Abstract
Panicum maximum is planted extensively in tropical and subtropical areas, due to its high-quality forage and high biomass yield. This study aims to assess the varied metabolic dynamics of P. maximum subject to different pollution-related wastewater levels, thus providing information for sustainable agriculture and soil restoration. We analyzed the primary and secondary metabolites in P. maximum subject to two different types of polluted wastewater (WW), compared to a control group. The alterations observed in the metabolite profiles were affected by several factors, including nutrient imbalances and oxidative stress induced by heavy metal accumulation. Initially, the increased nutrient availability stemming from wastewater treatment promoted plant growth; however, this positive effect was later diminished by the adverse impacts of heavy metals, which generated oxidative stress, resulting in metabolic disturbances and a decrease in the plant biomass. Importantly, the substantial increase in antioxidant enzymes, related to primary (e.g., sugars) and secondary metabolites (e.g., phenolics and flavonoids), underscores plants’ adaptive strategies to cope with stress. The increased biosynthesis of flavonoids and phenolic compounds is a protective mechanism against oxidative stress, which also improves the antimicrobial activity, following the activation of key biosynthetic pathways involved in their synthesis. These complex interactions among diverse metabolites suggest that plants exposed to polluted wastewater use various biochemical strategies to increase both their survival and defenses against pathogens. Collectively, these findings emphasize the significance of understanding how wastewater management practices can affect plant health, metabolic responses, and the broader implications for food safety and ecosystem stability.
1. Introduction
Guinea grass (Panicum maximum) is an economically important perennial species, among tropical and subtropical forage fodder plants, due to its high-quality feed production potential, as well as its biomass yield [1]. Given its wide adaptability to different soils and the potential for different types of environmental conditions, it has a role to play in numerous agricultural sectors, but it is most relevant to sustainable land management systems, which require quick plant establishment in otherwise unworkable timeframes [2].
Recent research highlights how regenerative agricultural techniques, not only enhance soil health by improving its structure and biodiversity, but also increase its carbon sequestration potential, thereby mitigating climate change effects [3,4,5]. In this regard, recent studies have revealed significant implications for sustainable agriculture and soil remediation, highlighting the adaptability of certain plant species in terms of addressing environmental challenges linked to wastewater use in agriculture. Recent research has shown that incorporating plants like P. maximum can enhance phytoremediation processes, whereby these plants take up and metabolize pollutants, thereby alleviating soil toxicity and enhancing soil health [6,7]. The variations in the primary and secondary metabolites in P. maximum suggest that it could be utilized for biomass production and that it has potential to synthesize valuable compounds that can improve soil quality and plant growth in polluted conditions. This aligns with findings from other studies that emphasize the importance of using wastewater for irrigation as a dual-purpose strategy to provide nutrients, while simultaneously enabling phytoremediation [8]. As such, the effects of environmental stressors (particularly water quality) on the growth and metabolic pathways in P. maximum can play a vital role in optimizing its use in agricultural conditions. Due to the heavy use of alternative water resources in agriculture, wastewater reuse has both potential benefits and constraints in terms of crop production [9]. Among various pollutants, wastewater, which is a byproduct of industrial and urban activities, adds to the major sources of pollution, which leads to soil health degradation and plant growth inhibition [10]. The composition of wastewater is diverse and influences the quality of effluent, according to its origin, the treatment process, etc., and it contains pollutant levels that are more important than others [11]. This variability can significantly impact the physicochemical characteristics of the soil, which are important for nutrient dynamics, as well as microbial communities, and are necessary to create a suitable rhizosphere environment that is beneficial to plant growth [9,12]. The presence of various contaminants in wastewater causes oxidative stress in plants, by significantly increasing the production of reactive oxygen species (ROS) and affecting their physiological and biochemical responses [13].
Metabolites are divided into primary and secondary metabolites, both of which are crucial for plant growth and adaptation [14]. Primary metabolites, including carbohydrates, amino acids, and lipids, play essential roles in supporting growth and energy production in plants. In contrast, secondary metabolites, such as phenolics and alkaloids, are crucial for the plant’s responses to stress and serve as defense mechanisms against various threats [14]. The changes in metabolite profiles in response to wastewater pollution and the study of metabolic and antioxidant responses to different pollution levels could provide valuable information on the resilience and adaptability of plants under stress and their ability to grow in degraded environments and could inform ways to reclaim polluted land [15].
Research is growing on how water pollution affects plant health and plant biochemical profiles. Despite some research having been carried out, there are not many in-depth studies focused on P. maximum and how its metabolites change when exposed to varying levels of wastewater pollution. Filling this knowledge gap is crucial for exploring the potential of P. maximum in phytoremediation efforts and in restoring polluted soils. Therefore, the present study aimed to investigate the variations in primary and secondary metabolites of P. maximum cultivated in diverse wastewater pollution conditions. We hypothesized that P. maximum shows shifted primary and secondary metabolite profiles in response to varying wastewater pollution levels, initially benefiting from nutrient availability, but later experiencing oxidative stress due to heavy metal accumulation, which triggers adaptive metabolic responses to promote plant survival and defense mechanisms. By analyzing the correlation between metabolite profiles, we aim to elucidate the potential implications for sustainable agricultural practices and soil remediation efforts.
2. Materials and Methods
2.1. Wastewater Sampling and Analysis
Water samples were gathered from two different irrigation sources (wastewater effluent), and fresh tap water irrigation was also applied (control). The wastewater samples were specifically collected from the El-Rahawy drain, at two different sources (WW1 and WW2) (located at 30°12′13.3″ N latitude and 31°03′54.3″ E longitude), in Giza, Egypt. This drain receives untreated sewage from the entire El-Giza governorate, as well as agricultural and domestic waste from the village of El-Rahawy. The wastewater samples were collected in plastic bottles and subsequently used for irrigation. The assessment of the elemental composition was conducted using inductively coupled plasma–mass spectrometry (ICP-MS; Finnigan Element XR, Scientific, Bremen, Germany), focusing on the detection of various salts and heavy metals present in the samples (Table 1).

Table 1.
Elemental analysis of wastewater from two different sources.
2.2. Plant Treatment
Healthy uniform seeds (obtained from the Agricultural Research Center (Giza, Egypt)) were planted in pots containing sterilized sand soil (70%) and Tref EGO substrates (Moerdijk, Netherlands; 30%). The plant seeds were planted in pots (30 cm diameter) filled with 1.5 kg of clay soil. The pots were divided into 3 groups, representing the following treatments: (i) freshwater (control), (ii) wastewater source 1 (WW1), and (iii) wastewater source 2 (WW2). The three pots served as biological replicates for each treatment. Daily measurements of the pot weight were taken to maintain the water level above 60% of the field capacity throughout the experiment. The plants were grown in a controlled environment chamber, with temperature settings of 21/18 °C and a 16 h light/8 h dark photoperiod, with a light intensity of 350 μmol PAR m−2 s−1. After four weeks of growth, the fresh weight (FW) and dry weight (DW) of the shoots were recorded and stored at −80 °C for subsequent analysis.
2.3. Determination of Minerals Accumulation in Plants
The accumulation of minerals in both the soil and plant samples was analyzed through total reflection X-ray fluorescence spectrometry (TXRF; Bruker Nano GmbH, Berlin, Germany), inductively coupled plasma–optical emission spectroscopy (ICP-OES), and the Kjeldahl method, targeting nutrients (e.g., K, S, and Zn), heavy metals (e.g., Cd and Pb), and nitrogen [16,17,18].
