Hydrochemical Characteristics, Controlling Factors and Strontium Enrichment Sources of Groundwater in the Northwest Plain of Shandong Province, China
Abstract
1. Introduction
2. Study Area
2.1. Natural Conditions
2.2. Hydrological and Geological Conditions
3. Materials and Methods
3.1. Field Sampling
3.2. Laboratory Sample Analysis
3.3. Data Analysis and Methods
4. Results
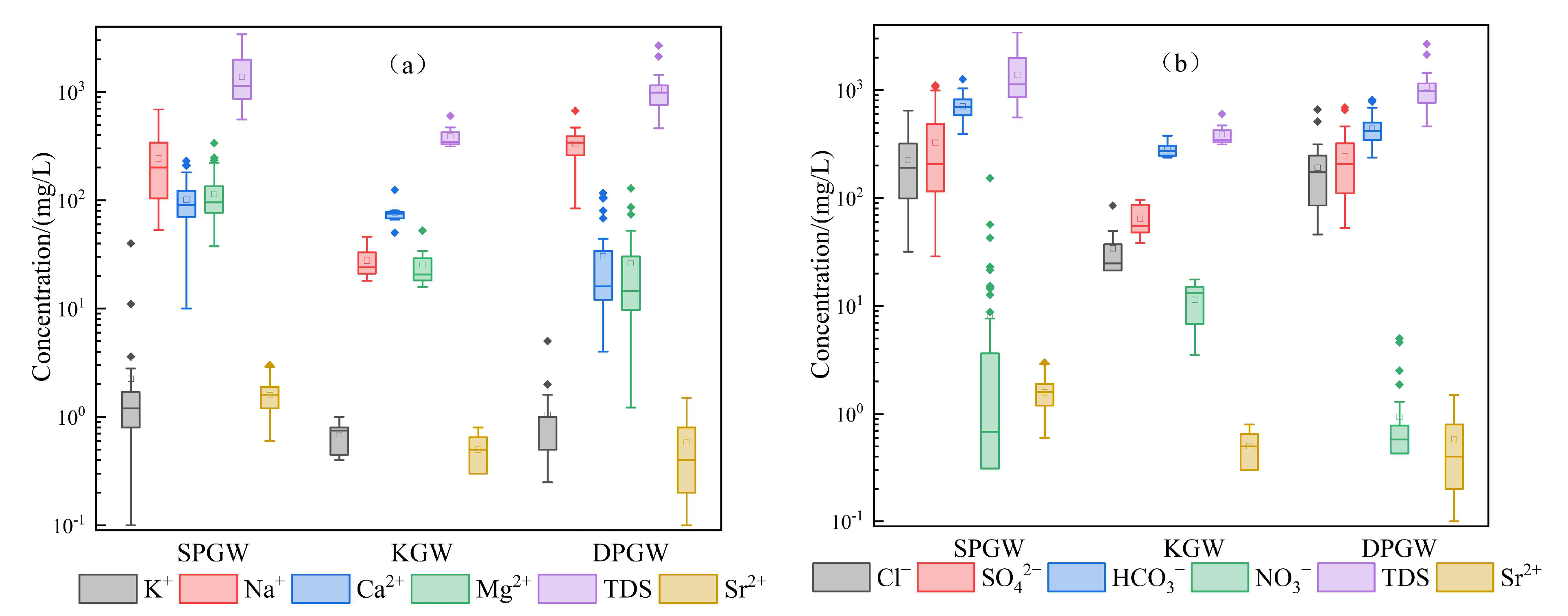
4.1. Hydrochemical Characteristics
4.1.1. General Hydrochemistry of Groundwater
4.1.2. Hydrochemical Type
4.2. Controlling Factors of Groundwater Hydrochemistry
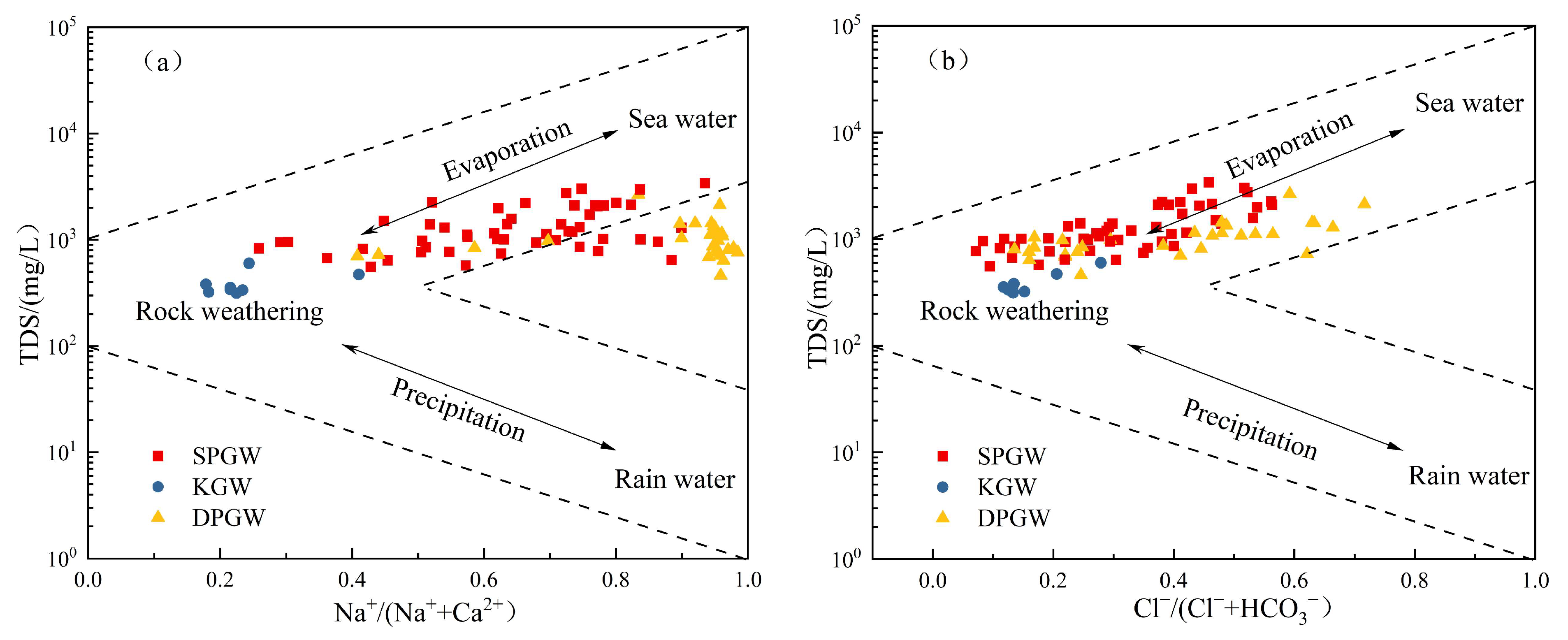
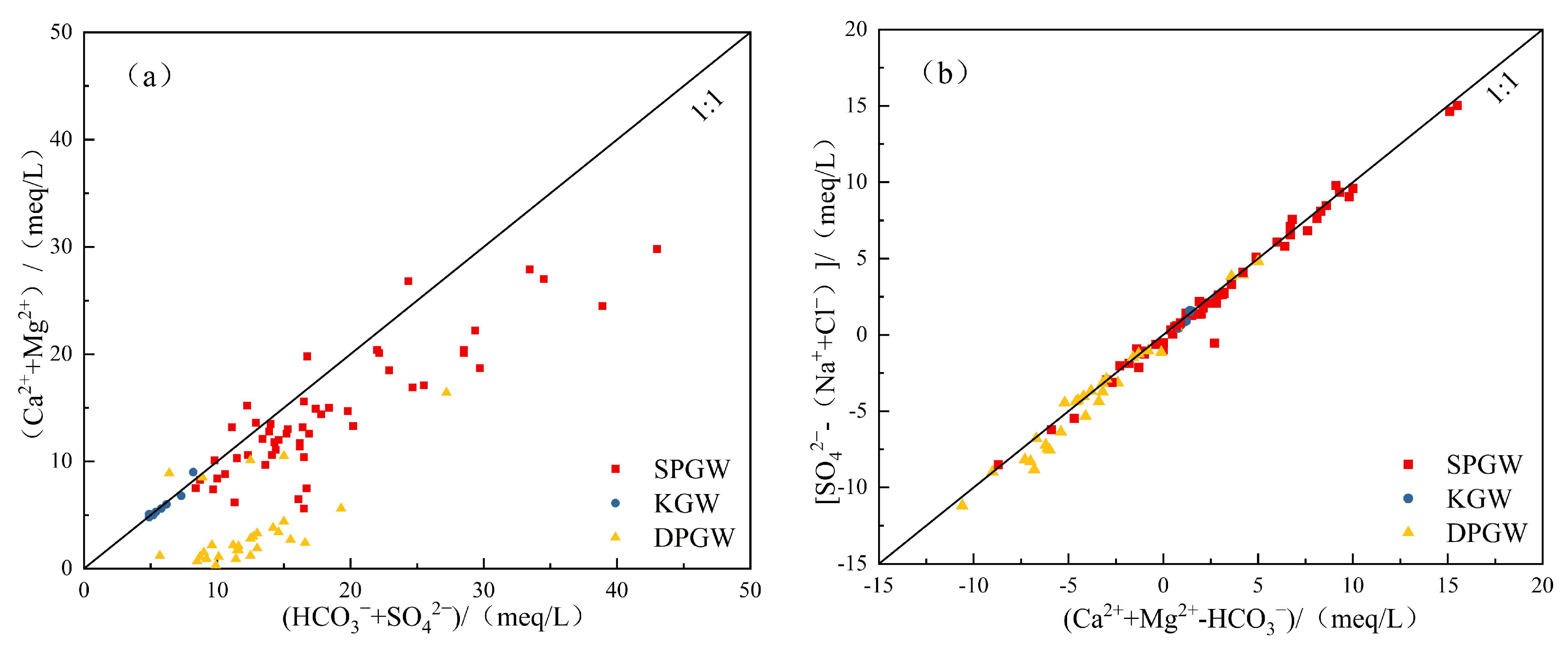
4.2.1. Dissolution
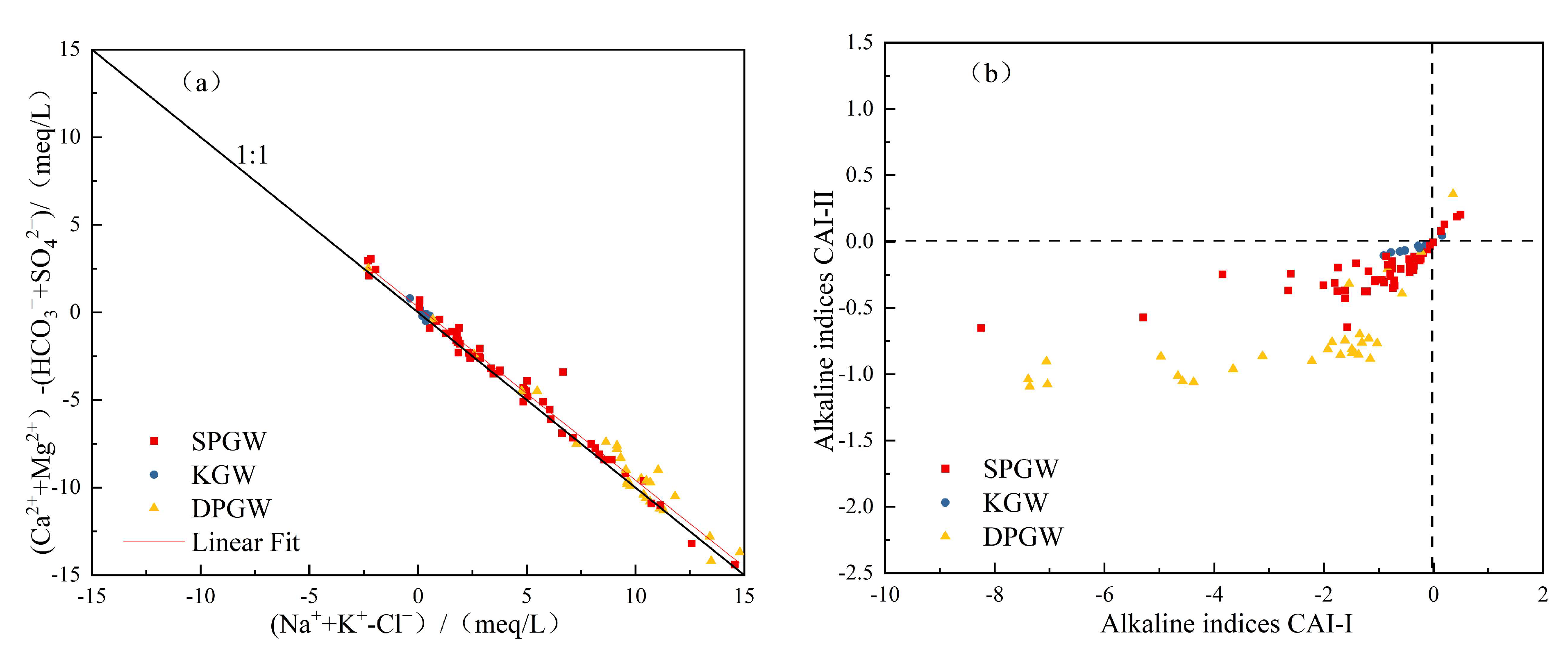
4.2.2. Alternating Adsorption of Cations
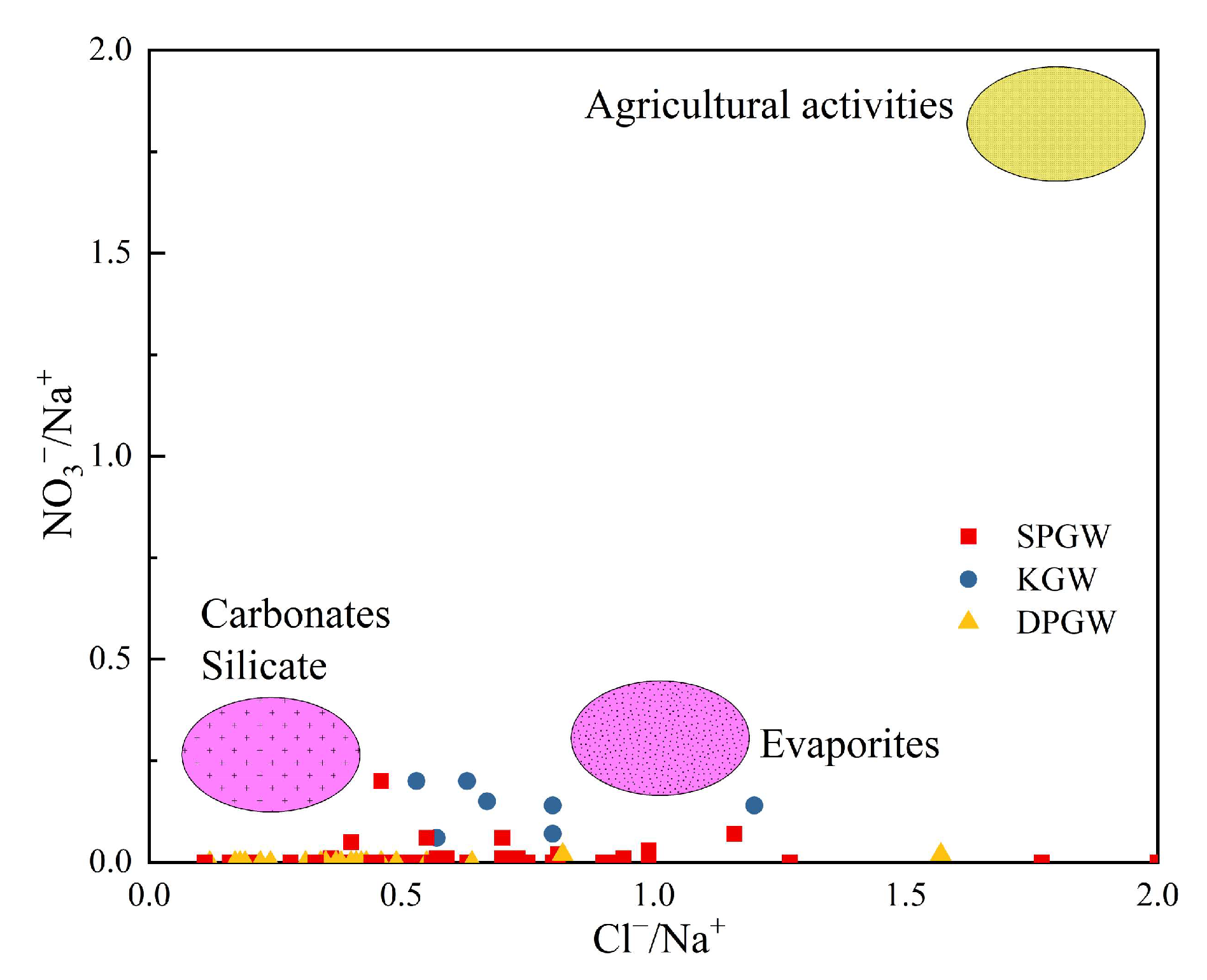
4.2.3. Impact of Human Activities
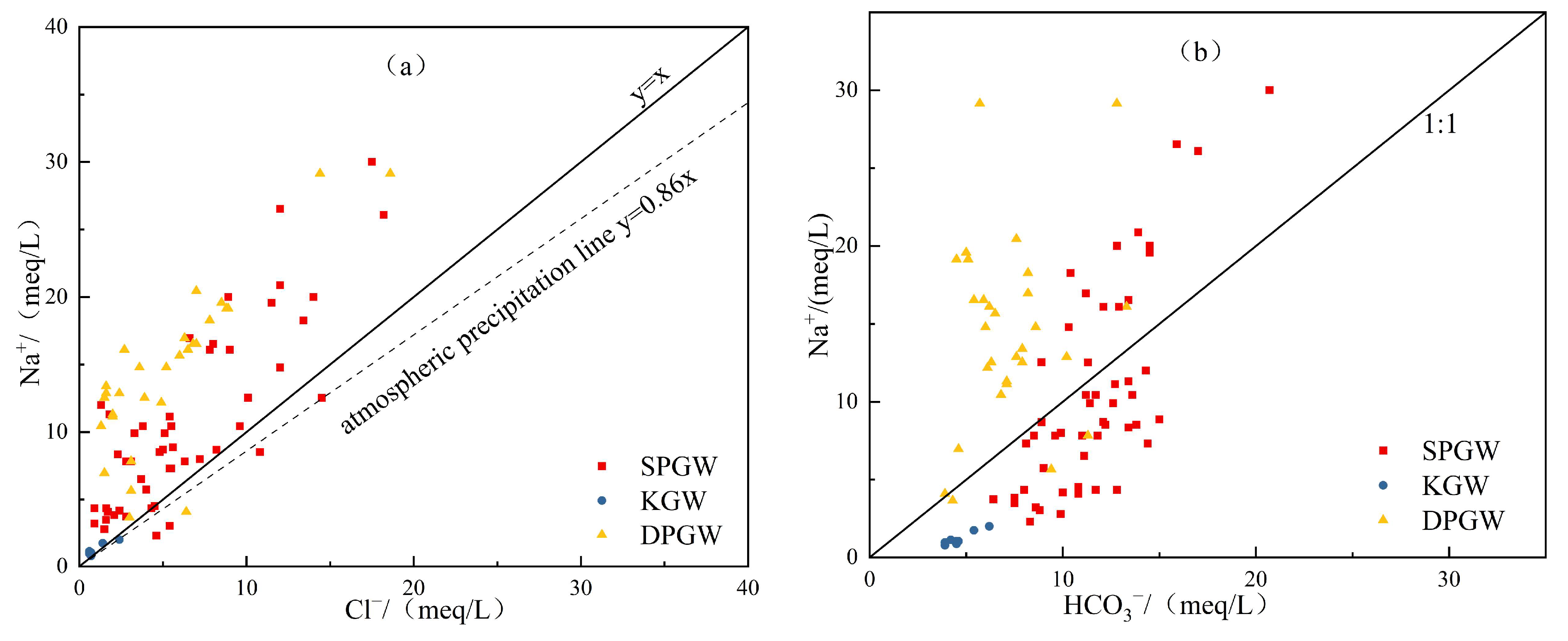
4.2.4. Ion Ratio
5. Discussion
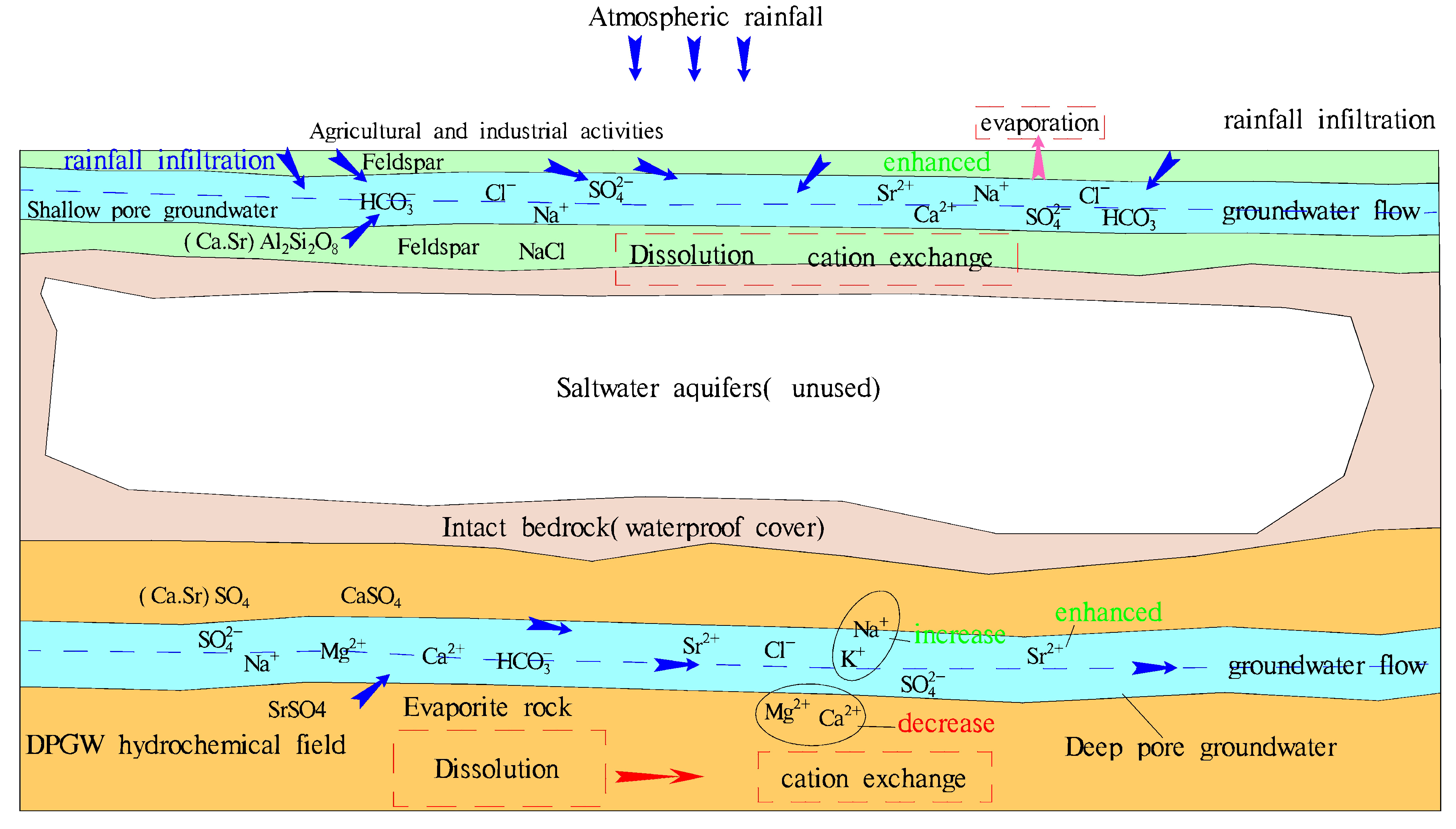
5.1. Controlling Factors of Groundwater’s Hydrochemical Characteristics
5.2. Origin of Strontium Ions in Groundwater
5.3. Suggestions for Groundwater Development and Utilization
6. Conclusions
- (1)
- There are significant differences in different groundwater types in the northwest plain of Shandong Province, China. Shallow groundwater is mainly brackish–saline water, deep groundwater is mainly brackish water, with some fresh water, and karst groundwater is fresh water. The shallow pore water is rich in sources and diverse in hydrochemistry. The cation content mainly comprises the Na·Mg type, Ca·Na type and Ca·Mg type and the anion content mainly comprises the HCO3 type, followed by the HCO3·Cl type and SO4·HCO3·Cl type. The cations in deep pore water are mainly of the Na type, followed by the Ca·Na·Mg type, and the anionic types are relatively complex, mainly including the HCO3·Cl and HCO3·Cl·SO4 types. The hydrochemical types of karst are relatively simple, mainly including the HCO3-Ca·Mg type and HCO3-Ca type.
- (2)
- The main components of groundwater sources in the NPSP are derived from water–rock interaction, and rock mineral leaching plays a dominant role, which is more obvious in karst water, and the influencing factors of evaporation and concentration are highly present in shallow and deep pore water. Shallow pore water mainly comprises dissolved silicate minerals, deep pore water mainly contains dissolved evaporative minerals, largely gypsum, and karst water is mainly composed of dissolved carbonate minerals. Cation exchange exists in these three kinds of groundwater, and a positive exchange is the main effect. Natural geological conditions and good water source protection measures mean that human activities have relatively little influence on the water chemical formation process.
