1. Introduction
Green microalgae are photoautotrophic, unicellular organisms that are found in water and soil [
1,
2]. Lately, green algae have received significant consideration from the scientific community, not only because of their vital role in influencing nutrient cycles, supporting biodiversity, sustaining life, and contributing to the overall functioning and ecological balance of ecosystems, but also for their great potential in commercial applications in the food industry, cosmetology, pharmacology, or nanomaterials production [
3,
4,
5]. As photosynthetic organisms, they convert sunlight and carbon dioxide to organic matter via photosynthesis, supplying energy and nutrients to numerous other organisms since they constitute the base of many aquatic food chains. This process contributes to the regulation of greenhouse gas levels and the mitigation of the effects of climate change [
6]. Green microalgae are responsible for a substantial amount of oxygen production on the planet, which is vital for the survival of aquatic life and for overall atmospheric balance.
Nanoparticles (NPs) have practically been part of the natural environment, in soil, waters, and the atmosphere [
7]. Despite this, the drastic increase in production and applications of engineered nanomaterials over the last two decades has raised concerns about the potential for increasing quantities ending up in natural bodies and their impact on ecosystems [
8].
Zinc oxide (ZnO) NPs are one of the most commonly utilized nanoparticles in the industry. ZnO NPs find extensive applications across various sectors due to their multifunctionality. They exhibit UV protection properties, making them suitable for sunscreens and cosmetic products [
9]. They also manifest antimicrobial properties and are thus used in coatings for surfaces in healthcare settings, textiles, and even food packaging [
10]. Zinc oxide NPs can also serve as catalysts for different chemical reactions due to their high surface area and reactivity. They can also be used in optoelectronic devices like solar cells, LEDs (light-emitting diodes), and sensors due to their semiconducting properties [
11].
NP characteristics, such as size, shape, and surface, not only provide them with unique properties for diverse industrial applications but have also been found to influence their environmental fate and impact on living organisms [
12]. External environmental factors like ionic strength, pH, and temperature can modify the physicochemical properties of NPs and therefore impact their interaction with biota [
11]. NPs usually end up in natural water bodies either directly, through intentional or unintentional release, or via inefficient treatment and capture in wastewater treatment plants [
13].
Various research studies have demonstrated the toxic effect of various types of nanoparticles like ZnO and TiO
2 on microalgae [
14,
15]. NPs can cause damage to algal cells through different mechanisms, including protein binding, mitochondrial damage, adhesion, or shading by blocking sunlight from reaching the cells [
16,
17]. They can also generate reactive oxygen species (ROS) that are responsible for causing oxidative stress to cells [
18].
In this study, focus was put on
Scenedesmus rubescens, a green microalga that is globally present in freshwater and wastewater [
19] and has been used in novel industrial applications like biodiesel production [
20].
S. rubescens is used as a model species for toxicity since it is found to be resistant to toxic effects induced by the presence of NPs and to exhibit minimal or even no growth inhibition [
15]. Other studies have also demonstrated an important or partial resistance of microalgal species of the Scenedesmus genus to different types of pollutants, like pesticides [
21,
22].
Nutrients play a crucial role in algal cellular metabolism. Nitrogen is a fundamental component of amino acids, which are the building blocks of proteins. Algal cells require nitrogen to synthesize proteins, which are crucial for their growth, structural integrity, and various physiological processes [
23]. Moreover, nitrogen is a constituent of chlorophyll, the pigment responsible for photosynthesis. Chlorophyll is the main pigment in green microalgae that confers their characteristic color. Finally, nitrogen is involved in various metabolic pathways within cells, including the synthesis of essential compounds like vitamins, hormones, and secondary metabolites crucial for cell function and adaptation to environmental changes [
24]. Nitrates are the most important source of inorganic nitrogen in aquatic environments, along with nitrites and ammonium [
25].
In our previous work, the nitrate content of the growing medium was reported to impact the toxicity of ZnO NPs, upon
Chlorococcum sp. growth [
26]. The scope of this study was to assess the short-term response of
S. rubescens microalga to ZnO NPs, a freshwater genus that has been found to be more robust compared to
Chlorococcum sp. [
15]. Moreover,
S. rubescens was cultured at different initial nitrate concentrations to assess the combined effects of nutrient availability and ZnO NP toxicity on algal growth. Therefore, our results could give insight into the behavior of
S. rubescens under simultaneous exposure to two potential stress factors, nitrates and ZnO nanoparticles.
2. Materials and Methods
2.1. Microalgae and Culture Medium
Scenedesmus rubescens (SAG 5.95) freshwater microalga was obtained from the Culture Collection of Algae at the University of Göttingen (SAG), Göttingen, Germany.
