Sediments of Hydropower Plant Water Reservoirs Contaminated with Potentially Toxic Elements as Indicators of Environmental Risk for River Basins
Abstract
1. Introduction
2. Materials and Methods
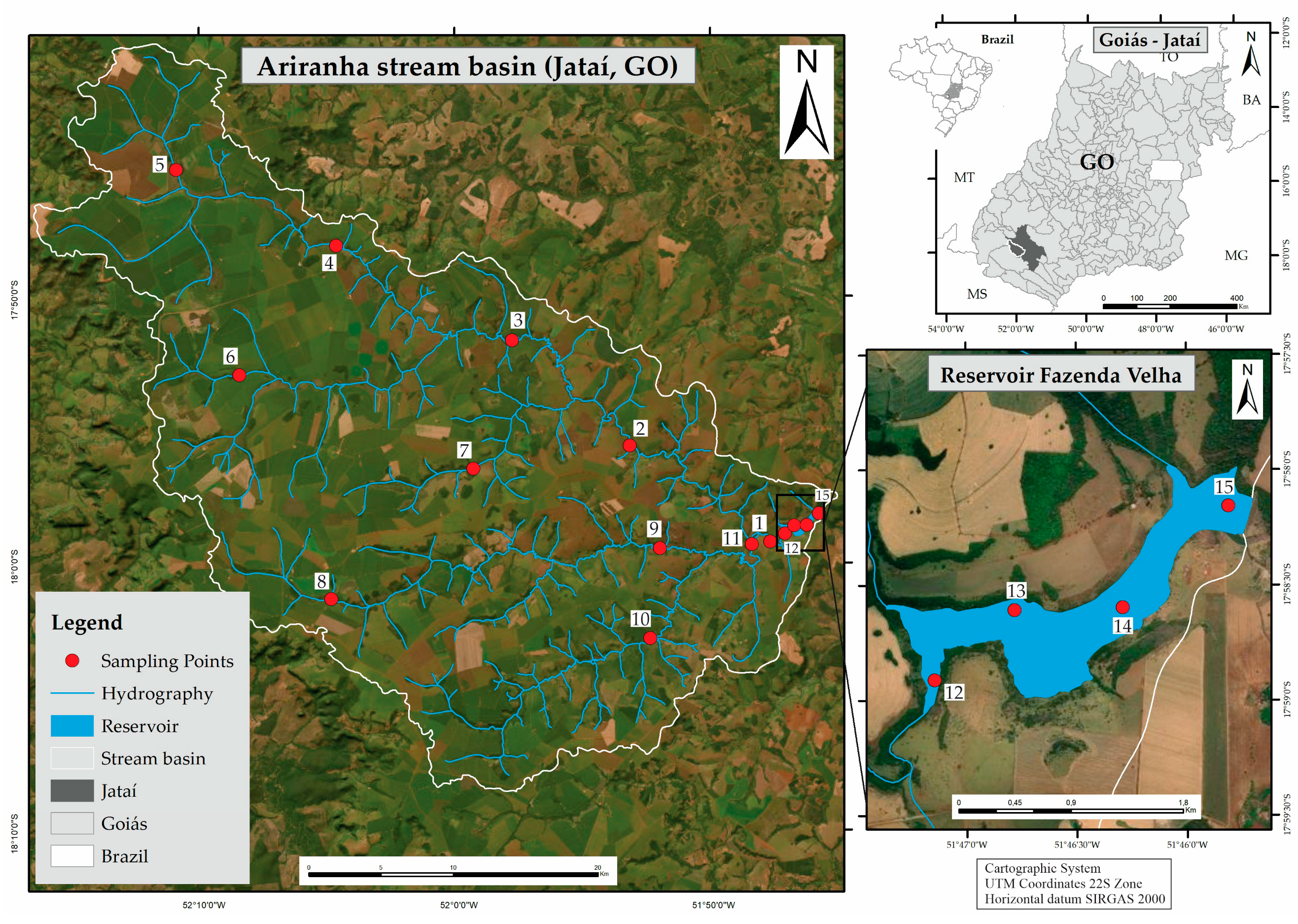
2.1. Location and Characterization of Study Area
2.2. Sediment Sampling and Sample Preparation Method
2.3. Physicochemical Analysis
2.4. Statistical Analysis
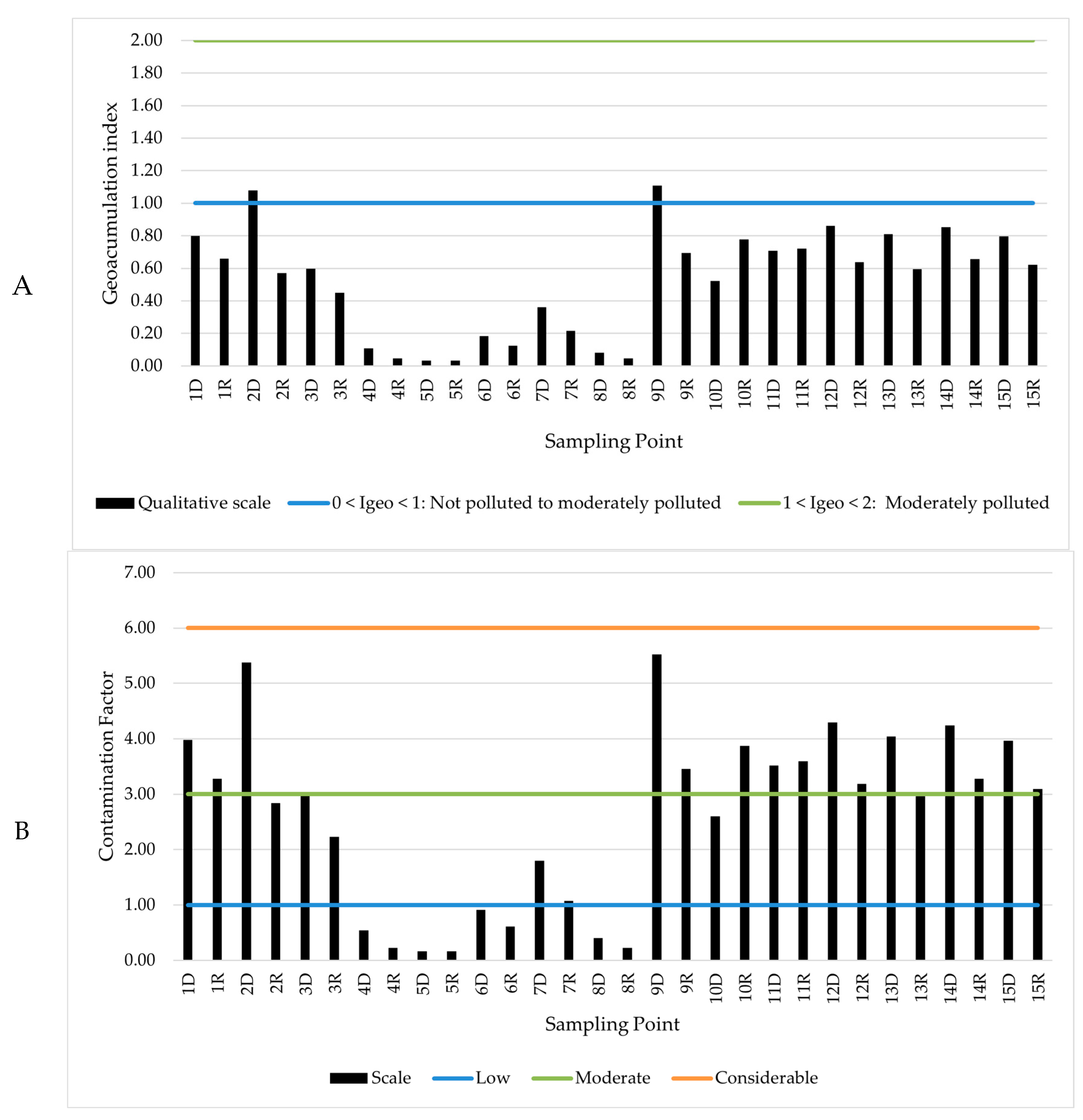
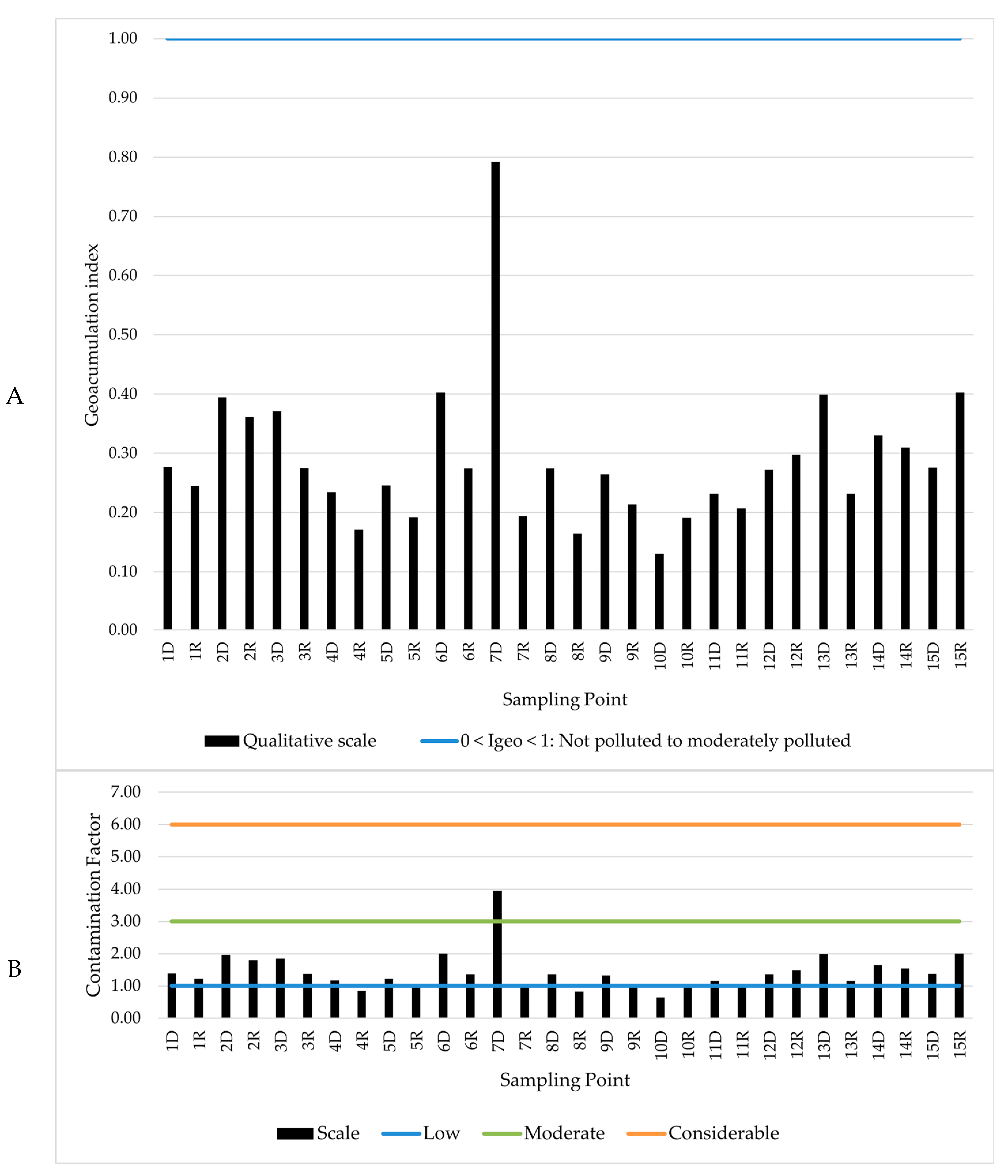
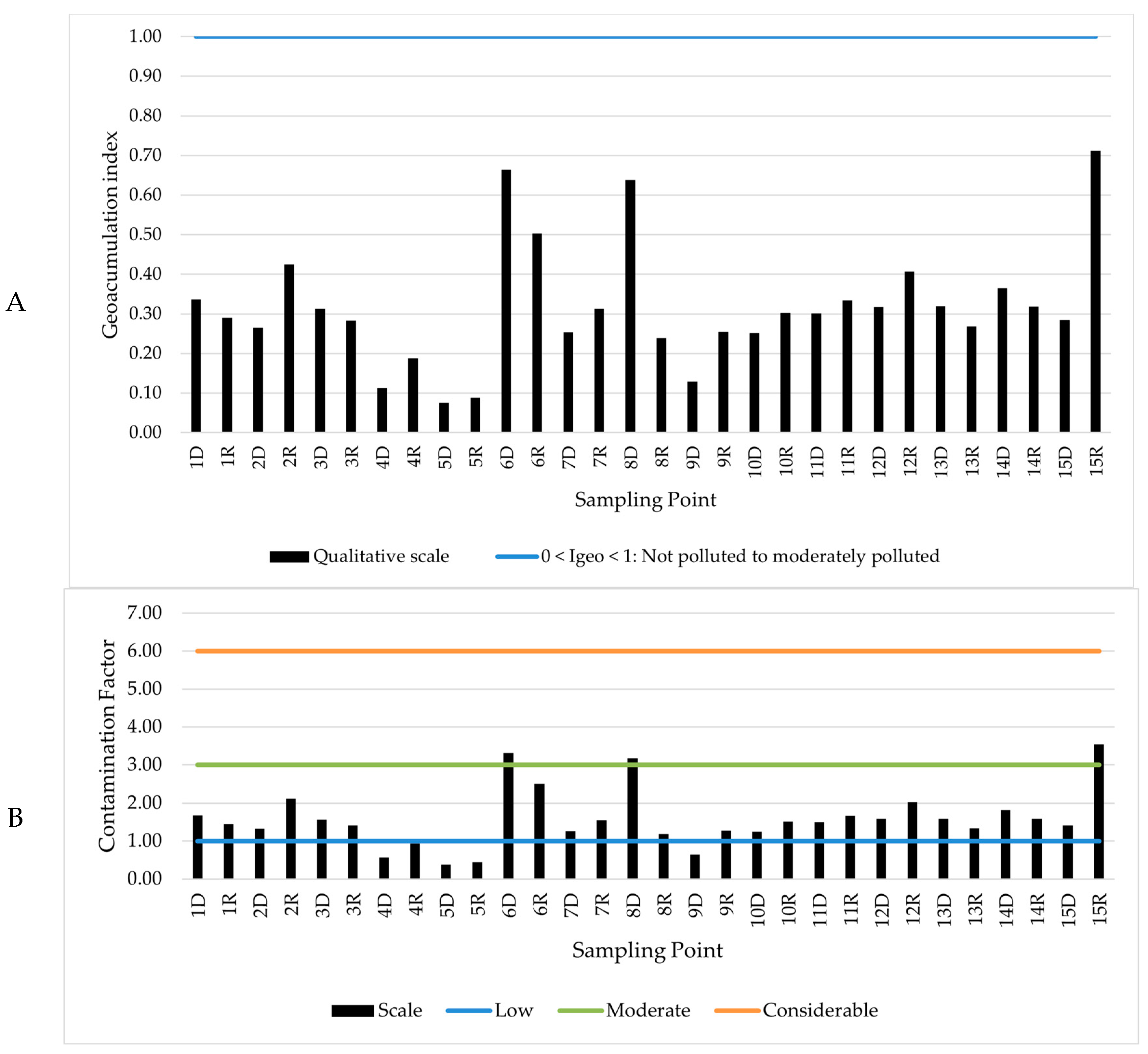
2.5. Assessment of Contamination Level of Sediments
3. Results and Discussion
3.1. Physicochemical Analysis
3.2. Distribution and Fate of PTEs in Stream Basin
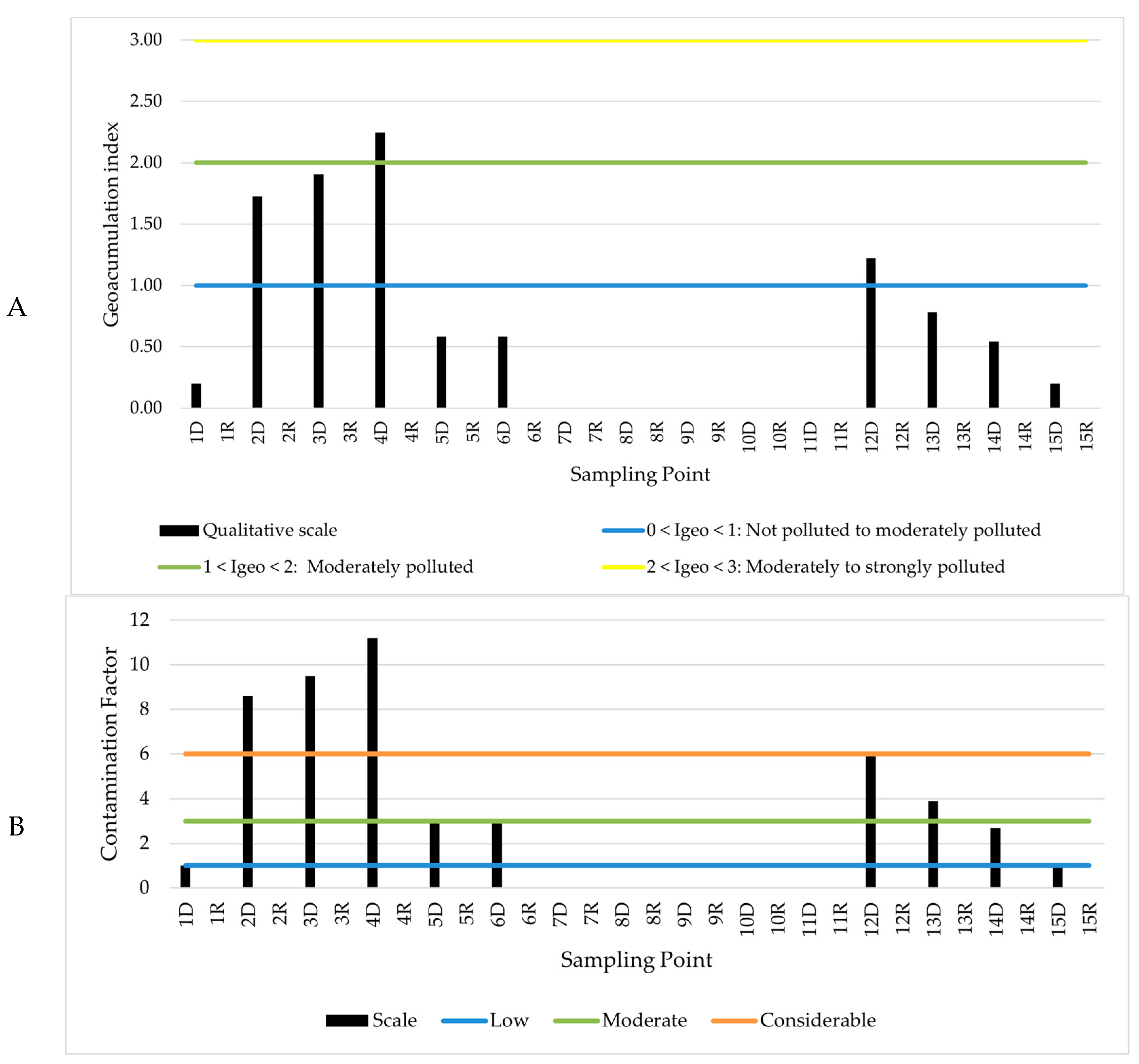
3.3. Qualitative Analysis of Sediment Contamination
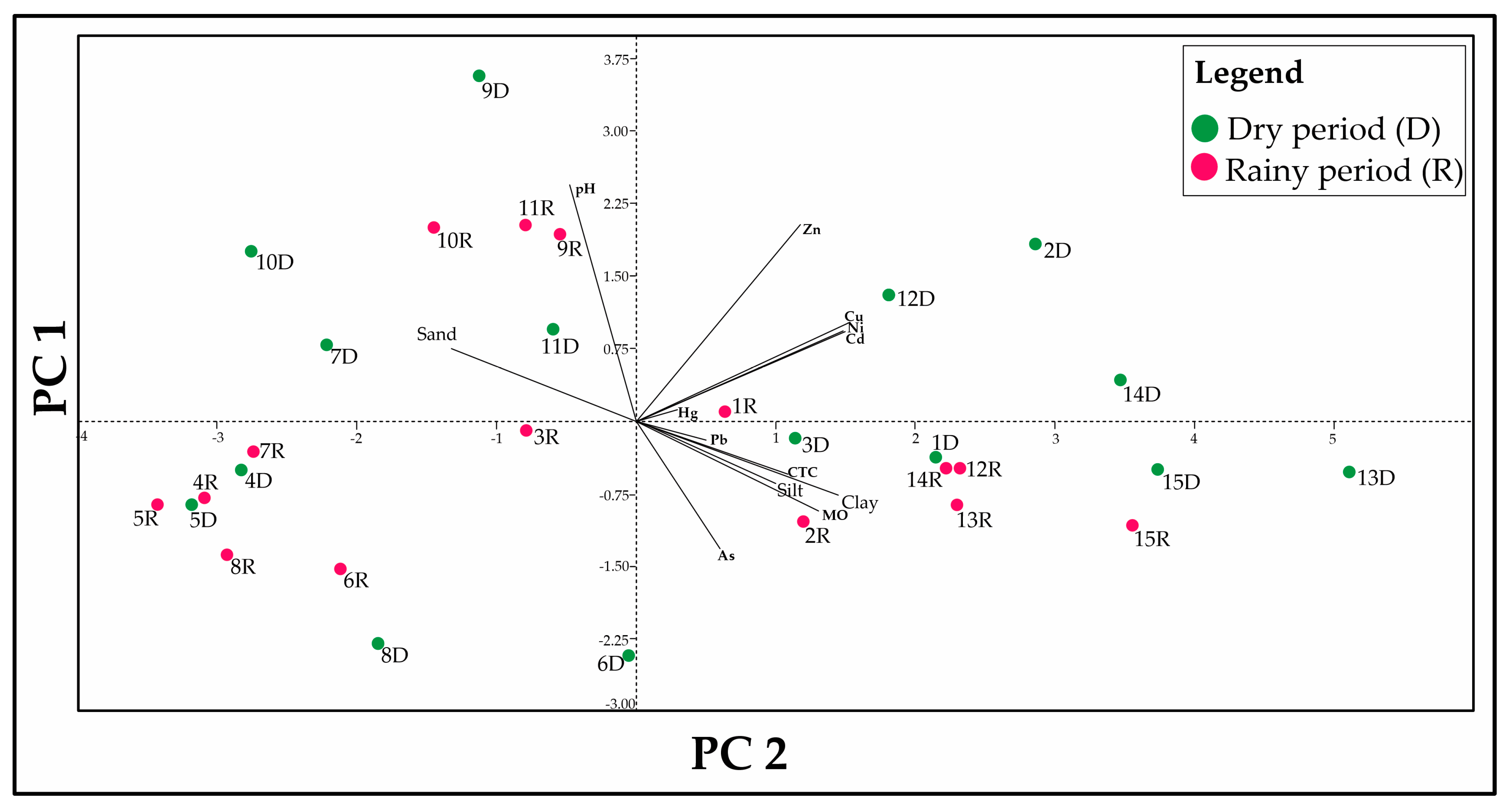
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canpolat, Ö.; Varol, M.; Okan, Ö.Ö.; Eriş, K.K. Sediment Contamination by Trace Elements and the Associated Ecological and Health Risk Assessment: A Case Study from a Large Reservoir (Turkey). Environ. Res. 2022, 204, 112145. [Google Scholar] [CrossRef] [PubMed]
- Cüce, H.; Kalipci, E.; Ustaoğlu, F.; Dereli, M.A.; Türkmen, A. Integrated Spatial Distribution and Multivariate Statistical Analysis for Assessment of Ecotoxicological and Health Risks of Sediment Metal Contamination, Ömerli Dam (Istanbul, Turkey). Water Air Soil. Pollut. 2022, 233, 199. [Google Scholar] [CrossRef]
- Varol, M.; Sünbül, M.R.; Aytop, H.; Yılmaz, C.H. Environmental, Ecological and Health Risks of Trace Elements, and Their Sources in Soils of Harran Plain, Turkey. Chemosphere 2020, 245, 125592. [Google Scholar] [CrossRef]
- Güzel, B.; Canlı, O.; Aslan, E. Spatial Distribution, Source Identification and Ecological Risk Assessment of POPs and Heavy Metals in Lake Sediments of Istanbul, Turkey. Mar. Pollut. Bull. 2022, 175, 113172. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological Risk Assessment of Heavy Metals in Sediment and Human Health Risk Assessment of Heavy Metals in Fishes in the Middle and Lower Reaches of the Yangtze River Basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- Shi, W.; Li, T.; Feng, Y.; Su, H.; Yang, Q. Source Apportionment and Risk Assessment for Available Occurrence Forms of Heavy Metals in Dongdahe Wetland Sediments, Southwest of China. Sci. Total Environ. 2022, 815, 152837. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.F.; Ling, T.Y.; Nyanti, L.; Gerunsin, N.; Wong, Y.E.; Kho, L.P. Assessment of Heavy Metals in Water, Sediment, and Fishes of a Large Tropical Hydroelectric Dam in Sarawak, Malaysia. J. Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Swarnalatha, K.; Letha, J.; Ayoob, S.; Nair, A.G. Risk Assessment of Heavy Metal Contamination in Sediments of a Tropical Lake. Environ. Monit. Assess. 2015, 187, 322. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Chen, Z.; Zhou, X.; Lu, X.; Liu, J. Pollution Characteristics and Source Analysis of Heavy Metals in Surface Sediments of Luoyuan Bay, Fujian. Environ. Res. 2022, 203, 111911. [Google Scholar] [CrossRef]
