Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Setting
2.2. Regional Geology and Hydrogeology
2.3. Groundwater Sampling and Field Measurement
2.4. Laboratory Analysis
2.5. Accuracy of Chemical Analysis
2.6. Geospatial Analysis
2.7. Groundwater Quality Index
- Assigning a weight for each groundwater quality parameter: Weights (wi) were assigned to various water quality parameters based on their relative importance to human consumption [34]. Nitrates and fluorides were given the highest weight of 5 due to the vital role they played in groundwater quality evaluation and their significant human health impacts [33,35]. Sodium and potassium were given the least weight because they are less significant in groundwater quality assessment. Table 1 shows the weights assigned to each groundwater quality parameter.
- Computation of relative weight: Equation (2) below was used to calculate the relative weight (Wi):where Wi is the relative weight; wi denotes the weights assigned for each parameter; n denotes the total number of quality parameters; and i is the ith parameter.
- Water quality rating scale: The rating scale (qi) for each parameter was computed by dividing the determined concentration of each parameter (ci) and its respective water quality standard (Si) recommended by World Health Organization [72], all multiplied by 100. The qi for all the parameters was computed using Equation (3) below:where qi denotes the quality rating; ci denotes the concentration of each groundwater quality parameter in mg/L; and Si is the WHO guideline value for each parameter.
- Sub-index and groundwater quality index computation: The sub-index (SIi) for each parameter and overall groundwater quality index (GWQI) were calculated using Equations (4) and (5):where SIi denotes the sub-index of the ith water quality parameter; qi denotes the quality rating of the ith parameter; n is the total number of water quality parameters; and GWQI is the overall groundwater quality index. The groundwater was classified based on portability in Table 2.
2.8. Hydrochemical Analysis
3. Results and Discussion
3.1. Hydrogen Ion Concentration (pH)
3.2. Total Dissolved Solids (TDS)
3.3. Total Hardness of Water (TH)
3.4. Calcium (Ca2+) and Magnesium (Mg2+)
3.5. Sodium (Na+) and Potassium (K+)
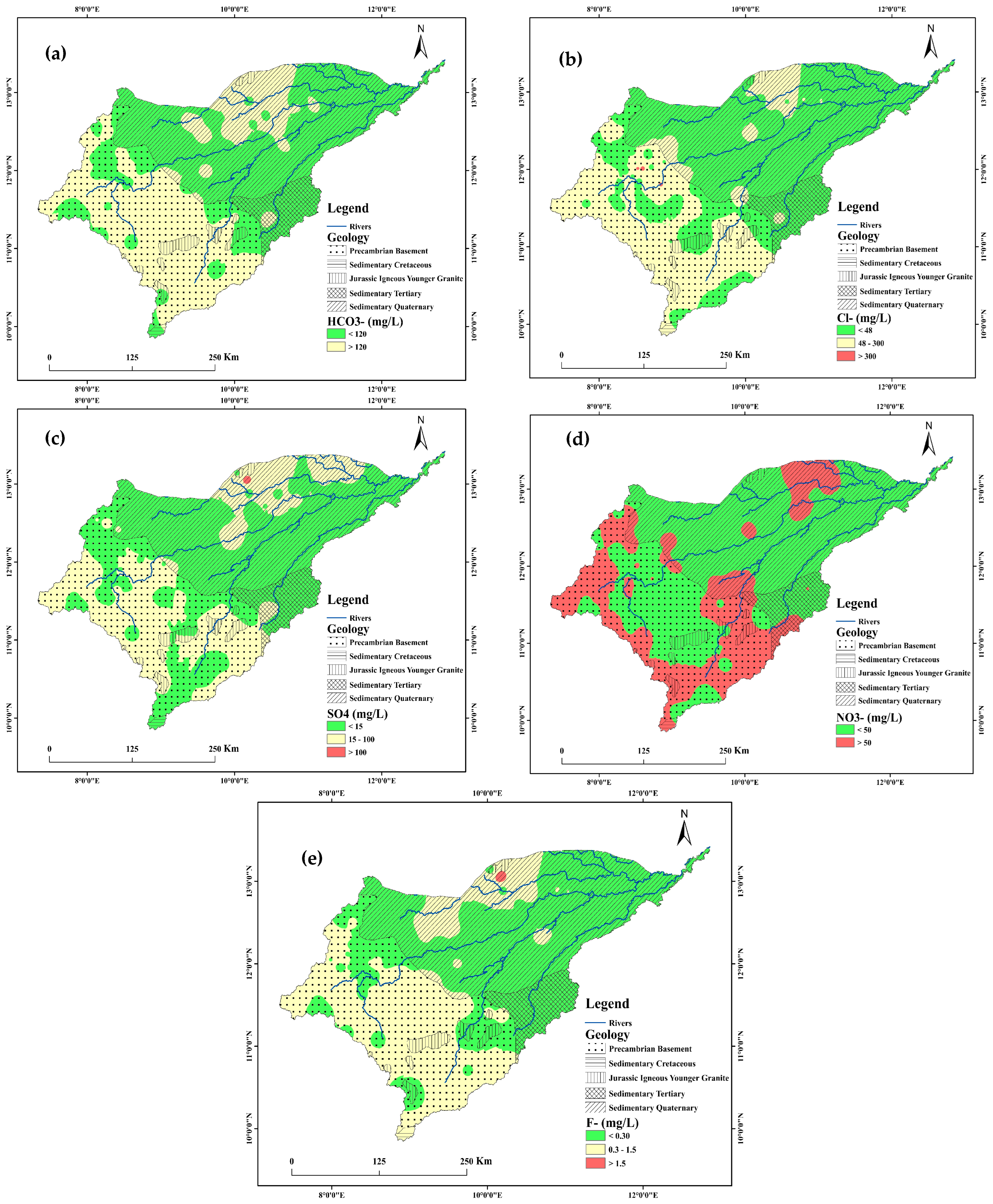
3.6. Bicarbonate (HCO−3)
3.7. Chloride (Cl−) and sulfate (SO42−)
3.8. Nitrate (NO3−) and Fluoride (F−)
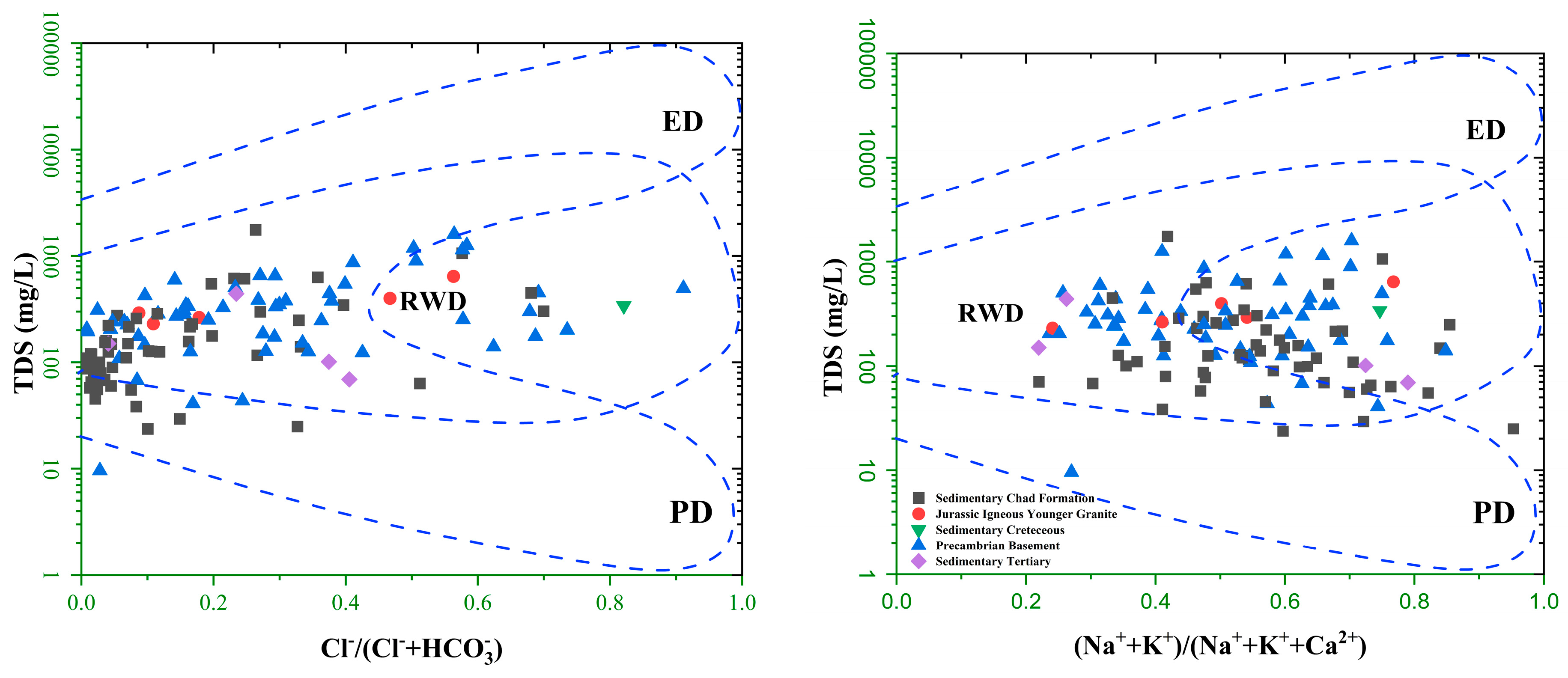
3.9. Geochemical Mechanism of Groundwater
3.10. Groundwater Types and Hydrogeochemical Evolution
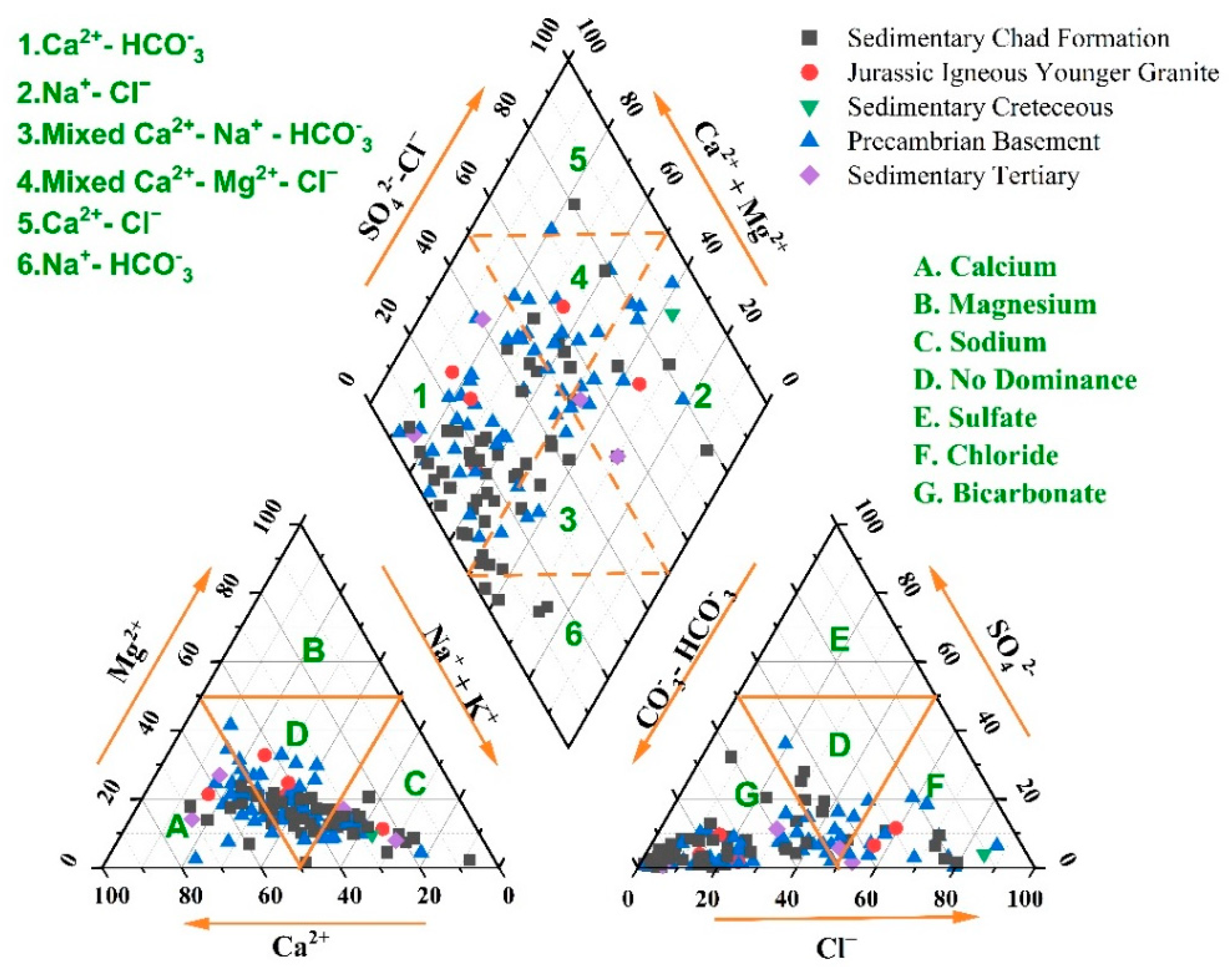
3.10.1. Piper Plot
