A Kinetic Study on Chronic Response of Activated Sludge to Diclofenac by Respirometry
Abstract
1. Introduction
2. Materials and Methods
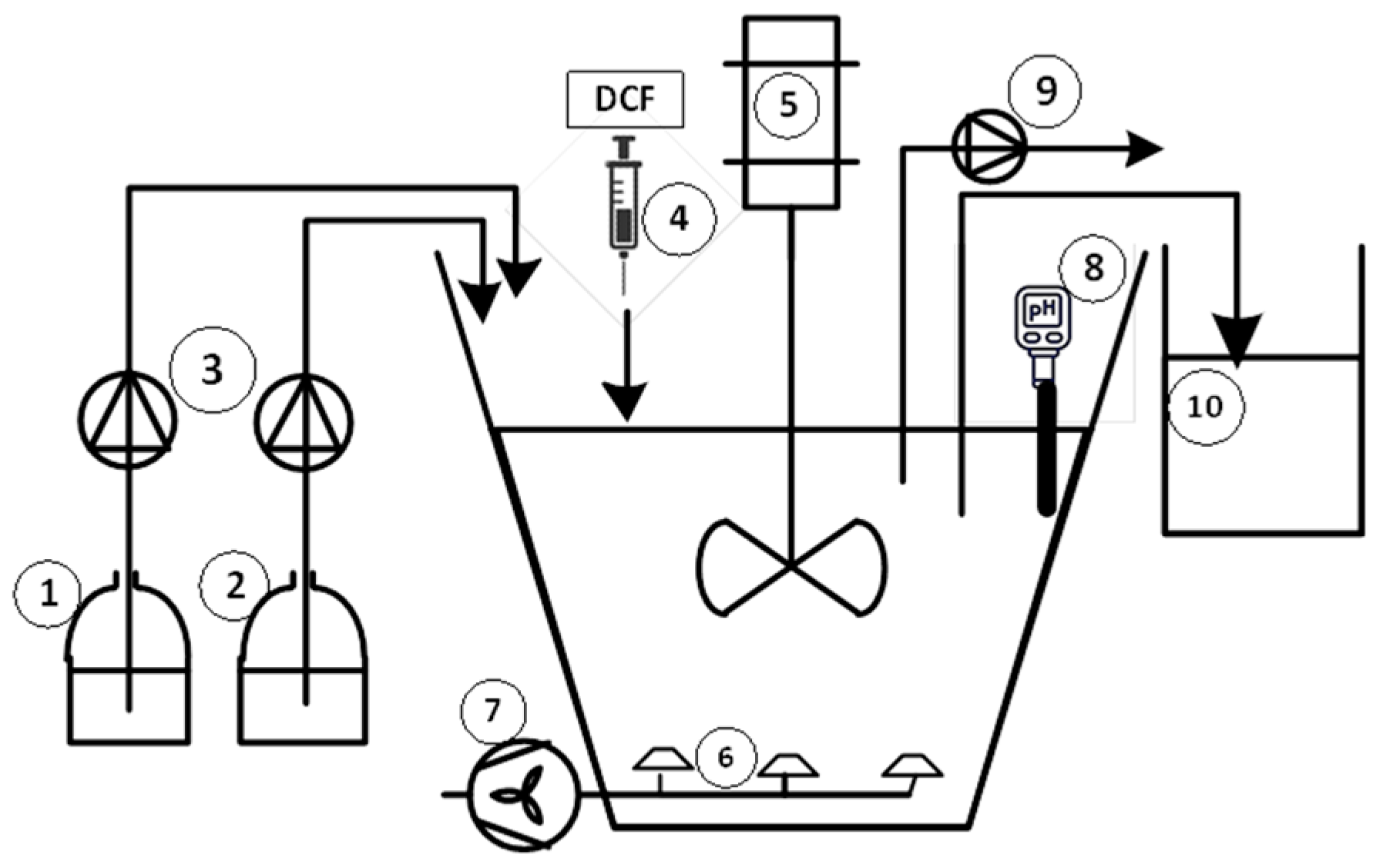
2.1. Experimental Setup
2.2. Respirometric Analyses and Modelling
2.3. Analytical Measurements
3. Results and Discussion
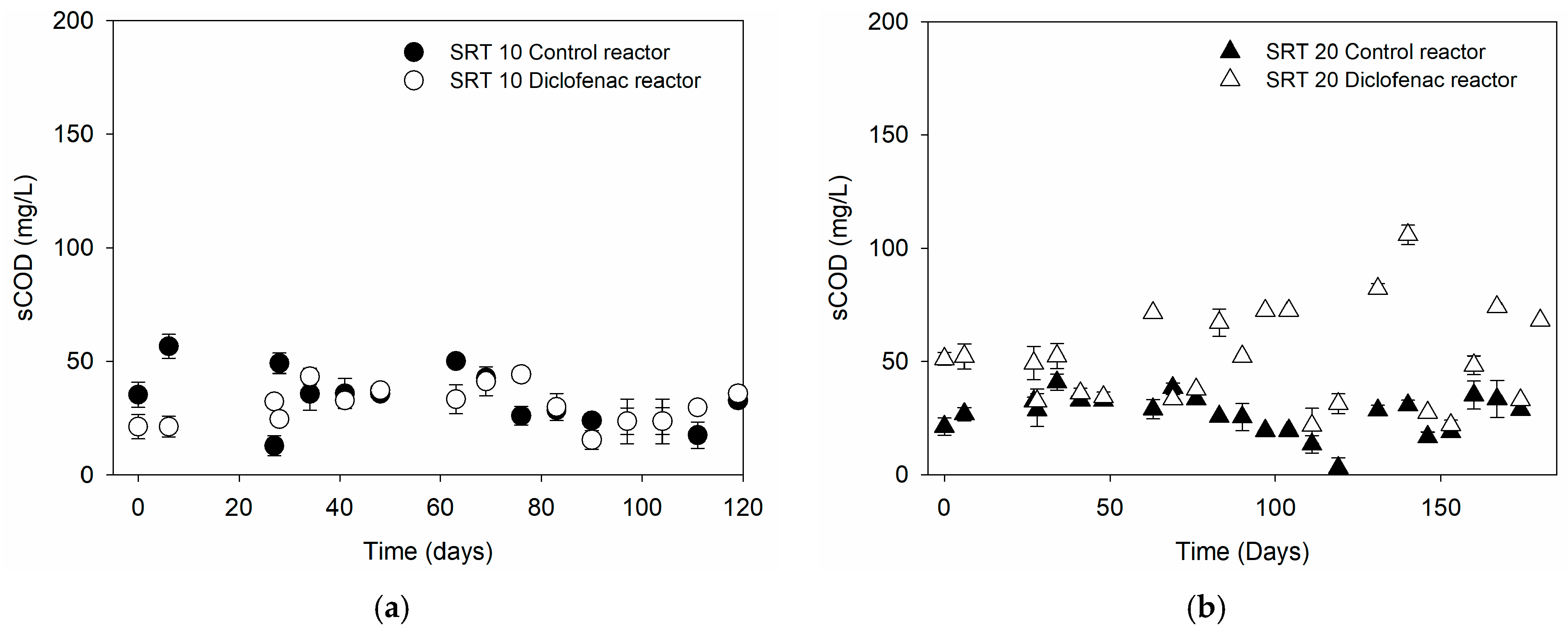
3.1. Impact of Diclofenac on Reactor Performance
3.2. Impact of SRT on Diclofenac Removal Performance
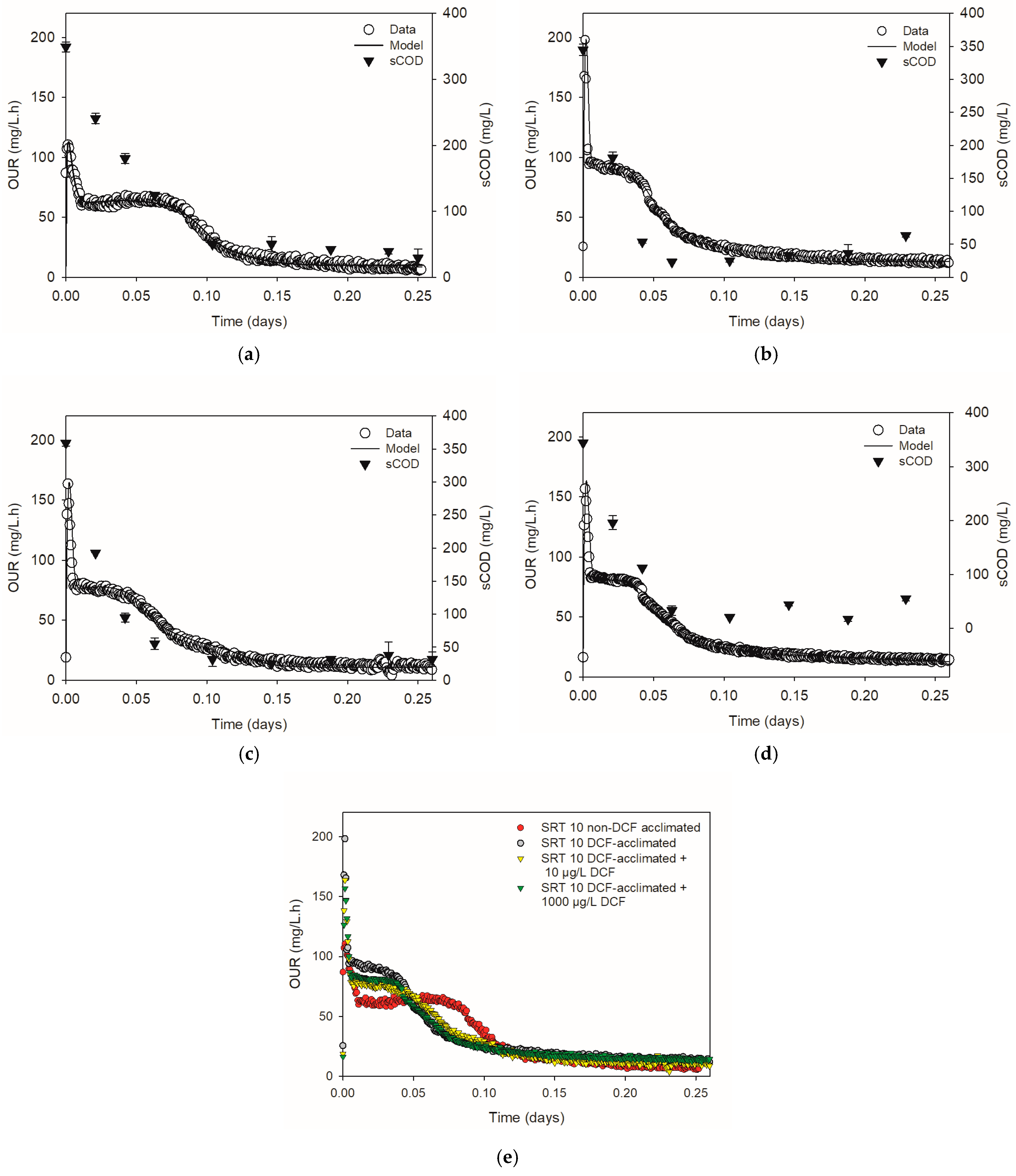
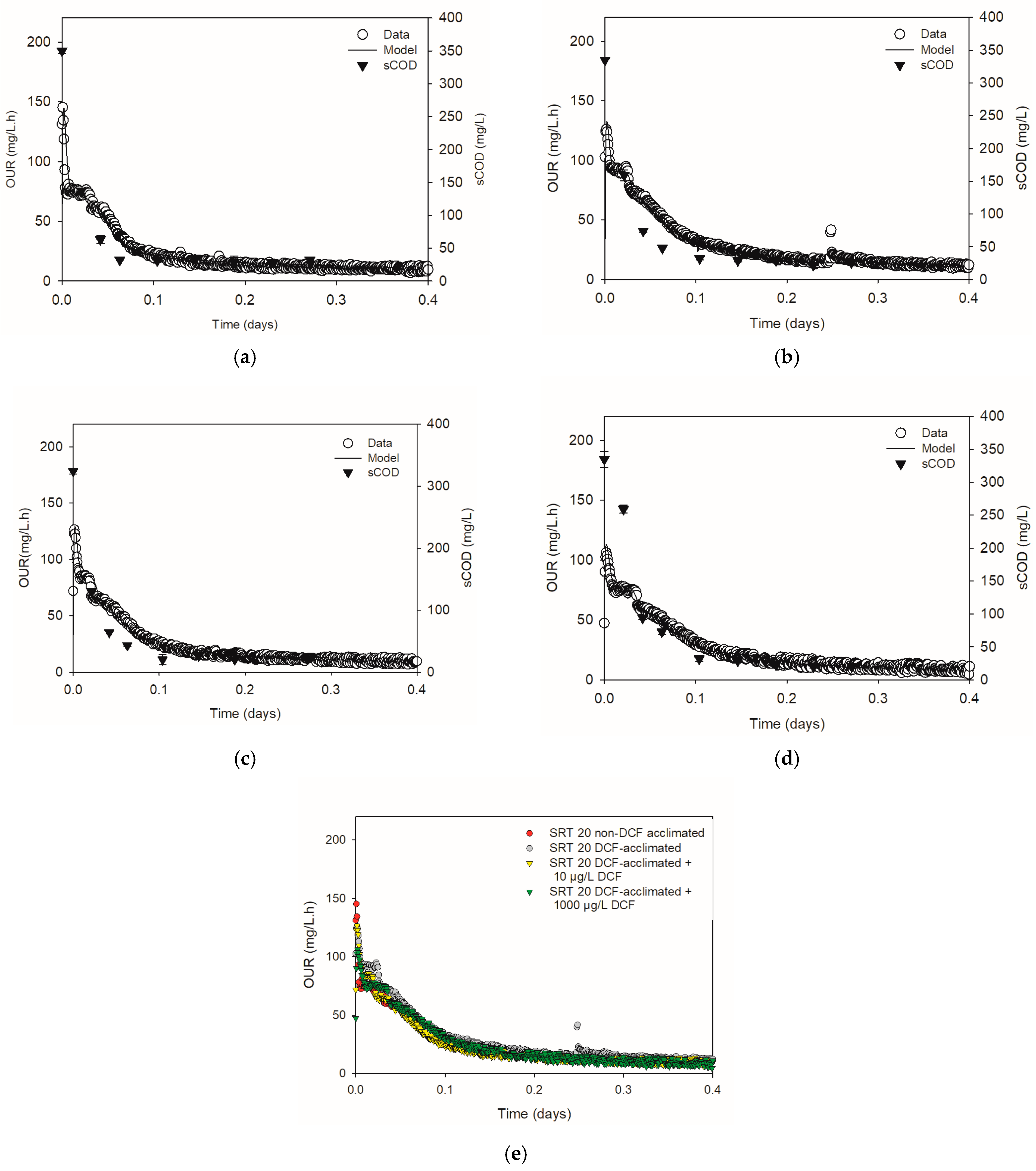
3.3. Respirometric Tests
3.4. Chronic Effect of Diclofenac on Microbial Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Green, G.A. Understanding NSAIDs: From Aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Rigueto, C.V.T.; Rosseto, M.; Nazari, M.T.; Ostwald, B.E.P.; Alessandretti, I.; Manera, C.; Piccin, J.S.; Dettmer, A. Adsorption of diclofenac sodium by composite beads prepared from tannery wastes-derived gelatin and carbon nanotubes. J. Environ. Chem. Eng. 2021, 9, 105030. [Google Scholar] [CrossRef]
- Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
- González-Pérez, D.M.; Pérez, J.I.; Gómez, M.A. Behaviour of the main nonsteroidal anti-inflammatory drugs in a membrane bioreactor treating urban wastewater at high hydraulic-and sludge-retention time. J. Hazard. Mater. 2017, 336, 128–138. [Google Scholar] [CrossRef]
- Matsuo, H.; Sakamoto, H.; Arizono, K.; Shinohara, R. Behavior of pharmaceuticals in waste water treatment plant in Japan. Bull. Environ. Contam. Toxicol. 2011, 87, 31–35. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Heyd, A.; Eikemper, R.; Köhler, H.R.; Schwaiger, J. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2004, 68, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Haap, T.; Triebskorn, R.; Köhler, H.R. Acute effects of diclofenac and DMSO to Daphnia magna: Immobilisation and hsp70-induction. Chemosphere 2008, 73, 353–359. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Cogni, D.; Riva, C.; Provini, A. An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicol. Vitr. 2009, 23, 935–942. [Google Scholar] [CrossRef]
- Adedara, I.A.; Awogbindin, I.O.; Afolabi, B.A.; Ajayi, B.O.; Rocha, J.B.T.; Farombi, E.O. Hazardous impact of diclofenac exposure on the behavior and antioxidant defense system in Nauphoeta cinerea. Environ. Pollut. 2020, 265, 115053. [Google Scholar] [CrossRef] [PubMed]
- Penha, L.C.d.C.; Rola, R.C.; Martinez, C.B.d.R.; Martins, C.d.M.G. Effects of anti-inflammatory diclofenac assessed by toxicity tests and biomarkers in adults and larvae of Danio rerio. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108955. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.I.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239, 40–47. [Google Scholar] [CrossRef]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Le Milbeau, C.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Xie, X.; Liang, G.; Cai, X.; Zhang, X.; Wang, Z. Fabrication of vessel–like biochar–based heterojunction photocatalyst Bi2S3/BiOBr/BC for diclofenac removal under visible LED light irradiation: Mechanistic investigation and intermediates analysis. J. Hazard. Mater. 2020, 391, 121407. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Ahkola, H.S.; Knuutinen, J.S.; Herve, S.H. Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Environ Sci Pollut. Res. 2016, 23, 7985–7997. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Oral, O.; Kantar, C. Diclofenac removal by pyrite-Fenton process: Performance in batch and fixed-bed continuous flow systems. Sci. Total Environ. 2019, 664, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Civelek, H. Biodegradability of Diclofenac under Aerobic Conditions. Master’s Thesis, Istanbul Technical University, Istanbul, Turkey, 2014. [Google Scholar]
- Ciftcioglu, B.; Demirkaya, E.; Salih, E.; Soylu, D.; Ozyildiz, G.; Zengin, G.E.; Guven, D.; Pala-Ozkok, I.; Insel, G.; Cokgor, E.; et al. Insights into the acute effect of anti-inflammatory drugs on activated sludge systems with high solids retention time. Environ. Technol. 2020, 42, 3920–3931. [Google Scholar] [CrossRef]
- Pala-Ozkok, I.; Ubay-Cokgor, E.; Jonas, D.; Orhon, D. Kinetic and microbial response of activated sludge community to acute and chronic exposure to tetracycline. J. Hazard. Mater. 2019, 367, 418–426. [Google Scholar] [CrossRef]
- Katipoglu-Yazan, T.; Ubay-Cokgor, E.; Orhon, D. Significance of enriched culture on the assessment of the acute inhibitory impact of sulfamethoxazole on nitrifying biomass. J. Chem. Technol. Biotechnol. 2023, 98, 706–717. [Google Scholar] [CrossRef]
- ISO 8192:2007; Water Quality—Test for Inhibition of Oxygen Consumption by Activated Sludge for Carbonaceous and Ammonium Oxidation. International Organization for Standardization: Geneva, Switzerland, 2009.
- Henze, M.; Gujer, W.; Mino, T.; van Loosdrecht, M.C. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Orhon, D.; Cokgor, E.U.; Insel, G.; Karahan, O.; Katipoglu, T. Validity of monod kinetics at different sludge ages—Peptone biodegradation under aerobic conditions. Biores. Technol. 1999, 100, 5678–5686. [Google Scholar] [CrossRef]
- Insel, G.; Orhon, D.; Vanrolleghem, P.A. Identification and modelling of aerobic hydrolysis—Application of optimal experimental design. J. Chem. Technol. Biotechnol. 2003, 78, 437–445. [Google Scholar] [CrossRef]
- Brun, R.; Reichert, P.; Künsch, R.K. Practical identifiability analysis of large environmental simulation models. Water Resour. Res. 2001, 37, 1015–1030. [Google Scholar] [CrossRef]
- Reichert, P.; Ruchti, J.; Simon, S. Aquasim 2.0; Swiss Federal Institute for Environmental Science and Technology (EAWAG): Duebendorf, Switzerland, 1998. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association. Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- ISO 6060; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1986.
- Topuz, E.; Sari, S.; Ozdemir, G.; Aydin, E.; Pehlivanoglu-Mantas, E.; Okutman Tas, D. Optimization of ultrasonic assisted diclofenac extraction from wastewater treatment plant sludge. J. Chromatogr. B 2014, 958, 48–54. [Google Scholar] [CrossRef]
- Bouju, H.; Nastold, P.; Beck, B.; Hollender, J.; Corvini, P.F.X.; Wintgens, T. Elucidation of biotransformation of diclofenac and 4′ hydroxydiclofenac during biological wastewater treatment. J. Hazard. Mater. 2016, 301, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Fatehifar, M.; Borghei, S.M. Application of moving bed biofilm reactor in the removal of pharmaceutical compounds (diclofenac and ibuprofen). J. Environ. Chem. Eng. 2018, 6, 5530–5535. [Google Scholar] [CrossRef]
- Tang, K.; Rosborg, P.; Rasmussen, E.S.; Hambly, A.; Madsen, M.; Jensen, N.M.; Hansen, A.A.; Sund, C.; Andersen, H.G.; Torresi, E.; et al. Impact of intermittent feeding on polishing of micropollutants by moving bed biofilm reactors (MBBR). J. Hazard. Mater. 2021, 403, 123536. [Google Scholar] [CrossRef]
- Zhao, J.; Xin, M.; Zhang, J.; Sun, Y.; Luo, S.; Wang, H.; Wang, Y.; Bi, X. Diclofenac inhibited the biological phosphorus removal: Performance and mechanism. Chemosphere 2020, 243, 125380. [Google Scholar] [CrossRef]
- Kujawa-Roeleveld, K.; Schuman, E. Biodegradability and Fate of Pharmaceutical Impact Compounds in Different Treatment Processes, Global Change and Ecosystems, 018530—SWITCH Sustainable Water Management in the City of the Future Integrated Project; Environmental Technology: Wageningen, The Netherlands, 2008. [Google Scholar]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Contreras, C.R.; Lopez, D.; Leiva, A.M.; Dominguez, C.; Bayona, J.M.; Vidal, G. Removal of organic pollutants in wastewater treated by activated sludge and constructed wetlands: A case study. Water 2019, 11, 2515. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Scheytt, T.; Mersmann, P.; Lindsta, R.; Heberer, T. Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef]
- Dereszewska, A.; Cytawa, S. Effect of diclofenac concentration on activated sludge conditions in a biological wastewater treatment plant. Water 2023, 15, 1838. [Google Scholar] [CrossRef]
- Demirkaya, E.; Ciftcioglu, B.; Ozyildiz, G.; Zengin, G.E.; Pala-Ozkok, I.; Cokgor, E.; Okutman-Tas, D. Comprehensive evaluation of starter culture impact on the bioreactor performance and microbial kinetics. Biochem. Eng. J. 2021, 177, 108233. [Google Scholar] [CrossRef]
- Grady, C.P.L., Jr.; Smets, B.F.; Barbeau, D.S. Variability in kinetic parameter estimates: A review of possible causes and a proposed terminology. Water Res. 1996, 30, 742–748. [Google Scholar] [CrossRef]
- Pala-Ozkok, I.; Rehman, A.; Kor-Bicakci, G.; Ural, A.; Schilhabel, M.B.; Ubay-Cokgor, E.; Jonas, D.; Orhon, D. Effect of sludge age on population dynamics and acetate utilization kinetics under aerobic conditions. Bioresour. Technol. 2013, 143, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Güven, D.; Cokgor, E.; Orhon, D. Respirometric evaluation and modeling of the impact of continuous benzo[a]anthracene feeding on activated sludge. J. Chem. Technol. Biotechnol. 2019, 94, 2621–2629. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Pala-Ozkok, I.; Ural, A.; Jonas, D.; Orhon, D.; Ubay-Cokgor, E. Is the chronic impact of sulfamethoxazole different for slow growing culture? The effect of culture history. Bioresour. Technol. 2016, 206, 65–76. [Google Scholar] [CrossRef]
- Orhon, D.; Cokgor, E.U.; Katipoglu, T.; Insel, G.; Karahan, O. Fate of 2,6-dihydroxybenzoic acid and its inhibitory impact on the biodegradation of peptone under aerobic conditions. Bioresour. Technol. 2010, 101, 2665–2671. [Google Scholar] [CrossRef]

| Time (Days) | SRT 10 Days | SRT 20 Days | ||
|---|---|---|---|---|
| Before Feeding (%) | After Feeding (%) | Before Feeding (%) | After Feeding (%) | |
| 27 | 1.2 | 1.1 | - | 1.8 |
| 48 | 3.0 | 1.8 | 2.5 | 3.3 |
| 63 | - | 1.5 | 1.5 | 2.0 |
| 69 | 1.0 | 1.0 | 1.3 | 2.3 |
| 76 | 2.3 | 1.6 | 1.3 | 1.8 |
| 83 | 1.2 | 1.3 | 1.5 | 1.6 |
| 90 | 1.1 | 1.0 | 1.8 | 1.9 |
| State Variables | Symbol | Unit | SRT 10 Days | SRT 20 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-DCF-Acclimated Biomass | DCF-Acclimated Biomass | DCF-Acclimated Biomass + 10 μg/L DCF | DCF-Acclimated Biomass + 1000 μg/L DCF | Non-DCF-Acclimated Biomass | DCF-Acclimated Biomass | DCF-Acclimated Biomass + 10 μg/L DCF | DCF-Acclimated Biomass + 1000 μg/L DCF | |||
| Maximum specific growth rate for XH | 1/day | 7.0 | 7.0 | 7.0 | 6.2 | 5.5 | 5.5 | 5.5 | 5.5 | |
| Half-saturation constant for growth of XH | KS | mg COD/L | 25 | 9 | 9 | 9 | 11 | 11 | 11 | 11 |
| Maximum hydrolysis rate for SH1 | kh1 | 1/day | 0.92 | 1.92 | 1.92 | 1.92 | 0.60 | 0.75 | 0.75 | 0.75 |
| Hydrolysis half-saturation constant for SH1 | KX | g COD/g COD | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.01 | 0.01 |
| Maximum hydrolysis rate for SH2 | kh2 | 1/day | 1.6 | 1.1 | 1.1 | 1.1 | 3.0 | 2.5 | 2.5 | 2.5 |
| Hydrolysis half-saturation constant for SH2 | KXX | g COD/g COD | 0.01 | 0.01 | 0.01 | 0.01 | 0.036 | 0.025 | 0.025 | 0.025 |
| Endogenous decay rate for XH | bH | 1/day | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Yield coefficient for XH | YH | g COD/g COD | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 |
| Volatile suspended solids | XT | mg COD/L | 2160 | 2380 | 2075 | 1980 | 2145 | 2960 | 2500 | 2250 |
| Initial active heterotrophic biomass | XH | mg COD/L | 1200 | 1650 | 1300 | 1425 | 1370 | 1220 | 1125 | 975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civelek Yoruklu, H.; Topuz, E.; Aydin, E.; Cokgor, E.; Zengin, G.E. A Kinetic Study on Chronic Response of Activated Sludge to Diclofenac by Respirometry. Water 2024, 16, 2898. https://doi.org/10.3390/w16202898
Civelek Yoruklu H, Topuz E, Aydin E, Cokgor E, Zengin GE. A Kinetic Study on Chronic Response of Activated Sludge to Diclofenac by Respirometry. Water. 2024; 16(20):2898. https://doi.org/10.3390/w16202898
Chicago/Turabian StyleCivelek Yoruklu, Hulya, Emel Topuz, Egemen Aydin, Emine Cokgor, and Gulsum Emel Zengin. 2024. "A Kinetic Study on Chronic Response of Activated Sludge to Diclofenac by Respirometry" Water 16, no. 20: 2898. https://doi.org/10.3390/w16202898
APA StyleCivelek Yoruklu, H., Topuz, E., Aydin, E., Cokgor, E., & Zengin, G. E. (2024). A Kinetic Study on Chronic Response of Activated Sludge to Diclofenac by Respirometry. Water, 16(20), 2898. https://doi.org/10.3390/w16202898