- Soares, F.R.; de Oliveira, R.G.; Rocha Sobrinho, H.M. da O Impacto Dos Metais Pesados Na Patogênese e Progressão Da Doença de Alzheimer. Rev. Eletrônica Acervo Saúde 2022, 15, e9454. [Google Scholar] [CrossRef]
- do Nascimento, L.P.; Reis, D.A.; Roeser, H.M.P.; Santiago, A.d.F. Avaliação Geoquímica de Metais Em Sistemas Fluviais Afetados Por Atividades Antrópicas No Quadrilátero Ferrífero. Eng. Sanitária E Ambient. 2018, 23, 767–778. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Hu, Y.; Chen, G.; Xu, J. The Influence of Anthropogenic Activities on Heavy Metal Pollution of Estuary Sediment from the Coastal East China Sea in the Past Nearly 50 Years. Mar. Pollut. Bull. 2022, 181, 113872. [Google Scholar] [CrossRef] [PubMed]
- Annammala, K.V.; Mohamad, N.A.; Sugumaran, D.; Masilamani, L.S.; Liang, Y.Q.; Jamal, M.H.; Yusop, Z.; Yusoff, A.R.M.; Nainar, A. Sediment Clues in Flood Mitigation: The Key to Determining the Origin, Transport, and Degree of Heavy Metal Contamination. Hydrol. Res. 2021, 52, 91–106. [Google Scholar] [CrossRef]
- Reymond, D.J.; Sudalaimuthu, K. Geospatial Visualization and Seasonal Variation of Heavy Metals in River Sediments. Glob. J. Environ. Sci. Manag. 2023, 9, 309–322. [Google Scholar]
- Shou, Y.; Zhao, J.; Zhu, Y.; Qiao, J.; Shen, Z.; Zhang, W.; Han, N.; Núñez-Delgado, A. Heavy Metals Pollution Characteristics and Risk Assessment in Sediments and Waters: The Case of Tianjin, China. Environ. Res. 2022, 212, 113162. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, B. Historical Records of Heavy Metal Accumulation in Sediments and the Relationship with Agricultural Intensification in the Yangtze–Huaihe Region, China. Sci. Total Environ. 2008, 399, 113–120. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Pinedo-Hernández, J.; Marrugo-Negrete, J.; Díez, S. Human Health Impacts of Exposure to Metals through Extreme Consumption of Fish from the Colombian Caribbean Sea. Environ. Geochem. Health 2018, 40, 229–242. [Google Scholar] [CrossRef]
- Lazăr, N.N.; Simionov, I.A.; Petrea, Ș.M.; Iticescu, C.; Georgescu, P.L.; Dima, F.; Antache, A. The Influence of Climate Changes on Heavy Metals Accumulation in Alosa Immaculata from the Danube River Basin. Mar. Pollut. Bull. 2024, 200, 116145. [Google Scholar] [CrossRef]
- Machado da Silva Acioly, T.; Francisco da Silva, M.; Iannacone, J.; Viana, D.C. Levels of Potentially Toxic and Essential Elements in Tocantins River Sediment: Health Risks at Brazil’s Savanna-Amazon Interface. Sci. Rep. 2024, 14, 18037. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Vieira, L.C.G.; Bernardi, J.V.E.; Moraes, L.d.C.; Rodrigues, Y.O.S.; de Souza, J.P.R.; de Souza, J.R.; Bastos, W.R.; Passos, C.J.S.; Dórea, J.G. Ecological Risk of Mercury in Bottom Sediments and Spatial Correlation with Land Use in Neotropical Savanna Floodplain Lakes, Araguaia River, Central Brazil. Environ. Res. 2023, 238, 117231. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dai, J.; Zhang, H.; Wan, Z.; Xu, J. Potentially Toxic Elements in Lake Sediments in China: Spatial Distribution, Ecological Risks, and Influencing Factors. Sci. Total Environ. 2023, 868, 161596. [Google Scholar] [CrossRef] [PubMed]
