Effects of Aquaculture and Thalassia testudinum on Sediment Organic Carbon in Xincun Bay, Hainan Island
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. SOC, TN, C/N Ratio, δ13C, and Enzyme Activity
2.3. Bacteria Diversity
2.4. Statistical Analysis
3. Results
3.1. SOC, TN, C/N Ratio, δ13C, and Enzyme Activity
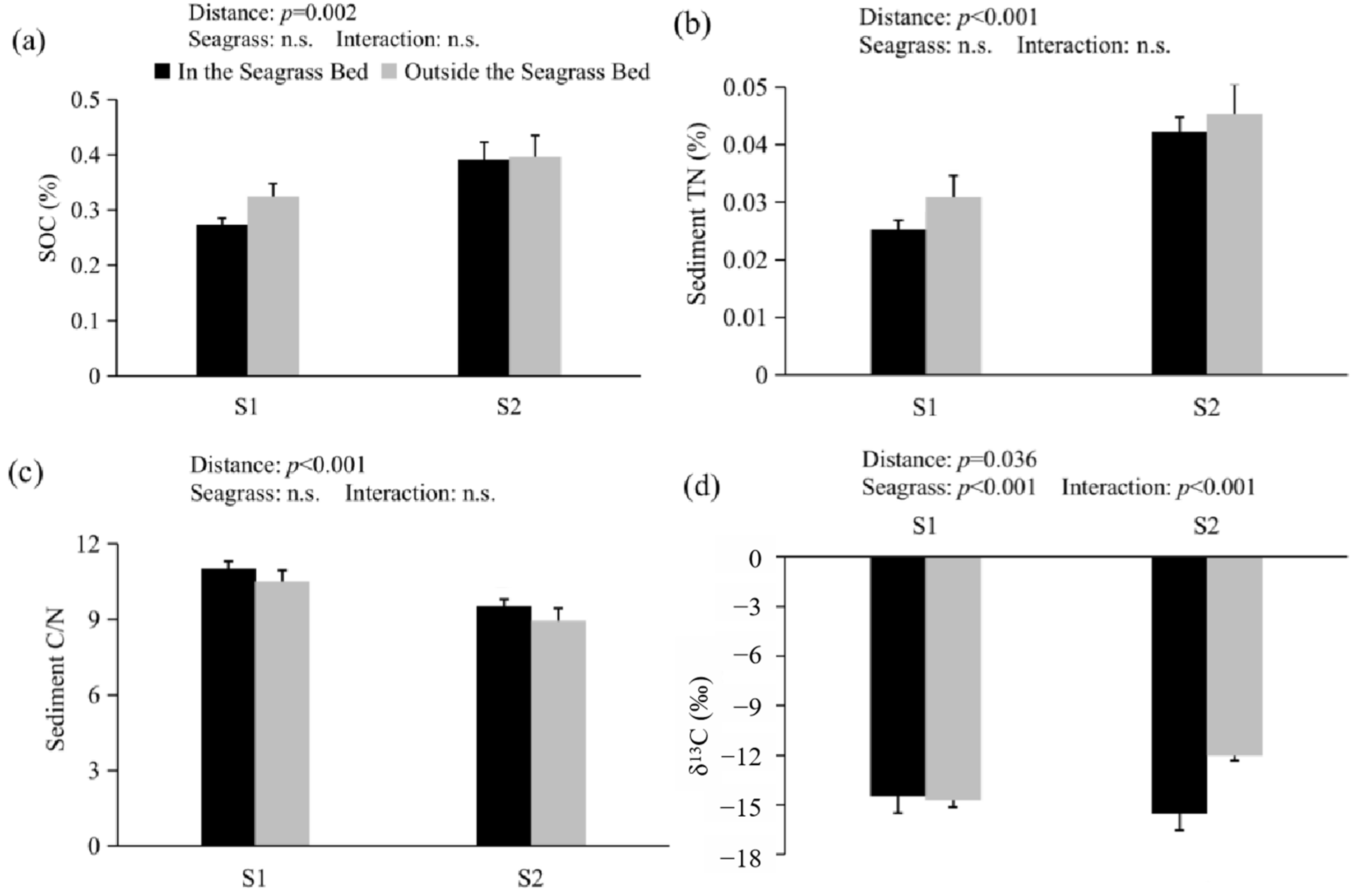
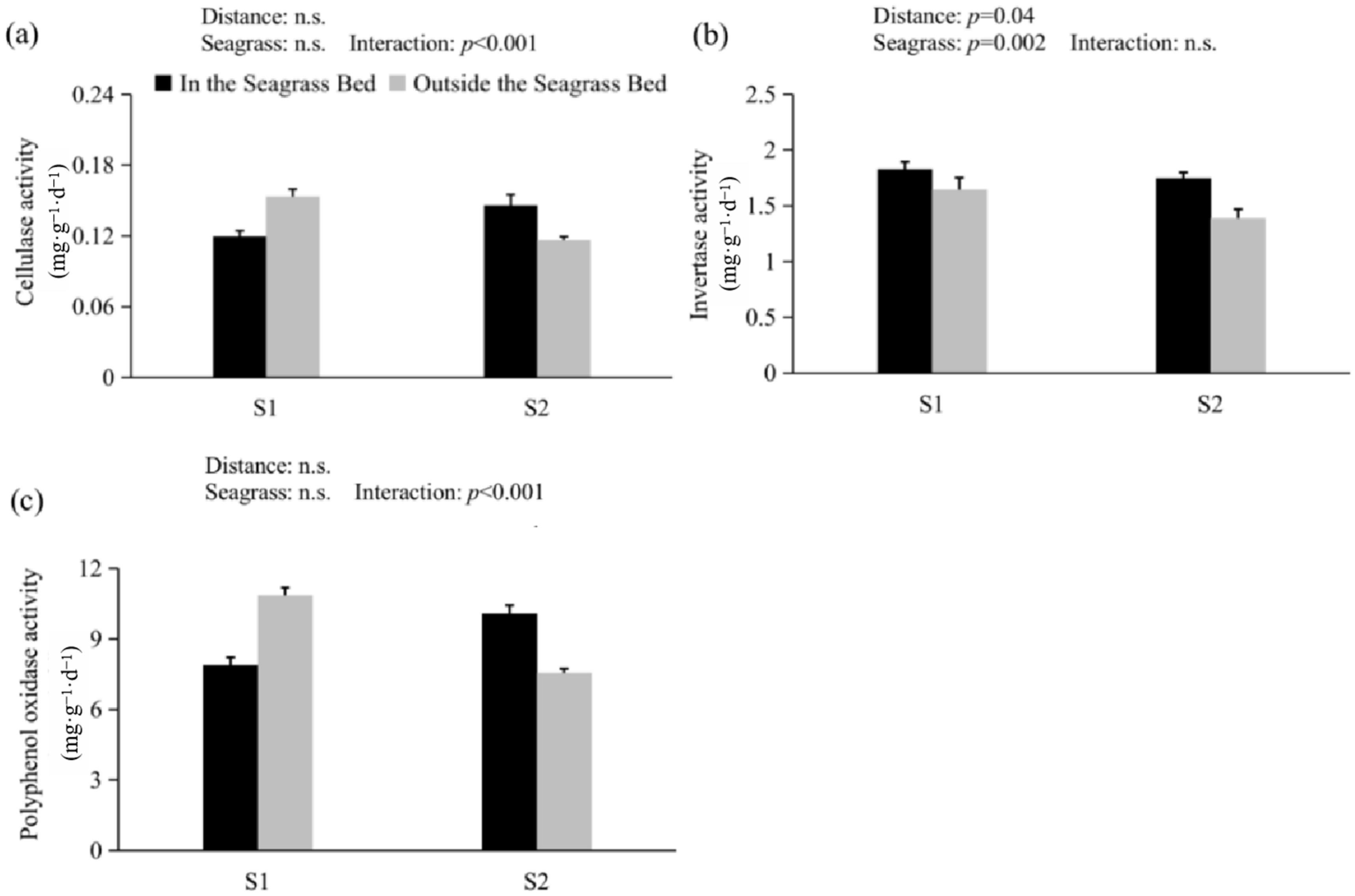
| In the Seagrass Bed | Outside the Seagrass Bed | |||
|---|---|---|---|---|
| SOC Content | δ13C | SOC Content | δ13C | |
| Sediment TN | 0.959 ** | −0.213 | 0.901 ** | 0.256 |
| Sediment C/N ratio | −0.731 ** | 0.465 | −0.031 | −0.249 |
| Cellulase activity | 0.747 ** | 0.126 | −0.323 | −0.103 |
| Invertase activity | 0.099 | 0.515 | −0.039 | 0.131 |
| Polyphenol oxidase activity | 0.471 * | 0.395 | −0.099 | 0.166 |
| S1 | S2 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Variables | In the Seagrass Bed | Outside the Seagrass Bed | In the Seagrass Bed | Outside the Seagrass Bed | Distance | Seagrass | Distance & Seagrass |
| SOC | 0.27 ± 0.01 | 0.33 ± 0.03 | 0.39 ± 0.02 | 0.40 ± 0.04 | 0.002 ** | 0.333 | 0.407 |
| TN | 0.025 ± 0.002 | 0.031 ± 0.002 | 0.042 ± 0.004 | 0.045 ± 0.005 | <0.001 ** | 0.228 | 0.713 |
| C/N | 11.02 ± 0.29 | 10.52 ± 0.27 | 9.52 ± 0.42 | 8.95 ± 0.50 | <0.001 ** | 0.173 | 0.926 |
| δ13C | −14.49 ± 0.24 | −14.71 ± 0.44 | −15.53 ± 0.42 | −12.03 ± 0.31 | 0.036 * | <0.001 ** | <0.001 ** |
| Cellulase activity | 0.12 ± 0.00 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.12 ± 0.00 | 0.382 | 0.741 | <0.001 ** |
| Invertase activity | 1.83 ± 0.07 | 1.65 ± 0.05 | 1.75 ± 0.11 | 1.39 ± 0.08 | 0.04 * | 0.002 ** | 0.262 |
| Polyphenol oxidase activity | 7.89 ± 0.34 | 10.85 ± 0.36 | 10.09 ± 0.34 | 7.56 ± 0.18 | 0.089 | 0.492 | <0.001 ** |
3.2. The Microbes in Sediments
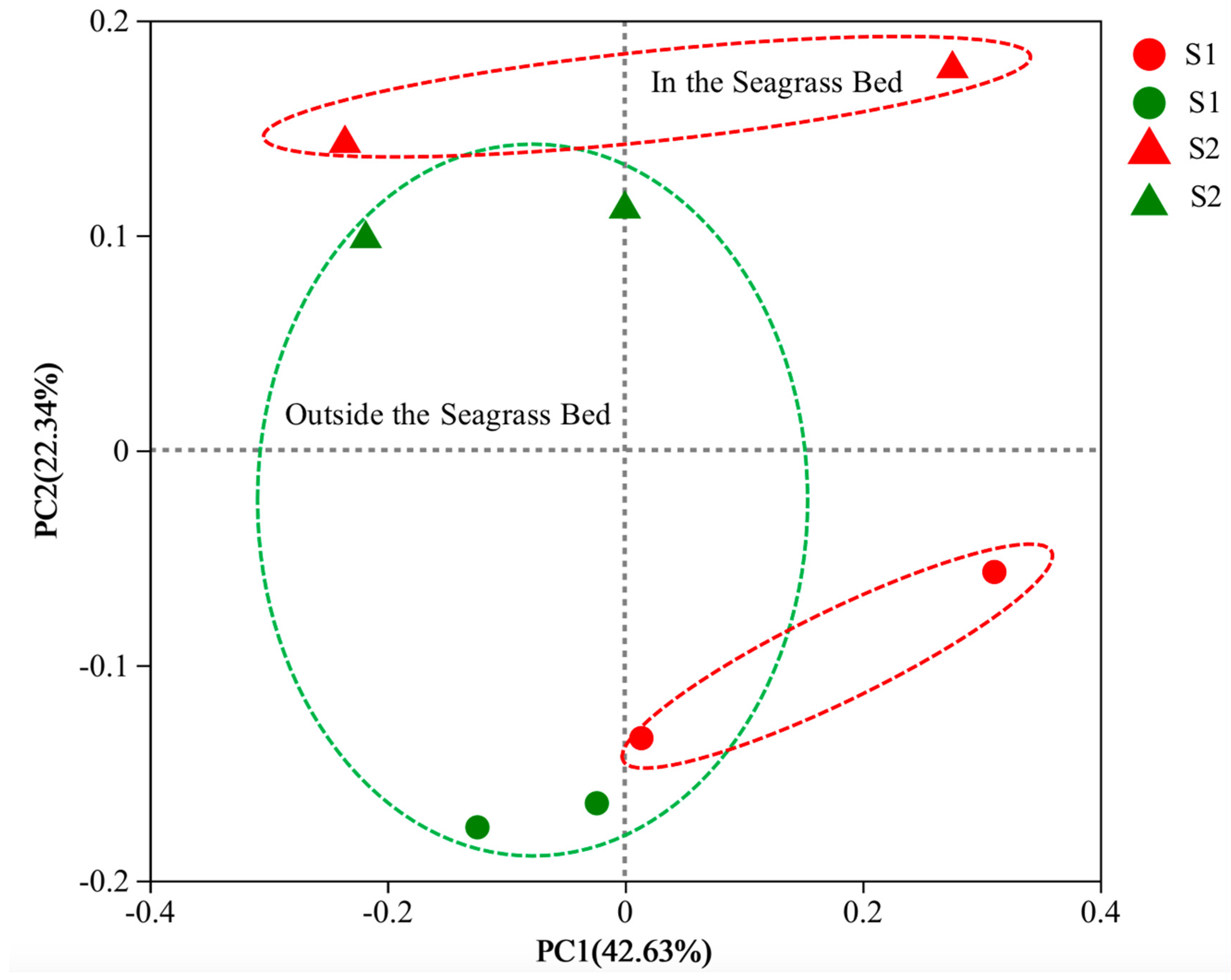
3.2.1. Alpha Diversity and Beta Diversity
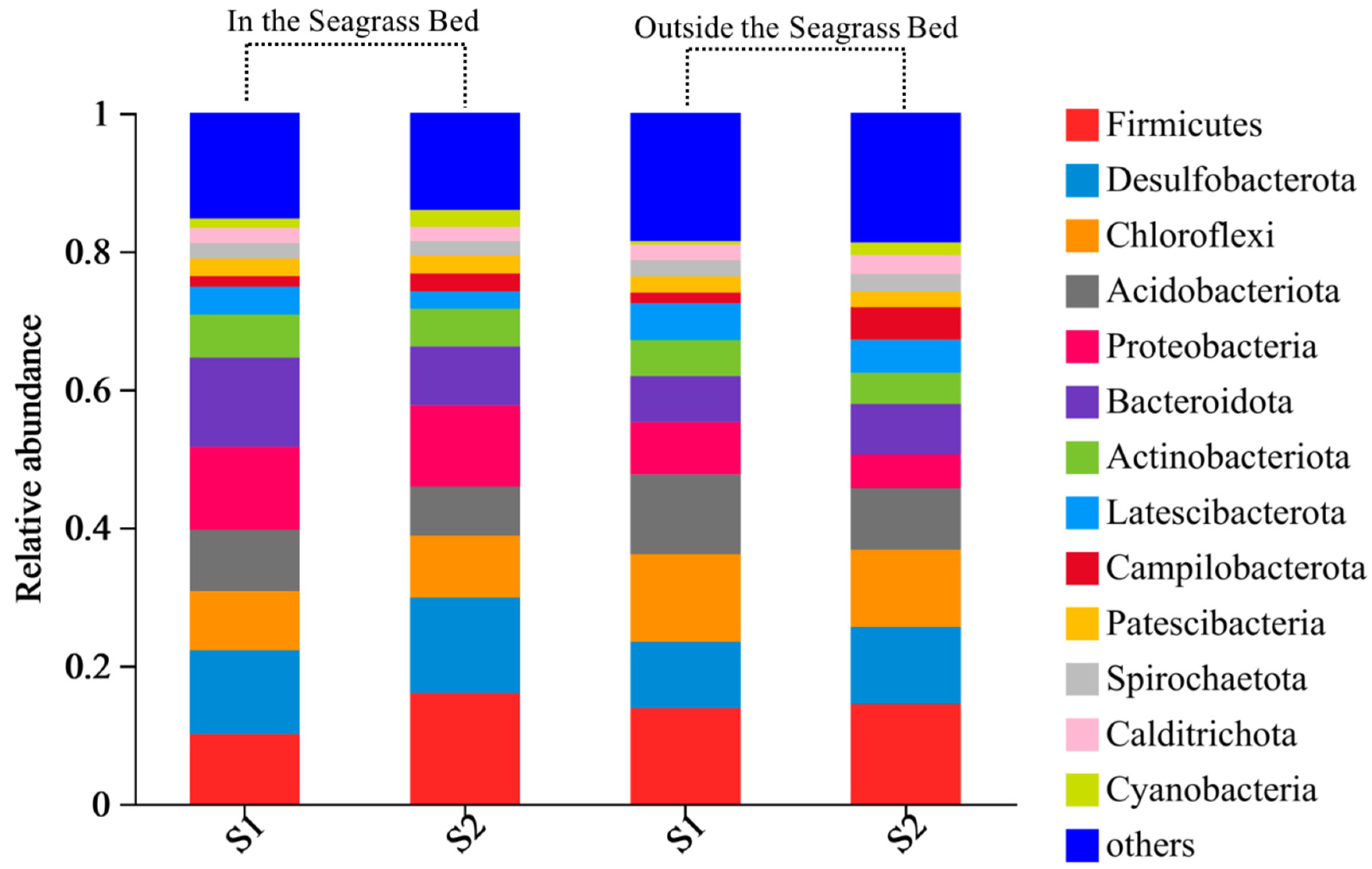
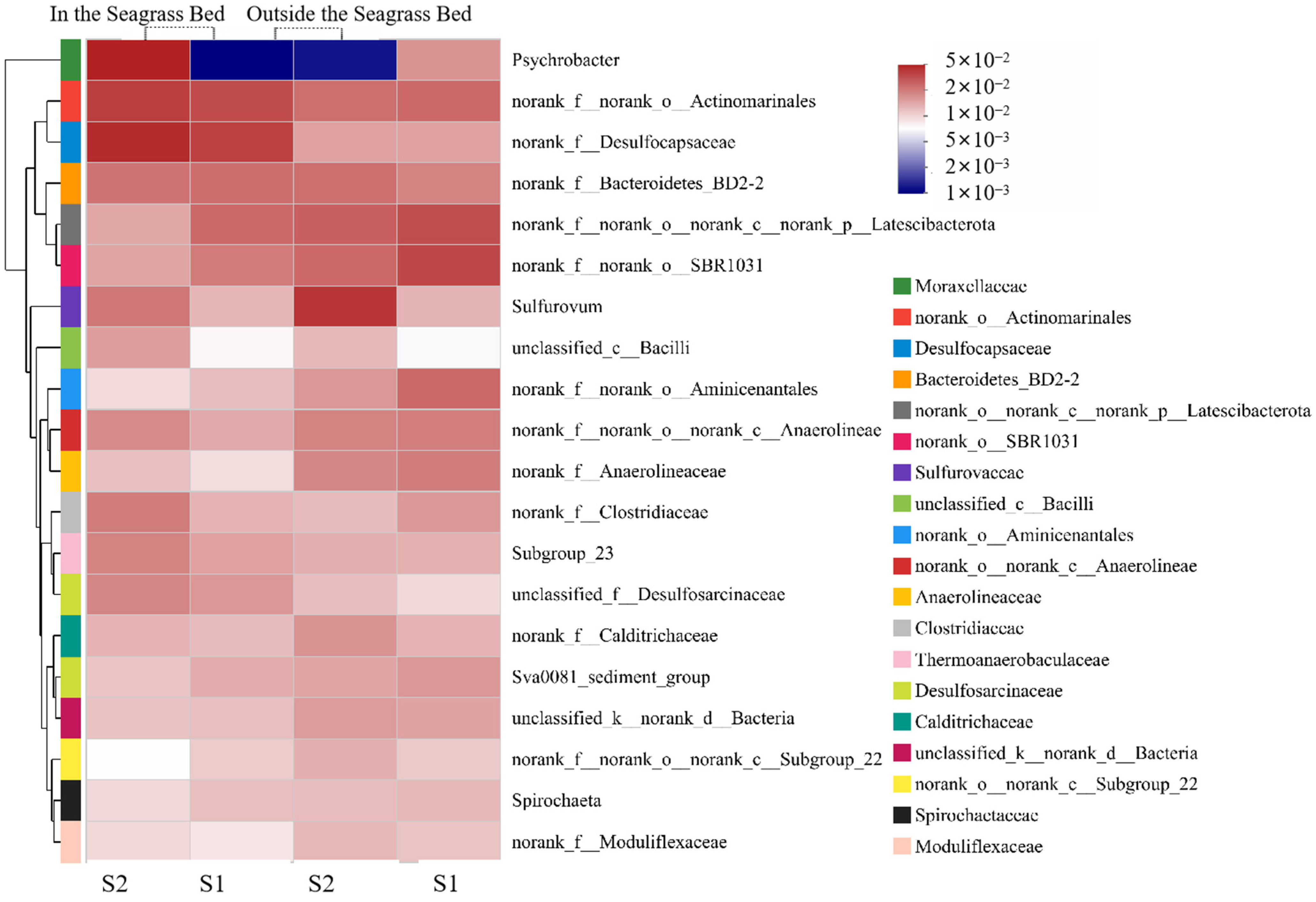
3.2.2. Microbial Community Structure
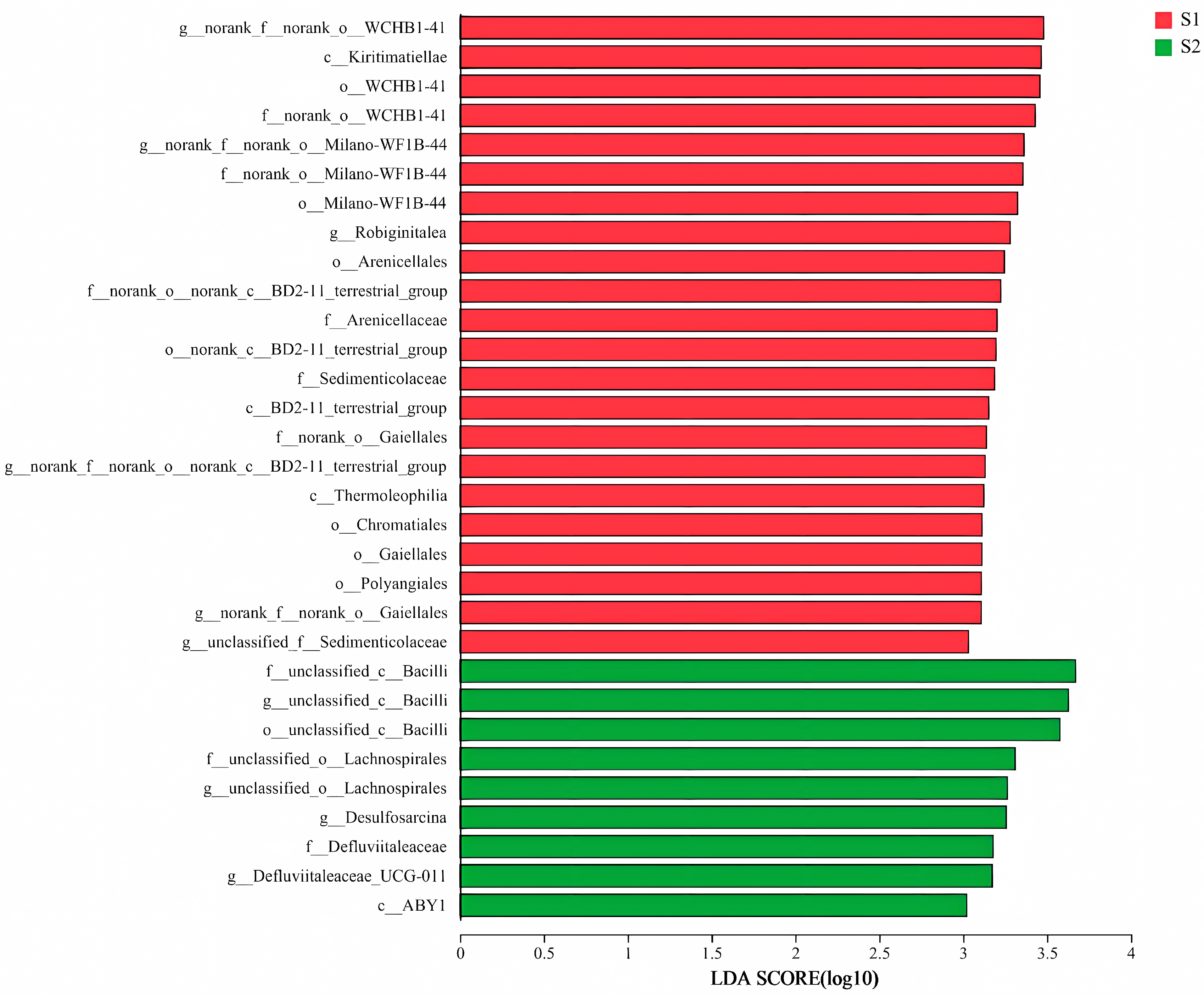
3.2.3. Linear Discriminant Analysis (LDA) Effect Size Analysis
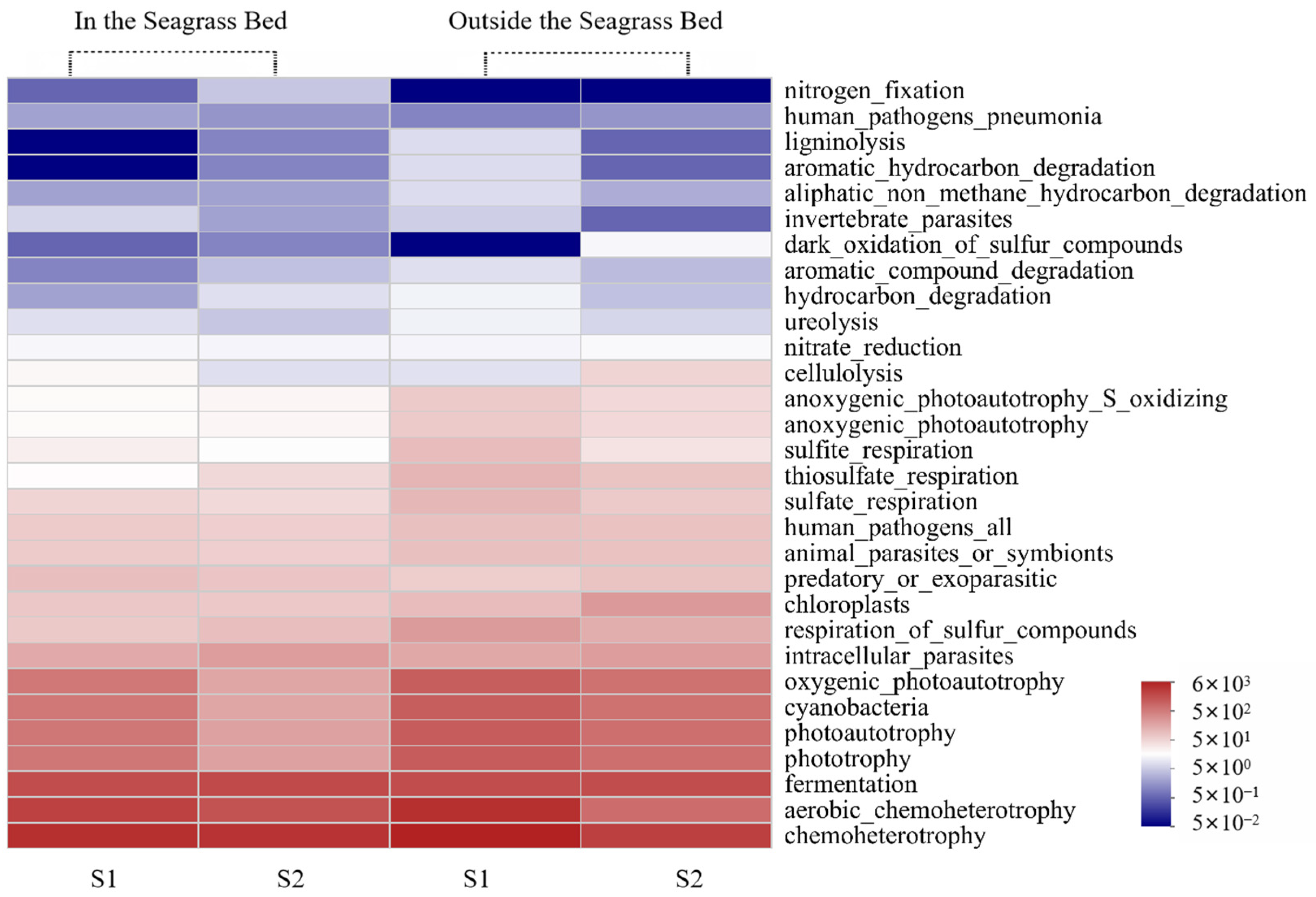
3.2.4. Function Prediction Analysis of Bacteria
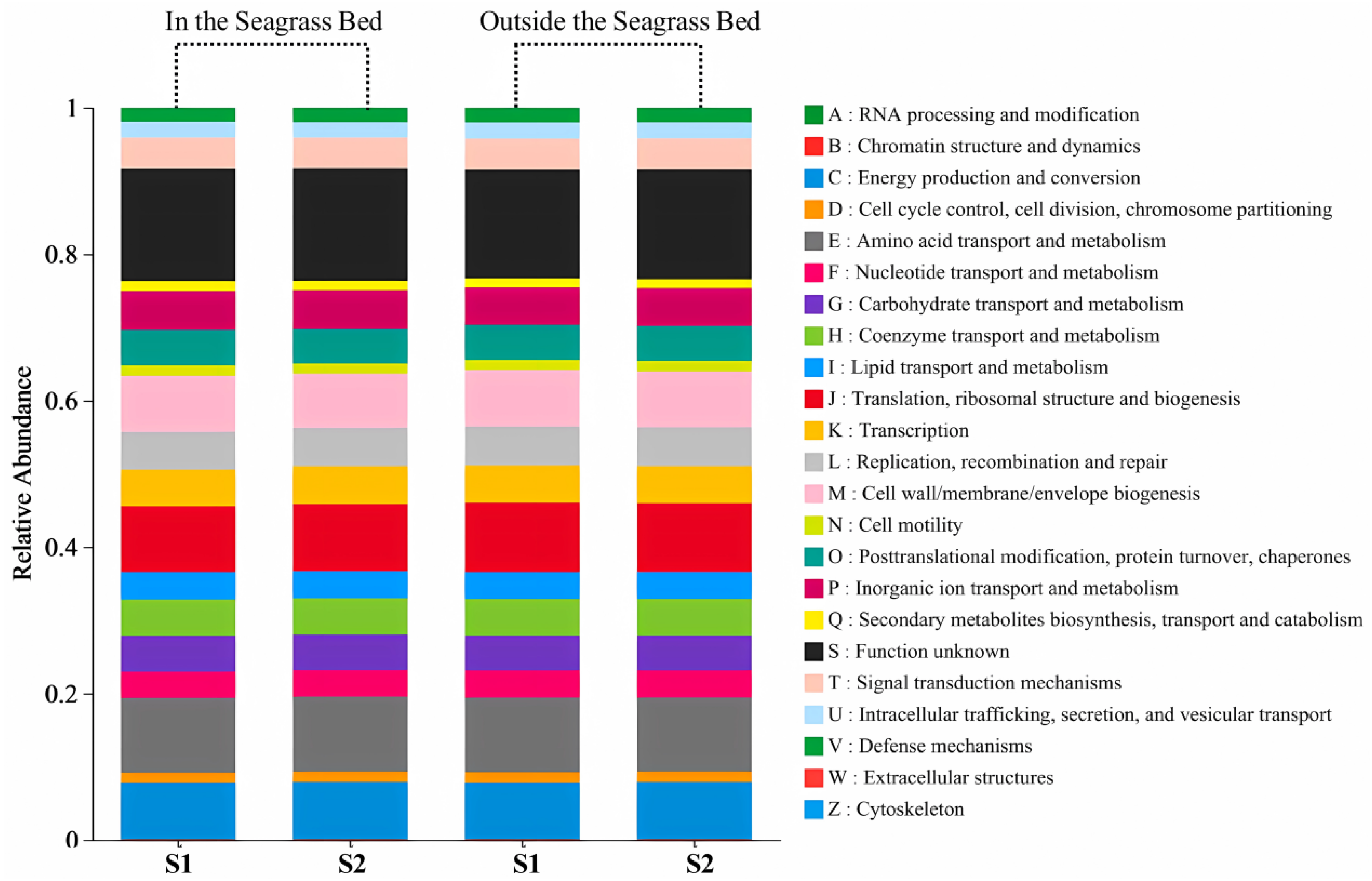
Function Prediction by PICRUSt
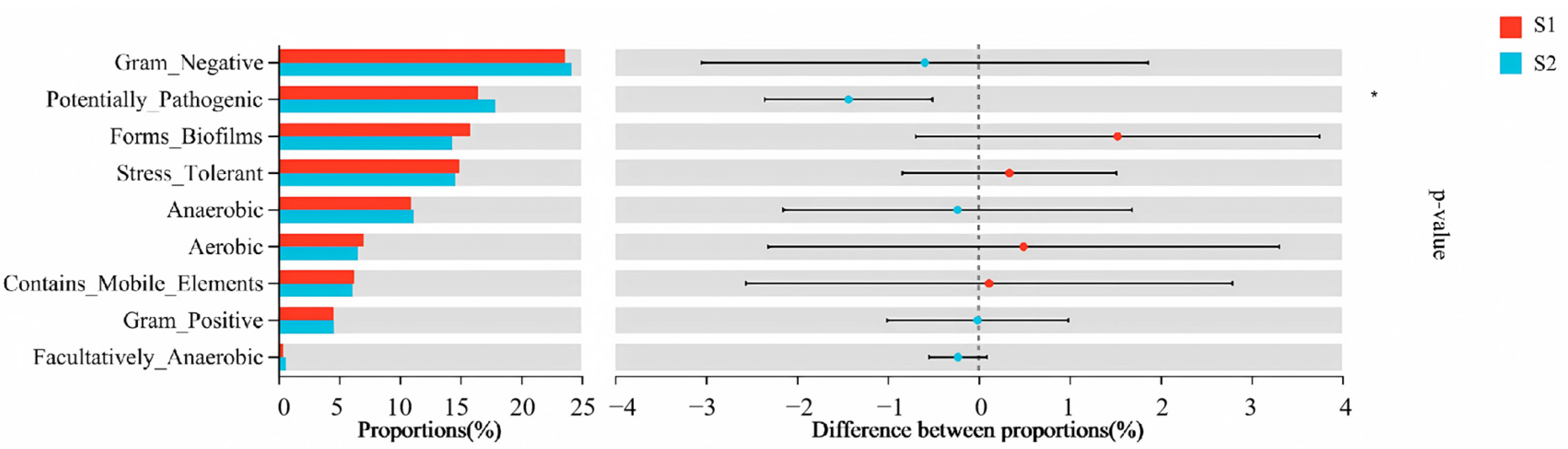
Phenotypic Prediction by BugBase
Function Prediction by FAPROTAX
