Abstract
Hexachlorobenzene (HCB) is one of the most persistent environmental pollutants of global concern. Ni/Fe nanoparticles, with their small particle size, large surface area, and high reactivity, are a promising candidate for HCB degradation. In this work, we investigated the kinetics and products of the dechlorination of HCB by Ni/Fe nanoparticles and how the presence of heavy metal ions Cd(Ⅱ) and Zn(Ⅱ) influences the reaction. It is found that 400 μg/L HCB can be rapidly removed by 7.5 g/L Ni/Fe nanoparticles and the removal percentage reaches 99% in 48 h. The removal is facilitated by adsorption and sequential dechlorination of HCB, producing PCB, 1,2,3,4-TeCB, and 1,2,3-TCB as the main products, with 1,2,3,5/1,2,4,5-TeCB, 1,2,4-TCB, and 1,2-DCB as the minor products. The addition of heavy metal ions Cd(Ⅱ) and Zn(Ⅱ) does not significantly affect the removal rate of HCB but hinders the adsorption and degradation of the byproducts through competitive adsorption. Additionally, the concentration of both Cd(Ⅱ) and Zn(Ⅱ) decreases rapidly and achieves over 98% removal in 4 h. Our study reveals that Ni/Fe nanoparticles can remove HCB and heavy metals Cd(Ⅱ) and Zn(Ⅱ) concurrently, with the extent of HCB dechlorination reduced compared to that without heavy metal. These findings may inform the application of Ni/Fe nanoparticles in the treatment of water bodies and soil contaminated by both halogenated aromatics and heavy metal.
1. Introduction
Hexachlorobenzene (HCB), a typical chlorinated aromatic compound and an important chemical intermediate, had been widely used in fungicides, military pyrotechnic smokes, and synthetic rubber peptizing agents in the past [1,2]. It is a persistent, bioaccumulative, and toxic compound that may damage human organs and cause a lot of diseases including dermatitis, severe numbness in the cardiovascular and nervous systems, and carcinogenesis [3,4,5,6]. Although most countries have banned the production and usage of HCB since the beginning of the 21st century, HCB is still prevalent in the environment due to its extensive historical use, ongoing unintentional emission, and long half-life. The current global burden of HCB on the environment was estimated to be 10,000–26,000 tons [4]. It has been detected in most parts of the ecosystem, including water, soil, air, animals, plants, and even anthropologically in human milk [7]. Thus, appropriate remediation technologies are in urgent need for HCB-contaminated water and soil.
In recent years, zero-valent Fe nanoparticles have drawn much attention as a promising reagent for the chemical degradation of chlorinated pollutants, because of their ease of synthesis, large surface area, and effectiveness in the treatment of a variety of organic pollutants and heavy metals [8]. Nevertheless, several drawbacks of the material have significantly limited its application. Firstly, Fe nanoparticles oxidize easily upon contact with air or in aqueous solution, forming a layer of iron oxide or hydroxide on the surface of the nanoparticles, significantly reducing their reactivity. Additionally, the reduction of 1,2,4,5-tetrachlorobenzene by Fe nanoparticles was shown to be slow and incomplete, with a half-life of 36 h and detection of only a small subset of the theoretical byproducts [9]. To counter these problems, an effective method is to deposit a second, less reactive metal on the surface of iron to form bimetallic nanoparticles. The second metal acts as a catalyst to enhance the electron-donating ability of Fe as well as protects the surface of the nanoparticles from being oxidized. Many metals have been shown to effectively catalyze dechlorination reactions when coupled with iron, such as Pt, Cu, Pd, and Ag [10,11,12,13,14]. However, only Pd/Fe and Cu/Fe nanoparticles have been applied to the treatment of HCB [10,14]. Nickel is the ninth most produced metal in the world and a typical hydrogenolysis catalyst with the ability to catalyze the hydrodechlorination of a variety of chloroaromatics such as 2,4-dichlorophenol, triclosan, and Aroclor 1242 [15,16,17,18]. Therefore, Ni/Fe nanoparticles hold great promise to serve as a commercially viable and effective reagent for HCB dechlorination.
Contaminated water bodies and soil usually contain a mixture of organic and metallic pollutants [19]. Examples are farmlands in post-mining areas and leachate from landfills, where the main heavy metal pollutants identified are Cd, Ni, Cu, As, Hg, Pb, and Zn [20]. Thus, it is necessary to consider the influence of co-existing heavy metal ions when developing remediation technologies for HCB. Most optimally, the heavy metal ions may be removed concurrently with the organic pollutants. Although there are a few studies on the effects of various ions on the degradation of chlorinated compounds by metallic nanoparticles, the influence of most heavy metal ions on the dechlorination of HCB is not well understood [16,21].
In this study, we synthesize Ni/Fe nanoparticles via chemical deposition and determine their surface characteristics with BET, TEM, and SEM. We then investigate the reaction kinetics and mechanism of HCB dechlorination by Ni/Fe nanoparticles and study the influence of heavy metals Cd(Ⅱ) and Zn(Ⅱ) on the removal and degradation of HCB. The change in heavy metal concentration is also monitored to determine whether they can be simultaneously removed by the nanoparticles.