- Dirbaba, N.; Yan, X.; Wu, H.; Colebrooke, L.; Wang, J. Occurrences and Ecotoxicological Risk Assessment of Heavy Metals in Surface Sediments from Awash River Basin, Ethiopia. Water 2018, 10, 535. [Google Scholar] [CrossRef]
- Liu, S.; Peng, B.; Li, J. Ecological Risk Evaluation and Source Identification of Heavy Metal Pollution in Urban Village Soil Based on XRF Technique. Sustainability 2022, 14, 5030. [Google Scholar] [CrossRef]
- Lotz, T.; Opp, C. Ranking of Basin-Scale Factors Affecting Metal Concentrations in River Sediment. Appl. Sci. 2022, 12, 2805. [Google Scholar] [CrossRef]
- Ismukhanova, L.; Choduraev, T.; Opp, C.; Madibekov, A. Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan. Appl. Sci. 2022, 12, 11474. [Google Scholar] [CrossRef]
- Sánez, J.; Froehner, S.; Falcão, F. Use of Biomarkers Indices in a Sediment Core to Evaluate Potential Pollution Sources in a Subtropical Reservoir in Brazil. Geochemistry 2013, 73, 555–563. [Google Scholar] [CrossRef]
- Nogueira, P.F.; Cabral, J.B.P.; Camara, M.A.B. Qualitative Evaluation of the Risk of Heavy Metal Pollution in the Sediments of the Foz Do Rio Claro Hydroelectric Plant. Rev. Do Dep. Geogr. 2021, 41, e172916. [Google Scholar] [CrossRef]
- Birro, S.O.G.; Ross, J.L.S.; Cabral, J.B.P. Analysis of Environmental Fragility in the Area of Direct Influence of the Espora Hydroelectric Power Plant in Corrente River, Goiás, Brazil. J. Geogr. Spat. Plan. 2021, 22, 140–163. [Google Scholar] [CrossRef]
- Cabral, J.B.P.; Nogueira, P.F.; Becegato, V.A.; Becegato, V.R.; Paulino, A.T. Environmental Assessment and Toxic Metal-Contamination Level in Surface Sediment of a Water Reservoir in the Brazilian Cerrado. Water 2021, 13, 1044. [Google Scholar] [CrossRef]
- Cabral, J.B.P.; Oliveira, S.F.; dos Santos, F.F.; Becegato, V.A.; Becegato, V.R.; Paulino, A.T. Potentially Toxic Metal Environmental Pollution in Sediments of a Model Hydroelectric Plant Water Reservoir in Brazil. Environ. Earth Sci. 2021, 80, 506. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskula, J.; Siepak, M. Heavy Metals in Bottom Sediments of Reservoirs in the Lowland Area of Western Poland: Concentrations, Distribution, Sources and Ecological Risk. Water 2018, 11, 56. [Google Scholar] [CrossRef]
- Smal, H.; Ligęza, S.; Pranagal, J.; Gmitrowicz-Iwan, J. Speciation and Risk Assessment of Zn, Pb, and Cd in Bottom Sediments of Two Small Upland Dam Reservoirs, Poland. J. Environ. Manag. 2022, 322, 116041. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.P.; Kalamdhad, A.S. Mobility and Risk Assessment of Heavy Metals in Benthic Sediments Using Contamination Factors, Positive Matrix Factorisation (PMF) Receptor Model, and Human Health Risk Assessment. Environ. Sci. Pollut. Res. 2023, 30, 7056–7074. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskuła, J. Heavy Metals in River Sediments: Contamination, Toxicity, and Source Identification—A Case Study from Poland. Int. J. Environ. Res. Public. Health 2022, 19, 10502. [Google Scholar] [CrossRef]
- Jaskuła, J.; Sojka, M. Assessment of Spatial Distribution of Sediment Contamination with Heavy Metals in the Two Biggest Rivers in Poland. Catena 2022, 211, 105959. [Google Scholar] [CrossRef]