- (3)
- The distribution of strontium-rich groundwater is the highest in shallow pore water, where the average Sr2+ content reaches 1.59 mg/L, while the average Sr2+ content in deep pore water and karst water is 0.58 mg/L and 0.50 mg/L, respectively. The strontium in shallow pore groundwater mainly comes from the enrichment of groundwater runoff, and its sources are abundant, though it mainly derives from silicic rock. Deep pore groundwater mainly comprises evaporative minerals containing strontium, and karst water is mainly derived from carbonate rock dissolution with similar characteristics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample Number | Type | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | TH | TDS | pH | Sr2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | SPGW | 1.60 | 86.00 | 90.18 | 46.17 | 99.26 | 110.47 | 390.53 | 0.52 | 415.33 | 639.83 | 7.4 | 1.1 |
| S2 | SPGW | 1.30 | 132.00 | 112.22 | 78.98 | 141.80 | 211.33 | 549.18 | 21.55 | 605.48 | 981.27 | 7.4 | 1.7 |
| S3 | SPGW | 3.60 | 196.00 | 210.42 | 113.00 | 382.86 | 218.54 | 744.44 | 0.55 | 990.79 | 1508.78 | 7.0 | 2.3 |
| S4 | KGW | 0.80 | 24.00 | 76.15 | 18.23 | 24.82 | 38.42 | 280.69 | 9.68 | 265.21 | 339.94 | 7.8 | 0.5 |
| S5 | KGW | 0.80 | 24.00 | 76.15 | 21.87 | 21.27 | 62.44 | 274.59 | 3.95 | 280.22 | 355.28 | 7.8 | 0.5 |
| S6 | KGW | 1.00 | 20.00 | 80.16 | 24.30 | 24.82 | 81.65 | 274.59 | 3.52 | 300.24 | 380.89 | 8.0 | 0.6 |
| S7 | KGW | 0.80 | 46.00 | 124.25 | 34.02 | 85.08 | 96.06 | 378.32 | 17.67 | 450.36 | 600.54 | 7.6 | 0.7 |
| S8 | SPGW | 0.40 | 80.00 | 52.10 | 59.54 | 56.72 | 43.23 | 457.65 | 42.70 | 375.30 | 574.01 | 8.0 | 1.2 |
| S9 | SPGW | 1.80 | 70.00 | 148.30 | 94.77 | 191.43 | 165.70 | 536.98 | 0.86 | 760.61 | 949.17 | 7.4 | 1.8 |
| S10 | SPGW | 1.00 | 240.00 | 78.16 | 94.77 | 194.98 | 240.15 | 683.42 | 0.45 | 585.47 | 1200.58 | 8.1 | 2.1 |
| S11 | DPGW | 0.75 | 440.00 | 20.04 | 13.37 | 315.51 | 341.01 | 274.59 | <0.20 | 105.08 | 1288.69 | 8.3 | 0.8 |
| S12 | SPGW | 2.20 | 200.00 | 148.30 | 92.34 | 177.25 | 302.59 | 738.34 | 0.75 | 750.60 | 1304.84 | 7.4 | 1.1 |
| S13 | SPGW | 1.20 | 150.00 | 80.16 | 104.49 | 131.17 | 196.92 | 677.32 | 3.53 | 630.50 | 1013.63 | 7.7 | 1.6 |
