Pilot Study on the Possibility of Improving Water Treatment Sludge Management in Almaty
Abstract
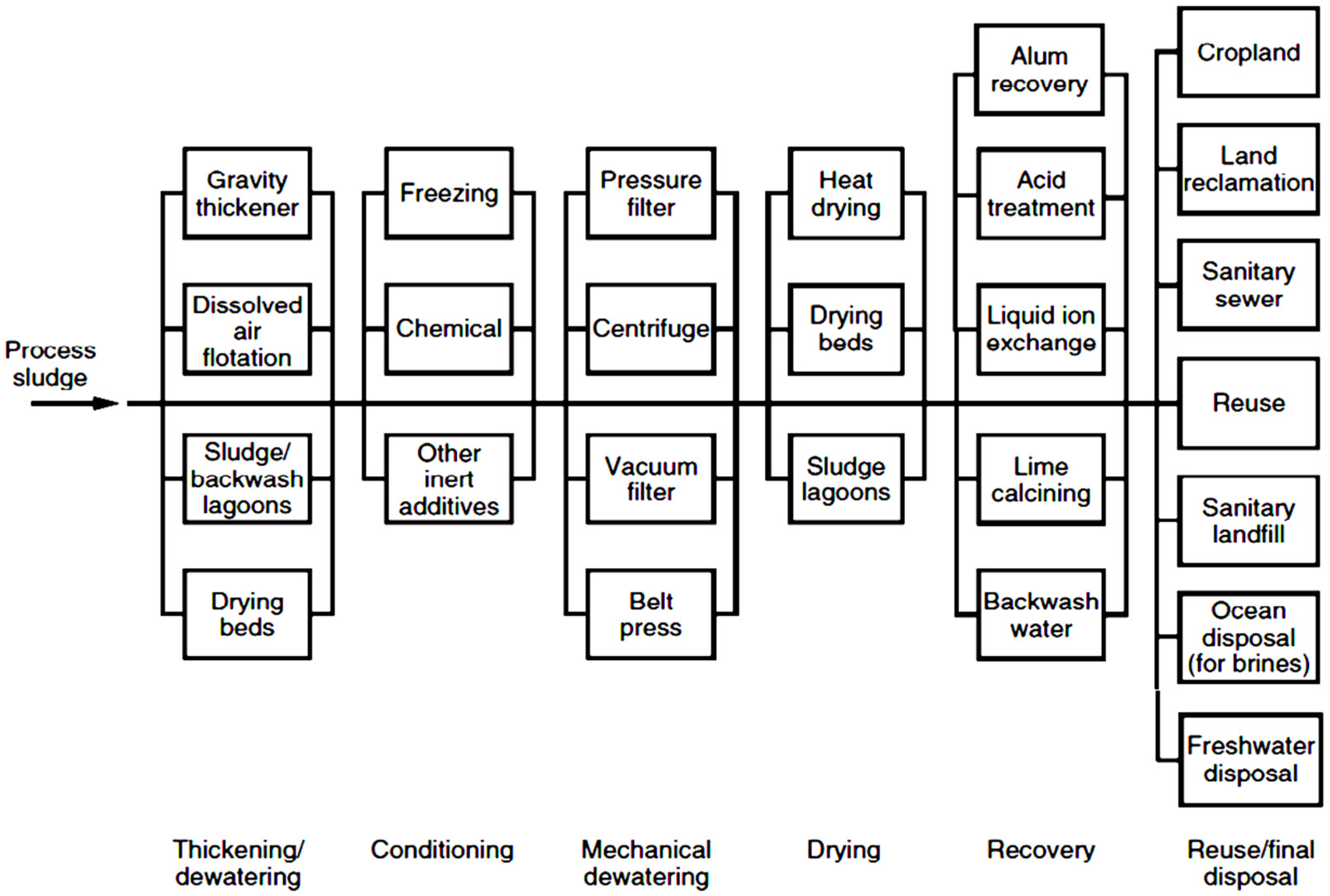
1. Introduction
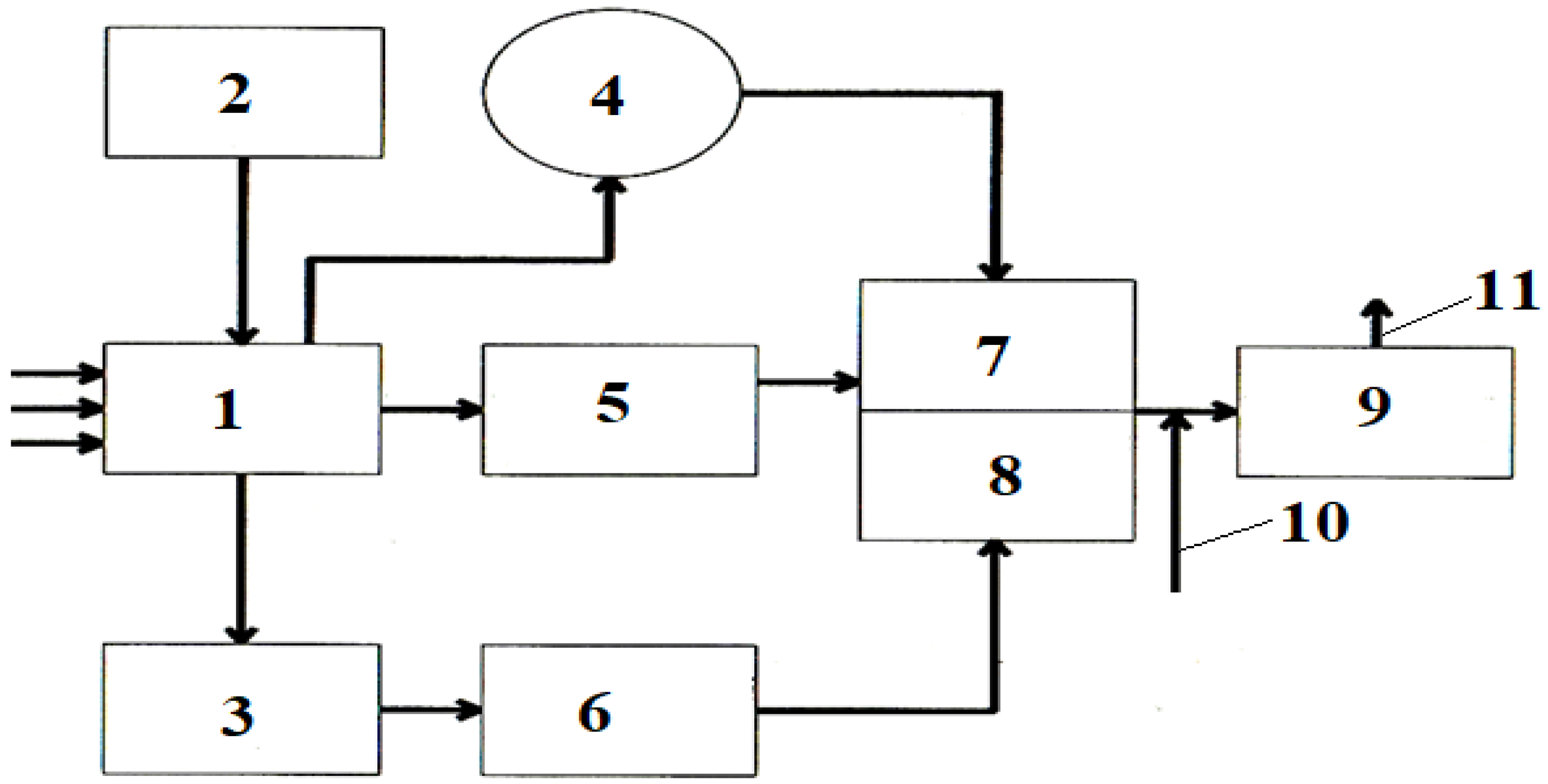
2. Materials and Methods
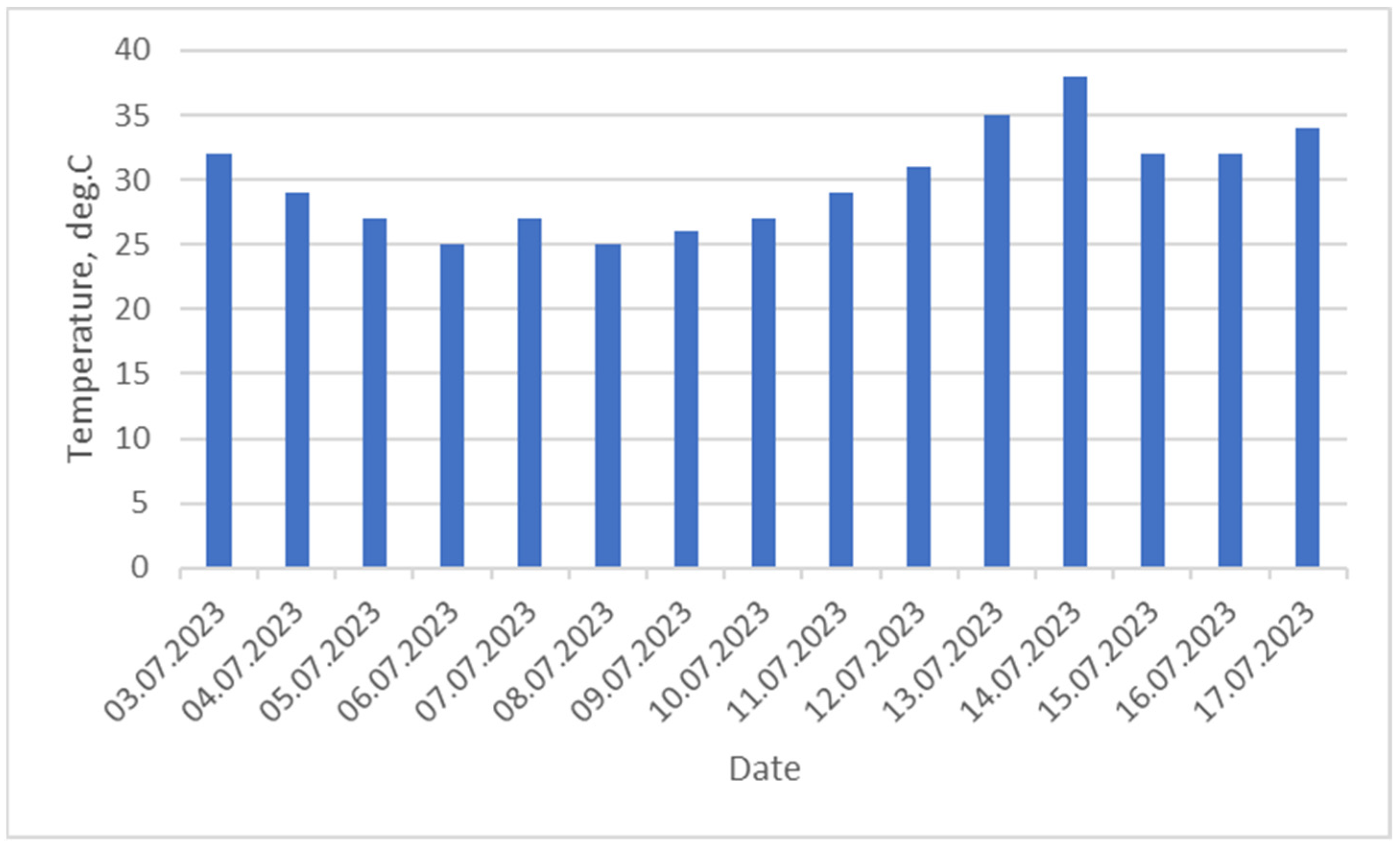
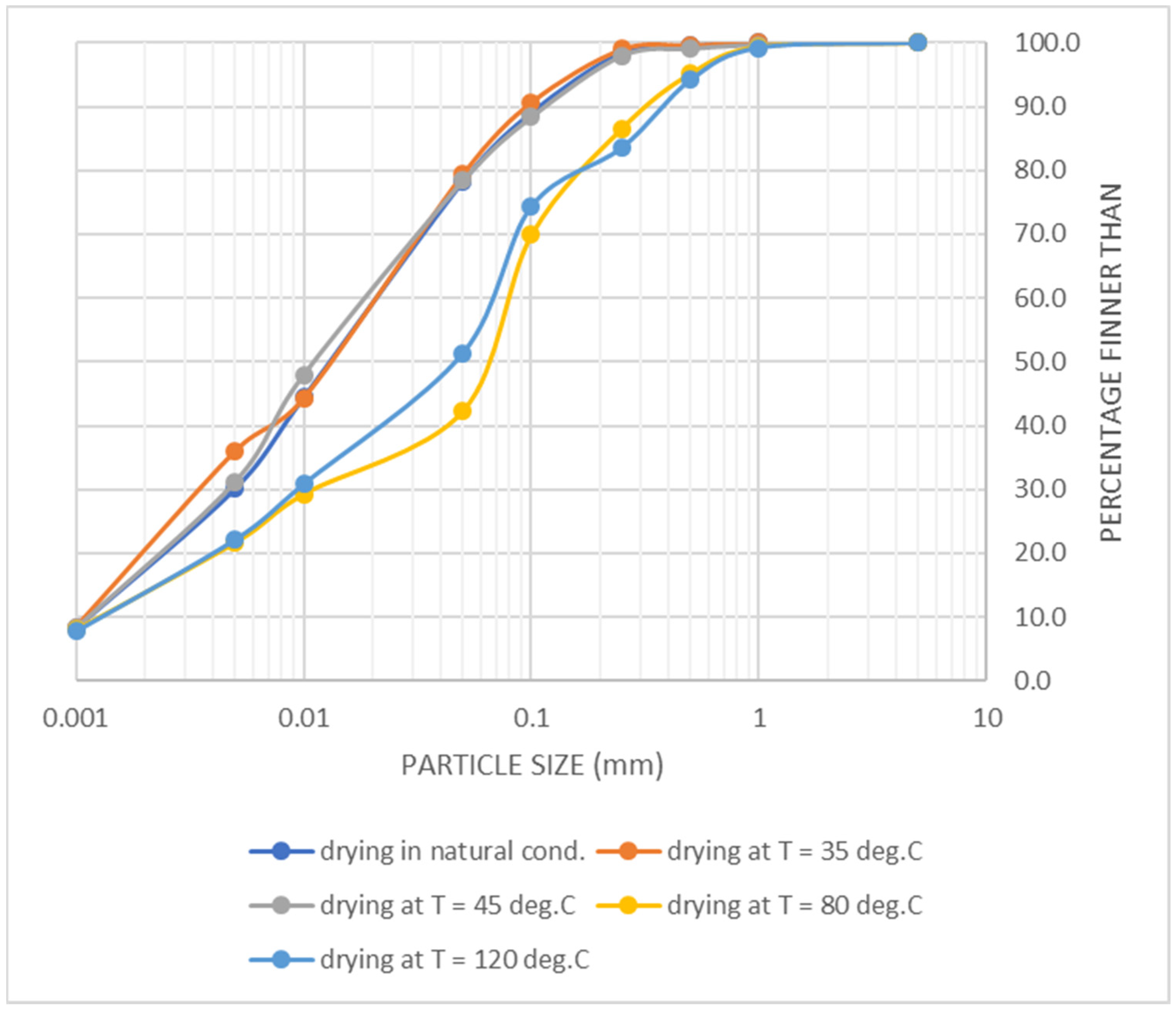
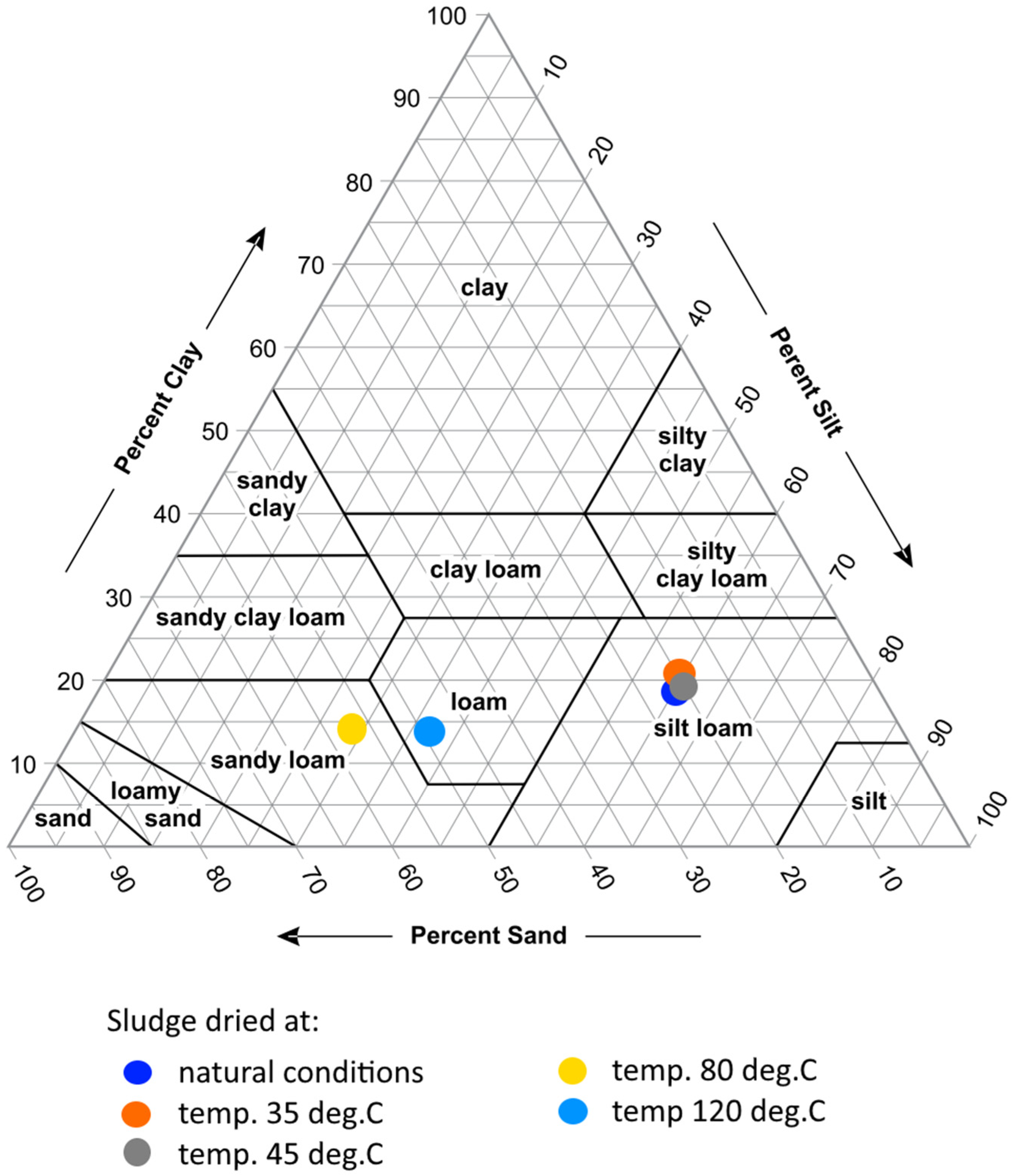
- Drying the sludge under natural conditions. The drying of sludge in summer was carried out in the open air on a concrete roofed surface, at an average air temperature of 25–38 °C, for 15 days with periodic turning.
- Drying the sludge on the heated floor at a temperature of 35 °C. The temperature in the dryer was controlled using a thermostat. The drying time was 13 days.