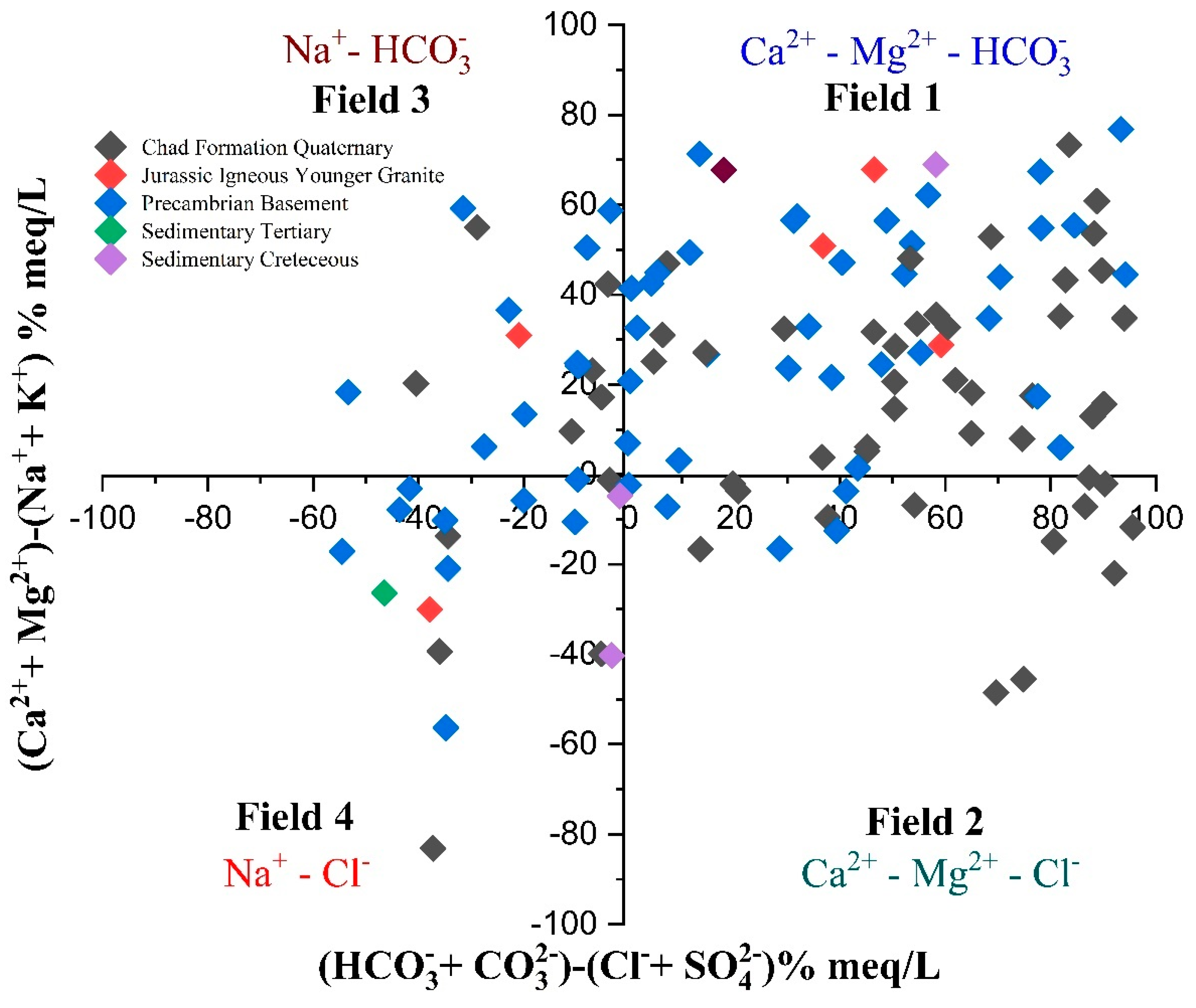
3.10.2. Chadha Diagram
3.11. Groundwater Quality Index
4. Conclusions
- The order of the abundance of the major cations and anions in the groundwater samples is: Ca2+ > Na+ > K+ > Mg2+ and HCO−3 > Cl− > NO3− > SO42− > F−, respectively. More than 90% of groundwater samples have Na+, Ca2+, Mg2+, K+, Cl−, and SO42− and total hardness within the WHO [72] maximum permissible limits. However, some locations show high F− and NO3− concentrations, largely in the Precambrian basement region and a few locations in the sedimentary formation parts of the study area.
- The chemistry of the major ions in the groundwater samples of the study area is predominantly (92%) influenced by weathering/rock–water interaction.
- Ca2+-Mg2+-HCO−3 is the most prevalent hydrochemical facies of groundwater in KYB accounting for more than half (59%) of the groundwater samples. The order of dominance of the groundwater type of the study region is Ca2+-Mg2+-HCO−3 > Na+-Cl− > Na+-HCO−3 > Ca2+-Mg2+-SO42−-Cl−. The Na+-HCO−3 groundwater type may promote fluoride dissolution, perhaps contributing to fluoride enrichment in groundwater in some parts of the Precambrian basement complex and the sedimentary Chad formation of the study area. The Piper trilinear plot findings agree with the Chadha diagram results.
