Abstract
The spread of sargassum on beaches in Africa, Brazil, Central America, and the Caribbean has increased to become a social, environmental, and economic problem. In recent years, the presence of biomass on the coasts of the Mexican Caribbean has been recorded as ≈2360 m3 Km−1, reaching up to 200 m wide in the northern part of the coasts. Its removal from the coast and, later, the continent is one of the strategies implemented to mitigate its impact on land. Several studies have reported the seasonality of and geographic variation in sorbed metals in sargasso. However, it is unknown whether these metals can mobilize or remain in sargassum tissue once they reach accumulation sites. This study included seawater, sargassum tissue as a consortium, and S. natans and S. fluitans, as well as the leachate generated in the process of degradation per se and percolated by rain. Of the 10 metals evaluated (As, B, Fe, Zn, Mn, Cd, Al, Ni, Cu, and Pb for water, tissue, and leachate), only B is recurrent in water from the north of the Mexican Caribbean, in addition to traces of Al and Fe. Meanwhile, in tissue, the results coincide with those of previous studies, where As is recurrent, although its concentration varies with the mentioned variability. The leachate showed that four to eight metals of those present in the tissue were detected, including As, Fe, and Al, which represent a potential impact on coastal systems and infiltration into shallow water table areas.
1. Introduction
Macroalgae represent an important ecosystem because they can provide shelter, food, and habitats for some fish and invertebrates, and they are considered environmental bioindicators; for example, the presence of oil has been recorded in the tissue of some species of the Sargassum genus. In the same sense, there is a hypothesis that the effects of climate change, as well as variations and deviations in marine currents, have promoted conditions of overproduction and displacement from their point of origin (North Equatorial Recirculation Region) to the Caribbean [1,2].
Since 2011, several points in the Mexican Caribbean began to receive atypical quantities of pelagic sargasso, and since 2014, extremely large quantities (2360 tons in December) have been recorded along the entire coast of the state of Quintana Roo, Mexico, with a record of more than 10 million tons in 2019 [1,3].
A deterioration of coastal ecosystems on the Mexican Caribbean coast has been observed due to urban and tourist development. In addition to these causes, a new one has been added in the past decade, namely, the massive arrival of a sargasso (Sargassum spp.) [3,4]-like consortium, which mainly consists of S. fluitans III and S. natans (I and VIII). Since there is evidence that the large quantities of sargasso arriving can cover nearly 200 m of area around the coast, the extension and persistence of a sargasso surface strip can cause alterations in the direct marine environment, including limited light, alterations in pH, dissolved oxygen, and turbidity, and increased concentrations of organic matter, compromising water quality [3].
Until now, thousands of tons have continued to be reported annually. Fortunately, there are satellite tools for monitoring this, which allows organizations to execute established plans for the management of this marine resource [1,5]. The Ministry of the Environment and Natural Resources [6] stipulated technical and management guidelines for attention to the contingency caused by sargasso in the Mexican Caribbean and the Gulf of Mexico, establishing strategies for the collection of sargasso in containment sites and beaches. However, given the excessive quantities that surpass the capacities of the management plan, the accumulation of sargassum residues has been maintained both at beach points and the inland of the continent, which continues to represent a risk of the alteration of the environment where it is deposited.
The process of degradation and stabilization of organic matter results in the production of impacting agents, such as the generation of leachate, which is the liquid generated from the biochemical disintegration of organic waste, surface runoff, and rain infiltration that, due to gravity, crosses the thickness of the materials, carrying with it compounds in dissolved or suspended form [7,8]. Leachate is considered a highly polluting waste, so it has a negative impact on the environment [9]. It is characterized by a high content of organic matter, macro-components, and trace elements (metals), which cause adverse effects, such as eutrophication, which leads to a trophic alteration of surface and groundwater bodies. The same happens with metals, which can be assimilated by primary producers (bioaccumulation), magnifying their accumulation through the food chain [10,11,12].
There is evidence that sorption and desorption mechanisms of compounds can occur in the tissue of the genus Sargassum spp. during its development as a pelagic biomass. This process involves the fixation of nutrients, as well as carbon dissolved in the marine environment [13]. Consequently, it contributes to the formation of carboxylic groups, complex carbohydrates, and alginates in its tissue. This last polymer is believed to confirm the porosity and permeability of the tissue. That is, it contributes to the absorption or rejection of elements that are foreign to the needs of biomass development. Among them are cationic species, such as some metals [13,14,15].
