1. Introduction
With the continuous improvement of people’s living standards, urban residents have more and more requirements for the quality of their drinking water, and the problem of water smell has gradually attracted widespread attention. The sources of odor in water are numerous, including the direct discharge of wastewater, the use of various water treatment agents, and the growth and metabolism of organisms. The metabolic products of algae, actinomycetes, etc., are an important cause of water odor. Due to an excess of nutrients, the balance of the freshwater ecosystem is destroyed, and the excessive growth of algae continues to secrete and produce various secondary metabolites that have an odor, which significantly affects the quality of drinking water. With the advancement of odor detection technology, research into the composition characteristics of odorants and advanced treatment technologies are helpful in improving the quality of drinking water.
Odor is often used as the main reference index for the public assessment of water quality. In recent years, the odor problem in drinking water has often been caused by the abnormal activity of algae and the discharge of agricultural and chemical production wastewater. The smell of drinking water has a direct effect on the drinkability of the water and odorous substances can be easily detected below the odor threshold in the polar regions. The odor concentration in drinking water sources has often increased, affecting the safety of human drinking water. Therefore, it is necessary to continuously improve the existing odor removal technology and explore more reasonable and efficient treatment methods to solve the current odor problem. Odors in drinking water are a common problem and conventional water treatment has a low removal rate for typical odors such as geosmin (GSM) and 2-methylisoboneol (2-MIB). The traditional process has a limited impact on odor removal and produces by-products. Adsorption treatment using powdered activated carbon and granular activated carbon is economical and feasible, but still has some disadvantages, such as not being easy to regenerate. Activated carbon fibers are easy to regenerate and do not produce secondary pollution, but raw-material production is complicated, and the cost is relatively high. Zeolite, attapulgite and synthetic ceramic adsorbents have their own advantages, but the technology is not yet mature. Advanced oxidation technology has good removal effects but is relatively expensive and easily produces by-products. Biological treatment has good removal effects, fewer by-products and no new pollution, but it is susceptible to environmental factors and the stability of the corresponding colonies needs to be controlled. The combined process of advanced oxidation technology and adsorption technology has a better effect on odor removal than that of a single process, with complementary advantages, and can better solve the problems of cost, by-products, secondary pollution, etc., thus it has good application prospects for odor removal.
Therefore, waterworks are faced with the challenge of eliminating odors. The control and elimination of odors in waterworks has become a hot topic in the field of water treatment and is also one of the problems that need to be urgently solved to ensure the safety of drinking water. Through continuous research and innovation in odor removal technology, a number of important advances have been achieved. These technologies include physical, chemical, and biological methods and have certain practical feasibilities and effectiveness. Understanding and mastering the progress in research on odor removal technology is of great importance to improving the degree of odor removal by waterworks and protecting public water needs and health. Therefore, based on the current situation of odor in drinking water, this article introduces the classification and identification of the sources of odors in waterworks, and focuses on the latest odor removal technologies in waterworks, including adsorption technology, chemical oxidation technology, biodegradation technology and combined technology.
2. Methodology
The scientific documents related to this review are mainly from the CNKI database, Web of SCI database and ScienceDirect database.
Several combinations of the following keywords were applied during the database search: waterworks; olfactory removal; adsorption; chemical oxidation; biodegradation; and combined technology. All references were original articles published before January 2024, mainly from the last 15 years. We removed duplicates and selected articles based on title, keyword, abstract, and relevance, and ultimately selected 58 papers with the highest relevance to odor removal in waterworks, covering the classification and identification of the sources of odors in waterworks, related technologies for odor removal and practical applications of odor removal technologies which met our inclusion criteria, and we acknowledge that some papers may have been omitted. However, we believe that all of the studies collected faithfully represent the current state of knowledge on the topic.
Our results consist of three main parts. The first part is the classification and identification of the sources of odors in water treatment plants, which cites 8 references. The second part mainly introduces the technologies related to odor removal, including adsorption technology, chemical oxidation technology, biodegradation technology, and combined technology, and 41 references are cited in this part, including the related references cited in the two tables. The third part mainly introduces the practical applications of odor removal technologies, including practical application scenarios, considerations, and prospects, and 9 references are cited in this part.
3. Classification and Identification of the Source of Odors in Water Treatment Plants
3.1. Odor Classification
The smell of drinking water is divided into three categories and thirteen types, including eight types of olfactory odors, four types of taste odors, and one type of oral and nasal sensations, of which olfactory smell is the most important [
1]. Odorous odors and their odor-causing substances are shown in
Figure 1. Odorous smells can be divided into soil, mold, fishy smell, fishy/rotten smell, grass smell, fruit smell, drug smell, chlorine/ozone smell, and chemical reagent taste. The smell of mold and the smell of fish are the two most common types of odors in water. The smell is mainly caused by geosmin (GSM) and 2-methylisoborneol (2-MIB). GSM and 2-MIB are two commonly detected odorants, both of which belong to the group of saturated cyclic tertiary alcohol terpenoids, which are difficult to biodegrade under natural conditions and are difficult to effectively remove with commonly used oxidizing agents. The odor threshold concentration (OTC) of GSM and 2-MIB is (1–10) ng/L and (5–10) ng/L, respectively, which can be easily detected even at low concentrations [
2]. A fishy smell is usually caused by amines and unsaturated aldehydes produced by algae growth and decay. Currently, the odorants present in fishy smells include trimethylamine, dimethylamine, 2,4-heptadienal, 2,4-decadienal, 2,4,7-decadienal, 2,6-nonadienal, etc. [
3].
Additionally, sulfur-containing compounds are the main cause of fishy odors/spoilage. Due to the low OTC of sulfur-containing compounds, their foul smell is easy to detect. The common sulfur-containing compounds mainly include methyl meriol (MT), dimethyl thiamine (DMS), dimethyl disulfide (DMDS), dimethyl trithioether (DMTS), and other dimethyl polysulfide compounds, etc. These sulfur-containing substances are mainly the products of microbial anaerobic metabolism, which can promote the emergence of putrid-odor problems. Vegetable and fruity flavors are caused by aldehydes, ketones, and alcohols. The smell of chemical reagents and medicines is mainly caused by the discharge of pollutants generated by human life and production into water bodies. Common chemical odorants include bromomethane, triiodomethane, phenol, 2,4-dichlorophenol, and other halogenated hydrocarbons and phenolic compounds [
4].
3.2. Source of Odors
There are a variety of odors in water, and their sources can be divided into two categories: one is endogenous odors caused by natural factors, and the other is exogenous odors caused by human factors [
5]. Natural factors refer to the metabolites produced by algae and microorganisms in the water and the odorous gases produced by sediments such as humus in the water that dissolve in the water and cause odor problems in the water [
6]. Phytoplankton such as cyanobacteria, green algae, chrysoalgae, microcystis, and diatoms can produce heteroodorous substances, mainly from their extracellular metabolic secretions, such as dimethyltrithioether (DMTS), β-cyclocitral, β-ionone, 2-methylisobornol (2-MIB), geosmin (GSM)., etc. These substances produce odors such as decay, rot, mold, etc., in the water body. In addition, minerals such as iron and manganese, which are precipitated from the rocky soil around the water body, cause the water body to contain a large number of dissolved solids, which leads to an odor in the water.
