Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies
Abstract
1. Introduction
2. Research Methodology
- -
- physical methods of wastewater treatment (sedimentation, filtration, adsorption, etc.) + microplastics,
- -
- chemical methods of wastewater treatment (coagulation, ozonation, Fenton process, etc.) + microplastics,
- -
- biological methods of wastewater treatment (activated sludge, constructed wetlands, etc.) + microplastics
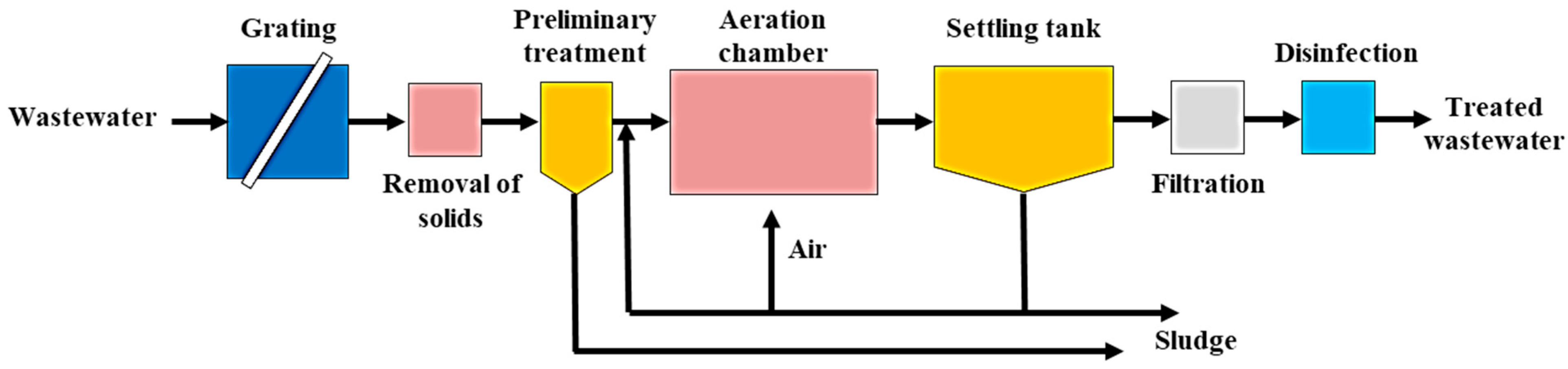
3. Characteristics of Microplastics in Wastewater Treatment Plants
4. Removal Efficiency and Fate of Microplastics in Existing Wastewater Treatment Plants
4.1. First-Stage (Pre-Treatment) in MPs Removal
4.2. Second Stage Treatment (Biological) in the Removal of MPs/NPs
4.2.1. Biological Methods of Wastewater Treatment
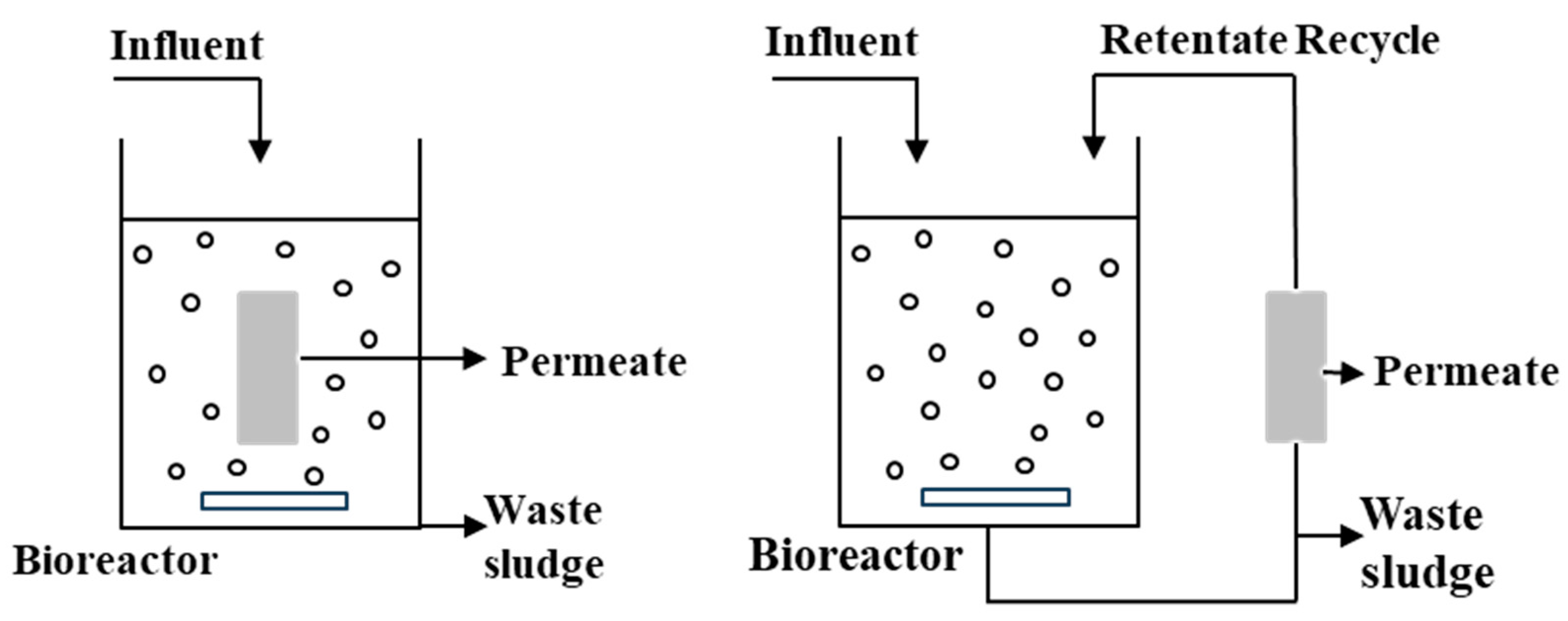
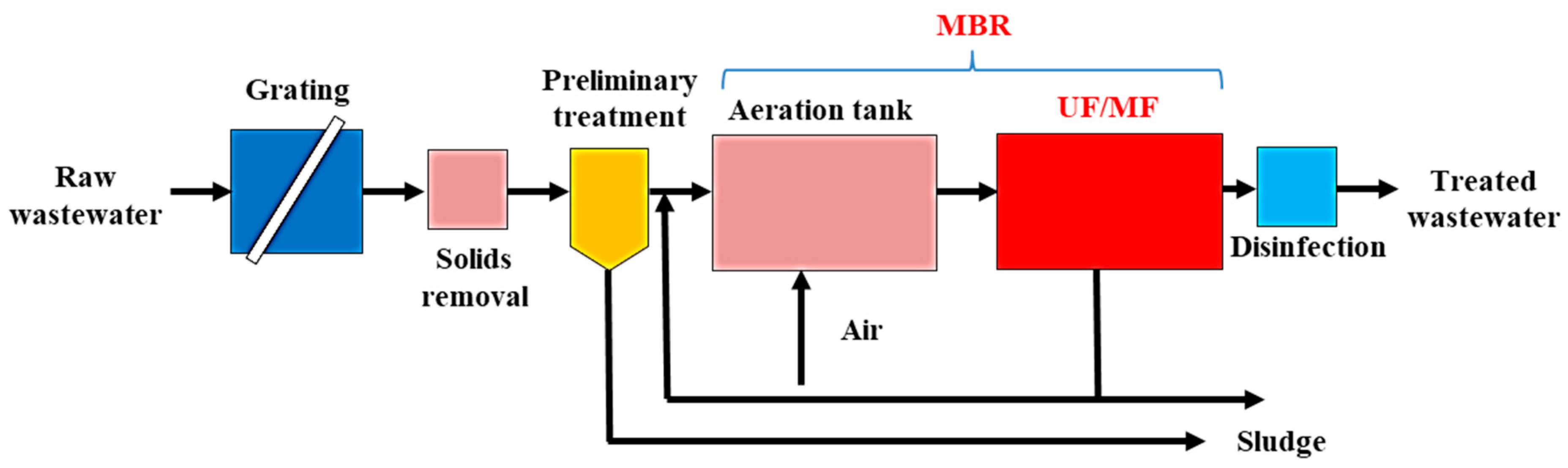
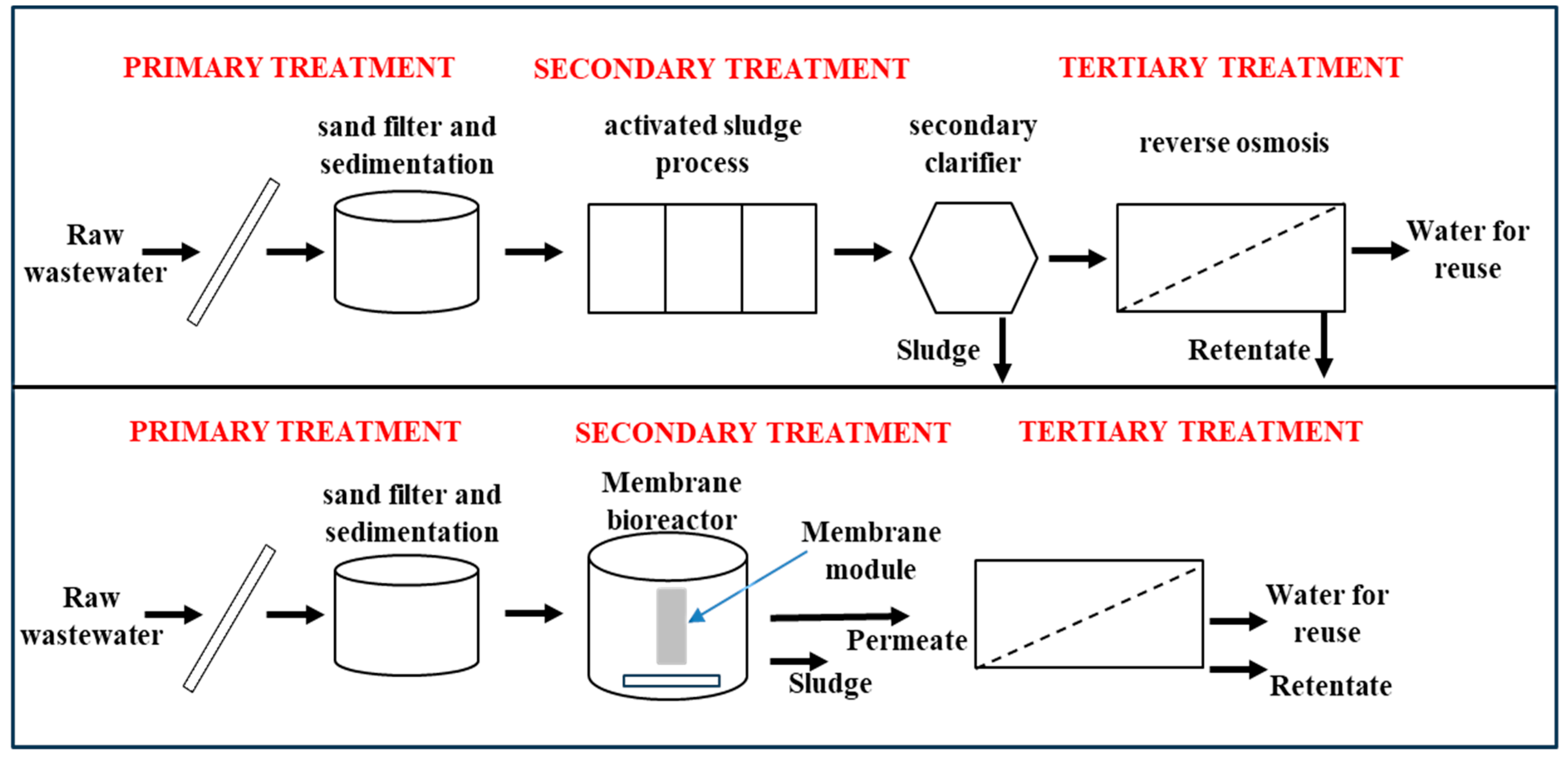
4.2.2. Membrane Bioreactors
4.3. Tertiary (Final) Treatment in the MPs Removal
4.3.1. Biological Methods of Tertiary Treatment
4.3.2. Coagulation
4.3.3. Classical Filtration Processes
4.3.4. Membrane Filtration
4.3.5. Oxidation Processes
5. Summary
6. Conclusions and Future Prospects
Funding
Conflicts of Interest
List of Abbreviations
| A2O | anaerobic-anoxic-oxygen | PC | polycarbonate |
| AnMBR | anaerobic membrane bioreactor | PE | polyethylene |
| AOPs | advanced oxidation processes | PES | polyester |
| AS | activated sludge | PET | polyethylene terephthalate |
| BAF | biologically active filter | PEQ | population equivalent |
| BPA | bisphenol A | PP | polypropylene |
| CB | conductivity band | PS | polystyrene |
| CW | constructed wetland | PS acrylic | acrylic polystyrene |
| DAF | dissolved air flotation | PUR/PU | polyurethane |
| DF | disc filter | PVA | polyvinyl acetate |
| EC | electrocoagulation | PV acrylate | polyvinyl acrylate |
| eCB− | the conduction band electrons | PVC | polyvinyl chloride |
| EDCs | endocrine disrupting compounds | PVDF | polyvinylidene fluoride |
| EPS | extracellular polymeric substances | PVAL | polyvinyl alcohol |
| EVA | ethylene vinyl acetate | PVE | polyvinyl ethylene |
| FTIR | Fourier-transform infrared spectroscopy | PVF | polyvinyl fluoride |
| GAC | granular activated carbon | R | removal rate |
| GF | granular filter | RO | reverse osmosis |
| HDPE | high-density polyethylene | RSF | rapid sand filter |
| HRT | hydraulic retention time | SAN | styrene acrylonitrile |
| hVB+ | valence band holes | SBS | styrene-butadiene-styrene |
| iMBR | an immersed module MBR—a membrane module submerged in the bioreactor | SEBS | styrene-ethylene-butadiene-styrene |
| IMS | integrated membrane system | sMBR | side-stream MBR—a membrane module outside the bioreactor |
| MBR | membrane bioreactor | SRT | sludge retention time |
| MF | microfiltration | TMP | transmembrane pressure |
| MPs | microplastics | UF | ultrafiltration |
| NF | nanofiltration | U.S. | the United States of America |
| NPs | nanoplastics | UV | ultraviolet |
| PA | polyamides | UV d | UV disinfection |
| PAC | polyaluminum chloride | UV-VIS | ultraviolet–visible |
| PAM | polyacrylamide | VB | valence band |
| WWTPs | wastewater treatment plants |
References
- Plastics Europe. 2024. Available online: www.plasticseurope.org (accessed on 19 February 2024).
- Arpia, A.A.; Chen, W.-H.; Ubando, A.T.; Naqvi, S.R.; Culaba, A.B. Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environmental effects: A state-of-the-art review. J. Hazard. Mater. 2021, 418, 126381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Kim, J.-G.; Kim, H.-B.; Choi, J.H.; Tsang, Y.F.; Baek, K. Occurrence and removal of microplastics in wastewater treatment plants and drinking water purification facilities: A review. Chem. Eng. J. 2021, 410, 128381. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Badola, N.; Bahuguna, A.; Sasson, Y.; Chauhan, J.S. Microplastics removal strategies: A step toward finding the solution. Front. Environ. Sci. Eng. 2022, 16, 7. [Google Scholar] [CrossRef]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W.-M. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process Saf. Environ. Prot. 