For all biomass, when decomposition occurs outdoors, as is the case with Sargassum spp., the material is broken down into several states, generating gasses, leachate drainage, and residual material, which can occur due to the interaction of various factors, such as changes in humidity, temperature, and microorganisms that take advantage of the available substrate [16,17].
Some authors have mentioned that there is variability over time in the individual incidence of certain metals in the sargasso biomass. In this regard, the evaluation of the presence of metals present in the sargasso tissue, as well as those present in the generated leachate, is of the utmost importance, since it can represent an impact on infiltration at accumulation sites [18,19].
Olguin-Maciel et al. [20] reported that the decomposition of one ton of sargassum can generate up to nearly 600 L of leachate in one month of accumulation, which, in addition to organic matter, contains various metals, with arsenic being the most concentrated (about 6 g/ton). These records correspond to the content and decomposition of the sargasso that arrived in 2020. In that year, the arrival of 40,000 tons was recorded at the highest bloom peaks. That is, there was a probability of generating around 24 million L of leachate in that year.
The structure of the region is mainly made up of permeable and soluble carbonate rocks, which, when dissolved, leave little residue, generating soils with significantly reduced thickness. This results in the presence of a mature karst aquifer system with wide fissures, fractures, and caves, causing high vulnerability in the aquifer due to its characteristics, which allow the infiltration and dragging of pollutants. The processes of attenuation of pollutants, such as retention, mineralization, and absorption, are null or inefficient, so contamination is exacerbated [21].
To date, there are no studies on the impact of sargasso leachate on aquifers or on their composition, so the physicochemical characterization and analysis of metals in the leachate produced by the degradation of sargasso is of utmost importance. The contribution of this information will make it possible to model the impact that sargasso leachate may have on the groundwater of the karstic aquifer in the coastal zone of Quintana Roo in the future.
The information presented below is the second part of a project that was previously presented in the article “Assessment of Leachate Generated by Sargassum spp. in the Mexican Caribe: Part 1 Spatial Variations“ [22], which evaluated the leachate generated and its conditions in terms of freshness in relation to the distance from the coastline and as dry residue that has moved to continental deposits as a Sargassum spp. consortium, as well as to evaluate the influence of the species on this degradation.
On this occasion, using the same methodology as that mentioned in the first part, with the same sargasso collections and procedures and with the objective of visualizing the mobility and incidence of the metals that are present, the metals’ transition from sargassum tissue to leachates was analyzed, and their presence in the collected water was verified. This study was carried out to contribute information to improve strategic management plans and the possible impacts that can be generated in the aquifer system of the coastal zone.
2. Material and Methods
2.1. Study Sites
The collection of sargassum samples was carried out in two periods: from September 17 to 24, 2020, and in April and May 2021 in the northern area of the Mexican Caribbean coast, and fresh and residual collection areas were included (Figure 1). Five sampling points were selected, with four being offshore: in the municipality of Puerto Morelos, samples were collected before the sargassum containment barrier (AB) and after the sargassum containment barrier (DB) in an area called Punta Brava. Beach samples (PL) and dry accumulation in inland deposits (DP) were also collected. The AL collection was carried out 6–19 km away from the coast in front of the municipalities of Benito Juárez and Isla Mujeres.
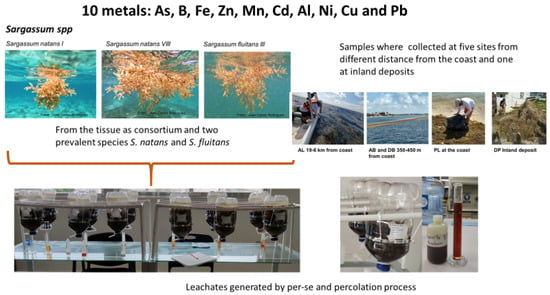
Figure 1.
Graphic representation of the evaluation of metals transported from sargassum tissue to the generated leachate from collected fresh (AL: 6–19 Km; AB and DB: 350–450 m off-shore; PL: on shore) and residual (DP: deposit inland) sargassum during two periods in 2020 and 2021.
Two types of samples were defined: (a) fresh, with sargassum floating adrift, taking the beach as a reference (AB, DB, and AL), and (b) residual, with sargassum that ran aground on the beach and accumulated in deposits within the coastal area (PL and DP). At the same time as the collection of the tissue, water samples were also collected as indicated by the Standard Methods for the Examination of Water and Wastewater, and in situ pH, salinity, and temperature readings were taken using a field probe (brand: Conductronic model Conductronic18).