2. Materials and Experiments
2.1. Chemicals
The standard materials of chlorinated benzenes were purchased from CNW Technologies GmbH (Dusseldorf, Germany). Deoxygenated–deionized water was used for solution preparation. Other reagents were from commercial companies of China, including FeCl3, NaBH4, Cd (NO3)2, ZnSO4, NaOH (analytical pure), thick HCl (superior pure), hexane (chromatographically pure), and anhydrous ethanol (chromatographically pure). All reagents were above analytical grade.
2.2. Equipment
The instruments used in this work include the following: (1) H-8100 transmission electron microscope (Hitachi, Tokyo, Japan), (2) Quanta FEG 450 thermal field emission environmental scanning electron microscope (FEI, Hillsboro, OR, USA), (3) Autosorb-1-type ratio surface and porosity analyzer (Quantachrome Instruments, Boynton Beach, FL, USA), (4) SHA-B-type water bath oscillator (Ronghua Instrument, Jintan, Jiangsu, China), (5) DHG-9070A-type drying box (Shanghai Yiheng Technology Co., Ltd., Shanghai, China), (6) C-MAG HS4S25 magnetic stirrer (IKA, Staufen, Germany) and (7) AP-9908S vacuum pump (Tianjin Altsnes Instrument Co., Ltd., Tianjin, China).
Gas chromatography was conducted on a GC 2010 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a DB-5 capillary column from Agilent Technologies, Santa Clara, CA, USA (30 m × 0.25 mm × 0.25 μm film thickness, (5%-phenyl)-methylpolysiloxane non-polar column). High-purity helium (purity 99.999%) was used in the analysis with a flow rate of 1 mL/min. The injector temperatures were set at 250 °C. The GC oven temperature was programmed as follows: the temperature was held at 60 °C for 1 min, then increased at 5 °C/min to 200 °C, which was held for 3 min; it was then increased at 30 °C/min to 250 °C and held for 2 min. The detector temperature was 300 °C.
2.3. Experiment Design
2.3.1. Synthesis and Characterization of Ni/Fe Nanoparticles
All glassware was soaked with 1:1 HNO3 for 48 h, rinsed with tap water and deionized water, and air-dried. In a nitrogen flow, 1.6 mol/L NaBH4 aqueous solution was slowly added into an equal volume of 1.0 mol/L FeCl3·6H2O (containing V/V = 30% anhydrous ethanol) solution. After all NaBH4 was added, the reaction was stirred for 5 min to afford iron nanoparticles via the following reaction:
4Fe3+ + 3BH4− + 9H2O → 4Fe↓ + 3H2BO3− + 12H+ + 6H2
The generated black solid particles were transferred to a sand core funnel with a built-in 0.2 µm filter membrane and were filtered and washed with deoxygenated deionized water and then with anhydrous ethanol 2-to-3 times. The black particles were then quickly transferred to the reaction bottle and dried at 50 °C overnight. After cooling, the bottle was sealed carefully to protect the nano-Fe particles from exposure to air.
Nanoscale Ni/Fe particles were prepared by the deposition of NiCl2 ethanol solution onto nanoscale Fe particles. As a typical procedure, 0.2 g of nanoscale Fe was added to 5 mL of 32 mM NiCl2 in a 12 mL vial and the solution was stirred for several minutes. As Ni(Ⅱ) was reduced, a thin layer of Ni was deposited on the surface of Fe, which gave nano-Ni/Fe bimetallic particles. After being washed for several times with deoxygenated deionized water to neutrality, the freshly prepared nanoparticles were immediately used in the degradation experiments.
The synthesized Fe and Ni/Fe nanoparticles were characterized by the BET method to find their specific surface areas. The experiment was performed by the Analytics and Chemistry Laboratory of China University of Geosciences (Beijing, China). The particles were also imaged by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) to determine their nanostructures.
2.3.2. Degradation Experiments
To investigate the nano-Ni/Fe degradation of HCB in a heavy-metal-free condition and in the presence of single or double heavy metals, we set up four systems of simulated polluted water: a heavy-metal-free system (HCB), a system with added Cd(Ⅱ) (HCB + Cd), a system with added Zn(Ⅱ) (HCB + Zn), and that with both Cd(Ⅱ) and Zn(Ⅱ) (HCB + Cd + Zn). In each system, the initial concentration of HCB was 400 μg/L with 0.1% acetone. For the heavy-metal-containing samples, analytical pure Cd (NO3)2/ZnSO4 was added during preparation to obtain Cd2+/Zn2+ concentrations of 5 mg/L.
The experiments were conducted in 12 mL reaction bottles. To a 5 mL sample solution, an excess of nano-Ni/Fe particles (7.5 g/L) was added. The solution pH was not adjusted. The oxygen in the headspace was immediately purged with N2 for seconds. The reaction was conducted in a shaking table at 120 rpm in the dark (25 ± 2 °C). Each run was performed in duplicate. At selected time intervals, an aliquot of the liquid phase was drawn from the reaction vial and the organic products were extracted with hexane. The extract was analyzed by gas chromatography and the experimental results took the average of three parallel samples.