| S14 | SPGW | 2.80 | 450.00 | 116.23 | 134.87 | 407.68 | 487.50 | 884.79 | 12.73 | 845.68 | 2061.57 | 7.4 | 3.0 |
| S15 | SPGW | 1.70 | 100.00 | 122.24 | 69.26 | 56.72 | 72.05 | 781.06 | 0.35 | 590.47 | 822.08 | 7.2 | 1.3 |
| S16 | SPGW | 2.80 | 204.00 | 78.16 | 113.00 | 198.52 | 67.24 | 915.30 | 0.86 | 660.53 | 1135.85 | 7.4 | 2.3 |
| S17 | SPGW | 1.20 | 196.00 | 70.14 | 92.34 | 170.16 | 28.82 | 842.08 | 1.61 | 555.44 | 993.40 | 7.8 | 2.4 |
| S18 | SPGW | 0.80 | 228.00 | 72.14 | 109.35 | 180.80 | 206.53 | 768.85 | 0.55 | 630.50 | 1191.84 | 7.8 | 1.8 |
| S19 | SPGW | 1.50 | 180.00 | 116.23 | 85.05 | 223.34 | 206.53 | 585.79 | 1.43 | 640.51 | 1120.71 | 7.5 | 1.8 |
| S20 | SPGW | 0.80 | 240.00 | 120.24 | 102.06 | 134.71 | 292.98 | 713.93 | 153.00 | 720.58 | 1408.26 | 7.8 | 1.1 |
| S21 | SPGW | 11.00 | 180.00 | 74.15 | 72.90 | 109.90 | 124.88 | 671.22 | 23.16 | 485.39 | 939.09 | 7.8 | 0.8 |
| S22 | SPGW | 0.80 | 260.00 | 44.09 | 64.40 | 63.81 | 158.50 | 817.67 | 0.91 | 375.30 | 1010.42 | 8.0 | 0.8 |
| S23 | SPGW | 0.60 | 390.00 | 38.08 | 55.89 | 233.97 | 235.35 | 683.42 | 0.20 | 325.26 | 1304.30 | 8.2 | 0.8 |
| S24 | SPGW | 0.50 | 180.00 | 44.09 | 111.78 | 99.26 | 211.33 | 720.04 | 3.64 | 570.46 | 1019.80 | 8.2 | 1.5 |
| S25 | SPGW | 1.00 | 480.00 | 90.18 | 153.09 | 425.40 | 557.15 | 848.18 | 0.44 | 855.68 | 2138.29 | 7.7 | 1.8 |
| S26 | SPGW | 1.00 | 184.00 | 100.20 | 103.28 | 255.24 | 196.92 | 604.10 | 0.40 | 675.54 | 1150.59 | 7.8 | 1.5 |
| S27 | SPGW | 1.40 | 340.00 | 180.36 | 134.87 | 425.40 | 569.16 | 628.51 | 14.88 | 1005.80 | 1988.49 | 7.4 | 1.6 |
| S28 | SPGW | 1.00 | 420.00 | 110.22 | 157.95 | 475.03 | 600.38 | 634.61 | 15.33 | 925.74 | 2104.90 | 7.6 | 1.9 |
| S29 | SPGW | 2.50 | 600.00 | 176.35 | 221.13 | 645.19 | 840.53 | 1037.34 | 1.11 | 1351.08 | 3018.28 | 7.5 | 2.5 |
| S30 | SPGW | 1.80 | 192.00 | 98.20 | 66.83 | 81.54 | 148.89 | 817.67 | 0.28 | 520.42 | 1009.21 | 7.8 | 0.8 |
| S31 | SPGW | 1.60 | 96.00 | 82.16 | 78.98 | 85.08 | 110.47 | 610.20 | <0.20 | 530.42 | 768.59 | 7.8 | 1.3 |
| S32 | SPGW | 1.00 | 74.00 | 86.17 | 37.67 | 31.91 | 52.83 | 524.77 | <0.20 | 370.30 | 556.86 | 7.6 | 0.6 |
| S33 | KGW | 0.50 | 26.00 | 74.15 | 15.80 | 21.27 | 48.03 | 256.28 | 14.33 | 250.20 | 335.72 | 8.1 | 0.3 |
| S34 | KGW | 0.40 | 18.00 | 70.14 | 19.44 | 24.82 | 48.03 | 237.98 | 14.89 | 255.20 | 322.20 | 7.8 | 0.3 |
| S35 | KGW | 0.40 | 22.00 | 66.13 | 18.23 | 21.27 | 48.03 | 237.98 | 11.96 | 240.19 | 314.51 | 8.1 | 0.3 |
| S36 | SPGW | 0.10 | 64.00 | 98.20 | 65.61 | 53.18 | 76.85 | 604.10 | 0.25 | 515.41 | 669.73 | 7.2 | 0.8 |
| S37 | SPGW | 0.30 | 100.00 | 52.10 | 91.13 | 152.44 | 86.45 | 488.16 | 8.80 | 505.40 | 742.89 | 7.9 | 1.2 |
| S38 | SPGW | 0.40 | 180.00 | 46.09 | 47.39 | 106.35 | 134.48 | 518.67 | 0.26 | 310.25 | 782.13 | 8.1 | 0.6 |
| S39 | SPGW | 40.00 | 240.00 | 66.13 | 207.77 | 340.32 | 403.45 | 829.87 | 0.53 | 1020.82 | 1721.66 | 7.4 | 2.1 |
| S40 | SPGW | 0.40 | 288.00 | 230.46 | 185.90 | 514.03 | 626.79 | 689.53 | 56.75 | 1341.07 | 2254.58 | 7.3 | 2.1 |
| S41 | SPGW | 2.50 | 690.00 | 42.08 | 336.56 | 620.38 | 1071.07 | 1263.11 | 0.52 | 1491.19 | 3403.03 | 7.4 | 2.4 |
| S42 | SPGW | 1.00 | 200.00 | 162.32 | 91.13 | 290.69 | 365.03 | 543.08 | 7.64 | 780.62 | 1396.85 | 7.8 | 1.0 |
| S43 | SPGW | 1.50 | 53.00 | 132.26 | 80.19 | 163.07 | 134.48 | 506.47 | <0.20 | 660.53 | 831.04 | 7.4 | 1.6 |
| S44 | SPGW | 1.50 | 370.00 | 90.18 | 193.19 | 276.51 | 787.69 | 738.34 | 0.70 | 1020.82 | 2097.56 | 7.8 | 1.7 |
| S45 | SPGW | 0.60 | 256.00 | 88.18 | 125.15 | 191.43 | 341.01 | 774.95 | 0.38 | 735.59 | 1398.72 | 7.7 | 1.3 |
| S46 | SPGW | 0.50 | 94.00 | 78.16 | 98.42 | 60.27 | 182.51 | 659.02 | <0.20 | 600.48 | 853.86 | 8.2 | 1.3 |
| S47 | SPGW | 0.50 | 100.00 | 72.14 | 85.05 | 31.91 | 115.27 | 713.93 | <0.20 | 530.42 | 770.66 | 7.8 | 1.7 |
| S48 | SPGW | 1.50 | 380.00 | 118.24 | 172.53 | 283.60 | 725.25 | 817.67 | 2.60 | 1005.80 | 2101.05 | 7.8 | 1.9 |
| S49 | SPGW | 1.50 | 460.00 | 152.30 | 246.65 | 496.30 | 991.82 | 781.06 | 14.49 | 1396.12 | 2761.24 | 7.5 | 2.9 |
| S50 | DPGW | 0.80 | 296.00 | 4.01 | 1.22 | 85.08 | 110.47 | 463.75 | 0.63 | 15.01 | 760.40 | 8.4 | 0.2 |
| S51 | DPGW | 0.50 | 390.00 | 14.03 | 25.52 | 223.34 | 206.53 | 500.36 | 0.43 | 140.11 | 1151.73 | 8.3 | 1.4 |
| S52 | DPGW | 0.50 | 296.00 | 8.02 | 9.72 | 56.72 | 110.47 | 622.40 | 0.57 | 60.05 | 803.78 | 8.2 | 0.4 |
| S53 | DPGW | 1.60 | 180.00 | 68.14 | 86.27 | 109.90 | 177.71 | 689.53 | <0.20 | 525.42 | 978.39 | 7.8 | 1.3 |
| S54 | DPGW | 1.00 | 130.00 | 80.16 | 74.12 | 109.90 | 148.89 | 573.59 | 0.43 | 505.40 | 841.29 | 7.4 | 1.5 |
| S55 | DPGW | 1.00 | 370.00 | 36.07 | 10.94 | 95.72 | 105.67 | 811.57 | 0.58 | 135.11 | 1036.19 | 7.2 | 0.6 |
| S56 | DPGW | 0.50 | 420.00 | 20.04 | 41.31 | 276.51 | 326.60 | 500.36 | 0.42 | 220.18 | 1345.94 | 7.8 | 0.4 |
| S57 | DPGW | 0.50 | 280.00 | 12.02 | 10.94 | 173.71 | 139.29 | 372.22 | 0.60 | 75.06 | 812.49 | 8.0 | 0.9 |
| S58 | SPGW | 2.60 | 168.00 | 108.22 | 92.34 | 194.98 | 43.23 | 878.69 | 0.45 | 650.52 | 1062.70 | 7.9 | 1.8 |
| S59 | DPGW | 0.25 | 240.00 | 8.02 | 3.65 | 46.09 | 81.65 | 414.94 | 0.29 | 35.03 | 638.68 | 8.4 | 0.2 |
| S60 | DPGW | 0.50 | 288.00 | 14.03 | 18.23 | 138.26 | 158.50 | 384.43 | 0.43 | 110.09 | 869.89 | 8.3 | 0.4 |
| S61 | SPGW | 0.70 | 168.00 | 50.10 | 76.55 | 191.43 | 120.08 | 494.26 | <0.20 | 440.35 | 862.56 | 7.8 | 1.7 |
| S62 | DPGW | 1.25 | 450.00 | 34.07 | 20.66 | 301.33 | 461.09 | 305.10 | 0.58 | 170.14 | 1430.34 | 7.8 | 0.9 |
| S63 | DPGW | 1.25 | 440.00 | 44.09 | 19.44 | 311.96 | 437.07 | 311.20 | 0.33 | 190.15 | 1419.05 | 7.8 | 0.9 |
| S64 | SPGW | 2.40 | 288.00 | 140.28 | 95.99 | 358.05 | 408.26 | 543.08 | 0.36 | 745.60 | 1577.36 | 7.8 | 1.5 |
| S65 | DPGW | 0.50 | 260.00 | 10.02 | 4.86 | 70.90 | 100.86 | 433.24 | 0.67 | 45.04 | 711.60 | 8.3 | 0.3 |
| S66 | DPGW | 0.50 | 288.00 | 12.02 | 6.08 | 53.18 | 105.67 | 482.06 | 0.80 | 55.04 | 762.21 | 8.4 | 0.2 |
| S67 | DPGW | 1.50 | 370.00 | 14.03 | 12.15 | 230.43 | 254.56 | 378.32 | 0.86 | 85.07 | 1082.60 | 8.0 | 0.2 |
| S68 | DPGW | 1.00 | 380.00 | 20.04 | 8.51 | 241.06 | 273.77 | 360.02 | 0.58 | 85.07 | 1113.71 | 8.1 | 0.2 |
| S69 | DPGW | 1.00 | 380.00 | 18.04 | 15.80 | 248.15 | 278.57 | 329.51 | 0.49 | 110.09 | 1116.79 | 8.0 | 0.3 |
| S70 | DPGW | 1.00 | 360.00 | 16.03 | 30.38 | 212.70 | 312.20 | 396.63 | 0.77 | 165.13 | 1142.48 | 8.2 | 0.6 |
| S71 | DPGW | 0.70 | 160.00 | 6.01 | 10.94 | 53.18 | 52.83 | 280.69 | 0.30 | 60.05 | 461.80 | 8.4 | 0.2 |
| S72 | DPGW | 2.00 | 670.00 | 26.05 | 52.25 | 510.48 | 653.21 | 347.81 | 2.52 | 280.22 | 2129.59 | 8.5 | 0.7 |
| S73 | DPGW | 0.60 | 256.00 | 14.03 | 4.86 | 70.90 | 76.85 | 433.24 | 0.54 | 55.04 | 687.82 | 8.6 | 0.2 |
| S74 | DPGW | 5.00 | 670.00 | 116.23 | 128.79 | 659.37 | 691.63 | 781.06 | 0.78 | 820.66 | 2671.04 | 7.4 | 1.4 |
| S75 | DPGW | 1.50 | 470.00 | 24.05 | 14.58 | 248.15 | 432.27 | 463.75 | 1.87 | 120.10 | 1435.39 | 7.8 | 0.4 |
| S76 | DPGW | 1.00 | 340.00 | 14.03 | 14.58 | 127.62 | 211.33 | 524.77 | 0.78 | 95.08 | 983.58 | 7.8 | 0.2 |
| S77 | DPGW | 1.00 | 308.00 | 6.01 | 7.29 | 56.72 | 168.11 | 482.06 | 1.30 | 45.04 | 838.31 | 8.3 | 0.1 |
| S78 | DPGW | 0.80 | 94.00 | 104.21 | 44.96 | 226.88 | 120.08 | 237.98 | 5.01 | 445.36 | 724.92 | 7.4 | 0.7 |
| S79 | DPGW | 0.90 | 84.00 | 106.21 | 38.88 | 106.35 | 220.94 | 262.39 | 4.61 | 425.34 | 702.58 | 7.5 | 0.7 |
| S80 | KGW | 0.70 | 40.00 | 50.10 | 52.25 | 49.63 | 91.26 | 329.51 | 15.29 | 340.27 | 471.48 | 7.4 | 0.8 |
| S81 | DPGW | 1.00 | 340.00 | 12.02 | 29.16 | 184.34 | 321.80 | 366.12 | <0.20 | 150.12 | 1080.73 | 8.2 | 0.6 |