2.4. Nutritional Components of Plants
The concentration of the total polyphenols and flavonoids in fresh shoot samples was quantified using the standards of gallic acid and quercetin, respectively, based on the methodologies established by Zhang et al. [19] and Chang et al. [20]. The total protein content was determined using the Bicinchoninic Acid (BCA) method, which involves mixing the sample with reagents that react to form a colored product, with the color intensity proportional to the protein amount in the sample and quantification of the absorbance at 562 nm [21]. The crude fiber levels were assessed using digestion procedures, following the AOAC guidelines [22]. The tannin content was analyzed via solvent extraction with HCl (1% v/v) in methanol, subsequently treated with vanillin–HCl, and measured at 500 nm [23]. The ash content was determined by weighting the samples post-incineration at 550 °C for approximately 7 h, as outlined by Czaja et al. [24]. The alkaloids were evaluated using liquid chromatography, coupled with tandem mass spectrometry (LC-MS/MS) methods, as detailed by Babič et al. [25], with sample extraction performed in a solution of acetonitrile and ammonium carbonate (1:1 v/v). The saponin content was analyzed spectrophotometrically by measuring the optical density at 544 nm, following the reaction with phenolic aldehyde and H2SO4 [26].
2.5. Sugar Analysis
The sugars in the shoot samples were extracted using a TAE buffer (50 mM, pH 7.5) containing polyclar, sodium azide, PMSF, sodium bisulfite, mannitol, and mercaptoethanol, followed by centrifugation. The supernatant was processed using a mixed-bed Dowex column. The quantification of the sugars, such as glucose, sucrose, and fructose, was performed via high-performance anion-exchange chromatography, coupled with pulsed amperometric detection (HPAEC-PAD), according to the methods outlined by Verspreet et al. [27]. The sugar separation utilized a CarboPac MA1 column, with specified NaOH gradients. An internal standard, maltotriose, was used to ensure the quality of the extraction and purification [28]. The total soluble sugars were measured spectrophotometrically, following their extraction with 80% ethanol and their treatment with the anthrone reagent. The absorbance was recorded at 625 nm, using a microplate reader (Synergy Mx, BioTek, Santa Clara, CA, USA) [29,30].
2.6. Oxidative Stress Assessment
To evaluate the levels of malondialdehyde (MDA) in shoot tissues, samples were homogenized in 80% ethanol, then centrifuged, and reacted with thiobarbituric acid (TBA) to generate a chromogenic product, with the absorbance measured at various wavelengths [31]. The hydrogen peroxide (H2O2) content was determined using trichloroacetic acid and by monitoring the oxidation of Fe2+ [32].
2.7. Overall Antioxidant Measurement
The total antioxidant capacity in the shoot samples was assessed using the ferric reducing/antioxidant power (FRAP) assay, measuring the absorbance at 600 nm [33]. HPLC was also used to determine the reduced glutathione (GSH) and ascorbate (ASC) levels [34,35]. The antioxidant enzyme activities, including the activities of superoxide dismutase (SOD), peroxidase (POX), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and glutathione peroxidase (GPX), were measured using various spectrophotometric methods [32,36,37,38,39,40,41]. The reduction in 2-hydroxy-ethyl-disulfide determined the Glutaredoxin (Grx) activity, while the thioredoxin (Trx) content was assessed by NADPH oxidation [42,43].
2.8. Assessment of the Content of Anthocyanins, Phenolics, and Flavonoids, and Their Metabolism
To measure the anthocyanin content, shoot homogenization in 99:1 v/v methanol/HCl solution and spectrophotometric determination of the absorbance at 550 nm were performed following the procedure by Wagner et al. [44]. Quantification of the individual phenolic acids and flavonoids was performed using HPLC, with a LiChrosorb Si-60 column, as well as a diode array detector (DAD), as reported by Hamad et al. [45]. The samples were prepared in an acetone water mixture and processed using HPLC subject to specified conditions, including a water-based mobile phase containing formic acid. The calculation involved calibration curves developed from standard compounds.
Hamad et al. [45] reported that cinnamic acid was detected at 290 nm, using a sodium borate buffer, to quantify the PAL activity in plant samples. The rate of the PAL enzyme activity is expressed as the amount of enzyme that converts 1 mole of cinnamic acid, per second, per milligram of protein (nkatal mg–1 of protein). In order to measure the C4H activity, the method by Jadhav et al. [46] was used, and the absorbance at 340 nm after sample homogenization was recorded. To determine the total content of the CoA esters of phenolic acids, an enzymatic assay involving ligase synthesis was carried out, according to which the formation of CoA esters bound by phenols groups was monitored using UV–vis spectroscopy [47]. The chalcone synthase (CHS) activity in the shoot samples was measured using a colorimetric assay that quantifies the production of chalcone from malonyl-CoA and p-coumaroyl-CoA, reflecting flavonoid biosynthesis [48]. The enzyme activity is expressed in terms of the nmol of the chalcone produced per minute, per mg of protein.
2.9. Antimicrobial Evaluation
The antimicrobial efficacy of the shoot samples was evaluated against Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa, Staphylococcus saprophyticus, Streptococcus salivarius, Salmonella typhimurium, Proteus vulgaris, Enterobacter aerogenes, Serratia marcescens, Salmonella typhimurium, Candida albicans, Candida glabrata, Aspergillus flavus, and Enterococcus faecalis, using the disc diffusion method (DDM), as described by Agusa et al. [49]. In brief, a bacterial suspension (108 CFU mL−1) was evenly spread on Mueller–Hinton agar. Discs of filter paper containing the plant extract were placed on the agar, with established antibiotic-positive controls and a solvent-negative control. The presence of inhibition zones indicated the antimicrobial activity in the extracts. The diameters of these zones were measured for further analysis [50].
2.10. Statistical Approach
The statistical evaluations were conducted using SPSS software (SPSS Inc., Chicago, IL, USA). A one-way ANOVA and Tukey’s Honest Significant Difference (HSD) post hoc tests were performed, maintaining a significance threshold of 5% (p < 0.05). The NCSS program was used to perform Ward’s method of cluster analysis (version 21.0.3. Kaysville, UT, USA). The results are presented as the mean of triplicate analyses (n = 3).
3. Results
3.1. Soil Physicochemical Characteristics
The hierarchical cluster analysis revealed distinct shifts in the physicochemical properties of the soil subject to various wastewater pollution conditions, in which the WW-treated soils and control were clustered into two different groups (Figure 1).
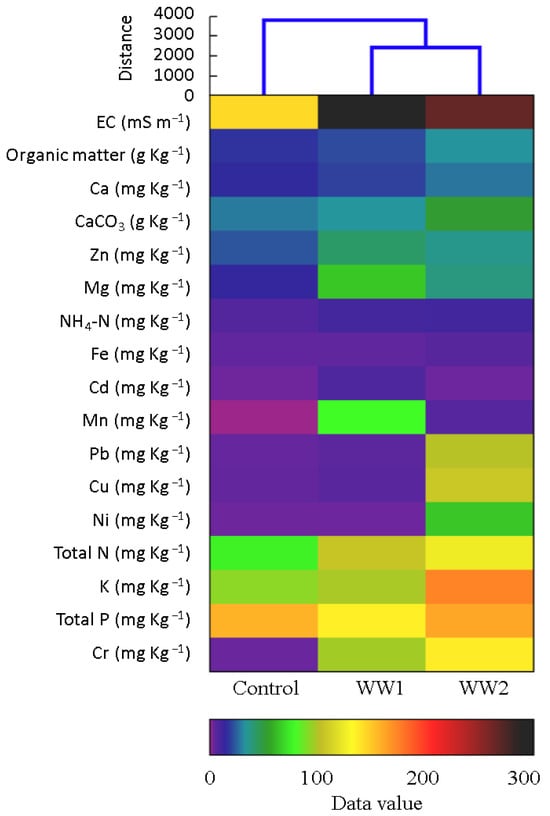
Figure 1.