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Han, Q.Y.; Bouma, T.J.; Brun, F.G.; Suykerbuyk, W.; van Katwijk, M.M. Resilience of Zostera noltii to burial or erosion disturbances. Mar. Ecol. Prog. Ser. 2012, 449, 133–143. [Google Scholar] [CrossRef][Green Version]
- Whitfield, A.K. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources forfishes in estuaries. Rev. Fish. Biol. Fisher. 2017, 27, 75–110. [Google Scholar] [CrossRef]
- Du, J.G.; Hu, W.J.; Nagelkerken, I.; Sangsawang, L.; Loh, K.H.; Ooi, J.L.S.; Liao, J.J.; Zheng, X.Q.; Qiu, S.T.; Chen, B. Seagrass meadows provide multiple benefits to adjacent coral reefs through various microhabitat functions. Ecosyst. Health Sust. 2020, 6, 1812433. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Nordlund, L.M.; Cullen-Unsworth, L.C. Seagrass meadows support global fisheries production. Conserv. Lett. 2019, 12, e12566. [Google Scholar] [CrossRef]
- Koch, E.W.; Barbier, E.B.; Silliman, B.R.; Reed, D.J.; Perillo, G.M.E.; Hacker, S.D.; Granek, E.F.; Primavera, J.; Muthiga, N.; Polasky, S.; et al. Non-linearity in ecosystem services: Temporal and spatial variability in coastal protection. Front. Ecol. Environ. 2009, 7, 29–37. [Google Scholar] [CrossRef]
- Nepf, H.M. Flow and Transport in Regions with Aquatic Vegetation. Annu. Rev. Fluid. Mech. 2012, 44, 123–142. [Google Scholar] [CrossRef]
- Guannel, G.; Ruggiero, P.; Faries, J.; Arkema, K.; Pinsky, M.; Gelfenbaum, G.; Guerry, A.; Kim, C.K. Integrated modeling framework to quantify the coastal protection services supplied by vegetation. J. Geophys. Res. Oceans 2015, 120, 324–345. [Google Scholar] [CrossRef]
- Bradley, K.; Houser, C. Relative velocity of seagrass blades: Implications for wave attenuation in low-energy environments. J. Geophys. Res. 2009, 114, 1–13. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Q.P. Eulerian-Lagrangian flow-vegetation interaction model using immersed boundary method and OpenFOAM. Adv. Water Resour. 2019, 126, 176–192. [Google Scholar] [CrossRef]
- Duarte, C.M.; Kennedy, H.; Marbà, N.; Hendriks, I. Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies. Ocean Coast. Manag. 2013, 83, 32–38. [Google Scholar] [CrossRef]
- Kaladharan, P.; Zacharia, P.U.; Thirumalaiselvan, S.; Anasukoya, A.; Lavanya, R.; Sikkander Batcha, S.M. Blue carbon stock of seagrass meadows of Gulf of Mannar and Palk Bay off Coromandel Coast, south India. Indian J. Fish. 2020, 67, 149–153. [Google Scholar] [CrossRef]