3. Results and Discussion
3.1. Micro-Appearance Analysis
Figure 1 shows the TEM images of Fe nanoparticles prepared in water, v/v 30% ethanol, and v/v 50% ethanol. The particles formed a characteristic chain-like structure, a typical behavior due to magnetic attraction between the metal particles. The diameter of the particles was in the range of 20–100 nm. It was found that the addition of ethanol changed the size and morphology of the nanoparticles under otherwise identical experimental conditions. As shown in the TEM images, the particle size tends to be larger and more severely clustered without ethanol. The addition of ethanol caused the particles to be more dispersed, but there was no significant difference upon increasing the ethanol concentration from 30% to 50%. Thus, to maximize the adsorption capacity of the nanoparticles while saving cost, 30% ethanol was used as the solvent for the FeCl3·6H2O solution during the synthesis of the nanoparticles.
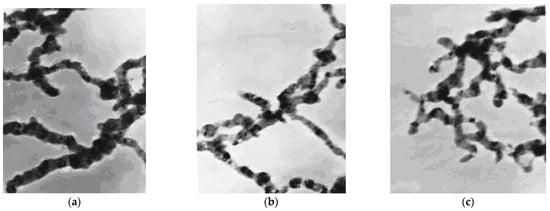
Figure 1.
Transmission electron microscopy (TEM) images of nano-Fe particles (a) in water, (b) in 30% ethanol, (c) in 50% ethanol (Magnification: 80,000×).
SEM images of Fe and Ni/Fe nanoparticles (Figure 2) showed that they had similar diameters, in the range of 20–60 nm, which was in line with the results obtained by Zhang et al. (20–100 nm) and by Naser et al. (40–50 nm) [15,22]. The nanoparticles form chain-like assemblies, where the Fe nanoparticles appeared as smooth “fiber”, while the Ni/Fe nanoparticles (2%) appeared slightly more “granular”. The formation of the chain-like structure was thought to be driven by magnetic attraction between the nanoparticles due to the ferromagnetic properties of Ni and Fe metal [23]. The surface area of the synthesized Fe and Ni/Fe nanoparticles were characterized by the BET method and was found to be 25.41 m2/g for the Fe nanoparticles and 27.70 m2/g for the Ni/Fe nanoparticles. The similarity in surface area indicated that they may have a similar adsorption capacity. It is worth noting that the size and morphology of the nanoparticles are largely dependent on the synthesis conditions, such as the solvent used, the type and concentration of the ferric salt and the reductant, etc., which in turn affected the reactivity of the obtained nanoparticles [23,24,25].
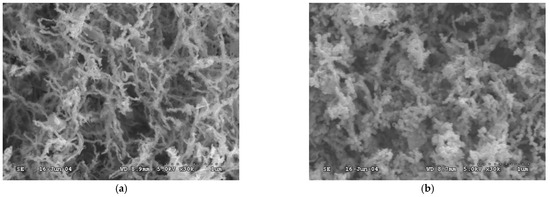
Figure 2.
Scanning electron microscope (SEM) images of (a) Fe nanoparticles and (b) Ni/Fe nanoparticles (2%).
3.2. Dechlorination of HCB by Nanoscale Ni/Fe
Figure 3 shows the degradation of HCB to chlorobenzenes in water over time. The concentration of HCB dropped rapidly in the first 4 h (removal percentage: 79%) and then gradually decreased, reaching 99% removal in 48 h. Judging from the mass balance, the initial drop in concentration was due to adsorption onto the nanoparticles, followed by a period of slower removal dominated by dechlorination [12]. The main products of HCB dechlorination were pentachlorobenzene (PCB), tetrachlorobenzenes (TeCBs), and trichlorobenzenes (TCBs), with a trace amount of 1,2-dichlorobenzene (DCB). Among the three isomers of TeCB, 1,2,3,4-TeCB was the main product, and 1,2,3,5- and 1,2,4,5-TeCB were only produced in small amounts. For TCB, 1,2,3-TCB was the main product, with very little 1,2,4-TCB detected in 72 h of reaction. The formation of the n-chlorobenzene series suggests a mechanism of sequential dechlorination where HCB was degraded from n-Cl- to (n-1)-Cl-benzenes. In the first 4 h, PCB was the main product, whose concentration first increased and then decreased, peaking at 12 h. TeCBs exhibited a similar trend of variation, but peaked at 24 h, before which their concentration was lower than that of PCB, indicating that they were formed further down the reaction series.
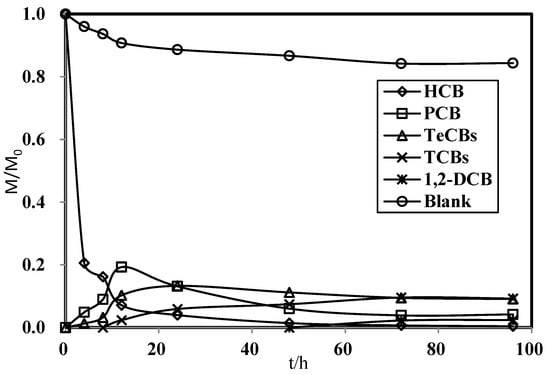
Figure 3.