Human factors refer to the smell of pollutants that enter the water body as a result of various human production and living behaviors. A large number of substances containing nitrogen, phosphorus, sulfur, phenols, thioethers, chemical reagents, pesticides, and other pollutants are discharged into the natural environment without proper treatment, which directly causes the water body to have an obvious and strong odor. Odorous substances such as hydrogen sulfide, thioether, and mercaptan, which are produced in discharged sulfur-containing domestic wastewater, production wastewater, and livestock and poultry wastewater under the action of anaerobic microbial metabolic activities, are the main causes of water odor [
7]. At the same time, the use of disinfectants in the water treatment process also leads to odors. For example, the use of chlorine disinfectants produces chloramine compounds, aldehydes, chlorophenols, and haloforms, and other disinfection by-products, which leads to odor problems in drinking water and affects consumers’ sensory experience. Ozone oxidation often produces aldehydes with different carbon numbers, which leads to a variety of different odors such as aroma, fruits, plastic, and fish [
8].
4. Odor Removal Technology
4.1. Adsorption Technology
4.1.1. Activated Carbon Adsorption Technology
Activated carbon is a black, porous substance that is created by carbonizing and activating hard coal and wood chips as raw materials. Activated carbon has good organic matter adsorption and other properties due to its rich pore structure, large specific surface area, and a significant number of functional group structures. It can effectively remove smells from water. Currently, there are two types of commonly used activated carbon: granular carbon (GAC) and powdered carbon (PAC). PAC is widely used in the emergency treatment of odors in seasonal water sources due to its remarkable effects, convenient dosage, flexible application, and effective separation in traditional water treatment processes. The results of research by Liu Cheng et al. [
9] showed that the optimal dosage of activated carbon was 15 mg/L, the optimal contact time was more than 30 min, At the same time, the amount of coagulant was increased appropriately. In this case, the removal rates for microcystin, GSM, and 2-MIB were 90%, 86%, and 93%, respectively. PAC can remove trace organic matter very effectively and can be flexibly applied to various processes in aquatic plants, but the effect is different at different process points, as shown in
Table 1. Zhang Jianfeng et al. [
10] used raw water used for production in a water treatment plant in Tianjin and raw water imported from a control tank as test raw water to conduct coagulation and adsorption tests, and studied the adsorption effect of powdered activated carbon applied at different process points. The test results showed that adding powdered activated carbon before adding polyaluminum chloride had the best effect on the removal of odors from the raw water used for production, and adding 15 mg/L of powdered activated carbon to the raw water from the inlet of the regulation tank had the best effect on the treating the odor after stirring for 5 min and allowing the water to stand for 2 h. However, the adsorption effect of PAC is greatly affected by the natural organic matter (NOM) in the raw water, which can easily cause secondary pollution, and the required dosage is high, which is easily affected by placement and longer adsorption treatment times.
GAC is mainly used where eutrophication is severe and long-term odor elimination is required. GAC is renewable and easy to use and manage. Therefore, it is often used for the control of odors in water from a filter. When the concentration of odorous compounds is high and PAC needs to be used continuously over a long period of time to solve odor problems, GAC is often the more economical method [
11]. Zhang et al. [
12] investigated granular activated carbon’s (GAC) adsorption of two algal odorants in water; this study demonstrated that GAC is an excellent adsorbent for the removal of aqueous dimethyl trisulfide and β-cyclocitral. Within 48 h, 100 mg/L GAC could remove 91.1% of 0.5 mg/L dimethyl trisulfide and 96.9% of 0.5 mg/L β-cyclocitral, respectively. Cheng Yin et al. [
13] investigated the adsorption mechanism of dimethyl trisulfide and cyclocitraldehyde with granular activated carbon. At a GAC dosage of 100 mg/L, the removal rates of the two substances could reach 91% and 96%, respectively, at an initial concentration of 500 µg/L after 48 h of adsorption.
Table 1.
Comparison of advantages and disadvantages of PAC in different processes [
14].
Table 1.
Comparison of advantages and disadvantages of PAC in different processes [
14].
| Dosing Point | Process Parameters | Removal Rate | Advantage | Shortcoming | Reference |
|---|
| Raw water pump house suction well feed | PAC dosage is 15 mg/L; Mixing time: 5 min | GSM concentration reduced to less than 6 ng/L | Increase contact time; well mixed | There is the problem of competing adsorption during the subsequent flocculation process. When the competitive adsorption phenomenon is severe, the adsorption effect is reduced. The service life of the pump impeller may be affected | [10] |
| Initial concentration: [GSM] = 34 ng/L; PAC dosage: 15 mg/L | 90.8~97.6% removal of GSM | [15] |
| Flocculation container before addition | Initial concentration: [GSM] = 109,628 ng/L; [2-MIB] = 92.509 ng/L; PAC dosage: 20 mg/L; Adsorption time:
30 min | 96.97% removal of GSM and 81.96% removal of 2-MIB | PAC can diffuse quickly, bind to flocculants, and separate from water during filtration processes. The contact adsorption time is suitable | Flocculants and PAC can form flocs, which can reduce the adsorption efficiency of PAC | [16] |
| Initial concentration: [2-MIB] = 200–350 ng/L; PAC dosage: 40 mg/L | 47% removal of olfactory odor | [17] |
| Front end of feed filter | Initial concentration: [chloroform ] = 15.5 μg/L; PAC dosage: 20 mg/L | 63.2% removal of chloroform | Effectively eliminates adsorption competition and reduces the burden on the coagulation process. It has good adsorption effect on small molecules that cannot be effectively removed in the coagulation process and improves water quality | PAC penetrates the filter layer resulting in insufficient adsorption time | [18] |
4.1.2. Other Adsorption Technology
In addition to the use of activated carbon adsorption technology to eliminate odors from waterworks, other adsorbents also raise concerns. Zeolite is easier to regenerate than activated carbon, has a greater stability during regeneration, and is not influenced by humus, oxide, or water hardness [
19]. Amin et al. [
20] studied the effectiveness of ozonation on the removal of phenol and COD using four H-type zeolites under different operating conditions. The results showed that the combination of zeolite and ozone can effectively remove phenol and COD. Zeolite mainly serves as an adsorbent, providing a surface for the reaction between ozone and phenol. Han Shanshan et al. [
21] investigated the adsorption ability of attapulgite to remove the odorants 2-MIB and GSM from water. The research results showed that the removal rates of GSM and 2-MIB in water were about 30% and 26%, respectively, when 2-MIB and GSM in water reached ng/L value. The removal effect was improved to varying degrees.
