Research on CeO2 Activated Carbon Electrode Capacitance Method for Sulfate Removal from Mine Water
Abstract
1. Introduction
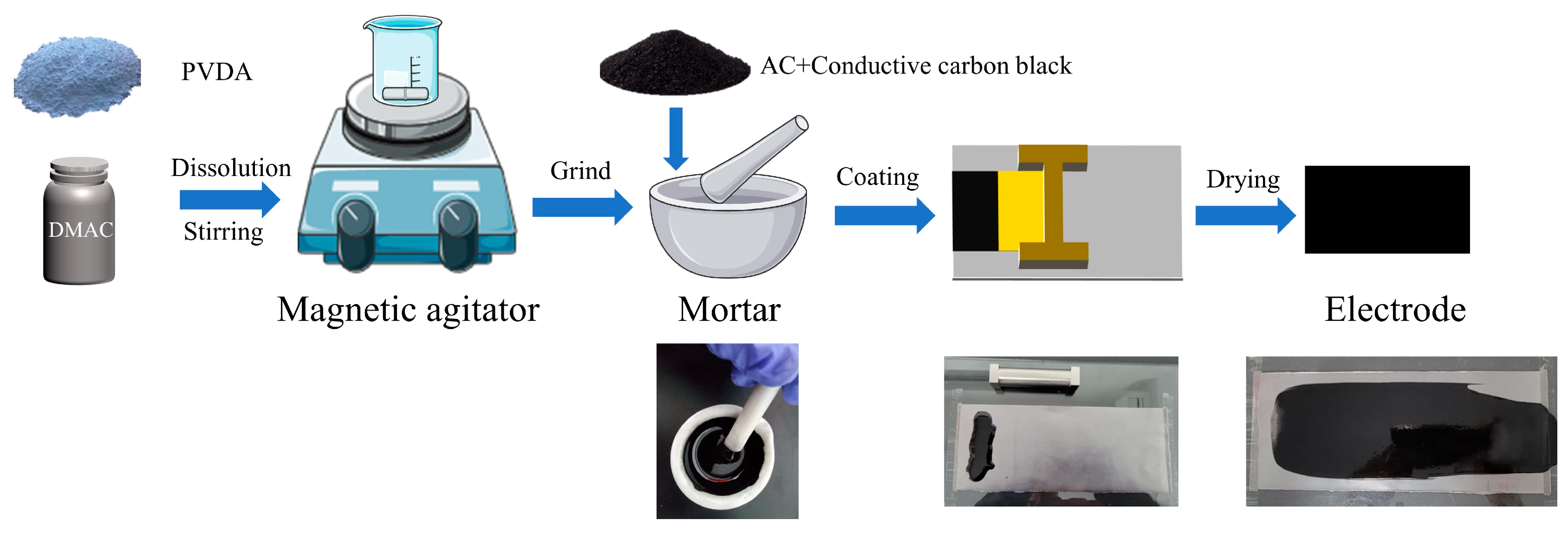
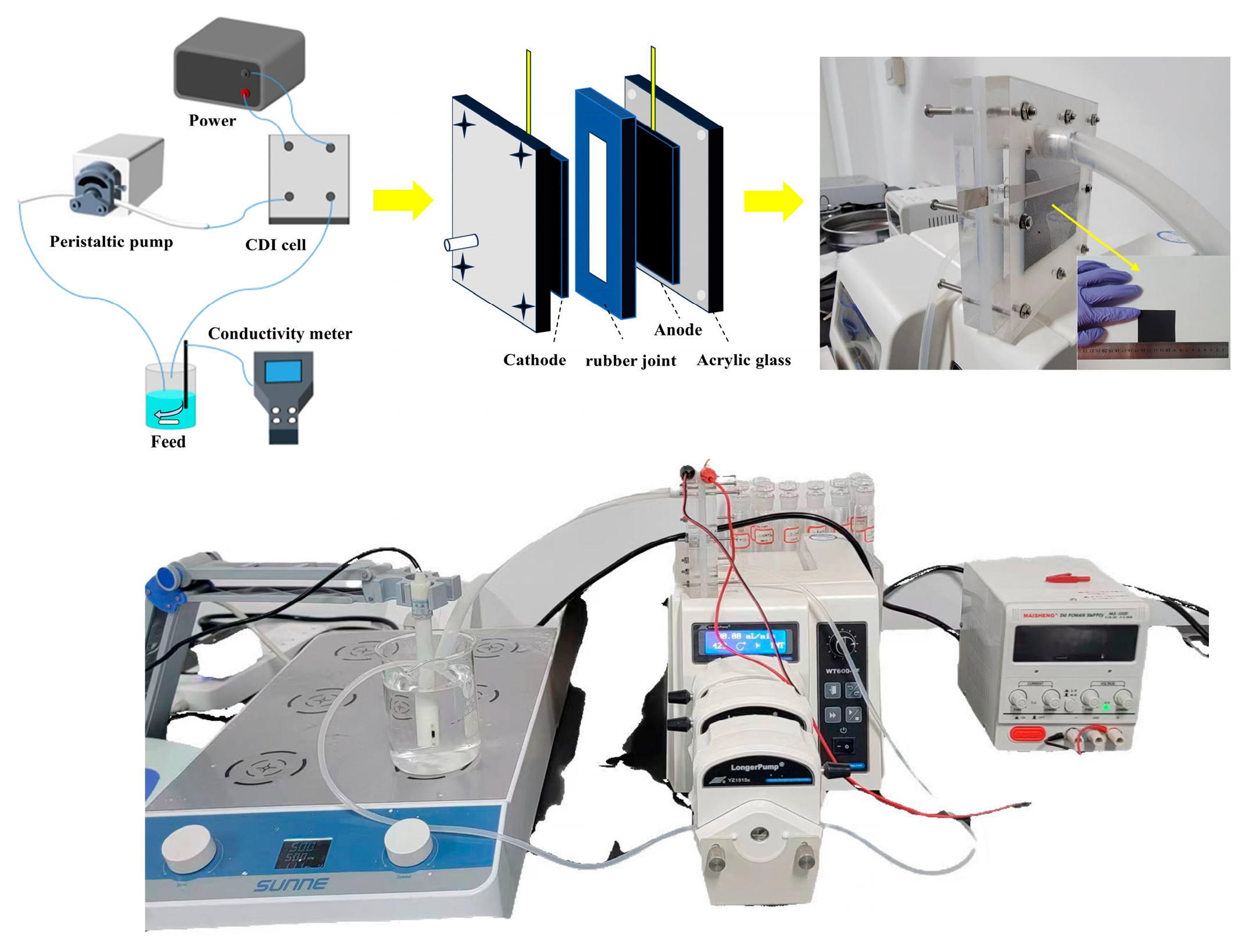
2. Materials and Methods
3. Results and Discussion
3.1. Analysis of CeO2 Activated Carbon Materials’ Properties
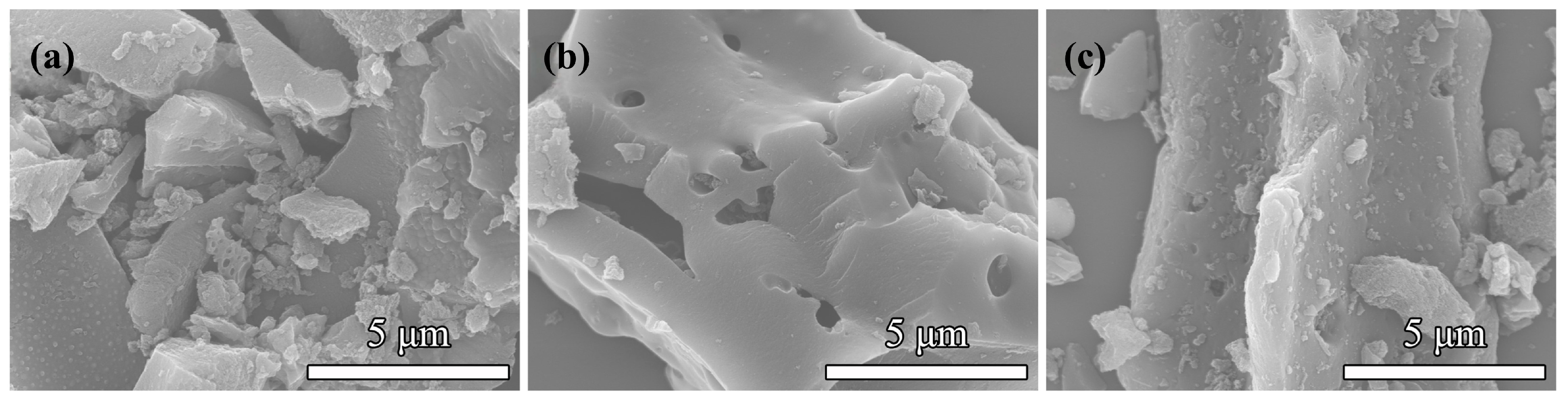
3.1.1. Microstructure and Phase Composition of CeO2 Activated Carbon Materials
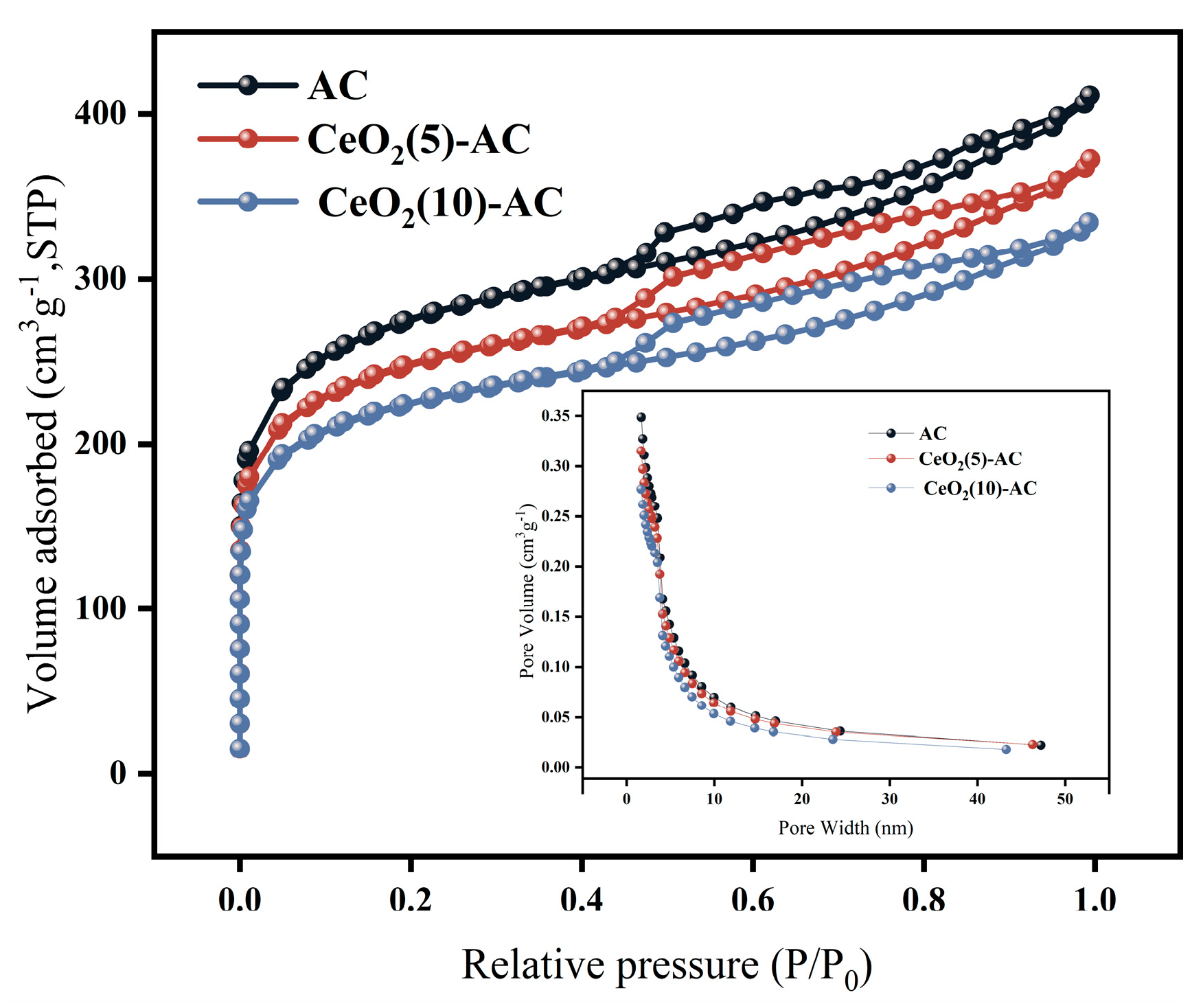
3.1.2. Specific Surface Area of the CeO2 Activated Carbon Material
3.1.3. Wettability of the CeO2 Activated Carbon Electrode Material
3.2. Analysis of CeO2 Activated Carbon Materials’ Properties
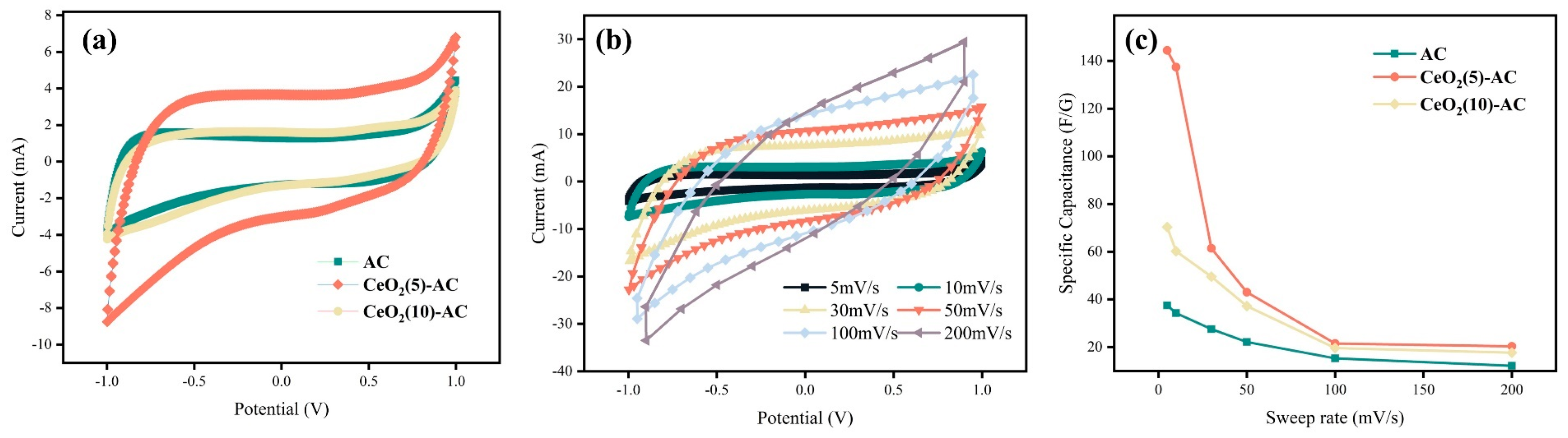
3.2.1. Cyclic Voltammetry Curve Characteristic Analysis
3.2.2. Electrochemical Impedance Spectroscopy
3.3. Effect of the CeO2 Mass Ratio on the Electro-Adsorption Properties of SO42−
3.4. Effects of Different CDI Process Parameters on the Electro-Adsorption Properties of SO42−
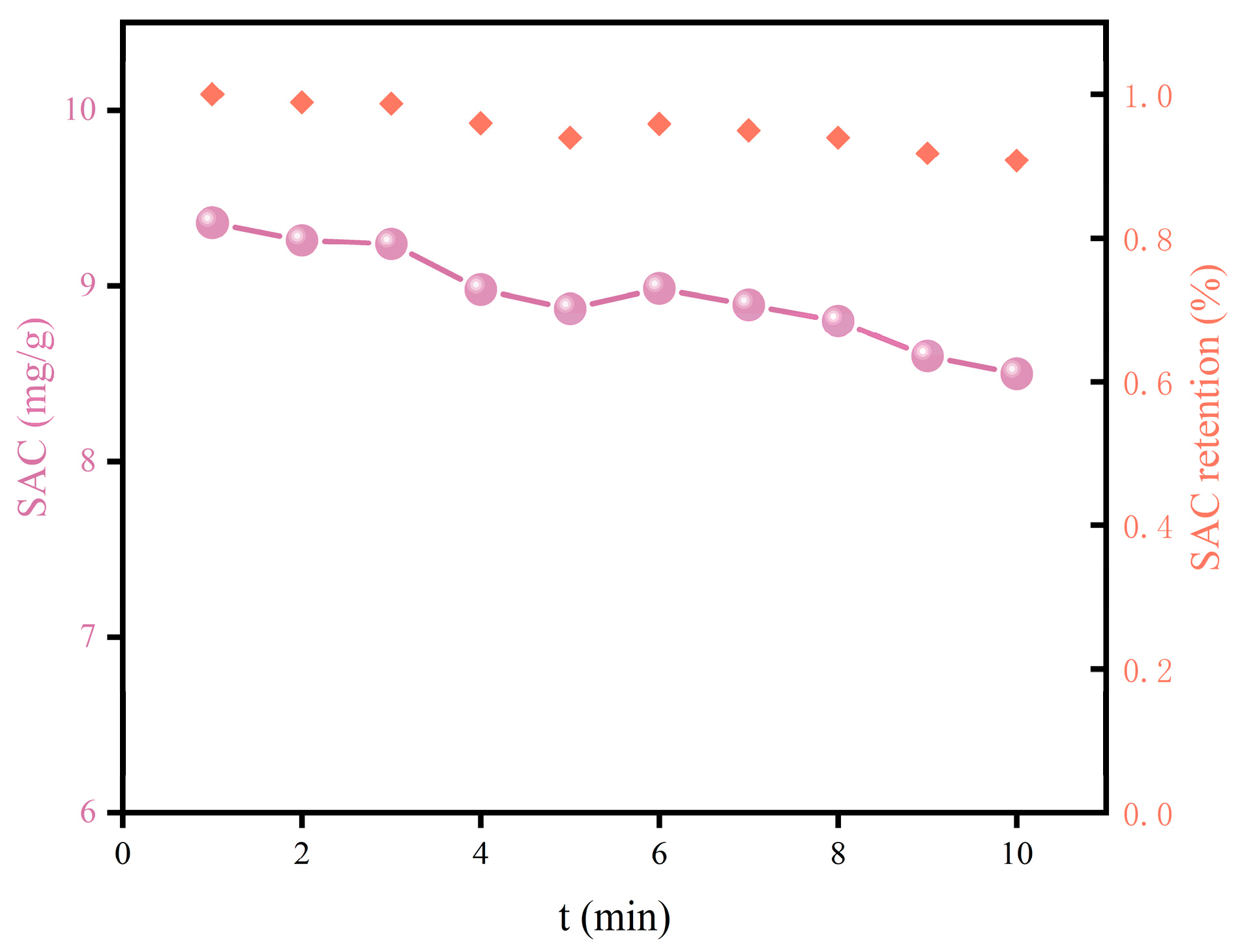
3.5. Cyclic Performance
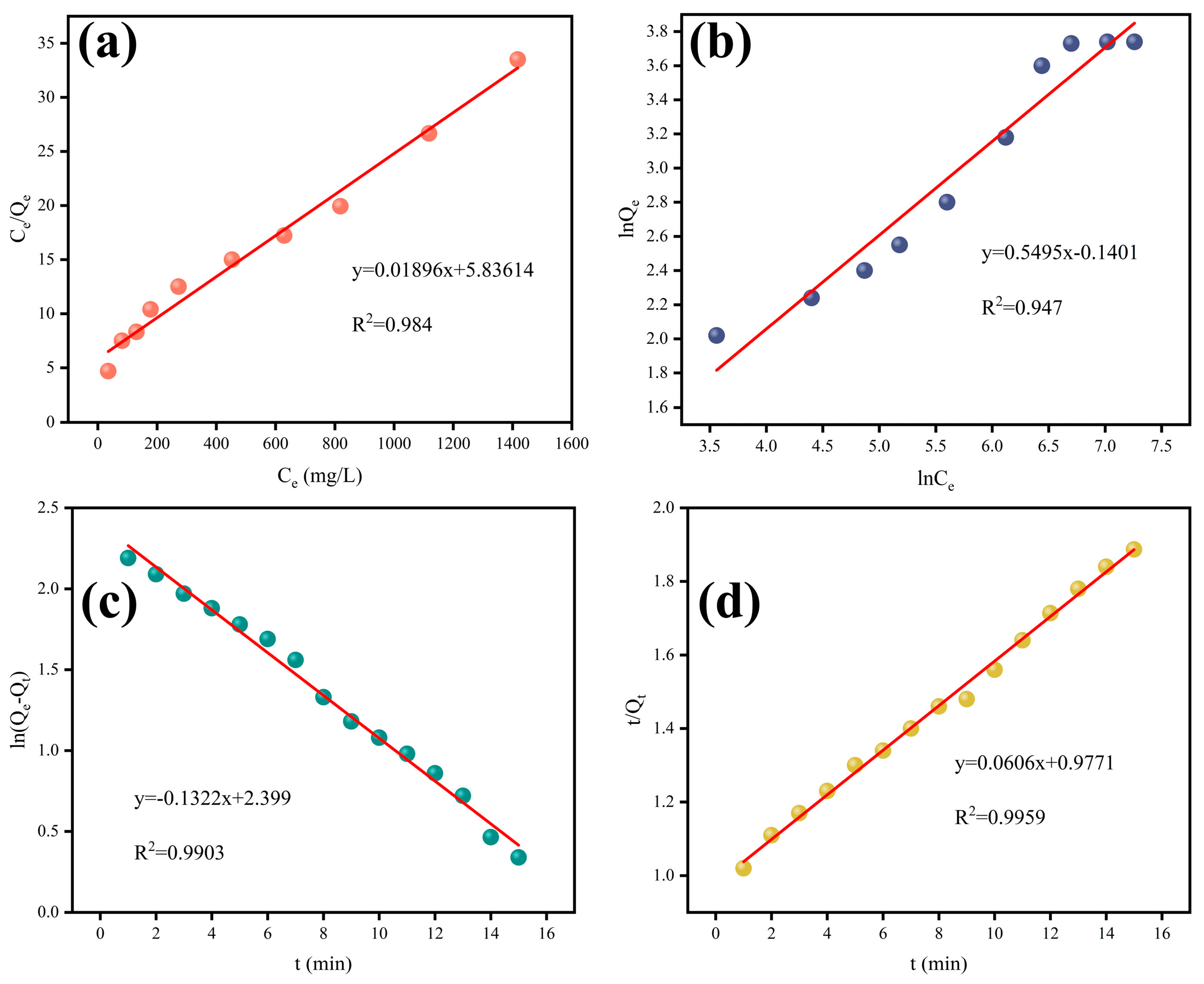
3.6. Electrosorption Isotherm and Kinetic Studies
3.7. Mine Water Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oncel, M.S.; Muhcu, A.; Demirbas, E.; Kobya, M. A comparative study of chemical precipitation and electrocoagulation for treatment of coal acid drainage wastewater. J. Environ. Chem. Eng. 2013, 1, 989–995. [Google Scholar] [CrossRef]
- Zuo, K.C.; Kim, J.; Jain, A.; Wang, T.X.; Verduzco, R.; Long, M.C.; Li, Q.L. Novel Composite Electrodes for Selective Removal of Sulfate by the Capacitive Deionization Process. Environ. Sci. Technol. 2018, 52, 9486–9494. [Google Scholar] [CrossRef]