Action time of HCB dechlorination by Ni/Fe.
The reductive dechlorination of chlorinated organic compound by Ni/Fe nanoparticles proceeds through catalytic hydrogenation, where Fe acts as the reductant and loses electrons to become Fe2+ [26]. H+ in water gains electrons and becomes activated [H], which is adsorbed onto the catalytic Ni surface. Meanwhile, the C-Cl bond in the chlorinated hydrocarbon (RCl) adsorbed on the surface of the nanoparticles breaks, with the Cl atom being replaced by an H atom, converting RCl into RH and Cl−. Based on byproduct analysis, a reaction pathway is proposed for the degradation of HCB by Ni/Fe nanoparticles (Figure 4). HCB loses one chlorine atom to form PCB, which undergoes further dechlorination to form 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-TeCB, as well as 1,2,3-TCB and 1,2,4-TCB. The final product was 1,2-DCB. The highly selective ortho-hydrogenation is thought to be primarily due to the steric hindrance of the chlorine atoms [9].
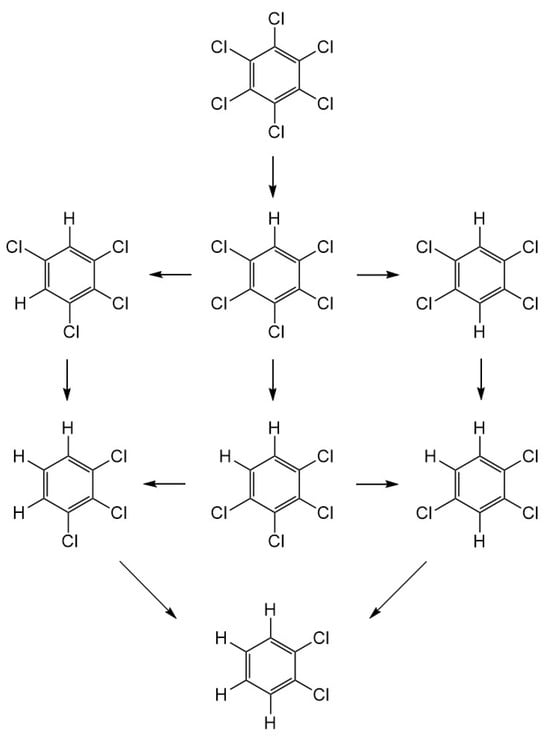
Figure 4.
Pathway of degradation of hexachlorobenzene by Ni/Fe nanoparticles.
Several other studies have investigated the rate and byproducts of HCB removal by Fe-based bimetallic nanoparticles. Zhu et al. showed that 4 g/L Cu/Fe nanoparticles in 200 μg/L HCB solution with pH = 4 buffer facilitated a 85% removal percentage in 12 h [14]. Shih et al. demonstrated that with 2 g Pd/Fe nanoparticles in 15 mL HCB solution (the initial concentration was not provided), the removal percentage of HCB reached 70% in 24 h and then plateaued [10]. In our study, 93% removal of HCB was achieved in 12 h with 7.5 g/L Ni/Fe nanoparticles in 400 μg/L HCB solution, which was faster than the rates reported in both studies. In terms of product distribution, the main products obtained by Zhu et al. and in the present study after 96 h of reaction were TeCB and TCB, whereas Shih et al. reported that DCB was the main product after 100 h of reaction, suggesting that more complete degradation was achieved with Pd/Fe nanoparticles. The differences may be attributed to different initial concentrations of reactants, reaction conditions, and catalytic abilities of the coating metals.
Theoretical calculations suggested that the activation energies of removing chlorine atoms become greater as the number of Cl substituents decreases [27]. Therefore, the degradation of DCB to mono-substituted CB and benzene may be difficult in the current reaction conditions. Nevertheless, the less-substituted chlorobenzenes exhibit lower toxicity and are more easily removed by microorganisms, so chemical degradation by nanoparticles may alleviate the toxic stress on the ecosystem and assist the self-repair of the environment.
3.3. Dechlorination of HCB in the Presence of Heavy Metals
3.3.1. HCB Dechlorination by Ni/Fe Nanoparticles in the Presence of a Single Heavy Metal
Figure 5 illustrates the concentrations of HCB and its degradation products at different time intervals for three reaction systems: (1) with no heavy metal, (2) with Cd(Ⅱ), and (3) with Zn(Ⅱ). As is apparent in Figure 5a, HCB was removed rapidly by Ni/Fe nanoparticles in all reaction systems, and the addition of heavy metal did not significantly affect the removal rate. Figure 5b shows that the concentration of PCB first increased then decreased in all reaction systems, peaking at 12 h. The addition of Cd(Ⅱ) elevated the PCB concentration throughout the experiment, which may be attributed to competitive adsorption between PCB and Cd(Ⅱ) [15]. On the contrary, the addition of Zn(Ⅱ) showed almost no effect on the concentration of PCB.