4.2. Chemical Oxidation Technology
4.2.1. Ozonation Technology
As a highly efficient and widely used oxidizer, ozone is stronger than sodium hypochlorite and hydrogen peroxide in water treatment and produces fewer toxic by-products than other oxidizers. Ozone oxidation has obvious effects on the removal of GSM, 2-MIB, and other odorants. Liu Xiwen et al. [
22] studied the efficiency of three common oxidizing agents, including sodium hypochlorite, potassium manganese and ozone, in removing GMS, 2-MIB, 1-octen-3-ol, 2-isopropyl-3-methoxypyrazine, 2-isobutyl-3-methoxypyrazine, β-cyclocitric aldehyde, 2,4,6-trichloroanisole and 3-Methylindole by oxidation. The results showed that ozone oxidation can effectively remove these eight typical odorants; except for the removal rate of 2-MIB and GMS, which is 55–70%, the removal rate of other odorants is more than 90%, and sodium hypochlorite and potassium permanganate only have a removing effect on some odorants. This shows that, of the three, ozone is the most suitable oxidizing agent for removing typical odorous substances.
Odor removal by ozone oxidation was influenced by the amount of ozone, the initial pH of the solution, and the initial concentration of the solution. Gu Yurong et al. [
23] studied the removal efficiency and influencing factors of ozone oxidation on 2-MIB and GSM, and found that, at an ozone concentration of 2 mg/L, the removal rates of 2-MIB and GSM were 75.1% and 88.3%, respectively, after a 15 min reaction. The removal rates of 2-MIB and GSM increased with the increase in ozone dose and initial pH and decreased with the increase in initial ozone concentration. It was found that the presence of an appropriate amount of NOM in water improves the efficiency of the ozone oxidation of odors. However, ozonation alone has the disadvantages of requiring a high dosage, long contact reaction time, and the easy formation of bromate and other by-products. Therefore, when eliminating odors in waterworks, methods such as catalytic ozone oxidation or a combination with other methods are often used to promote the formation of free hydroxyl radicals (·OH) and improve the utilization rate. Wang et al. [
24] coupled ozone with granular activated carbon (GAC) to study the 2-MIB removal pattern in water by this process and found that the removal efficiency of 2-MIB was enhanced by about 55%.
4.2.2. Ultraviolet Advanced Oxidation Technology
The advanced UV oxidation odor removal technology stimulates free radicals with strong oxidation ability under UV light radiation to break down the odor substances in the water. Common technologies include ultraviolet/ozone (UV/O
3), ultraviolet/persulfate (UV/PS), ultraviolet/hydrogen peroxide (UV/H
2O
2), ultraviolet/chlorine (UV/Cl), and ultraviolet/titanium dioxide (UV/TiO
2). The UV/O
3 process can treat odors in water and reduce the production of by-products. The advantages and disadvantages of each advanced UV oxidation technology are listed in
Table 2. Zoschke, Kristin et al. [
25] found that UV/O
3 had a high efficiency in removing odors in raw water and minimizing the generation of disinfection by-products. Tan et al. [
26] found that, at a H
2O
2 concentration of 7.5 mg/L, a UV intensity of 400 mJ/cm
2, and a GMS concentration of 300 ng/L, the highest GSM removal rate is 97.14%. UV/PS can generate more selective and faster sulfate radicals (
·). Yue Siyang et al. [
27] found that both UV/H
2O
2 and UV/PS could effectively degrade 2-MIB, and in pure water systems, the degradation effect of UV/PS was better. T.K. Kim et al. [
28] found that the UV/Cl system completely removed GSM within 40 min and 2-MIB within 2 h, while there was no significant change in the two odorants during the chlorination process alone. He et al. [
29] studied the degradation of 2-MIB by the UV/TiO
2 system and the results showed that the photocatalytic UV/TiO
2 system could effectively remove the odorous substance 2-MIB from water and the degradation rate of 2-MIB after UV irradiation for 60 min achieved 95%. Although UV advanced oxidation technology has better degradation ability for olfactory substances, the problems of low light energy utilization, high energy consumption, and easy to produce by-products limit its full application.
4.2.3. Other Oxidation Technologies
In addition to ozone oxidation technology and advanced UV oxidation technology, some other oxidation technologies have been found to have good odor removal effects. Tian Jiayu et al. [
37] studied the removal effect of potassium permanganate (PM) activated by sodium bisulfite (BS) on 2-MIB and GSM, and the results showed that, under the conditions of multiple applications, a reagent concentration ratio and PM dosage of 1:5 and 50 mol/L, the removal efficiency of 2-MIB and GSM could reach more than 96% and 98%, respectively. Although potassium permanganate has a low price and is not easily formed as a by-product, its degradability to 2-MIB and GSM is weak. Using BS to activate PM effectively improves its removal effect and shows good potential in removing drinking water odors. Sun Xin et al. [
38] found that the advanced oxidation technology of ultrasonic (US) activated persulfate (PS) had the best degradation effect when the concentration of 2-MIB and GSM was 100 ngL
−1, which was 88.7% and 93%, respectively, corresponding to 3%. The US/PS process affects both sulfate radicals and hydroxyl radicals in odor removal and has a clear effect on odor removal. Wei Jie et al. [
39] used an Fe
2+-activated persulfate system (PDS) to degrade 2-MIB and GSM. Under pH 3.0 conditions, a PDS concentration of 1.0 mmol/L, and an Fe
2+ concentration of 1.0 mmol/L, the degradation rates of 2-MIB and GSM are 99.10% and 83.08%, respectively. The active ingredient in the Fe
2+/PDS system is Fe (IV), which has good application prospects for the degradation of 2-MIB and GSM. The degradation of pyrazine odorants by conventional processes is limited, and there is an urgent need to develop efficient methods for degrading pyrazines in response to the current problems of pyrazine-induced odor and potentially unsafe drinking water. He et al. [
40] successfully investigated and synthesized N-doped Mn
3O
4 composites (Mn-nN) and explored their application in catalytic ozonation for the degradation of pyrazine odorants, revealing that the Mn-nN catalysts have great potential in the treatment of olfactory problems caused by pyrazines in water, providing a new direction for the improvement of water treatment processes.
4.3. Biodegradation Technology
Bacteria and other microorganisms degrade olfactory substances through direct degradation, utilization of secondary substrates, cell lysis, predation, and biosorption flocculation to remove odor from water [
41]. Compared with adsorption technology and chemical oxidation technology, biodegradation technology does not require the addition of chemical agents to eliminate odors in water and has low operating costs. In recent years, biodegradation technology has become a research focus for odor elimination. Various strains have been found to have a degrading effect on odors, particularly 2-MIB. The degradation effects of different strains on 2-MIB are shown in
Table 3. The main organisms capable of degrading 2-MIB and GSM are bacteria, including a few fungi and predatory protozoa, which are mainly removed through metabolic processes such as degradation, co metabolism, synergistic degradation, and enzymatic degradation [
42]. Yuan Rongfang et al. [
43] inoculated 2-MIB and GSM-degrading bacteria onto the surface of the biological filter, and the removal rates of 2-MIB and GMS in the biological filter were 75% and 78%, respectively, while the removal rates of the two odorous substances were in the uninoculated biological filters were only 43% and 23%, respectively. Although inoculation with 2-MIB and GSM-degrading bacteria can significantly increase the degradation of odorants, if the concentration of odorants in the influent is too high, it is difficult for simple biological filter technology to degrade odorants to standard requirements. The concentration of odorous substances in the water can be effectively reduced through increased ozonation in the pre-biofilter stage. Yuan Rongfang et al. [
43] adopted the combined ozone-seeded biofilter process and the removal rates of 2-MIB and GSM were 84% and 94%, respectively. In addition, odor removal by biological filters also has the disadvantages of long film holding time, poor treatment effects at low temperatures, the need for new reaction structures, and high renovation costs for the water system.