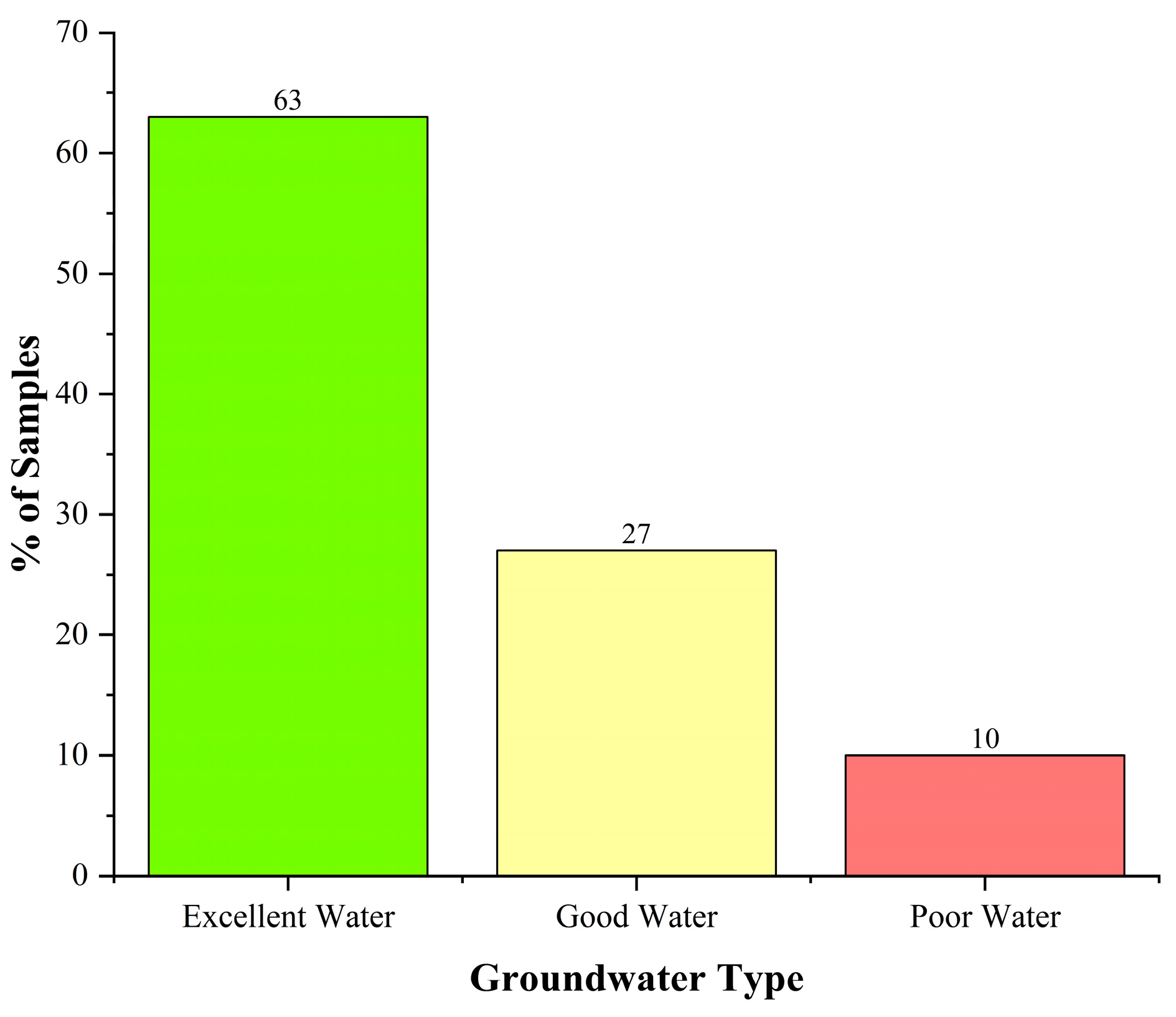
- Based on GWQI, the groundwater in the study area is generally of excellent (63%) to good quality (27%) with only 10% exhibiting poor quality. The Precambrian basement complex region of the study basin has the most significant presence of good and poor water quality classes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganiyu, S.A.; Mabunmi, A.A.; Olurin, O.T.; Adeyemi, A.A.; Jegede, O.A.; Okeh, A. Assessment of microbial and heavy metal contamination in shallow hand-dug wells bordering Ona River, Southwest Nigeria. Environ. Monit. Assess. 2021, 193, 126. [Google Scholar] [CrossRef]
- Kawo, N.S.; Karuppannan, S. Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J. African Earth Sci. 2018, 147, 300–311. [Google Scholar] [CrossRef]
- Namara, R.E.; Hanjra, M.A.; Castillo, G.E.; Ravnborg, H.M.; Smith, L.; Van Koppen, B. Agricultural water management and poverty linkages. Agric. Water Manag. 2010, 97, 520–527. [Google Scholar] [CrossRef]
- Solangi, G.S.; Siyal, A.A.; Babar, M.M.; Siyal, P. Groundwater quality evaluation using the water quality index (WQI), the synthetic pollution index (SPI), and geospatial tools: A case study of Sujawal district, Pakistan. Hum. Ecol. Risk Assess. 2020, 26, 1529–1549. [Google Scholar] [CrossRef]
- Li, P.Y.; Qian, H.; Wu, J.H.; Ding, J. Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci. Eng. 2010, 3, 282–291. [Google Scholar] [CrossRef]
- Mostafa, M.G.; Uddin, S.M.H.; Haque, A.B.M.H. Assessment of hydro-geochemistry and groundwater quality of Rajshahi City in Bangladesh. Appl. Water Sci. 2017, 7, 4663–4671. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Badmus, B.S.; Olurin, O.T.; Ojekunle, Z.O. Evaluation of seasonal variation of water quality using multivariate statistical analysis and irrigation parameter indices in Ajakanga area, Ibadan, Nigeria. Appl. Water Sci. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Trabelsi, R.; Zouari, K. Coupled geochemical modeling and multivariate statistical analysis approach for the assessment of groundwater quality in irrigated areas: A study from North Eastern of Tunisia. Groundw. Sustain. Dev. 2019, 8, 413–427. [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Akurugu, B.A.; Manu, E.; Aliou, A.S. Assessment of groundwater quality and the main controls on its hydrochemistry in some Voltaian and basement aquifers, northern Ghana. Groundw. Sustain. Dev. 2020, 10, 100296. [Google Scholar] [CrossRef]
- Rivett, M.O.; Miller, A.V.; MacAllister, D.J.; Fallas, A.; Wanangwa, G.J.; Mleta, P.; Phiri, P.; Mannix, N.; Monjerezi, M.; Kalin, R.M. A conceptual model based framework for pragmatic groundwater-quality monitoring network design in the developing world: Application to the Chikwawa District, Malawi. Groundw. Sustain. Dev. 2018, 6, 213–226. [Google Scholar] [CrossRef]
- Goes, B.J.M. Estimate of shallow groundwater recharge in the Hadejia-Nguru Wetlands, semi-arid northeastern Nigeria. Hydrogeol. J. 1999, 7, 294–304. [Google Scholar] [CrossRef]
- Goni, I.B.; Sheriff, B.M.; Kolo, A.M.; Ibrahim, M.B. Assessment of nitrate concentrations in the shallow groundwater aquifer of Maiduguri and environs, Northeastern Nigeria. Sci. African 2019, 4, e00089. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.; Hayder, G.; Baloo, L.; Abubakar, S.; Ghaleb, A.A.; Lawal, I.M.; Noor, A.; Umaru, I.; Almahbashi, N.M. Water quality hazard assessment for hand dug wells in Rafin Zurfi, Bauchi State, Nigeria. Ain Shams Eng. J. 2020, 11, 983–999. [Google Scholar] [CrossRef]
- Wali, S.U.; Alias, N.; Bin, S. Reevaluating the hydrochemistry of groundwater in basement complex aquifers of Kaduna Basin, NW Nigeria using multivariate statistical analysis. Environ. Earth Sci. 2021, 80, 208. [Google Scholar] [CrossRef]
- Balogun, M.A.; Anumah, A.O.; Adegoke, K.A.; Maxakato, N.W. Environmental Health Impacts and Controlling Measures of Anthropogenic Activities on Groundwater Quality in Southwestern Nigeria; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 194. [Google Scholar] [CrossRef]
- Ocheri, M.; Odoma, L.; Umar, N. Groundwater Quality in Nigerian Urban Areas: A Review. Glob. J. Sci. Front. Res. 2014, 14, 35–46. Available online: http://journalofscience.org/index.php/GJSFR/article/view/1293 (accessed on 14 February 2024).