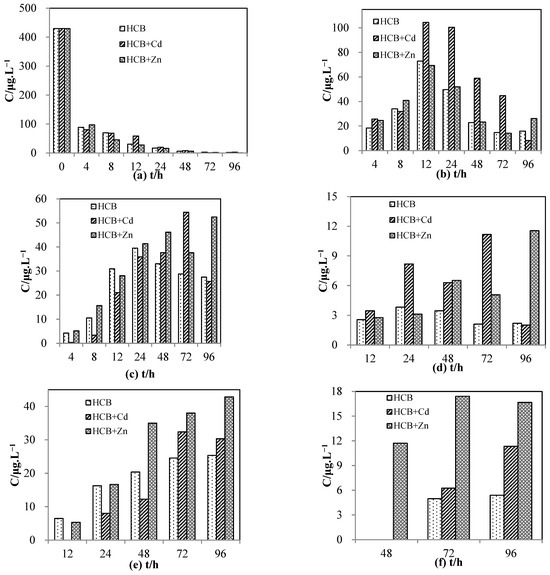
Figure 5.
Change in the concentrations of hexachlorobenzene and its degradation products over time. (a) hexachlorobenzene, (b) pentachlorobenzene, (c) 1,2,3,4-tetrachlorobenzene, (d) 1,2,3,5/1,2,4,5-tetrachlorobenzene, (e) 1,2,3-trichlorobenzene, (f) 1,2-dichlorobenzene.
Figure 5c shows that the concentration of 1,2,3,4-TeCB first increased and then decreased, peaking at 24 h for the system with HCB only and 72 h and 48 h for the systems with added Cd(Ⅱ) and Zn(Ⅱ), respectively. The delayed peaks indicated that the addition of heavy metal slowed down the degradation. Furthermore, the concentration of 1,2,3,4-TeCB was lower in the beginning of the reaction in the system with Cd(Ⅱ), but rose to a high level as the reaction proceeded. It reached a maximum of 54.42 μg/L in the system with Cd(Ⅱ) and 52.46 μg/L in the system with Zn(Ⅱ), which were higher than the maximum of the heavy-metal-free system (35.89 μg/L). This phenomenon suggested that with added heavy metals, the generation of TeCB was slowed down at the beginning of the reaction. As the reaction went on, the removal of TeCB via adsorption and dechlorination became the dominant process, which was also slowed down by the addition of heavy metal, leading to an increased concentration. 1,2,3,5/1,2,4,5-TeCB was produced in a small amount and its variation was more irregular (Figure 5d). However, the delay of the maximum and the increased concentration in the later part of the reaction can still be observed.
Figure 5e shows that the concentration of 1,2,3-TCB was initially lower with added Cd(Ⅱ) and Zn(Ⅱ). It then increased, surpassing that without added heavy metal and staying at a high level. The concentration plateaued at 72 h for the system with added Cd(Ⅱ), whereas it kept increasing until the end of the experiment with added Zn(Ⅱ). The observation that the maximum of TCB was delayed or even unobtainable in the time frame of the reaction indicated that its removal was inhibited by the addition of heavy metals.
Figure 5f shows that 1,2-DCB was produced at a low level, with only a trace amount detected at 48 h for the system with added Zn(Ⅱ) and 72 h for the other two systems. The concentration of DCB increased with added Cd(Ⅱ) and Zn(Ⅱ), suggesting that the heavy metals affected its adsorption efficiency.
The changing trends of the byproducts agree with previously published results on the catalytic dechlorination of 2,4-dichlorophenol over Ni/Fe nanoparticles, where addition of an inhibitory substance (humic acid) delayed the peak of the byproduct o-chlorophenol and lowered the initial concentration of phenol, the final product [15]. On the other hand, the lack of change in the removal rate of HCB may be explained by its non-polarity. It is hypothesized that HCB may occupy different active sites on the surface of the nanoparticles from charged ions and polar organic molecules, therefore experiencing less adsorptive competition. This is supported by previous studies where the presence of Na+ and Mg2+ was shown to have no impact on HCB’s degradation rate [21].
3.3.2. HCB Dechlorination by Ni/Fe Nanoparticles in the Presence of Multiple Heavy Metals
Figure 6 shows the concentration of HCB and its degradation products in a heavy-metal-free system and in a system with both Cd(Ⅱ) and Zn(Ⅱ). As shown in Figure 6a, the concentration of HCB was lower with Cd(Ⅱ) + Zn(Ⅱ), and the removal percentage in 4 h increased from 79% to 88%. It seems that the co-existence of Cd(Ⅱ) and Zn(Ⅱ) did not affect the removal rate of HCB but promoted it slightly.