- Braga, C.d.C.; Cabral, J.B.P.; Lopes, S.M.F.; Oliveira, S.F.; Rocha, I.R. da Qualidade Dos Sedimentos Em Relação à Presença de Metais Pesados No Reservatório Da Usina Hidrelétrica de Caçu—GO. Rev. Bras. Geogr. Física 2018, 11, 959–972. [Google Scholar] [CrossRef]
- Cabral, J.B.P.; Gentil, W.B.; Ramalho, F.L.; Braga, C.C.; Becegato, V.A.; Paulino, A.T. Harmful Effects of Potentially Toxic Elements in Soils of Cerrado Biomes. Water Air Soil. Pollut. 2023, 234, 334. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento. Acompanhamento Safra Brasileira: Graos (Safra 2023/24–11 Levantamento), 1st ed.; Conab: Brasília, DF, Brazil, 2023; Volume 1. [Google Scholar]
- Costa, H.C.; Marcuzzo, F.; Ferreira, O.M.; Andrade, L.R. Espacialização e Sazonalidade Da Precipitação Pluviométrica Do Estado de Goiás e Distrito Federal (Seasonality and Spatial Distribution of Rainfall in the State of Goiás and Federal District). Rev. Bras. Geogr. Física 2012, 5, 87. [Google Scholar] [CrossRef]
- Filizola, H.F.; Gomes, M.A.F.; Souza, M.D. Manual de Procedimentos de Coleta de Amostras Em Áreas Agricolas Para Análise Da Qualidade Ambiental: Solo, Água e Sedimentos; Embrapa: Brasília, Brazil, 2006; ISBN 8585771437. [Google Scholar]
- Usepa. SW-846 Method 3051A—Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; U.S. Environmental Protection Agency: Washington, DC, USA, 2007; p. 30. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Abbireddy, C.O.R.; Clayton, C.R.I. A Review of Modern Particle Sizing Methods. Proc. Inst. Civ. Eng.—Geotech. Eng. 2009, 162, 193–201. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Hongyu, K.; Sandanielo, V.L.M.; Junior, G.J.d.O. Principal Component Analysis: Theory, Interpretations and Applications. Eng. Sci. 2015, 5, 1. [Google Scholar]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution Indices as Useful Tools for the Comprehensive Evaluation of the Degree of Soil Contamination–A Review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.; Amorós, J.A.; Pérez-de-los-Reyes, C.; García, F.J.; Moreno, M.M.; Sánchez-Ormeño, M.; Higueras, P. Influence of the Soil PH in the Uptake and Bioaccumulation of Heavy Metals (Fe, Zn, Cu, Pb and Mn) and Other Elements (Ca, K, Al, Sr and Ba) in Vine Leaves, Castilla-La Mancha (Spain). J. Geochem. Explor. 2017, 174, 79–83. [Google Scholar] [CrossRef]
- Guo, W.; Huo, S.; Xi, B.; Zhang, J.; Wu, F. Heavy Metal Contamination in Sediments from Typical Lakes in the Five Geographic Regions of China: Distribution, Bioavailability, and Risk. Ecol. Eng. 2015, 81, 243–255. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Jiang, M.; Yang, Z.; Cui, F.; Zhu, M.; Shen, L.; Hu, L. Effects of Sediment Geochemical Properties on Heavy Metal Bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y.; Zhang, Y. Distribution and Bioavailability of Heavy Metals in Different Particle-Size Fractions of Sediments in Taihu Lake, China. Chem. Speciat. Bioavailab. 2012, 24, 205–215. [Google Scholar] [CrossRef]
- Asomba, H.C.; Ezewudo, B.I.; Okeke, C.J.; Islam, M.d.S. Grain Size Analysis and Ecological Risk Assessment of Metals in the Sediments of Konsin River and Igboho Dam Reservoir, Oyo State, Nigeria, under Agricultural Disturbances. Environ. Monit. Assess. 2023, 195, 378. [Google Scholar] [CrossRef]
