Coupled Electrolysis–Microfiltration System for Efficient Phosphorus Removal and Recovery in the Form of Iron Phosphate Compounds from Wastewater
Abstract
1. Introduction
2. Materials and Methods
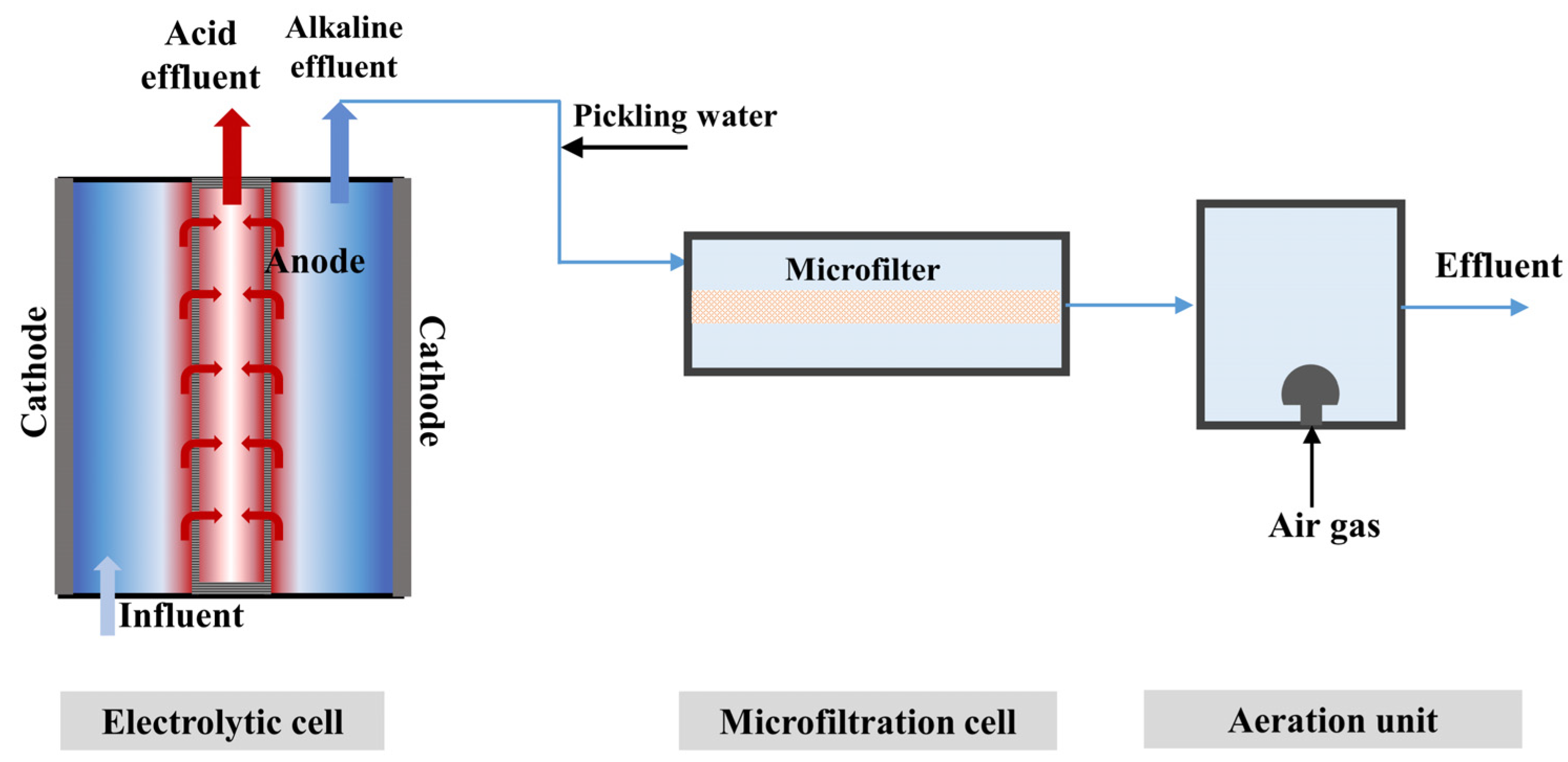
2.1. Experimental Equipment
2.2. Characterizations and Analytical Methods
3. Results
3.1. Effect of Water Quality Parameters on P Recovery
3.1.1. Initial pH
3.1.2. Initial Phosphorus Concentration
3.1.3. Fe/P Molar Ratio
3.2. Effect of Current Density on Phosphorus Recovery
3.3. Effect of Coexisting Ions (HCO3− and SiO42−)
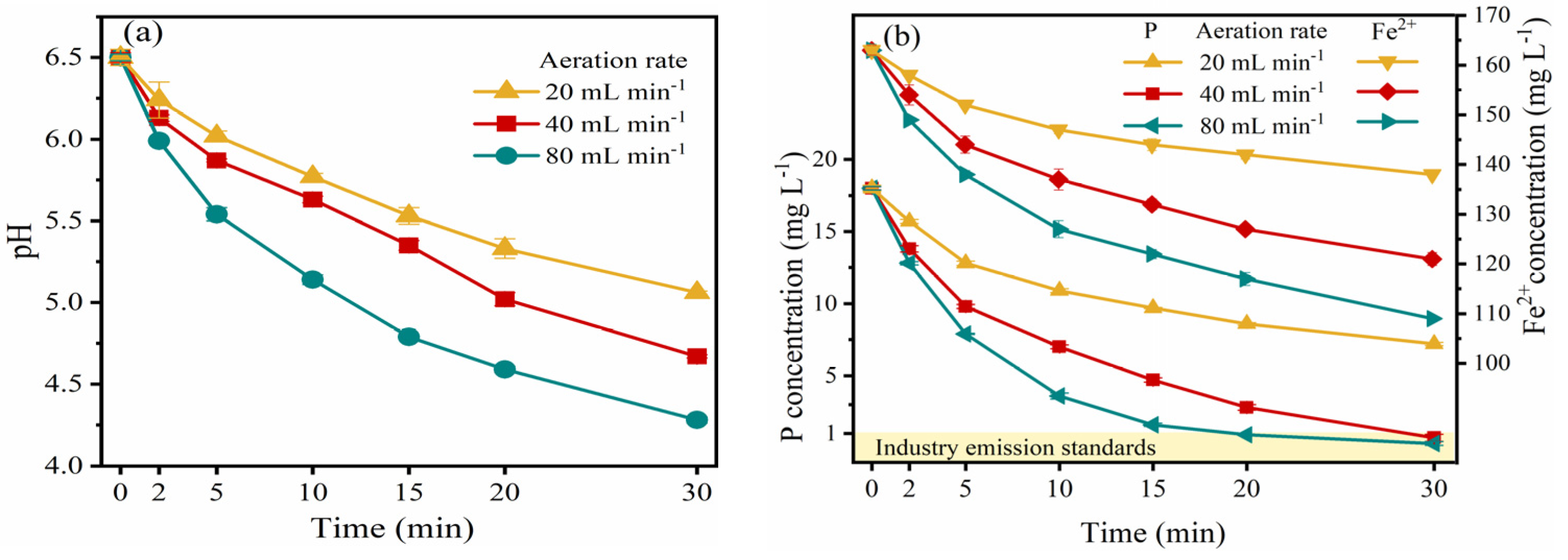
3.4. The Role of Aeration Unit
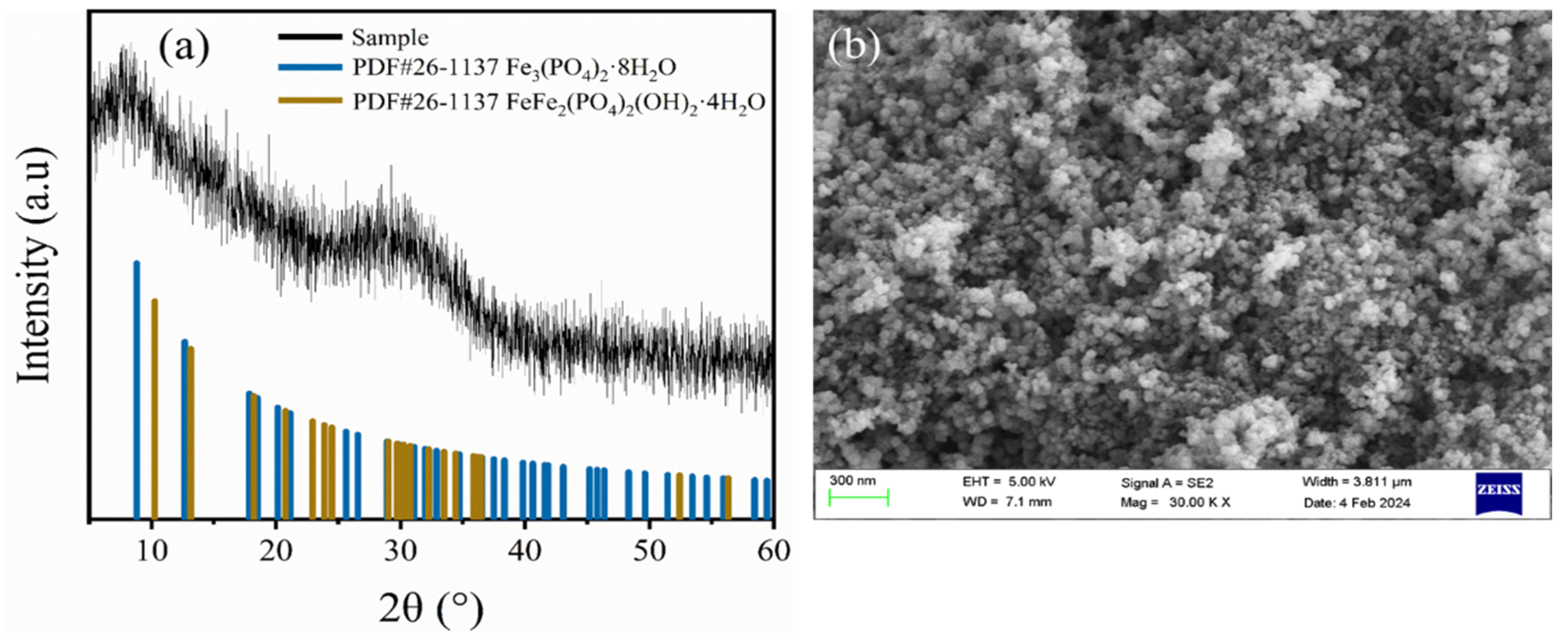
3.5. Analytical Characterization of Phosphorus-Containing Sediments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, M.; Shi, C.; Hao, L.; Wang, X.; Guo, X.; Liu, R.; Hao, X. Sustainable development of phosphorus recovery: From a product perspective. Sustain. Prod. Consum. 2023, 41, 275–290. [Google Scholar] [CrossRef]
- Langhans, C.; Beusen, A.H.W.; Mogollón, J.M.; Bouwman, A.F. Phosphorus for Sustainable Development Goal target of doubling smallholder productivity. Nat. Sustain. 2022, 5, 57–63. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Zhong, H.; Kim, Y.-N.; Smith, C.; Robinson, B.; Dickinson, N. Seabird guano and phosphorus fractionation in a rhizosphere with earthworms. Appl. Soil Ecol. 2017, 120, 197–205. [Google Scholar] [CrossRef]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically mediated precipitation of phosphate minerals for phosphorus removal and recovery: Progress and perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, Q.; Liu, D.; Wu, J.; Hao, Y.; Jiang, B. Integrating divided electrolysis-microfiltration process for energy-efficient phosphorus recovery in the form of calcium phosphate. Sep. Purif. Technol. 2022, 301, 121922. [Google Scholar] [CrossRef]