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Bodzek, M.; Pohl, A. Removal of microplastics in unit processes used in water and wastewater treatment: A review. Arch. Environ. Prot. 2022, 48, 102–128. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Sørensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Foshtomi, M.Y.; Oryan, S.; Taheri, M.; Bastami, K.D.; Zahed, M.A. Composition and abundance of microplastics in surface sediments and their interaction with sedimentary heavy metals, PAHs and TPH (total petroleum hydrocarbons). Mar. Pollut. Bull. 2019, 149, 110655. [Google Scholar] [CrossRef]
- Singla, M.; Díaz, J.; Broto-Puig, F.; Borrós, S. Sorption and release process of polybrominated diphenyl ethers (PBDEs) from different composition microplastics in aqueous medium: Solubility parameter approach. Environ. Pollut. 2020, 262, 114377. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Leusch, F.D.L. Wastewater treatment plant effluent as a source of microplastics: Review of the fate, chemical interactions and potential risks to aquatic organisms. Water Sci. Technol. 2016, 74, 2253–2269. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake—Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, H.; Peng, J.; Wang, Y.; Xiong, X.; Wu, C.; Lam, P.K.S. Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018, 630, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, L.D.K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Velázquez, D.; Edo, C.; Carretero, O.; Gago, J.; Barón-Sola, Á.; Hernández, L.E.; Yousef, I.; Quesada, A.; Leganés, F.; et al. Fibers spreading worldwide: Microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020, 722, 137904. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Alvim, C.B.; Mendoza-Roca, J.A.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ren, S.-Y.; Ni, H.-G. Incidence of microplastics in personal care products: An appreciable part of plastic pollution. Sci. Total Environ. 2020, 742, 140218. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, X.; Gu, J.; Kobayashi, N.; Yuan, H.; Chen, Y. Chemical recycling of waste plastics: Current challenges and perspectives. Fundam. Res. 2024, in press. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021, 162, 111847. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.D.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.-Y.; Park, J.; Zhou, J.L.; Yoon, M.-H. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of Microplastic Mass and Removal Rates at Wastewater Treatment Plants Applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- González-Camejo, J.; Morales, A.; Peña-Lamas, J.; Lafita, C.; Enguídanos, S.; Seco, A.; Martí, N. Feasibility of rapid gravity filtration and membrane ultrafiltration for the removal of microplastics and microlitter in sewage and wastewater from plastic industry. J. Water Process Eng. 2023, 51, 103452. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Hidayaturrahman, H.; Lee, T.-G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Lykkemark, J.; Andersen, T.R.; Vollertsen, J. Permeable pavements: A possible sink for tyre wear particles and other microplastics? Sci. Total Environ. 2023, 869, 161770. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester textiles as a source of microplastics from households: A mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic Bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The colors of the ocean plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jian, Y.; Xue, Y.; Hou, Q.; Wang, L. Microplastics in the wastewater treatment plants (WWTPs): Occurrence and removal. Chemosphere 2019, 235, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, S.; Ji, C.; Li, F.; Wu, H. Microplastics aggravate the bioaccumulation and toxicity of coexisting contaminants in aquatic organisms: A synergistic health hazard. J. Hazard. Mater. 2022, 424, 127533. [Google Scholar] [CrossRef]
- Vuori, L.; Ollikainen, M. How to remove microplastics in wastewater? A cost-effectiveness analysis. Ecol. Econ. 2022, 192, 107246. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Gauchotte-Lindsay, C. Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period. Water Res. 2019, 163, 114909. [Google Scholar] [CrossRef]