BG-11 (BlueGreen-11) was chosen as the culture medium for this study, and its composition is described thoroughly in our previous work [
26]. In order to investigate the effect of NO
3− on microalgae exposed to nanoparticles, four different concentrations of NO
3− were tested, namely 0, 70, 200, and 300 mg NO
3−/L, resulting in four different compositions of the culture medium.
2.2. Nanoparticles
ZnO nanopowder was acquired from Sigma-Aldrich, St. Louis, MO, USA (catalog number 544906) containing ZnO nanoparticles with a nominal particle size less than 100 nm, as provided by the manufacturer. A total of two separate stock solutions were prepared, one at a concentration of 8.1 g ZnO NPs/L and the other at 81 mg ZnO NPs/L, both in deionized water. Prior to each application, ZnO NPs underwent 30 min of ultrasonication using an ultrasound bath (Transsonic TI-H-5, Elma Hans Schmidbauer GmbH & Co., Ltd., KG, Singen, Germany) to disperse possible large agglomerates.
2.3. ZnO NP Exposure and Microalgal Growth
The response of
Scenedesmus rubescens to ZnO NPs was assessed based on OECD toxicity guideline number 201 [
27]. Before each experiment, an
S. rubescens preculture was prepared as follows. Four 2 L solutions of BG-11 medium were prepared, each containing one of the four different nitrate concentrations. The BG-11 medium was sterilized using an autoclave (121 °C, sterilization time 20 min) and inoculated with an algal cell concentration from the stock solution.
The following parameters were applied to the preculture. Continuous stirring was provided with a magnetic stirrer, and constant aeration was delivered through an air pump (model type HP-400, Sunsun, Zhejiang, China, flow rate of air 3 L/min). The supplied air was filtered using a Nylon syringe filter with a nominal pore diameter of 0.22 µm. Finally, irradiation was constant by means of artificial lighting, with an intensity of 100 μmol m−2 s−1).
All experiments were conducted using 250 mL Erlenmeyer flasks. Precultures were allowed to proliferate until they reached their logarithmic growth phase. After that, a quantity of
S. rubescens cells were withdrawn and directly added to the Erlenmeyer flasks, which contained sterile medium with the same nitrate concentration that was used in the preculture. The starting cell concentration in all test cultures was 10
4 cells/mL and the culture volume was 100 mL [
27]. Additionally, varying amounts of ZnO nanoparticles from the two stock solutions were introduced in all test cultures except the control culture, resulting in four different starting concentrations of ZnO NPs, specifically 0.081, 0.81, 8.1, and 81 mg ZnO NPs/L. Moreover, foil was used in order to cover the flasks and prevent contamination of biological origin. Samples were kept static without stirring, continuously irradiated with a 100 μmol m
−2 s
−1 artificial lighting source similarly to the preculture, and placed inside a walk-in incubator room.
In summary, for every composition of the BG-11 medium, five distinct samples were prepared: a control culture only containing S. rubescens cells (no ZnO NPs), and four samples with one of the four aforementioned ZnO NP concentrations. Triplicates were used for each sample for error minimization.
2.4. Analytical Methods
All experiments were run for 96 h, while sampling took place every 24 h starting from 0 to 96 h. Four different parameters were examined to evaluate microalgal growth and nitrate consumption: cell number, NO3− content, UV-Vis-NIR absorption, and pH. All the flasks were vigorously stirred prior to each sampling.
Cell concentration was quantified in triplicate via optical microscopy (model DMLB, Leica Microsystems GmbH, Wetzlar, Germany). More precisely, sample preparation consisted of mixing a 1 mL volume with 100 µL of Lugol’s iodine solution within a Neubauer hemocytometer (0.1 mm, 0.0025 mm2, Optic Labor, Görlitz, Germany). Lugol is commonly used for its capacity to facilitate the microscopic observation of cells by immobilizing them. Cell concentration stood as the primary indicator for biomass determination due to its high accuracy.
The nitrate content in the solution was determined using ion chromatography (Metrohm 850 Professional IC, Metrohm, Herisau, Switzerland). Filtration of samples took place prior to measurement using a syringe filter (0.22 µm), while dilution with deionized water was carried out when necessary.