- Tokatli, C.; Islam, M.S. Spatiotemporal Variations and Bio-Geo-Ecological Risk Assessment of Heavy Metals in Sediments of a Wetland of International Importance in Turkey. Arab. J. Geosci. 2022, 15, 121. [Google Scholar] [CrossRef]
- Vargas González, H.H.; Arreola Lizárraga, J.A.; García Hernández, J.; Mendoza Salgado, R.A.; Zenteno Savín, T.; Méndez Rodríguez, L.C. Calidad de Sedimentos Asociada a Actividades Antrópicas En Lagunas Costeras Semiáridas Subtropicales de La Costa Central Este Del Golfo de California. Rev. Int. Contam. Ambient. 2017, 33, 7–22. [Google Scholar] [CrossRef]
- Rosini, D.N.; Becegato, V.A.; Dalalibera, A.; Vilela, P.B.; Xavier, J.d.A.; Duminelli, E.C. Avaliação Da Qualidade Dos Sedimentos Em Áreas Agrícolas Do Município de Bom Retiro/SC. Rev. Ibero-Am. Ciências Ambient. 2020, 11, 79–93. [Google Scholar] [CrossRef]
- Souza, A.M.; Salviano, A.M.; Melo, J.F.B.; Felix, W.P.; Belém, C.S.; Ramos, P.N. Seasonal Study of Concentration of Heavy Metals in Waters from Lower São Francisco River Basin, Brazil. Braz. J. Biol. 2016, 76, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, D.; Aleksander-Kwaterczak, U.; Pociecha, A.; Szarek-Gwiazda, E.; Waloszek, A.; Wilk-Woźniak, E. Small Effects of a Large Sediment Contamination with Heavy Metals on Aquatic Organisms in the Vicinity of an Abandoned Lead and Zinc Mine. Environ. Monit. Assess. 2013, 185, 9825–9842. [Google Scholar] [CrossRef] [PubMed]
- Fabijańczyk, P.; Zawadzki, J.; Wojtkowska, M. Geostatistical Study of Spatial Correlations of Lead and Zinc Concentration in Urban Reservoir. Study Case Czerniakowskie Lake, Warsaw, Poland. Open Geosci. 2016, 8, 484–492. [Google Scholar] [CrossRef]
- Maj-Zajezierska, K.; Koszelnik, P. Contamination of Bottom Sediments by Lead, Zinc and Cadmium in Rzeszow Reservoir. Environ. Prot. Nat. Resour. 2018, 29, 10–15. [Google Scholar] [CrossRef]
- Gerolin, C.R.; Pupim, F.N.; Sawakuchi, A.O.; Grohmann, C.H.; Labuto, G.; Semensatto, D. Microplastics in Sediments from Amazon Rivers, Brazil. Sci. Total Environ. 2020, 749, 141604. [Google Scholar] [CrossRef]
- Kim, B.S.M.; Salaroli, A.B.; Ferreira, P.A.d.L.; Sartoretto, J.R.; de Mahiques, M.M.; Figueira, R.C.L. Spatial Distribution and Enrichment Assessment of Heavy Metals in Surface Sediments from Baixada Santista, Southeastern Brazil. Mar. Pollut. Bull. 2016, 103, 333–338. [Google Scholar] [CrossRef]
- Bueno Guerra, M.B.; de Oliveira, C.; Rocha de Carvalho, M.; Silva, A.O.; Santana Alvarenga, I.F.; Barbosa, M.V.; Feitosa, M.M.; Penido, E.S.; Valentim dos Santos, J.; Carbone Carneiro, M.A.; et al. Increased Mobilization of Geogenic Arsenic by Anthropogenic Activities: The Brazilian Experience in Mining and Agricultural Areas. Curr. Opin. Environ. Sci. Health 2023, 33, 100472. [Google Scholar] [CrossRef]
- Rojos, S.; Aguilar, G.; Sepúlveda, B.; Pavez, O. Dinámica de La Concentración de Cobre, Plomo, Mercurio y Arsénico En Sedimentos Del RíoCOPIAPÓ, CHILE. Rev. Int. Contam. Ambient. 2019, 35, 361–370. [Google Scholar] [CrossRef]