- Singh, S.; Lal, M.M.; Southgate, P.C.; Wairiu, M.; Singh, A. Blue carbon storage in Fijian seagrass meadows: First insights into carbon, nitrogen and phosphorus content from a tropical southwest Pacific Island. Mar. Pollut. Bull. 2022, 176, 113432. [Google Scholar] [CrossRef] [PubMed]
- Kaladharan, P.; Anasukoya, A. Shrinking seagrass meadows-observations from four Lagoons of Laccadive Archipelago. J. Mar. Boil. Ass. India 2019, 61, 47–51. [Google Scholar] [CrossRef]
- Burkholder, J.A.M.; Tomasko, D.A.; Touchette, B.W. Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 2007, 350, 46–72. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Hughes, A.R.; Kimbro, D.L. Loss of ‘blue carbon’ from coastal salt marshes following habitat disturbance. PLoS ONE 2013, 8, e69244. [Google Scholar] [CrossRef]
- Ralph, P.J.; Durako, M.J.; Enríquez, S.; Collier, C.J.; Doblin, M.A. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; van Belzen, J.; Herman, P.M.J.; van Katwijk, M.M.; Lamers, L.P.M.; van Leenat, P.J.M.; Bouma, T.J. Low-canopy seagrass beds still provide important coastal protection service. PLoS ONE 2013, 8, e62413. [Google Scholar] [CrossRef]
- Campbell, J.E.; Lacey, E.A.; Decker, R.A.; Crooks, S.; Fourqurean, J.W. Carbon storage in seagrass beds of Abu Dhabi, United Arab Emirates. Estuar. Coast. 2015, 38, 242–251. [Google Scholar] [CrossRef]
- Bedulli, C.; Lavery, P.S.; Harvey, M.; Duarte, C.M.; Serrano, O. Contribution of seagrass blue carbon toward carbon neutral policies in a touristic and environmentally-friendly island. Front. Mar. Sci. 2020, 7, 1. [Google Scholar] [CrossRef]
- Kennedy, H.; Beggins, J.; Duarte, C.M.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochem. Cycl. 2010, 24, GB4026. [Google Scholar] [CrossRef]
- Duarte, C.M.; Marbà, N.; Gacia, E.; Fourqurean, J.W.; Beggins, J.; Barrón, C.; Apostolaki, E.T. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochem. Cy. 2010, 24, GB4032. [Google Scholar] [CrossRef]
- Barrón, C.; Apostolaki, E.; Duarte, C. Dissolved organic carbon release by marine macrophytes. Biogeosci. Discuss. 2012, 9, 1529–1555. [Google Scholar] [CrossRef]
- Liu, S.L.; Jiang, Z.J.; Zhou, C.Y.; Wu, Y.C.; Arbi, I.; Zhang, J.P.; Huang, X.P.; Trevathan-Tackett, S.M. Leaching of dissolved organic matter from seagrass leaf litter and its biogeochemical implications. Acta Oceanol. Sin. 2018, 37, 84–90. [Google Scholar] [CrossRef]