- Drying the sludge on the heated floor at a temperature of 45 °C. The drying time was 10.5 days.
- Drying the sludge in an oven at 80 °C. The drying cabinet ShS-40-02 SPU (Smolenskoye SKTB SPU, Smolensk, Russia) is designed for the drying, heat treatment and testing of materials, products and samples. The sludge inside the dryer was heated to 80 °C. The drying time was 3 days.
- Drying the sludge in an oven at a temperature of 120 °C. The sludge inside the dryer was heated to 120 °C. The drying time was 3 days.
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global Water Scarcity Including Surface Water Quality and Expansions of Clean Water Technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, X.; Qin, H.; Lin, H.; Chhuon, K.; Chen, Q. Research Progress of Tap Water Treatment Process. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 052025. [Google Scholar] [CrossRef]
- Anjithan, K. Management Practices of Water Treatment Sludge in Sri Lanka and Re-use Potential of Sludge Material. Master’s Thesis, University of Moratuwa, Homagama, Sri Lanka, 2016. [Google Scholar]
- Qrenawi, L.I.; Rabah, F.K.J. Sludge Management in Water Treatment Plants: Literature Review. Int. J. Environ. Waste Manag. 2021, 27, 93. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q. Constructive Approaches Toward Water Treatment Works Sludge Management: An International Review of Beneficial Reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization of Water Treatment Plant’s Sludge and Its Safe Disposal Options. Procedia Environ. Sci. 2016, 35, 950–955. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking Water Treatment Residuals: A Review of Recent Uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Technology Transfer Handbook: Management of Water Treatment Plant Residuals. EPA/625/R-95/008. 1996. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=115424 (accessed on 2 August 2024).