- Mancuso, M.A.; de Azevedo, F.C.G.; Wastowski, A.D.; Fioreze, M. Determination of the Chemical Properties of Water and Sediments in Agricultural Land Use by Using Energy Dispersive X-Ray Fluorescence Spectrometry. Geol. USP. Série Científica 2016, 16, 85. [Google Scholar] [CrossRef][Green Version]
- de Carvalho, V.G.B.; do Nascimento, C.W.A.; Biondi, C.M. Potencial de Fertilizantes e Corretivos No Aporte de Micronutrientes Ao Solo. Rev. Bras. Cienc. Solo 2012, 36, 931–938. [Google Scholar] [CrossRef][Green Version]
- Parra, R.R.; Roeser, H.M.P.; Leite, M.G.P.; Nalini Jr, H.A.; Guimarães, A.T.A.; Pereira, J.C.; Friese, K. Influência Antrópica Na Geoquímica de Água e Sedimentos Do Rio Conceição, Quadrilátero Ferrífero, Minas Gerais-Brasil. Geochim. Bras. 2007, 21, 36–49. [Google Scholar]
- Kanashiro, A.M.; Xie, J. Evaluation of Chemical Properties of Dredged Materials, Paranagua Port, Brazil, According to Dredging Legislation CONAMA 454/2012. Univers. J. Manag. 2016, 4, 246–254. [Google Scholar] [CrossRef][Green Version]
- Ogino, C.M.; Costa Junior, G.; Popova, N.D.; Martines Filho, J.G. Poder de Compra, Preço e Consumo de Fertilizantes Minerais: Uma Análise Para o Centro-Oeste Brasileiro. Rev. Econ. E Sociol. Rural 2021, 59, e220367. [Google Scholar] [CrossRef]
- Baird, C. Química Ambiental, 2nd ed.; Bookman: Porto Alegre, Brazil, 2002. [Google Scholar]
- Ren, J.; Wen, Z. Distribution Characteristics of Cadmium and Arsenic in the Typical Vegetable Soil and the Sediment of Its Irrigation Sources in Xijiang River Basin at the End of the Farming Slack Period. Bull. Environ. Contam. Toxicol. 2022, 108, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Merga, L.B.; Mengistie, A.A.; Alemu, M.T.; Van den Brink, P.J. Biological and Chemical Monitoring of the Ecological Risks of Pesticides in Lake Ziway, Ethiopia. Chemosphere 2021, 266, 129214. [Google Scholar] [CrossRef]
- Vinhal-Freitas, I.C.; Corrêa, G.F.; Wendling, B.; Bobuľská, L.; Ferreira, A.S. Soil Textural Class Plays a Major Role in Evaluating the Effects of Land Use on Soil Quality Indicators. Ecol. Indic. 2017, 74, 182–190. [Google Scholar] [CrossRef]
- Guilherme, L.R.G.; Marques, J.J.; Pierangeli, M.A.P.; Zuliane, D.Q.; Campos, M.L.; Marchi, G. Elementos-Traço Em Solos e Sistemas Aquáticos. In Tópicos em Ciência do Solo; Torrado-Vidal, P., Alleoni, L.R.F., Cooper, M., Silva, A.P., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2005; pp. 345–390. [Google Scholar]
- Dragović, S.; Mihailović, N.; Gajić, B. Heavy Metals in Soils: Distribution, Relationship with Soil Characteristics and Radionuclides and Multivariate Assessment of Contamination Sources. Chemosphere 2008, 72, 491–495. [Google Scholar] [CrossRef]
- Akbar, M.J.; Al-Tamimi, O.S. Ecological Indices of the Heavy Metals in the Soil of Shewasoor Sub-Basin, Kirkuk NE Iraq. Open Sci. J. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- Ferreira Batista, D.; Cabral, J.B.P. Avaliação qualitativa dos níveis de contaminação por elementos potencialmente tóxicos na bacia do ribeirão santo antônio em Iporá-GO. Geoambiente -Line 2023, 45, 24–50. [Google Scholar]