- Hao, X.; Yu, W.; Yuan, T.; Wu, Y.; van Loosdrecht, M.C.M. Unravelling key factors controlling vivianite formation during anaerobic digestion of waste activated sludge. Water Res. 2022, 223, 118976. [Google Scholar] [CrossRef]
- Wilfert, P.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C.M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef]
- Rao, S.R.; Varadaraju, U.V. Hydrothermal synthesis of LiFePO4 nanorods composed of nanoparticles from vivianite precursor and its electrochemical performance for lithium ion battery applications. Bull. Mater. Sci. 2015, 38, 1385–1388. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, S.T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. 2019, 26, 20183–20207. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bligh, M.W.; Liang, P.; Waite, T.D.; Huang, X. Phosphorus removal by in situ generated Fe(II): Efficacy, kinetics and mechanism. Water Res. 2018, 136, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-L.; Sidik, F. Harvesting of cyanobacteria and phosphorus by electrocoagulation-flocculation-flotation: Role of phosphorus precipitation in cell separations and organics destabilization. Water Res. 2024, 259, 121868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ba, X.; Zhao, Z.; Wang, J.; Yang, Q.; Liu, Y.; Bo, J. Environmentally Friendly Fabrication of Ti/RuO2-IrO2-SnO2-Sb2O5 Anode with In Situ Incorporation of Reduced TiO2 Interlayer. J. Electrochem. Soc. 2021, 168, 053502. [Google Scholar] [CrossRef]
- Shyla, B.; Nagendrappa, G. A simple spectrophotometric method for the determination of phosphate in soil, detergents, water, bone and food samples through the formation of phosphomolybdate complex followed by its reduction with thiourea. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 497–502. [Google Scholar] [CrossRef]