2.2. COLSAR Design and Operation to Generate Leachate
From the methodology described by Leal-Bautista et al. [22], collectors (COLSAR) were built using transparent polyethylene terephthalate (PET) plastic containers with a volume of 6 L, as presented in Figure 1. They were subsequently attached to a support using 1” PVC pipes with the following dimensions: 110 cm long × 15 cm wide × 37.5 cm high. The assembly of the leachate collectors was kept inside the laboratory of the Water Science Unit at a room temperature of 24 °C.
We evaluated two degradation processes: (a) per se for all of the fresh samples and (b) a percolation process. In the first assessment, we evaluated the volume generated by one kilogram of sargassum, as presented in Figure 2. The per se process occurred through the natural degradation of sargassum without adding water; on the other hand, for the percolation process, ultrapure water (resistivity: 18.2 MΩ·cm 25 °C; total organic carbon (TOC): ≤5 ppb) was used. The volume of water used was based on the average rainfall in the collection months from the last ten years in the state of Quintana Roo (see the Supplementary Information), which represented 84 mL of water added per day at each COLSAR for 10 days.
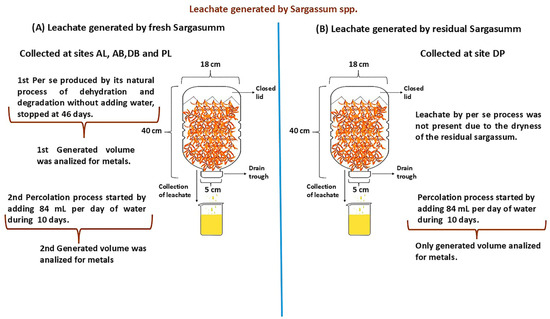
Figure 2.
The assembly of the leachate collectors was kept inside the laboratory of the Water Science Unit at 24 °C. The figure shows the two degradation processes: (A) the per se process for all fresh samples (AL, AB, DB, and PL); (B) the percolation process for AL, AB, DB, PL, and DP. The percolation process started when the per se process showed a decrease in the generated leachate volume.
The leachate was collected in a graduated cylinder, stored in high-density polyethylene (HDPE) bottles, and refrigerated at 4 °C for further laboratory analysis.
2.3. Leachate Collection and Characterization
The leachate was obtained through two different processes, as described in Part 1 [22] of this assessment; a) the first was generation through the natural transformation of sargassum; the resulting leachate was called leachate per se. This process was maintained until the leachate stopped draining liquid. The DP sample did not present leachate per se because the sargasso in these conditions had already undergone a dehydration process at the deposit sites, so it was dry. Once completed, b) the leachate was obtained by simulating rainfall, and it was called percolation leachate. For this purpose, reagent type I water was used. The volume of water used was based on the average rainfall in the collection months from the last ten years in the state of Quintana Roo [23,24].
The collected leachate was placed in high-density polyethylene (HDPE) bottles and stored in a refrigerator at 4 °C for subsequent characterization. At the end of each of the leachate generation processes, the following values were determined: the pH according to NMX-AA-008-SCFI-2016 [25], and the determination of conductivity (EC) as indicated by NMX-AA-093-SCFI-2000 [26]. Both readings were taken with a multisonde model PC18 from Conductronic.
2.4. Determination of Metals in Water from the Sargassum Collection Site and Sargassum as Tissue
The presence of metals was reported in sargassum collected in the Caribbean as a consortium and as individual species in 2020 and 2021 in [3,4], indicating the recurrent presence of As, B, Al, Cu, Fe, and Zn. On the other hand, the analysis of Cd and Pb showed that they were below the limits of detection. Moreover, Cipolloni et al. [27] reported the presence of metals in S. fluitans III and S. natans (I and VIII), which resulted in contamination. Thus, in this assessment, we focused on the same species and metals, including Ni and Mn.
To determine the number of metals that prevailed in the leachate generated by sargassum, we analyzed the water collected at the site when the sargassum was collected. Sargassum tissue was analyzed in the form of a Sargassum spp. consortium for 2020 and 2021, and the species of S. natans and S. fluitans were only separated in the 2021 collection. Finally, the leachate generated through the per se and percolation processes can be seen in Figure 2.
