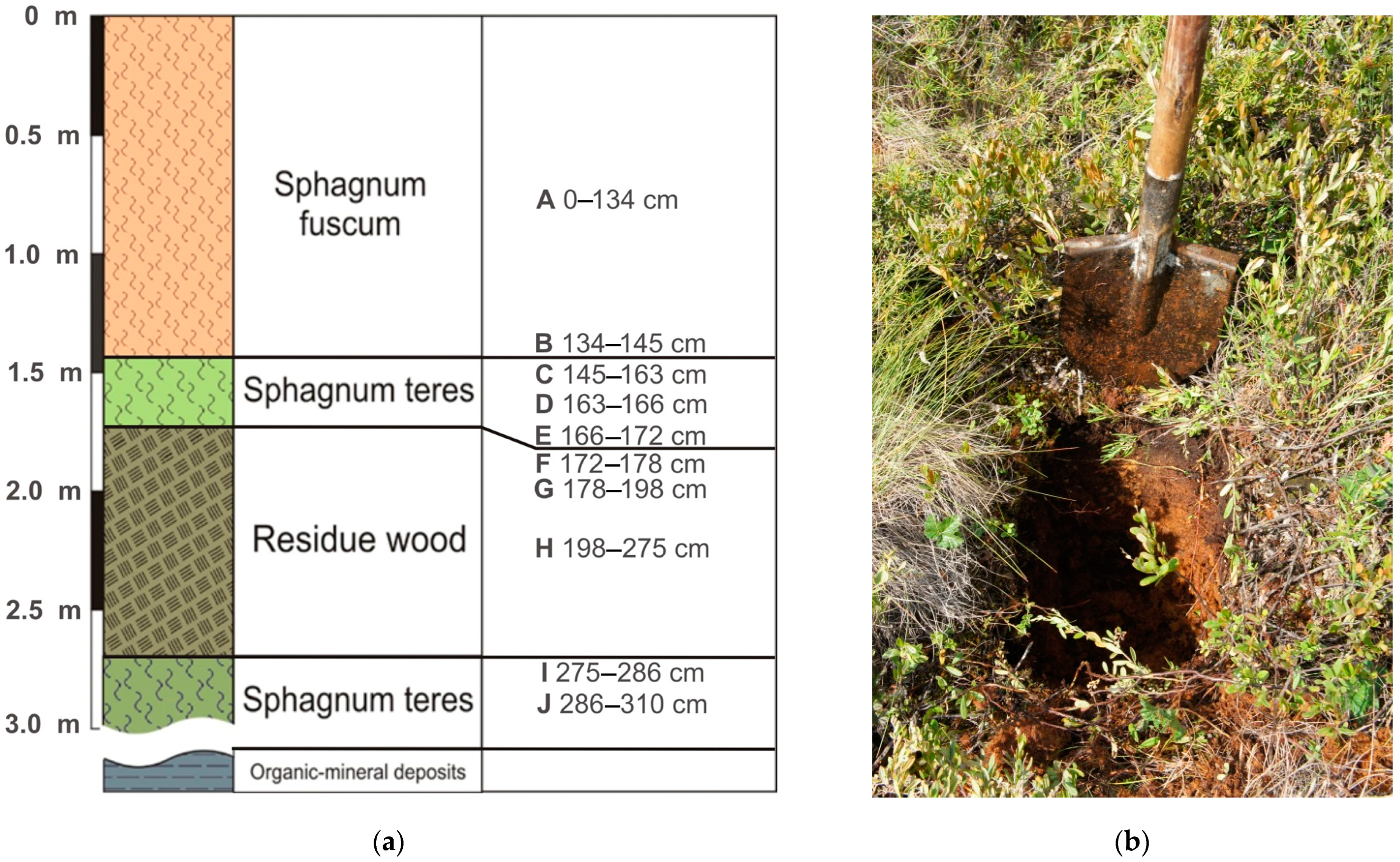
3.1.1. Chemical Composition of Bog and Pore Waters
According to the prevailing ions, bog water belongs to the chloride-sulfate class of the calcium group; according to alkaline–acid conditions, to the acid class; according to the value of total dissolved solids (TDS), to ultra-fresh water (
Table 1). The low pH values (3.4) of bog water are due to the decomposition of organic substances, leading to the release of CO
2, fulvic and humic acids and other organic matter (OM) compounds into the water. The distribution of Eh-pH parameters and the main ions in the pore water of the upper part of the section up to 163 cm (horizons A–C) is presented in
Table 1. Compared to the bog water, in the pore water, pH increases up to 5.5, and Eh decreases to the negative value of −134 mV (horizon C). A significant change in the TDS of pore water also occurs in horizon C due to an increase in the concentrations of anions NO
3−, HCO
3−, SO
42− and Ca
2+. It is obvious that the chemical composition of pore water changes strongly, and it becomes sulfate-nitrate-hydrocarbonate of the calcium group.