Heat map generated by hierarchical cluster analysis using Ward’s method, showing the variations in the physicochemical properties of the soil affected by differing wastewater pollution conditions. Each row represents a distinct soil sample, while each column corresponds to specific physicochemical parameters. The color gradient, ranging from 0 (low values) to 300 (high values), indicates the magnitude of change in the soil properties subject to various wastewater conditions. This analysis highlights the relationship between the treatment conditions and the impact on soil quality, offering insight into the effects of wastewater contamination on soil health. The heat map facilitates easy identification of particular patterns and trends associated with different pollution levels.
Compared to the control, the EC levels increased by 108% for WW1 and 84% for WW2 (p < 0.05). The organic matter showed an increase of 24% and 48% in the WW1 and WW2 treatments compared to the control, respectively (p < 0.05). The CaCO3 levels in soils treated with WW2 were significantly elevated, by 45%, compared to the control (p < 0.05). The total nitrogen increased by 41% and 66%, and the NH4-N concentration exhibited an increment of 58% and 65%, in the samples treated with WW1 and WW2, respectively (p < 0.05). The total phosphorus non-significantly decreased for WW1 (by 13%) and increased for WW2 (by 3%) compared to the control (p ≥ 0.05). The potassium and calcium levels showed significant increases in response to WW2, which displayed the highest concentrations (p < 0.05). The heavy metal concentrations, especially Pb, Cd, Cu, and Cr, were higher in the samples treated with the polluted water sources compared to the control samples. In this regard, Pb increased by 85% for WW1 and 4085% for WW2, Cd by 5487% for WW1 and 267% for WW2, Cu by 89% for WW1 and 3749% for WW2, and Cr by 6445% for WW1 and 9182% for WW2.
3.2. Plant Biomass and Sugar Content
Both the fresh and dry weight of the plants decreased under polluted conditions. Accordingly, the fresh weight reduced by 23% for WW1 and 48% for WW2, and the dry weight decreased by 27% and 59% for WW1 and WW2 compared to the control plants, respectively (p < 0.05) (Figure 2).
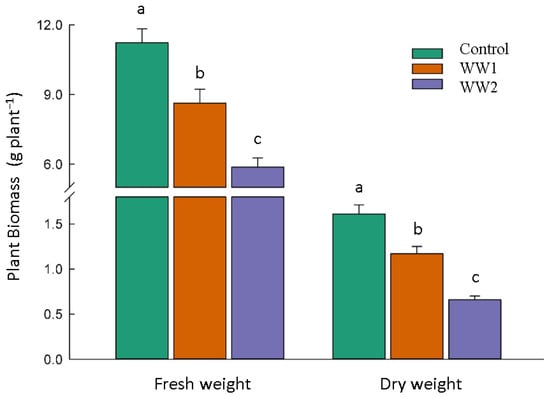
Figure 2.
Plant biomass in response to diverse polluted wastewater (WW) treatments. The bars and error bars represent the average biomass values and standard deviation, respectively. At a 5% probability level, Tukey’s HSD test reveals that the means sharing the same letter are not significantly different.
3.3. Mineral and Heavy Metal Concentration in Plants
The concentrations of minerals and heavy metals in P. maximum were influenced by the source of the irrigation (Table 2). Accordingly, in comparison to the control, the P and S levels in the shoot tissues significantly decreased by 30% and 38% for WW2, respectively (p < 0.05). The K content remained relatively stable in WW1, with a slight non-significant decrease of 7% (p ≥ 0.05), while a more pronounced reduction of 25% occurred in the samples treated with WW2 (p < 0.05). The heavy metal accumulation in plants varied drastically, with Pb increasing by 179% in the samples treated with WW1 and a striking 4476% in the samples treated with WW2 (p < 0.05). In contrast, Cd significantly increased by 1030% for WW1 (p < 0.05), but showed no significant change for WW2 (p ≥ 0.05). However, the Zn levels did not change in the samples treated with WW1, and the Zn content significantly increased, by 24%, in the samples treated with WW2. Moreover, the Cu concentrations increased considerably in WW2-treated plants, which was 7.4 times higher than the concentration in the control treatment. Cr, Ni, Ca, Mg, Mn, and Fe displayed diverse trends, with Cr showing a substantial increase of 16,565% in the samples treated with WW2, while a decrease of 38% occurred in the samples treated with WW2, compared to the control samples (Table 2). The data reflect significant variations in the mineral and heavy metal accumulation in P. maximum, due to the contrasting pollution levels in the wastewater sources.

Table 2.
Mineral and heavy metal concentration in plants (mean ± standard deviation) subject to diverse polluted wastewater (WW) treatments.
According to Figure 3, however, the fructose content in the shoot tissues was impacted by the WW treatments (p by WW t. glucose, sucrose, and total soluble sugar content were significantly increased in WW-treated plants (p < 0.05)). In this regard, glucose and sucrose increased by 59% and 50% for WW1 and 42% and 34% for WW2, respectively. These improvements resulted in a higher content of total soluble sugars in WW-treated plants, which was 62% higher for WW1 and 46% higher for WW2, than the control plants (p < 0.05). Moreover, the total sugars significantly increased, by 21%, for WW1 (p < 0.05), while WW2 showed a non-significant increase (3%) compared to the control plants (p ≥ 0.05).
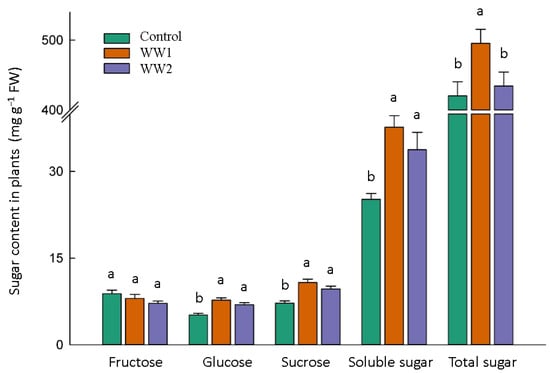
Figure 3.
Sugar content in plants subject to diverse wastewater (WW) pollution treatments. The bars and error bars represent the average sugar content values and standard deviation, respectively. At a 5% probability level, Tukey’s HSD test reveals that the data sharing the same letter(s) are not significantly different.
3.4. Oxidative Marker Content
Oxidative stress markers, including H2O2 and MDA, were notably elevated in the samples subject to polluted wastewater conditions, with WW2 exhibiting the highest increase, indicating an increased oxidative stress level in the plants subjected to wastewater pollution. H2O2 increased by 47% for WW1 and 83% for WW2. MDA increased by 132% for WW1 and 99% for WW2 compared to the control samples (Figure 4).
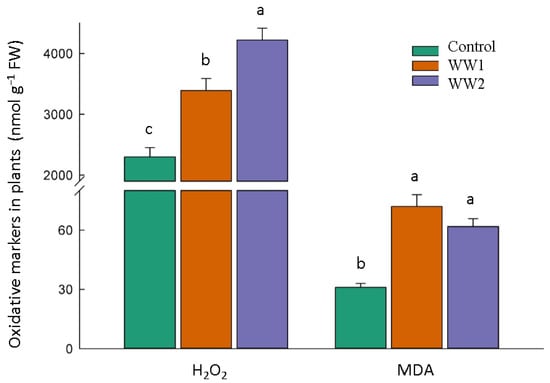
Figure 4.