- Tesfaldet, Z.O.; van Staden, J.F.; Stefan, R.I. Sequential injection spectrophotometric determination of iron as Fe(II) in multi-vitamin preparations using 1,10-phenanthroline as complexing agent. Talanta 2004, 64, 1189–1195. [Google Scholar] [CrossRef]
- Oickle, A.M.; Goertzen, S.L.; Hopper, K.R.; Abdalla, Y.O.; Andreas, H.A. Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant. Carbon 2010, 48, 3313–3322. [Google Scholar] [CrossRef]
- Crutchik, D.; Sánchez, A.; Garrido, J.M. Simulation and experimental validation of multiple phosphate precipitates in a saline industrial wastewater. Sep. Purif. Technol. 2013, 118, 81–88. [Google Scholar] [CrossRef]
- Ekama, G.A.; Wentzel, M.C.; Loewenthal, R.E. Integrated chemical–physical processes kinetic modelling of multiple mineral precipitation problems. Water Sci. Technol. 2006, 53, 65–73. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef]
- Emerson, S.; Widmer, G. Early diagenesis in anaerobic lake sediments—II. Thermodynamic and kinetic factors controlling the formation of iron phosphate. Geochim. Cosmochim. Acta 1978, 42, 1307–1316. [Google Scholar] [CrossRef]
- Cerozi, B.d.S.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xie, Y.; Yang, L.; Yang, G.; Han, F.; Chen, K.; Shao, P.; He, G.; Luo, X. Precise recovery of highly-purified iron phosphate from complex lithium extraction slag leach solution: Theory guiding experiment. Sep. Purif. Technol. 2024, 347, 127605. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, X.; Qi, X.; Li, N.; Tian, J.; Qiu, B.; Xu, K.; Qu, D. Recovery of phosphate from aqueous solutions via vivianite crystallization: Thermodynamics and influence of pH. Chem. Eng. J. 2018, 349, 37–46. [Google Scholar] [CrossRef]
- Jones, A.M.; Griffin, P.J.; Collins, R.N.; Waite, T.D. Ferrous iron oxidation under acidic conditions—The effect of ferric oxide surfaces. Geochim. Cosmochim. Acta 2014, 145, 1–12. [Google Scholar] [CrossRef]