- Bouillon, S.; Moens, T.; Dehairs, F. Carbon sources supporting benthic mineralization in mangrove and adjacent seagrass sediments (Gazi Bay, Kenya). Biogeosciences 2004, 1, 311–333. [Google Scholar] [CrossRef]
- Barrón, C.; Apostolaki, E.T.; Duarte, C.M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Front. Mar. Sci. 2014, 1, 42. [Google Scholar] [CrossRef]
- Enríquez, S.; Schubert, N. Direct contribution of the seagrass Thalassia testudinum to lime mud production. Nat. Commun. 2014, 5, 3835. [Google Scholar] [CrossRef]
- Perry, C.T.; Salter, M.A.; Morgan, K.M.; Harborne, A.R. Census Estimates of Algal and Epiphytic Carbonate Production Highlight Tropical Seagrass Meadows as Sediment Production Hotspots. Front. Mar. Sci. 2019, 6, 120. [Google Scholar] [CrossRef]
- Cao, H.; Sun, H.; Yang, H.; Sun, B.; Zhao, Q.G. A review soil enzyme activity and its indication for soil quality. Chin. J. Appl. Environ. Biol. 2003, 9, 105–109. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, Z.J.; Huang, X.P.; Zhang, J.P. Effect of environmental stress on non-structural carbohydrates reserves and transfer in seagrasses. Acta Ecol. Sin. 2012, 32, 6242–6250. [Google Scholar] [CrossRef][Green Version]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marba, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Liu, S.L.; Jiang, Z.J.; Zhang, J.P.; Wu, Y.C.; Huang, X.P.; Macreadie, P.I. Sediment microbes mediate the impact of nutrient loading on blue carbon sequestration by mixed seagrass meadows. Sci. Total. Environ. 2017, 599–600, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally signifificant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Racchetti, E.; Bartoli, M.; Soana, E.; Longhi, D.; Christian, R.R.; Pinardi, M.; Viaroli, P. Influence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification. Biogeochemistry 2011, 103, 335–354. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Colman, B.P.; Bernhardt, E.S. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob. Chang. Biol. 2013, 19, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.R.; Fourqurean, J.W. Carbon storage in seagrass soils: Long-term nutrient history exceeds the effects of near-term nutrient enrichment. Biogeosciences 2016, 13, 313–321. [Google Scholar] [CrossRef]