4.4. Combined Technology
4.4.1. Ozone-Activated Carbon Technology
The mechanism of combined ozone-activated carbon technology is that ozone first oxidizes and decomposes macromolecular organics and adsorbs activated carbon to remove compounds or the by-products of ozonation. Combined use can effectively extend the service life of activated carbon, reduce the by-products generated by ozone, and improve the odor removal effect. The results of research by Sun Ting et al. [
48] showed that the advanced ozone-activated carbon treatment process in a water plant in Henan Province had the highest removal rates of the odorants 2-MIB and GSM, up to 98.0% and 49.3%, respectively. The removal effect for GSM was lower than that for 2-MIB, which may be due to the low concentration of GSM in the absorbed water, and no carcinogenic bromides were generated in the entire water treatment process. The biological activated carbon can extend the adsorption of activated carbon by removing organic matter adsorbed by the activated carbon due to its microbial degradation. Chen Siying et al. [
49] added a certain concentration of geosin and dimethylisobornyl to the water in Taihu Lake, so that the concentration of the two substances in the raw water reached 300 ng/L. When 1.0 mg/L of ozone was added, the concentrations of 2-MIB and GSM in the water treated with the ozone-biological activated carbon process were below the detection threshold and corresponded to the water quality standard. By combining ozone–biological activated carbon, various mechanisms such as activated carbon adsorption, ozone oxidation, biodegradation, and ozone sterilization and disinfection can come into full effect and effectively break down odorous substances in the water.
4.4.2. Potassium Permanganate—Powdered Activated Carbon Technology
The odor removal rate of potassium permanganate and powdered activated carbon in water is much higher than using either on their own. Potassium permanganate can oxidize and decompose some odor-causing substances in water, and its reaction product, hydrated manganese dioxide, has good adsorption, coagulation and catalytic effects [
11], which can help reduce the odor in water, but an excessive addition of potassium permanganate can change the color of the water. The combined technology of potassium permanganate and powdered activated carbon can achieve a synergistic effect. Potassium permanganate causes organic matter in the water to oxidize and polymerize on the surface of the activated carbon, which improves the adsorption capacity of the activated carbon. The use of powdered activated carbon accelerates the production of manganese dioxide, the product of potassium permanganate reduction and avoids excessive manganese concentrations in the water. Li Weiguang et al. [
50] studied the odor removal effect of combined potassium permanganate–powder activated carbon technology on B tributary water in Taihu Lake. The results showed that, when the potassium permanganate dosage was 0.5 mg/L and the powdered activated carbon dosage was 40 mg/L, the odor threshold of submerged water was only five and the removal rate reached 98.8%. Peng Xiaojun et al. [
51] conducted an algae and odor removal experiment by combining potassium permanganate with powdered activated carbon. At a potassium permanganate dosage of 1.2 mg/L and a powdered activated carbon dosage of 15 mg/L, the odor removal rate was 91.9%. The combination of potassium permanganate and powdered activated carbon is an efficient technology for eliminating odors in drinking water; the order of application of which cannot be changed in waterworks. In addition, after introducing potassium permanganate into the flocculation tank, activated carbon should be added to the tank before it enters the flocculation tank, otherwise the effect will be worse [
52].
5. Practical Application of Olfactory Removal Technology
5.1. Application Scenarios and Considerations for Different Technologies
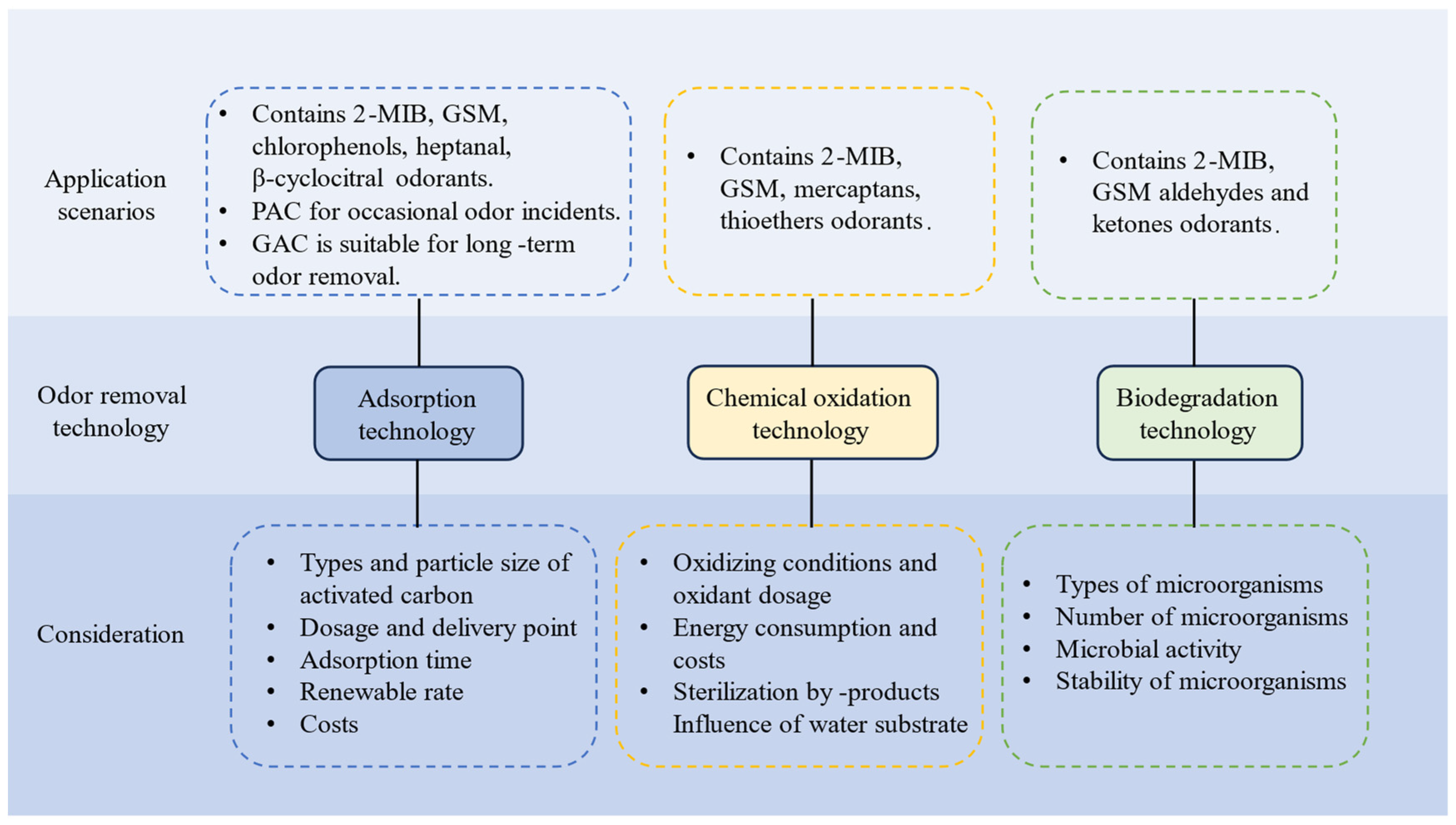
The application scenarios and considerations of different technologies are shown in
Figure 2. Activated carbon adsorption technology can remove most olfactory substances including 2-MIB, GMS, chlorophenols, heptanal, β-cyclocitral, and other olfactory substances, but it cannot effectively adsorb thiols and thioethers. PAC and GAC are two common activated carbons used in engineering applications, and PAC has the advantages of cheapness and flexibility in the deodorization of olfactory substances, which can be used in all aspects of the water treatment process, due to the large variation of concentration of the olfactory substances in raw water. The concentration of odor-causing substances in the raw water varies greatly, and the dosage of PAC is also related to the turbidity of the raw water, the type of odor-causing substances in the water, the variety of activated carbon, the contact time, and other factors, and the adsorption effect is also dependent on the concentration and characteristics of the natural organic matter (NOM) in the water. Cook studied the olfactory situation in four water supply plants in Australia and concluded that, for GSM to be below the olfactory threshold, the minimum PAC dosage required was 21–29 mg/L, and for 2-MIB to be below the olfactory threshold, the minimum PAC dosage required was 39–55 mg/L [