- Jia, Q.-L.; Chen, H.; Zhao, X.; Li, L.; Nie, Y.-H.; Ye, J.-F. Removal of microplastics by different treatment processes in Shanghai large municipal wastewater treatment plants. Environ. Sci. 2019, 40, 4105–4112. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.-S.; Sun, J.; Dai, X.; Ni, B.-J. Revealing the mechanisms of polyethylene microplastics affecting anaerobic digestion of waste activated sludge. Environ. Sci. Technol. 2019, 53, 9604–9613. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.-T.; Huang, Q.-S.; Ni, B.-J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Res. 2019, 163, 114881. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O. Microplastics in wastewater treatment plants: Occurrence, fate and identification. Process Saf. Environ. Prot. 2021, 146, 77–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Nandakumar, V.K.; Palani, S.G.; Varma, M.R.R. Interactions between microplastics and unit processes of wastewater treatment plants: A critical review. Water Sci. Technol. 2022, 85, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Michielssen, M.R.; Michielssen, E.R.; Ni, J.; Duhaime, M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016, 2, 1064–1073. [Google Scholar] [CrossRef]
- Ranade, V.V.; Bhandari, V.M. Industrial Wastewater Treatment, Recycling and Reuse; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- de Sena, R.F.; Tambosi, J.L.; Genena, A.K.; Moreira, R.d.F.P.M.; Schröder, H.F.; José, H.J. Treatment of meat industry wastewater using dissolved air flotation and advanced oxidation processes monitored by GC–MS and LC–MS. Chem. Eng. J. 2009, 152, 151–157. [Google Scholar] [CrossRef]
- Bui, X.-T.; Vo, T.-D.-H.; Nguyen, P.-T.; Nguyen, V.-T.; Dao, T.-S.; Nguyen, P.-D. Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies. Environ. Technol. Innov. 2020, 19, 101013. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Foglia, A.; Akyol, Ç.; Cipolletta, G.; Benedetti, M.; Regoli, F.; Eusebi, A.L.; Sabbatini, S.; Tseng, L.Y.; Katsou, E.; et al. Microplastics in real wastewater treatment schemes: Comparative assessment and relevant inhibition effects on anaerobic processes. Chemosphere 2021, 262, 128415. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and improving microplastic removal during water treatment: Impact of coagulation and flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Shammas, N.K.; Bennett, G.F. Principles of Air Flotation Technology. In Flotation Technology; Wang, L.K., Shammas, N.K., Selk, W.A., Aulenbach, D.B., Eds.; Handbook of Environmental Engineering; Humana Press: Totowa, NJ, USA, 2010; pp. 1–47. [Google Scholar]
- Harrison, J.P.; Sapp, M.; Schratzberger, M.; Osborn, A.M. Interactions between microorganisms and marine microplastics: A call for research. Mar. Technol. Soc. J. 2011, 45, 12–20. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa, S.D. Microplastic degradation by bacteria in aquatic ecosystem. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 431–467. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Arossa, S.; Martin, C.; Rossbach, S.; Duarte, C.M. Microplastic removal by Red Sea giant clam (Tridacna maxima). Environ. Pollut. 2019, 252, 1257–1266. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Nash, S.M.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Corona, E.; Martin, C.; Marasco, R.; Duarte, C.M. Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front. Mar. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-based biopolymer for nano- and microplastic removal: A possible biosolution for wastewater treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, C.M.; Narayan, V. Biological wastewater treatment and bioreactor design: A review. Sustain. Environ. Res. 2019, 29, 33. [Google Scholar] [CrossRef]