- Cabral, J.B.P.; Nogueira, P.F.; Ramalho, F.L.; Maldonado, F.D.; Becegato, V.A.; Paulino, A.T. Environmental Contamination Levels Based on Geochemical Properties of Soils from Direct Influence Areas of Hydroelectric Power Plants. Arab. J. Geosci. 2023, 16, 327. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A Critical Review on Various Remediation Approaches for Heavy Metal Contaminants Removal from Contaminated Soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-G.; Gao, Y.-P.; Chen, F.; Huang, H.-H.; Yu, S.-H.; Jordan, R.W.; Jiang, S.-J. Risk Assessment of Heavy Metal and Pesticide Mixtures in Aquatic Biota Using the DGT Technique in Sediments. Water Res. 2022, 224, 119108. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, X.; Qiu, Y.; Cheng, J.; Chen, Y.; Wang, L.; Zhang, L.; Zhao, S.; Wang, X.; Sun, C. Characterization and Risk Assessment of Heavy Metals in River Sediments on the Western Bank of Taihu Lake, China. Bull. Environ. Contam. Toxicol. 2022, 109, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, L.; Liang, T.; Zhang, Y.; Li, J.; Xiao, J.; Dong, L.; Zhang, H. Geostatistical Analyses and Co-Occurrence Correlations of Heavy Metals Distribution with Various Types of Land Use within a Watershed in Eastern QingHai-Tibet Plateau, China. Sci. Total Environ. 2019, 653, 849–859. [Google Scholar] [CrossRef]
- Zhou, P.; Zeng, D.; Wang, X.; Tai, L.; Zhou, W.; Zhuoma, Q.; Lin, F. Pollution Levels and Risk Assessment of Heavy Metals in the Soil of a Landfill Site: A Case Study in Lhasa, Tibet. Int. J. Environ. Res. Public. Health 2022, 19, 10704. [Google Scholar] [CrossRef]
- Campos, M.L.; Guilherme, L.R.G.; Marques, J.J.G.d.S.e.M.; Curi, N.; Araújo, A.S.A.; Miquelluti, D.J.; Lopes, C.; Spiazzi, F.R. Arsenate and Cadmium Content in Soils of the Cerrado Bioma. Rev. Bras. Cienc. Solo 2013, 37, 281–286. [Google Scholar] [CrossRef][Green Version]
- de Menezes, M.D.; Bispo, F.H.A.; Faria, W.M.; Gonçalves, M.G.M.; Curi, N.; Guilherme, L.R.G. Modeling Arsenic Content in Brazilian Soils: What Is Relevant? Sci. Total Environ. 2020, 712, 136511. [Google Scholar] [CrossRef]




| Quality References (mg kg−1) | Potentially Toxic Elements | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cu | Hg | Ni | Zn | Pb | As | |
| Quality reference values | 0.34 | 19.93 | 0.10 | 6.14 | 17.25 | 8.27 | 2.31 |
| Level I contamination | 0.60 | 35.70 | 0.17 | 18.0 | 123.0 | 35.0 | 5.90 |
| Level II contamination | 3.50 | 197.0 | 0.49 | 35.9 | 315.0 | 91.3 | 17.0 |
| Minimum | Mean | Maximum | Stand. Dev. | CV | |
|---|---|---|---|---|---|
| pH | 3.90 | 4.50 | 5.40 | 0.44 | 12.8 |
| OM (%) | 6.30 | 16.35 | 31.5 | 7.89 | 46.2 |
| CEC (cmol dm−3) | 2.30 | 8.36 | 19.5 | 3.81 | 23.8 |
| Clay (%) | 7.00 | 22.1 | 59.0 | 13.1 | 63.0 |
| Silt (%) | 4.00 | 7.36 | 24.0 | 4.64 | 21.0 |
| Sand (%) | 30.0 | 70.2 | 88.0 | 16.60 | 200 |
| Zn (mg kg−1) | 2.79 | 45.1 | 95.3 | 27.8 | 128 |
| Cu (mg kg−1) | 2.79 | 73.5 | 166 | 51.9 | 210 |
| As (mg kg−1) | 0.87 | 3.67 | 8.20 | 1.74 | 10.5 |
| Hg (mg kg−1) | 0.10 | 0.49 | 1.12 | 0.36 | 136 |
| Ni (mg kg−1) | 1.87 | 14.5 | 30.3 | 8.84 | 41.5 |
| Pb (mg kg−1) | 5.39 | 11.9 | 32.7 | 4.99 | 33.4 |
| Cd (mg kg−1) | 0.19 | 5.43 | 11.3 | 3.35 | 15.5 |