Water samples were collected as directed by the Standard Methods for the Examination of Water and Wastewater [28] regarding containers, quantity, and preservation. All samples for water analysis were filtered using a nitrocellulose membrane with a pore size of 0.45 μm and kept in the cold (4 °C) until laboratory analysis. Prior to the analysis of metals, the concentrations of nitrites (NO2− mg/L) were evaluated with the colorimetric method through reaction with sulfanilamide, in accordance with the NMX-AA-099-SCFI-2006 standard [29]. Ammonium ion concentrations in saltwater or brackish water samples were analyzed using the phenyl method, while for freshwater (groundwater) samples, the salicylate method was used [30]. To determine nitrates (N-NO3− mg/L), the colorimetric brucine reaction method was used according to the EPA 352.1 technique [31]. The concentrations of total orthophosphates were determined with the ascorbic acid method, following the methodology of EPA-600/4-79-020 [32].
2.5. Metals in Sargasso
For the evaluation of metals in sargassum tissue, samples were digested in a MARS Xpress acid-digesting microwave; 0.50 g of biomass was weighed out, to which 9 mL of nitric acid (HNO3), 1 mL of hydrogen peroxide (H2 O2), and the amount of deionized water necessary to reach 25 mL were added. Each sample was brought to a final volume (mL) of 25 mL in a volumetric flask with deionized water. This methodology was adapted from protocol 3052 EPA for the microwave-assisted acid digestion of siliceous and organically based matrices [33]. For quantification, an inductively coupled optical plasma emission spectrometer (ICP-OES Perkin Elmer Optima 8000, Shelton, CT, USA) was used. A calibration curve was established for each analyte using a standard. This method was applied in the Sargassum spp. consortia from 2020 and 2021, as well as species of the S. natans and S. fluitans from 2021. All samples were analyzed in triplicate.
2.6. Metals in the Water and Leachate
These samples did not require digestion; they were acidified with 100 μL of ultrapure nitric acid and later directly introduced into the same equipment (ICP-OES Perkin Elmer Optima 8000) since they were in a liquid state; the results are expressed in mg/L for that reason. All analyses were performed in triplicate, standards were used, and the Syngistyx software (Version 3.0.4.3510) was used for ICP-OES.
From the establishment of the methodology to the analysis, the appropriate standards were used. Sigma Aldrich’s Multielement Standard Solution 6 for ICP (Lot: BCCC9249) and Perkin Elmer Pure’s Instrument Standard 4 (Lot#2-85 MKBY1) were used for calibration curves. A standard point calibration curve was used as a checking standard throughout the analysis to ensure proper operation of the equipment. In the absence of a certified reference material equal to the matrices, the most similar available was used, i.e., 1640 Trace Elements in Natural Water, which resulted in recovery percentages greater than 80%, ensuring compliance with the methodology in terms of precision and accuracy.
Subsequently, the data analysis was carried out with the Syngistyx software for ICP-OES. The results obtained are expressed in mg/L for the aqueous phase and in mg/Kg for solid-phase tissue.
3. Results
As mentioned, 10 metals were evaluated—As, B, Fe, Zn, Mn, Cd, Al, Ni, Cu, and Pb—in water, tissue, and leachate.
3.1. Metals in Water
The results for the water samples indicated their consistency with the marine characteristics, as presented in Table 1, where the salinity and pH characteristics of these conditions appear.

Table 1.
Water conditions at the sites where sargassum was collected.
The presence of nitrogen (nitrites, nitrates, and NH4) and phosphate was detected in very low concentrations in some of the collection sites during the first period. In the second period, they were reported as below the detectable level (BDL). These compounds were only considered to characterize the collected water and verify its marine origin.
In the case of the 10 metals evaluated, boron was consistently present at all sites in both periods in a range from 1.92 to 3.66 mg/L; the presence of aluminum and zinc was reported in two sites near the coast in only 2020, as shown in Table 2. Arsenic was not detected in the Mexican Caribbean water in either of the two collection periods (2020 and 2021).

Table 2.
Metals detected in water at the Mexican Caribbean sites.
3.2. Metals in Tissue
The metals detected in the Sargassum spp. consortium tissue are presented below for the consortium for 2020 and for the consortium and by species for 2021. The following 10 metals were also evaluated in all tissues: As, B, Fe, Zn, Mn, Cd, Al, Ni, Cu, and Pb, the results of which are shown in Table 3. The absence of lead (Pb) and the constant presence of As, Fe, Al, and Cd are noteworthy.

Table 3.
Metals in Sargassum spp. in 2020 and 2021 and by species.