- Kalčíková, G. Beyond ingestion: Adhesion of microplastics to aquatic organisms. Aquat. Toxicol. 2023, 258, 106480. [Google Scholar] [CrossRef] [PubMed]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Alvim, C.B.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. J. Chem. Eng. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Hongprasith, N.; Kittimethawong, C.; Lertluksanaporn, R.; Eamchotchawalit, T.; Kittipongvises, S.; Lohwacharin, J. IR microspectroscopic identification of microplastics in municipal wastewater treatment plants. Environ. Sci. Pollut. Res. 2020, 27, 18557–18564. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Liu, F.-F.; Liu, G.-Z.; Zhu, Z.-L.; Wang, S.-C.; Zhao, F.-F. Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 2019, 214, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L. Biological Wastewater Treatment; Instalator Polski: Warszawa, Poland, 1996. (In Polish) [Google Scholar]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect evaluation of microplastics on activated sludge nitrification and denitrification. Sci. Total Environ. 2020, 707, 135953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.; Zhang, D.; Zhu, X. Waste activated sludge fermentation for hydrogen production enhanced by anaerobic process improvement and acetobacteria inhibition: The role of fermentation pH. Environ. Sci. Technol. 2010, 44, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane processes for microplastic removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- Dvořák, L.; Svojitka, J.; Wanner, J.; Wintgens, T. Nitrification performance in a membrane bioreactor treating industrial wastewater. Water Res. 2013, 47, 4412–4421. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Song, K.; Zhou, Y. Performance evaluation of MBR in treating microplastics polyvinylchloride contaminated polluted surface water. Mar. Pollut. Bull. 2020, 150, 110724. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ji, M.; Zhai, H.; Liu, Y. Occurrence of phthalate esters and microplastics in urban secondary effluents, receiving water bodies and reclaimed water treatment processes. Sci. Total Environ. 2020, 737, 140219. [Google Scholar] [CrossRef]
- Kalčíková, G.; Alič, B.; Skalar, T.; Bundschuh, M.; Gotvajn, A.Ž. Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188, 25–31. [Google Scholar] [CrossRef]
- Mareddy, A.R. Technology in EIA. In Environmental Impact Assessment: Theory and Practice; Mareddy, A.R., Ed.; Butterwortha-Heinemanna: Oxford, UK, 2017; pp. 290–421. [Google Scholar] [CrossRef]
- Rocher, V.; Paffoni, C.; Gonçalves, A.; Guérin, S.; Azimi, S.; Gasperi, J.; Moilleron, R.; Pauss, A. Municipal wastewater treatment by biofiltration: Comparisons of various treatment layouts. Part 1: Assessment of carbon and nitrogen removal. Water Sci. Technol. 2012, 65, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nord, N.B.; Bester, K.; Vollertsen, J. Microplastics Removal from Treated Wastewater by a Biofilter. Water 2020, 12, 1085. [Google Scholar] [CrossRef]
- Helcoski, R.; Yonkos, L.T.; Sanchez, A.; Baldwin, A.H. Wetland soil microplastics are negatively related to vegetation cover and stem density. Environ. Pollut. 2020, 256, 113391. [Google Scholar] [CrossRef]
- Wang, Q.; Hernández-Crespo, C.; Santoni, M.; Van Hulle, S.; Rousseau, D.P. Horizontal subsurface flow constructed wetlands as tertiary treatment: Can they be an efficient barrier for microplastics pollution? Sci. Total Environ. 2020, 721, 137785. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hidayaturrahman, H.; Peera, S.G.; Lee, T.G. Elimination of microplastics at different stages in wastewater treatment plants. Water 2022, 14, 2404. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Din, M.F.M.; Taib, S.M.; Talaiekhozani, A.; Yadav, K.K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Katrivesis, F.K.; Karela, A.D.; Papadakis, V.G.; Paraskeva, C.A. Revisiting of coagulation-flocculation processes in the production of potable water. J. Water Process Eng. 2019, 27, 193–204. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Cescon, A.; Jiang, J.-Q. Filtration Process and Alternative Filter Media Material in Water Treatment. Water 2020, 12, 3377. [Google Scholar] [CrossRef]
- Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 µm microplastic particles from treated wastewater by a disc filter. Water 2019, 11, 1935. [Google Scholar] [CrossRef]