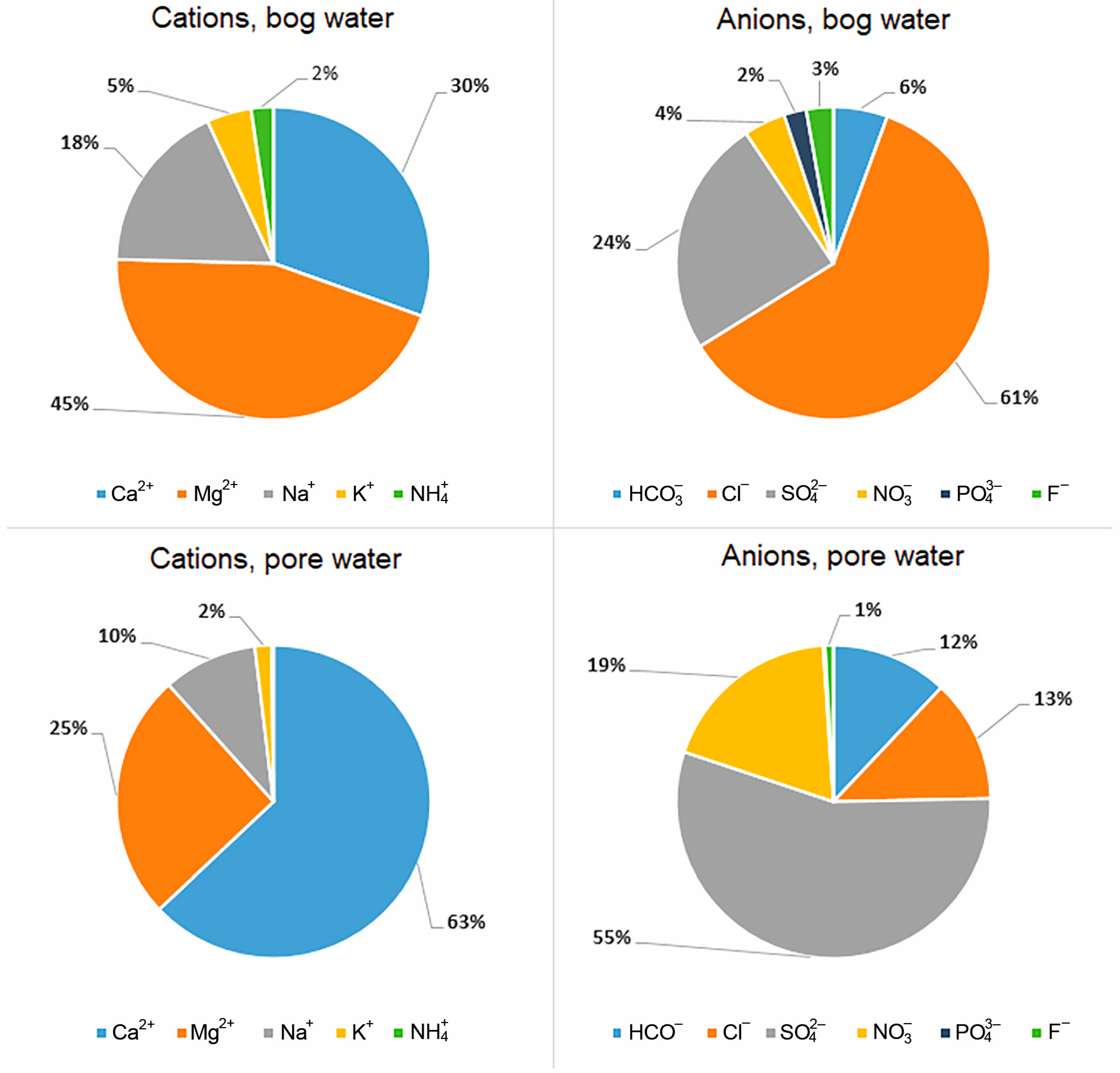
The increase in TDS with depth and the change in the relationship between the main cations and anions are a reflection of the diagenetic transformation of peat. The pie chart shows the ratios in % equivalent required to calculate ion-exchange and redox reactions, which allows one to more clearly see that Mg gives way to calcium with depth, and the chloride ion gives way to sulfate and nitrate (
Figure 2).