- Zhao, F.; Rahunen, N.; Varcoe, J.R.; Chandra, A.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C.T. Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ. Sci. Technol. 2008, 42, 4971–4976. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhou, Q.; Huang, J.; Vymazal, J.; Kuschk, P. Sulfate removal and sulfur transformation in constructed wetlands: The roles of filling material and plant biomass. Water Res. 2016, 102, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Leão, V.A. Batch and fixed-bed assessment of sulphate removal by the weak base ion exchange resin Amberlyst A21. J. Hazard. Mater. 2014, 280, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Cravotta Iii, C.A.; Trahan, M.K. Limestone drains to increase pH and remove dissolved metals from acidic mine drainage. Appl. Geochem. 1999, 14, 581–606. [Google Scholar] [CrossRef]
- Tang, W.W.; He, D.; Zhang, C.Y.; Waite, T.D. Optimization of sulfate removal from brackish water by membrane capacitive deionization (MCDI). Water Res. 2017, 121, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Nurmesniemi, E.T.; Hu, T.; Rajaniemi, K.; Lassi, U. Sulphate removal from mine water by precipitation as ettringite by newly developed electrochemical aluminium dosing method. Desalination Water Treat. 2021, 217, 195–202. [Google Scholar] [CrossRef]
- Zhu, M.; Yin, X.; Chen, W.; Yi, Z.; Tian, H. Removal of sulphate from mine waters by electrocoagulation/rice straw activated carbon adsorption coupling in a batch system: Optimization of process via response surface methodology. J. Water Reuse Desalination 2019, 9, 163–172. [Google Scholar] [CrossRef]