- Bodzek, M. Membrane separation techniques—Removal of inorganic and organic admixtures and impurities from water environment—Review. Arch. Environ. Prot. 2019, 45, 4–19. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Rajca, M. Membranes in water and wastewater disinfection—Review. Arch. Environ. Prot. 2019, 45, 3–18. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system. Case Stud. Chem. Environ. Eng. 2021, 3, 100075. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumée, L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano- and microplastics. J. Membr. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Chen, Z.; Ma, B.; Chen, J.P. Ultrafiltration membrane fouling by microplastics with raw water: Behaviors and alleviation methods. Chem. Eng. J. 2021, 410, 128174. [Google Scholar] [CrossRef]
- Yahyanezhad, N.; Bardi, M.J.; Aminirad, H. An evaluation of microplastics fate in the wastewater treatment plants: Frequency and removal of microplastics by microfiltration membrane. Water Pract. Technol. 2021, 16, 782–792. [Google Scholar] [CrossRef]
- Luogo, B.D.P.; Salim, T.; Zhang, W.; Hartmann, N.B.; Malpei, F.; Candelario, V.M. Reuse of water in laundry applications with micro- and ultrafiltration ceramic membrane. Membranes 2022, 12, 223. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Z.-R.; Li, W.-H.; Yan, X.; Wang, L.-K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.-J. Revisiting microplastics in landfill leachate: Unnoticed tiny microplastics and their fate in treatment works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, J.; Lu, J.; Wang, J.; Zhang, C. Fate of microplastics in a coastal wastewater treatment plant: Microfibers could partially break through the integrated membrane system. Front. Environ. Sci. Eng. 2022, 16, 96. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Tian, L.; Kolvenbach, B.; Corvini, N.; Wang, S.; Tavanaie, N.; Wang, L.; Ma, Y.; Scheu, S.; Corvini, P.F.-X.; Ji, R. Mineralisation of 14C-labelled polystyrene plastics by Penicillium variabile after ozonation pre-treatment. New Biotechnol. 2017, 38, 101–105. [Google Scholar] [CrossRef]
- Chen, R.; Qi, M.; Zhang, G.; Yi, C. Comparative experiments on polymer degradation technique of produced water of polymer flooding oilfield. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012208. [Google Scholar] [CrossRef]
- Aljuboury, D.A.; Palaniandy, P.; Aziz, H.B.A.; Feroz, S. A Review on the Fenton Process for Wastewater Treatment. J. Innov. Eng. 2014, 2, 4. [Google Scholar]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef]
- Feng, H.-M.; Zheng, J.-C.; Lei, N.-Y.; Yu, L.; Kong, K.H.-K.; Yu, H.-Q.; Lau, T.-C.; Lam, M.H.W. Photoassisted Fenton degradation of polystyrene. Environ. Sci. Technol. 2011, 45, 744–750. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. Nano-photocatalysis in water and wastewater treatment. Desalination Water Treat. 2021, 243, 51–74. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, Y.; Zhang, C.; Zhu, S.; Qin, L.; Wang, W.; He, D.; Zhou, Y.; Luo, H.; Qin, F. Recent Advances in Photocatalytic Degradation of Plastics and Plastic-Derived Chemicals. J. Mater. Chem. A 2021, 9, 13402–13441. [Google Scholar] [CrossRef]
- Ali, S.S.; Qazi, I.A.; Arshad, M.; Khan, Z.; Voice, T.C.; Mehmood, C.T. Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes. Environ. Nanotechnol. Monit. Manag. 2016, 5, 44–53. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Y.; Song, S.; Dong, X.; Yu, X. High photocatalytic degradation activity of polyethylene containing polyacrylamide grafted TiO2. Polym. Degrad. Stab. 2013, 98, 1754–1761. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Zebger, I.; Goikoetxea, A.B.; Jensen, S.; Ogilby, P.R. Degradation of vinyl polymer films upon exposure to chlorinated water: The pronounced effect of a sample’s thermal history. Polym. Degrad. Stab. 2003, 80, 293–304. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628–629, 740–747. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef]
| Location | Capacity [m3/Year] | Purification Process | Inflow [MPs/L] | Outflow [MPs/L] | Discharge [MPs/d] | R [%] |
|---|---|---|---|---|---|---|