Oxidative marker content in plants subject to diverse wastewater (WW) pollution treatments. The bars and error bars represent the average malondialdehyde (MDA) and hydrogen peroxide (H2O2) content and standard deviation, respectively. At a 5% probability level, Tukey’s HSD test reveals that the data sharing the same letter(s) are not significantly different.
3.5. Antioxidant Enzymes and Molecules
The effects of the two different wastewater sources on the antioxidant molecules and enzyme activities in P. maximum were significant when compared to the control conditions (Table 3). The total antioxidant capacity increased by 126% in the samples treated with WW1 and 44% in the samples treated with WW2 relative to the control levels (p < 0.05). The total tocopherol level in the plants was enhanced by 73% and 23% in the plants treated with WW1 and WW2, respectively. The ascorbate and glutathione concentrations showed a notable increase of 140% and 146% in the samples treated with WW1 and 55% and 51% in the samples treated with WW2 compared to the control samples, respectively (p < 0.05). The activity of POX, CAT, SOD, DHAR, GR, and GPX enzymes rose by 73%, 67%, 70%, 82%, 88%, and 47% in the samples treated with WW1 (p < 0.05), respectively, while they increased non-significantly in the samples treated with WW2 (p ≥ 0.05). For APX and Trx, the activity rose by 75% and 128% in the samples treated with WW1 and by 22% and 43% in the samples treated with WW2 compared to the control, respectively (p < 0.05).

Table 3.
Total antioxidant capacity (µmol Trolex g−1 FW), total tocopherols (ng g−1 FW), antioxidant molecule content (µmol g−1 FW), and the activity of antioxidant enzymes (μmol min−1 mg−1 protein) in plants (mean ± standard deviation) subject to diverse wastewater (WW) treatments.
3.6. Flavone and Phenolic Acid Content
The investigation of the phenolic acid and flavone content in P. maximum subject to varying wastewater pollution conditions revealed significant alterations compared to the control group (Figure 5). The caffeic acid concentration increased by 79% in the samples treated with WW1 and by 31% in the samples treated with WW2 relative to the control, while the gallic acid level exhibited a substantial increase of 79% in the samples treated with WW1 and 30% in the samples treated with WW2 (p < 0.05). Additionally, the quercetin level soared by 132% in the samples treated with WW1 and by 60% in the samples treated with WW2 compared to the control values (p < 0.05), indicating a marked enhancement in the flavonoid content in response to wastewater exposure. Protocatechuic acid also showed a significant increase, rising by 249% in the samples treated with WW1 and 158% in the samples treated with WW2 (p < 0.05). These findings reveal that exposure to wastewater significantly enhances the accumulation of key phenolic acids and flavones in P. maximum, with WW1 yielding the highest increases across several parameters. The hierarchical cluster analysis, depicted in Figure 1, further illustrates the differential accumulation of these metabolites subject to contrasting wastewater conditions, highlighting the variability in plant responses to pollution levels. The heat map visually demonstrates the differentially altered metabolite profiles, especially under the WW2 treatment, providing insight into the adaptive reactions by P. maximum to environmental stressors (Figure 5).
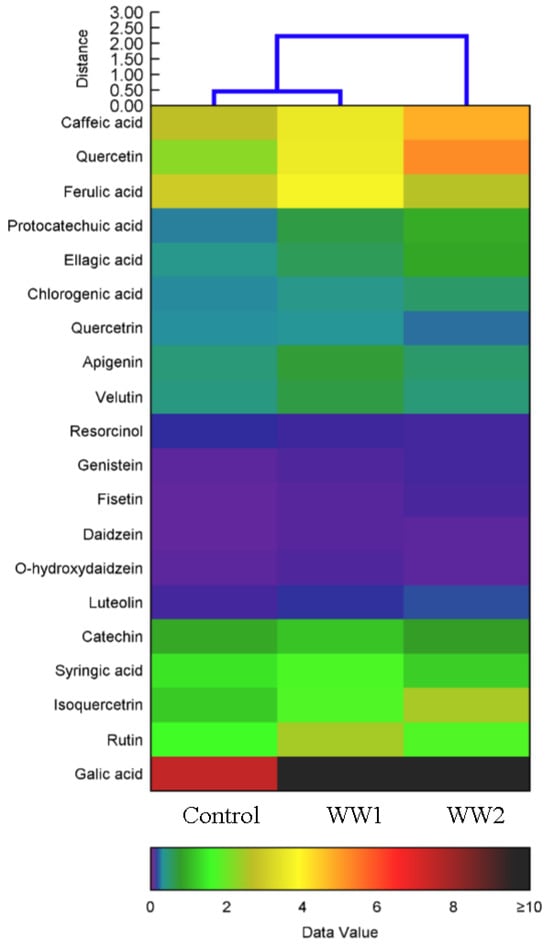
Figure 5.
Heat map generated by hierarchical cluster analysis using Ward’s method, showing the differential shifts in the phenolic acid and flavonoid content in plants exposed to various wastewater pollution conditions. Each row corresponds to a specific plant sample, while each column represents different phenolic compounds and flavones assessed in the study. The heat map visually captures the intensity of the alterations in the compound concentrations, highlighting the variations in the metabolite profiles associated with distinct levels of wastewater contamination. The accompanying color gradient at the bottom of the panel ranges from 0 (indicating minimal content) to 10 (indicating maximal content), providing a clear visual reference for interpreting the varying concentrations of these phytochemicals.
3.7. Anthocyanin Content and Its Metabolism
The analysis of the anthocyanin content and its related metabolic parameters in plants subject to varying wastewater pollution conditions indicated significant changes compared to the control (Table 4). The anthocyanin levels increased markedly in the samples treated with WW2, reaching 86 ng g−1, which represents an increase of 40% over the control value (p < 0.05), while the samples treated with WW1 showed a non-significant decrease. Additionally, the phenylalanine content demonstrated a notable increase, rising by 97% in the samples treated with WW1 and 45% in the samples treated with WW2 compared to the control samples (p < 0.05). The levels of p-coumaric acid also showed significant variation, increasing by 36% in the samples treated with WW2, while the WW1 samples exhibited a slight non-significant decrease. Furthermore, the naringenin content in plants increased dramatically in the samples given the WW1 treatment, an increase of 154% compared to the control (Table 4). These results show that wastewater exposure significantly alters the anthocyanin content and associated metabolic pathways in P. maximum subject to pollution conditions.

Table 4.
Anthocyanin content and its metabolism in plants (mean ± standard deviation) subject to diverse wastewater (WW) pollution treatments.
3.8. Nutritive Values
The effects of diverse wastewater pollution conditions on the nutritional values of P. maximum were assessed, with notable variations observed in regard to several parameters when compared to the control conditions (Table 5). The findings indicate that both the WW1 and WW2 treatments enhanced the antimicrobial activity of the plant extracts, with variations dependent on the source and type of microorganism tested. Specifically, the antimicrobial activity against S. epidermidis increased dramatically by 500% in the samples treated with WW1, reaching 22.8 mm and were further elevated to 24.8 mm in the samples treated with WW2, showcasing a considerable increase of 560% over the control value of 3.8 mm (p < 0.05). Similarly, P. aeruginosa exhibited enhanced susceptibility, with minimal inhibitory concentrations, rising from 12.1 mm in the control group to 25.5 mm in the samples treated with WW1 and 27.5 mm in the samples treated with WW2, reflecting increases of 110% and 128%, respectively (p < 0.05). The extracts also showed notable activity against S. typhimurium, with an increase from 6.6 mm in the control group to 19.6 mm in the samples treated with WW1 and 23.1 mm in the samples treated with WW2, corresponding to increases of 196% and 250%, respectively (p < 0.05). The heat map visually demonstrates that the exposure to polluted wastewater led to differentially altered antimicrobial activities, providing insight into the potential of P. maximum as a source of effective antimicrobial agents when subject to environmental stresses (Figure 6).