UV-Vis-NIR absorption spectrum was obtained with a Specord 210 Plus spectrophotometer (Analytik Jena, Jena, Germany), where 3 mL of each sample was analyzed using a quartz cuvette. UV–Visible absorbance is a valuable indicator for biomass growth and ZnO NP presence and stability. pH was determined using a pH-meter (pH 300/310 waterproof Hand-held pH, Oakton Instruments, Singapore).
Finally, the interactions between the ZnO NPs and algal surfaces was evaluated by scanning electron microscopy (SEM) analysis (microscope model JEOL 6300, JEOL Ltd., Akishima, Japan) and light microscopy (model DMLB, Leica Microsystems GmbH, Wetzlar, Germany). All cultures were subjected to an elemental analysis after 96 h. Specifically, SEM samples were prepared as follows: 1 to 2 mL of algal solution was transferred on slides, dehydrated inside an oven, and then glued to SEM stubs with colloidal silver and sputter-coated with gold–palladium through a gold ion sputter coater (JEOL, JFC 1100 Fine Coat, JEOL Ltd., Akishima, Japan). The electronic beam of the SEM was set at 20 kV, and a minimum of three fields per sample were observed at various magnifications ranging from 500 to 14,000×.
2.5. Statistical Analysis
The following equation was used to calculate the specific growth rate (μ) from the growth phase:
where X
t is the number of cells at time t (days) and X
0 is the initial cell number at time t
0.
The homogeneity of variance was assessed using Levene’s test of equality of error variances, and the significance of differences between the parameters was tested by running a Kruskal–Wallis test (p < 0.05). Additionally, the Friedman test (p < 0.05) was used in order to evaluate significant alterations in the growth of all cultures and different concentrations of ZnO NPs, at 24, 48, 72, and 96 h. Statistical analysis was conducted using IBM SPSS Statistics 26.0 software.
3. Results and Discussion
The biomass concentration expressed in cells/mL for
S. rubescens cultivated in modified BG-11 medium and subjected to various concentrations of ZnO NPs can be seen in
Figure 1. Cell number results show that the highest biomass growth for
S. rubescens was obtained with the initial concentration of nitrates of 300 mg/L in the culture medium, followed by 70, 0, and 200 mg/L of NO
3−, respectively. The enhanced cell number observed at 300 mg/L of NO
3− implies that this concentration of nitrates is closer to the optimum one for
S. rubescens growth. Further studies are required to define the optimum nitrate concentration for the specific alga. In cultures containing nitrates at 0, 70, and 300 mg/L, a minor decrease in cell number occurs at 48 h of exposure for some of the samples. In previous studies by our team [
17] investigating the toxic effect of ZnO NPs on
Scenedesmus rubescens, an increasing tendency in the cell concentration was observed after 48 h. This might be attributed to the adaptation period of
S.
rubescens to new cultivation conditions. Despite the importance of nitrogen in microalgae proliferation and the fact that nitrates were the unique source of nitrogen in our experiments, nitrate availability was found to affect biomass growth in a non-linear way, as demonstrated by other studies as well [
15,
24,
26]. Extracellular as well as intracellular parameters could be the reason for this phenomenon. In fact, the complexity of the extracellular environment (culture medium composition, nanoparticles, irradiation) and the number of interactions between them, as well as the particularity of microalgal metabolism, do not allow us to conclude on the origins of this phenomenon. In order to better understand the mechanism behind this observation, more in-depth research is needed with a focus on cells’ metabolic processes. Moreover, even in the absence of nitrogen, algal cells were able to grow and achieve cell numbers up to 41.0 ± 9.5 × 10
4 cells/mL for microalgae exposed to 81 mg/L of ZnO NPs. It is possible that a limited quantity of nitrogen was made available in the test culture following the lysis of dead cells and the consequent release of organic and inorganic matter containing nitrogen. When in N-starvation, cells tend to adapt their metabolism in order to cope with this particular stressor. Some microalgae, like
Tetraselmis sp., are found to produce and accumulate certain types of macromolecules, especially carbohydrates [
23]. Moreover, the results of
S. rubescens biomass concentration demonstrate its resistance to ZnO NP exposure. Other studies [
15,
28] have also reported the resistance of
S. rubescens to NP exposure. It is possible that the morphological characteristics of this genus, such as its highly resistant cell walls [
19], could be among the parameters impacting its behavior. Many species of microalgae are resistant to nanoparticles, especially at low nanoparticle concentrations, and nanoparticles have even been used in order to trigger an increase in biomass concentration and lipid content [
29].
