1999, 107, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Gottlieb, M.; Clute, E.; Pongsiri, M.J.; Sherman, J.; Obrams, G.I. Breast cancer and pesticides in Hawaii: The need for further study. Environ. Health Perspect. 1997, 105 (Suppl. S3), 679–683. [Google Scholar] [PubMed]
- Aschengrau, A.; Paulu, C.; Ozonoff, D. Tetrachloroethylene-contaminated drinking water and the risk of breast cancer. Environ. Health Perspect. 1998, 106 (Suppl. S4), 947–953. [Google Scholar] [PubMed]
- Gallagher, L.G.; Webster, T.F.; Aschengrau, A.; Vieira, V.M. Using residential history and groundwater modeling to examine drinking water exposure and breast cancer. Environ. Health Perspect. 2010, 118, 749–755. [Google Scholar] [CrossRef]
- Peremiquel-Trillas, P.; Benavente, Y.; Martín-Bustamante, M.; Casabonne, D.; Pérez-Gómez, B.; Gómez-Acebo, I.; Oliete-Canela, A.; Diéguez-Rodríguez, M.; Tusquets, I.; Amiano, P. Alkylphenolic compounds and risk of breast and prostate cancer in the MCC-Spain study. Environ. Int. 2019, 122, 389–399. [Google Scholar] [CrossRef]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Mérida-Ortega, Á.; Rothenberg, S.J.; Cebrián, M.E.; López-Carrillo, L. Breast cancer and urinary metal mixtures in Mexican women. Environ. Res. 2022, 210, 112905. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Yang, M.; Tan, C.; Chu, W. The occurrence, characteristics, transformation and control of aromatic disinfection by-products: A review. Water Res. 2020, 184, 116076. [Google Scholar] [CrossRef]
- Liu, M.; Graham, N.; Wang, W.; Zhao, R.; Lu, Y.; Elimelech, M.; Yu, W. Spatial assessment of tap-water safety in China. Nat. Sustain. 2022, 5, 689–698. [Google Scholar] [CrossRef]
- Rizzuto, S.; Jones, K.C.; Zhang, H.; Baho, D.L.; Leu, E.; Nizzetto, L. Critical assessment of an equilibrium-based method to study the binding of waterborne organic contaminants to natural dissolved organic matter (DOM). Chemosphere 2021, 285, 131524. [Google Scholar] [CrossRef]
- Ma, L.; Yates, S.R. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review. Sci. Total Environ. 2018, 640, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-Y.; Du, Y.; Wu, Q.-Y.; Zhao, X.; Tang, X.; Chen, Z. Differences in dissolved organic matter between reclaimed water source and drinking water source. Sci. Total Environ. 2016, 551, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, Q.-Y.; Zhao, X.; Du, Y.; Huang, H.; Shi, X.-L.; Hu, H.-Y. Transformation of anti-estrogenic-activity related dissolved organic matter in secondary effluents during ozonation. Water Res. 2014, 48, 605–612. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Mirzaee, S.A.; Martinez, S.S.; Rachoń, D.; Hoseinzadeh, M.; Jaafarzadeh, N. Environmental exposure to nonylphenol and cancer progression Risk–A systematic review. Environ. Res. 2020, 184, 109263. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef]
- He, H.; Luo, N.; Huang, B.; Li, B.; Zhang, Z.; Xu, Z.; Pan, X. Optical characteristics and cytotoxicity of dissolved organic matter in the effluent and sludge from typical sewage treatment processes. Sci. Total Environ. 2020, 725, 138381. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer Oxidative stress-its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Jenie, R.I.; Amalina, N.D.; Ilmawati, G.P.N.; Utomo, R.Y.; Ikawati, M.; Khumaira, A.; Kato, J.-Y.; Meiyanto, E. Cell cycle modulation of CHO-K1 cells under genistein treatment correlates with cells senescence, apoptosis and ROS level but in a dose-dependent manner. Adv. Pharm. Bull. 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Wilson, S.T.; Zalatan, F. Physiological melatonin inhibition of human breast cancer cell growth in vitro: Evidence for a glutathione-mediated pathway. Cancer Res. 1997, 57, 1909–1914. [Google Scholar] [PubMed]
- Yeung, K.; Moore, N.; Sun, J.; Taylor-Edmonds, L.; Andrews, S.; Hofmann, R.; Peng, H. Thiol Reactome: A Nontargeted Strategy to Precisely Identify Thiol Reactive Drinking Water Disinfection Byproducts. Environ. Sci. Technol. 2023, 57, 18722–18734. [Google Scholar] [CrossRef]
- Fan, L.; Strasser-Weippl, K.; Li, J.-J.; St Louis, J.; Finkelstein, D.M.; Yu, K.-D.; Chen, W.-Q.; Shao, Z.-M.; Goss, P.E. Breast cancer in China. Lancet Oncol. 2014, 15, e279–e289. [Google Scholar] [CrossRef]
- Peng, S.; Dong, S.; Gong, C.; Chen, X.; Du, H.; Zhan, Y.; Yang, Z. Evidence-based identification of breast cancer and associated ovarian and uterus cancer risk components in source waters from high incidence area in the Pearl River Basin, China. Sci. Total Environ. 2023, 903, 166060. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Center, N. China Cancer Registry Annual Report 2019; People’s Medical Publishing House: Beijing, China, 2021. [Google Scholar]
- Dong, S.; Page, M.A.; Massalha, N.; Hur, A.; Hur, K.; Bokenkamp, K.; Wagner, E.D.; Plewa, M.J. Toxicological comparison of water, wastewaters, and processed wastewaters. Environ. Sci. Technol. 2019, 53, 9139–9147. [Google Scholar] [CrossRef]
- Zepp, R.G.; Sheldon, W.M.; Moran, M.A. Dissolved organic fluorophores in southeastern US coastal waters: Correction method for eliminating Rayleigh and Raman scattering peaks in excitation–emission matrices. Mar. Chem. 2004, 89, 15–36. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Davey, J.C.; Bodwell, J.E.; Gosse, J.A.; Hamilton, J.W. Arsenic as an endocrine disruptor: Effects of arsenic on estrogen receptor–mediated gene expression in vivo and in cell culture. Toxicol. Sci. 2007, 98, 75–86. [Google Scholar] [CrossRef]
- Choe, S.-Y.; Kim, S.-J.; Kim, H.-G.; Lee, J.H.; Choi, Y.; Lee, H.; Kim, Y. Evaluation of estrogenicity of major heavy metals. Sci. Total Environ. 2003, 312, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef]
- Scorei, I.R. Boron compounds in the breast cancer cells chemoprevention and chemotherapy. In Breast Cancer—Current and Alternative Therapeutic Modalities; InTech: London, UK, 2011; p. 540. [Google Scholar]
- Veselik, D.J.; Divekar, S.; Dakshanamurthy, S.; Storchan, G.B.; Turner, J.M.; Graham, K.L.; Huang, L.; Stoica, A.; Martin, M.B. Activation of estrogen receptor-α by the anion nitrite. Cancer Res. 2008, 68, 3950–3958. [Google Scholar] [CrossRef]
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of fluoride on endocrine tissues and their secretory functions--review. Chemosphere 2020, 260, 127565. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Plewa, M.J.; Wagner, E.D.; Wei, X.; Bokenkamp, K.; Hur, K.; Jia, A.; Liberatore, H.K.; Lee, C.-F.T.; Shirkhani, R. Drivers of disinfection byproduct cytotoxicity in US drinking water: Should other DBPs be considered for regulation? Environ. Sci. Technol. 2021, 56, 392–402. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Altenburger, R. Approaches to assessing combination effects of oestrogenic environmental pollutants. Sci. Total Environ. 1999, 233, 131–140. [Google Scholar] [CrossRef]
- Sommer, S.; Fuqua, S.A. Estrogen Receptor and Breast Cancer; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 339–352. [Google Scholar]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef]
- Baghoth, S.; Sharma, S.; Amy, G. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation–emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef]
- Zito, P.; Podgorski, D.C.; Johnson, J.; Chen, H.; Rodgers, R.P.; Guillemette, F.; Kellerman, A.M.; Spencer, R.G.; Tarr, M.A. Molecular-level composition and acute toxicity of photosolubilized petrogenic carbon. Environ. Sci. Technol. 2019, 53, 8235–8243. [Google Scholar] [CrossRef]
- Osburn, C.L.; Handsel, L.T.; Peierls, B.L.; Paerl, H.W. Predicting sources of dissolved organic nitrogen to an estuary from an agro-urban coastal watershed. Environ. Sci. Technol. 2016, 50, 8473–8484. [Google Scholar] [CrossRef]
- Powers, L.C.; Luek, J.L.; Schmitt-Kopplin, P.; Campbell, B.J.; Magen, C.; Cooper, L.W.; Gonsior, M. Seasonal changes in dissolved organic matter composition in Delaware Bay, USA in March and August 2014. Org. Geochem. 2018, 122, 87–97. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Q.; Zhuang, W.-E.; Wang, H.; Chen, W. Seasonal changes in the chemical composition and reactivity of dissolved organic matter at the land-ocean interface of a subtropical river. Environ. Sci. Pollut. Res. 2019, 26, 24595–24608. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Boyer, J.N.; Jaffé, R. Evaluating the distribution of terrestrial dissolved organic matter in a complex coastal ecosystem using fluorescence spectroscopy. Cont. Shelf Res. 2013, 66, 136–144. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Uchimiya, M.; Ohno, T.; He, Z. Pyrolysis temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes. J. Anal. Appl. Pyrolysis 2013, 104, 84–94. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Zhou, Y.; Wang, H.; Lu, J. Limited Cu (II) binding to biochar DOM: Evidence from C K-edge NEXAFS and EEM-PARAFAC combined with two-dimensional correlation analysis. Sci. Total Environ. 2020, 701, 134919. [Google Scholar] [CrossRef]