Although the concentration of metals detected varied by site and period, their absence and presence stood out because the tissue was the sorbent matrix. In this way, lead was not detected in any tissue when analyzed either as a consortium or as a species. The most recurrent metals were As, B, Fe, Zn, Al, and Cu. Manganese was only detected in 2020 and was not present in 2021 in the analyses of both the consortium and the species.
3.3. Metals in Leachate
In the case of the leachate obtained for the same periods, results were obtained from the per se process and the percolation process. The fewest metals were detected in 2021 for percolation leachate 4 as a consortium and for S. natans. Of the 10 metals detected in tissue, eight of them were present in a minimum of four mobile phases in the leachate. As was a recurrent element detected in the greatest amount in comparison with the other metals, whose variations could result in them being detected in the smallest amounts, as shown in Table 4. For more details, see the Supplementary Information.

Table 4.
Presence of metals in Sargassum spp. and in the processes of leachate generation per se and through percolation.
3.3.1. Metals: Leachate Generation Per Se as a Consortium and by Species
The results obtained from the leachate that was generated per se (Figure 3, Figure 4 and Figure 5) demonstrated the presence of As, B, Zn, Mn, Cd, and Ni in 2020, and in 2021, As, B, Al, and Cu were detected. The presence of arsenic (As) in both collections was significant, since it was present in high concentration at the study sites. The presence of boron (B) was related to the origin of Sargassum spp., since it is of marine origin, and this metal is one of the constituent elements of seawater, causing it to be found in a higher concentration at the AL site (1.20 mg/L).
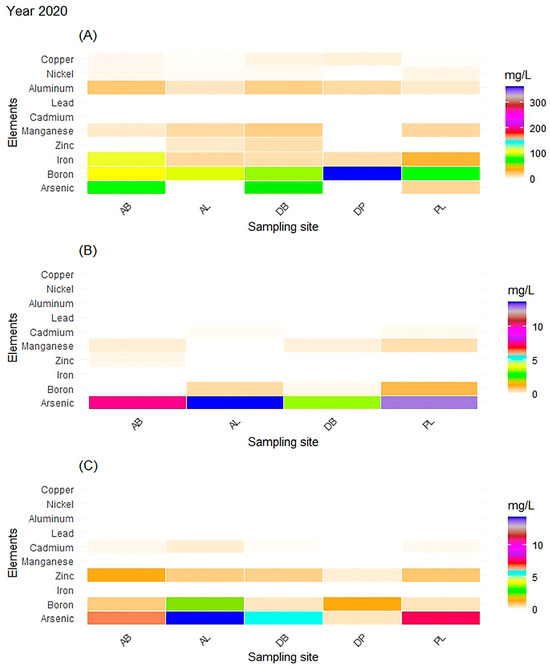
Figure 3.
Year 2020: All results represent the evaluation of the consortium; (A) concentration of metals in the tissue of Sargassum spp. (consortium), (B) concentration of metals in leachate generated per se, and (C) concentration of metals in leachate generated percolation. The heat plots show the concentration of metals by site, where the presence of As in the leachate in the consortia in both stages was notorious, and its presence was relevant to the AL site.
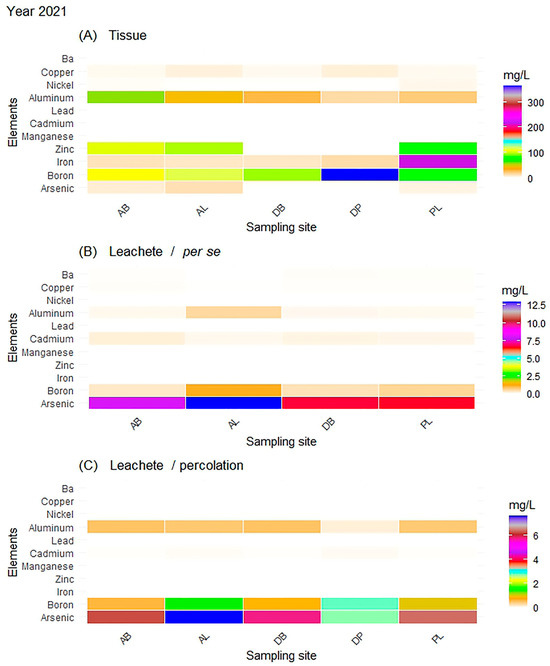
Figure 4.
Year 2021: All results represent the evaluation of the consortium: (A) concentration of metals in the tissue of Sargassum spp. (consortium), (B) concentration of metals in the leachate generated per se, and (C) concentration of metals in the leachate through percolation.