| S82 | SPGW | 0.80 | 88.00 | 10.02 | 95.99 | 74.45 | 120.08 | 457.65 | 15.11 | 420.34 | 642.01 | 8.0 | 1.3 |
| S83 | SPGW | 0.80 | 276.00 | 38.08 | 44.96 | 46.09 | 105.67 | 872.59 | 0.27 | 280.22 | 957.77 | 7.8 | 0.9 |
| S84 | SPGW | 1.70 | 104.00 | 208.42 | 38.88 | 159.53 | 100.86 | 659.02 | 2.03 | 680.54 | 954.04 | 7.0 | 1.5 |
| S85 | SPGW | 0.60 | 228.00 | 68.14 | 120.29 | 116.99 | 422.66 | 695.63 | 0.87 | 665.53 | 1315.20 | 8.0 | 1.3 |
| S86 | SPGW | 1.00 | 370.00 | 164.33 | 170.10 | 319.05 | 790.09 | 787.16 | 0.31 | 1110.89 | 2218.25 | 7.5 | 2.1 |
| S87 | SPGW | 1.00 | 460.00 | 100.20 | 166.46 | 315.51 | 730.06 | 884.79 | 0.68 | 935.75 | 2228.15 | 7.3 | 1.7 |
| S88 | SPGW | 1.50 | 610.00 | 104.21 | 234.50 | 425.40 | 1104.69 | 970.22 | 0.26 | 1225.98 | 2978.98 | 7.2 | 2.0 |
References
- Gao, M.; Li, X.; Qian, J.; Wang, Z.; Hou, X.; Gui, C.; Bai, Z.; Fu, C.; Li, J.; Zuo, X. Hydrochemical Characteristics and Controlling Factors of Shallow and Deep Groundwater in the Heilongdong Spring Basin, Northern China. Sustainability 2023, 15, 15447. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J. Clean. Prod. 2021, 282, 125416. [Google Scholar] [CrossRef]
- Li, Z.; Coles, A.; Xiao, J. Groundwater and streamflow sources in China’s Loess Plateau on catchment scale. CATENA 2019, 181, 104075. [Google Scholar] [CrossRef]
- Qu, S.; Duan, L.; Mao, H.; Wang, C.; Liang, X.; Luo, A.; Huang, L.; Yu, R.; Miao, P.; Zhao, Y. Hydrochemical and isotopic fingerprints of groundwater origin and evolution in the Urangulan River basin, China’s Loess Plateau. Sci. Total Environ. 2023, 866, 161377. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yun, S.; Yu, S.; Kim, K.; Lee, J.; Lee, S. The combined use of self-organizing map technique and fuzzy c-means clustering to evaluate urban groundwater quality in Seoul metropolitan city, South Korea. J. Hydrol. 2019, 569, 685–697. [Google Scholar] [CrossRef]
- Alvarez, M.; Funes, D.; Dapeña, C.; Bouza, P. Origin and hydrochemical characteristics of groundwater in the Northeastern Patagonia, Argentina: The relationship with geomorphology and soils. Environ. Earth Sci. 2020, 79, 503. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Ke, X.; Ou, A.; Wang, D. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water 2022, 14, 1874. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Qian, H.; Gao, Y. Groundwater Chemistry Regulated by Hydrochemical Processes and Geological Structures: A Case Study in Tongchuan, China. Water 2018, 10, 338. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Wang, M.; Li, Y.; Shi, M.; Zhang, H.; Ma, Y. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ. Earth Sci. 2019, 78, 76. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Cai, W.; Wang, Z.; Li, C. Numerical investigation on the effects of geological parameters and layered subsurface on the thermal performance of medium-deep borehole heat exchanger. Renew. Energy 2020, 149, 384–399. [Google Scholar] [CrossRef]
- Zhai, Y.; Zheng, F.; Zhao, X.; Xia, X.; Teng, Y. Identification of hydrochemical genesis and screening of typical groundwater pollutants impacting human health: A case study in Northeast China. Environ. Pollut. 2019, 252, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, X.; Bush, R. Chemical weathering and CO2 consumption in the Lower Mekong River. Sci. Total Environ. 2014, 472, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Luo, Q.; Wu, J.; Xu, S.; Wu, J. Identification of the long-term variations of groundwater and their governing factors based on hydrochemical and isotopic data in a river basin. J. Hydrol. 2021, 592, 125604. [Google Scholar] [CrossRef]
- Dai, L.; Mei, M.; Chu, C.; Lo, E. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gan, S.; Li, J.; Dong, Z.; Long, Q.; Qiu, S.; Zhou, Y.; Lu, C. Groundwater Chemistry and Pollution in a Changing Environment: Monitoring, Mechanisms, and Remediations. J. Chem. 2021, 2021, 5547924. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Zhai, Y.; Su, Y.; Liu, C. An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei. Water 2021, 13, 699. [Google Scholar] [CrossRef]
- Zuo, R.; Teng, Y.; Wang, J. Modeling migration of strontium in sand and gravel aquifer in the candidate VLLW disposal site. J. Radioanal. Nucl. Chem. 2009, 281, 653–662. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Han, P.-F.; Wang, J.; Wan, L. Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China. Sustainability 2023, 15, 5091. [Google Scholar] [CrossRef]
- Peng, H.; Zou, P.; Ma, C.; Xiong, S.; Lu, T. Elements in potable groundwater in Rugao longevity area, China: Hydrogeochemical characteristics, enrichment patterns and health assessments. Ecotoxicol. Environ. Saf. 2021, 218, 112279. [Google Scholar] [CrossRef]
- Athamena, A.; Gaagai, A.; Aouissi, H.A.; Burlakovs, J.; Bencedira, S.; Zekker, I.; Krauklis, A.E. Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability 2023, 15, 20. [Google Scholar] [CrossRef]
- DZ/T 0064; Methods for Analysis of Groundwater Quality. Ministry of Natural Resources, China: Beijing, China.