- Liu, S.L.; Jiang, Z.J.; Zhang, J.P.; Wu, Y.C.; Lian, Z.L.; Huang, X.P. Effect of nutrient enrichment on the source and composition of sediment organic carbon in tropical seagrass beds in the South China Sea. Mar. Pollut. Bull. 2016, 110, 274–280. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Blum, L.K. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 2011, 8, 987–993. [Google Scholar] [CrossRef]
- Kearns, P.J.; Angell, J.H.; Howard, E.M.; Deegan, L.A.; Stanley, R.H.R.; Bowen, J.L. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nat. Commun. 2016, 7, 12881. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.; Qiu, C.Y.; Zeng, W.X.; Chen, S.Q.; Zhao, M.Q.; Shi, Y.F.; Zhang, X.L. Sedimnt carbon sequestration and driving factors in seagrass beds from Hainan Island and the Xisha Islands. Processes 2023, 11, 456. [Google Scholar] [CrossRef]
- Grigorakis, K.; Rigos, G. Aquaculture effects on environmental and public welfare-the case of Mediterranean mariculture. Chemosphere 2011, 85, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Ferriss, B.E.; Reum, J.C.P.; Mcdonald, P.S.; Farrell, D.M.; Harvey, C.J. Evaluating trophic and non-trophic effects of shellfish aquaculture in a coastal estuarine foodweb. ICES J. Mar. Sci. 2016, 73, 429–440. [Google Scholar] [CrossRef]
- Huang, D.J.; Huang, X.P.; Huang, Z.G. Spatiotemporal variation of TN & TP contents in Enhalus acoroides and responses to nutrient load in Xincun Bay, Hainan. Mar. Envrion. Sci. 2010, 29, 40–43. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.P.; Huang, X.P.; Jiang, Z.J. Physiological responses of the seagrass Thalassia hemprichii (Ehrenb.) Aschers as indicators of nutrient loading. Mar. Pollu. Bull. 2014, 83, 508–515. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, Z.J.; Zhang, G.X. The discussion of ecological status ans sustainable use of seagrass beds in Xincun Bay. Ocean Dev. Manage. 2011, 11, 74–78. (In Chinese) [Google Scholar]
- Zhang, Y.P.; Yu, S.; Huang, D.J.; Zhou, Z.B. Characteristics of tide, tidal current and their effects on nutrients in Xincun Lagoon, Hainan Island. J. Mar. Sci. 2022, 40, 69–82. (In Chinese) [Google Scholar]
- Guo, J.Y. Changes in Seagrass Blue Carbon Storage and Its Management in Xincun Bay, Hainan. Master’s Thesis, Hainan Tropical Ocean University, Sanya, China, 2023. (In Chinese). [Google Scholar]