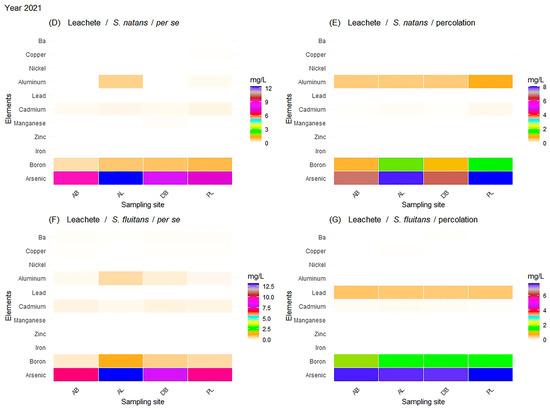
Figure 5.
Year 2021: All results represent the evaluation of the leachate by species: (D) concentration of metals in the leachate generated by S. natans through the per se process, (E) concentration of metals in the leachate generated by S. natans through the percolation process, (F) concentration of metals in the leachate generated by S. fluitans through the per se process, and (G) concentration of metals in the leachate generated by S. fluitans through the percolation process.
In the case of the species S. natans and S. fluitans, As, B, Al, Cd, and Cu were present, and Mn was added, though it was not detected in the consortium. As was still present in higher concentrations at the AL site (12.36 mg/L), and it had a value similar to that obtained in the leachate from the consortium through the per se process. Similarly, the AB site had an amount of 9.06 mg/L, while the DB and PL sites had an average concentration of 7.47 mg/L. This showed that the presence of some metals decreased with their proximity to the coast.
3.3.2. Metals: Leachate Generated through Percolation as a Consortium and by Species
In this case, the presence of metals decreased in the percolation process compared with that in the per se process; in the 2020 consortium, five species were detected, while by 2021, this number had decreased to four. In both cases, the metal As was predominant over the other metals in the five study sites, and it was also found in higher concentrations at the AL site in Both 2020 (14.34 mg/L ± 0.5) and 2021 (12.96 ± 0.04 mg/L). Cd, Zn, and Cu not only decreased in concentration but also did not appear in both periods, as was the case with the metals Zn and Cu, which were only present in the 2020 consortium.
Figure 5 shows that, in the dilution process during the generation of the leachate of S. natans through percolation, there was a reduction in the concentration of metals, as expected. However, As continued to have the highest concentration in the high-sea (AL) and beach (PL) sites, with values of 7.94 ± 0.6 and 8.01 ± 0.4 mg/L. For sites AB and DB, its presence was reduced, presenting values of 7.14 ± 0.3 mg/L to 8.19±0.1. So, its presence was recurrent in both the consortium and the species.
The metal As was present in both types of leachate and at all study sites. The AB, DB, and PL sites presented similar concentrations regardless of whether the leachate was generated per se or through percolation. As expected, a clear decrease in the concentration of metals was observed. With respect to the other sites, it was still the leachate generated at the AL site that registered the highest concentration of As (12.37 ± 0.08 mg/L), while the DP site had the lowest concentration. In the case of sites AB, DB, and PL, similar values of As from 9.06 ± 0.3 mg/L to 7.02 ± 0.02 mg/L were presented.
The concentration of B varied between the types of leachate. In the leachate generated per se, there was a lower concentration of the metal at the AL site (0.54 ± 0.04 mg/L), while the leachate generated through percolation presented the highest concentration (2.32 ± 0.04 mg/L) with respect to the other sites. For the rest of the metals, the results obtained showed a clear difference in concentration depending on the type of leachate, in addition to differences among the study sites. Regarding species, it was observed that the highest concentration of As in S. natans was in the leachate generated per se at the AL site (13.67 ± 0.4 mg/L).
4. Discussion
The results obtained in this work are consistent with the values reported for metal sorption and transport by other authors in [18,34,35]. It should be noted that the metal As was the one found in the highest concentration in sargassum tissue. The concentration of metals in leachate is related to the concentration of metals in sargassum tissue. The results obtained showed that the presence of metals in the water collected at the same sites was lower than that reported in Sargassum spp. and its species, but the numbers reported in the leachate generated through the per se and percolation processes were different. In water, the presence of B was consistently reported due to its marine origin, since, aside from the six major ions that are found in conservative amounts in the chemistry of seawater, boron represents 0.01% by weight [36].
However, the lack of presence of metals such as As, Fe, