Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants
Abstract
1. Introduction
2. Biological Methods for Wastewater Treatment
2.1. Microbial Biodegradation of Emerging Pollutants
2.2. Installation of Floating Wetlands for Wastewater Treatment
| Name of Pollutants | Plant Species | Water Matrix | Degradation % | References |
|---|---|---|---|---|
| Iron, nickel, manganese, lead, and chromium | Phragmites australis and Brachia mutica | Polluted river water | 79.05, 91.4, 91.8, 36.14, and 85.19 | [109] |
| Ammonia, chromium, and total ammonia nitrogen | Chrysopogon zizanioides L. | Industrial wastewater | 40.29–50 | [110] |
| Sewage and industrial wastewater | Brachiaria mutica, Cannabis indica, Leptochloa fusca, Phragmites australis, Rhaphiolepis indica, and Typha domingensis | Ponds and industrial wastewater | 60 and 40 | [111] |
| Hexavalent chromium | Brachiaria mutica | Industrial wastewater | 53 | [112] |
| Copper, nickel, manganese, zinc, lead, and iron | Phragmites australis | Textile wastewater | 77.5, 73.3, 89.7, 81, 70, and 65.5 | [113] |
| COD, TN, ammoniacal nitrogen, nitrate nitrogen, TP, and phosphate ion | Canna sp. | Synthetic wastewater | 91.3. 58.3, 58.3, 92, 79.5, and 67.7 | [114] |
| COD, BOD, ammonia nitrogen, and orthophosphate | Cyperus sp. and Heliconia sp. | Polluted fishpond water | 33.96, 29.41, 27.80, and 28.44 | [115] |
| Nitrogen, phosphorus, organic matter, and coliform | Phragmites sp. | Domestic wastewater | 93, 100, 99.6, and 99.9 | [116] |
| COD, BOD, and TSS | Eichhornia crassipes, Eichhornia paniculate, polygonum ferrugineum, and Borreria scabiosoides | Dairy wastewater | 74.8, 86.4, and 84.8 | [117] |
| Hydrocarbons, COD, BOD, TOC, and phenol | Phragmites australis | Diesel-oil-contaminated water | 95.8, 98.6, 97.7, 95.2, and 98.9 | [118] |
| COD, BOD, colors, and trace metals | Phragmites australis | Textile industry wastewater | 92, 91, 86, and 87 | [119] |
| Oil, COD, and BOD | Brachiara mutica and Phragmites australis | Oil field wastewater | 97, 93, and 97 | [120] |
| BOD, TSS, nitrogen and phosphorus | Carex virgata | Domestic wastewater | 100, 100, 93, and 93 | [121] |
| Nitrogen and phosphorus | Agrostis alba, Canna generalis, Carex stricta, Iris ensata, and Panicum virgatum | Nursery wastewater | 59.6 and 64.7 | [122] |
| TP, TSS, BOD, TOC, turbidity, and DOC | Juncus maritimus | Saline wastewater | 86, 82, 78, 55, 53, and 19 | [123] |
| Total phosphorus (TP) and total nitrogen (TN) | Pontederia cordata and Juncus effusus | Agricultural runoff | 90 and 84 | [124] |
| Phenolic compounds, TOC, and TN | Cyperus alternifolius and Vetiveria zizanioides | Olive mill wastewater | 98.8, 95.3, 82.7, and 98.8 | [125] |
| Total nitrogen and total phosphorus | Iris wilsonii | Municipal wastewater | 57.6 and 46.7 | [126] |
| Ammonium, BOD, TN, TP, iron, lead, copper, and nickel | Eichhornia crassipes | Polluted lake water | 97.4, 75, 82, 84.2, 62.5, 88.9, 81.7, and 80.4 | [127] |
| BOD, COD, TN, ammonium, nitrate, phosphate, and sulfate | Spirodela polyrhiza | Septage effluent | 68.43, 64.29, 66.41, 81.87, 58.02, 60.48, and 64.45 | [128] |
3. Physiochemical Methods for Wastewater Treatment
3.1. Physical Method
3.1.1. Sedimentation
3.1.2. Degasification
3.1.3. Filtration
3.1.4. Aeration
3.2. Chemical Methods
3.2.1. Adsorption
3.2.2. Chemical Precipitation
3.2.3. Flocculation and Coagulation
3.2.4. Ion Exchange
3.2.5. Ozonation
| Name of Treatment | Micropollutants | Water Matrix | Removal Efficiency (%) | References |
|---|---|---|---|---|
| Sedimentation | Phosphorus Nitrogen | Municipal wastewater | 72.43 98.63 | [184] |
| Oils | Oily wastewater | 82 | [185] | |
| Phenolic compounds | Olive mill wastewater | 76.2 | [186] | |
| Phosphorus Volatile fatty acids | Organic wastewater | 31 | [187] | |
| Ferric chloride Phosphorus Nitrogen | Sewage effluent | 80 70 40 | [188] | |
| Colors | Antibiotic fermentation wastewater | 97.3 | [137] | |
| Toxic phenolic compounds | Olive mill wastewater | 71 | [186] | |
| Degasification | Methane Hydrogen sulfide | Anerobic treated wastewater | 94 88 | [189] |
| Phosphate | Animal manure wastewater | 80–86 | [142] | |
| Dust Carbon monoxide Nitrogen oxides | Industrial wastewater | 20 59.4 55.1 | [190] | |
| Nitrogen | Coal gasification wastewater | 81.23 | [138] | |
| Organic compounds | Anaerobic wastewater | 90 | [191] | |
| Chromium | Synthetic and industrial wastewater | 92.6 | [192] | |
| Organic matter | Sugar industry wastewater | 79 | [193] | |
| Filtration | Phenol Sodium sulfate Ferrous sulfate Sulfuric acid Sodium hydroxide Potassium titanium | Synthetic and industrial wastewater | 100 | [143] |
| Conventional pollutants | Swine wastewater | 99 | [194] | |
| Color Total Nitrogen | Textile wastewater | 98.4 86.1 | [195] | |
| Microplastics | Sewage wastewater | 96 | [144] | |
| Phosphorus Organic carbon Heavy metals | Urban road runoff | 84.1–97.4 | [145] | |
| Copper | Acid mine drainage | 100 | [146] | |
| Dye/salt mixtures | Textile wastewater | 99.84 | [147] | |
| p-chloroaniline | Industrial wastewater | 50 | [149] | |
| Free DNA Antibiotic resistance genes | Domestic wastewater | 99.80 | [196] | |
| Adsorption | Manganese | Agricultural wastewater | 99 | [197] |
| Heavy metal ions | Domestic wastewater | 99 | [198] | |
| Dyes (basic violet and red) | Textile wastewater | 77 and 93 | [199] | |
| TetrabromobispenolA | Industrial wastewater | 90 | [200] | |
| Bisphenol A | Hospital effluents | 100 | [201] [202] | |
| Estrone 17β-estradiol 17α--ethinylestradiol | Laboratory wastewater | 86 94 94 | [203] | |
| Cadmium | Industrial wastewater | 86 | [204] | |
| Chromium | Industrial wastewater | 96 | [205] | |
| Lead | Tannery wastewater | 99.12 | [206] | |
| Zinc | Domestic wastewater | 93.3 | [21] | |
| Copper Iron Lead Nickel Cadmium | Agricultural and industrial wastewater | 98.54 99.25 87.17 96.95 73.54 | [207] | |
| Chemical precipitation | Chromium Copper Lead Zinc | Contaminated river water | 99.8 | [208] |
| Zinc | Industrial wastewater | 99–99.3 | [209] | |
| Fluoride Ammonia nitrogen Phosphate | Synthetic wastewater | 91 58 97 | [165] | |
| Lead | Industrial wastewater | 99.4 | [210] | |
| Silicon | Pulping whitewater | 93–95 | [211] | |
| Polycyclic aromatic hydrocarbons Micropollutants | Domestic wastewater | 80–100 | [212] | |
| Copper | Textile wastewater | 80.2 | [213] | |
| Lead | Contaminated river water | 94 | [214] | |
| Cobalt | Industrial wastewater | 99.9 | [215] | |
| Copper | Textile wastewater | 92 | [216] | |
| Flocculation and coagulation | Iron, phosphorus, and aluminum | Tannery wastewater | 99 | [217] |
| Reactive and acid | Dye bath effluents | 98 | [218] | |
| Sulfur | Industrial dying wastewater | 100 | [219] | |
| Arsenic Mercury Lead | Mature landfill leachate | 46 9 85 | [220] | |
| Microplastics Humic acid | Synthetic wastewater | 98.2 97.9 | [221] | |
| Colors | Tannery wastewater | 95 | [222] | |
| Total organic carbon Color | Textile effluents | 82 70 | [223] | |
| Turbidity Total organic carbon | Vegetable oil refinery wastewater | 100 98 | [224] | |
| Ion exchange | Arsenic | Domestic wastewater | 100 | [225] |
| Nickel Zinc | Synthetic wastewater | 98 | [226] | |
| Chromium | Synthetic wastewater | 93 | [227] | |
| Chromium | Tannery wastewater | 95 | [228] | |
| Nickel Vanadium | Hospital effluents | 98 | [229] | |
| Hexavalent chromium | Tannery wastewater | 98.5 | [230] | |
| Cadmium Lead | Mango peel wastewater | 72.46 76.26 | [231] | |
| Thallium Chloride | Industrial wastewater | 98 90 | [232] | |
| Methylene blue | Textile wastewater | 97.02 | [233] | |
| Ozonation | Colors | Tannery wastewater | 100 | [234] |
| Nitrogenous heterocyclic compounds Total nitrogen | Coal gasification wastewater | 95.6 80.6 | [235] | |
| Ibuprofen | Synthetic wastewater | 99 | [236] | |
| Proteins Polysaccharides | Organic wastewater | 100 42 | [237] | |
| Non-polar pollutants | Synthetic wastewater | 95 | [238] | |
| Metolachlor | Organic wastewater | 82 | [239] | |
| Atrazine Metolachlor Nonylphenol | Organic wastewater | 75 78 100 | [240] | |
| Diclofenac Sulfamethoxazole | Pharmaceutical industrial wastewater | 100 95 | [241] | |
| Diclofenac Sulfamethoxazole 17-α-Ethynylestradiol | Pharmaceutical industrial wastewater | 100 | [242] | |
| Ibuprofen Ciprofloxacin | Pharmaceutical industrial wastewater | 100 88 | [243] | |
| 2,4–Dichlorophenol 2,4,6–Trichlorophenol Phenazone | Synthetic wastewater | 98 98 79 | [244] |
4. Nanotechnology for Wastewater Treatment
| Name of Emerging Pollutant | Name of Nanomaterials | Characteristics | Removal Efficiency % | References |
|---|---|---|---|---|
| Organic dyes | PVA/PAA/GO-COOH@AgNPs | High catalytic activity, easy to recycle, perform efficiently at room temperature, inexpensive | 99.8 | [267] |
| Dyes | Bismuth oxychloride | Controllable shape, perform at various temperatures (low–high), large surface area, ecofriendly | 85.31 | [268] |
| 4-nitrophenol and 2-nitroaniline | PVA/PAA/Fe3O4/MXene@AgNP | Excellent structure, high thermal stability and good magnetic properties, able to be reused and high catalytic activity | 72.55 and 88.8 | [269] |
| Phosphorus and nitrogen | Carbon-based nanomaterials | Easy to synthesize, ecofriendly, high adsorption capacity, high enzymatic and catalytic activity | 24.1–42.7 | [270] |
| Organic matter and personal care products | TiO2 and ZnO | Diverse range of particle sizes, grow in clusters, inexpensive, high sorption capacity, and perform in different temperatures efficiently | 43.8–55.3 | [271] |
| Methylene blue | Rod-shaped manganese oxide | High adsorption capacity, perform efficiently at pH 8.0 and room temperature, ecofriendly, inexpensive, high degradation ability | 99.8 | [272] |
| Congo red dye | Silica composite (Si-IL) and silica-coated magnetite (Fe3O4-Si-IL) composites | Excellent adsorption capacity, high catalytic activity, diverse range of sizes, good magnetic and thermal properties | 100 | [273] |
| Chromium, arsenic, and lead | Single-walled carbon nanotubes | Reduced pore size, smoother surface, and high rejection ability | 96.8, 87.6, and 30.3 | [274] |
| Diazinon, phosalone, and chlorpyrifos | Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate | Excellent absorbance, easy to synthesize, inexpensive, ecofriendly, and easy to recycle | 99, 98, and 96 | [275] |
| Methomyl | Cu/Cu2O/CuO hybrid nanoparticles | Efficient in extreme environmental conditions, good reusability, ecofriendly, high adsorption ability, good catalytic activity, and easy to synthesize | 91 | [276] |
| Chlorpyriphos | ZnO | Highly dependent on pH, good thermodynamic properties, economical, and environmentally friendly | 56 | [277] |
| Ciprofloxacin | Fe3O4/red mud nanoparticles | High removal efficiency, depend on pH, contact time, and temperature, high adsorption capacity, and able to reuse | 30–100 | [278] |
| Naproxen | Silica and magnetic nanoparticle-decorated graphene oxide (GO-MNPs-SiO2) | Perform efficiently in optimum conditions, good adsorption ability, inexpensive, and environmentally friendly | 83–94 | [279] |
| Lead | TiO2 | High catalytic activity, average crystalline size, large surface area, and easy to recycle | 82.53 | [280] |
| Dimethoate | Graphene-oxide-supported graphitic carbon nitride microflowers decorated by silver nanoparticles | Grow in crystals, high adsorption ability, good removal efficiency, and long reaction time | 93 | [281] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Ahmad, S.; Dongming, C.; Zhong, G.; Liu, J. Microbial Technologies Employed for Biodegradation of Neonicotinoids in the Agroecosystem. Front. Microbiol. 2021, 12, 759439. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-Based Remediation as an Effective Approach for Removal of Environmental Pollutants—A Review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, H.W.; Bhatt, P. Microbial Adaptation and Impact into the Pesticide’s Degradation. Arch. Microbiol. 2022, 204, 288. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.S.; Mahdi, A.J.; Bilal, M.; Sohail, H.M.; Ali, N.; Iqbal, H.M. Environmental Impact and Pollution-Related Challenges of Renewable Wind Energy Paradigm—A Review. Sci. Total Environ. 2019, 683, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The Ecotoxicological Effects of Microplastics on Aquatic Food Web, from Primary Producer to Human: A Review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks, and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging Pollutants Removal Through Advanced Drinking Water Treatment: A Review on Processes and Environmental Performances Assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and Prospects of Advanced Oxidation Water Treatment Processes Using Catalytic Nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-Related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Borah, P.; Devi, P. Priority and emerging pollutants in water. In Inorganic Pollutants in Water; Kumar, M., Borah, P., Devi, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. [Google Scholar] [CrossRef]
- Vargas-Berrones, K.; Bernal-Jácome, L.; de León-Martínez, L.D.; Flores-Ramírez, R. Emerging Pollutants (EPs) in Latin América: A Critical Review of Under-Studied EPs, Case of Study—Nonylphenol. Sci. Total Environ. 2020, 726, 138493. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Chandrasekaran, M.; Ahmad, H.W. Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review. Microorganisms 2023, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- Arlos, M.J.; Parker, W.J.; Bicudo, J.R.; Law, P.; Hicks, K.A.; Fuzzen, M.L.; Andrews, S.A.; Servos, M.R. Modeling the Exposure of Wild Fish to Endocrine Active Chemicals: Potential Linkages of Total Estrogenicity to Field-Observed Intersex. Water Res. 2018, 139, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Cverenkárová, K.; Vojs Staňová, A.; Fehér, M.; Tamáš, M.; Škulcová, A.B.; Gál, M.; Naumowicz, M.; Špalková, V.; Bírošová, L. Hospital Wastewater—Source of Specific Micropollutants, Antibiotic-Resistant Microorganisms, Viruses, and Their Elimination. Antibiotics 2021, 10, 1070. [Google Scholar] [CrossRef]
- Vilela, C.L.S.; Bassin, J.P.; Peixoto, R.S. Water Contamination by Endocrine Disruptors: Impacts, Microbiological Aspects and Trends for Environmental Protection. Environ. Pollut. 2018, 235, 546–559. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Barceló, D. Mitigation of Bisphenol A Using an Array of Laccase-Based Robust Bio-Catalytic Cues—A Review. Sci. Total Environ. 2019, 689, 160–177. [Google Scholar] [CrossRef]
- Bao, Q.; Huang, L.; Xiu, J.; Yi, L.; Ma, Y. Study on the Treatment of Oily Sludge in Oil Fields with Lipopeptide/Sophorolipid Complex Bio-Surfactant. Ecotoxicol. Environ. Saf. 2021, 212, 111964. [Google Scholar] [CrossRef]
- Al-Asgah, N.A.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.; Allam, H.Y. Haematological and Biochemical Parameters and Tissue Accumulations of Cadmium in Oreochromis niloticus Exposed to Various Concentrations of Cadmium Chloride. Saudi J. Biol. Sci. 2015, 22, 543–550. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of Heavy Metal Ions from Aqueous Solutions Using Carbon Aerogel as an Adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Ahmad, H.W.; Ahmad, S.; Maqsood, U. Sustainable Application of Microwave-Assisted Extraction for the Recovery of Bioactive Components from Agro-Waste. In Proceedings of the 2nd International Conference on Precision and Sustainable Agriculture Under Climate Change, Rahim Yar Khan, Pakistan, 22–24 February 2024. [Google Scholar]
- Alaguprathana, M.; Poonkothai, M.; Al-Ansari, M.M.; Al-Humaid, L.; Kim, W. Cytogenotoxicity assessment in Allium cepa roots exposed to methyl orange treated with Oedogonium subplagiostomum AP1. Environ. Res. 2022, 2013, 113612. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Z.; Wen, Q.; Yang, B.; Pan, Y. Evaluation of a hybrid process of magnetic ion-exchange resin treatment followed by ozonation in secondary effluent organic matter removal. Sci. Total Environ. 2021, 754, 142361. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Tian, H.; Wu, Q.; Yi, Y.; Yan, X.; Liu, B. Mechanism of Bio-Electrokinetic Remediation of Pyrene Contaminated Soil: Effects of an Electric Field on the Degradation Pathway and Microbial Metabolic Processes. J. Hazard. Mater. 2021, 422, 126959. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Zafeiraki, E.; Manea-Karga, E.; Kouretas, D.; Tekos, F.; Skaperda, Z.; Doumpas, N.; Machera, K. Bioaccumulation of Organic and Inorganic Pollutants in Fish from Thermaikos Gulf: Preliminary Human Health Risk Assessment Assisted by a Computational Approach. J. Xenobiot. 2024, 14, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Polińska, W.; Kotowska, U.; Kiejza, D.; Karpińska, J. Insights into the Use of Phytoremediation Processes for the Removal of Organic Micropollutants from Water and Wastewater; A Review. Water 2021, 13, 2065. [Google Scholar] [CrossRef]
- Piai, L.; Blokland, M.; van der Wal, A.; Langenhoff, A. Biodegradation and Adsorption of Micropollutants by Biological Activated Carbon from a Drinking Water Production Plant. J. Hazard. Mater. 2020, 388, 122028. [Google Scholar] [CrossRef]
- Popova, S.; Tsenter, I.; Garkusheva, N.; Beck, S.E.; Matafonova, G.; Batoev, V. Evaluating (Sono)-Photo-Fenton-Like Processes with High-Frequency Ultrasound and UVA LEDs for Degradation of Organic Micropollutants and Inactivation of Bacteria Separately and Simultaneously. J. Environ. Chem. Eng. 2021, 9, 105249. [Google Scholar] [CrossRef]
- Ouarda, Y.; Tiwari, B.; Azaïs, A.; Vaudreuil, M.-A.; Ndiaye, S.D.; Drogui, P.; Tyagi, R.D.; Sauvé, S.; Desrosiers, M.; Buelna, G. Synthetic Hospital Wastewater Treatment by Coupling Submerged Membrane Bioreactor and Electrochemical Advanced Oxidation Process: Kinetic Study and Toxicity Assessment. Chemosphere 2018, 193, 160–169. [Google Scholar] [CrossRef]
- Di Marcantonio, C.; Chiavola, A.; Bains, A.; Singhal, N. Effect of Oxic/Anoxic Conditions on the Removal of Organic Micropollutants in the Activated Sludge Process. Environ. Technol. Innov. 2020, 20, 101161. [Google Scholar] [CrossRef]
- Adar, E.; Ilhan, F.; Aygun, A. Different Methods Applied to Remove Pollutants from Real Epoxy Paint Wastewater: Modeling Using the Response Surface Method. Sep. Sci. Technol. 2022, 57, 492–507. [Google Scholar] [CrossRef]
- Nabgan, W.; Jalil, A.A.; Nabgan, B.; Ikram, M.; Ali, M.W.; Lakshminarayana, P. A State of the Art Overview of Carbon-Based Composites Applications for Detecting and Eliminating Pharmaceuticals Containing Wastewater. Chemosphere 2022, 288, 132535. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.; Kumar, P.S.; Rathi, B.S. A Review on Recent Trends in the Removal of Emerging Contaminants from Aquatic Environment Using Low-Cost Adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C. Removal of Emerging Contaminants from Wastewater Using Advanced Treatments: A Review. Environ. Chem. Lett. 2022, 20, 43. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S. Preferential Removal of Pesticides from Water by Molecular Imprinting on TiO2 Photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Mapelli, F.; Scoma, A.; Michoud, G.; Aulenta, F.; Boon, N.; Borin, S.; Kalogerakis, N.; Daffonchio, D. Biotechnologies for Marine Oil Spill Cleanup: Indissoluble Ties with Microorganisms. Trends Biotechnol. 2017, 35, 860–870. [Google Scholar] [CrossRef]
- Arslan, M.; Afzal, M.; Anjum, N.A. Constructed and Floating Wetlands for Sustainable Water Reclamation. Sustainability 2022, 14, 1268. [Google Scholar] [CrossRef]
- Mani, S.; Chowdhary, P.; Zainith, S. Microbes Mediated Approaches for Environmental Waste Management. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–36. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of Endocrine System in the Regulation of Female Insect Reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhatt, P.; Ahmad, H.W.; Cui, D.; Guo, J.; Zhong, G. Enzymes Involved in the Bioremediation of Pesticides. In Industrial Applications of Microbial Enzymes; CRC Press: Boca Raton, FL, USA, 2022; pp. 133–168. [Google Scholar] [CrossRef]
- Sriram, N.; Reetha, D.; Saranraj, P. Biological Degradation of Reactive Dyes by Using Bacteria Isolated from Dye Effluent Contaminated Soil. Middle-East J. Sci. Res. 2013, 17, 1695–1700. [Google Scholar] [CrossRef]
- Thangaraj, S.; Bankole, P.O.; Sadasivam, S.K. Microbial Degradation of Azo Dyes by Textile Effluent Adapted Enterobacter hormaechei Under Microaerophilic Condition. Microbiol. Res. 2021, 250, 126805. [Google Scholar] [CrossRef]
- Adedayo, O.; Javadpour, S.; Taylor, C.; Anderson, W.; Moo-Young, M. Decolourization and Detoxification of Methyl Red by Aerobic Bacteria from a Wastewater Treatment Plant. World J. Microbiol. Biotechnol. 2004, 20, 545–550. [Google Scholar] [CrossRef]
- Meerbergen, K.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Isolation and Screening of Bacterial Isolates from Wastewater Treatment Plants to Decolorize Azo Dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kiayi, Z.; Lotfabad, T.B.; Heidarinasab, A.; Shahcheraghi, F. Microbial Degradation of Azo Dye Carmoisine in Aqueous Medium Using Saccharomyces cerevisiae ATCC 9763. J. Hazard. Mater. 2019, 373, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zheng, X.; Zhang, D.; Iqbal, W.; Liu, C.; Yang, B.; Zhao, X.; Lu, X.; Mao, Y. Microbial Characterization of Heavy Metal Resistant Bacterial Strains Isolated from an Electroplating Wastewater Treatment Plant. Ecotoxicol. Environ. Saf. 2019, 181, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly Isolated Bacillus sp. PS-6 Assisted Phytoremediation of Heavy Metals Using Phragmites communis: Potential Application in Wastewater Treatment. Bioresour. Technol. 2021, 320, 124353. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Celebioglu, A.; Sarioglu, O.F.; Uyar, T.; Tekinay, T. Encapsulation of Living Bacteria in Electrospun Cyclodextrin Ultrathin Fibers for Bioremediation of Heavy Metals and Reactive Dye from Wastewater. Colloids Surf. B Biointerfaces 2018, 161, 169–176. [Google Scholar] [CrossRef]
- Ibrahim, S.; Zulkharnain, A.; Zahri, K.N.M.; Lee, G.; Convey, P.; Gomez Fuentes, C.; Sabri, S.; Khalil, K.; Alias, S.; Gonzales-Rocha, G. Effect of Heavy Metals and Other Xenobiotics on Biodegradation of Waste Canola Oil by Cold-Adapted Rhodococcus sp. AQ5-07. Rev. Mex. Ing. Quim. 2020, 19, 1041–1052. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.P.; Alba-Elías, F. Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef]
- Da Silva, A.P.A.; De Oliveira, C.D.L.; Quirino, A.M.S.; Da Silva, F.D.M.; De Aquino Saraiva, R.; Silva-Cavalcanti, J.S. Endocrine Disruptors in Aquatic Environment: Effects and Consequences on the Biodiversity of Fish and Amphibian Species. Aquat. Sci. Technol. 2018, 6, 35–51. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Ondon, B.S.; Liu, Y.; Wang, H. Degradation Performance and Microbial Community Analysis of Microbial Electrolysis Cells for Erythromycin Wastewater Treatment. Biochem. Eng. J. 2019, 146, 1–9. [Google Scholar] [CrossRef]
- Xue, W.; Li, F.; Zhou, Q. Degradation Mechanisms of Sulfamethoxazole and Its Induction of Bacterial Community Changes and Antibiotic Resistance Genes in a Microbial Fuel Cell. Bioresour. Technol. 2019, 289, 121632. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Yang, Q.; Chen, Y.; Du, B. Evolution of Microbial Community and Drug Resistance During Enrichment of Tetracycline-Degrading Bacteria. Ecotoxicol. Environ. Saf. 2019, 171, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, G.; Manage, P.M. Removal of Ciprofloxacin (CIP) by Bacteria Isolated from Hospital Effluent Water and Identification of Degradation Pathways. Int. J. Med. Pharm. Drug Res. 2018, 2, 37–47. Available online: http://dr.lib.sjp.ac.lk/handle/123456789/6985 (accessed on 10 April 2024). [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Arasu, M.V. Effective Degradation of Tetracycline by Manganese Peroxidase Producing Bacillus velezensis Strain Al-Dhabi 140 from Saudi Arabia Using Fibrous-Bed Reactor. Chemosphere 2020, 268, 128726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Chen, Z.; Wang, G. Simultaneous Degradation of Triazophos, Methamidophos and Carbofuran Pesticides in Wastewater Using an Enterobacter Bacterial Bioreactor and Analysis of Toxicity and Biosafety. Chemosphere 2020, 261, 128054. [Google Scholar] [CrossRef]
- Mavriou, Z.; Alexandropoulou, I.; Melidis, P.; Karpouzas, D.G.; Ntougias, S. Biotreatment and Bacterial Succession in an Upflow Immobilized Cell Bioreactor Fed with Fludioxonil Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 3774–3786. [Google Scholar] [CrossRef]
- Avila, R.; Peris, A.; Eljarrat, E.; Vicent, T.; Blánquez, P. Biodegradation of Hydrophobic Pesticides by Microalgae: Transformation Products and Impact on Algae Biochemical Methane Potential. Sci. Total Environ. 2021, 754, 142114. [Google Scholar] [CrossRef]
- Ramya, K.; Vasudevan, N. Biodegradation of Synthetic Pyrethroid Pesticides Under Saline Conditions by a Novel Halotolerant Enterobacter ludwigii. Desalin. Water Treat. 2020, 173, 255–266. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Rene, E.R.; Kumar, A.J.; Chen, S. Mechanism of Allethrin Biodegradation by a Newly Isolated Sphingomonas trueperi Strain CW3 from Wastewater Sludge. Bioresour. Technol. 2020, 305, 123074. [Google Scholar] [CrossRef] [PubMed]
- Al-Mur, B.A.; Pugazhendi, A.; Jamal, M.T. Application of Integrated Extremophilic (Halo-Alkalo-Thermophilic) Bacterial Consortium in the Degradation of Petroleum Hydrocarbons and Treatment of Petroleum Refinery Wastewater Under Extreme Condition. J. Hazard. Mater. 2021, 413, 125351. [Google Scholar] [CrossRef]
- Jamal, M.T.; Pugazhendi, A. Degradation of Petroleum Hydrocarbons and Treatment of Refinery Wastewater Under Saline Condition by a Halophilic Bacterial Consortium Enriched from Marine Environment (Red Sea), Jeddah, Saudi Arabia. 3 Biotech 2018, 8, 276. [Google Scholar] [CrossRef]
- Patel, K.; Patel, M. Improving Bioremediation Process of Petroleum Wastewater Using Biosurfactants Producing Stenotrophomonas sp. S1VKR-26 and Assessment of Phytotoxicity. Bioresour. Technol. 2020, 315, 123861. [Google Scholar] [CrossRef] [PubMed]
- Kachieng’a, L.; Momba, M. The Synergistic Effect of a Consortium of Protozoan Isolates (Paramecium sp., Vorticella sp., Epistylis sp. and Opercularia sp.) on the biodegradation of petroleum hydrocarbons in wastewater. J. Environ. Chem. Eng. 2018, 6, 4820–4827. [Google Scholar] [CrossRef]
- Tian, X.; Wang, X.; Peng, S.; Wang, Z.; Zhou, R.; Tian, H. Isolation, Screening, and Crude Oil Degradation Characteristics of Hydrocarbons-Degrading Bacteria for Treatment of Oily Wastewater. Water Sci. Technol. 2018, 78, 2626–2638. [Google Scholar] [CrossRef] [PubMed]
- Abo-State, M.; Riad, B.; Bakr, A.; Aziz, M.A. Biodegradation of naphthalene by Bordetella avium isolated from petroleum refinery wastewater in Egypt and its pathway. J. Radiat. Res. Appl. Sci. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of Pulsed Electric Fields and High Pressure Homogenization on the Aqueous Extraction of Intracellular Compounds from the Microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Stroppa, N.; Onelli, E.; Pilu, S.; Baldi, A.; Rossi, L. Bioaccumulation of Heavy Metals from Wastewater Through a Typha latifolia and Thelypteris palustris Phytoremediation System. Chemosphere 2020, 241, 125018. [Google Scholar] [CrossRef]
- Bhatt, P.; Ahmad, S.; Joshi, S.; Bhatt, K. Recent Advancement in Microbial Enzymes and Their Industrial Applications. In Industrial Applications of Microbial Enzymes; Bhatt, P., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–17. [Google Scholar] [CrossRef]
- Dey, P.; Malik, A.; Mishra, A.; Singh, D.K.; von Bergen, M.; Jehmlich, N. Mechanistic Insight to Mycoremediation Potential of a Metal Resistant Fungal Strain for Removal of Hazardous Metals from Multimetal Pesticide Matrix. Environ. Pollut. 2020, 262, 114255. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Zhang, Y.; Carvalho, P.N.; Lv, T.; Arias, C.; Brix, H.; Chen, Z. Microbial Density and Diversity in Constructed Wetland Systems and the Relation to Pollutant Removal Efficiency. Water Sci. Technol. 2016, 73, 679–686. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An Overview of Microbial Fuel Cell Usage in Wastewater Treatment, Resource Recovery and Energy Production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial Fuel Cells: An Overview of Current Technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Ferreira, L.; Rosales, E.; Danko, A.S.; Sanromán, M.A.; Pazos, M.M. Bacillus thuringiensis: A Promising Bacterium for Degrading Emerging Pollutants. Process Saf. Environ. Prot. 2016, 101, 19–26. [Google Scholar] [CrossRef]
- Li, K.; Xu, A.; Wu, D.; Zhao, S.; Meng, T.; Zhang, Y. Degradation of Ofloxacin by a Manganese-Oxidizing Bacterium Pseudomonas sp. F2 and Its Biogenic Manganese Oxides. Bioresour. Technol. 2021, 328, 124826. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Mahanty, B.; Yoon, S.; Kim, C.-G. Degradation of the Long-Resistant Pharmaceutical Compounds Carbamazepine and Diatrizoate Using Mixed Microbial Culture. J. Environ. Sci. Health A 2016, 51, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Thapa, B.; Yang, X.; Gertsen, H.; Salánki, T.; Geissen, V.; Garbeva, P. Decay of Low-Density Polyethylene by Bacteria Extracted from Earthworm’s Guts: A Potential for Soil Restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y. Application of a Heavy Metal-Resistant Achromobacter sp. for the simultaneous immobilization of cadmium and degradation of sulfamethoxazole from wastewater. J. Hazard. Mater. 2021, 402, 124032. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Dick, W.A.; Ye, X.; Chen, K.; Kost, D.; Chen, L. Bioremediation of Hydrocarbon Degradation in a Petroleum-Contaminated Soil and Microbial Population and Activity Determination. Chemosphere 2017, 169, 124–130. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Paramasivan, M.; Ahmad, S. Review on arbuscular mycorrhizal fungi mediated alleviation of arsenic stress. Int. Biodeterior. Biodegrad. 2024, 194, 105872. [Google Scholar] [CrossRef]
- Akram, M.A.; Asad, M.J.; Mahmood, R.T.; Shah, M.B.; Nazir, S.; Khan, J.; Rashid, K.; Aslam, S.; Rawalpindi, P. First Report on the Biodegradation of Direct Flavine 5-G and Reactive Red S3B Textile Dyes by Piptoporus betulinus. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2023, 66, 138–143. [Google Scholar]
- Uqaili, A.A.; Usman, G.; Bhatti, U.; Nasir, H.; Zia, R.; Akram, M.A.; Jawad, F.A.; Farid, A.; Abdelgawwad, M.R.; Almutairi, S.M.; et al. Bioinformatics, RNA sequencing, and targeted bisulfite sequencing analyses identify the role of PROM2 as a diagnostic and prognostic biomarker. Am. J. Transl. Res. 2023, 15, 5389. [Google Scholar] [PubMed]
- Li, J.; Shaikh, S.N.; Uqaili, A.A.; Nasir, H.; Zia, R.; Akram, M.A.; Jawad, F.A.; Sohail, S.; Abdelgawwad, M.R.; Almutairi, S.M.; et al. A pan-cancer analysis of pituitary tumor-transforming 3, pseudogene. Am. J. Transl. Res. 2023, 15, 5408. [Google Scholar]
- Xiong, J.Q.; Govindwar, S.; Kurade, M.B.; Paeng, K.J.; Roh, H.S.; Khan, M.A.; Jeon, B.H. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere 2019, 218, 551–558. [Google Scholar] [CrossRef]
- Asiandu, A.P.; Wahyudi, A. Phycoremediation: Heavy metals green-removal by microalgae and its application in biofuel production. J. Environ. Treat. Tech. 2021, 9, 647–656. [Google Scholar]
- Jasrotia, S.; Kansal, A.; Kishore, V.V.N. Arsenic phyco-remediation by Cladophora algae and measurement of arsenic speciation and location of active absorption site using electron microscopy. Microchem. J. 2014, 114, 197–202. [Google Scholar] [CrossRef]
- Abioye, O.P.; Keji, Y.D.; Aransiola, S.A.; Oyewole, O. Phycoremediation of manganese by Spirogyra and Richterella species isolated from pond. J. Glob. Agric. Ecol. 2015, 2, 78–83. [Google Scholar]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2021, 29, 41909–41922. [Google Scholar] [CrossRef]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and Biostimulation of Hydrocarbon Degradation and the Microbial Community in a Petroleum-Contaminated Soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Tirpak, R.A.; Tondera, K.; Tharp, R.; Borne, K.E.; Schwammberger, P.; Ruppelt, J.; Winston, R.J. Optimizing Floating Treatment Wetland and Retention Pond Design through Random Forest: A Meta-Analysis of Influential Variables. J. Environ. Manag. 2022, 312, 114909. [Google Scholar] [CrossRef]
- Landmann, J.; Hammer, T.C.; Günther, H.; Hildebrandt, A. Large-Scale Investigation of Wave Dampening Characteristics of Organic, Artificial Floating Islands. Ecol. Eng. 2022, 181, 106691. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Colares, G.S.; Lutterbeck, C.A.; Dell’Osbel, N.; Machado, Ê.L.; Rodrigues, L.R. Floating Treatment Wetlands in Domestic Wastewater Treatment as a Decentralized Sanitation Alternative. Sci. Total Environ. 2021, 773, 145609. [Google Scholar] [CrossRef] [PubMed]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed Floating Wetlands: A Review of Research, Design, Operation, and Management Aspects, and Data Meta-Analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Rottle, N.; Bowles, M.; Andrews, L.; Engelke, J. Constructed Floating Wetlands: A “Safe-to-Fail” Study with Multi-Sector Participation. Restor. Ecol. 2022, 31, e13672. [Google Scholar] [CrossRef]
- Chance, L.M.G.; Van Brunt, S.C.; Majsztrik, J.C.; White, S.A. Short- and Long-Term Dynamics of Nutrient Removal in Floating Treatment Wetlands. Water Res. 2019, 159, 153–163. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic Technology as Decentralised System for Domestic Wastewater Treatment and Vegetable Production in Urban Agriculture: A Review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef]
- Benvenuti, T.; Hamerski, F.; Giacobbo, A.; Bernardes, A.M.; Zoppas-Ferreira, J.; Rodrigues, M.A. Constructed Floating Wetland for the Treatment of Domestic Sewage: A Real-Scale Study. J. Environ. Chem. Eng. 2018, 6, 5706–5711. [Google Scholar] [CrossRef]
- Pavlidis, G.; Zotou, I.; Karasali, H.; Marousopoulou, A.; Bariamis, G.; Tsihrintzis, V.A.; Nalbantis, I. Performance of Pilot-Scale Constructed Floating Wetlands in the Removal of Nutrients and Pesticides. Water Resour. Manag. 2021, 36, 399–416. [Google Scholar] [CrossRef]
- Han, W.; Luo, G.; Luo, B.; Yu, C.; Wang, H.; Chang, J.; Ge, Y. Effects of Plant Diversity on Greenhouse Gas Emissions in Microcosms Simulating Vertical Constructed Wetlands with High Ammonium Loading. J. Environ. Sci. 2019, 77, 229–237. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.-S.; Deng, W.-J.; Ying, G.-G. Removal of Steroid Hormones and Biocides from Rural Wastewater by an Integrated Constructed Wetland. Sci. Total Environ. 2019, 660, 358–365. [Google Scholar] [CrossRef]
- Gholipour, A.; Zahabi, H.; Stefanakis, A.I. A Novel Pilot and Full-Scale Constructed Wetland Study for Glass Industry Wastewater Treatment. Chemosphere 2020, 247, 125966. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.R.; Santos, F.; Ferreira, A.C.F.; Lourinha, I.; Basto, M.C.P.; Mucha, A.P. Can Veterinary Antibiotics Affect Constructed Wetlands Performance during Treatment of Livestock Wastewater? Ecol. Eng. 2017, 102, 583–588. [Google Scholar] [CrossRef]
- Mustapha, H.I.; Van Bruggen, H.J.J.A.; Lens, P.N. Vertical Subsurface Flow Constructed Wetlands for the Removal of Petroleum Contaminants from Secondary Refinery Effluent at the Kaduna Refining Plant (Kaduna, Nigeria). Environ. Sci. Pollut. Res. 2018, 25, 30451–30462. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of Floating Wetlands for the Treatment of Polluted Water of River Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar] [CrossRef]
- Effendi, H.; Margaretha, J.; Krisanti, M. Reducing Ammonia and Chromium Concentration in Batik Wastewater by Vetiver (Chrysopogon zizanioides L.) grown in floating wetland. Appl. Ecol. Environ. Res. 2018, 16, 2947–2956. [Google Scholar] [CrossRef]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating Treatment Wetlands as a Suitable Option for Large-Scale Wastewater Treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- Akram, A.; Tara, N.; Khan, M.A.; Abbasi, S.A.; Irfan, M.; Arslan, M.; Afzal, M. Enhanced Remediation of Cr6+ in Bacterial-Assisted Floating Wetlands. Water Environ. J. 2020, 34, 970–978. [Google Scholar] [CrossRef]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd_Allah, E.F.; et al. Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Gupta, P.; Ann, T.-W.; Lee, S.-M. Use of Biochar to Enhance Constructed Wetland Performance in Wastewater Reclamation. Environ. Eng. Res. 2016, 21, 36–44. [Google Scholar] [CrossRef]
- Somprasert, S.; Mungkung, S.; Kreetachat, N.; Imman, S.; Homklin, S. Implementation of an Integrated Floating Wetland and Biofilter for Water Treatment in Nile Tilapia Aquaculture. J. Ecol. Eng. 2021, 22, 146–152. [Google Scholar] [CrossRef]
- Saeed, T.; Afrin, R.; Al-Muyeed, A.; Miah, M.J.; Jahan, H. Bioreactor Septic Tank for On-Site Wastewater Treatment: Floating Constructed Wetland Integration. J. Environ. Chem. Eng. 2021, 9, 105606. [Google Scholar] [CrossRef]
- Queiroz, R.d.C.S.d.; Lôbo, I.P.; Ribeiro, V.d.S.; Rodrigues, L.B.; Almeida Neto, J.A.d. Assessment of Autochthonous Aquatic Macrophytes with Phytoremediation Potential for Dairy Wastewater Treatment in Floating Constructed Wetlands. Int. J. Phytoremediat. 2020, 22, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Fahid, M.; Arslan, M.; Shabir, G.; Younus, S.; Yasmeen, T.; Rizwan, M.; Siddique, K.; Ahmad, S.R.; Tahseen, R.; Iqbal, S. Phragmites australis in Combination with Hydrocarbons Degrading Bacteria Is a Suitable Option for Remediation of Diesel-Contaminated Water in Floating Wetlands. Chemosphere 2020, 240, 124890. [Google Scholar] [CrossRef] [PubMed]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-Site Performance of Floating Treatment Wetland Macrocosms Augmented with Dye-Degrading Bacteria for the Remediation of Textile Industry Wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with Bacteria in Floating Treatment Wetlands Positively Modulates the Phytoremediation of Oil Field Wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Park, J.B.; Sukias, J.P.; Tanner, C.C. Floating Treatment Wetlands Supplemented with Aeration and Biofilm Attachment Surfaces for Efficient Domestic Wastewater Treatment. Ecol. Eng. 2019, 139, 105582. [Google Scholar] [CrossRef]
- Spangler, J.T.; Sample, D.J.; Fox, L.J.; Albano, J.P.; White, S.A. Assessing Nitrogen and Phosphorus Removal Potential of Five Plant Species in Floating Treatment Wetlands Receiving Simulated Nursery Runoff. Environ. Sci. Pollut. Res. 2019, 26, 5751–5768. [Google Scholar] [CrossRef]
- Cicero-Fernandez, D.; Expósito-Camargo, J.; Peña-Fernandez, M. Efficacy of Juncus maritimus Floating Treatment Saltmarsh as Anti-Contamination Barrier for Saltwater Aquaculture Pollution Control. Water Sci. Technol. 2022, 85, 2811–2826. [Google Scholar] [CrossRef]
- Spangler, J.T.; Sample, D.J.; Fox, L.J.; Owen, J.S., Jr.; White, S.A. Floating treatment wetland aided nutrient removal from agricultural runoff using two wetland species. Ecol. Eng. 2019, 127, 468–479. [Google Scholar] [CrossRef]
- Goren, A.Y.; Yucel, A.; Sofuoglu, S.C.; Sofuoglu, A. Phytoremediation of Olive Mill Wastewater with Vetiveria zizaniodes (L.) Nash and Cyperus alternifolius L. Environ. Technol. Innov. 2021, 24, 102071. [Google Scholar] [CrossRef]
- Huang, Z.; Kong, F.; Li, Y.; Xu, G.; Yuan, R.; Wang, S. Advanced Treatment of Effluent from Municipal Wastewater Treatment Plant by Strengthened Ecological Floating Bed. Bioresour. Technol. 2020, 309, 123358. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, M.S.; Ismail, K.; Aboagye, D.; Ismail, M.M.; Sobhi, M.; Stefanakis, A.I. Effect of Design and Operational Parameters on Nutrients and Heavy Metal Removal in Pilot Floating Treatment Wetlands with Eichhornia crassipes Treating Polluted Lake Water. Environ. Sci. Pollut. Res. 2021, 28, 25664–25678. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Chand, N.; Suthar, S. Septage Effluent Treatment Using Floating Constructed Wetland with Spirodela polyrhiza: Response of Biochar Addition in the Support Matrix. Nat.-Based Solut. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Millar, G.J.; Ayoko, G.A.; Zhu, J.; Zhu, R.; Liang, X.; He, H.; Xi, Y. Degradation of 2,4-Dichlorophenol Using Palygorskite-Supported Bimetallic Fe/Ni Nanocomposite as a Heterogeneous Catalyst. Appl. Clay Sci. 2019, 168, 276–286. [Google Scholar] [CrossRef]
- Samal, S. Effect of Shape and Size of Filler Particle on the Aggregation and Sedimentation Behavior of the Polymer Composite. Powder Technol. 2020, 366, 43–51. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.; Jang, D.; Jung, S.; Jin, J. Study of Flow Characteristics in a Secondary Clarifier by Numerical Simulation and Radioisotope Tracer Technique. Appl. Radiat. Isot. 2005, 63, 519–526. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The Removal of Microplastics in the Wastewater Treatment Process and Their Potential Impact on Anaerobic Digestion Due to Pollutants Association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Anthony, E.T.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. A Critical Review on the Occurrence of Resistomes in the Environment and Their Removal from Wastewater Using Apposite Treatment Technologies: Limitations, Successes, and Future Improvement. Environ. Pollut. 2020, 263, 113791. [Google Scholar] [CrossRef]
- Amosa, M.K.; Jami, M.S.; Alkhatib, M.A.F.R.; Tajari, T.; Jimat, D.N.; Owolabi, R.U. Turbidity and suspended solids removal from high-strength wastewater using high surface area adsorbent: Mechanistic pathway and statistical analysis. Cogent Eng. 2016, 3, 1162384. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Effects of Advanced Treatment Systems on the Removal of Antibiotic Resistance Genes in Wastewater Treatment Plants from Hangzhou, China. Environ. Sci. Technol. 2013, 47, 8157–8163. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Lin, L.; Li, X.-Y.; Leung, K.M.Y. Removal of emerging contaminants from wastewater during chemically enhanced primary sedimentation and acidogenic sludge fermentation. Water Res. 2020, 175, 115646. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.-P.; Sun, D.-Z. Treatment of Antibiotic Fermentation Wastewater by Combined Polyferric Sulfate Coagulation, Fenton, and Sedimentation Process. J. Hazard. Mater. 2009, 168, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Zhu, H.; Xu, C.; Li, K.; Wang, D. Enhanced Nitrogen Removal from Coal Gasification Wastewater by Simultaneous Nitrification and Denitrification (SND) in an Oxygen-Limited Aeration Sequencing Batch Biofilm Reactor. Bioresour. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Z.; Cheng, Y.; Drioli, E.; Wang, Z.; Zhang, F.; Cui, Z. Development and emerging application of membrane degassing technology. Adv. Membr. 2023, 3, 100076. [Google Scholar] [CrossRef]
- Bandara, W.M.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Removal of residual dissolved methane gas in an upflow anaerobic sludge blanket reactor treating low-strength wastewater at low temperature with degassing membrane. Water Res. 2011, 45, 3533–3540. [Google Scholar] [CrossRef]
- Saidou, H.; Korchef, A.; Moussa, S.B.; Amor, M.B. Struvite precipitation by the dissolved CO2 degasification technique: Impact of the airflow rate and pH. Chemosphere 2009, 74, 338–343. [Google Scholar] [CrossRef]
- Zhang, T.; Li, P.; Fang, C.; Jiang, R. Phosphate recovery from animal manure wastewater by struvite crystallization and CO2 degasification reactor. Ecol. Chem. Eng. 2014, 21, 89. [Google Scholar] [CrossRef]
- Sun, M.; An, J.; Pan, Z.; Feng, G.; Fan, X.; Song, C.; Wang, T. Enhanced Organic Wastewater Treatment Performance in Electrochemical Filtration Process of Coal-Based Carbon Membrane via the Simple Fe2+ Addition. Sep. Purif. Technol. 2021, 276, 119418. [Google Scholar] [CrossRef]
- Shen, M.; Hu, T.; Huang, W.; Song, B.; Zeng, G.; Zhang, Y. Removal of Microplastics from Wastewater with Aluminosilicate Filter Media and Their Surfactant-Modified Products: Performance, Mechanism, and Utilization. Chem. Eng. J. 2021, 421, 129918. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lim, B.-R.; Hur, J.; Kim, H.-S.; Shin, H.-S. Combined Dual-Size Foam Glass Media Filtration Process with Micro-Flocculation for Simultaneous Removal of Particulate and Dissolved Contaminants in Urban Road Runoff. J. Environ. Manag. 2021, 277, 111475. [Google Scholar] [CrossRef]
- Menzel, K.; Barros, L.; García, A.; Ruby-Figueroa, R.; Estay, H. Metal Sulfide Precipitation Coupled with Membrane Filtration Process for Recovering Copper from Acid Mine Drainage. Sep. Purif. Technol. 2021, 270, 118721. [Google Scholar] [CrossRef]
- Jiang, M.; Ye, K.; Deng, J.; Lin, J.; Ye, W.; Zhao, S.; Van der Bruggen, B. Conventional Ultrafiltration as an Effective Strategy for Dye/Salt Fractionation in Textile Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 10698–10708. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.P.; Khanal, S.N.; Bakke, R. A simple anaerobic and filtration combined system for domestic wastewater treatment. Water-Energy Nexus 2020, 3, 41–45. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Ma, J.; Xu, S.; Wu, Z. Development of an Electrochemical Ceramic Membrane Filtration System for Efficient Contaminant Removal from Waters. Environ. Sci. Technol. 2018, 52, 4117–4126. [Google Scholar] [CrossRef]
- Ghernaout, D. The hydrophilic/hydrophobic ratio vs. dissolved organics removal by coagulation—A review. J. King Saud Univ. Sci. 2014, 26, 169–180. [Google Scholar] [CrossRef]
- Asadi, A.; Verma, A.; Yang, K.; Mejabi, B. Wastewater treatment aeration process optimization: A data mining approach. J. Environ. Manag. 2017, 203, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kusiak, A.; Zeng, Y.; Wei, X. Modeling and optimization of a wastewater pumping system with data-mining methods. Appl. Energy 2016, 164, 303–311. [Google Scholar] [CrossRef]
- Ghernaout, D. Aeration Process for Removing Radon from Drinking Water—A Review. Appl. Eng. 2019, 3, 32–45. [Google Scholar]
- Uggetti, E.; Hughes-Riley, T.; Morris, R.H.; Newton, M.I.; Trabi, C.L.; Hawes, P.; Puigagut, J.; García, J. Intermittent aeration to improve wastewater treatment efficiency in pilot-scale constructed wetland. Sci. Total Environ. 2016, 559, 212–217. [Google Scholar] [CrossRef]
- Iskurt, C.; Keyikoglu, R.; Kobya, M.; Khataee, A. Treatment of coking wastewater by aeration assisted electrochemical oxidation process at controlled and uncontrolled initial pH conditions. Sep. Purif. Technol. 2020, 248, 117043. [Google Scholar] [CrossRef]
- Jehawi, O.H.; Abdullah, S.R.S.; Kurniawan, S.B.; Ismail, N.I.; Idris, M.; Al Sbani, N.H.; Muhamad, M.H.; Hasan, H.A. Performance of pilot Hybrid Reed Bed constructed wetland with aeration system on nutrient removal for domestic wastewater treatment. Environ. Technol. Innov. 2020, 19, 100891. [Google Scholar] [CrossRef]
- Skouteris, G.; Rodriguez-Garcia, G.; Reinecke, S.; Hampel, U. The use of pure oxygen for aeration in aerobic wastewater treatment: A review of its potential and limitations. Bioresour. Technol. 2020, 312, 123595. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, S. Adsorption of heavy metals: A review. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 2267–2281. [Google Scholar]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015, 13, 699–708. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.; Ndamitso, M.; Abdulkareem, S.; Shuaib, D.; Mohammed, A.; Sumaila, A. The role of kaolin and kaolin/ZnO nanoadsorbents in adsorption studies for tannery wastewater treatment. Sci. Rep. 2020, 10, 13068. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, T.; Meng, Y.; Cheng, Y.; Lu, J.; Wang, H. Novel graphene oxide/aminated lignin aerogels for enhanced adsorption of malachite green in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125281. [Google Scholar] [CrossRef]
- Son, D.-J.; Kim, W.-Y.; Jung, B.-R.; Chang, D.; Hong, K.-H. Pilot-scale anoxic/aerobic biofilter system combined with chemical precipitation for tertiary treatment of wastewater. J. Water Process Eng. 2020, 35, 101224. [Google Scholar] [CrossRef]
- Altaş, L.; Büyükgüngör, H. Sulfide removal in petroleum refinery wastewater by chemical precipitation. J. Hazard. Mater. 2008, 153, 462–469. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing contaminants from tannery wastewater by chemical precipitation using CaO and Ca (OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Nyström, F.; Nordqvist, K.; Herrmann, I.; Hedström, A.; Viklander, M. Removal of metals and hydrocarbons from stormwater using coagulation and flocculation. Water Res. 2020, 182, 115919. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.-Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Karam, A.; Bakhoum, E.S.; Zaher, K. Coagulation/flocculation process for textile mill effluent treatment: Experimental and numerical perspectives. Int. J. Sustain. Eng. 2021, 14, 983–995. [Google Scholar] [CrossRef]
- John, D.; Yesodharan, S.; Achari, V.S. Integration of coagulation-flocculation and heterogeneous photocatalysis for the treatment of pulp and paper mill effluent. Environ. Technol. 2022, 43, 443–459. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Liu, Z.; Lompe, K.M.; Mohseni, M.; Bérubé, P.R.; Sauvé, S.; Barbeau, B. Biological ion exchange as an alternative to biological activated carbon for drinking water treatment. Water Res. 2020, 168, 115148. [Google Scholar] [CrossRef]
- Martins, V.L.; Ogden, M.D.; Jones, M.R.; Trowsdale, S.A.; Hall, P.J.; Jensen, H.S. Opportunities for coupled electrochemical and ion-exchange technologies to remove recalcitrant micropollutants in water. Sep. Purif. Technol. 2020, 239, 116522. [Google Scholar] [CrossRef]
- Amini, A.; Kim, Y.; Zhang, J.; Boyer, T.; Zhang, Q. Environmental and economic sustainability of ion exchange drinking water treatment for organics removal. J. Clean. Prod. 2015, 104, 413–421. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Li, D.; Ning, X.-a.; Yuan, Y.; Hong, Y.; Zhang, J. Ion-exchange polymers modified bacterial cellulose electrodes for the selective removal of nitrite ions from tail water of dyeing wastewater. J. Environ. Sci. 2020, 91, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, S.; Wang, B.; Huang, J.; Deng, S.; Yu, G.; Wang, Y. Modelling of emerging contaminant removal during heterogeneous catalytic ozonation using chemical kinetic approaches. J. Hazard. Mater. 2019, 380, 120888. [Google Scholar] [CrossRef] [PubMed]
- Grace Pavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Mainardis, M.; Buttazzoni, M.; De Bortoli, N.; Mion, M.; Goi, D. Evaluation of ozonation applicability to pulp and paper streams for a sustainable wastewater treatment. J. Clean. Prod. 2020, 258, 120781. [Google Scholar] [CrossRef]
- Khalifa, O.; Banat, F.; Srinivasakannan, C.; AlMarzooqi, F.; Hasan, S.W. Ozonation-assisted electro-membrane hybrid reactor for oily wastewater treatment: A methodological approach and synergy effects. J. Clean. Prod. 2021, 289, 125764. [Google Scholar] [CrossRef]
- He, W.; Wang, Q.; Zhu, Y.; Wang, K.; Mao, J.; Xue, X.; Shi, Y. Innovative technology of municipal wastewater treatment for rapid sludge sedimentation and enhancing pollutants removal with nano-material. Bioresour. Technol. 2021, 324, 124675. [Google Scholar] [CrossRef]
- Rubi, H.; Fall, C.; Ortega, R. Pollutant removal from oily wastewater discharged from car washes through sedimentation–coagulation. Water Sci. Technol. 2009, 59, 2359–2369. [Google Scholar] [CrossRef]
- Khoufi, S.; Feki, F.; Sayadi, S. Detoxification of olive mill wastewater by electrocoagulation and sedimentation processes. J. Hazard. Mater. 2007, 142, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, R.-h.; Yang, Z.-y.; Li, X.-y. Effect of coagulant on acidogenic fermentation of sludge from enhanced primary sedimentation for resource recovery: Comparison between FeCl3 and PACl. Chem. Eng. J. 2017, 325, 681–689. [Google Scholar] [CrossRef]
- Poon, C.S.; Chu, C. The use of ferric chloride and anionic polymer in the chemically assisted primary sedimentation process. Chemosphere 1999, 39, 1573–1582. [Google Scholar] [CrossRef]
- Lee, E.; Rout, P.R.; Kyun, Y.; Bae, J. Process optimization and energy analysis of vacuum degasifier systems for the simultaneous removal of dissolved methane and hydrogen sulfide from anaerobically treated wastewater. Water Res. 2020, 182, 115965. [Google Scholar] [CrossRef] [PubMed]
- Shchokin, V.; Ezhov, V.; Shchokina, O.; Chasova, E. Degasification and removal of dust at mass explosions in pits using a humate reagent in the internal and external storage. Ukr. J. Ecol. 2021, 11, 132–138. [Google Scholar]
- Bandara, W.; Ikeda, M.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Introduction of a degassing membrane technology into anaerobic wastewater treatment. Water Environ. Res. 2013, 85, 387–390. [Google Scholar] [CrossRef]
- Dias, D.; Lapa, N.; Bernardo, M.; Ribeiro, W.; Matos, I.; Fonseca, I.; Pinto, F. Cr (III) removal from synthetic and industrial wastewaters by using co-gasification chars of rice waste streams. Bioresour. Technol. 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Satoh, H.; Bandara, W.M.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Enhancement of organic matter degradation and methane gas production of anaerobic granular sludge by degasification of dissolved hydrogen gas. Bioresour. Technol. 2017, 244, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of antibiotic resistance genes from swine wastewater by membrane filtration treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef]
- Badawi, A.K.; Zaher, K. Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- Slipko, K.; Reif, D.; Woegerbauer, M.; Hufnagl, P.; Krampe, J.; Kreuzinger, N. Removal of extracellular free DNA and antibiotic resistance genes from water and wastewater by membranes ranging from microfiltration to reverse osmosis. Water Res. 2019, 164, 114916. [Google Scholar] [CrossRef] [PubMed]
- Rudi, N.N.; Muhamad, M.S.; Te Chuan, L.; Alipal, J.; Omar, S.; Hamidon, N.; Hamid, N.H.A.; Sunar, N.M.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, e05049. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Yan, T.; Shen, J.; Zhang, J.; Zhang, D. Selective Capacitive Removal of Heavy Metal Ions from Wastewater over Lewis Base Sites of S-Doped Fe–N–C Cathodes via an Electro-Adsorption Process. Environ. Sci. Technol. 2021, 55, 7665–7673. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile wastewater treatment by agro-industrial waste: Equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Hu, Y.; Fang, Z.; Cheng, J.; Chen, Y. Adsorption characteristics of tetrabromobisphenol A onto sodium bisulfite reduced graphene oxide aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 781–788. [Google Scholar] [CrossRef]
- Zhao, Y.; Cho, C.-W.; Cui, L.; Wei, W.; Cai, J.; Wu, G.; Yun, Y.-S. Adsorptive removal of endocrine-disrupting compounds and a pharmaceutical using activated charcoal from aqueous solution: Kinetics, equilibrium, and mechanism studies. Environ. Sci. Pollut. Res. 2019, 26, 33897–33905. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Obaid, A.Y.; Asiri, N.A.; Salam, M.A. Adsorption behavior of estrogenic compounds on carbon nanotubes from aqueous solutions: Kinetic and thermodynamic studies. J. Ind. Eng. Chem. 2014, 20, 916–924. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Owalude, S.O.; Tella, A.C. Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 377–388. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Abhinaya, R.; Thiruvengadaravi, K.; Baskaralingam, P.; Sivanesan, S. Lead (II) adsorption onto sulphuric acid treated cashew nut shell. Sep. Sci. Technol. 2011, 46, 2436–2449. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Blais, J.-F.; Mercier, G. Heavy metals removal from acidic and saline soil leachate using either electrochemical coagulation or chemical precipitation. J. Environ. Eng. 2006, 132, 545–554. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of lead from lead battery recycling plant wastewater. Ind. Eng. Chem. Res. 2002, 41, 1579–1582. [Google Scholar] [CrossRef]
- Huuha, T.S.; Kurniawan, T.A.; Sillanpää, M.E. Removal of silicon from pulping whitewater using integrated treatment of chemical precipitation and evaporation. Chem. Eng. J. 2010, 158, 584–592. [Google Scholar] [CrossRef]
- Ates, H.; Argun, M.E. Removal of PAHs from leachate using a combination of chemical precipitation and Fenton and ozone oxidation. Water Sci. Technol. 2018, 78, 1064–1070. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Soklun, H.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Zhu, L. A green strategy for simultaneous Cu (II)-EDTA decomplexation and Cu precipitation from water by bicarbonate-activated hydrogen peroxide/chemical precipitation. Chem. Eng. J. 2019, 370, 1298–1309. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Mercier, G.; Blais, J.-F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Chen, X.; Du, D.; Wu, R.; Qu, G.; Guo, X.; Jia, H.; Wang, T. Non-thermal plasma oxidation of Cu (II)-EDTA and simultaneous Cu (II) elimination by chemical precipitation. J. Environ. Manag. 2019, 248, 109237. [Google Scholar] [CrossRef] [PubMed]
- Anouzla, A.; Abrouki, Y.; Souabi, S.; Safi, M.; Rhbal, H. Colour and COD removal of disperse dye solution by a novel coagulant: Application of statistical design for the optimization and regression analysis. J. Hazard. Mater. 2009, 166, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Golob, V.; Vinder, A.; Simonič, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dye. Pigment. 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Bidhendi, G.N.; Torabian, A.; Ehsani, H.; Razmkhah, N. Evaluation of industrial dyeing wastewater treatment with coagulants and polyelectrolyte as a coagulant aid. J. Environ. Health Sci. Eng. 2007, 4, 29–36. [Google Scholar]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205, 208–215. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suta, T.; Usuelli, M.; Handschin, S.; Canelli, G.; Bagnani, M.; Mezzenga, R. Sustainable removal of microplastics and natural organic matter from water by coagulation–flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021, 55, 8848–8858. [Google Scholar] [CrossRef]
- Irfan, M.; Butt, T.; Imtiaz, N.; Abbas, N.; Khan, R.A.; Shafique, A. The removal of COD, TSS and colour of black liquor by coagulation–flocculation process at optimized pH, settling and dosing rate. Arab. J. Chem. 2017, 10, S2307–S2318. [Google Scholar] [CrossRef]
- Torres, N.H.; Souza, B.S.; Ferreira, L.F.R.; Lima, A.S.; Dos Santos, G.N.; Cavalcanti, E.B. Real textile effluents treatment using coagulation/flocculation followed by electrochemical oxidation process and ecotoxicological assessment. Chemosphere 2019, 236, 124309. [Google Scholar] [CrossRef]
- Khouni, I.; Louhichi, G.; Ghrabi, A.; Moulin, P. Efficiency of a coagulation/flocculation–membrane filtration hybrid process for the treatment of vegetable oil refinery wastewater for safe reuse and recovery. Process Saf. Environ. Prot. 2020, 135, 323–341. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Ponprasath, R.; Rohan, K.; Jahnavi, N. An effective separation of toxic arsenic from aquatic environment using electrochemical ion exchange process. J. Hazard. Mater. 2021, 412, 125240. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Edebali, S.; Pehlivan, E. Evaluation of Amberlite IRA96 and Dowex 1× 8 ion-exchange resins for the removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2010, 161, 161–166. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaser, Z.A.; Hamoda, M.F. Removal of nickel and vanadium from desalination brines by ion-exchange resins. Desalin. Water Treat 2019, 157, 148–156. [Google Scholar] [CrossRef]
- Alvarado, L.; Torres, I.R.; Chen, A. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater. Sep. Purif. Technol. 2013, 105, 55–62. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Long, J.; Jiang, D.; Liu, J.; Li, S.; Qi, J.; Zhang, P.; Wang, J.; Gong, J. Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J. Hazard. Mater. 2017, 333, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sharma, G.; Thakur, R. Pectin@ zirconium (IV) silicophosphate nanocomposite ion exchanger: Photo catalysis, heavy metal separation and antibacterial activity. Chem. Eng. J. 2015, 267, 235–244. [Google Scholar] [CrossRef]
- Poznyak, T.; Bautista, G.L.; Chaírez, I.; Córdova, R.I.; Ríos, L.E. Decomposition of toxic pollutants in landfill leachate by ozone after coagulation treatment. J. Hazard. Mater. 2008, 152, 1108–1114. [Google Scholar] [CrossRef]
- Zhu, H.; Han, Y.; Ma, W.; Han, H.; Ma, W. Removal of selected nitrogenous heterocyclic compounds in biologically pretreated coal gasification wastewater (BPCGW) using the catalytic ozonation process combined with the two-stage membrane bioreactor (MBR). Bioresour. Technol. 2017, 245, 786–793. [Google Scholar] [CrossRef]
- Quero-Pastor, M.; Garrido-Perez, M.; Acevedo, A.; Quiroga, J. Ozonation of ibuprofen: A degradation and toxicity study. Sci. Total Environ. 2014, 466, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, L.; Chen, Y.; Cheng, Y.; Liu, Y.; Zha, X. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Morales, J.; Gómez, M.J.; Herrera-López, S.; Fernández-Alba, A.R.; García-Calvo, E.; Rosal, R. Energy efficiency for the removal of non-polar pollutants during ultraviolet irradiation, visible light photocatalysis and ozonation of a wastewater effluent. Water Res. 2013, 47, 5546–5556. [Google Scholar] [CrossRef]
- Restivo, J.; Órfão, J.; Armenise, S.; Garcia-Bordejé, E.; Pereira, M. Catalytic ozonation of metolachlor under continuous operation using nanocarbon materials grown on a ceramic monolith. J. Hazard. Mater. 2012, 239, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Restivo, J.; Órfão, J.J.; Pereira, M.F.; Garcia-Bordejé, E.; Roche, P.; Bourdin, D.; Houssais, B.; Coste, M.; Derrouiche, S. Catalytic ozonation of organic micropollutants using carbon nanofibers supported on monoliths. Chem. Eng. J. 2013, 230, 115–123. [Google Scholar] [CrossRef]
- Martins, R.C.; Cardoso, M.; Dantas, R.F.; Sans, C.; Esplugas, S.; Quinta-Ferreira, R.M. Catalytic studies for the abatement of emerging contaminants by ozonation. J. Chem. Technol. Biotechnol. 2015, 90, 1611–1618. [Google Scholar] [CrossRef]
- Pocostales, P.; Álvarez, P.; Beltrán, F. Catalytic ozonation promoted by alumina-based catalysts for the removal of some pharmaceutical compounds from water. Chem. Eng. J. 2011, 168, 1289–1295. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: In situ ATR-FTIR studies. Appl. Catal. B Environ. 2010, 97, 340–346. [Google Scholar] [CrossRef]
- Lv, A.; Hu, C.; Nie, Y.; Qu, J. Catalytic ozonation of toxic pollutants over magnetic cobalt-doped Fe3O4 suspensions. Appl. Catal. B Environ. 2012, 117, 246–252. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current Trends in the Application of Nanomaterials for the Removal of Emerging Micropollutants and Pathogens from Water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in municipal drinking water—A review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Phromsatit, T.; Boonyuen, S.; Andou, Y. Synthesis of silver nanoparticles/porphyrin/reduced graphene oxide hydrogel as dye adsorbent for wastewater treatment. FlatChem 2020, 23, 100174. [Google Scholar] [CrossRef]
- Kariim, I.; Abdulkareem, A.; Abubakre, O. Development and characterization of MWCNTs from activated carbon as adsorbent for metronidazole and levofloxacin sorption from pharmaceutical wastewater: Kinetics, isotherms and thermodynamic studies. Sci. Afr. 2020, 7, e00242. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Shkir, M.; Palanivel, B.; Khan, A.; Kumar, M.; Chang, J.-H.; Mani, A.; AlFaify, S. Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: An impact of Ni doping. Chemosphere 2022, 291, 132687. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-T.; Nguyen, T.T.-N.; Huynh, T.T.-T.; Vo, T.T.-T.; Nguyen, T.T.-N.; Nguyen, D.-T.; Dang, V.-S.; Dang, C.-H.; Nguyen, T.-D. Biosynthesis of silver and gold nanoparticles using aqueous extract from Crinum latifolium leaf and their applications forward antibacterial effect and wastewater treatment. J. Nanomater. 2019, 2019, 8385935. [Google Scholar] [CrossRef]
- Najafpoor, A.; Norouzian-Ostad, R.; Alidadi, H.; Rohani-Bastami, T.; Davoudi, M.; Barjasteh-Askari, F.; Zanganeh, J. Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J. Mol. Liq. 2020, 303, 112640. [Google Scholar] [CrossRef]
- Rupa, E.J.; Kaliraj, L.; Abid, S.; Yang, D.-C.; Jung, S.-K. Synthesis of a zinc oxide nanoflower photocatalyst from sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Huang, X.; Yang, J.; Wang, J.; Bi, J.; Xie, C.; Hao, H. Design and synthesis of core–shell Fe3O4@ PTMT composite magnetic microspheres for adsorption of heavy metals from high salinity wastewater. Chemosphere 2018, 206, 513–521. [Google Scholar] [CrossRef]
- Bhatt, P.; Chaudhary, P.; Ahmad, S.; Bhatt, K.; Chandra, D.; Chen, S. Recent advances in the application of microbial inoculants in the phytoremediation of xenobiotic compounds. In Unravelling Plant-Microbe Synergy; Chandra, D., Bhatt, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–48. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A.; Badawy, M.E.; Abdelgaleil, S.A. Green low cost nanomaterial produced from Moringa oleifera seed waste for enhanced removal of chlorpyrifos from wastewater: Mechanism and sorption studies. J. Environ. Chem. Eng. 2021, 9, 105376. [Google Scholar] [CrossRef]
- Guzmán-Trampe, S.; Ceapa, C.D.; Manzo-Ruiz, M.; Sánchez, S. Synthetic biology era: Improving antibiotic’s world. Biochem. Pharmacol. 2017, 134, 99–113. [Google Scholar] [CrossRef]
- Su, H.-C.; Liu, Y.-S.; Pan, C.-G.; Chen, J.; He, L.-Y.; Ying, G.-G. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: From drinking water source to tap water. Sci. Total Environ. 2018, 616, 453–461. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622, 293–305. [Google Scholar] [CrossRef]
- Fang, T.; Wang, H.; Cui, Q.; Rogers, M.; Dong, P. Diversity of potential antibiotic-resistant bacterial pathogens and the effect of suspended particles on the spread of antibiotic resistance in urban recreational water. Water Res. 2018, 145, 541–551. [Google Scholar] [CrossRef]
- Essawy, A.A.; Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.; Kamel, M.M. Green synthesis of spongy Nano-ZnO productive of hydroxyl radicals for unconventional solar-driven photocatalytic remediation of antibiotic enriched wastewater. J. Environ. Manag. 2020, 271, 110961. [Google Scholar] [CrossRef]
- Cai, W.; Weng, X.; Chen, Z. Highly efficient removal of antibiotic rifampicin from aqueous solution using green synthesis of recyclable nano-Fe3O4. Environ. Pollut. 2019, 247, 839–846. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, W.; Zhang, H.; Jia, Y. Strong pyro-catalysis of shape-controllable bismuth oxychloride nanomaterial for wastewater remediation. Appl. Surf. Sci. 2020, 513, 145630. [Google Scholar] [CrossRef]
- Huang, X.; Wang, R.; Jiao, T.; Zou, G.; Zhan, F.; Yin, J.; Zhang, L.; Zhou, J.; Peng, Q. Facile preparation of hierarchical AgNP-loaded MXene/Fe3O4/polymer nanocomposites by electrospinning with enhanced catalytic performance for wastewater treatment. ACS Omega 2019, 4, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, Q.; Guo, F.; Sun, X.; Zhang, J.; Chen, Y. Impacts of carbon-based nanomaterials on nutrient removal in constructed wetlands: Microbial community structure, enzyme activities, and metabolism process. J. Hazard. Mater. 2021, 401, 123270. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Johnston, M.; Wang, G.-S.; Huang, C. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO2 and ZnO. Sci. Total Environ. 2018, 625, 1321–1329. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, B.; Wang, R.; Zhang, L.; Jiao, T.; Liu, Z. Facile preparation of rod-like MnO nanomixtures via hydrothermal approach and highly efficient removal of methylene blue for wastewater Treatment. Nanomaterials 2018, 9, 10. [Google Scholar] [CrossRef]
- Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. Novel magnetic silica-ionic liquid nanocomposites for wastewater treatment. Nanomaterials 2019, 10, 71. [Google Scholar] [CrossRef]
- Gupta, S.; Bhatiya, D.; Murthy, C. Metal removal studies by composite membrane of polysulfone and functionalized single-walled carbon nanotubes. Sep. Sci. Technol. 2015, 50, 421–429. [Google Scholar] [CrossRef]
- Bandforuzi, S.R.; Hadjmohammadi, M.R. Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate for enhanced removal and trace determination of three organophosphorus pesticides from natural waters. Anal. Chim. Acta 2019, 1078, 90–100. [Google Scholar] [CrossRef]
- Tony, M.; Mansour, S.A. Microwave-assisted catalytic oxidation of methomyl pesticide by Cu/Cu2O/CuO hybrid nanoparticles as a Fenton-like source. Int. J. Environ. Sci. Technol. 2020, 17, 161–174. [Google Scholar] [CrossRef]
- ul Haq, A.; Saeed, M.; Usman, M.; Naqvi, S.A.R.; Bokhari, T.H.; Maqbool, T.; Ghaus, H.; Tahir, T.; Khalid, H. Sorption of chlorpyrifos onto zinc oxide nanoparticles impregnated Pea peels (Pisum sativum L): Equilibrium, kinetic and thermodynamic studies. Environ. Technol. Innov. 2020, 17, 100516. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Beduk, F.; Ulvi, A. Removal of antibiotics from aqueous solution by using magnetic Fe3O4/red mud-nanoparticles. Sci. Total Environ. 2019, 670, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nodeh, M.K.; Radfard, M.; Zardari, L.A.; Rashidi Nodeh, H. Enhanced removal of naproxen from wastewater using silica magnetic nanoparticles decorated onto graphene oxide; parametric and equilibrium study. Sep. Sci. Technol. 2018, 53, 2476–2485. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Deng, X.; Chen, R.; Zhao, Z.; Cui, F.; Xu, X. Graphene oxide-supported graphitic carbon nitride microflowers decorated by sliver nanoparticles for enhanced photocatalytic degradation of dimethoate via addition of sulfite: Mechanism and toxicity evolution. Chem. Eng. J. 2021, 425, 131683. [Google Scholar] [CrossRef]
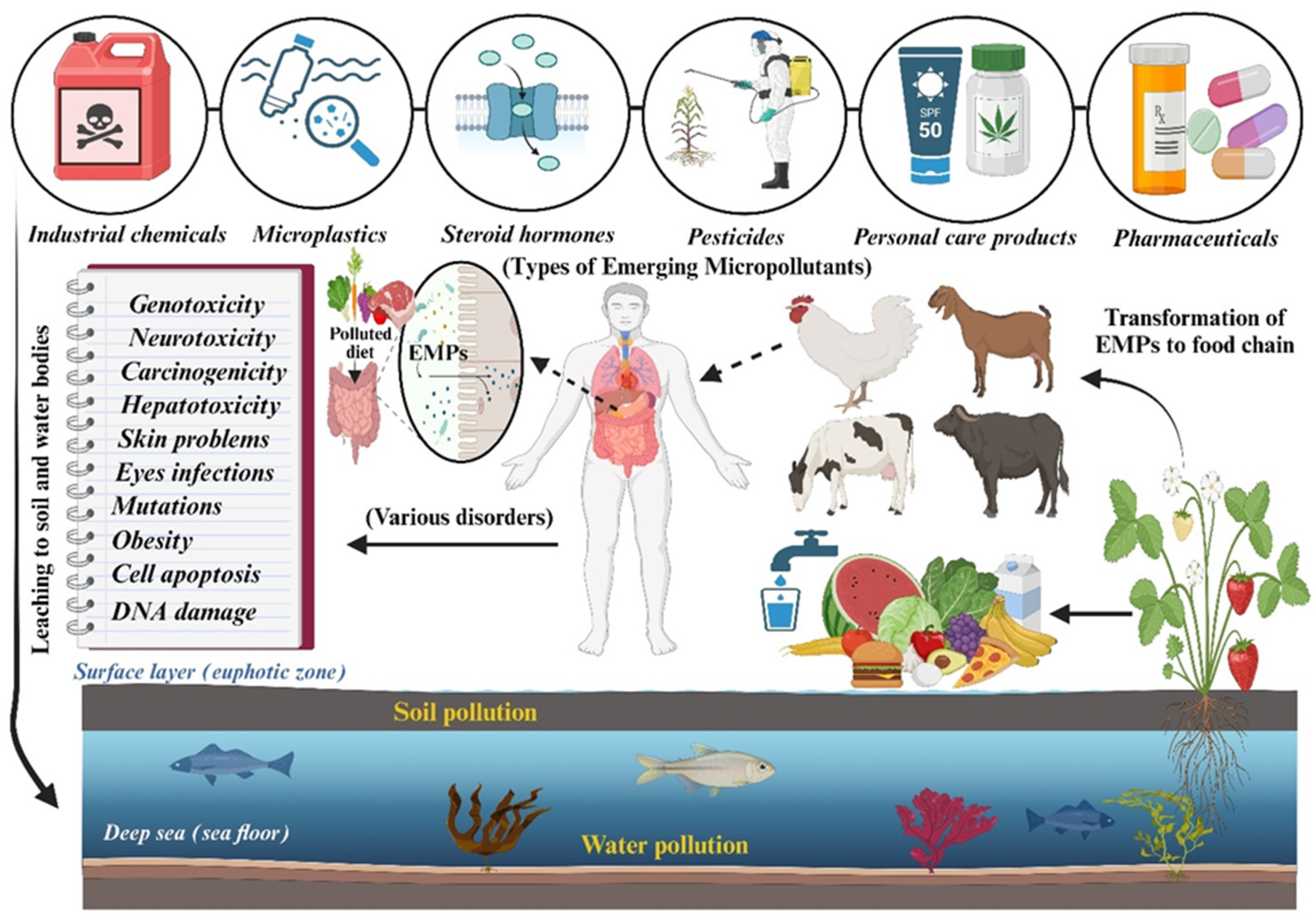
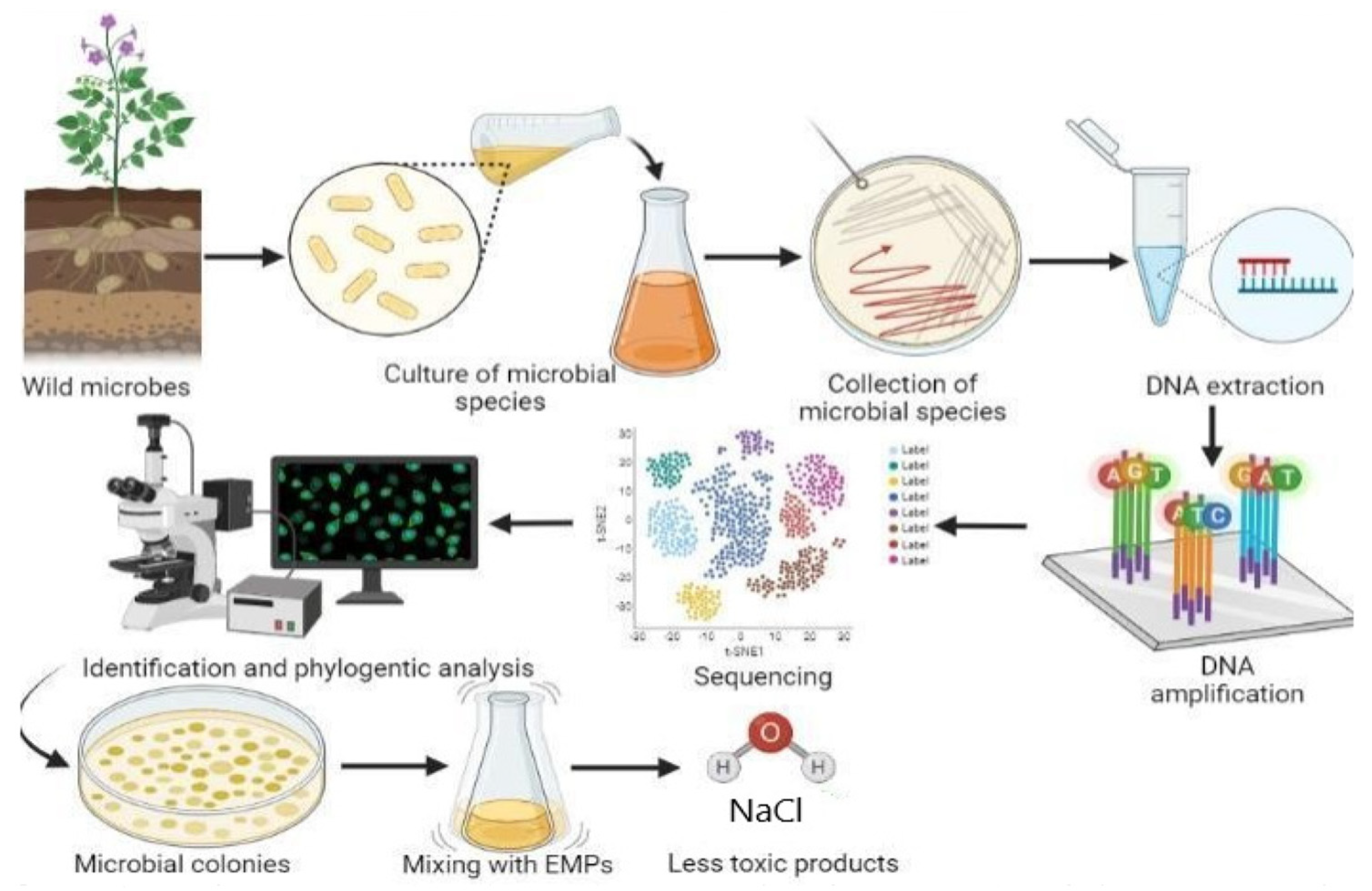
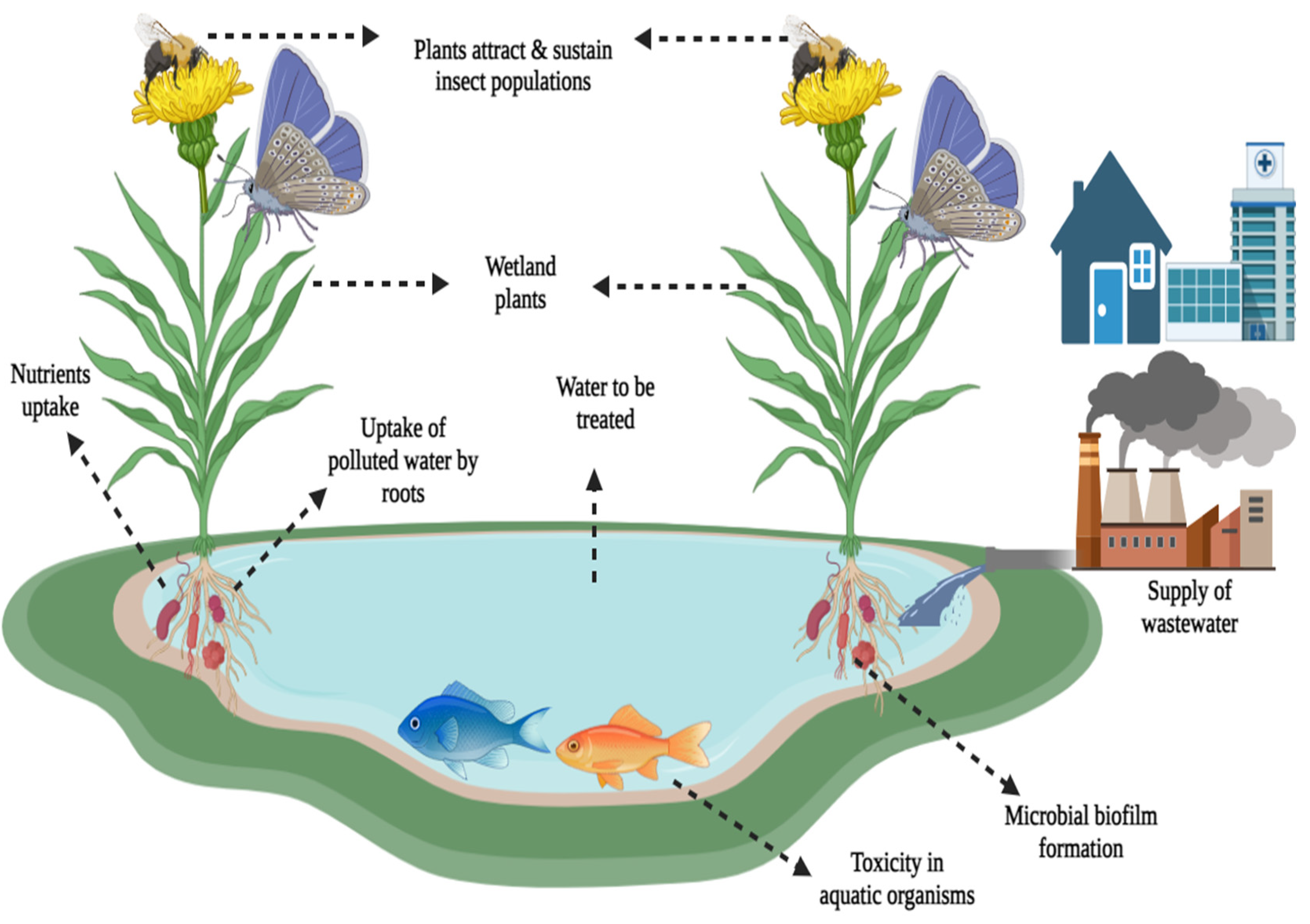
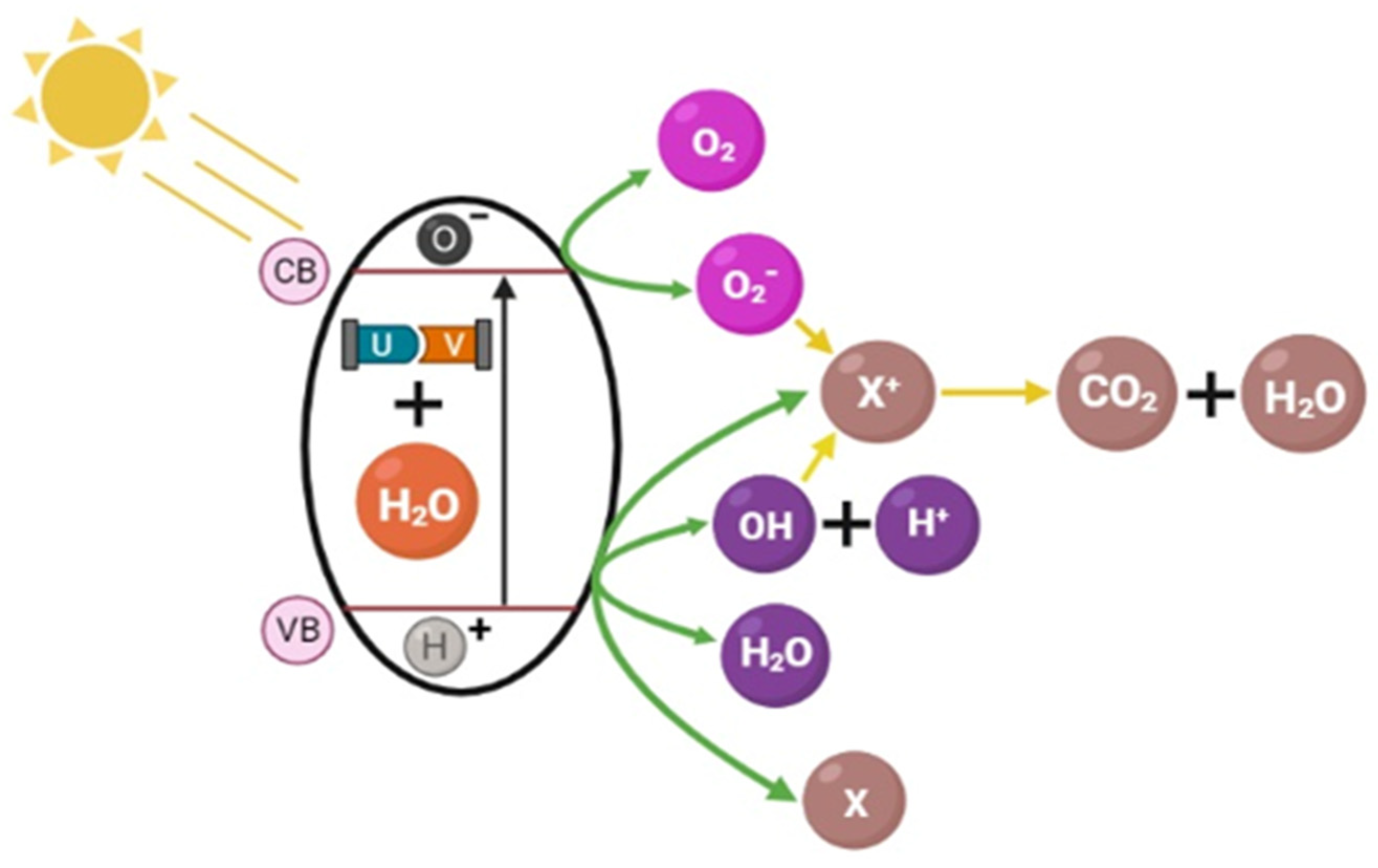
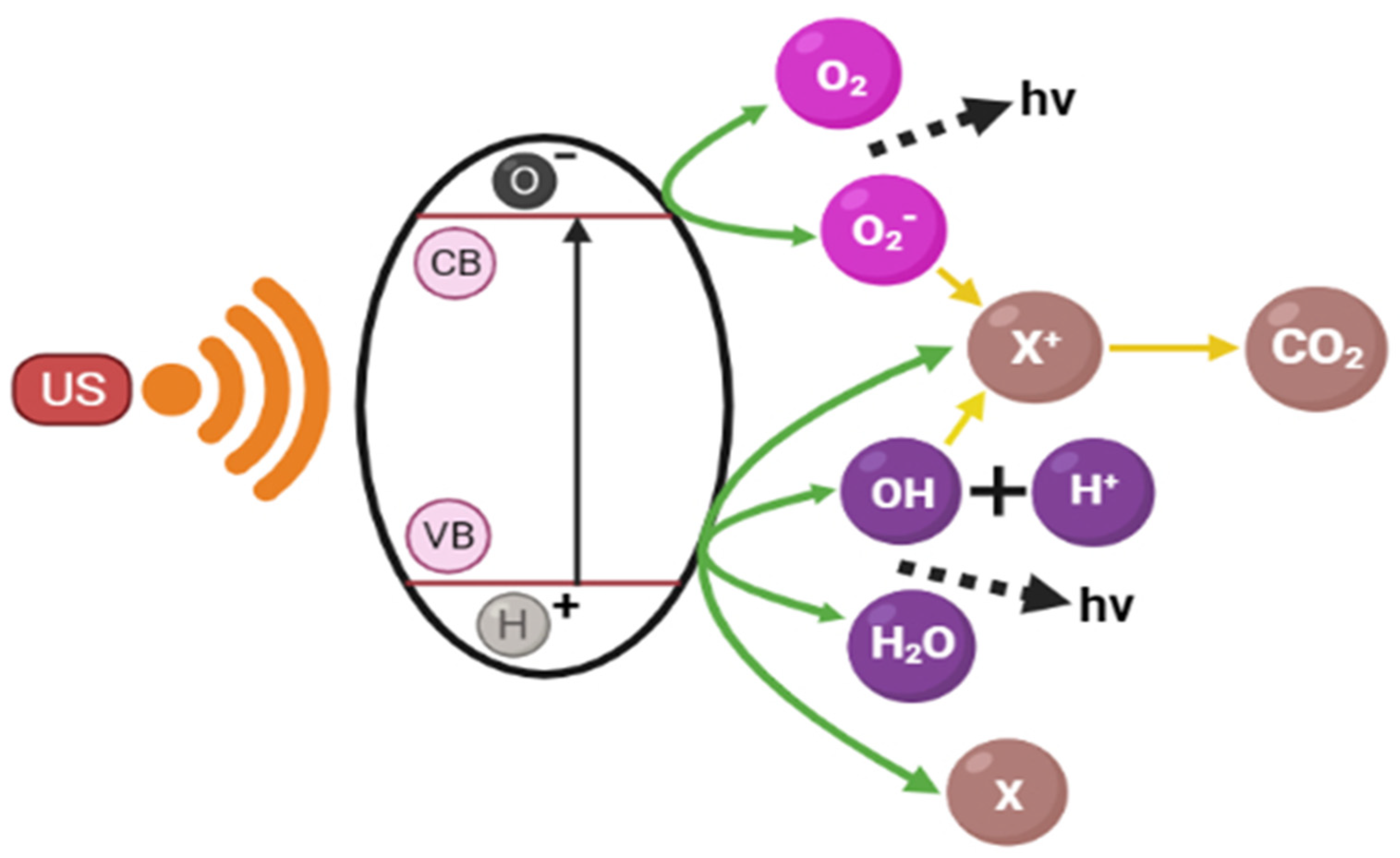
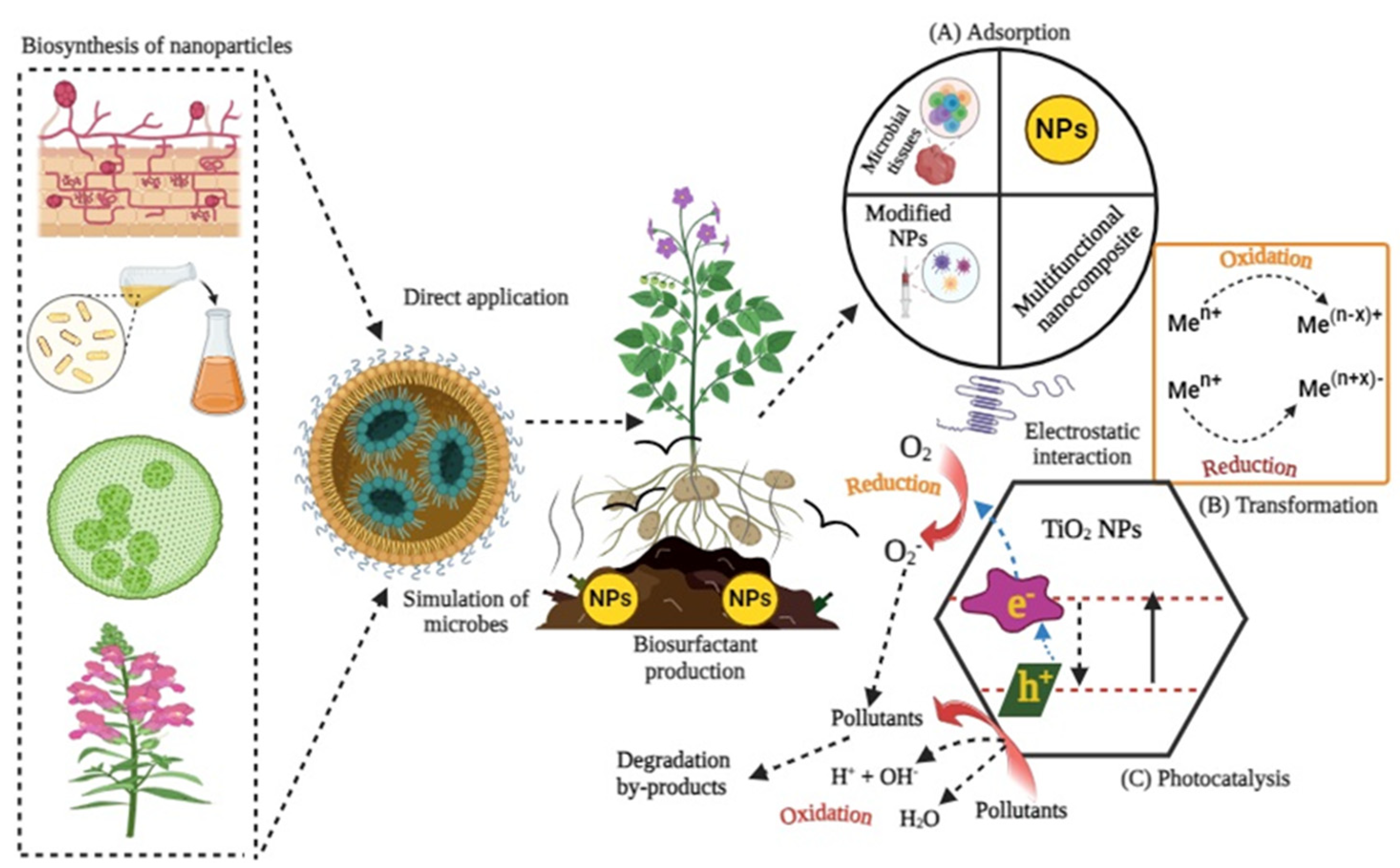
| Name of Emerging Pollutant | Name of Microbial Species | Source of Isolation | Culture Conditions | Degradation Efficiency % | References |
|---|---|---|---|---|---|
| Dyes | Pseudomonas fluorescens, Bacillus sp., and Escherichia coli | Dye contaminated soil | Temperature 30 ± 1 °C Nutrient agar Shaken 110 rpm | 43, 15, 90 | [42] |
| Azo dyes | Enterobacter hormaechei SKB 16 | Textile-effluent-polluted soil | Temperature 37 °C Nutrient agar Shaken 115 rpm | 98 | [43] |
| Methyl red | Vibrio logei and Pseudomonas nitroreducens | Wastewater | Temperature 25 °C Minimal medium Shaken 110 rpm | 65–82 | [44] |
| Monoazo and diazo dyes | Acinetobacter sp. and Klebsiella sp. | Activated sludge | Temperature 30 °C Minimal medium Shaken 120 rpm | 80 | [45] |
| Carmoisine | Saccharomyces cerevisiae ATCC 9763 | Commercial | Temperature 30 °C Yeast extract peptone dextrose Shaken 180 rpm | 100 | [46] |
| Copper, nickel, manganese, cobalt, and dichromate | Bacillus sp., Shewanella sp., Lysinibacillus sp., and Acinetobacter sp. | Sludge | Temperature 30 °C Luria broth medium Shaken 120 rpm | 90–100 | [47] |
| Iron, copper, zinc, cadmium, manganese, nickel, and lead | Bacillus sp. PS-6 | Industrial wastewater | Temperature 35 °C Luria broth medium Shaken 120 rpm | 44.12–89.46 | [48] |
| Nickel, chromium, and textile dyes | Lysinibacillus sp. | Wastewater | Temperature 30 °C Nutrient broth medium Shaken 150 rpm | 70, 58, 82 | [49] |
| Zinc, cobalt, nickel, lead, copper, chromium, mercury, arsenic, and silver | Rhodococcus sp. AQ5-07 | Oil-polluted soil | Temperature 10 °C Tween-peptone agar Shaken 150 rpm | 80–100 | [50] |
| Chromium, lead, iron, cobalt, nickel, manganese, zinc, copper, and aluminum | Agaricus bisporus | Commercial | Temperature 25 °C | 80–98 | [51] |
| Sulfamethoxazole | Escherichia coli JM109 and Chlorella sorokiniana | Fish breeding tank | Temperature 37 °C Temperature 28 °C | 54.34 | [52] |
| Erythromycin | Geobacter sp. and Acetoanaerobium sp. | Wastewater | - | 99 | [53] |
| Sulfamethoxazole | Shewanella sp. Alcaligenes sp., Pseudomonas sp., and Achromobacter sp. | Wastewater | Temperature 30 °C | 85.1 | [54] |
| Tetracycline | Shewanella sp., Bacillus sp., and Pseudomonas sp. | Seed sludge | Temperature 30 °C Luria–Bertani medium Agitation 150 rpm | 95 | [55] |
| Ciprofloxacin | Lactobacillus gesseri, Enterobacter sp., Bacillus sp., Bacillus subtilius, and Micrococcus luteus | Hospital effluent water | Temperature 28 °C Luria–Bertani medium Agitation 100 rpm | 100 | [56] |
| Tetracycline | Bacillus velezensis strain Al-Dhabi 140 | Municipal soil sludge | Temperature 37 °C Minimal medium Agitation 150 rpm | 100 | [57] |
| Triazophos, methamidophos, and carbofuran | Enterobacter sp. strain Z1 | Wastewater | Temperature 37 °C, pH 7 | 100, 100, 98.7 | [58] |
| Fludioxonil | Betaproteobacteria sp., Chloroflexi sp., Planctomycete sp., Firmicutes sp., Empedobacter sp., Sphingopyxis sp., and Rhodopseudomonas sp. | Fungicide wastewater | Room temperature Agitation 120 rpm | 95.4 | [59] |
| Chlorpyriphos, oxadiazon, and cypermethrin | Chlorella sp. and Scenedesmus sp. | Contaminated semiopen photobioreactor | Temperature 25 °C pH 7.5 Agitation 120 rpm | 97, 88, 74 | [60] |
| Deltamethrin, cyfluthrin, cypermethrin, permethrin, and lambda-cyhalothrin | Enterobacter ludwigii | Industrial wastewater | Temperature 30 °C Saline condition pH 7 | 90 | [61] |
| Allethrin | Sphingomonas trueperi | Wastewater sludge | Temperature 30 °C pH 7.0 Inoculum concentration 100 mg/L | 93 | [62] |
| Anthracene, phenanthrene, fluorene, naphthalene, pyrene, benzo(e)pyrene, benzo(k)fluoranthene, and benzo(a)pyrene | Ochrobactrum sp., Bacillus sp., Marinobacter sp., Pseudomonas sp., Martelella sp., Stenotrophomonas sp., and Rhodococcus sp. | Wastewater | Temperature 55 °C pH 9 Agitation 150 rpm Salt concentration 100 g/L | 100, 100, 100, 100, 93, 60, 55, 51 | [63] |
| Phenanthrene and fluorene | Ochrobactrum halosaudis strain CEES1, Stenotrophomonas maltophilia CEES2, Achromobacter xylosoxidans CEES3 and Mesorhizobium halosaudis CEES4 | Red Sea saline water and sediment samples | Temperature 37 °C pH 7 | 90 | [64] |
| Naphthalene, phenanthrene, fluoranthene, pyrene, total petroleum hydrocarbons, and phenolic compounds | Stenotrophomonas sp. S1VKR-26 | Polluted Damanganga river | Temperature 37 °C pH 7 Incubation time 7 days | 93, 86, 92, 98.3, 72.33, 93.06 | [65] |
| Petroleum hydrocarbons | Paramecium sp., Vorticella sp., Epistylis sp. and Opercularia sp. | Wastewater | Temperature 25 °C pH 7 Incubation time 16 days | 70 | [66] |
| Crude oil, crude oil alkanes, pristane, and phytane | Pseudomonas sp. and Bacillus sp. | Oil-polluted sediment | Temperature 30 °C pH 7 Incubation time 14 days | 80.64, 76.30, 46.75, 78.23 | [67] |
| Naphthalene | Bordetella avium | Petroleum refinery wastewater | Temperature 30 °C pH 7.5 Naphthalene concentration 100 to 500 mg/L Incubation time 10 days | 100 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, H.W.; Bibi, H.A.; Chandrasekaran, M.; Ahmad, S.; Kyriakopoulos, G.L. Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water 2024, 16, 2893. https://doi.org/10.3390/w16202893
Ahmad HW, Bibi HA, Chandrasekaran M, Ahmad S, Kyriakopoulos GL. Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water. 2024; 16(20):2893. https://doi.org/10.3390/w16202893
Chicago/Turabian StyleAhmad, Hafiz Waqas, Hafiza Aiman Bibi, Murugesan Chandrasekaran, Sajjad Ahmad, and Grigorios L. Kyriakopoulos. 2024. "Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants" Water 16, no. 20: 2893. https://doi.org/10.3390/w16202893
APA StyleAhmad, H. W., Bibi, H. A., Chandrasekaran, M., Ahmad, S., & Kyriakopoulos, G. L. (2024). Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water, 16(20), 2893. https://doi.org/10.3390/w16202893