- Oladunni, J.; Zain, J.H.; Hai, A.; Banat, F.; Bharath, G.; Alhseinat, E. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 2018, 207, 291–320. [Google Scholar] [CrossRef]
- Chu, M.; Tian, W.; Zhao, J.; Zhang, D.; Zou, M.; Lu, Z.; Jiang, J. Dual-activated biochar with a multichannel structure enhanced electrosorption capacity of capacitive deionization for sulfate removal from mining wastewater. Desalination 2023, 556, 116588. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Tang, W.W.; Kovalsky, P.; Cao, B.C.; He, D.; Waite, T.D. Fluoride Removal from Brackish Groundwaters by Constant Current Capacitive Deionization (CDI). Environ. Sci. Technol. 2016, 50, 10570–10579. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Porada, S.; Bryjak, M.; van der Wal, A.; Biesheuvel, P.M. Effect of electrode thickness variation on operation of capacitive deionization. Electrochim. Acta 2012, 75, 148–156. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, H.T.; Wu, C.X.; Wang, Y.S.; Li, W. A study of electrosorption selectivity of anions by activated carbon electrodes in capacitive deionization. Desalination 2015, 369, 46–50. [Google Scholar] [CrossRef]
- Dai, K.; Shi, L.; Fang, J.; Zhang, D.; Yu, B. NaCl adsorption in multi-walled carbon nanotubes. Mater. Lett. 2005, 59, 1989–1992. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.Y.; Zhang, L. A comparative study on electrosorption behavior of carbon nanotubes electrodes fabricated via different methods. Chem. Phys. Lett. 2016, 649, 15–18. [Google Scholar] [CrossRef]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization with carbon aerogel electrodes: Carbonate, sulfate, and phosphate. Int. SAMPE Tech. Conf. 1995, 27, 294–304. [Google Scholar]