| Australia | 1.12 × 108 | First stage | - | 1.5 | 4.60 × 108 | - |
| Australia | 4.75 × 106– 1.753 × 107 | I and II stage/RO | - | 0.21–0.28 | 3.60 × 106–1.00 × 107 | - |
| Sweden | 1.88 × 106 | First and second stage | 15.1 | 0.00825 | 4.25 × 104 | 99.9 |
| France | 8.76 × 107 | I and II stage (biofilter) | 293 | 35 | 8.40 × 109 | 88.1 |
| Scotland | 9.52 × 107 | I and II stage | 15.7 | 0.25 | 6.52 × 107 | 98.4 |
| Netherlands | 3.37 × 106–2.63 × 108 | I and II stage | - | 55–81 | 7.48 × 108 | 94 |
| USA | 7.89 × 107 | I and II stage | - | 0.023 | 4.97 × 106 | - |
| Germany | 1.9 × 105–1.40 × 105 | I and II stage | - | 0.08–7.52 | 4.19 × 104–1.24 × 107 | - |
| Germany | 1.3 × 107 | I and II stage/final filtration, | - | 0.01–0.38 | 2.79 × 105–2.62 × 106 | - |
| Australia | 6.21 × 106 | I and II stage | - | 0.4 | 8.16 × 105 | - |
| Denmark | - | I and II stage | 2223–10,044 | 29–447 | - | - |
| Finland | 3.65 × 106 | I and II stage | 57.6 | 1 | 1.00 × 107 | 98.3 |
| Finland | 8.0 × 105 | I and II stage/BAF | 610 | 13.5 | 3.65 × 109 | - |
| Finland | I and II stage/(BAF, DF, MBR, RSF) | - | 0.02–0.3 | 1.26 × 106–6.59 × 107 | - | |
| Finland | 1.10 × 103 | I stage/MBR(pilot) | 57.6 | 0.4 | - | 99.3 |
| Germany | 1.13 × 104 | I and II stage | - | 80.4 | 2.47 × 106 | - |
| The Netherlands | 2.03 × 106 | I degree/MBR | 68 | 51 | 2.84 × 108 | 25 |
| Netherlands | 9240–720 × 103 PEQ | I and II stage | 68–910 | 51–81 | - | 72 |
| USA | 8.58 × 105 | I and II stage | 0.004–0.195 | 5.28 × 104–1.49 × 107 | - | |
| USA | 1.4 × 108–5.51 × 108 | I and II stage | 1 | 8.8 × 10−4 | 9.3 × 105 | 99.9 |
| USA | - | I stage/AnMBR | 91 | 0.5 | - | 99.4 |
| USA | 4.75 × 106–7.77 × 107 | I and II stage/(GF, BAF) | - | 0.009–0.127 | 1.01 × 105–9.63 × 106 | - |
| USA | 1.30 × 106–3.13 × 108 | I and II stage/gravity filter. | - | 0–2.43 × 10−4 | 0–2.08 × 102 | 97.2 |
| USA | 6.23 × 105 | I and II stage/GF | 91 | 2.6 | 4.43 × 106 | |
| USA | 9.13 × 108 | I and II stage | 133 | 5.9 | 1.48 × 1010 | 95.6 |
| Denmark | - | I and II stop./RSF | 81.49 | 19 | - | - |
| Israel | 30 × 103 | I and II degree/filter.sand + Cl2 | 64.78 | 1.97 | 5.91 × 107 | 97.0 |
| Spain | 210 × 103 PEQ | I and II degree | 12.43 | 1.23 | 6.7 × 106 | 90.3 |
| Spain | 29,777 PEQ | I and II stage/(MBR, RSF) | 4.40 | 092–1.08 | 12.96 × 106 | 75.5–79.0 |
| South Korea | 20.840–26.545 | I and II stage/O3/RSF | 4200–5840 | 33–66 | 8.8 × 108–1.37 × 109 | 98.9–99.2 |
| South Korea | 40 × 103 | I stage/RSF, UV d | 114–216 | 0.26–0.48 | 2.9 × 109 | 99.8 |
| China | 3.1 × 106 | I and II stage/UV d | 126 | 30.6 | - | 75 |
| China | 3.5 × 106 | I and II stage | 37.9 | 1.57–13.69 | - | - |
| Italy | 80,000 PEQ | I and II stage/UF | 3.6 | 0.76 | 4.15 × 107 | 86 |
| Thailand | 130 × 103 | I and II stage/UF | 77 | 2.33 | 2.8 × 108 | 97 |
| Turkey | 87,500 | I and II stage | 135.3 | 8.5 | 5.25 × 108 | 93.7 |
| Spain | 70,417 PEQ | I and II stage/RSF/UV d | 3.78 | 1.38 | 1.7 × 107 | 63.4 |
| Location | Concentration in the Inflow [MPs/L] | Fibres [%] | Granules [%] | Spheres [%] | Films [%] | Foams [%] | Fragments [%] |
|---|---|---|---|---|---|---|---|
| Northern California, USA | 0.195 | 90 | - | - | - | 1 | 9 |
| 0.022 | 91 | - | - | - | - | 9 | |
| 0.064 | 58 | - | 0 | 4 | 4 | 35 | |
| 0.092 | 94 | - | - | 2 | - | 4 | |
| 0.072 | 59 | - | - | - | - | 41 | |
| 0.127 | 78 | - | - | 3 | - | 18 | |
| 0.047 | 100 | - | - | - | - | - | |
| Northern Ohio, USA | 0.042 | 8 | - | 4 | 15 | 4 | 70 |
| Central New York, USA | 0.019 | 68 | - | - | 3 | - | 28 |
| 0.08 | 58 | - | 1 | 8 | 2 | 30 | |
| Eastern Wisconsin, USA | 0.007 | 39 | - | 2 | 5 | 1 | 53 |
| 0.017 | 15 | - | 2 | 6 | - | 77 | |
| West New York, USA | 0.009 | 68 | - | 5 | 2 | 5 | 21 |
| 0.047 | 68 | - | 5 | 2 | 5 | 21 | |
| East New York, USA | 0.004 | 13 | - | 6 | 13 | 3 | 65 |
| New Jersey, USA | 0.028 | - | - | - | - | - | - |
| Sydney, Australia | 0.280 | 80 | 20 | - | - | - | - |
| 0.480 | 66 | 34 | - | - | - | - | |
| 1500 | - | - | - | - | - | - | |
| Hong Kong, China | 2060 | 71 | - | - | 3 | 26 | - |
| 1010 | 55 | - | 1 | - | 19 | 25 | |
| Scotland | 15.7 | 18.5 | - | 3 | 9.9 | 1.3 | 67.3 |