- Han, Q.Y.; Zeng, W.X.; Ye, J.H.; Qiu, C.Y.; Shi, Y.F.; Zhao, M.Q. Characteristics of morphological and physiological indicators of Thalassia hemprichii and environmental factors in Xincun Bay, Hainan Island. Prog. Fisher. Sci. 2023, 44, 225–238. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Burke, M.K.; Dennison, W.C.; Moore, K.A. Non-structural carbohydrate reserves of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1996, 137, 195–201. [Google Scholar] [CrossRef]
- Kenworthy, J.W.; Thayer, G.W. Production and decomposition of the roots and rhizomes of seagrasses, Zostera Marina and Thalassia testudinum, in temperate and subtropical marine ecosystems. B. Mar. Sci. 1984, 35, 364–379. [Google Scholar] [CrossRef]
- Luo, H.X.; Liu, S.L.; Jiang, Z.J.; Wu, Y.C.; Huang, X.P. Organic carbon composition and microbial transformation of seagrass meadow and its responses to eutrophication. Chin. Sci. Bull. 2021, 66, 4649–4663. [Google Scholar] [CrossRef]
- Touchette, B.W.; Burkholder, J.A.M. Carbon and nitrogen metabolism in the seagrass, Zostera marine L.: Environmental control of enzymes involved in carbon allocation and nitrogen assimilation. J. Exp. Mar. Biol. Ecol. 2007, 350, 216–233. [Google Scholar] [CrossRef]
- Liu, S.L.; Jiang, Z.J.; Wu, Y.C.; Zhang, J.P.; Zhao, C.Y.; Huang, X.P. Mechanisms of sediment carbon sequestration in seagrass meadows and its responses to eutrophication. Chin. Sci. Bull. 2012, 62, 3309–3318. [Google Scholar] [CrossRef][Green Version]
- Hemminga, M.A.; Mateo, M.A. Stable carbon isotopes in seagrasses: Variability in ratio and use in ecology studies. Mar. Ecol. Prog. Ser. 1996, 140, 285–298. [Google Scholar] [CrossRef]
- Park, H.; Choy, E.; Lee, K. Trophic transfer between coastal habitats in a seagrass-dominated marcrotidal embayment system as determined by stable isotope and fatty acid signatures. Mar. Freshwater Res. 2013, 64, 1169–1183. [Google Scholar] [CrossRef]
- Jaschinski, S.; Hansen, T.; Sommer, U. Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. 2008, 6, 12–15. [Google Scholar] [CrossRef]
- Moncreiff, C.A.; Sullivan, M.J. Trophic importance of epiphytic algae in subtropical seagrass beds: Evidence from multiple stable isotope analyses. Mar. Ecol. Prog. Ser. 2001, 215, 93–106. [Google Scholar] [CrossRef]