An increase in HCO
3− concentrations in water down the peat section from 2.4 to 22.0 ppm was established. The content of the hydrocarbonate ion in the peat section is low and averages 16% of the total anions. For SO
42−, a sharp increase in its concentration with depth from 8.5 to 80 ppm is also observed. The sulfate ion is one of the main anions in pore water and averages 51% of the total anions in the section. The concentration of Cl
− practically does not change along the depth; however, in the range of 0–18 cm (horizon A), an increase in chlorine from 15.4 to 29.9 ppm is noted. Chlorine is on average 35% of the total anions. The content of Ca
2+ in the pore water increases along the depth from 7.5 to 51 ppm. Calcium is the main cation; on average, it accounts for 55% of the total of all cations. In the distribution of Mg
2+, Na
+ and K
+ cations, there is no clearly expressed trend of their increase with depth (
Table 1), except an increase in K
+ concentration in the depth of 0–18 cm (horizon A) to 7 ppm, which may be associated with active mineralization of peat-forming plants.
An increase with depth in the concentration of NO
3−, one of the products of mineralization of OM during diagenesis, from 1.9 to 35 ppm was established. The decrease in ammonium nitrogen NH
4+, on the contrary, is not so noticeable with depth. Microbiological activity is responsible for the oxidation of ammonia to nitrate (2):
For PO43−, concentrations decrease with depth from 0.52 to 0.17 ppm. Such a behavior may be due to the formation of iron phosphates in the reducing intervals of the peat bog. However, in comparison with mineral deposits, the content of phosphorus in the pore water is quite high, which reflects the processes of anaerobic degradation of organic matter.
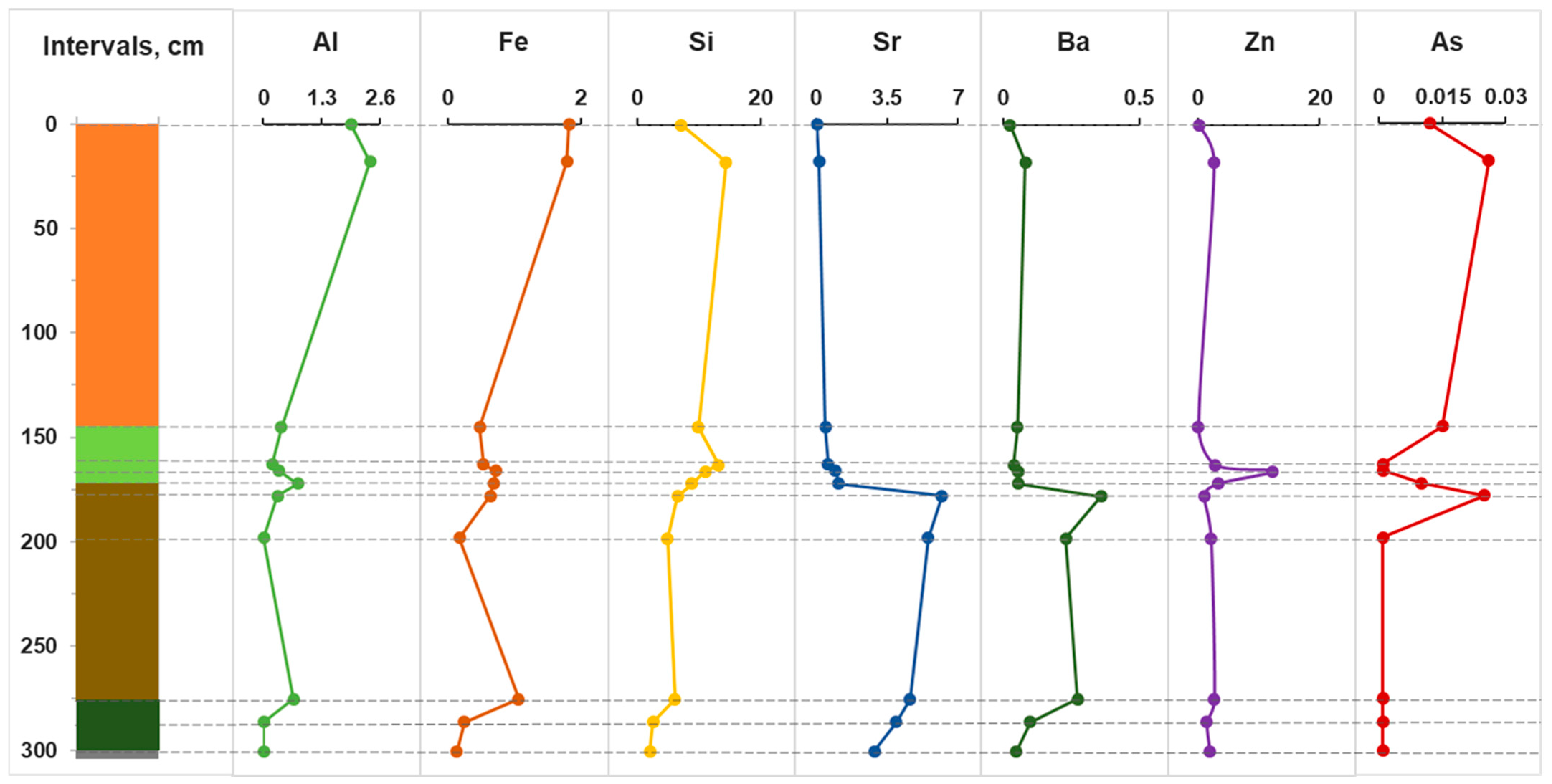
Figure 3 shows the distribution of some trace elements in pore water to the full depth of the section across the horizons. It should be noted that in pore water, the reducing environment in horizons C-J is co-stored, the pH reaches neutral values of 7.0, and the Eh minimum (−200 mV) occurs at a depth of 200 cm. Intense changes occur in the range of 160–200 cm, as can be seen from the example of all trace elements. Significant increases in concentrations are recorded for Al, Fe and Si. It is worth noting the distinct peaks for Sr, Ba, Zn and As. It is known that aluminum can play the role of an abiogenic indicator, i.e., terrigenous continental runoff [