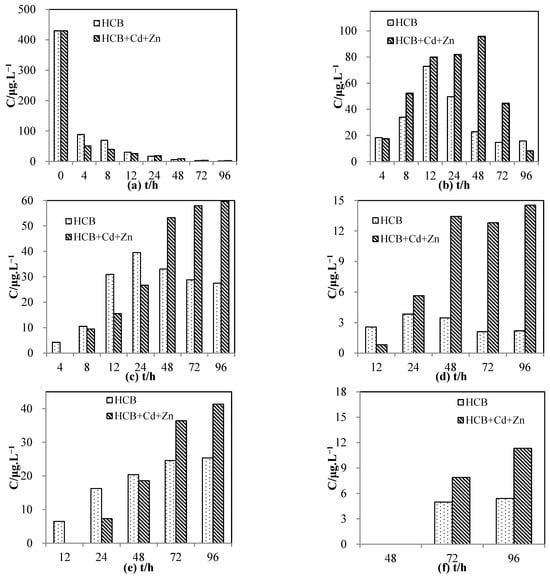
Figure 6.
Change in the concentrations of hexachlorobenzene and its products over the reaction time. (a) hexachlorobenzene, (b) pentachlorobenzene, (c) 1,2,3,4-tetrachlorobenene, (d) 1,2,3,5/1,2,4,5-tetrachlorobenzene, (e) 1,2,3-trichlorobenzene, (f) 1,2-dichlorobenzene.
Figure 6b shows that the concentration of PCB peaked at 48 h with Cd(Ⅱ) + Zn(Ⅱ), and stayed at a high level from 12 h to 48 h. The delayed peak and plateauing were not observed in the systems with a single heavy metal, suggesting that the reaction was further slowed down by the addition of both heavy metals. The variation in 1,2,3,4-TeCB in the system with Cd(Ⅱ) + Zn(Ⅱ) resembled that with a single heavy metal, except that the concentration kept increasing until the end of the experiment instead of peaking at a certain point like in the system with Cd(Ⅱ) (Figure 6c). This implies that TeCB’s degradation was further inhibited by the addition of both heavy metals and therefore could not reach a maximum in the time frame of the experiment. Moreover, as can be seen from Figure 6c,d, the maximum concentration of 1,2,3,4-TeCB and 1,2,4,5/1,2,3,5-TeCB was higher than that of the systems with a single heavy metal, suggesting that adsorptive competition was more pronounced in the presence of both heavy metals.
The concentration of 1,2,3-TCB with the addition of both heavy metals was the lowest among all four systems in the beginning. It then kept rising and became the second highest, only slightly lower than the system with Zn(Ⅱ) (Figure 6e). A very low amount of 1,2,4-TCB was also detected during the experiment. For 1,2-DCB, a small amount was produced and its variation with the addition of two heavy metals was similar to that with Cd(Ⅱ) (Figure 6f).
Overall, the addition of two heavy metals caused notable changes in the variation in the chlorobenzenes. Specifically, the removal of HCB was slightly accelerated, while the adsorption and dechlorination reactions involving PCB, TeCB, and TCB were further hindered compared to the systems with a single heavy metal. The variation in DCB did not differ much from that of the system with Cd(Ⅱ). The observed phenomena show that the effects of multiple influencing ions are not merely additive but complex, varying for different organic molecules. One of the possible causes is that different adsorbates have distinct preferences for binding sites. Additionally, the adsorbed species are known to alter the surface characteristics of the nanoparticles and modify their adsorption capacity.
3.3.3. Change in Heavy Metal Concentration
Figure 7 shows the normalized concentration of Cd(Ⅱ) and Zn(Ⅱ) in the reaction system containing a single type of heavy metal and the reaction system with both heavy metals.
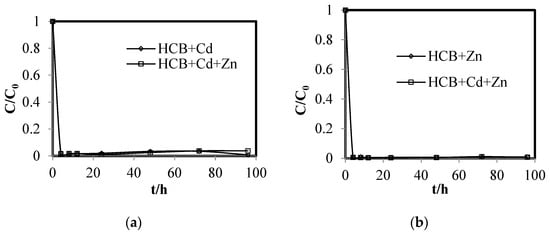
Figure 7.
Normalized concentrations of (a) Cd(Ⅱ) and (b) Zn(Ⅱ) in the reaction systems.
In all cases, the concentration of the heavy metals decreased rapidly, reaching a removal percentage of over 98% in 4 h, and remained at a low level. The removal of Zn(Ⅱ) was slightly better than that of Cd(Ⅱ). Since the standard reduction potentials of Zn(Ⅱ) and Cd(Ⅱ) are more negative or very close to that of iron, the main removal mechanism is believed to be adsorption or surface complex formation instead of metal displacement [28]. The finding that the heavy metals can be effectively removed by Ni/Fe nanoparticles suggests that competition between the heavy metals and CBs for adsorption sites may be the main cause for the reduced rate of dechlorination.
Our results demonstrate that HCB can be quickly removed by Ni/Fe nanoparticles via adsorption and sequential dechlorination, producing PCB, 1,2,3,4-TeCB, and 1,2,3-TCB as the main products, with 1,2,3,5/1,2,4,5-TeCB, 1,2,4-TCB, and 1,2-DCB detected in trace amounts. The effect of heavy metal ions on the reaction rate and product composition was examined by analyzing the variation in concentration for all products, where a low initial concentration, increased maximum, and the delay or absence of peaks were identified as signals of inhibited adsorption and degradation. It can be seen from the experiment results that the addition of heavy metals has little effect on HCB’s removal but affects the adsorption and degradation of the byproducts. The presence of both Cd(Ⅱ) and Zn(Ⅱ) further reduces the extent of the sequential dechlorination process. On the other hand, the removal of heavy metal ions was not affected, probably because the amount of HCB added was low and thus had no noticeable effect on the adsorption of metal ions to the nanoparticles. Future investigation was required to clarify how the change in reaction conditions, such as starting HCB and heavy metal concentration, pH, temperature, etc., affects the reaction.