The homogeneity hypothesis of the variances between the different ZnO NP concentrations was tested using the Kruskal–Wallis test for each sapling time. The homogeneity was rejected for the 24 and 96 h of exposure to a nitrate concentration of 200 mg/L (Levene’s test p-values 0.01 and 0.02, respectively). Differences in distribution in cell concentration were also significant between the different ZnO NP concentrations according to the Kruskal–Wallis (KW) test for the 24 and 96 h of exposure time (KW test p-values < 0.001 in all four periods of exposure). Regarding the time evolution of cell concentration, the null hypothesis of equal media across time was rejected for all the different ZnO NP concentrations (Freidman test p-values < 0.001 for all concentrations).
The growth rates of algal cultures for all tested modified media and ZnO NP concentrations have been calculated and are presented in
Table 1. The results demonstrate that the growth rate in all experiments decreases over time, with higher values observed at 24 h after the beginning of each experiment, and a progressive decrease occurring after every 24 h interval. The highest growth rate was displayed by cultures with an initial nitrate concentration of 300 mg/L, followed by cultures at 70, 0, and 200 mg/L, in accordance with cell number results, reaching 4.03 ± 0.09, 3.31 ± 0.33, 2.94 ± 0.14, and 0.79 ± 0.01, respectively, over the first 24 h of the assay.
Overall, the total decrease in growth rate over the period of the experiments varied from 47 to 70% for 0, 70, and 300 mg/L of nitrates, with most cultures exhibiting an average decrease of 68% of their initial growth rate at the end of the toxicity assays. Microalgae cultured with 200 mg/L of NO3− showed minimal to no decrease in their initial growth rate over 96 h.