53]. Then, comparing the behavior of Al, As and Zn, we can conclude that they are a consequence of the anthropogenic load on the bog ecosystem in the 20th–21st centuries. In addition, As is often associated with sulfides, primarily pyrite; therefore, an increase in its content in the pore water of the upper horizons may also be a consequence of the dissolution of sulfides.
It is worth mentioning the high concentrations of Al and Fe in bog water, the maximum allowable concentrations (MACs) of which for drinking water are 0.5 and 0.3 ppm, respectively. They form strong complexes with organic acids (TOC 128 mg/L), which may be the reason for their high concentrations. It should be noted that the extremely high concentrations of zinc in the pore water of the D horizon are up to 10 ppm (10 times higher than the MAC). High zinc contamination of oligotrophic bogs of the Baraba forest steppe was also noted earlier [
13,
44]. Thus, the destruction of a number of minerals and the diagenesis of peaty vegetation residues leads to an increase in the concentrations of Al, Fe and P in the slightly acidic (pH 4–4.5) pore water of the upper part (horizon A). The increase in the total P content in the upper intervals is a consequence of the active destruction of OM under aerobic conditions.
To identify sources and transformation of matter in bog water, Pearson’s correlation coefficients were calculated (
Table 2). A strong positive correlation of Cu-Tl, Zn-V and Mn-As pairs confirms the anthropogenic load on the bog ecosystem. The most mobile element in bog waters is Zn, which is indicated by high values of Cc at the level of 26.8 (
Table 3). The strong inverse correlation of the Al-B pair may be due to different sources of matter. Boron is a biophilic element, and its content in the ashes of dead plants is many times higher than that in the lithosphere and soils; aluminum is due to terrigenous drift, as mentioned above. In bog waters, the NH
4+/NO
3− ratio decreases with depth, which is a reflection of OM destruction processes. This is indicated by rather high values of Cc for NO
3− at the level of 7.3 (
Table 3).