- Stephen, U.N.; Celestine, O.O.; Solomon, O.O. Analysis of hydrogeochemical facies in groundwater of upper part of Cross River Basin, southeastern Nigeria. J. African Earth Sci. 2017, 131, 145–155. [Google Scholar] [CrossRef]
- Wali, S.U.; Dankani, I.M.; Abubakar, S.D.; Gada, M.A.; Usman, A.A.; Shera, I.M.; Umar, K.J. Review of groundwater potentials and groundwater hydrochemistry of semi-arid Hadejia-Yobe Basin, North-Eastern Nigeria. J. Geol. Res. 2020, 2, 20–33. [Google Scholar] [CrossRef]
- Masood, A.; Aslam, M.; Bao, Q.; Warish, P.; Sarfaraz, K. Integrating water quality index, GIS and multivariate statistical techniques towards a better understanding of drinking water quality Bureau of Indian Standards. Environ. Sci. Pollut. Res. 2021, 29, 26860–26876. [Google Scholar] [CrossRef] [PubMed]
- Omonona, O.V.; Amah, J.O.; Olorunju, S.B.; Waziri, S.H.; Ekwe, A.C.; Umar, D.N.; Olofinlade, S.W. Hydrochemical characteristics and quality assessment of groundwater from fractured Albian carbonaceous shale aquifers around Enyigba-Ameri, southeastern Nigeria. Environ. Monit. Assess. 2019, 191, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nalami, Z.M.; Ibrahim, M.; Usman, A. The Effect of Hadejia- Jama’ are River Basin Development Authority on Dry Season Farming in Kura Local Government Area of Kano State, Nigeria. Sci. Pap. Manag. Econ. Eng. Agric. Rural. Dev. 2019, 19, 215–220. [Google Scholar]
- Ezugwu, C.K.; Onwuka, O.S.; Egbueri, J.C.; Unigwe, C.O.; Ayejoto, D.A. Multi-criteria approach to water quality and health risk assessments in a rural agricultural province, southeast Nigeria. HydroResearch 2019, 2, 40–48. [Google Scholar] [CrossRef]
- Wagh, V.M.; Mukate, S.V.; Panaskar, D.B.; Muley, A.A.; Sahu, U.L. Study of groundwater hydrochemistry and drinking suitability through Water Quality Index (WQI) modelling in Kadava river basin, India. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Q.; Xiao, D.; Zhu, Y.; Yin, S.; Zhang, Y. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical Evaluation of Groundwater Quality for Drinking and Irrigation Purposes and Integrated Interpretation with Water Quality Index Studies. Environ. Process. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Kalin, A.; Long, R.M. Application of hydrogeochemical modelling for validation of hydrologie flow modelling in the Tucson basin aquifer, Arizona, United States of America. In Proceedings of the a Final Research Co-ordination Meeting Held, Vienna, Austria, 1–4 June 1993; pp. 147–178. [Google Scholar]
- Kalin, R.M. Manual on mathematical models in isotope hydrogeology. In Chapter 4: Basic Concepts and Formulations for Isotope-geochemical Process Investigations, Procedures and Methodologies of Geochemical Modelling of Groundwater Systems; IAEA: Vienna, Autria, 1995; pp. 155–206. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Manual+on+mathematical+models+in+isotope+hydrogeology#0 (accessed on 14 February 2024).
- Marini, L. Geological Sequestration of Carbon Dioxide: Thermodynamics, Kinetics, and Reaction Path Modeling; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ahmed, N.; Bodrud-Doza, M.; Islam, S.D.; Choudhry, M.A.; Muhib, M.I.; Zahid, A.; Hossain, S.; Moniruzzaman, M.; Deb, N.; Bhuiyan, M.A. Hydrogeochemical evaluation and statistical analysis of groundwater of Sylhet, north-eastern Bangladesh. Acta Geochim. 2019, 38, 440–455. [Google Scholar] [CrossRef]
- Mgbenu, C.N.; Egbueri, J.C. The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl. Water Sci. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Singh, A.K.; Singh, A.K.; Singh, M.P. Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh, India. Appl. Water Sci. 2017, 7, 1609–1623. [Google Scholar] [CrossRef]
- Egbueri, J.C. Heavy Metals Pollution Source Identification and Probabilistic Health Risk Assessment of Shallow Groundwater in Onitsha, Nigeria. Anal. Lett. 2020, 53, 1620–1638. [Google Scholar] [CrossRef]
- Vaiphei, S.P.; Kurakalva, R.M.; Sahadevan, D.K. Water quality index and GIS-based technique for assessment of groundwater quality in Wanaparthy watershed, Telangana, India. Environ. Sci. Pollut. Res. 2020, 27, 45041–45062. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Taloor, A.K. Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundw. Sustain. Dev. 2020, 10, 100288. [Google Scholar] [CrossRef]
- Aladejana, J.A.; Kalin, R.M.; Sentenac, P.; Hassan, I. Groundwater quality index as a hydrochemical tool for monitoring saltwater intrusion into coastal freshwater aquifer of Eastern Dahomey Basin, Southwestern Nigeria. Groundw. Sustain. Dev. 2021, 13, 100568. [Google Scholar] [CrossRef]
- Olasoji, S.O.; Oyewole, N.O.; Abiola, B.; Edokpayi, J.N. Water quality assessment of surface and groundwater sources using a water quality index method: A case study of a peri-urban town in southwest, Nigeria. Environments 2019, 6, 23. [Google Scholar] [CrossRef]
- Qasemi, M.; Darvishian, M.; Nadimi, H.; Gholamzadeh, M.; Afsharnia, M.; Farhang, M.; Allahdadi, M.; Darvishian, M.; Zarei, A. Characteristics, water quality index and human health risk from nitrate and fluoride in Kakhk city and its rural areas, Iran. J. Food Compos. Anal. 2023, 115, 104870. [Google Scholar] [CrossRef]
- Aladejana, J.A.; Kalin, R.M.; Sentenac, P.; Hassan, I. Assessing the impact of climate change on groundwater quality of the shallow coastal aquifer of eastern dahomey basin, Southwestern Nigeria. Water 2020, 12, 224. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Paramasivam, S.K.; Karuppannan, S.; Ravichandran, N.; Selvaraj, P. A GIS-based evaluation of hydrochemical characterisation of groundwater in hard rock region, South Tamil Nadu, India. Arab. J. Geosci. 2020, 13, 1–22. [Google Scholar] [CrossRef]
- Machiwal, D.; Cloutier, V.; Güler, C.; Kazakis, N. A review of GIS-integrated statistical techniques for groundwater quality evaluation and protection. Environ. Earth Sci. 2018, 77, 1–30. [Google Scholar] [CrossRef]