- Fichot, C.G.; Benner, R. The spectral slope coefficient of chromophoric dissolved organic matter (S275–295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnol. Oceanogr. 2012, 57, 1453–1466. [Google Scholar] [CrossRef]
- Dos Santos, S.R.; Schellekens, J.; da Silva, W.T.L.; Buurman, P.; Boim, A.G.F.; Vidal-Torrado, P. Selective sorption and desorption of DOM in podzol horizons—FTIR and Py-GC/MS of leachates from a column experiment. Sci. Total Environ. 2022, 826, 154144. [Google Scholar] [CrossRef] [PubMed]
- Eusterhues, K.; Rennert, T.; Knicker, H.; Kogel-Knabner, I.; Totsche, K.U.; Schwertmann, U. Fractionation of organic matter due to reaction with ferrihydrite: Coprecipitation versus adsorption. Environ. Sci. Technol. 2011, 45, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kaal, J.; Liang, J.; Zhang, Y.; Wei, S.; Wang, D.; Green, N.W. Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci. Total Environ. 2017, 603, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, N.; Ghalla, H.; Brandán, S.A.; Bardak, F.; Flakus, H.; Atac, A.; Oujia, B. Experimental FTIR and FT-Raman and theoretical studies on the molecular structures of monomer and dimer of 3-thiopheneacrylic acid. J. Mol. Struct. 2017, 1135, 209–221. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.-H. Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Barapatre, A.; Meena, A.S.; Mekala, S.; Das, A.; Jha, H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016, 86, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Limer, J.L.; Speirs, V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H. The therapeutic potential of phytoestrogens. Expert Opin. Investig. Drugs 2000, 9, 1829–1840. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, Z.; Frenkel, K.; Klein, C.B.; Costa, M. The role of nickel and nickel-mediated reactive oxygen species in the mechanism of nickel carcinogenesis. Environ. Health Perspect. 1994, 102 (Suppl. S3), 281–284. [Google Scholar]
- Brody, J.G.; Aschengrau, A.; McKelvey, W.; Swartz, C.H.; Kennedy, T.; Rudel, R.A. Breast cancer risk and drinking water contaminated by wastewater: A case control study. Environ. Health 2006, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Herrera, N.; Gracia-Lavedan, E.; Pollan, M.; Aragonés, N.; Boldo, E.; Perez-Gomez, B.; Altzibar, J.M.; Amiano, P.; Zabala, A.J.; Ardanaz, E. Ingested nitrate and breast cancer in the Spanish Multicase-Control Study on Cancer (MCC-Spain). Environ. Health Perspect. 2016, 124, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Bhasker, T.V.; Gowda, N.; Mondal, S.; Krishnamoorthy, P.; Pal, D.; Mor, A.; Bhat, S.K.; Pattanaik, A. Boron influences immune and antioxidant responses by modulating hepatic superoxide dismutase activity under calcium deficit abiotic stress in Wistar rats. J. Trace Elem. Med. Biol. 2016, 36, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Canturk, Z.; Tunali, Y.; Korkmaz, S.; Gulbaş, Z. Cytotoxic and apoptotic effects of boron compounds on leukemia cell line. Cytotechnology 2016, 68, 87–93. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Dong, S.; Gong, C.; Chen, X.; Du, H.; Zhan, Y.; Ye, C. Identification of Breast-Cancer-Associated Properties of Drinking Water under a Composite-Toxicity Perspective of Mixed Contaminants: A Case Study in a High-Prevalence Area of China. Water 2024, 16, 702. https://doi.org/10.3390/w16050702
Peng S, Dong S, Gong C, Chen X, Du H, Zhan Y, Ye C. Identification of Breast-Cancer-Associated Properties of Drinking Water under a Composite-Toxicity Perspective of Mixed Contaminants: A Case Study in a High-Prevalence Area of China. Water. 2024; 16(5):702. https://doi.org/10.3390/w16050702
Chicago/Turabian StylePeng, Shuhan, Shengkun Dong, Chang Gong, Xiaohong Chen, Hongyu Du, Yuehao Zhan, and Changxin Ye. 2024. "Identification of Breast-Cancer-Associated Properties of Drinking Water under a Composite-Toxicity Perspective of Mixed Contaminants: A Case Study in a High-Prevalence Area of China" Water 16, no. 5: 702. https://doi.org/10.3390/w16050702
APA StylePeng, S., Dong, S., Gong, C., Chen, X., Du, H., Zhan, Y., & Ye, C. (2024). Identification of Breast-Cancer-Associated Properties of Drinking Water under a Composite-Toxicity Perspective of Mixed Contaminants: A Case Study in a High-Prevalence Area of China. Water, 16(5), 702. https://doi.org/10.3390/w16050702