- Kohli, D.K.; Bhartiya, S.; Singh, A.; Singh, R.; Singh, M.K.; Gupta, P.K. Capacitive deionization of ground water using carbon aerogel based electrodes. Desalination Water Treat. 2016, 57, 26871–26879. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, H.Y.; Moon, J.K.; Won, H.J.; Shul, Y.G. Electrosorption of uranium ions on activated carbon fibers. J. Radioanal. Nucl. Chem. 2011, 287, 833–839. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, C.; Li, Y.; Cheng, G.; Liu, F. Removal of nitrate from drinking water by ACF electrosorption. Chin. J. Environ. Eng. 2016, 10, 3510–3514. [Google Scholar] [CrossRef]
- Li, H.B.; Lu, T.; Pan, L.K.; Zhang, Y.P.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773–6779. [Google Scholar] [CrossRef]
- Liu, P.Y.; Yan, T.T.; Shi, L.Y.; Park, H.S.; Chen, X.C.; Zhao, Z.G.; Zhang, D.S. Graphene-based materials for capacitive deionization. J. Mater. Chem. A 2017, 5, 13907–13943. [Google Scholar] [CrossRef]
- Cao, Z.; Hu, S.; Yu, J.; Wang, L.; Yang, Q.; Song, H.; Zhang, S. Enhanced capacitive deionization of toxic metal ions using nanoporous walnut shell-derived carbon. J. Environ. Chem. Eng. 2022, 10, 108245. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. A short review of activated carbon assisted electrosorption process: An overview, current stage and future prospects. J. Hazard. Mater. 