- Sebei, A.; Slama, T.; Helali, M.A. Hydrochemical characterization and geospatial analysis of groundwater quality in Cap Bon region, northeastern Tunisia. Environ. Earth Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Rivett, M.O.; Budimir, L.; Mannix, N.; Miller, A.V.; Addison, M.J.; Moyo, P.; Wanangwa, G.J.; Phiri, O.L.; Songola, C.E.; Nhlema, M.; et al. Responding to salinity in a rural African alluvial valley aquifer system: To boldly go beyond the world of hand-pumped groundwater supply? Sci. Total Environ. 2019, 653, 1005–1024. [Google Scholar] [CrossRef]
- Al-Jawad, J.Y.; Al-Jawad, S.B.; Kalin, R.M. Decision-making challenges of sustainable groundwater strategy under multi-event pressure in arid environments: The Diyala River Basin in Iraq. Water 2019, 11, 2160. [Google Scholar] [CrossRef]
- Mahammad, S.; Islam, A. Evaluating the groundwater quality of Damodar Fan Delta (India) using fuzzy-AHP MCDM technique. Appl. Water Sci. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Bernard, E.; Ayeni, N. Physicochemical Analysis of Groundwater Samples of Bichi Local Government Area of Kano State of Nigeria. World Environ. 2012, 2, 116–119. [Google Scholar] [CrossRef]
- Hamidu, H.; Halilu, F.B.; Yerima, K.M.; Garba, L.M.; Suleiman, A.A.; Kankara, A.I.; Abdullahi, I.M. Heavy metals pollution indexing, geospatial and statistical approaches of groundwater within Challawa and Sharada industrial areas, Kano City, North-Western Nigeria. SN Appl. Sci. 2021, 3, 690. [Google Scholar] [CrossRef]
- Suleiman, A.; Ibrahim, A.; Abdullahi, U. Statistical Explanatory Assessment of Groundwater Quality in Gwale LGA, Kano State, Northwest Nigeria. Hydrospatial Anal. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Usman, F.U.; Aliyu, B. Impact of Pit Latrine on groundwater quality in some communities of Nguru town, Nguru Local Government area, Yobe State, Nigeria. East African Sch. Multidiscip. Bull. 2020, 3, 218–225. [Google Scholar] [CrossRef]
- Waziri, M.; Ogugbuaja, V.O. Interrelationships between physicochemical water pollution indicators: A case study of River Yobe-Nigeria. Am. Jouran Sci. Ind. Res. 2010, 1, 76–80. [Google Scholar]
- Adeyeri, O.E.; Lawin, A.E.; Laux, P.; Ishola, K.A.; Ige, S.O. Analysis of climate extreme indices over the Komadugu-Yobe basin, Lake Chad region: Past and future occurrences. Weather Clim. Extrem. 2019, 23, n100194. [Google Scholar] [CrossRef]
- Goes, B.J.M. Effects of river regulation on aquatic macrophyte growth and floods in the Hadejia-Nguru wetlands and flow in the Yobe River, northern Nigeria; implications for future water management. River Res. Appl. 2002, 18, 81–95. [Google Scholar] [CrossRef]
- Ahmed, S.D.; Agodzo, S.K.; Adjei, K.A.; Deinmodei, M.; Ameso, V.C. Preliminary investigation of flooding problems and the occurrence of kidney disease around Hadejia-Nguru wetlands, Nigeria and the need for an ecohydrology solution. Ecohydrol. Hydrobiol. 2018, 18, 212–224. [Google Scholar] [CrossRef]
- Adeyeri, O.E.; Laux, P.; Lawin, A.E.; Ige, S.O.; Kunstmann, H. Analysis of hydrometeorological variables over the transboundary komadugu-yobe basin, West Africa. J. Water Clim. Chang. 2020, 11, 1339–1354. [Google Scholar] [CrossRef]
- Avbovbo, A.A.; Ayoola, F.O.; Osahon, G.A. Depositional and Structural Styles in Chad Basin of Northeastern Nigeria. Am. Assoc. Pet. Geol. Bull. 1986, 70, 1787–1798. [Google Scholar] [CrossRef]
- McCurry, P. Pan-african orogeny in northern Nigeria. Bull. Geol. Soc. Am. 1971, 82, 3251–3262. [Google Scholar] [CrossRef]
- Obaje, N.G.; Wehner, H.; Hamza, H.; Scheeder, G. New geochemical data from the Nigerian sector of the Chad basin: Implications on hydrocarbon prospectivity. J. African Earth Sci. 2004, 38, 477–487. [Google Scholar] [CrossRef]
- Obaje, N.G. Geology and Mineral Resources of Nigeria; Springer: Berlin/Heidelberg, Germany, 2011; Volume 131. [Google Scholar]
- Lopez, T.; Antoine, R.; Kerr, Y.; Darrozes, J.; Rabinowicz, M.; Ramillien, G.; Cazenave, A.; Genthon, P. Subsurface Hydrology of the Lake Chad Basin from Convection Modelling and Observations; Springer: Amsterdam, The Netherlands, 2016; Volume 37. [Google Scholar] [CrossRef]
- Bura, B.; Goni, I.B.; Sheriff, B.M.; Gazali, A.K. Occurrence and distribution of fluoride in groundwater of chad formation aquifers in Borno state, Nigeria. Int. J. Hydrol. 2018, 2, 528–537. [Google Scholar] [CrossRef]
- Ola-Buraimo, A.O.; Abdulganiyu, Y. Palynology and stratigraphy of the Upper Miocene Chad Formation, Bornu Basin, northeastern Nigeria. J. Palaeogeogr. 2017, 6, 108–116. [Google Scholar] [CrossRef]
- Adegoke, O.S.; Agumanu, A.E.; Benkhelil, M.J.; Ajayi, P.O. New stratigraphic, sedimentologic and structural data on the kerri-kerri formation, Bauchi and Borno States, Nigeria. J. African Earth Sci. 1986, 5, 249–277. [Google Scholar] [CrossRef]
- Wali, S.U.; Alias, N.; Harun, S.B. Quality reassessment using water quality indices and hydrochemistry of groundwater from the Basement Complex section of Kaduna Basin, NW Nigeria. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Vassolo, S.; Daïra, D. Lake Chad: Sustainable Water Managemen; Report No. 4; BGR: Berlin, Germany, 2012. [Google Scholar]
- Edmunds, W.M.; Fellman, E.; Goni, I.B.; Prudhomme, C. Spatial and temporal distribution of groundwater recharge in northern Nigeria. Hydrogeol. J. 2002, 10, 205–215. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Fellman, E.; Goni, I.B. Lakes, groundwater and palaeohydrology in the Sahel of NE Nigeria: Evidence from hydrogeochemistry. J. Geol. Soc. London. 1999, 156, 345–355. [Google Scholar] [CrossRef]
- Akujieze, C.N.; Coker, S.J.L.; Oteze, G.E. Groundwater in Nigeria—A millennium experience—Distribution, practice, problems and solutions. Hydrogeol. J. 2003, 11, 259–274. [Google Scholar] [CrossRef]
- Edet, A.E.A.; Nganje, T.N.; Ukpong, A.J. Groundwater chemistry and quality of Nigeria: A status review. Afr. J. Environ. Sci. Technol. 2012, 5, 1152–1169. [Google Scholar] [CrossRef]
- Le Coz, M.; Genthon, P.; Adler, P.M. Multiple-Point Statistics for Modeling Facies Heterogeneities in a Porous Medium: The Komadugu-Yobe Alluvium, Lake Chad Basin. Math. Geosci. 2011, 43, 861–878. [Google Scholar] [CrossRef]
- Carter, R.C.; Alkali, A.G. Shallow groundwater in the northeast arid zone of Nigeria. Q. J. Eng. Geol. 1996, 29, 341–355. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; p. 1360. ISBN 978-087553-013-0. Available online: http://www.standardmethods.org/ (accessed on 14 February 2024).