| Factor | p-Value | |
|---|---|---|
| FC | Elements | 3.660552 × 10−40 |
| Point | 2.592930 × 10−5 | |
| Residues | NA | |
| Igeo | Elements | 3.478105 × 10−40 |
| Point | 2.559377 × 10−5 | |
| Residues | NA |
| pH | OM | CEC | Clay | Silt | Sand | Zn | Cu | As | Hg | Ni | Pb | Cd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||||
| OM | −0.53 | 1 | |||||||||||
| CEC | −0.38 | 0.49 | 1 | ||||||||||
| Clay | −0.43 | 0.66 | 0.56 | 1 | |||||||||
| Silt | −0.14 | 0.36 | 0.30 | 0.56 | 1 | ||||||||
| Sand | 0.37 | −0.65 | −0.52 | −0.95 | −0.79 | 1 | |||||||
| Zn | 0.33 | 0.34 | 0.31 | 0.44 | 0.24 | −0.42 | 1 | ||||||
| Cu | −0.01 | 0.65 | 0.55 | 0.68 | 0.43 | −0.68 | 0.88 | 1 | |||||
| As | −0.23 | 0.41 | 0.21 | 0.34 | 0.24 | −0.35 | 0.00 | 0.19 | 1 | ||||
| Hg | −0.19 | 0.25 | 0.06 | 0.12 | −0.09 | −0.06 | 0.10 | 0.14 | −0.17 | 1 | |||
| Ni | −0.11 | 0.67 | 0.61 | 0.67 | 0.34 | −0.63 | 0.85 | 0.95 | 0.12 | 0.33 | 1 | ||
| Pb | −0.13 | 0.23 | −0.05 | 0.23 | 0.10 | −0.22 | 0.14 | 0.23 | 0.27 | 0.16 | 0.23 | 1 | |
| Cd | 0.00 | 0.61 | 0.46 | 0.70 | 0.46 | −0.70 | 0.86 | 0.97 | 0.30 | 0.08 | 0.90 | 0.37 | 1 |
| Degrees of correlation | Perfect | Strong | Fair | Weak | Null | ||||||||
| Eigenvalue | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| % Variance | 6.23 | 1.94 | 1.34 | 1.15 |
| 47.9 | 14.9 | 10.3 | 8.91 | |
| pH | −0.11 | 0.58 | −0.30 | 0.09 |
| OM | 0.31 | −0.22 | 0.19 | 0.04 |
| CEC | 0.26 | −0.13 | 0.07 | −0.41 |
| Clay | 0.35 | −0.19 | −0.04 | −0.07 |
| Silt | 0.24 | −0.16 | −0.39 | −0.11 |
| Sand | −0.35 | 0.20 | 0.17 | 0.09 |
| Zn | 0.28 | 0.49 | −0.03 | 0.00 |
| Cu | 0.37 | 0.24 | 0.00 | −0.01 |
| As | 0.14 | −0.32 | −0.30 | 0.40 |
| Hg | 0.07 | 0.03 | 0.73 | 0.06 |
| Ni | 0.36 | 0.22 | 0.19 | −0.06 |
| Pb | 0.12 | −0.05 | 0.11 | 0.78 |
| Cd | 0.37 | 0.22 | −0.08 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, J.B.P.; Gentil, W.B.; Ramalho, F.L.; de Barcelos, A.A.; Becegato, V.A.; Paulino, A.T. Sediments of Hydropower Plant Water Reservoirs Contaminated with Potentially Toxic Elements as Indicators of Environmental Risk for River Basins. Water 2024, 16, 2733. https://doi.org/10.3390/w16192733
Cabral JBP, Gentil WB, Ramalho FL, de Barcelos AA, Becegato VA, Paulino AT. Sediments of Hydropower Plant Water Reservoirs Contaminated with Potentially Toxic Elements as Indicators of Environmental Risk for River Basins. Water. 2024; 16(19):2733. https://doi.org/10.3390/w16192733
Chicago/Turabian StyleCabral, João Batista Pereira, Wanderlubio Barbosa Gentil, Fernanda Luisa Ramalho, Assunção Andrade de Barcelos, Valter Antonio Becegato, and Alexandre Tadeu Paulino. 2024. "Sediments of Hydropower Plant Water Reservoirs Contaminated with Potentially Toxic Elements as Indicators of Environmental Risk for River Basins" Water 16, no. 19: 2733. https://doi.org/10.3390/w16192733
APA StyleCabral, J. B. P., Gentil, W. B., Ramalho, F. L., de Barcelos, A. A., Becegato, V. A., & Paulino, A. T. (2024). Sediments of Hydropower Plant Water Reservoirs Contaminated with Potentially Toxic Elements as Indicators of Environmental Risk for River Basins. Water, 16(19), 2733. https://doi.org/10.3390/w16192733