53]. From an economic point of view, powdered activated carbon can be used to dispose of occasional olfactory events [
54]. GAC is a porous substance with a large adsorption capacity, which has the advantages of a strong adsorption capacity, ease of control, and low cost, and is capable of removing most of the organic substances and some inorganic substances from the water body. When activated carbon is needed for a long period of time to solve the problem of olfactory odors, granular activated carbon is a better choice. For the treatment of low concentration of odor-causing substances, granular activated carbon can be used for a long time. Zeolite has the advantages of being highly selective and easier to regenerate than activated carbon, but its cost is expensive and there is no report of this technology being put into practical application. In practical applications, the influence of the type of activated carbon, particle size, dosage, dosage point, and adsorption time should be considered, and the desorption of the adsorbent, recyclability, and the cost of desorption are also issues that need to be considered in the subsequent application of physical adsorption in the actual treatment process.
Conventional chemical oxidation technology is not effective in removing olfactory odors, but ozone oxidation technology has a better degradation effect on olfactory substances such as GSM and 2-MIB. Compared to activated carbon adsorption, chemical oxidation has better removal effects on thiols and thioethers [
55]. The effectiveness of UV-based advanced oxidation for the removal of GSM and 2-MIB from water has been widely demonstrated, but the technology requires a large investment in equipment and is still in the experimental stage, and how to balance the relationship between the production and operation costs and the treatment effect, as well as how to solve the problem of disinfection by-products, still remains to be solved. Zoschke, Kristin et al. [
25] compared the effectiveness and economics of UVU, UV/O
3, and UV/H
2O
2 for the removal of GSM and 2-MIB and showed that UV and O
3 had the highest combined efficiency and UV/H
2O
2 had the highest treatment cost for treating taste and odor compounds in raw water. Khajouei, Golnoosh et al. [
33] showed that the UV/Cl process consumes less energy compared to the UV/H
2O
2 and UV/PS processes. The research of oxidation technology mainly focuses on the oxidizing agent and oxidation conditions, and the dosage of the oxidizing agent is determined according to the target removal rate, which lacks the exploration of the removal mechanism, and cannot be directly applied to the removal of different water sources, different seasons, different causes, and different olfactogenic substances. In practical applications, energy consumption, cost, formation of disinfection by-products, and the influence of water matrix on the degradation process should be considered, and the joint use of oxidation with adsorption and biological methods should be considered to take advantage of their respective advantages in removing pollutants from water.
When biological action exists in the water purification process, it can play a role in partially removing odors. Microorganisms in bio-activated carbon filters and sand filters can absorb and degrade some of the odor-causing substances. However, biological methods need to control the stability of the growth of adapted colonies, otherwise the effect is very little; usually, the concentration of odor-causing substances is low, and thus the number of microorganisms in the biological treatment process section is not controlled, and the olfactory compounds can only be utilized as secondary substrates. The use of biological treatments alone to control olfactory odors in water is not yet mature in water purification engineering technology [
42]. Therefore, there are fewer studies on biodegradation technologies to control olfactory odor. Throughout the biodegradation process, attention should be paid to cultivating and screening out specialized microorganisms with strong targeting abilities and fast degradation rates and ensuring the activity and number of degrading microorganisms in different seasons. Combined processes will be the focus of future practical application, and in the future, with a variety of joint processes, we must pay attention to the coherence of the process based on the chemical and biological processes which have been combined; We should consider whether the previous process creates an unfavorable environment for subsequent microorganisms to survive, and must strictly control the parameters of each stage of the treatment engineering process.