Thus, this study revealed significant diagenetic transformations in peat, evidenced by changes in total dissolved solids (TDS) and the shifting relationship between major cations and anions in water. This transformation is reflected in the transition from chloride-sulfate to sulfate-nitrate-hydrocarbonate of the calcium group with increasing depth.
3.1.2. Chemical Composition of Peat and Underlying Sediments
The terrestrial sources of inorganic solids in the peats may include both mineral matter physically incorporated from the underlying sediments and elements that were added to the peats by water–rock interactions [
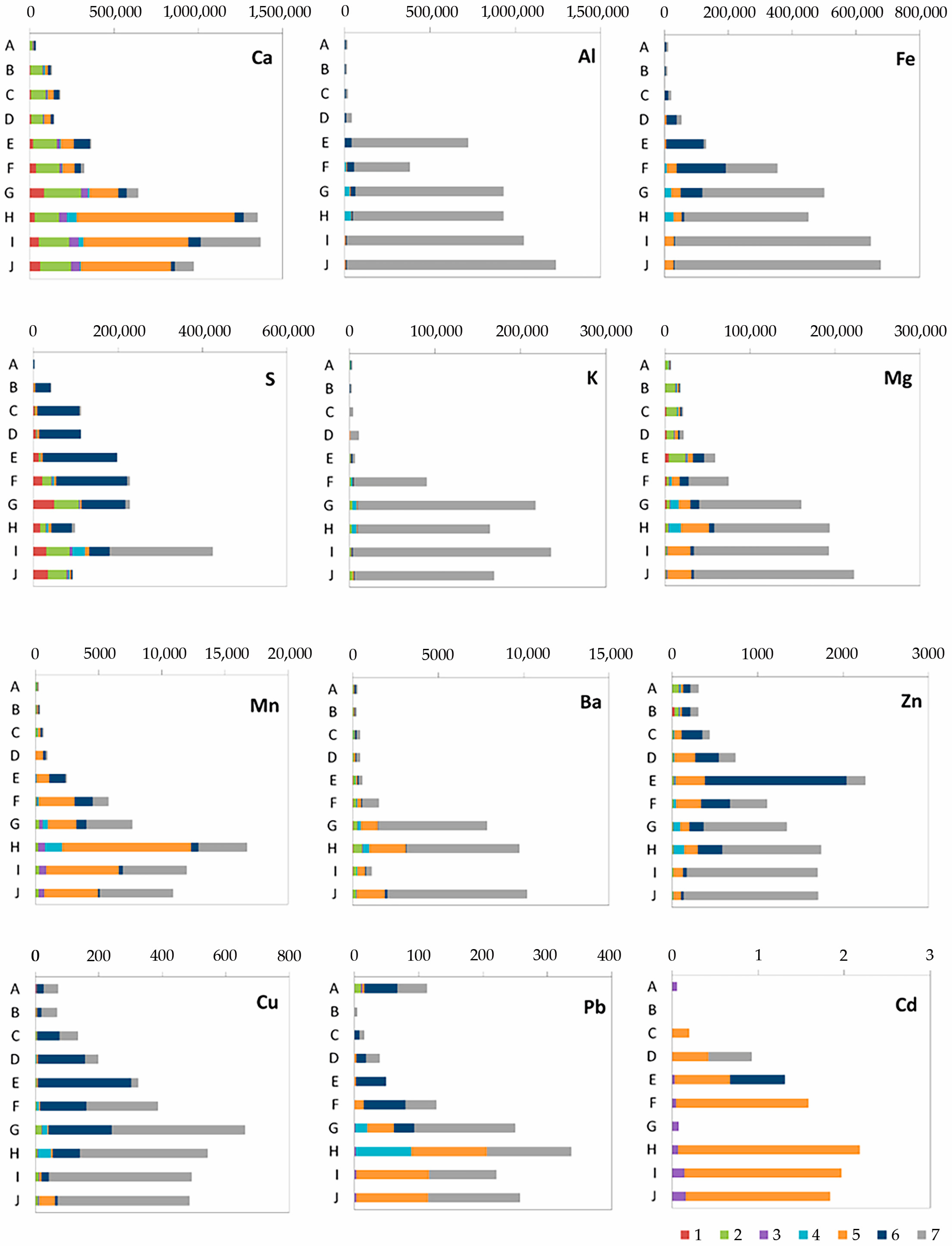
34]. The average chemical composition of the ash part of the peat deposits is shown in
Table 4. We divided all the elements into five groups for the data description: (a) lithophilic metals of the Al group (Al, K, Na, Li, Ba); (b) siderophilic metals of the Fe group (Fe, Mn, Cr, Ni); (c) lithophilic elements of the Ca group (Ca, Mg); (d) chalcophilic elements (As, Cd, Cu, Zn, Pb); (e) lithophilic and siderophilic elements of mixed valence forming cations and anions (Ti, V, U, Mo).