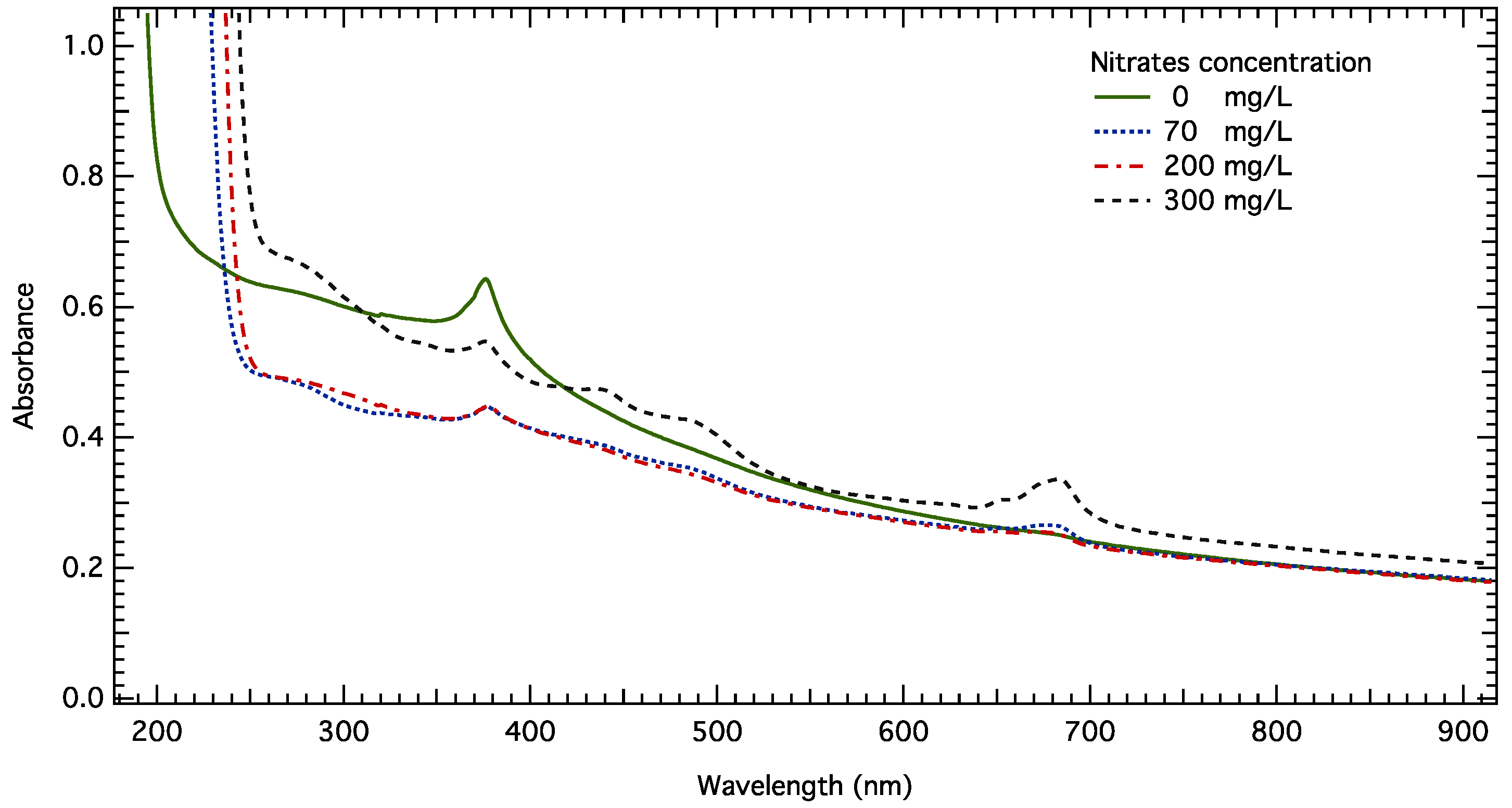
The UV-Vis-NIR spectra of
S. rubescens cultures after 96 h of contact at 81 mg/L under different nitrate concentrations are shown in
Figure 2. There are three areas of interest in the obtained spectra. First, a broad band with peaks at 435 nm and 485 nm is attributed to carotenoids. These peaks can be seen in the cultures containing 300 mg/L of NO
3−, manifesting the highest biomass concentration, and are also visible in the 70 mg/L NO
3− cultures, which had the second highest cell number. Carotenoids are secondary pigments that are usually present in smaller quantities than chlorophyll a. They are synthesized by cells to absorb light, a source of energy used in photosynthesis. They are also important antioxidants, thus protecting functional macromolecules inside the cell from oxidative stress [
30]. Different species including
S. rubescens have been found to increase their carotenoid content after exposure to different xenobiotics [
31]. Nevertheless, in our case, their appearance in UV-Vis spectra acts primarily as an indicator of algal growth, but a contribution due to nanoparticle-induced stress could not be excluded.
Secondly, a band peaking at about 680 nm, as mentioned in the literature as well [
32,
33], is used for biomass detection. This band increases over time, indicating biomass growth. Its intensity is primarily due to chlorophyll-a absorption and to biomass turbidity. UV-Vis spectra are in accordance with cell number values. Microalgae cultured at 300 mg/L of NO
3− exhibit the most intense and broad band due to their higher biomass concentration. It should be noted that for the sample exposed to 0 mg/L of NO
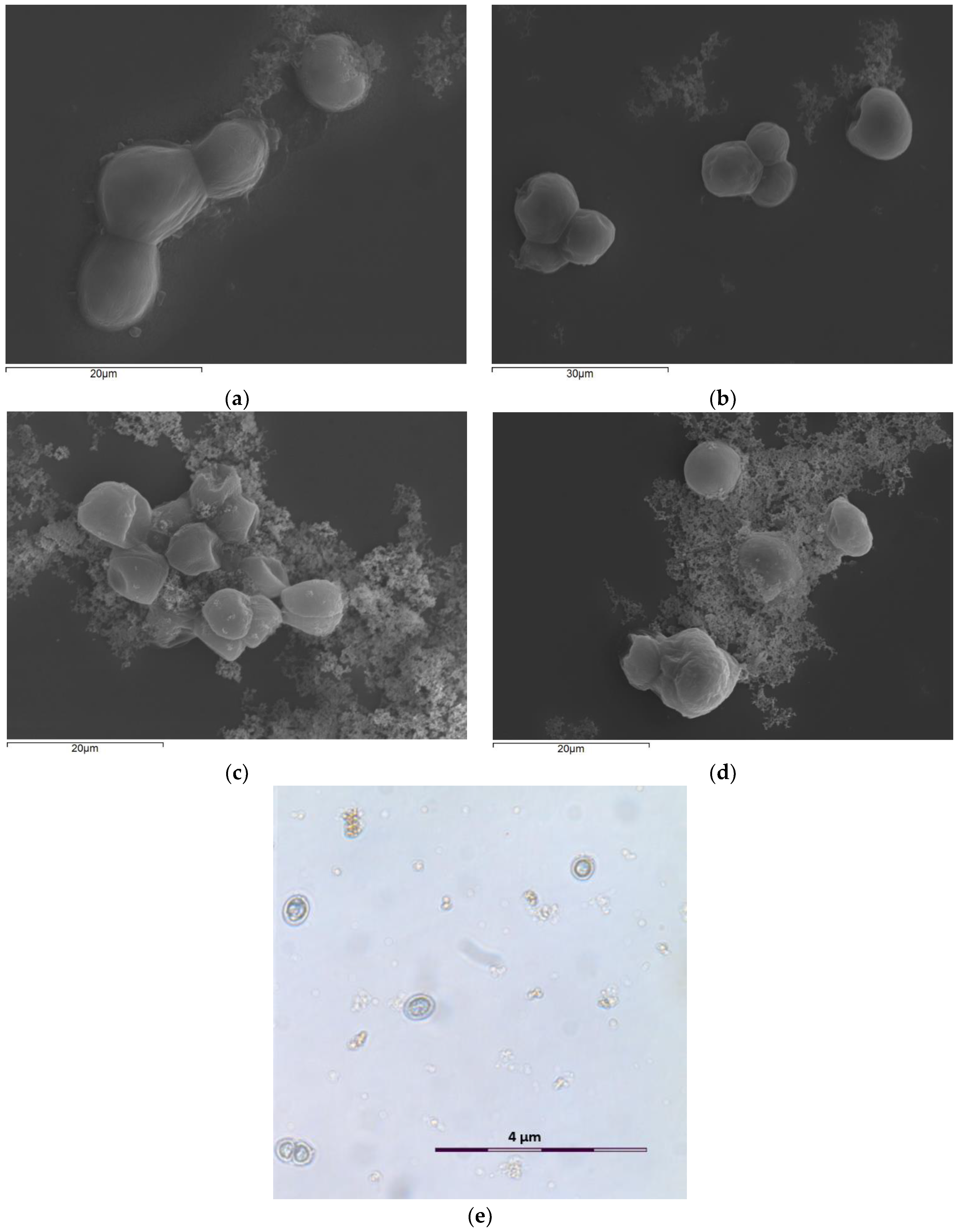