4. Conclusions
Our study has investigated the kinetics and products of HCB dechlorination by Ni/Fe nanoparticles, and the influence of added heavy metal ions Cd(Ⅱ) and Zn(Ⅱ) on the reaction. It is found that HCB can be rapidly removed by Ni/Fe nanoparticles via adsorption and sequential dechlorination. The main products are pentachlorobenzene, 1,2,3,4-tetrachlorobenzene, and 1,2,3-trichlorobenzene, with 1,2,3,5/1,2,4,5-tetrachlorobenzene, 1,2,4-trichlorobenzene, and 1,2-dichlorobenzene as the minor products. The addition of Cd(Ⅱ) and Zn(Ⅱ) has minimal effect on the removal rate of HCB, but inhibits the adsorption and degradation of the products, as can be seen in the changes in their concentration–time profiles. The inhibitory effect may be attributed to competitive adsorption between the heavy metals and the chlorobenzenes. The presence of both heavy metals generally caused greater inhibition than a single type of heavy metal. On the other hand, the heavy metals were effectively removed by Ni/Fe nanoparticles in all reaction systems, with the removal of Zn(Ⅱ) slightly better than that of Cd(Ⅱ). The results show that Ni/Fe nanoparticles can remove HCB and heavy metal ions concurrently, with the degradation of hexachlorobenzene made slower by the presence of heavy metals. These findings are poised to guide further applications of bimetallic nanoparticles in the treatment of water and soil with mixed pollutants.
Author Contributions
Conceptualization, Y.H., S.L. and Q.W.; Data curation, Y.H. and Q.W.; Formal analysis, Y.H., Q.W. and G.H.; Funding acquisition, S.L.; Investigation, Y.H., Q.W. and G.H.; Methodology, Y.H. and G.H.; Resources, S.L.; Supervision, S.L.; Visualization, Q.W.; Writing—original draft, Y.H.; Writing—review and editing, S.L., Q.W., G.H., X.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Key Research and Development Program of China (No. 2023YFC3709104) and the Research Fund of China Geological Survey (DD20230754).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Jingyan Hao, Lei Yang, and Longlong Zhang for their kind help during this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Böhm, L.; Grančič, P.; Scholtzová, E.; Heyde, B.J.; Düring, R.-A.; Siemens, J.; Gerzabek, M.H.; Tunega, D. Adsorption of the Hydrophobic Organic Pollutant Hexachlorobenzene to Phyllosilicate Minerals. Environ. Sci. Pollut. Res. 2022, 30, 36824–36837. [Google Scholar] [CrossRef] [PubMed]
- Pala, N.; Jiménez, B.; Roscales, J.L.; Bertolino, M.; Baroni, D.; Figuerola, B.; Avila, C.; Corsolini, S. First Evidence of Legacy Chlorinated POPs Bioaccumulation in Antarctic Sponges from the Ross Sea and the South Shetland Islands. Environ. Pollut. 2023, 329, 121661. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E. Global Hexachlorobenzene Emissions. Chemosphere 2001, 43, 167–182. [Google Scholar] [CrossRef]
- Barber, J.L.; Sweetman, A.J.; Van Wijk, D.; Jones, K.C. Hexachlorobenzene in the Global Environment: Emissions, Levels, Distribution, Trends and Processes. Sci. Total Environ. 2005, 349, 1–44. [Google Scholar] [CrossRef]
- Fan, J.; Liu, C.; Guo, D.; Han, L.; Zhang, C.; Jin, H. Biochar and Biochar-Polylactic Acid Composite Enhance Biodegradation of Hexachlorobenzene in Soil by Altering Microbial Community. Appl. Soil Ecol. 2022, 177, 104521. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Zhang, Z.-F.; Yang, P.-F.; Li, Y.-F.; Cai, M.; Kallenborn, R. Pesticides in the Atmosphere and Seawater in a Transect Study from the Western Pacific to the Southern Ocean: The Importance of Continental Discharges and Air-Seawater Exchange. Water Res. 2022, 217, 118439. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T.; Li, J.; Pan, H.; Li, Q.; Miao, G.; Gong, J. Organochlorine Pesticides in the Air around the Taihu Lake, China. Environ. Sci. Technol. 2004, 38, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Qian, T.-T.; Jiang, H. Bimetallic Fe Nanoparticles: Recent Advances in Synthesis and Application in Catalytic Elimination of Environmental Pollutants. Chem. Eng. J. 2014, 236, 448–463. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Yang, X.; Gu, C.; Zhang, Y.; Jiang, X. Degradation of Highly-Chlorinated Benzenes by Nanoscale Iron and Palladized Iron in Aqueous System. Environ. Sci. 2011, 32, 692–698. [Google Scholar]