- Yao, Y.; Lyu, J.; Li, X.; Chen, C.; Verpoort, F.; Wang, J.; Pan, Z.; Kou, Z. A review of efficient electrocatalysts for the oxygen evolution reaction at large current density. DeCarbon 2024, 5, 100062. [Google Scholar] [CrossRef]
- Sun, W.; Ma, L.; Hu, Y.; Dong, Y.; Zhang, G. Hydrogen bubble flotation of fine minerals containing calcium. Min. Sci. Technol. 2011, 21, 591–597. [Google Scholar] [CrossRef]
- Wang, S.-N.; Chen, Y.-H.; Ge, R.; Cao, J.-S.; Ni, B.-J.; Fang, F. Revealing the hydrodynamic effects on phosphorus recovery as vivianite in stirring and aeration systems through PIV experiments and theoretical calculations. Chem. Eng. J. 2023, 475, 146454. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Behrens, M. Coprecipitation: An excellent tool for the synthesis of supported metal catalysts—From the understanding of the well known recipes to new materials. Catal. Today 2015, 246, 46–54. [Google Scholar] [CrossRef]
- Ma, J.; Wang, R.; Wang, X.; Zhang, H.; Zhu, B.; Lian, L.; Lou, D. Drinking water treatment by stepwise flocculation using polysilicate aluminum magnesium and cationic polyacrylamide. J. Environ. Chem. Eng. 2019, 7, 103049. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Yuan, F.; Yang, Q. Optimization and control strategies of aeration in WWTPs: A review. J. Clean. Prod. 2023, 418, 138008. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, S.; Yuan, J.; You, Y.; Zhang, Y. Characteristics of microbial denitrification under different aeration intensities: Performance, mechanism, and co-occurrence network. Sci. Total Environ. 2021, 754, 141965. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Ya, V.; Leewiboonsilp, N.; Choo, K.-H.; Noophan, P.; Li, C.-W. Electrochemical crystallization for phosphate recovery from an electronic industry wastewater effluent using sacrificial iron anodes. J. Clean. Prod. 2020, 276, 124234. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Wang, X.; Chen, Z.; Zheng, X.; Chen, Y. Advanced treatment of phosphorus-containing tail water by Fe–Mg–Zr layered double hydroxide beads: Performance and mechanism. J. Environ. Manag. 2021, 296, 113203. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Kalisz, B.; Rodziewicz, J.; Janczukowicz, W. Separation and recovery of elements from drainage water arising in soilless tomato cultivation—Application of electrocoagulation. Sep. Purif. Technol. 2025, 354, 128805. [Google Scholar] [CrossRef]





| Parameters | Concentration |
|---|---|
| Fe2+ | 2.3 mol/L |
| Total Fe | 2.5 mol/L |
| pH | ~0 |
| Conductivity | 10.2 mS cm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Wang, L.; Liu, W.; Liu, X.; Liu, D. Coupled Electrolysis–Microfiltration System for Efficient Phosphorus Removal and Recovery in the Form of Iron Phosphate Compounds from Wastewater. Water 2024, 16, 3397. https://doi.org/10.3390/w16233397
Yan H, Wang L, Liu W, Liu X, Liu D. Coupled Electrolysis–Microfiltration System for Efficient Phosphorus Removal and Recovery in the Form of Iron Phosphate Compounds from Wastewater. Water. 2024; 16(23):3397. https://doi.org/10.3390/w16233397
Chicago/Turabian StyleYan, Hengfei, Lifeng Wang, Weiping Liu, Xiaofeng Liu, and Di Liu. 2024. "Coupled Electrolysis–Microfiltration System for Efficient Phosphorus Removal and Recovery in the Form of Iron Phosphate Compounds from Wastewater" Water 16, no. 23: 3397. https://doi.org/10.3390/w16233397
APA StyleYan, H., Wang, L., Liu, W., Liu, X., & Liu, D. (2024). Coupled Electrolysis–Microfiltration System for Efficient Phosphorus Removal and Recovery in the Form of Iron Phosphate Compounds from Wastewater. Water, 16(23), 3397. https://doi.org/10.3390/w16233397