Table 5.
Nutritional value of plants (mean ± standard deviation) subject to diverse wastewater (WW) pollution treatments.
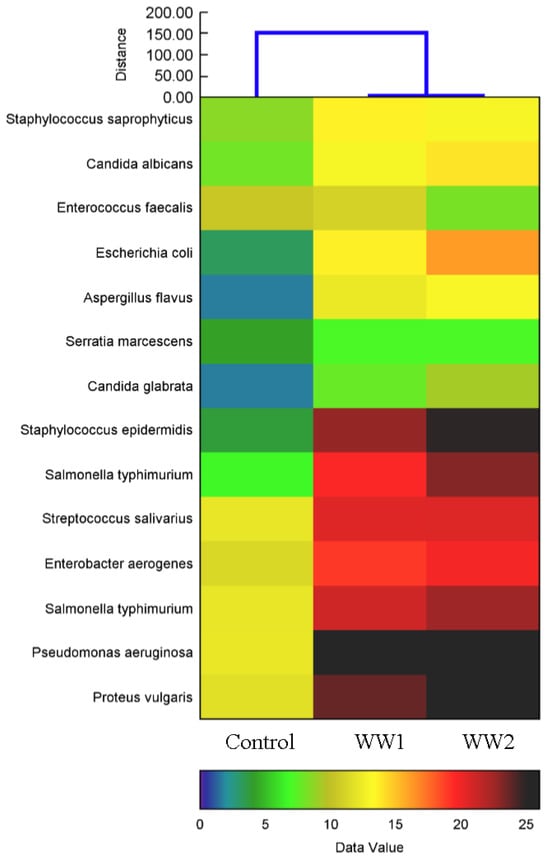
Figure 6.
Heat map generated by hierarchical cluster analysis using Ward’s method, showing the differential antimicrobial activity of plant extracts subjected to various wastewater pollution conditions. Each row of the heat map represents a distinct plant extract, while each column corresponds to the different wastewater treatment conditions applied in the present study. The map highlights variations in antimicrobial efficacy, as indicated by the diameter of the inhibition zones measured on agar plates following exposure to specific microbial strains. The color gradient at the bottom of the panel, ranging from 0 (indicating no inhibition) to 25 mm (indicating maximal inhibition), provides a quantitative scale for interpreting the results.
3.9. Antimicrobial Activity
The evaluation of the antimicrobial activity in the P. maximum extracts subjected to different wastewater sources revealed significant improvements compared to the control (Figure 6). The findings indicate that both the WW1 and WW2 treatments enhanced the antimicrobial activity in the plant extracts, with the variations dependent on the source and type of microorganism tested. Specifically, the antimicrobial activity against S. epidermidis increased dramatically by 500% in the samples treated with WW1, reaching 22.8 mm, and were further elevated to 24.8 mm in the samples treated with WW2, showcasing a considerable increase of 560% over the control value of 3.8 mm (p < 0.05). Similarly, P. aeruginosa exhibited enhanced susceptibility, with minimal inhibitory concentrations, rising from 12.1 mm in the control group to 25.5 mm in the samples treated with WW1 and 27.5 mm in the samples treated with WW2, reflecting increases of 110% and 128%, respectively (p < 0.05). The extracts also showed notable activity against S. typhimurium, with an increase from 6.6 mm in the control group to 19.6 mm in the samples treated with WW1 and 23.1 mm in the samples treated with WW2, corresponding to increases of 196% and 250%, respectively (p < 0.05). The heat map visually demonstrates that the exposure to polluted wastewater led to differentially altered antimicrobial activities, providing insight into the potential of P. maximum as a source of effective antimicrobial agents when subject to environmental stresses (Figure 6).
4. Discussion
The present study shows that various levels of polluted wastewater significantly affect the complex networks governing the production of primary and secondary metabolites in plants. Complex interactions, such as fluctuating nutrient levels, osmotic stress, and the plant’s defense machinery related to toxic conditions, were responsible for the observed variations in the metabolite profiles.
The observed increases in organic matter, total N, and CaCO3 in the soil indicated improved nutrient availability, which supported plant nutrient absorption. Wastewater contains high levels of certain nutrients, such as N, Mg, K, and Mn, which might create an imbalance in plant nutrition. In a related study, Shtull-Trauring et al. [51] found that the use of polluted wastewater for irrigating fields can lead to nutrient imbalances in the soil due to the leaching of excess nutrients. Additionally, this practice can elevate the levels of salts, heavy metals, and organic pollutants, which pose risks to both crop and soil health. This imbalance may interfere with essential metabolic processes, like photosynthesis and respiration, ultimately impacting the production of vital carbohydrates and energy molecules [52]. For example, the elevated N levels found in the effluent from the wastewater treatment plant likely led to higher concentrations of amino acids, such as proline, in the treated plants. This buildup can be seen as the plant’s response to osmotic stress, as proline serves as an osmoprotectant [53]. Additionally, research has shown that exposure to high concentrations of heavy metals, like Cu and Cd, can disrupt nitrogen metabolism in plants, negatively affecting the synthesis of amino acids and proteins [54].
Moreover, the increasing heavy metal concentrations in the present research (e.g., Pb, Cd, Cu, and Cr) indicate a striking level of risk of metal toxicity in the soil. The dual effects of nutrient enrichment and heavy metal toxicity present a complex problem for plant health. Heavy metal pollution can significantly inhibit the plant biomass through several physiological mechanisms, among which, one of the main impacts is the disruption of essential physiological processes, including photosynthesis, by damaging chloroplasts and reducing chlorophyll synthesis [55]. While soil nutrient enrichment can initially support plant growth, heavy metal accumulation can result in metabolic disruptions, oxidative damage, and impaired development, as observed by the reduced biomass in plants. Over time, heavy metal exposure can lead to a decline in plant health and productivity and a threat to food safety and ecosystem health [56]. It has been suggested that heavy metal accumulation in plants can create a cycle of degradation, where subsequent crop cycles suffer from a compounding effect of metal toxicity, leading to a decline in agricultural productivity and further ecological imbalances [57]. Albqmi et al. [58] also noted the same trends, asserting that these heavy metals can induce oxidative damage, interfering with the physiological processes of plants. The significant reduction in the biomass of WW-treated plants is likely related to the imposed heavy metal stress, which can affect water availability in the soil and nutrient uptake by plants, further leading to growth decline [54]. Heavy metals can hinder root development and functionality, restricting the plant’s ability to absorb water and nutrients, which subsequently adversely affects biomass production [59]. The high uptake of Cd and Ni in WW1-treated plants, and Pb, Cr, Cu, Ni, and Zn in WW2-treated plants, indicates that P. maximum can accumulate these metals, potentially threatening plant health and subsequent trophic levels through bioaccumulation. Increased metal uptake is often associated with altered nutrient dynamics and stress responses, which can manifest as impaired plant function and growth inhibition [60]. The evidence presented thus far supports the idea that the mitigation of metal toxicity impacts by employing strategies, such as phytoremediation, where specific plant species are used to extract or stabilize heavy metals, can be proposed because of their potential to restore contaminated soils and protect the health of the ecosystem [61].