- Shih, Y.; Chen, Y.-C.; Chen, M.; Tai, Y.; Tso, C.-P. Dechlorination of Hexachlorobenzene by Using Nanoscale Fe and Nanoscale Pd/Fe Bimetallic Particles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 84–89. [Google Scholar] [CrossRef]
- Nie, X.; Liu, J.; Yue, D.; Zeng, X.; Nie, Y. Dechlorination of Hexachlorobenzene Using Lead–Iron Bimetallic Particles. Chemosphere 2013, 90, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, W. Subcolloidal Fe/Ag Particles for Reductive Dehalogenation of Chlorinated Benzenes. Ind. Eng. Chem. Res. 2000, 39, 2238–2244. [Google Scholar] [CrossRef]
- Nie, X.; Liu, J.; Zeng, X.; Yue, D. Rapid Degradation of Hexachlorobenzene by Micron Ag/Fe Bimetal Particles. J. Environ. Sci. 2013, 25, 473–478. [Google Scholar] [CrossRef]
- Zhu, N.; Luan, H.; Yuan, S.; Chen, J.; Wu, X.; Wang, L. Effective Dechlorination of HCB by Nanoscale Cu/Fe Particles. J. Hazard. Mater. 2010, 176, 1101–1105. [Google Scholar] [CrossRef]
- Zhang, Z.; Cissoko, N.; Wo, J.; Xu, X. Factors Influencing the Dechlorination of 2,4-Dichlorophenol by Ni–Fe Nanoparticles in the Presence of Humic Acid. J. Hazard. Mater. 2009, 165, 78–86. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, X.; Owens, G.; Chen, Z. Simultaneous Removal of Mixed Contaminants Triclosan and Copper by Green Synthesized Bimetallic Iron/Nickel Nanoparticles. Sci. Total Environ. 2019, 695, 133878. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, S.; Baig, S.A.; Tang, J.; Xu, X. Catalytic Dechlorination of Aroclor 1242 by Ni/Fe Bimetallic Nanoparticles. J. Colloid Interface Sci. 2012, 385, 160–165. [Google Scholar] [CrossRef] [PubMed]
- USGS. Mineral Commodity Summaries 2023; Mineral Commodity Summaries; USGS: Reston, VA, USA, 2023; p. 210.
- Zhang, Y.; Liu, J.; Zhou, Y.; Gong, T.; Wang, J.; Ge, Y. Enhanced Phytoremediation of Mixed Heavy Metal (Mercury)–Organic Pollutants (Trichloroethylene) with Transgenic Alfalfa Co-Expressing Glutathione S-Transferase and Human P450 2E1. J. Hazard. Mater. 2013, 260, 1100–1107. [Google Scholar] [CrossRef]
- Zhao, W.; Gu, C.; Ying, H.; Feng, X.; Zhu, M.; Wang, M.; Tan, W.; Wang, X. Fraction Distribution of Heavy Metals and Its Relationship with Iron in Polluted Farmland Soils around Distinct Mining Areas. Appl. Geochem. 2021, 130, 104969. [Google Scholar] [CrossRef]
- Su, Y.; Hsu, C.-Y.; Shih, Y. Effects of Various Ions on the Dechlorination Kinetics of Hexachlorobenzene by Nanoscale Zero-Valent Iron. Chemosphere 2012, 88, 1346–1352. [Google Scholar] [CrossRef]
- Naser, R.; Shahwan, T. Comparative Assessment of the Decolorization of Aqueous Bromophenol Blue Using Fe Nanoparticles and Fe-Ni Bimetallic Nanoparticles. Desalination Water Treat. 2019, 159, 346–355. [Google Scholar] [CrossRef]
- Zhang, L.; Manthiram, A. Chains Composed of Nanosize Metal Particles and Identifying the Factors Driving Their Formation. Appl. Phys. Lett. 1997, 70, 2469–2471. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Yan, Q.; Chen, Y. Reactions of Bivalent Metal Ions with Borohydride in Aqueous Solution for the Preparation of Ultrafine Amorphous Alloy Particles. J. Phys. Chem. 1993, 97, 8504–8511. [Google Scholar] [CrossRef]
- Mitov, M.; Popov, A.; Dragieva, I. Nanoparticles Produced by Borohydride Reduction as Precursors for Metal Hydride Electrodes. J. Appl. Electrochem. 1999, 29, 59–63. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of Trichloroethylene to Hydrocarbons Using Bimetallic Nickel-Iron Nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Garbou, A.M.; Liu, M.; Zou, S.; Yestrebsky, C.L. Degradation Kinetics of Hexachlorobenzene over Zero-Valent Magnesium/Graphite in Protic Solvent System and Modeling of Degradation Pathways Using Density Functional Theory. Chemosphere 2019, 222, 195–204. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W. Sequestration of Metal Cations with Zerovalent Iron Nanoparticless A Study with High Resolution X-ray Photoelectron Spectroscopy (HR-XPS). J. Phys. Chem. C 2007, 111, 6939–6946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).