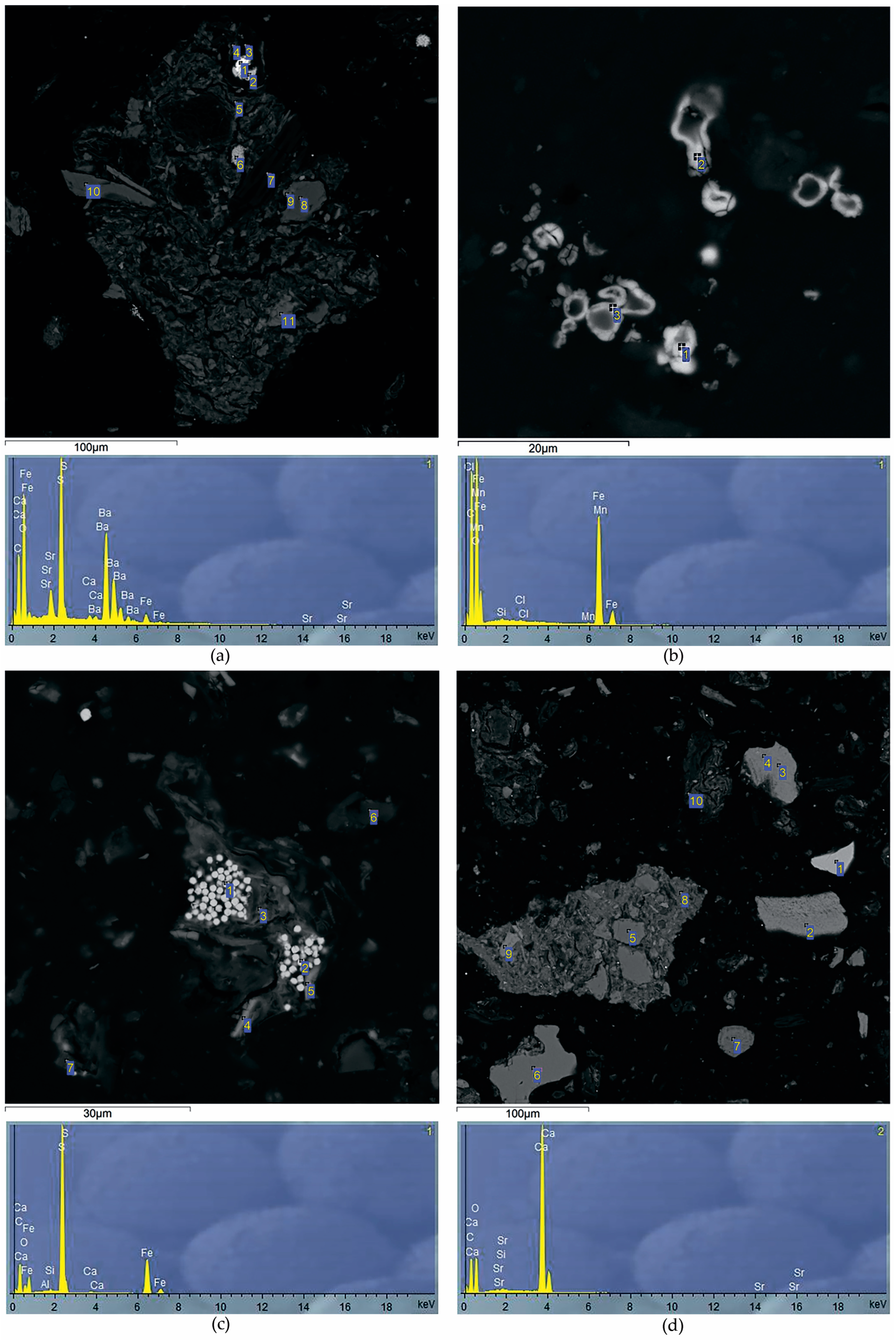
Since Al is one of the main components of peat ash, with an increase in ash content in peatland sections, Al concentrations increase synchronously. It was found that the minimum contents of Al (0.2 ppm), K (0.9%), Na (0.04 ppm) and Li (0.6 ppm) are characteristic of the upper peat horizons. According to the distribution similarity of Al, K, Na and Li in the Ubinskoye peatland, it can be concluded that these elements have a common source of input with atmospheric precipitation in the composition of silicate and aluminum silicate material. An increase in Ba content from 23 to 250 ppm in the lower peat horizons indicates the formation of authigenic Ba minerals in the diagenesis such as barite and barytocelestite detected in the peat interval 163–198 cm (horizons D-G) via the SEM method (
Table 5,
Figure 4a). The deposition mechanism of authigenic barite in the sediment is similar to the formation of Fe-Mn nodules. However, barite does not precipitate at the redox boundary, where Fe(II) and Mn(II) are oxidized and precipitated, but at the boundary of a sharp decrease in sulfate ions in bog waters. The precipitation zone of diagenetic barite is located just below the redox boundary, where active processes of bacterial sulfate reduction begin. The inclusion of Sr in the composition of barites clearly indicates their authigenic (diagenetic) nature—during the deposition process, such barites capture part of Sr from groundwater. In addition, the increase in the Ba/Al ratio from 0.0092 to 0.0125 in the grass transition type of peat indicates the leaching of barium from the underlying high-ash sites of peat and its deposition up the section.
All elements of the Fe group are closely correlated with each other, which reflects a similar direction of biogeochemical migration of elements both during the formation of the peat deposit of the Ubinskoye bog and during the diagenesis. Along with biochemical factors, the alkaline–acid and redox conditions of the medium play a huge role in their geochemistry. The upper peat intervals have oxidative medium conditions (+ 114 mV) and present an oxidative geochemical barrier on which elements of mixed valence are concentrated. Therefore, a significant increase in the content of Fe (1.4%), Cr (32 mg/kg), Ni (15 mg/kg), partially Mn (100 mg/kg) and Co (1.9 mg/kg) in the upper peat horizons can be associated with oxidation of their reduced forms coming with bog waters from the lower intervals of peatland with reducing conditions. In strong reducing conditions, Fe, Mn and Co actively change to a divalent state. Thus, the presence of mobile Fe and an unlimited reserve of carbonate ions leads to the formation of authigenic Fe carbonates, in particular siderite, during the diagenesis (