- Garcias-Bonet, N.; Eguíluz, V.M.; Díaz-Rúa, R.; Duarte, C.M. Host-association as major driver of microbiome structure and composition in Red Sea seagrass ecosystems. Environ. Microbiol. 2020, 23, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere microbiomes of European seagrasses are selected by the plant, but are not species specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Arnosti, C. Microbial extracellular enzymes and the marine carbon cycle. Annu. Rev. Mar. Sci. 2011, 3, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.; Liu, D.Y. Macroalgae blooms and their effects on seagrass ecosystems. J. Ocean U. China 2014, 13, 791–798. [Google Scholar] [CrossRef]
- VolKman, J.K.; Barrett, S.M.; Blackburn, S.I.; Mansour, M.P.; Sikes, E.l.; Gelin, F. Microalgal biomarkers: A review of recent research developments. Org. Geochem. 1998, 29, 1163–1179. [Google Scholar] [CrossRef]
- Bååth, E. The use of neutral lipid fatty acids to indicate the physiological conditions of soil fungi. Microb. Ecol. 2003, 24, 373–383. [Google Scholar] [CrossRef]
- Fahimipour, A.K.; Kardish, M.R.; Eisen, J.A.; Lang, J.M.; Green, J.L.; Stachowicz, J.J. Global-scale structure of the eelgrass microbiome. Appl. Environ. Microb. 2017, 83, e03391-16. [Google Scholar] [CrossRef]
- Piñeiro-Juncal, N.; Kaal, J.; Moreira, J.C.F.; Martínez Cortizas, A.; Lambais, M.R.; Otero, X.L.; Mateo, M.A. Cover loss in a seagrass Posidonia oceanica meadow accelerates soil organic matter turnover and alters soil prokaryotic communities. Org. Geochem. 2021, 151, 104140. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Atwood, T.B.; Seymour, J.R.; Fontes, M.L.S.; Sanderman, J.; Nielsen, D.A.; Connolly, R.M. Vulnerability of seagrass blue carbon to microbial attack following exposure to warming and oxygen. Sci. Total Environ. 2019, 686, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Brysch, K.; Schneider, C.; Fuchs, G.; Widdel, F. Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch. Microbiol. 1987, 148, 264–274. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Alberto, B.G.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Lu, Y.H. A review of syntrophic fatty acids oxidation in anoxic paddy soil. Microbiol. China 2013, 40, 109–122. (In Chinese) [Google Scholar] [CrossRef]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- Taylor, C.R.; Hardiman, E.M.; Ahmad, M.; Sainsbury, P.; Norris, P.; Bugg, T. Isolation of bacterial strains able to metabolize lignin from screening of environmental samples. J. Appl. Microbiol. 2012, 113, 521–530. [Google Scholar] [CrossRef]