- Peng, H.; Hou, Q.; Zen, M.; Huang, C.; Shi, H.; Pi, P.; Pan, Y. Hydrochemical Characteristics and Controlling Factors of Groundwater in the Leizhou Peninsula. Environ. Sci. 2021, 42, 5374–5384. [Google Scholar]
- Xiao, Y.; Liu, K.; Hao, Q.; Xiao, D.; Zhu, Y.; Yin, S.; Zhang, Y. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Wei, H.; Liang, X.; Liu, S.; Liu, M.; Xiao, C. Hydrochemical Evolution of Groundwater in Dehui, China. Water 2020, 12, 3378. [Google Scholar] [CrossRef]
- Gibbs, R. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1890. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, S.; Wang, B. Hydrochemistry characteristics of groundwater with the influence of spatial variability and water flow in Hetao Irrigation District, China. Environ. Sci. Pollut. Res. 2022, 29, 71150–71164. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhao, Z.; Tao, F.; Liu, B.; Tao, Z.; Gao, S.; Zhang, L. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Q. Groundwater Chemical Characteristics and Controlling Factors in a Region of Northern China with Intensive Human Activity. Int. J. Environ. Res. Public Health 2020, 17, 9126. [Google Scholar] [CrossRef]
- Peng, H.; Yang, W.; Ferrer, A.; Xiong, S.; Li, X.; Niu, G.; Lu, T. Hydrochemical characteristics and health risk assessment of groundwater in karst areas of southwest China: A case study of Bama, Guangxi. J. Clean. Prod. 2022, 341, 130872. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H.; Dan, J. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resour. Res. 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Sami, K. Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J. Hydrol. 1992, 139, 27–48. [Google Scholar] [CrossRef]
- Thimonier, A.; Schmitt, M.; Waldner, P.; Schleppi, P. Seasonality of the Na/Cl ratio in precipitation and implications of canopy leaching in validating chemical analyses of throughfall samples. Atmos. Env. 2008, 42, 9106–9117. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, C.; Zheng, L.; Zhang, L.; Fu, X.; Chen, S.; Chen, Y.; Hu, J. Evaluating the genesis and dominant processes of groundwater salinization by using hydrochemistry and multiple isotopes in a mining city. Environ. Pollut. 2021, 283, 117381. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, Y. Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci. Total Environ. 2015, 508, 155–165. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, M.; Zhang, Z.; Liu, T.; He, J. Hydrochemical characteristics and possible controls of the surface water in ranwu lake basin. Environ. Sci. 2020, 41, 4003–4010. (In Chinese) [Google Scholar]
- Su, C. Formation Mechanism of Strontium-Rich Groundwater in Xintian County, Hunan Province. Ph.D. Thesis, China University of Geosciences, Shenzhen, China, 2022. (In Chinese). [Google Scholar]
- Chen, C. Distribution Characteristics and Formation Mechanism of Strontium Rich and Metasilicic Acid Rich Groundwater in Muwen River Groundwater System. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2020. (In Chinese). [Google Scholar]




| Index | Detection Limit (mg/L) |
|---|---|
| pH | / |
| TH | 3 mg/L (CaCO3) |
| TDS | / |
| K+ | 0.5 |
| Na+ | 0.6 |
| Ca2+ | 4 |
| Mg2+ | 3 |
| Cl− | 3 |
| SO42− | 10 |
| HCO3− | 5 |
| NO3− | 0.2 |
| Type | Index | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | TH | TDS | pH | Sr2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPGW | AV | 2.24 | 242.80 | 101.18 | 113.95 | 224.73 | 326.37 | 706.64 | 8.14 | 721.95 | 1382.29 | 7.65 | 1.59 |
| CV | 2.50 | 0.63 | 0.48 | 0.53 | 0.70 | 0.89 | 0.23 | 2.87 | 0.41 | 0.50 | 0.04 | 0.36 | |
| Min | 0.10 | 53.00 | 10.02 | 37.67 | 31.91 | 28.82 | 390.53 | 0.00 | 280.22 | 556.86 | 7.00 | 0.60 | |
| MD | 1.20 | 200.00 | 90.18 | 95.99 | 191.43 | 206.53 | 695.63 | 0.68 | 660.53 | 1135.85 | 7.70 | 1.60 | |
| Max | 40.00 | 690.00 | 230.46 | 336.56 | 645.19 | 1104.69 | 1263.11 | 153.00 | 1491.19 | 3403.03 | 8.20 | 3.00 | |
| DPGW | AV | 1.05 | 332.76 | 30.41 | 26.19 | 191.19 | 244.13 | 440.82 | 0.94 | 183.77 | 1069.72 | 8.02 | 0.58 |
| CV | 0.82 | 0.42 | 1.06 | 1.09 | 0.74 | 0.67 | 0.33 | 1.27 | 1.02 | 0.42 | 0.05 | 0.71 | |
| Min | 0.25 | 84.00 | 4.01 | 1.22 | 46.09 | 52.83 | 237.98 | 0.00 | 15.01 | 461.80 | 7.20 | 0.10 | |
| MD | 1.00 | 340.00 | 16.03 | 14.58 | 173.71 | 206.53 | 414.94 | 0.58 | 110.09 | 983.58 | 8.10 | 0.40 | |
| Max | 5.00 | 670.00 | 116.23 | 128.79 | 659.37 | 691.63 | 811.57 | 5.01 | 820.66 | 2671.04 | 8.60 | 1.50 | |
| KGW | AV | 0.68 | 27.50 | 77.15 | 25.52 | 34.12 | 64.24 | 283.74 | 11.41 | 297.74 | 390.07 | 7.83 | 0.50 |
| CV | 0.32 | 0.36 | 0.27 | 0.48 | 0.66 | 0.35 | 0.17 | 0.46 | 0.23 | 0.25 | 0.03 | 0.39 | |
| Min | 0.40 | 18.00 | 50.10 | 15.80 | 21.27 | 38.42 | 237.98 | 3.52 | 240.19 | 314.51 | 7.40 | 0.30 | |
| MD | 0.75 | 24.00 | 75.15 | 20.66 | 24.82 | 55.24 | 274.59 | 13.15 | 272.72 | 347.61 | 7.80 | 0.50 | |
| Max | 1.00 | 46.00 | 124.25 | 52.25 | 85.08 | 96.06 | 378.32 | 17.67 | 450.36 | 600.54 | 8.10 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, X.; Zhao, J.; Liu, S.; Zhang, Y.; Liu, J.; Gao, Z. Hydrochemical Characteristics, Controlling Factors and Strontium Enrichment Sources of Groundwater in the Northwest Plain of Shandong Province, China. Water 2024, 16, 550. https://doi.org/10.3390/w16040550
Chen J, Wu X, Zhao J, Liu S, Zhang Y, Liu J, Gao Z. Hydrochemical Characteristics, Controlling Factors and Strontium Enrichment Sources of Groundwater in the Northwest Plain of Shandong Province, China. Water. 2024; 16(4):550. https://doi.org/10.3390/w16040550
Chicago/Turabian StyleChen, Jingpeng, Xiaohua Wu, Jichu Zhao, Shuai Liu, Yuqi Zhang, Jiutan Liu, and Zongjun Gao. 2024. "Hydrochemical Characteristics, Controlling Factors and Strontium Enrichment Sources of Groundwater in the Northwest Plain of Shandong Province, China" Water 16, no. 4: 550. https://doi.org/10.3390/w16040550
APA StyleChen, J., Wu, X., Zhao, J., Liu, S., Zhang, Y., Liu, J., & Gao, Z. (2024). Hydrochemical Characteristics, Controlling Factors and Strontium Enrichment Sources of Groundwater in the Northwest Plain of Shandong Province, China. Water, 16(4), 550. https://doi.org/10.3390/w16040550