Primary metabolites (e.g., sugars) exhibited significant alterations in response to the WW treatments. The high glucose and sucrose levels in response to the WW treatments emphasize the function of these sugars as osmoprotectants and signaling molecules that help maintain the osmotic balance and protect cellular structures [62,63]. The increased synthesis of these soluble sugars in plants is driven by stress-responsive pathways that divert carbon flux towards osmolyte production, which is critical for stress tolerance [64]. The high production of primary metabolites (e.g., glucose) can lead to an increased flux through the shikimate pathway, which is considered an essential precursor route for several secondary metabolites (e.g., phenolics and flavonoids) [65]. This flux enhancement is important because many secondary compounds are synthesized from primary metabolism intermediates. Comparable results have been reported in plants grown in soil contaminated with wastewater, whether irrigated with treated wastewater or planted in soil affected by sewage waste [66,67].
Moreover, heavy metals induce oxidative damage by generating ROS, which can result in protein oxidation, lipid peroxidation, and DNA damage [68]. In the present study, the significant boost in oxidative markers, including H2O2 and MDA, serves as evidence of the extent of the oxidative damage. The disruption of photosynthesis due to oxidative stress reduces energy production and shifts resource allocation, influencing plant growth and development [69]. Furthermore, oxidative damage triggers enhanced antioxidant responses, which can divert metabolic resources away from growth and reproduction towards stress mitigation, thereby affecting plant production and fitness [70]. The regulatory framework that includes both enzymatic and non-enzymatic antioxidant systems works to maintain the levels of ROS in plant cells at a concentration that does not cause damage [69]. In this regard, plants counteract this damage by developing antioxidant systems and synthesizing secondary metabolites with antioxidative properties [71]. In fact, exposure to diverse pollutants in wastewater, including heavy metals and organic pollution, can induce the activation of plant defense mechanisms. This activation frequently leads to the reallocation of metabolic resources from primary pathways to the production of secondary metabolites, which are crucial for detoxification and stress tolerance [72]. The present research strongly supports this understanding, as evidenced by the significant improvement in phenolic compounds and flavonoid content in plants treated with wastewater. These compounds are recognized as antioxidants and metal chelators, suggesting a protective role against oxidative stress and heavy metal toxicity associated with pollutant sources [73]. The elevated levels of flavones and phenolic acids (e.g., quercetin and gallic acid) reflect the increased production of these compounds as a stress response [74]. The upregulation of the phenylpropanoid pathway, responsible for producing phenolic acids and flavonoids, represents a critical aspect of the plant’s response to stress, providing antioxidative, antimicrobial, and structural defense functions [75,76].
The polluted wastewater treatments had a significant effect on the activity of antioxidant enzymes in plants, especially in WW1-treated plants. It has already been reported that exposure to heavy metals generally found in wastewater can induce oxidative damage in plants, resulting in shifts in antioxidant enzyme activity [67,77]. For instance, the enzyme activities of CAT, SOD, and POX have been shown to increase in response to wastewater treatment, providing a protective adaptive strategy [78,79]. However, the effectiveness of these enzymatic responses can differ based on the specific types of pollutants present and their concentrations in the wastewater. This suggests that while plants possess robust defense mechanisms, their efficacy may be contingent upon the nature and severity of the environmental stressors they encounter.
The high level of anthocyanin content in WW2-treated plants indicates the activation of phenylpropanoid and flavonoid biosynthetic pathways. These pigments are not only involved in the antioxidant defense system, but also mitigate light-induced oxidative damage by absorbing excess light energy [80]. Critical enzymes involved in this pathway, including phenylalanine ammonia-lyase, chalcone synthase, and cinnamate-4-hydroxylase, serve as essential regulators. Their upregulation indicates an enhanced biosynthetic activity in response to stress [76]. Additionally, the observed reduction in the total protein content, alongside an increase in crude fiber and the total phenols, highlights the dual impact of stress, namely hindering protein synthesis, while improving the structural defense mechanisms [81]. The elevated level of total phenols aligns with the enhanced synthesis of phenolic compounds, providing antioxidative protective roles against stress [82].
The increased antimicrobial activity of the plant extracts in response to polluted wastewater treatments can be linked to high levels of secondary metabolites with antimicrobial properties in the present study, including flavonoids, phenolics, and alkaloids [83]. These compounds are known for their ability to disrupt microbial cell walls and inhibit their growth, thus improving the plant’s defense mechanisms against pathogens [84]. For example, the increased synthesis of flavonoids in stressed plants can boost the antimicrobial efficacy because of their ability to form complexes with extracellular and soluble proteins and bacterial cell walls [75]. Moreover, the increased saponin synthesis in WW2-treated plants can be translated into the higher antimicrobial activity in the plant extracts, since saponins are glycoside compounds with detergent-like properties that can lyse microbial cell membranes [85]. The diverse array of secondary metabolites can work synergistically to improve the antimicrobial efficacy. For instance, phenolic acids and flavonoids can potentiate the effects of each other, leading to more effective inhibition of microbial growth [86].
A major limitation of the present research is that the controlled environment conditions for plant growth may not accurately replicate the variable conditions found in real-world agricultural settings. Factors such as fluctuating temperatures, varying light conditions, and external stresses, like biotic interactions and abiotic factors, are absent in this controlled setup, potentially limiting the applicability of the findings to practical agricultural contexts. Therefore, complementary studies in field conditions are essential for validating the results and understanding their relevance in dynamic farming environments.
5. Conclusions
The present research revealed the strong effects of polluted wastewater on plant metabolic dynamics, highlighting the role of nutrient enrichment and heavy metal toxicity in primary and secondary metabolite production. The metabolite profiles experienced changes resulting from various factors, such as the nutrient imbalance and oxidative stress, induced by the accumulation of heavy metals. Wastewater treatment initially supplied the plants with more nutrients that supported plant growth. However, this was counteracted by the negative influence of the heavy metals, which caused oxidative stress, leading to disruptions to plant metabolism and a reduction in the plant biomass. Notably, there was an increase in antioxidant enzymes, related to primary metabolites (sugars) and secondary metabolites (phenolics and flavonoids), indicating various adaptive mechanisms against stress employed by plants. The increase in flavonoids and phenolic compounds is a protective response to oxidative damage, as well as a way to improve the antimicrobial activity, aligned with the activation of key biosynthetic pathways accountable for their synthesis. This complex interplay among the metabolites indicates that plants responding to contaminated wastewater employ a sophisticated network of biochemical strategies that facilitate both survival and defense mechanisms against pathogens. Overall, the findings underscore the importance of understanding how wastewater management practices can influence plant health, metabolic responses, and the potential implications for food safety and ecosystem integrity.
Author Contributions
Conceptualization, H.S. and Y.A.H.; data curation, H.S., M.K.O., E.A.E. and M.S.S.; investigation, M.K.O.; methodology, H.S., M.K.O., M.S.S. and Y.A.H.; resources, A.M.A., E.A.E. and M.S.S.; software, M.K.O.; validation, A.M.A., E.A.E. and M.S.S.; visualization, A.M.A.; writing—original draft, H.S. and Y.A.H.; writing—review and editing, Y.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project number (RSPD2024R931), King Saud University, Riyadh, Saud Arabia.