| Vancouver, Canada | 31.1 | 65.6 | 0.45 | 5.4 | 0.2 | 0.22 | 28.1 |
| Xiamem, China | 6.55 | 17.7 | 49.8 | 2.5 | - | - | 30 |
| Beijing, China | 12.03 | 85.92 | 14.08 | - | - | - | - |
| Polymer | Abbreviation | Inflow [MPs/L] | Outflow [MPs/L] |
|---|---|---|---|
| Polyethylene | PE | 0.03–1.05 | 0.00–0.67 |
| Polypropylene | PP | 0.02–1.42 | 0.00–0.22 |
| Polyamide | PA | 0.06–0.71 | 0.00–0.06 |
| Polyester | PES | 0.22–6.31 | 0.07–1.33 |
| Polystyrene | PS | 0.00–0.41 | 0.00–0.08 |
| Polyethylene terephthalate | PET | 0.01–0.63 | 0.00–0.16 |
| Polyurethane | PUR/PU | 0.07–1.40 | 0.00–0.02 |
| Polyvinyl chloride | PVC | 0.12–1.65 | 0.00 |
| Polyvinyl acetate | PVA | 0.26–0.50 | 0.00–0.01 |
| Ethylene vinyl acetate | EVA | 0.00–0.01 | 0.00 |
| Polyacrylates | – | 0.06–0.40 | 0.00–0.03 |
| Polyvinyl ethylene | PVE | 0.09 | 0.00 |
| Polyvinyl fluoride | PVF | 0.09 | 0.00 |
| Styrene-butadiene-styrene | SBS | 0.02 | 0.00 |
| Styrene-ethylene-butadiene-styrene | SEBS | 0.06 | 0.00 |
| Styrene acrylonitrile | SAN | 0.01 | 0.00 |
| Polyvinyl alcohol | PVAL | 0.03 | 0.00 |
| Polyethylene and polypropylene | PE&PP | 0.09 | 0.01 |
| Acrylic polystyrene | PS acrylic | 0.30 | 0.00 |
| Polyvinyl acrylate | PV acrylate | 0.09 | 0.00 |
| Acrylonitrile-butadiene | – | 0.80 | 0.01 |
| Ethyl acrylate | – | 0.14 | 0.01 |
| Ethylene-propylene | – | 0.28 | 0.00 |
| Type of Treatment Process | Removal Efficiency [%] |
|---|---|
| Primary settling tank | 47.8 |
| Aerated sand trap + primary settling tank | 58.8 |
| Coarse screen + fine screen + sand trap + primary settling tank | 40.7 |
| Dissolved air flotation (DAF) | 95.0 |
| Type of Treatment | Removal Efficiencies [%] |
|---|---|
| Activated sludge + secondary settling tank | 60.0 |
| Primary settling tank + subsequent biological treatment stages | 68.3 |
| Aerobic biological reactor + secondary settling tank | 74.8 |
| Aerated tank + secondary settling tank | 84.0 |
| Anaerobic + anoxic + aerobic processes | 54.4 |
| Anaerobic + anoxic + aerobic processes | 16.0 |
| Method | Type of Stream | Raw Wastewater [MPs/L] | Cleaned Wastewater [MPs/L] | Removal Rate [%] |
|---|---|---|---|---|
| 10 µm disc filter | After the second-stage treatment | 0.5 | 0.3 | 40.0 |
| 20 µm disc filter | After the second-stage treatment | 2.0 | 0.03 | 98.5 |
| Rapid sand filter | After the second-stage treatment | 0.7 | 0.02 | 97.1 |
| Flotation | After the second-stage treatment | 2.0 | 0.1 | 95.0 |
| Membrane bioreactor | After the first-stage treatment | 6.9 | 0.005 | 99.9 |
| Membrane bioreactor | After the first-stage treatment | 57.6 | 0.4 | 99.4 |
| Treatment Type | Efficiency [%] | Type of Removed MPs |
|---|---|---|
| MBR, AS, and settling tank | 83.1–91.9 | Fragments |
| AS and clarification | 92 | Fragments, fibres |
| AS | 93.8 | Microgranules |
| AS | 89.8 | Microgranules |
| MBR | 79.01 | Fibres, PP, PS |
| A2O | 71.67 ± 11.58 | No data available |
| AS, sedimentation | 64 | Fibres |
| MBR | 99 | Fragments, PVC fibres |
| CW | 97 | Fragments, fibres |
| AS | 52 | PE < 100 µm |
| Biological aerated filter | 99 | PE 100–300 µm |
| A2O | 54.4 | - |
| A2O | 28.1 | PET, PE, PES, PAN, PAA |
| AS | 66.7 | PS |
| MBR | 99.9 | 20–100 μm MPs |
| MBR | 97.6 | PES fibres and PE fragments |
| A2O | 93.7 | PE, PP, PE |
| MBR | 99.4 | PES, PE, PA and PP |
| AS | 98.3 | Different types of MPs |
| AS | 75–91.9 | Different types of MPs |
| Submerged MBR | 100.0 | - |
| Submerged anaerobic MBR | 99.4 | - |
| Submerged MBR (KUBOTA) | 100 | - |
| Type of Process | Method Advantages | Method Disadvantages | Effectiveness in MPs Removal | |
|---|---|---|---|---|
| Physical treatment technologies for MPs removal | Adsorption | Adsorbents with high surface area and porosity effectively retain even large MPs. | Spherical MP particles with a diameter of 10 μm are adsorbed to a lesser extent. | Low to moderate effectiveness |
| Density separation | Enables the removal of low-density solid particles. | Heavy salts are very expensive and some of them are hazardous. Not useful for large-scale particulate removal. | Moderate to high effectiveness | |