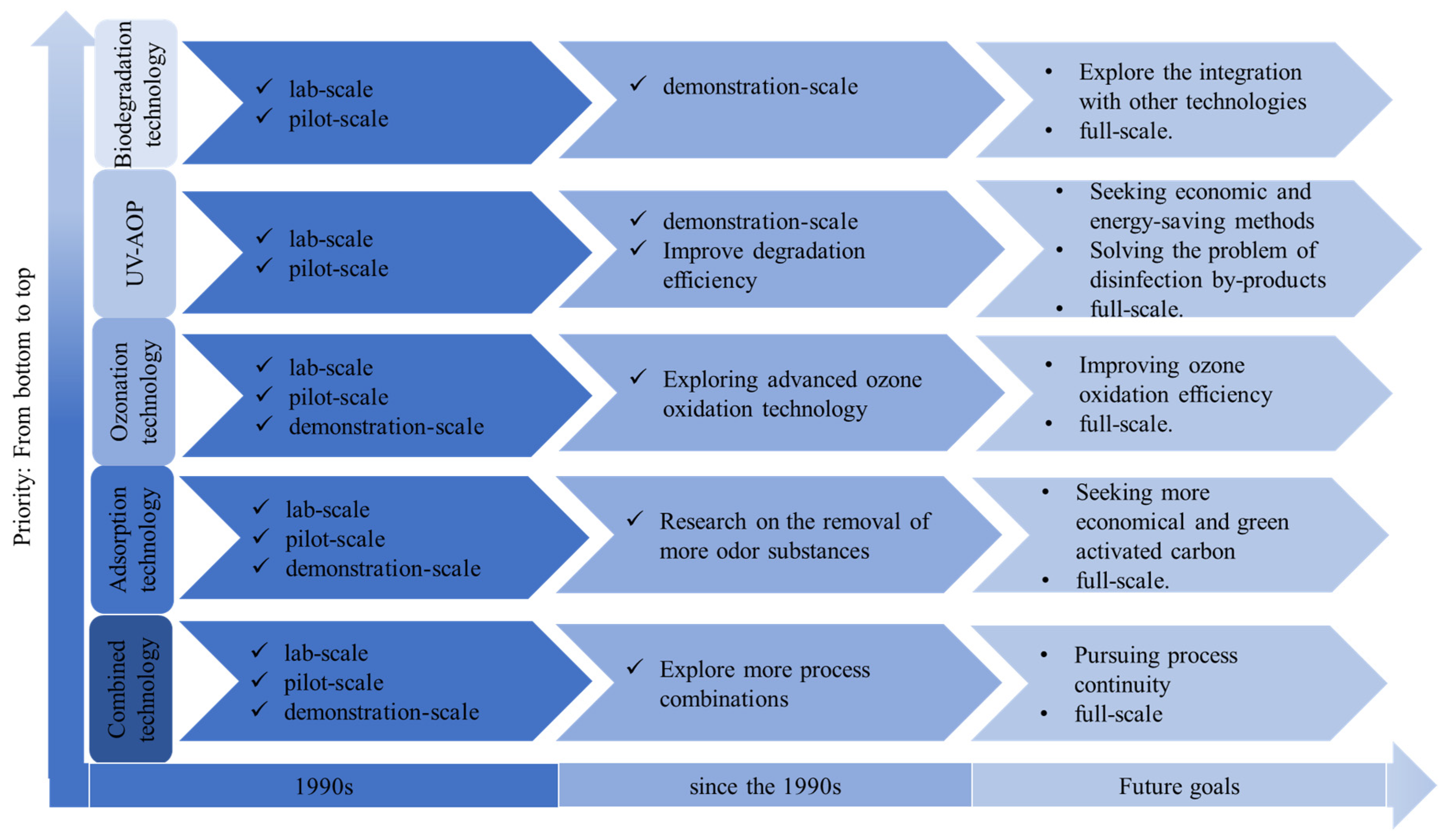
5.2. Prospects for the Practical Application of Different Technologies
In recent years, different technologies for olfactory odor removal have been extensively studied at both the laboratory scale and the pilot scale, and
Figure 3 shows a roadmap for the development of olfactory odor removal technologies. A single technology often fails to remove the olfactory odor completely, and the combination of technologies is the final embodiment of the synergistic promotion effect among the technologies, which has a good research value and application prospects both at the experimental scale, the pilot scale, and in practical applications. Jiang et al. [
32] found that the UV/H
2O
2-bio-activated carbon (BAC) process can play a stronger oxidizing role in advanced oxidation in a medium-scale study. Using the physical adsorption and biological effects of activated carbon, it has a better removal effect on odorous substances with long-term stable operation, and the concentrations of GSM and 2-MIB are lower than 5 ng/L after treatment. The focus of future research on the topic will be the efficient combination of different processes. Activated carbon adsorption is still the most practical deodorization method, and has been used in many practical applications for the removal of olfactory odors. Huang et al. [
56] used activated carbon adsorption technology as the main means of olfactory odor removal in a water plant, and the results showed that the removal rate of 2-MIB was increased by 48.9% after the modification, and the olfactory odor substances in the effluent water were effectively controlled. However, the NOM in the water weakens the adsorption capacity of activated carbon, and research on the modification of activated carbon and the development of new adsorbent materials and other topics will be the focus of further research into adsorption technology to remove odorous substances. Chemical oxidation technology has also achieved good deodorization results at the laboratory scale and the medium scale. Xu et al. [
57] studied the removal effects of algae on 2-MIB by combining pre-ozone treatment, coagulation, precipitation, filtration, and post-oxidation systems at the pilot scale, and they verified that moderate pre-ozone treatment and enhanced coagulation combined with the post-oxidation process can effectively remove filamentous cyanobacteria and 2-MIB.However, balancing the relationship between production cost and treatment effect and solving the problem of disinfection by-products are obstacles to its practical application. The research and application of bioexplanatory technology mostly tends to be combined with other technologies. Gao et al. [
58] showed that the ozone bioactivated carbon (BAC) process has a better effect on the removal of 2-MIB, and the pilot study showed that, with an ozone dosage of 0.8–1.5 mg/L, the removal rate of 2-MIB by pre-ozone was 25–50%, and the removal rate by post-ozone was 35–55%, and the removal rate of BAC was 35–55%. The removal rate of 2-MIB by pre-ozone was 25~50%, that of 2-MIB by post-ozone was 35~55%, and that of 2-MIB by BAC was 30~40%. The biodegradation method is not selective; therefore, considering the screening and cultivation of specialized deodorizing strains would be a new way to study this method.
6. Conclusions
This article first introduces the classification of odors, and then introduces the source of and removal technologies for the odor, such as: B. Earth mud smell, fishy smell/spoilage smell, vegetation smell/fruit smell, medicinal smell, chlorine smell/ozone smell, and chemical reagents. 2-Methylisobornol (2-MIB) and Geosmin (GSM) are two typical odors, and the elimination of these two odors has been studied. Activated carbon technology can effectively remove odors in water through good adsorption, but its adsorption effect is easily affected by NOM and it has some defects such as being unable to easily regenerate the adsorbent, a large dosage, and a long adsorption time. Ozone oxidation technology has a good effect on removing a variety of odorous substances, but bromate and other by-products are easily produced. Advanced UV oxidation technology ensures the efficient oxidative degradation of free radicals and is widely used in odor treatment. Although biodegradation technology has advantages such as low costs and no secondary pollution, it still has many technical problems which need to be further investigated in the actual application of waterworks. Ozone–activated carbon, potassium permanganate powder–activated carbon and other combined technologies can overcome the disadvantages of one-time use, have a synergistic effect in odor elimination, and have good application prospects in odor elimination by aquatic plants. In the future, appropriate measures should be taken according to the odor characteristics and economical, environmentally friendly, and efficient technologies should be selected to deal with the odor problems in waterworks.
