The COVID-19 Pandemic Impact of Hospital Wastewater on Aquatic Systems in Bucharest
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analysis
2.3. Statistically Analysis
3. Results and Discussion
3.1. Quantification of Hospital Wastewater Microbial Load
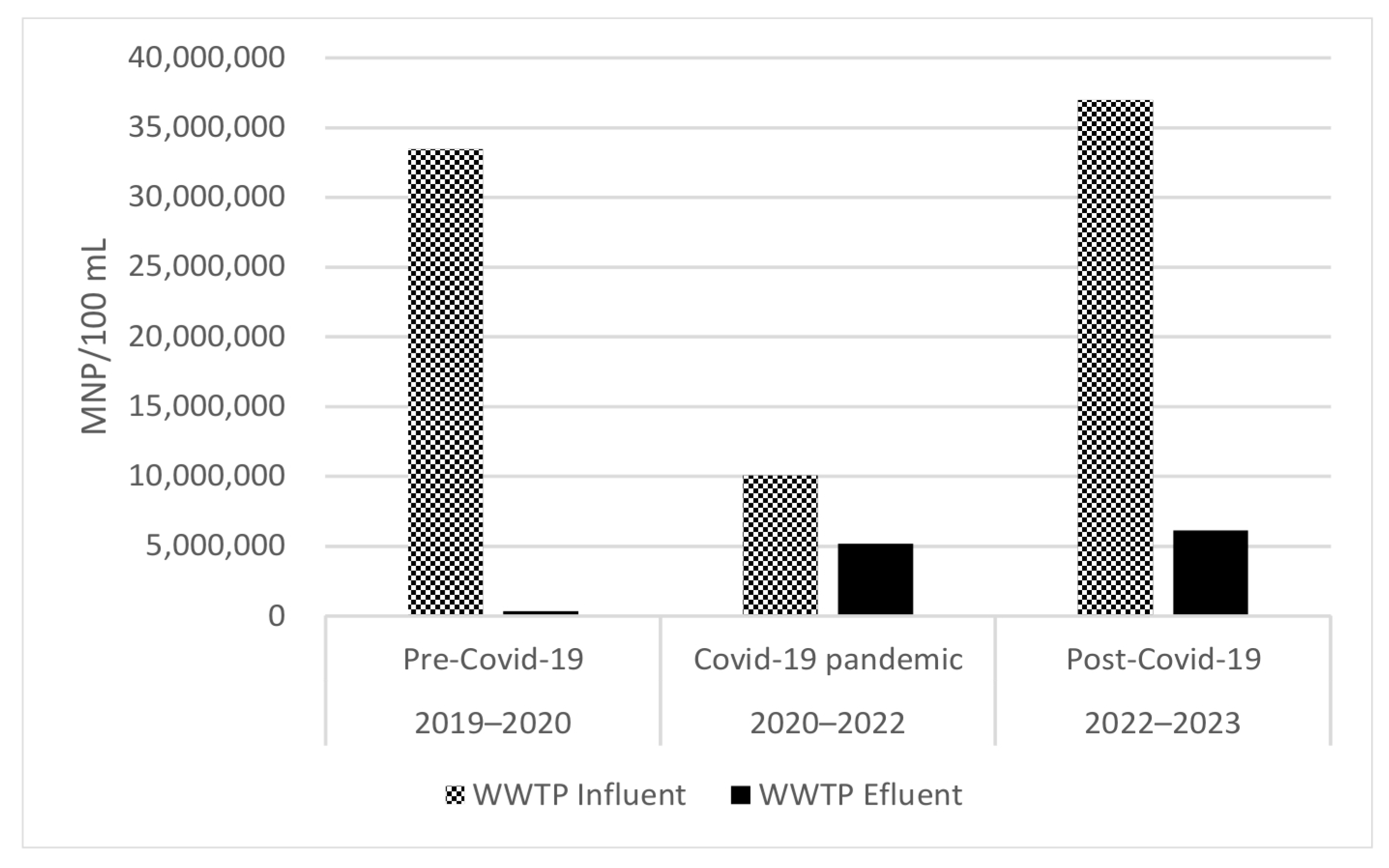
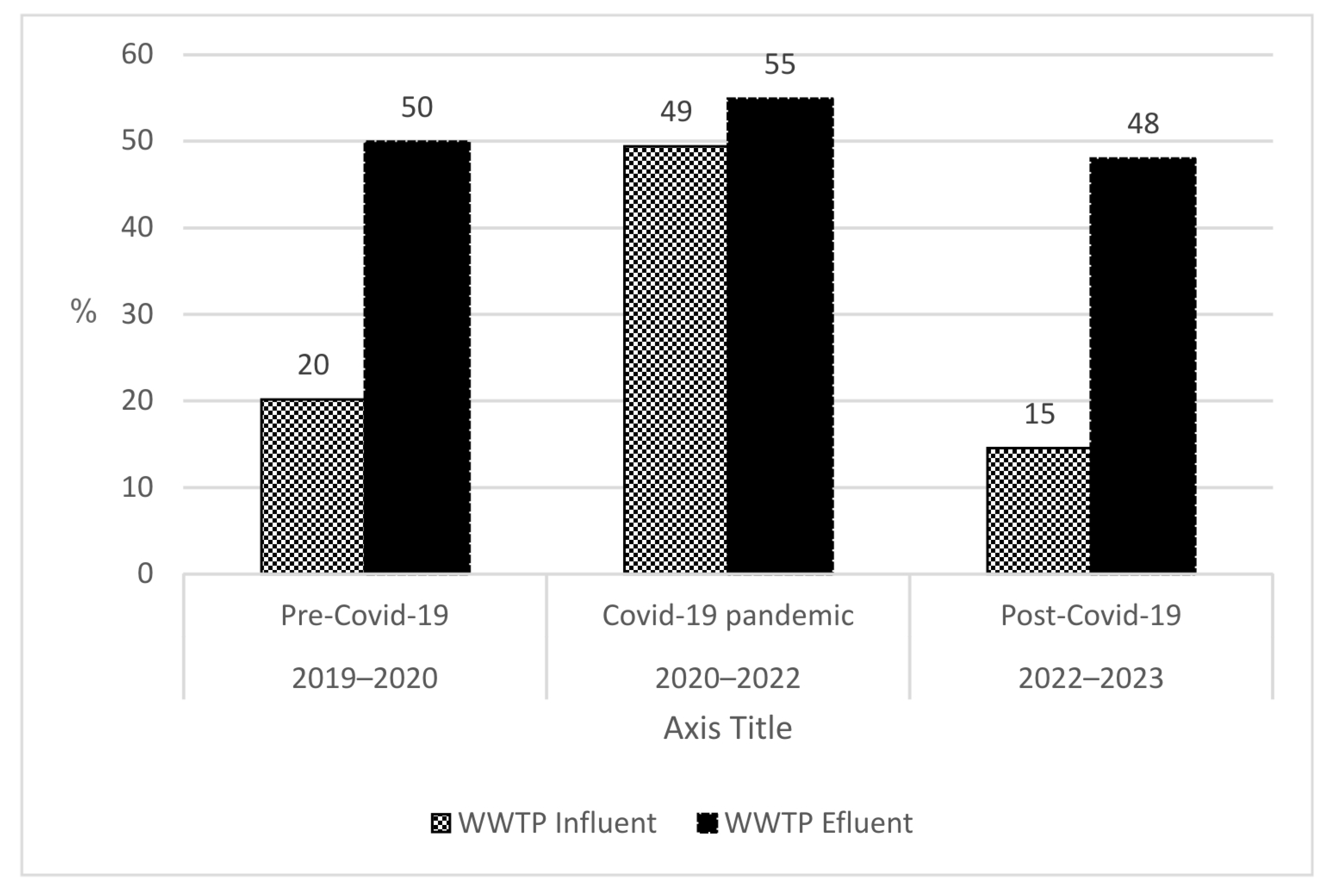
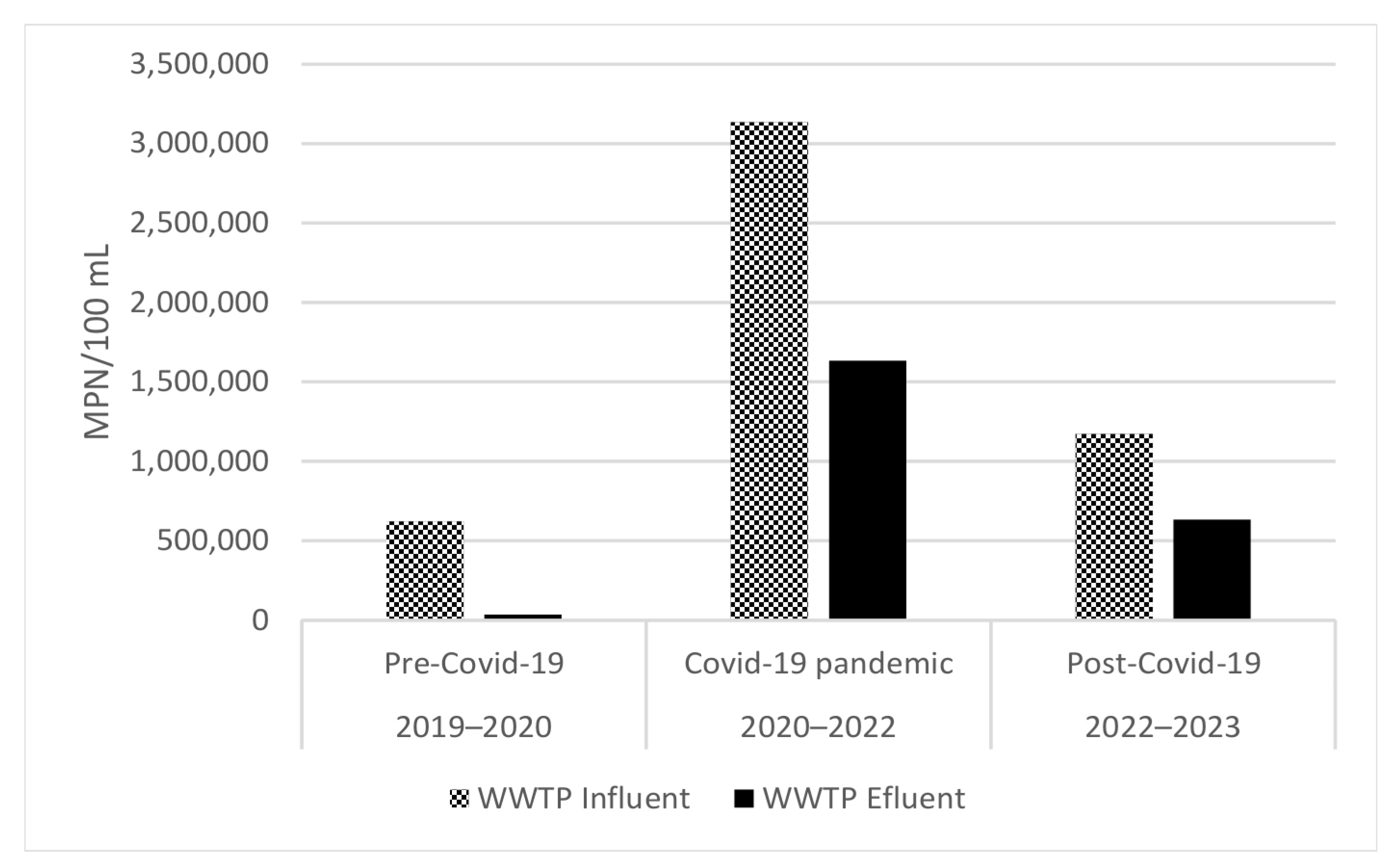
3.2. Microbial Load from WWTP
3.3. Quantification of Surface Water Microbial Load
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Armisen, T.; İnceoğlu, Ö.; Ouattara, N.K.; Anzil, A.; Verbanck, M.A.; Brion, N.; Servais, P. Seasonal variations and resilience of bacterial communities in a sewage polluted urban river. PLoS ONE 2014, 9, e92579. [Google Scholar] [CrossRef]
- Yazdian, H.; Jamshidi, S. Performance evaluation of wastewater treatment plants under the sewage variations imposed by COVID-19 spread prevention actions. J. Environ. Health Sci. Eng. 2021, 19, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Sidrach-Cardona, R.; Hijosa-Valsero, M.; Marti, E.; Balcazar, J.L.; Becares, E. Prevalence of antibiotic-resistant fecal bacteria in a river impacted by both an antibiotic production plant and urban treated discharges. Sci. Total Environ. 2014, 488–489, 220–227. [Google Scholar] [CrossRef]
- Ramírez-Coronel, A.A.; Mohammadi, M.J.; Majdi, H.S.; Zabibah, R.S.; Taherian, M.; Prasetio, D.B.; Gabr, G.A.; Asban, P.; Kiani, A.; Sarkohaki, S. Hospital wastewater treatment methods and its impact on human health and environments. Rev. Environ. Health 2023. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- European Court of Auditors. Available online: https://www.eca.europa.eu/lists/ecadocuments/sr19_21/sr_antimicrobial_resistance_ro (accessed on 20 September 2023).