- Adimalla, N.; Venkatayogi, S. Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl. Water Sci. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- WHO. A Global Overview of National Regulations and Standards for Drinking-Water Quality. 2018. Available online: http://apps.who.int/bookorders (accessed on 14 February 2024).
- Eyankware, M.O.; Aleke, C.G.; Selemo, A.O.I.; Nnabo, P.N. Hydrogeochemical studies and suitability assessment of groundwater quality for irrigation at Warri and environs. Niger delta basin, Nigeria. Groundw. Sustain. Dev. 2020, 10, 100293. [Google Scholar] [CrossRef]
- White, A.F.; Schulz, M.S.; Lowenstern, J.B.; Vivit, D.V.; Bullen, T.D. The ubiquitous nature of accessory calcite in granitoid rocks: Implications for weathering, solute evolution, and petrogenesis. Geochim. Cosmochim. Acta 2005, 69. [Google Scholar] [CrossRef]
- Davis, R.J.M.; DeWiest, S.N. Hydrogeology; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Sawyer, P.; McCarthy, C. Chemistry for Environmental Engineering and Science, 2nd ed.; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Bloise, A.; Critelli, T.; Catalano, M.; Apollaro, C.; Miriello, D.; Croce, A.; Barrese, E.; Liberi, F.; Piluso, E.; Rinaudo, C.; et al. Asbestos and other fibrous minerals contained in the serpentinites of the Gimigliano-Mount Reventino Unit (Calabria, S-Italy). Environ. Earth Sci. 2014, 71, 3773–3786. [Google Scholar] [CrossRef]
- Uddin, M.G.; Diganta, M.T.; Sajib, A.M.; Hasan, M.A.; Moniruzzaman, M.; Rahman, A.; Olbert, A.I.; Moniruzzaman, M. Assessment of hydrogeochemistry in groundwater using water quality index model and indices approaches. Heliyon 2023, 9, e19668. [Google Scholar] [CrossRef]
- Vaughan, D.J. Sulfide mineralogy and geochemistry: Introduction and overview. Rev. Mineral. Geochemistry 2006, 61, 1–5. [Google Scholar] [CrossRef]
- Akpata, E.S.; Danfillo, I.S.; Otoh, E.C.; Mafeni, J.O. Geographical mapping of fluoride levels in drinking water sources in Nigeria. Afr. Health Sci. 2009, 9, 227–233. [Google Scholar]
- Giwa, A.S.; Memon, A.G.; Ahmad, J.; Ismail, T.; Abbasi, S.A.; Kamran, K.; Wang, B.; Segun, B.; Seydou, H. Assessment of high fluoride in water sources and endemic fluorosis in the North-Eastern communities of Gombe State, Nigeria. Environ. Pollut. Bioavailab. 2021, 33, 31–40. [Google Scholar] [CrossRef]
- Malago, N.N.M.; Edikafubeni, J.; Alfred, M. Fluoride Levels in Surface and Groundwater in Africa: A Review. Am. J. Water Sci. Eng. 2017, 3, 1. [Google Scholar] [CrossRef]
- Waziri, M.; Musa, U.; Hati, S.S. Assessment of Fluoride Concentrations in Surface Waters and Groundwater Sources in Northeastern Nigeria. Resour. Environ. 2012, 2, 67–72. [Google Scholar] [CrossRef]
- Haritash, A.K.; Aggarwal, A.; Soni, J.; Sharma, K.; Sapra, M.; Singh, B. Assessment of fluoride in groundwater and urine, and prevalence of fluorosis among school children in Haryana, India. Appl. Water Sci. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Yadav, J.P.; Lata, S.; Kataria, S.K.; Kumar, S. Fluoride distribution in groundwater and survey of dental fluorosis among school children in the villages of the Jhajjar District of Haryana, India. Environ. Geochem. Health 2009, 31, 431–438. [Google Scholar] [CrossRef]
- Gibbs, J.R. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Adimalla, N.; Vasa, S.K.; Li, P. Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: An insight of controlling factors of fluoride enrichment. Model. Earth Syst. Environ. 2018, 4, 841–852. [Google Scholar] [CrossRef]




| Parameters | Units | WHO [72] | Weight (wi) | Relative Weight (Wi) |
|---|---|---|---|---|
| pH | / | 6.5–8.5 | 4 | 0.095 |
| TDS | mg/L | 1000 | 5 | 0.119 |
| TH | mg/L CaCO3 | 500 | 3 | 0.071 |
| Na+ | mg/L | 200 | 2 | 0.048 |
| K+ | mg/L | 12 | 2 | 0.048 |
| Ca2+ | mg/L | 75 | 3 | 0.071 |
| Mg2+ | mg/L | 50 | 3 | 0.071 |
| Cl− | mg/L | 300 | 4 | 0.095 |
| HCO3- | mg/L | 250 | 3 | 0.071 |
| SO42− | mg/L | 250 | 3 | 0.071 |
| NO3− | mg/L | 50 | 5 | 0.119 |
| F− | mg/L | 5 | 5 | 0.119 |
| Range of GWQI | Class of Water | Number of Samples | % of Samples |
|---|---|---|---|
| <50 | Excellent Water | 76 | 63 |
| 50–100 | Good Water | 32 | 27 |
| 100–200 | Poor Water | 12 | 10 |
| 200–300 | Very Poor Water | / | / |
| >300 | Unsuitable | / | / |
| Total | 120 | 100 |
| Parameters | Units | Maximum | Minimum | Mean | WHO [72] | PAMPL | |
|---|---|---|---|---|---|---|---|
| HDL | MPL | ||||||
| pH | / | 8.24 | 5.52 | 7.2 | 6.5–8.5 | 8.5 | / |
| EC | µS/cm | 2746 | 15 | 462 | 1000 | / | 5 |
| TDS | mg/L | 1757 | 10 | 296 | 1000 | 1500 | 2.5 |
| TH | mg/L CaCO3 | 704 | 0.8 | 138 | 100 | 500 | 2 |
| Na+ | mg/L | 285 | 2 | 36 | 200 | 250 | 2.5 |
| K+ | mg/L | 96 | 0.1 | 10 | 12 | / | 6 |
| Ca2+ | mg/L | 220 | 0.2 | 39 | 75 | 200 | 2 |
| Mg2+ | mg/L | 58 | 0.1 | 9.9 | 50 | 150 | 2.5 |
| HCO−3 | mg/L | 379 | 1.5 | 120 | 250 | 500 | 10.8 |
| Cl− | mg/L | 372 | 0.7 | 48 | 300 | 600 | 2.5 |
| SO42− | mg/L | 133 | 0.1 | 15 | 250 | 500 | / |