| Disc filters | Formation of slime cakes; floating MPs are especially removed. | Need to backwash. | Moderate to high effectiveness | |
| GAC Filtration | Removes small-sized MP particles. | GAC Filters Clogging. | Moderate to high effectiveness | |
| Gravel/Sedimentation | Low-cost process; effective for large MP particles | Multiple stages of purification are needed to remove small solid particles. | Low to moderate effectiveness | |
| Magnetic separation | Effective removal of smaller MPs; better for drinking water treatment. | MPs recovery from sludge is lower. | Moderate effectiveness | |
| Membrane filtration | High MP removal efficiency; suitable for various water sources; compact and modular design. | Prone to fouling and clogging; high operational costs; requires regular maintenance and replacement. | High effective | |
| Rapid sand filter | Low operating and maintenance costs. | Clogging reduces efficiency. Regular backwashing is necessary. | Moderate to high effectiveness | |
| Chemical treatment technologies for MPs removal | Classical Fenton process | Cost-effective and uses optimal reagents. | Limited to specific MP types; low efficiency. | Low effectiveness |
| Coagulation | Flexible operating conditions; simple to use; suitable for small MPs. | Not effective for large MPs; uses significant amounts of chemicals. | Moderate to high effectiveness | |
| Electrocoagulation | Effective sediment reduction; cost-effective; no secondary pollution. | Sacrificial anodes must be replaced many times; passivation of the cathode occurs; electrical energy is required. | Moderate to high effectiveness | |
| Electro-Fenton process | Environmentally friendly process; low reagent costs; less sludge production. | Further modifications are necessary to improve effectiveness. | Moderate effectiveness | |
| Ozonation | Effective tertiary treatment; removes MPs by changing properties/morphology. | Complex ozone production; high costs; potential environmental pollution. | Moderate to high effectiveness | |
| Photocatalytic degradation | Environmentally friendly; avoids excessive chemicals. | Generates secondary pollutants; energy-intensive. | Low to moderate effectiveness | |
| Photo-Fenton process | Highly efficient; no excessive catalyst/chemical use required. | Requires optimal pH maintenance; further research is needed. | Further research is needed | |
| Biological treatment technologies for MPs removal | Activated sludge (AS) | Well-established biological process. | Removal efficiency depends on microorganism activity and MP properties. | Varied removal |
| Anaerobic-anoxic-aerobic process (A2O) | Integrated treatment approach. | Highly variable removal efficiency based on conditions. | Varied removal | |
| Constructed wetlands (CWs) | Environmentally friendly and cost-effective; provides simultaneous removal of nutrients, organic matter, and MPs; minimal energy consumption. | Slower treatment process compared to mechanical or chemical methods; removal efficiency depends on many factors; requires large land areas. | Moderate effectiveness | |
| Membrane bioreactors (MBRs) | Combines biological treatment with membrane filtration. Highly efficient at removing MPs; produces high-quality effluent; capable of removing MPs of varying sizes due to the membrane’s fine pore size. | High operational and maintenance costs; membrane fouling can reduce efficiency and requires regular cleaning. | Very high effectiveness | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodzek, M.; Pohl, A.; Rosik-Dulewska, C. Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies. Water 2024, 16, 3574. https://doi.org/10.3390/w16243574
Bodzek M, Pohl A, Rosik-Dulewska C. Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies. Water. 2024; 16(24):3574. https://doi.org/10.3390/w16243574
Chicago/Turabian StyleBodzek, Michał, Alina Pohl, and Czesława Rosik-Dulewska. 2024. "Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies" Water 16, no. 24: 3574. https://doi.org/10.3390/w16243574
APA StyleBodzek, M., Pohl, A., & Rosik-Dulewska, C. (2024). Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies. Water, 16(24), 3574. https://doi.org/10.3390/w16243574