Table 5,
Figure 4a). In the section of the Ubinskoye peatland, siderite was found in the upper (0–145 cm) intervals of peat (
Figure 4b). The conditions for the formation and location of siderite in modern peatlands are rather complicated since the entire mineral system, and especially the upper intervals of peat, is unstable. The formation of the solid siderite phase is possible with partial loss of peat in CO
2 in deeper layers via the dissolution of bicarbonate iron ions due to a drop in the partial pressure of CO
2. Siderite formation occurs mainly in summer, when the process of oxidation of OM in the upper layers is significantly intensified. An increase in the concentration of hydrogen ions, even if the Eh values remain unchanged, shifts the mineral system towards the formation of siderite. In winter, partial dissolution of siderite in the upper layers of peat is quite possible, when the oxidation of OM is minimal, and bog water is somewhat enriched by dissolved oxygen and may have higher pH values.
Part of the reduced Fe(II) enters the upper layers of peat via a concentration gradient, where it is oxidized to Fe(III) to form iron oxides and hydroxides of various compositions. In the section of peat of the Ubinskoye bog, the presence of iron oxides is established only in the upper horizons of peat (0–134 cm), which are included in the zone of seasonal fluctuations in the bog water level. The source of chemically reactive iron for the formation of iron oxides may be peat alternations containing siderite. The solution of ferrobicarbonate with siderite in equilibrium during the dry season of the year can hydrolyze with sufficient oxygen supply with the precipitation of the solid phase Fe (OH)
2, which rapidly oxidizes to Fe
2O
3. In deeper, high-ash peat intervals, with an increase in the reducing settings, pyrite was established along the entire peatland section (
Table 5,
Figure 4c). Pyrite was formed in the early stages of diagenesis by the crystallization of amorphous Fe sulfides under the influence of H
2S in the process of bacterial sulfate reduction.