Data Availability Statement
The data will be made available on request.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2024R931), King Saud University, Riyadh, Saud Arabia.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Döndü Bilgin, F. Guinea Grass (Panicum maximum) Forage: A Review. MAS J. Appl. Sci. 2021, 6, 77–82. [Google Scholar] [CrossRef]
- Philp, J.N.M.; Vance, W.; Bell, R.W. Forage Options to Sustainably Intensify Smallholder Farming Systems on Tropical Sandy Soils: A Review. Agron. Sustain. Dev. 2019, 39, 30. [Google Scholar] [CrossRef]
- Jamali, M.; Bakhshandeh, E.; Yaghoubi Khanghahi, M.; Crecchio, C. Metadata analysis to evaluate environmental impacts of wheat residues burning on soil quality in developing and developed countries. Sustainability 2021, 13, 6356. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Murgese, P.; Strafella, S.; Crecchio, C. Soil biological fertility and bacterial community response to land use intensity: A case study in the Mediterranean area. Diversity 2019, 11, 211. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Cucci, G.; Lacolla, G.; Lanzellotti, L.; Crecchio, C. Soil fertility and bacterial community composition in a semiarid Mediterranean agricultural soil under long-term tillage management. Soil Use Manag. 2020, 36, 604–615. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Ximba, B.J.; Fatoki, O.S.; Opeolu, B.O. Assessment of the phytoremediation potential of Panicum maximum (guinea grass) for selected heavy metal removal from contaminated soils. Afr. J. Biotechnol. 2014, 13, 1979–1984. [Google Scholar] [CrossRef][Green Version]
- Hasan, H.; Shloul, T.; Alomari, B.; Alhadidi, L.; Mazahreh, N. Phytoremediation ability of Panicum maximum and Salicornia europaea irrigated with treated wastewater for salt elements in the soil. J. Saudi Soc. Agric. Sci. 2024, 23, 451–457. [Google Scholar] [CrossRef]
- Al Hamedi, F.H.; Kandhan, K.; Liu, Y.; Ren, M.; Jaleel, A.; Alyafei, M.A.M. Wastewater Irrigation: A Promising Way for Future Sustainable Agriculture and Food Security in the United Arab Emirates. Water 2023, 15, 2284. [Google Scholar] [CrossRef]
- Abegunrin, T.P.; Awe, G.O.; Idowu, D.O.; Adejumobi, M.A. Impact of Wastewater Irrigation on Soil Physico-Chemical Properties, Growth and Water Use Pattern of Two Indigenous Vegetables in Southwest Nigeria. CATENA 2016, 139, 167–178. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Aziz, T.; Zia-ur-Rehman, M.; Sabir, M.; Khalid, H. Sources and Composition of Waste Water: Threats to Plants and Soil Health. In Soil Science: Agricultural and Environmental Perspectives; Hakeem, K., Akhtar, J., Sabir, M., Eds.; Springer: Cham, Switzerland, 2016; p. 16. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Wolff, D.; Krah, D.; Dötsch, A.; Ghattas, A.K.; Wick, A.; Ternes, T.A. Insights into the Variability of Microbial Community Composition and Micropollutant Degradation in Diverse Biological Wastewater Treatment Systems. Water Res. 2018, 143, 313–324. [Google Scholar] [CrossRef]
- Nayek, S.; Gupta, S.; Saha, R.N. Metal Accumulation and Its Effects in Relation to Biochemical Response of Vegetables Irrigated with Metal Contaminated Water and Wastewater. J. Hazard. Mater. 2010, 178, 588–595. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023; p. 33. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Applications of Metabolomics for the Elucidation of Abiotic Stress Tolerance in Plants: A Special Focus on Osmotic Stress and Heavy Metal Toxicity. Plants 2023, 12, 269. [Google Scholar] [CrossRef]
- Yoshida, Y.; Marubodee, R.; Ogiso-Tanaka, E.; Iseki, K.; Isemura, T.; Takahashi, Y.; Tomooka, N. Salt Tolerance in Wild Relatives of Adzuki Bean, Vigna angularis (Willd.) Ohwi et Ohashi. Genet. Resour. Crop Evol. 2016, 63, 627–637. [Google Scholar] [CrossRef]
- Bamrah, R.K.; Vijayan, P.; Karunakaran, C.; Muir, D.; Hallin, E.; Stobbs, J.; Goetz, B.; Nickerson, M.; Tanino, K.; Warkentin, T.D. Evaluation of X-ray Fluorescence Spectroscopy as a Tool for Nutrient Analysis of Pea Seeds. Crop Sci. 2019, 59, 2689–2700. [Google Scholar] [CrossRef]
- Jaglan, J.; Jaglan, S.; Jaglan, P.; Jaglan, A. Inductively Coupled Plasma Optical Emission Spectroscopy Based Toxicological Risk Assessment of Cadmium and Lead in Tinospora cordifolia. Pharmacol. Res. Mod. Chin. Med. 2023, 7, 100246. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Analytical Chemistry). Determination of Crude Fiber. In Official Methods of Analysis; AOAC: Washington, DC, USA, 1980. [Google Scholar]
- Maxson, E.; Rooney, L. Evaluation of Methods for Tannin Analysis in Sorghum Grain. Cereal Chem. 1972, 49, 719. [Google Scholar]
- Czaja, T.; Sobota, A.; Szostak, R. Quantification of Ash and Moisture in Wheat Flour by Raman Spectroscopy. Foods 2020, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Babič, J.; Tavčar-Kalcher, G.; Celar, F.A.; Kos, K.; Červek, M.; Jakovac-Strajn, B. Ergot and Ergot Alkaloids in Cereal Grains Intended for Animal Feeding Collected in Slovenia: Occurrence, Pattern and Correlations. Toxins 2020, 12, 730. [Google Scholar] [CrossRef]
- Oganesyan, É.T. Mechanism of the Reaction of Triterpenoids with Sulfuric Acid. Chem. Nat. Compd. 1980, 16, 464–468. [Google Scholar] [CrossRef]
- Verspreet, J.; Pollet, A.; Cuyvers, S.; Vergauwen, R.; den Ende, W.; Delcour, J.A. A Simple and Accurate Method for Determining Wheat Grain Fructan Content and Average Degree of Polymerization. J. Agric. Food Chem. 2012, 60, 2102–2107. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Mohammed, A.E.; van Dijk, J.R.; Beemster, G.T.S.; Alotaibi, M.O.; Saleh, A.M. The Impact of Chromium Toxicity on the Yield and Quality of Rice Grains Produced under Ambient and Elevated Levels of CO2. Front. Plant Sci. 2023, 14, 1019859. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; AbdElgawad, H.; Verbruggen, E.; Korany, S.M.; Alsherif, E.A.; Beemster, G.T.S. Biofertilisation with a Consortium of Growth-Promoting Bacterial Strains Improves the Nutritional Status of Wheat Grain under Control, Drought, and Salinity Stress Conditions. Physiol. Plant. 2022, 174, e13800. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.; AbdElgawad, H.; Asard, H. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Hartley-Whitaker, J.; Ainsworth, G.; Vooijs, R.; Bookum, W.T.; Schat, H.; Meharg, A.A. Phytochelatins Are Involved in Differential Arsenate Tolerance in Holcus lanatus. Plant Physiol. 2001, 126, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Horemans, N.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate Influences the Plant Cell Cycle through a Glutathione-Independent Reduction Mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Shabbaj, I.I.; Abdelgawad, H.; Balkhyour, M.A.; Tammar, A.; Madany, M.M.Y. Elevated CO2 Differentially Mitigated Oxidative Stress Induced by Indium Oxide Nanoparticles in Young and Old Leaves of C3 and C4 Crops. Antioxidants 2022, 11, 308. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Reid, D.M. Leaf Senescence and Lipid Peroxidation: Effects of Some Phytohormones, and Scavengers of Free Radicals and Singlet Oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Kumar, K.; Khan, P. Age-Related Changes in Catalase and Peroxidase Activities in the Excised Leaves of Eleusine coracana Gaertn. cv PR 202 during Senescence. Exp. Gerontol. 1983, 18, 409–417. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate Quantification of Enzymes of the Plant Ascorbate–Glutathione Cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for Glutathione Peroxidase Activities in Cultured Plant Cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Lundberg, M.; Johansson, C.; Chandra, J.; Enoksson, M.; Jacobsson, G.; Ljung, J.; Johansson, M.; Holmgren, A. Cloning and Expression of a Novel Human Glutaredoxin (Grx2) with Mitochondrial and Nuclear Isoforms. J. Biol. Chem. 2001, 276, 26269–26275. [Google Scholar] [CrossRef]