| NO3− | mg/L | 314 | ND | 42 | 50 | 50 | 30 |
| F− | mg/L | 2.3 | ND | 0.3 | 1.5 | 1.5 | 2 |
| TDS (mg/L) | Class of Groundwater | % of Samples |
|---|---|---|
| <500 | Desirable for drinking | 85 |
| 500–1000 | Permissible for drinking | 10 |
| 1000–3000 | Useful for irrigation | 5 |
| >3000 | Unfit for drinking and irrigation | / |
| Water Class | TH as CaCO3 (mg/L) | % of Samples |
|---|---|---|
| Soft water | <75 | 43 |
| Moderately hard water | 75–150 | 18 |
| Hard water | 150–300 | 26 |
| Very hard water | > 300 | 13 |
| Sample Numbers | GWQI | Water Type | Sample Numbers | GWQI | Water Type |
|---|---|---|---|---|---|
| L1 | 79 | Good Water | L63 | 74 | Good Water |
| L2 | 49 | Excellent Water | L64 | 51 | Good Water |
| L3 | 74 | Good Water | L65 | 125 | Poor Water |
| L4 | 104 | Poor Water | L66 | 76 | Good Water |
| L5 | 41 | Excellent Water | L67 | 9 | Excellent Water |
| L6 | 82 | Good Water | L68 | 62 | Good Water |
| L7 | 20 | Excellent Water | L69 | 15 | Excellent Water |
| L8 | 18 | Excellent Water | L70 | 20 | Excellent Water |
| L9 | 24 | Excellent Water | L71 | 62 | Good Water |
| L10 | 82 | Good Water | L72 | 46 | Excellent Water |
| L11 | 39 | Excellent Water | L73 | 38 | Excellent Water |
| L12 | 29 | Excellent Water | L74 | 61 | Good Water |
| L13 | 18 | Excellent Water | L75 | 41 | Excellent Water |
| L14 | 17 | Excellent Water | L76 | 49 | Excellent Water |
| L15 | 19 | Excellent Water | L77 | 61 | Good Water |
| L16 | 14 | Excellent Water | L78 | 73 | Good Water |
| L17 | 156 | Poor Water | L79 | 37 | Excellent Water |
| L18 | 20 | Excellent Water | L80 | 67 | Good Water |
| L19 | 31 | Excellent Water | L81 | 91 | Good Water |
| L20 | 25 | Excellent Water | L82 | 38 | Excellent Water |
| L21 | 19 | Excellent Water | L83 | 14 | Excellent Water |
| L22 | 15 | Excellent Water | L84 | 79 | Good Water |
| L23 | 31 | Excellent Water | L85 | 52 | Good Water |
| L24 | 59 | Good Water | L86 | 64 | Good Water |
| L25 | 91 | Good Water | L87 | 38 | Excellent Water |
| L26 | 134 | Poor Water | L88 | 15 | Excellent Water |
| L27 | 170 | Poor Water | L89 | 18 | Excellent Water |
| L28 | 49 | Excellent Water | L90 | 15 | Excellent Water |
| L29 | 36 | Excellent Water | L91 | 16 | Excellent Water |
| L30 | 28 | Excellent Water | L92 | 13 | Excellent Water |
| L31 | 107 | Poor Water | L93 | 17 | Excellent Water |
| L32 | 150 | Poor Water | L94 | 107 | Poor Water |
| L33 | 14 | Excellent Water | L95 | 58 | Good Water |
| L34 | 37 | Excellent Water | L96 | 30 | Excellent Water |
| L35 | 27 | Excellent Water | L97 | 14 | Excellent Water |
| L36 | 65 | Good Water | L98 | 14 | Excellent Water |
| L37 | 107 | Poor Water | L99 | 32 | Excellent Water |
| L38 | 101 | Poor Water | L100 | 26 | Excellent Water |
| L39 | 23 | Excellent Water | L101 | 103 | Poor Water |
| L40 | 22 | Excellent Water | L102 | 24 | Excellent Water |
| L41 | 38 | Excellent Water | L103 | 23 | Excellent Water |
| TL42 | 83 | Good Water | L104 | 50 | Good Water |
| L43 | 34 | Excellent Water | L105 | 18 | Excellent Water |
| L44 | 64 | Good Water | L106 | 40 | Excellent Water |
| L45 | 49 | Excellent Water | L107 | 30 | Excellent Water |
| L46 | 32 | Excellent Water | L108 | 33 | Excellent Water |
| L47 | 58 | Good Water | L109 | 28 | Excellent Water |
| L48 | 71 | Good Water | L110 | 52 | Good Water |
| L49 | 23 | Excellent Water | L111 | 25 | Excellent Water |
| L50 | 32 | Excellent Water | L112 | 19 | Excellent Water |
| L51 | 39 | Excellent Water | L113 | 18 | Excellent Water |
| L52 | 27 | Excellent Water | L114 | 24 | Excellent Water |
| L53 | 40 | Excellent Water | L115 | 58 | Good Water |
| L54 | 54 | Good Water | L116 | 16 | Excellent Water |
| L55 | 110 | Poor Water | L117 | 12 | Excellent Water |
| L56 | 35 | Excellent Water | L118 | 11 | Excellent Water |
| L57 | 33 | Excellent Water | L119 | 35 | Excellent Water |
| L58 | 72 | Good Water | L120 | 94 | Good Water |
| L59 | 24 | Excellent Water | Maximum | 170 | / |
| L60 | 36 | Excellent Water | Minimum | 9 | / |
| L61 | 33 | Excellent Water | Mean | 48 | / |
| L62 | 84 | Good Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuaibu, A.; Kalin, R.M.; Phoenix, V.; Banda, L.C.; Lawal, I.M. Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region. Water 2024, 16, 601. https://doi.org/10.3390/w16040601
Shuaibu A, Kalin RM, Phoenix V, Banda LC, Lawal IM. Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region. Water. 2024; 16(4):601. https://doi.org/10.3390/w16040601
Chicago/Turabian StyleShuaibu, Abdulrahman, Robert M. Kalin, Vernon Phoenix, Limbikani C. Banda, and Ibrahim Mohammed Lawal. 2024. "Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region" Water 16, no. 4: 601. https://doi.org/10.3390/w16040601
APA StyleShuaibu, A., Kalin, R. M., Phoenix, V., Banda, L. C., & Lawal, I. M. (2024). Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region. Water, 16(4), 601. https://doi.org/10.3390/w16040601