A less noticeable increase in Mn contents than in Fe in the uppermost peat layers is due to the difference in the migration characteristics of these elements: the oxidation of dissolved iron (and as a consequence, its precipitation) occurs already with an Eh value ≥ 0, while the oxidation of Mn is observed at higher Eh values (+ 200… + 600 mV). The increase in manganese from 0.01 to 0.07 with depth indicates the predominance of its authigenic forms in the lower layers of the peatland, for example, as part of the digenetic carbonates, particularly calcite, which was found here according to SEM in the lower (163–172 cm) high-ash intervals (
Figure 4d).
The group of Ca, Sr and partially Mg is characterized by their inclusion in the composition of authigenic carbonates formed during the peat formation. Increased concentrations of Ca (six times) and Mg (ten times) were detected in the lowest peatland intervals. In the upper part of the peat, the contents of this group of elements are minimal. We assume that the increase in Ca and Mg contents is a consequence of the formation of authigenic carbonates, in particular calcite and dolomite which are associated with both the change in water regime and the water content of the peatland at the early stages of its formation in the Holocene, and with the calcite redeposition during the diagenesis. Thus, the distribution of Ca and Mg is differentiated in a certain way along the peatland section—the upper horizons represented by the upper peat are depleted in Ca and Mg in contrast to the underlying layers of peat and organomineral deposits. In addition, a decrease in the contents of Ca in the upper intervals is also possible due to its removal by near-surface runoff, which leads to the partial calcium depletion of the upper peat of the Ubinskoye bog.
An increase in the concentrations of chalcophilic elements (As, Cd, Cu, Zn, Pb) in the upper peat intervals of the Ubinskoye bog was also found (
Table 4). Thus, for the upper intervals of the peatland, the concentrations of these elements were as follows: Cu—47.1; Zn—101; As—17.2; Cd—0.68; Pb—45.6 ppm. The increase in the concentration of chalcophilic elements in the upper intervals of the Ubinskoye peatland is explained by the higher technogenic load on the bog ecosystem, specifically the proximity of the highway and a major urban area of the Ubinskoye rural community. One of the entering sources of this group of elements is atmospheric transport.
Uranium was not found in the peat material except for in the lowest intervals, where its concentrations were up to 2.3 ppm. In the diagenesis, U slightly migrates in strong reducing conditions and precipitates on a reducing geochemical barrier. It is likely that low ash values of the upper peat can also cause low U content.
The distribution of V in the diagenesis is defined by its multivalence—a change in the oxidation state in a wide range from +2 to +5. The data show a slight increase in concentrations of V in a peat deposit depth from 22 to 65 ppm from the range of 163 cm, which is also a consequence of an increase in reducing medium conditions. It is worth noting that in the uppermost interval of the peatland, high V levels were established (28 ppm). This can be preliminarily associated with a technogenic load on the bog ecosystem since the main sources of V are chemicals, pesticides and atmospheric precipitation. However, we believe that the main way V enters the upper bog is through aerosol precipitation.
Thus, the study of the chemical composition of peat and underlying sediments in the Ubinskoye bog enabled the identification of characteristic behavior patterns for distinct elemental groupings such as lithophilic metals, siderophilic metals, chalcophilic elements and mixed valence elements. The distribution of elements like Al, K, Na and Li suggests a common source from atmospheric precipitation, while an increase in Ba content indicates the diagenetic formation of authigenic Ba minerals.
Elements of the Fe group (Fe, Cr, Ni, Mn, Co) show a close correlation, reflecting biogeochemical migration during both peat formation and diagenesis. The study underscores the influence of alkaline–acid and redox conditions on their geochemistry, with the upper peat intervals exhibiting oxidative conditions and acting as a geochemical barrier.
The increase in concentrations of chalcophilic elements (As, Cd, Cu, Zn, Pb) in the upper peat layers is attributed to higher technogenic load, particularly due to the proximity to a highway and urban area. Atmospheric transport is identified as one of the sources for these elements.