3−, no absorbance was observed even though cells grown in this particular medium exhibited growth comparable to cultures with an initial concentration of nitrates of 200 mg/L. As discussed previously, despite the lack of nitrogen in the cultivation medium, algal cells were able to adapt their metabolism to this particular constraint and proliferate, but were unable to synthesize sufficient quantities of chlorophyll, and thus no absorption occurred. This affirmation was also verified by optical microscopy where cells in all different ZnO NP concentrations as well as in the control sample (without NPs) all appeared blue rather than green. A micrograph of
S. rubescens cells exposed to 81 mg/L of ZnO NPs after 96 h can be seen in
Figure 3e. Moro et al. [
31] also observed blue cells of
S. rubescens exposed to ibuprofen, a commonly used pharmaceutical compound. These observations combined suggest that the decrease in chlorophyll content of the cells is not solely observed in N-deficient environments, but rather under stressful conditions capable of impacting the cell’s metabolism and functioning.
Finally, the presence of zinc nanoparticles is verified by the peak at around 370 nm. The intensity of the peak due to ZnO NPs decreased over time in all cultures This peak is associated with electron transitions of ZnO nanoparticles from the valence to the conduction band [
34,
35], and its decrease is due to NP dissolution. For a nitrate concentration of 0 mg/L, the absorbance band attributed to ZnO NPs appears more intense and with greater area. This is attributed to the fact that a higher ionic strength in the culture medium along with sodium ions cause extensive aggregation between NPs [
15]. In the N-deficient medium, sodium ions and the overall ionic strength are lower due to the absence of NaNO
3, thus explaining our results.