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/Flocculation in Dewatering of Sludge: A Review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Drinking Water Treatment Plant Residuals Management. Technical Report; EPA 820-R-11-003. 2011. Available online: https://www.epa.gov/sites/default/files/2015-11/documents/dw-treatment-residuals-mgmt-tech-report-sept-2011.pdf (accessed on 29 July 2024).
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable Management of Water Treatment Sludge through 3‘R’ Concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Thomas, M.; Surapaneni, A.; Moon, E.M.; Milne, N.A. Beneficial Reuse of Water Treatment Sludge in the Context of Circular Economy. Environ. Technol. Innov. 2022, 28, 102651. [Google Scholar] [CrossRef]
- De Carvalho Gomes, S.; Zhou, J.L.; Li, W.; Long, G. Progress in Manufacture and Properties of Construction Materials Incorporating Water Treatment Sludge: A Review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Fiore, F.A.; Rodgher, S.; Koga Ito, C.Y.; Bardini, V.S.D.S.; Klinsky, L.M.G. Water Sludge Reuse as a Geotechnical Component in Road Construction: Experimental Study. Clean. Eng. Technol. 2022, 9, 100512. [Google Scholar] [CrossRef]
- Takao, T.W.; Bardini, V.S.; De Jesus, A.D.; Marchiori, L.; Albuquerque, A.; Fiore, F.A. Beneficial Use of Water Treatment Sludge with Stabilizers for Application in Road Pavements. Sustainability 2024, 16, 5333. [Google Scholar] [CrossRef]
- Almaty City. Summary of the Socio-Economic Development of the Region. Bureau of National statistics QUAZSTAT. Available online: https://stat.gov.kz/en/region/almaty/ (accessed on 29 July 2024).
- Almaty Climate. Available online: http://www.pogodaiklimat.ru/climate/36870.htm (accessed on 29 July 2024).
- Almaty Su. Sources of Water Supply. Available online: https://almatysu.kz/?page_id=661&lang=ru (accessed on 29 July 2024).
- Almaty Su. Main Water Treatment Plant. Available online: https://almatysu.kz/?page_id=2265&lang=ru (accessed on 29 July 2024).
- Miyanoshita, T.; Oda, N.; Hayashi, N.; Fujiwara, M.; Furumai, H. Economic Evaluation of Combined Treatment for Sludge from Drinking Water and Sewage Treatment Plants in Japan. J. Water Supply Res. Technol.-Aqua 2009, 58, 221–227. [Google Scholar] [CrossRef]
- State Standard GOST 56226-2014; Sewage Sludge. Methods of Sampling and Sample Preparation. Standardinform: Moscow, Russia, 2019. Available online: https://meganorm.ru/Data2/1/4293766/4293766633.pdf (accessed on 19 September 2024).
- PND F 16.2.2:2.3:3.27-02; Methodology for Measuring Moisture Content in Solid and Liquid Industrial and Consumer Waste, Sediment, Sludge, Activated Sludge, Bottom Sludge Using the Gravimetric Method. Federal State Institution Center for Environmental Control and Analysis: Moscow, Russia, 2002; 10p.
- ST RK GOST R 51592—2003; Water. General Requirements for Sampling. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2003; 77p.
- ST RK 3060-2017; Water Quality. Methods for Measuring Temperature, Transparency and Odor. Committee for Technical Regulation and Metrology of the Ministry of Investment and Development of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2017; 54p.
- ST RK ISO 10523-2013; Water Quality. Determination of pH. KazStandard RSE: Astana, Republic of Kazakhstan, 2013; 22p.
- ST RK 1498-2006; Water Quality. Determination of Permanganate Number. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2006; 10p.
- ST RK 1514-2006; Drinking Water. Methods for Determining Hardness. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2006; 24p.
- ST RK GOST R 51309—2003; Drinking water. Determination of Element Content by Atomic Spectrometry Methods. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2003; 24p.
- GOST 3351-74*; Drinking Water. Methods for Determining Taste, Smell, Color and Turbidity. State Committee of Standards of the Council of Ministers of the USSR: Moscow, Russia, 1974; 8p.
- GOST 31868-2012; Water. Methods for Determining Color. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2012; 12p.
- ST RK ISO 7890-3-2006; Water Quality. Determination of Nitrate. Part 3. Spectrometric Method Using Sulfosalicylic Acid. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2006; 12p.
- ST RK 1015-2000; Water. Gravimetric Method for Determining the Content of Sulfates in Natural Wastewater. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2000; 12p.
- ST RK 2727-2015; Water Quality. Method for Determination of Fluorides. Committee for Technical Regulation and Metrology of the Ministry of Investment and Development of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2015; 44p.
- GOST 4245-72; Drinking Water. Methods for Determining Chloride Content. State Committee of Standards of the Council of Ministers of the USSR: Moscow, Russia, 1972; 6p.