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Haushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process. Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Kulik, K.; Lenart-Boron, A.; Wyrzykowska, K. Impact of antibiotic pollution on the bacterial population within surface water with special focus on mountain rivers. Water J. 2023, 15, 975. [Google Scholar] [CrossRef]
- Johar, A.A.; Salih, M.A.; Abdelrahman, H.A.; Al Mana, H.; Hadi, H.A.; Eltai, N.O. Wastewater-based epidemiology for tracking bacterial diversity and antibiotic resistance in COVID-19 isolation hospitals in Qatar. J. Hosp. Infect. 2023, 141, 209–220. [Google Scholar] [CrossRef]
- Available online: https://www.eca.europa.eu/lists/ecadocuments/sr19_21/sr_antimicrobial_resistance_ro.pdf (accessed on 20 September 2023).
- Liang, Z.; Ziquan, L.; Liangqiang, L.; Xiaowei, L.; Jian, X.; Suli, H.; Yuhua, C.; Yulin, F.; Changfeng, P.; Tingting, C.; et al. Impact of COVID-19 pandemic on profiles of antibiotic-resistant genes and bacteria in hospital wastewater. Environ. Pollut. 2023, 334, 122133. [Google Scholar]
- Mukherjee, P.; Pramanick, P.; Zaman, S.; Mitra, A. Eco-restoration of River Ganga water quality during COVID-19 lockdown period using Total Coliform (TC) as proxy. NUJS J. Regul. Stud. 2020, 75–82. [Google Scholar]
- ISO 19458; Water Quality–Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Zwitzerland, 2007.
- ISO 9308-2; Water Quality–Detection and Enumeration of Coliform Organisms, Thermotolerant Coliform Organisms and Presumptive Escherichia coli—Part 2: Multiple Tube (Most Probable Number) Method. International Organization for Standardization: Geneva, Zwitzerland, 2014.
- ISO 7899-2; Water Quality–Detection and Enumeration of Intestinal Enterococci Part 2: Membrane Filtration Method. International Organization for Standardization: Geneva, Zwitzerland, 2008.
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Setia, R. Effects of COVID-19 pandemic lockdown on microbial and metals contaminations in a part of Thirumanimuthar River, South India: A comparative health hazard perspective. J. Hazard. Mater. 2021, 416, 3–11. [Google Scholar] [CrossRef]
- Manoiu, V.M.; Kubiak-Wojcicka, K.; Craciun, A.I.; Akman, C.; Akman, E. Water quality and water pollution in time of COVID-19: Positive and negative repercussions. Water 2022, 14, 1124. [Google Scholar] [CrossRef]
- Popa, L.I.; Gheorghe, I.; Czobor Barbu, I.; Surleac, M.; Paraschiv, S.; Marutescu, L.G.; Popa, M.; Gradisteanu Parcalabioru, G.; Talapan, D.; Nita, M.; et al. Multidrug resistant Klebsiella pneumoniae ST101 clone survival chain from inpatients to hospital effluent after chlorine treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef] [PubMed]
- Surleac, M.; Czobor Barbu, I.; Paraschiv, S.; Popa, L.I.; Gheorghe, I.; Marutescu, L.; Popa, M.; Sarbu, I.; Talapan, D.; Nita, M.; et al. Whole genome sequencing snapshot of multi-drug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS ONE 2020, 15, e0228079. [Google Scholar] [CrossRef] [PubMed]
- Hadi Jaber, A.S.; Rohani, I.; Al Hamid, A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11931. [Google Scholar]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Van Dongen, M.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containent. Future Sci. OA 2023, 7, FSO736. [Google Scholar] [CrossRef]
- Toc, D.A.; Mihaila, R.M.; Botan, A.; Bobohalma, C.N.; Risteiu, G.A.; Simut-Cacuci, B.N.; Steorobelea, B.; Troanca, S.; Junie, L.M. Enterococcus and COVID-19: The emergence of a perfect storm. Int. J. Transl. Med. 2022, 2, 220–229. [Google Scholar] [CrossRef]