Scanning electron and optical micrographs (
Figure 3) verify the form of ZnO NP agglomerations and their deposition on algal surfaces only in the two higher ZnO NP concentrations (8.1 and 81 mg/L ZnO NPs). The existence of ZnO nanoparticles in the cultures results in the development of larger algal aggregates, as a protection mechanism for microalgae to NP exposure. Many studies [
15,
36] have reported that the shape and surface structures of microalgae induced various sorption processes that led to different toxicities of ZnO NPs on them. The latter was further brought out by data obtained after SEM analysis (X-ray diffractometer spectrum), which indicated increased levels of zinc ions on the algal surface, especially when ZnO NPs were present in high concentrations. Optical microscope photos showed similar results to SEM images, in which the micrograph of the control culture displayed smooth spherical cells with intact cell membranes. On the contrary, algal cells exposed to ZnO NPs clumped together and were surrounded by NP agglomerates.
Changes in pH values (
Figure 4) were observed in all cultures, and its values increased over time. More precisely, as it has already been observed in previous studies as well, algal growth has been found to increase the pH of the culture medium [
26]. This is due to the fact that cells consume diluted CO
2 during photosynthesis at a rate higher than that of the dissolution of gaseous carbon dioxide into the medium. Furthermore, microalgae have the ability to release metabolites known as extracellular polymeric substances (EPSs), which can also induce variations in the pH of the solution [
37]. Therefore, the increase in pH values verifies the proliferation of
S. rubescens cells. The increase in pH due to cell growth is visible in cultures containing 70, 200, and 300 mg/L of NO
3− (
Figure 4b–d). In the nitrogen-deficient culture (
Figure 4a), a different pattern is observed, probably due to the adjusted cell metabolism, where after an initial increase over the first 24 h, pH slightly decreases over time. Overall, all cultures showed signs of stabilization of their pH over the last days of the experiments, a result that correlates with the trend in growth rate values presented before.
After 96 h of exposure, the pH in all cultures ranged from 8 to 11 (
Figure 4). pH 11 is a critical value above which cells are not able to function properly; thus, when this value is reached, a stabilization is observed as a result of cells trying to regulate their extracellular pH via the release of EPI into the environment. pH variations have a certain correlation with cell number variations as well. In fact, in samples containing 300 mg/L of nitrates, a rapid increase in biomass can be observed in the pH, which reaches a value of 10.5 after only 24 h of exposure. On the other hand, at 70 and 200 mg/L of NO
3−, the increase in pH is slower, and pH 10 is reached after 48 and 72 h, respectively. Samples at 70 mg/L of NO
3− exhibit a higher cell number and growth rate; therefore, the increase in pH in the first days of the experiment is sharper compared to samples at 200 mg/L of NO
3−. In general, pH acts as a macroscopic indicator of cell growth but cannot be used for the precise microscopic determination of cell concentration. For a given nitrate content, cells are found to exhibit similar growth in all NP exposure concentrations, whereas the pH is found to slightly decrease with increasing ZnO NP concentration. This result is not contradictory, since pH translates the macroscopic conditions of the solution, one of which is the proliferation of microalgae. The presence of ZnO NPs induces a slight acidification of the solution, thus explaining the differences in pH values between samples in the same nitrate concentration.
The pH of the medium is found to affect the agglomeration of ZnO NPs. As has been reported by Yung et al. [
38], ZnO NPs dissolved better at pH 7 than at pH 8. The formation of NP agglomerates may reduce the toxic effects of ZnO NPs since fewer NPs are in contact with microalgae.
The variation in nitrate content in the medium was evaluated through ion chromatography, as seen in
Figure 5. Nitrate content reduction in modified BG-11 is associated with biomass growth. Exposure to ZnO NPs for the same medium composition tends to induce a slight decrease in nitrate consumption by cells in the case of a nitrate concentration of 70 mg/L. Specifically, nitrate removal over 96 h decreased from 74% in the control to 53% in the 81 mg/L ZnO NP culture. On the other hand, in the cultures containing 200 and 300 mg/L of nitrates, the increase in ZnO NP concentration resulted in a less important decrease in nitrate removal; 23 to 20% and 50 to 45%, respectively. ZnO NPs are known to be quite soluble and to release Zn
2+ ions in the solution [
15]. Increased quantities of these ions could result in perturbations and interference in nutrient transport towards the cell [
39]. NPs themselves could also participate in this procedure by blocking some active sites on the cell walls [
40]. A similar effect on nitrate uptake by
Scenedesmus quadricauda has been reported by Awasthi et al. [
39] due to the presence of increasing zinc concentrations. On the other hand, in our case, this result did not seem to have a direct impact on biomass growth.