- ST RK 3068-2017; Water Quality. Gravimetric Method for Measuring Suspended Solids and Total Impurities. Committee for Technical Regulation and Metrology of the Ministry of Investment and Development of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2017; 32p.
- ST RK 1322-2005; Water Quality. Determination of Chemical Oxygen Demand (COD). Committee for Technical Regulation and Metrology of the Ministry of Investment and Development of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2005; 8p.
- ST RK ISO 5815-1-2010; Water Quality. Hydrosphere. Determination of Biochemical Oxygen Demand after n Days (BOD). Committee for Technical Regulation and Metrology of the Ministry of Industry and New Technologies: Astana, Republic of Kazakhstan, 2017; 58p.
- GOST 12536-2014; Soil. Methods for Laboratory Determination of Granulometric (Grain) and Microaggregate Composition. Standardinform: Moscow, Russia, 2014; 19p.
- Methodology NSAM 172-C; Method of Quantitative Chemical Analysis. Determination of Silicon, Titanium, Aluminum, Iron, Calcium, Magnesium, Manganese in Rocks, Ore and Non-Ore Mineral Raw Materials, Environmental Objects Using the Flame Atomic Absorption Method. Ministry of Natural Resources and Environment of the Russian Federation: Moscow, Russia, 2010; 32p.
- ST RK 2276-2013; Soils. Determination of the Mass Concentration of Elemental Sulfur Using the Sulfite Method. Committee for Technical Regulation and Metrology of the Ministry of Industry and New Technologies: Astana, Republic of Kazakhstan, 2013; 12p.
- GOST 26204-91; Soils. Determination of Mobile Compounds of Phosphorus and Potassium According to the Chirikov Method as Modified by CINAO. Standardization and Metrology Committee of the USSR: Moscow, Russia, 1991; 8p.
- ST RK 11047-2008; Soil Quality. Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc Content in Soil Extracts in Aqua Regia. Spectrometric Methods of Atomic Absorption in Flame and with Electrothermal Spray. Committee for Technical Regulations and Metrology at the Min. of Industry and Trade of the Republic of Kazakhstan: Astana, Republic of Kazakhstan, 2008; 20p.
- U.S. Department of Agriculture, Natural Resources Conservation Service. Soil Survey Manual; USDA: Washington, DC, USA, 2017; ISBN 978-1-973804-53-6. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/The-Soil-Survey-Manual.pdf (accessed on 29 July 2024).
- Ospanov, K.; Kuldeyev, E.; Kenzhaliyev, B.; Korotunov, A. Wastewater Treatment Methods and Sewage Treatment Facilities in Almaty, Kazakhstan. J. Ecol. Eng. 2022, 23, 240–251. [Google Scholar] [CrossRef]

| Parameter | Range | Drinking Water Standard |
|---|---|---|
| Temperature, °C | 0.8–13 | – |
| Turbidity, mg/L | 0.2–140 | 1.5 |
| Color, deg | 0–13 | 20 |