- Wolosiuk, R.A.; Crawford, N.A.; Yee, B.C.; Buchanan, B.B. Isolation of Three Thioredoxins from Spinach Leaves. J. Biol. Chem. 1979, 254, 1627–1632. [Google Scholar] [CrossRef]
- Wagner, G.J. Content and Vacuole/Extravacuole Distribution of Neutral Sugars, Free Amino Acids, and Anthocyanin in Protoplasts. Plant Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hamad, I.; Abdelgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.T.S.; Hegab, M.; Hagagy, N.; Selim, S.A. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.R.; Mahatma, M.K.; Jha, S.; Mahatma, L.; Parekh, V.B.; Jha, S.K. Changes in Phenylpropanoid Pathway during Compatible and Incompatible Interaction of Ricinus communis-Fusarium oxysporum f.sp. ricini. Ind. J. Agric. Biochem. 2013, 26, 56–60. [Google Scholar]
- Allina, S.M.; Pri-Hadash, A.; Theilmann, D.A.; Ellis, B.E.; Douglas, C.J. 4-Coumarate:Coenzyme A Ligase in Hybrid Poplar. Properties of Native Enzymes, cDNA Cloning, and Analysis of Recombinant Enzymes. Plant Physiol. 1998, 116, 743–754. [Google Scholar] [CrossRef]
- Beritognolo, I.; Magel, E.; Abdel-Latif, A.; Charpentier, J.P.; Jay-Allemand, C.; Breton, C. Expression of Genes Encoding Chalcone Synthase, Flavanone 3-Hydroxylase, and Dihydroflavonol 4-Reductase Correlates with Flavanol Accumulation during Heartwood Formation in Juglans nigra. Tree Physiol. 2002, 22, 291–300. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Yasunaga, G.; Iwata, H.; Subramanian, A.; Ismail, A.; Tanabe, S. Concentrations of Trace Elements in Marine Fish and Its Risk Assessment in Malaysia. Mar. Pollut. Bull. 2005, 51, 896–911. [Google Scholar] [CrossRef]
- Hagagy, N.; AbdElgawad, H. Rapeseed Plant: Biostimulation Effects of Plant Growth-Promoting Actinobacteria on Metabolites and Antioxidant Defense System under Elevated CO2 Conditions. J. Sci. Food Agric. 2024, 104, 51–62. [Google Scholar] [CrossRef]
- Shtull-Trauring, E.; Cohen, A.; Ben-Hur, M.; Israeli, M.; Bernstein, N. NPK in treated wastewater irrigation: Regional scale indices to minimize environmental pollution and optimize crop nutritional supply. Sci. Total Environ. 2022, 806, 150387. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Rabe, E.; Lovatt, C.J. Increased Arginine Biosynthesis during Phosphorus and Nitrogen Nutritional Stress in Tomato. J. Plant Nutr. 1986, 9, 905–916. [Google Scholar]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.L.; Epron, D.; Badot, P.M. Effect of Copper on Growth in Cucumber Plants (Cucumis sativus) and Its Relationships with Carbohydrate Accumulation and Changes in Ion Contents. Plant Sci. 2004, 166, 1213–1223. [Google Scholar] [CrossRef]
- Yuan, Z.; Cai, S.; Yan, C.; Rao, S.; Cheng, S.; Xu, F.; Liu, X. Research Progress on the Physiological Mechanism by Which Selenium Alleviates Heavy Metal Stress in Plants: A Review. Agronomy 2024, 14, 1787. [Google Scholar] [CrossRef]
- Sarma, H.; Dikshit, A.K.; Bandyopadhyay, M.; Mishra, S. Heavy Metals in Water: Presence, Removal and Safety. Water Air Soil Pollut. 2004, 174, 231–242. [Google Scholar]
- Vasilachi-Mitoseru, I.C.; Stoleru, V.; Gavrilescu, M. Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health. Plants 2023, 12, 3754. [Google Scholar] [CrossRef]
- Albqmi, M.; Selim, S.; Yaghoubi Khanghahi, M.; Crecchio, C.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; Al-Jaouni, S.K.; Hussein, S.; Warrad, M.; et al. Chromium (VI) Toxicity and Active Tolerance Mechanisms of Wheat Plant Treated with Plant Growth-Promoting Actinobacteria and Olive Solid Waste. ACS Omega 2023, 8, 32458–32467. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular Mechanisms of Metal Hyperaccumulation in Plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–952. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Dobra, J.; Motyka, V.; Dobrev, P.; Malbeck, J.; Prasil, I.T.; Haisel, D.; Gaudinova, A.; Havlova, M.; Gubis, J.; Vankova, R. Comparison of Hormonal Responses to Heat, Drought and Combined Stress in Tobacco Plants with Elevated Proline Content. J. Plant Physiol. 2010, 167, 1360–1370. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Al Kashgry, N.A.T.; Darwish, H.; Aljomiha, N.A.; Alharthi, S.; Alayafi, A.A.M.; Fallatah, A.M.; El-Deeb, B.A.; Abd El-Gawad, H.G.; Hewidy, M.; Al-Harbi, N.A.; et al. Silver nanoparticles alleviate the impact of soil contamination and wastewater irrigation on rosemary plants: Modulating of gene expression and secondary metabolites. Mater. Res. Express 2024, 11, 065009. [Google Scholar] [CrossRef]
- Hashem, H.A.; Hassanein, R.A.; El-Deep, M.H.; Shouman, A.I. Irrigation with industrial wastewater activates antioxidant system and osmoprotectant accumulation in lettuce, turnip and tomato plants. Ecotoxicol. Environ. Saf. 2013, 95, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar]
- Alsherif, E.A.; Yaghoubi Khanghahi, M.; Crecchio, C.; Korany, S.M.; Sobrinho, R.L.; AbdElgawad, H. Understanding the Active Mechanisms of Plant (Sesuvium portulacastrum L.) Against Heavy Metal Toxicity. Plants 2023, 12, 676. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, D.; Jerbi, B.; Medhioub, M.; Bousselmi, L.; Charef, A. Impact of Treated Urban Wastewater for Reuse in Agriculture on Crop Response and Soil Ecotoxicity. Environ. Sci. Pollut. Res. 2016, 23, 15877–15887. [Google Scholar] [CrossRef] [PubMed]
- Basiglini, E.; Pintore, M.; Forni, C. Effects of Treated Industrial Wastewaters and Temperatures on Growth and Enzymatic Activities of Duckweed (Lemna minor L.). Ecotoxicol. Environ. Saf. 2018, 153, 54–59. [Google Scholar] [CrossRef]
- Nowwar, A.I.; Farghal, I.I.; Ismail, M.A.; Elewa, I.S.; El-Lithy, M.E. Impact of Irrigation with Wastewater on Accumulation of Heavy Metals in Phaseolus vulgaris L. and Its Remediation. J. Soil Sci. Plant Nutr. 2023, 23, 761–777. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-Ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. Int. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, S.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of Flavonoids and Other Phenylpropanoid-Derived Natural Products. Part I. Chemical Diversity, Impacts on Plant Biology and Human Health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Osbourn, A.E. Saponins in Cereals. Phytochemistry 2003, 62, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).