2009, 170, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.S.; Obaid, M.; Mohamed, I.M.A.; Yousef, A.; Barakat, N.A.M. ZrO2 nanofibers/activated carbon composite as a novel and effective electrode material for the enhancement of capacitive deionization performance. RSC Adv. 2017, 7, 4616–4626. [Google Scholar] [CrossRef]
- Dou, C.; Zhai, S.Y.; Liu, Y.Y.; Chen, P.; Yin, D.; Huang, G.T.; Zhang, L.H. Chemical modification of carbon particles to enhance the electrosorption of capacitive deionization process. J. Water Reuse Desalination 2020, 10, 57–69. [Google Scholar] [CrossRef]
- Zouli, N. Ceria-Incorporated Activated Carbon Composite as Electrode Material for Capacitive Deionization. Int. J. Electrochem. Sci. 2022, 17, 22042. [Google Scholar] [CrossRef]
- You, S.K.; Yang, J.J.; Ahn, S.; Park, S.G. Synthesis of a spherical carbon-TiO2 composite as electrode material for capacitive deionization. Int. J. Electrochem. Sci. 2019, 14, 4683–4692. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.A.; Zhou, K.F.; Wu, P.C.; Hou, C.H. Enhanced desalination performance via mixed capacitive-Faradaic ion storage using RuO2-activated carbon composite electrodes. Electrochim. Acta 2019, 295, 769–777. [Google Scholar] [CrossRef]
- Fang, J.; Cao, Z.; Zhang, D.; Shen, X.; Ding, W.; Shi, L. Preparation and CO conversion activity of ceria nanotubes by carbon nanotubes templating method. J. Rare Earths 2008, 26, 153–157. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.J.; Xie, B.R.; Li, Y.A.; Zhan, S.H.; Tian, Y. Synergistic degradation of antimicrobial agent ciprofloxacin in water by using 3D CeO2/RGO composite as cathode in electro-Fenton system. J. Electroanal. Chem. 2017, 784, 6–12. [Google Scholar] [CrossRef]