- Rashid, G.M.M.; Taylor, C.R.; Liu, Y.; Zhang, X.; Bugg, T.D.H. Identification of manganese superoxide dismutase from Sphingobacterium sp. T2 as a novel bacterial enzyme for lignin oxidation. ACS Chem. Biol. 2015, 10, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Taylor, C.R.; Pink, D.; Burton, K.; Eastwood, D.; Bending, G.D.; Bugg, T.D.H. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol. BioSyst. 2010, 6, 815–821. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.Y.; Yang, S.; Wang, Z.R.; Feng, X.; Liu, H.Y.; Jiang, Y. Variations in soil bacterial taxonomic profiles and putative functions in response to straw incorporation combined with N fertilization during the maize growing season. Agr. Ecosyst. Environ. 2019, 283, 106578. [Google Scholar] [CrossRef]
- Gralka, M.; Szabo, R.; Stocker, R.; Cordero, O.X. Trophic interactions and the drivers of microbial community assembly. Curr. Biol. 2020, 30, R1176–R1188. [Google Scholar] [CrossRef]

| Samples | Shannon | Simpson | Ace | Chao1 | |
|---|---|---|---|---|---|
| In the seagrass bed | S1 | 6.65 ± 0.23 | 0.0038 ± 0.0011 | 4716.50 ± 802.28 | 4650.80 ± 545.54 |
| S2 | 6.11 ± 0.04 | 0.0093 ± 0.0004 | 4310.60 ± 506.39 | 4235.80 ± 285.69 | |
| Outside the seagrass bed | S1 | 6.65 ± 0.01 | 0.0038 ± 0.0001 | 4849.60 ± 152.75 | 4759.50 ± 30.23 |
| S2 | 6.69 ± 0.09 | 0.0044 ± 0.0000 | 5003.90 ± 233.42 | 4904.10 ± 165.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Che, W.; Zhao, H.; Ye, J.; Zeng, W.; Luo, Y.; Bai, X.; Zhao, M.; Shi, Y. Effects of Aquaculture and Thalassia testudinum on Sediment Organic Carbon in Xincun Bay, Hainan Island. Water 2024, 16, 338. https://doi.org/10.3390/w16020338
Han Q, Che W, Zhao H, Ye J, Zeng W, Luo Y, Bai X, Zhao M, Shi Y. Effects of Aquaculture and Thalassia testudinum on Sediment Organic Carbon in Xincun Bay, Hainan Island. Water. 2024; 16(2):338. https://doi.org/10.3390/w16020338
Chicago/Turabian StyleHan, Qiuying, Wenxue Che, Hui Zhao, Jiahui Ye, Wenxuan Zeng, Yufeng Luo, Xinzhu Bai, Muqiu Zhao, and Yunfeng Shi. 2024. "Effects of Aquaculture and Thalassia testudinum on Sediment Organic Carbon in Xincun Bay, Hainan Island" Water 16, no. 2: 338. https://doi.org/10.3390/w16020338
APA StyleHan, Q., Che, W., Zhao, H., Ye, J., Zeng, W., Luo, Y., Bai, X., Zhao, M., & Shi, Y. (2024). Effects of Aquaculture and Thalassia testudinum on Sediment Organic Carbon in Xincun Bay, Hainan Island. Water, 16(2), 338. https://doi.org/10.3390/w16020338