| Odor at 20 °C, points | nd. | 2 |
| pH | 7.7–8.4 | 6–9 |
| Permanganate index, mgO2/L | 0.42 | 5.0 |
| Total hardness, mgEq/L | 1.1–2.3 | 7.0 |
| Chlorides, mg/L | 0.6–7.3 | 350 |
| Sulfates, mg/L | 3.5–12.0 | 500 |
| Nitrates, mg/L | 1.2–4.4 | 45.0 |
| Fluorides, mg/L | 0.5–1.0 | 1.5 |
| Aluminum, mg/L | 0.02–0.25 | 0.5 |
| Boron, mg/L | 0.006–0.09 | 0.5 |
| Copper, mg/L | 0.02–0.2 | 1.0 |
| Iron, mg/L | 0.1–0.2 | 0.3 |
| Manganese, mg/L | <0.01 | 0.1 |
| Nickel, mg/L | <0.005 | 0.1 |
| Total microbial count, CFU/mL | 3–900 | 50 |
| Parameter | Minimum | Maximum | Average | Median | Percentile 25 | Percentile 75 |
|---|---|---|---|---|---|---|
| Temperature, °C | 8.4 | 22 | 13.34 | 10.5 | 9.425 | 18.2 |
| Turbidity, mg/L | 50.4 | 62.3 | 55.65 | 53.95 | 51.825 | 60.75 |
| Color, degree | 58 | 71 | 67.7 | 70 | 68.25 | 70 |
| Odor at 20°, points | 6 | 8 | 7.6 | 8 | 7.25 | 8 |
| pH | 7 | 7.5 | 7.33 | 7.35 | 7.3 | 7.4 |
| Permanganate index, mg O2/L | 3.9 | 4.9 | 4.5 | 4.55 | 4.275 | 4.775 |
| Total hardness, mgEq/L | 4.3 | 6.7 | 5.2 | 5 | 4.55 | 5.675 |
| Aluminum, mg/L | 0.32 | 0.61 | 0.534 | 0.56 | 0.53 | 0.5975 |
| Beryllium, mg/L | <0.0001 | <0.0001 | - | - | - | - |
| Boron, mg/L | 0.0001 | 0.004 | 0.00234 | 0.002 | 0.002 | 0.003 |
| Total iron, mg/L | 1.292 | 7.1 | 5.1451 | 5.8 | 4.775 | 6.18975 |
| Cadmium, mg/L | 0.00003 | 0.0004 | 0.00019 | 0.0001 | 0.0001 | 0.0004 |
| Manganese, mg/L | 0.00001 | 0.002 | 0.00141 | 0.0016 | 0.001325 | 0.001775 |
| Copper, mg/L | 0.0007 | 0.003 | 0.00227 | 0.002 | 0.002 | 0.003 |
| Nickel, mg/L | 0.00015 | 0.004 | 0.00113 | 0.0004 | 0.0003 | 0.001925 |
| Nitrates, mg/L | 0.03 | 1.6 | 0.59 | 0.43 | 0.04 | 1.05 |
| Lead, mg/L | 0.003 | 0.007 | 0.00505 | 0.005 | 0.004 | 0.006075 |
| Selenium, mg/L | 0.0002 | 0.0014 | 0.00055 | 0.0003 | 0.000233 | 0.00075 |
| Sulfates, mg/L | 30 | 54.3 | 42.78 | 44.05 | 37.7 | 47.725 |
| Fluorides, mg/L | 0.2 | 0.42 | 0.303 | 0.3 | 0.225 | 0.3775 |
| Chlorides, mg/L | 175.5 | 254.6 | 224.76 | 231.15 | 219.025 | 239.675 |
| Hexavalent chromium, mg/L | 0.02 | 0.042 | 0.0331 | 0.038 | 0.02375 | 0.04 |
| Residual chlorine, mg/L | 0.01 | 0.3 | 0.094 | 0.079 | 0.072 | 0.08675 |
| Total nitrogen, mg/L | 73.2 | 84.6 | 79.59 | 79.5 | 78.6 | 81.15 |
| Suspended substances, mg/L | 138.9 | 167.8 | 147.175 | 143.75 | 141.275 | 147.675 |
| COD, mg/L | 248.6 | 374.8 | 311.06 | 300.9 | 271.6 | 360.35 |
| BOD, mg/L | 218.4 | 416.8 | 336.15 | 342.2 | 318.7 | 375.375 |
| Total microbial count, CFU/mL | 7000 | 100,000 | 88,100 | 100,000 | 92,500 | 100,000 |
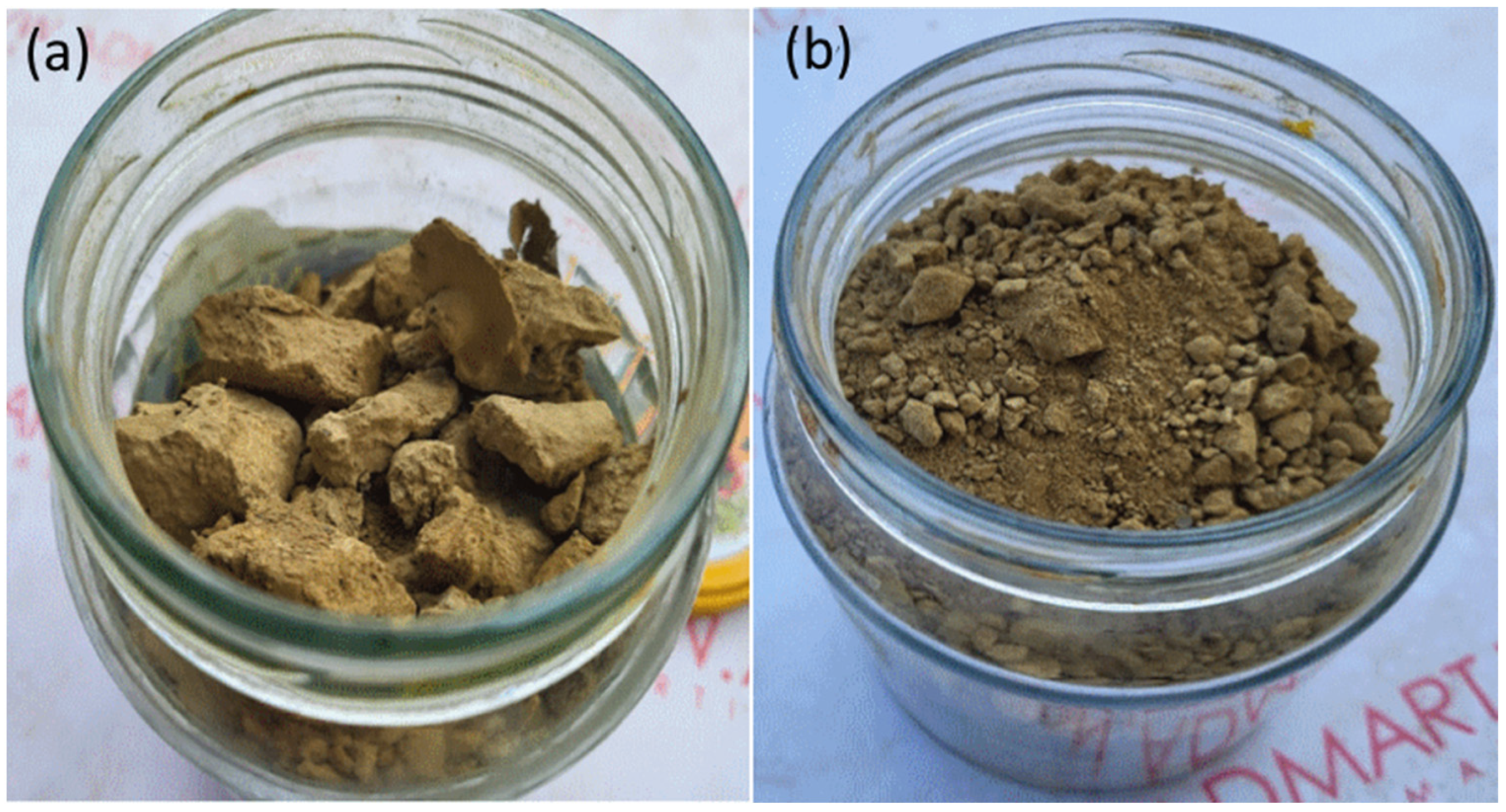
| Form of the Sludge | Moisture Content, % | Relative Volume |
|---|---|---|
| Raw sludge | 90 | 1.00 |
| Thickened sludge after 12 h of thickening | 84 | 0.6250 |