- Yousef, A.; Al-Enizi, A.M.; Mohamed, I.M.A.; El-Halwany, M.M.; Ubaidullah, M.; Brooks, R.M. Synthesis and characterization of CeO2/rGO nanoflakes as electrode material for capacitive deionization technology. Ceram. Int. 2020, 46, 15034–15043. [Google Scholar] [CrossRef]
- Adinaveen, T.; Vijaya, J.J.; Kennedy, L.J. Comparative Study of Electrical Conductivity on Activated Carbons Prepared from Various Cellulose Materials. Arab. J. Sci. Eng. 2016, 41, 55–65. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, D.Z.; Zhang, Z.X.; Hu, Y.; Quan, H.Y. Mesopore dominated capacitive deionization of N-doped hierarchically porous carbon for water purification. Sep. Purif. Technol. 2022, 290, 120912. [Google Scholar] [CrossRef]
- Chao, L.M.; Liu, Z.Y.; Zhang, G.X.; Song, X.N.; Lei, X.D.; Noyong, M.; Simon, U.; Chang, Z.; Sun, X.M. Enhancement of capacitive deionization capacity of hierarchical porous carbon. J. Mater. Chem. A 2015, 3, 12730–12737. [Google Scholar] [CrossRef]
- Song, X.; Fang, D.Z.; Huo, S.L.; Li, K.X. 3D-ordered honeycomb-like nitrogen-doped micro-mesoporous carbon for brackish water desalination using capacitive deionization. Environ. Sci.-Nano 2021, 8, 2191–2203. [Google Scholar] [CrossRef]
- Li, H.B.; Liang, S.; Li, J.; He, L.J. The capacitive deionization behaviour of a carbon nanotube and reduced graphene oxide composite. J. Mater. Chem. A 2013, 1, 6335–6341. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 2002, 40, 667–674. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, S.; Mao, H.; Zhou, H.; Wang, G.; Zhang, H.; Zhao, H. Pseudocapacitive desalination via valence engineering with spindle-like manganese oxide/carbon composites. Nano Res. 2021, 14, 4878–4884. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, D.; Ma, J.X.; Tang, W.W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)—Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Yan, T.T.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Trace-Fe-Enhanced Capacitive Deionization of Saline Water by Boosting Electron Transfer of Electro-Adsorption Sites. Environ. Sci. Technol. 2020, 54, 8411–8419. [Google Scholar] [CrossRef] [PubMed]
- Jande, Y.A.C.; Kim, W.S. Modeling the capacitive deionization batch mode operation for desalination. J. Ind. Eng. Chem. 2014, 20, 3356–3360. [Google Scholar] [CrossRef]
- Li, D.; Ning, X.A.; Yang, C.; Chen, X.; Wang, Y. Rich heteroatom doping magnetic carbon electrode for flow-capacitive deionization with enhanced salt removal ability. Desalination 2020, 482, 114374. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zhang, C.Y.; Bai, L.M.; Xie, B.H.; Gan, Z.D.; Xing, J.J.; Li, G.B.; Liang, H. Scaling behavior of iron in capacitive deionization (CDI) system. Water Res. 2020, 171, 115370. [Google Scholar] [CrossRef]






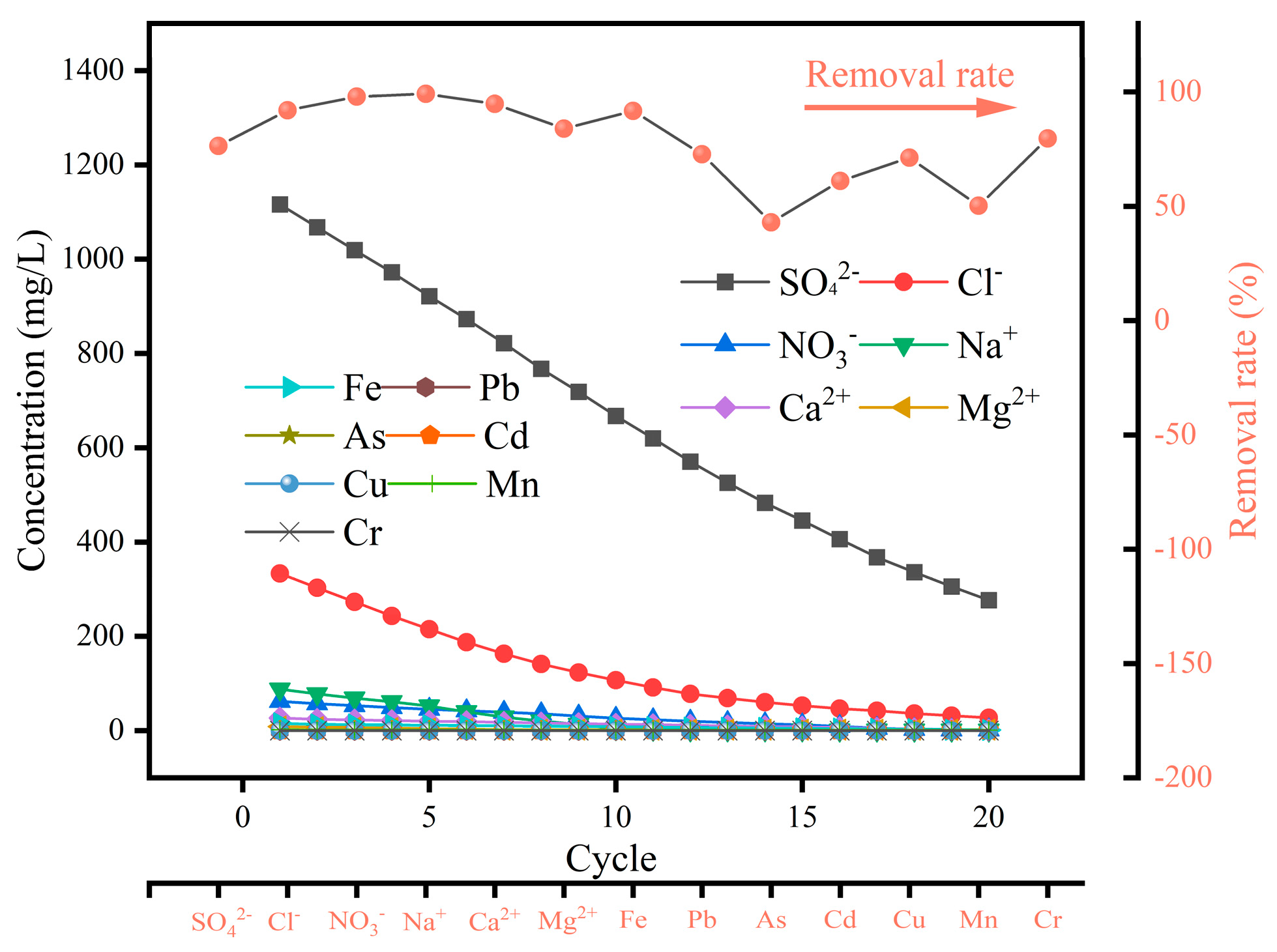
| Salt ions and heavy metals | SO42− | Cl− | NO3− | Na+ | Ca2+ | Mg2+ | Fe | Pb | As | Cd | Cr | Cu | Mn |
| Concentration (mg/L) | 1170 | 342 | 67 | 102 | 27.13 | 10.54 | 16.47 | 0.14 | 0.0512 | 0.0126 | 0.016 | 0.024 | 1.93 |
| Samples | SBET (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vtot (m3 g−1) | Vmicro (m3 g−1) | Vmeso (m3 g−1) | Vmeso/Vtot (%) | Mean Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|---|
| AC | 997.94 | 632.16 | 365.78 | 0.606 | 0.304 | 0.302 | 49.8 | 4.043 |
| CeO2(5)-AC | 898.11 | 510.24 | 387.87 | 0.547 | 0.211 | 0.336 | 61.4 | 4.098 |
| CeO2(10)-AC | 813.71 | 479.43 | 334.28 | 0.494 | 0.207 | 0.287 | 58.1 | 4.094 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| KL | Qm | R2 | n | KF | R2 |
| 0.0032 | 52.74 | 0.984 | 1.82 | 0.869 | 0.947 |
| Pseudo first-order | Pseudo second-order | ||||
| K1 | Qe | R2 | K2 | Qe | R2 |
| 0.1322 | 11.01 | 0.9903 | 0.0037 | 16.5 | 0.9959 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Zou, Y.; Condé, S.M.; Wang, X.; Dong, C. Research on CeO2 Activated Carbon Electrode Capacitance Method for Sulfate Removal from Mine Water. Water 2024, 16, 675. https://doi.org/10.3390/w16050675
Feng X, Zou Y, Condé SM, Wang X, Dong C. Research on CeO2 Activated Carbon Electrode Capacitance Method for Sulfate Removal from Mine Water. Water. 2024; 16(5):675. https://doi.org/10.3390/w16050675
Chicago/Turabian StyleFeng, Xiujuan, Yanjun Zou, Sékou Mohamed Condé, Xiaoqing Wang, and Chengliang Dong. 2024. "Research on CeO2 Activated Carbon Electrode Capacitance Method for Sulfate Removal from Mine Water" Water 16, no. 5: 675. https://doi.org/10.3390/w16050675
APA StyleFeng, X., Zou, Y., Condé, S. M., Wang, X., & Dong, C. (2024). Research on CeO2 Activated Carbon Electrode Capacitance Method for Sulfate Removal from Mine Water. Water, 16(5), 675. https://doi.org/10.3390/w16050675