- Ene, A.; Vasile, M.A.; Bahrim, G. Study of microbiological contamination level of surface water in MONITOX network areas before and after COVID-19 pandemic, Environmental Challenges in the Blak Sea basin: Impact on human health. Ann. “Dunarea De. Jos” Univ. Galati 2020, 43, 75–81. [Google Scholar]
- Barbu, I.C.; Barbu-Gheorghe, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial resistance in Romania: Updates on Gram-Negative ESCAPE pathogens in the clinical, veterinary, and aqautic sectors. Int. J. Mol. Sci. 2023, 24, 7892. [Google Scholar] [CrossRef]
- Berglund, F.; Molina-Rodriguez, D.; Gradisteanu-Pircalaboiu, G.; Blaak, H.; Chifiriuc, M.C.; Czobor-Barbu, I.; Flach, C.F.; Gheorghe-Barbu, I.; Marutescu, L.; Popa, M.; et al. The resistome and microbiome of wastewater treatment plant workers—The AWARE study. Environ. Int. 2023, 180, 108242. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G.; Popa, M.; Gheorghe-Barbu, I.; Czobor-Barbu, I.; Rodriguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.F.; Kemper, M.A.; Spieberger, B.; et al. Wastewater treatment plants, an “escape gate” for ESCAPE pathogens. Front. Microbiol. 2023, 14, 1193907. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-molina, D.; Mang, P.; Schmitt, H.; Chifiriuc, M.C.; Radon, K.; Wengenroth, L. Do wastewater treatment plants increase antibiotic resistant bacteria or genes in the environment? Protocol for a systematic review. Syst. Rev. 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Alistar, C.F.; Nica, I.C.; Nita-Lazar, M.; Vasile, G.G.; Gheorghe, S.; Croitoru, A.M.; Dolete, G.; Mihaescu, D.E.; Ficai, A.; Craciun, N.; et al. Antioxidative defense and gut microbial changes under pollution stress in Carassius gibelio from Bucharest lakes. Int. J. Environ. Res. Public Health 2022, 19, 7510. [Google Scholar] [CrossRef]
- Kroll, J.H.; Heald, C.L.; Cappa, C.D.; Farmer, D.F.; Fry, J.L.; Murphy, J.G.; Steiner, A. The complex chemical effects of COVID-19 shutdowns on air quality. Nat. Chem. 2020, 12, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, C.; Yu, W.; Han, J. Tracing COVID-19 drugs in the environment: Are we focusing on the right environmental compartment? Environ. Pollut. 2023, 339, 2–18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banciu, A.R.; Pascu, L.F.; Radulescu, D.M.; Stoica, C.; Gheorghe, S.; Lucaciu, I.; Ciobotaru, F.V.; Novac, L.; Manea, C.; Nita-Lazar, M. The COVID-19 Pandemic Impact of Hospital Wastewater on Aquatic Systems in Bucharest. Water 2024, 16, 245. https://doi.org/10.3390/w16020245
Banciu AR, Pascu LF, Radulescu DM, Stoica C, Gheorghe S, Lucaciu I, Ciobotaru FV, Novac L, Manea C, Nita-Lazar M. The COVID-19 Pandemic Impact of Hospital Wastewater on Aquatic Systems in Bucharest. Water. 2024; 16(2):245. https://doi.org/10.3390/w16020245
Chicago/Turabian StyleBanciu, Alina Roxana, Luoana Florentina Pascu, Dragos Mihai Radulescu, Catalina Stoica, Stefania Gheorghe, Irina Lucaciu, Florin Valentin Ciobotaru, Laura Novac, Catalin Manea, and Mihai Nita-Lazar. 2024. "The COVID-19 Pandemic Impact of Hospital Wastewater on Aquatic Systems in Bucharest" Water 16, no. 2: 245. https://doi.org/10.3390/w16020245
APA StyleBanciu, A. R., Pascu, L. F., Radulescu, D. M., Stoica, C., Gheorghe, S., Lucaciu, I., Ciobotaru, F. V., Novac, L., Manea, C., & Nita-Lazar, M. (2024). The COVID-19 Pandemic Impact of Hospital Wastewater on Aquatic Systems in Bucharest. Water, 16(2), 245. https://doi.org/10.3390/w16020245