Author Contributions
Conceptualization, H.D., Z.W., Y.S. and K.J.S.; methodology, H.D., Z.W., Y.S. and K.J.S.; validation, H.D., Z.W., Y.S. and K.J.S.; formal analysis, H.D. and Y.S.; investigation, H.D. and Y.S.; resources, Y.S. and K.J.S.; data curation, Y.S. and K.J.S.; writing—original draft preparation, H.D. and Y.S.; writing—review and editing, Y.S. and K.J.S.; visualization, Y.S. and K.J.S.; supervision, Y.S. and K.J.S.; project administration, Y.S. and K.J.S.; funding acquisition, Y.S. and K.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No.51508268), Natural Science Foundation of Jiangsu Province in China (No. BK20201362), and 2018 Six Talent Peaks Project of Jiangsu Province (JNHB-038).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shui, B.; Song, X.; Fan, W.; Duan, Z. Research progress on odor of drinking water and removal technology. Appl. Chem. Ind. 2022, 51, 3075–3081. [Google Scholar]
- Zhou, R.; Zhang, S.; Zheng, S.; Yang, Y.; Sun, S.; Lin, K. Study on the removal of 2-methylisoborneol and geosmin from water by activated carbon. J. China Urban Water Assoc. 2023, 65–68. [Google Scholar]
- Guo, Q.; Wang, C.; Yu, J.; Ding, C.; Chen, T.; Li, C.; Li, X.; Ma, W.; Yang, M. Research Progress on Typical Taste and Odor Problems in Drinking Water. China Water Wastewater 2020, 36, 82–88. [Google Scholar]
- Guo, Q.; Li, X.; Yu, J.; Zhang, H.; Zhang, Y.; Yang, M.; Lu, N.; Zhang, D. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry for the screening of potent swampy/septic odor-causing compounds in two drinking water sources in China. Anal. Methods 2015, 7, 2458–2468. [Google Scholar] [CrossRef]
- Shao, C.; Li, L.; Yu, S.; Hou, L. Contribution of algae on the occurrence of odor and taste compound 2-MIB in drinking water source of East Tai Contribution of algae on the occurrence of odor and taste compound 2-MIB in drinking water source of East Tai Lake. China Environ. Sci. 2014, 34, 2328–2333. [Google Scholar]
- Hu, T. Research Advances of Odorous Compounds and Its Analysis Methods in Drinking Water. Water Purif. Technol. 2019, 38, 11–14. [Google Scholar]
- Guo, Q.; Yu, K.; Yang, X.; Wen, H.; Zhang, Z.; Yu, H.; Li, D.; Zhang, M.; Yang, M. Identification of complex septic odorants in Huangpu River source water by combining the data from gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography using retention indices. Sci. Total Environ. 2016, 556, 36–44. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, K.; Yu, J.; Wang, C.; Wen, X.; Zhang, L.; Yang, M.; Xia, P.; Zhang, D. Simultaneous removal of multiple odorants from source water suffering from septic and musty odors: Verification in a full-scale water treatment plant with ozonation. Water Res. 2016, 100, 1–6. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Chen, W.; Wang, M. Study on PAC Pretreatment of High-algae Source Water. China Water Wastewater 2012, 28, 52–55. [Google Scholar]
- Zhang, J.; Zhang, Y.; Zhao, Y. Small-scale Study on Effect of Powdered Activated Carbon on Odor Removal of Drinking Raw Water. Tianjin Sci. Technol. 2020, 47, 74–76. [Google Scholar]
- Xie, G.; Chao, M.; Xu, H.; Hu, X.; Ding, W. Odor removal in raw water by potassium permanganate combined with powdered activated carbon. Water Technol. 2012, 6, 1–4. [Google Scholar]
- Zhang, K.; Gao, N.; Deng, Y.; Shui, M.; Tang, Y. Granular Activated Carbon (GAC) Adsorption of Two Algal Odorants, Dimethyl Trisulfide and β-Cyclocitral. Desalination 2011, 266, 231–237. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, N.; Zhang, K.; Na, A.; Rong, W.; Zhou, S. Study on the mechanism of granular activated carbon to adsorb dimethyl thrisulfide and β-cyclocitral. Technol. Water Treat. 2011, 37, 54–58. [Google Scholar]
- Jiang, X.; Wu, S.; Xuan, X.; Yan, X.; Chi, J. Application of powder activated carbon addition technology in water treatment process of water purification plant in China. Ind. Water Wastewater 2020, 51, 8–11. [Google Scholar]
- Zhou, D. Analysis of Odor-Causing Substances in Raw Water of the Ninth Water Treatment Plant and Its Removal Technology. J. China Urban Water Assoc. 2014, 20–24. [Google Scholar] [CrossRef]
- Qu, L.; Mai, J.; Yang, W.; Zhao, Z.; Wang, X.; Mai, X.; Li, J.; Sun, T. Removal of geosmin and 2-methylisoborneol from water by activated carbon enhanceocoagulation. Water Technol. 2015, 9, 17–21. [Google Scholar]
- Yin, Q.; Ni, X.; Ju, J.; Wang, J.; Xu, S. Taihu Lake Technological Process of Emergency Treatment for 2-MIB Pollution in Raw Water of East. Water Purif. Technol. 2019, 38, 84–86. [Google Scholar]
- Tang, M.; Ding, L.; Wu, Q.; Xing, P.; Wu, Z.; Xu, J. Pre-chlorination and powder activated carbon process for algae contained raw water purification. Water Wastewater Eng. 2015, 40–43. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Zhang, B.D.; Wang, Y.Z.; Kong, J. Research Progress in Removal Technology of Taste and Odor Compounds in Drinking Water. Shandong Chem. Ind. 2020, 49, 54–56. [Google Scholar]
- Amin, N.; Akhtar, J.; Rai, H. Screening of combined zeolite-ozone system for phenol and COD removal. Chem. Eng. J. 2010, 158, 520–527. [Google Scholar] [CrossRef]
- Han, S. Study on the Efficiency of Adsorption and Enhanced Coagulation of Odor Substances in Water by Attapulgite Soil. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2011. [Google Scholar]
- Liu, X.; Yan, H.; Han, Z.; Fang, H.; Tian, J. Research on the removal of 8 typical taste and odor compounds from source water by oxidation methods. Water Technol. 2020, 14, 1–7. [Google Scholar]
- Gu, Y.; Zhang, M.; Li, X.; Guo, X.; Zhang, Q.; Dong, Z.; Li, Y. Study on Factors Affecting Degradation of Typical Odor Compounds by Ozonation. Tianjin Sci. Technol. 2020, 47, 35–40. [Google Scholar]
- Wang, W.; Fan, Y.; Liu, G.; Liu, H. Study on the Removal Performance of 2-Methylisoborneol by O3-GAC Combined. Technol. Water Treat. 2017, 43, 33–37. [Google Scholar]
- Zoschke, K.; Dietrich, N.; Börnick, H.; Worch, E. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Res. 2012, 46, 5365–5373. [Google Scholar] [CrossRef]
- Tan, F.; Chen, H.; Wu, D.; Wang, N.; Gao, Z.; Wang, L. Optimization of geosmin removal from drinking water using UV/H2O2. J. Residuals Sci. Technol. 2016, 13, 23–30. [Google Scholar] [CrossRef][Green Version]
- Yue, S.; Zhang, A.; Xie, P.; Wang, Z.; Ma, J. Comparative Study on UV/Persulfate and UV/Hydrogen Peroxide for Degradation of 2-MIB and Geosmin. China Water Wastewater 2016, 32, 6–9. [Google Scholar]