| Sludge after dewatering | 63 | 0.2703 |
| Sludge after drying under natural conditions at temperatures 25–38 °C (15 d) | 2.1 | 0.1021 |
| Sludge after drying on a heated floor at a temperature of 35 °C (13.5 d) | 2.4 | 0.1025 |
| Sludge after drying on a heated floor at a temperature of 45 °C (12 d) | 2.2 | 0.1022 |
| Sludge after drying in an oven at 80 °C (3 d) | 2.9 | 0.1030 |
| Sludge after drying in an oven at 120 °C (3 d) | 2.45 | 0.1025 |
| Parameter | Minimum | Maximum | Average | Median | Percentile 25 | Percentile 75 |
|---|---|---|---|---|---|---|
| Aluminum, mg/kg | 0.94 | 13.8 | 6.281 | 3.97 | 3.0 | 10.4575 |
| Magnesium, mg/kg | 0.89 | 2.4 | 1.731 | 1.77 | 1.66 | 2.0575 |
| Phosphorus, mg/kg | 0.21 | 0.36 | 0.28625 | 0.29 | 0.235 | 0.34 |
| Sulfur, mg/kg | 0.002 | 0.89 | 0.2466 | 0.13 | 0.027 | 0.405 |
| Silicon, mg/kg | 50.24 | 147.6 | 92.094 | 76.75 | 57.65 | 130.3 |
| Potassium, mg/kg | 1.72 | 5.51 | 3.972 | 4.28 | 3.0325 | 5.1975 |
| Calcium, mg/kg | 71.8 | 80.6 | 76.094 | 76.49 | 73.095 | 78.55 |
| Titanium, mg/kg | 0.09 | 1.2 | 0.6408 | 0.69 | 0.6175 | 0.78 |
| Chromium, mg/L | 0.001 | 0.9 | 0.336 | 0.074 | 0.04175 | 0.67 |
| Manganese, mg/L | 0.015 | 1.5 | 0.7783 | 0.885 | 0.57 | 0.96 |
| Iron, mg/L | 2 | 7.54 | 5.007 | 5.38 | 2.625 | 7.1625 |
| Nickel, mg/L | 0.9 | 4.4 | 2.074 | 1.6 | 1.345 | 2.655 |
| Copper, mg/L | 0.9 | 3.87 | 2.181 | 1.77 | 1.5525 | 3.1725 |
| Zinc, mg/kg | 0.048 | 1.55 | 0.7126 | 0.755 | 0.1025 | 1.2675 |
| Bromine, mg/kg | 0.001 | 0.041 | 0.01725 | 0.008 | 0.0055 | 0.0335 |
| Strontium, mg/kg | 0.08 | 1.53 | 0.853 | 0.975 | 0.285 | 1.29 |
| Lead, mg/kg | 0.85 | 1.7 | 1.207 | 1.205 | 0.95 | 1.375 |
| Barium, mg/kg | 0.001 | 0.021 | 0.004775 | 0.0026 | 0.001 | 0.00425 |
| Indium, mg/kg | 0.001 | 0.0021 | 0.001586 | 0.002 | 0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ospanov, K.; Kuldeyev, E.; Andraka, D.; Alzhigitova, M. Pilot Study on the Possibility of Improving Water Treatment Sludge Management in Almaty. Water 2024, 16, 2849. https://doi.org/10.3390/w16192849
Ospanov K, Kuldeyev E, Andraka D, Alzhigitova M. Pilot Study on the Possibility of Improving Water Treatment Sludge Management in Almaty. Water. 2024; 16(19):2849. https://doi.org/10.3390/w16192849
Chicago/Turabian StyleOspanov, Kairat, Erzhan Kuldeyev, Dariusz Andraka, and Manat Alzhigitova. 2024. "Pilot Study on the Possibility of Improving Water Treatment Sludge Management in Almaty" Water 16, no. 19: 2849. https://doi.org/10.3390/w16192849
APA StyleOspanov, K., Kuldeyev, E., Andraka, D., & Alzhigitova, M. (2024). Pilot Study on the Possibility of Improving Water Treatment Sludge Management in Almaty. Water, 16(19), 2849. https://doi.org/10.3390/w16192849