- Kim, T.; Moon, B.; Kim, T.; Kim, M.; Zoh, K. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions. Chemosphere 2016, 162, 157–164. [Google Scholar] [CrossRef]
- He, Y.; Liu, L.; Zhang, Y.; Fang, Y.; Gu, Y.; Huang, Y.; Zhang, A. Mechanism for photocatalytic degradation of odor 2-methylisoborneol in drinking water. Tech. Equip. Environ. Pollut. Control. 2012, 6, 2533–2538. [Google Scholar]
- Zhang, Y.; Zhang, X.; Cao, Y.; Chen, S.; Liang, Q.; Song, C. Research Progress in Degradation of Odor Compounds Geosmin and 2-Methylisoborneol in Drinking Water by Advanced Oxidation Processes. Chemistry 2023, 86, 1451–1458. [Google Scholar]
- Ma, Z.; Song, X.; Zhang, X. Research progress of ultraviolet/persulfate advanced oxidation technology in drinking water treatment. Appl. Chem. Ind. 2022, 51, 1466–1471. [Google Scholar]
- Jiang, Q.; Wang, Y.; Tian, L.; Liu, Y.; Liu, J.; He, G.; Liu, J. Pilot-scale and mechanistic study of the degradation of typical odors and organic compounds in drinking water by a combined UV/H2O2-BAC process. Chemosphere 2022, 292, 133419. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, G.; Finklea, H.; Lin, L. UV/chlorine advanced oxidation processes for degradation of contaminants in water and wastewater: A comprehensive review. J. Environ. Chem. Eng. 2022, 10, 107508. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.K.; Zoh, K. Degradation kinetics and pathways of β-cyclocitral and β-ionone during UV photolysis and UV/chlorination reactions. J. Environ. Manag. 2019, 239, 8–16. [Google Scholar] [CrossRef]
- Shui, B.; Song, X.; Fan, W. Research progress and challenges of photocatalytic technology in water treatment. Chem. Ind. Eng. Prog. 2021, 40, 356–363. [Google Scholar]
- Antonopoulou, M.; Konstantinou, I. TiO2 photocatalysis of 2-isopropyl-3-methoxy pyrazine taste and odor compound in aqueous phase: Kinetics, degradation pathways and toxicity evaluation. Catal. Today 2015, 240, 22–29. [Google Scholar] [CrossRef]
- Tian, J.; Jin, S.; Han, Z.; Bai, X. Performance optimization of permanganate/bisulfite process for removing odor compounds in water. Ind. Water Treat. 2022, 42, 64–71. [Google Scholar]
- Sun, X.; Sun, J.; Li, P.; Tang, J.; Yang, Q.; Tang, X. Ultrasonically Activated Persulfate Degrades Typical Odors in Water. Environ. Sci. 2019, 40, 1811–1818. [Google Scholar]
- Wei, J.; Duan, J.; Zhang, Q.; Wang, Y.; Yang, J.; Dong, Z. Efficacy and Mechanism of Fe(I) Oxidized by Persulfate to Generate Fe(IV) for 2-Methylisoborneol and Geosmin Degradation. Water Purif. Technol. 2021, 40, 40–45. [Google Scholar]
- He, Y.; Li, J.; Tang, J.; Cheng, H.; Zeng, T.; He, Z.; Wang, D.; Wang, L.; Song, S.; Ma, J. Constructed electron-dense Mn sites in nitrogen-doped Mn3O4 for efficient catalytic ozonation of pyrazines: Degradation and odor elimination. Water Res. 2023, 247, 120823. [Google Scholar] [CrossRef]
- Han, Z.; Han, H.; Yan, H.; Liu, Y.; Li, R.; He, W. Review on the biological process for removing geosmin and 2-methylisoboneol from source water. Water Technol. 2016, 10, 7–12. [Google Scholar]
- Wang, F.; Chen, K.; Zhao, Y.; Cao, N.; Guo, Q.; Yu, J. Biodegradation Bacteria Screening and Initial Biodegradability Evaluation for 2-MIB in Drinking Water. Water Purif. Technol. 2018, 37, 1–4. [Google Scholar]
- Yuan, R.; Zhou, B.; Shi, C.; Gu, J.; Li, Y. Ozonation-lnoculated Biofiltration for Removal of the Typical Taste and Odor Compounds inDrinking Water. Res. Environ. Sci. 2013, 26, 800–806. [Google Scholar]
- Zhou, B.; Yuan, R.; Shi, C.; Yu, L.; Gu, J.; Zhang, C. Biodegradation of geosmin in drinking water by novel bacteria isolated from biologically active carbon. J. Environ. Sci. 2011, 23, 816–823. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Shi, C.; Yu, L.; Zhang, C.; Cuo, J. Biodegradation of 2-methylisoborneol by bacteria enriched from biological activated carbon. Front. Environ. Sci. Eng. 2012, 6, 701–710. [Google Scholar] [CrossRef]
- Du, K.; Liu, J.; Zhou, B.; Yuan, R. Isolation of bacteria capable of removing 2-methylisoborneol and effect of cometabolism carbon on biodegradation. Environ. Eng. Res. 2016, 21, 256–264. [Google Scholar] [CrossRef][Green Version]
- Zhou, M. Screening and Characteristics of Odor-Degrading Bacteria. Master’s Thesis, China University of Geosciences, Beijing, China, 2019. [Google Scholar]
- Sun, T.; Xu, H. Analysis of the removal effect of organic matter by ozone activated carbon process. China New Technol. New Prod. 2021, 62–66. [Google Scholar]
- Chen, S. Taste and Odor Treatment in Tai Lake by Ozonation/GAC Technology. J. Henan Sci. Technol. 2018, 151–152. [Google Scholar]
- Li, W.; Gao, Y.; Huang, X.; Sun, S.; Zhang, J. Study on Taste and Odor Removal in Drinking Water by Potassium Permanganate Combined with Powdered Activated Carbon. China Water Wastewater 2007, 23, 18–21. [Google Scholar]
- Peng, X.; Yang, Y.; Shen, Z. Experimental study on the removal of algae and odor with the combined potassium permanganate and powdered activated carbon. Environ. Eng. 2023, 41, 230–232. [Google Scholar]
- Cheng, X.; Zhang, Z.; Song, H. Solving odor pollution in water treatment plants by potassium permanganate and powdered activated carbon combination. Heilongjiang Environ. J. 2023, 36, 33–35. [Google Scholar]
- Cook, D.; Newcombe, G.; Sztajnbok, P. The application of powdered activated carbon for MIB and geosmin removal: Predicting PAC doses in four raw waters. Water Res. 2001, 35, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Long, C.; Li, A. Progress in Removal Technology of Taste and Odor Compounds-Geosmin and 2-MIB in Drinking Water Source. Environ. Sci. Technol. 2012, 35, 122–126. [Google Scholar]
- Zhang, K.; Wu, X.; Wu, J.; Zhang, S. Review on sulfur ether odorants in drinking water distribution system. Water Wastewater Eng. 2020, 46, 99–105. [Google Scholar]
- Huang, M.; Shao, Z.; Yang, S.; Zhang, W.; Yang, F.; Wu, Y. Application of Full-Flow Control Technology for Odorous Substance in a Water Treatment Plant in Shenzhen City. Water Purif. Technol. 2020, 39, 159–163. [Google Scholar]
- Xu, H.; Zhang, J.; Wang, W.; Li, Y.; Pei, H. Moderate Pre-Ozonation Coupled with a Post-Peroxone Process Remove Filamentous Cyanobacteria and 2-Mib Efficiently: From Bench to Pilot-Scale Study. J. Hazard. Mater. 2022, 424, 127530. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z. Treatment of Raw Water by Ozone-Biological Activated Carbon Process. Water Purif. Technol. 2014, 44–47. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).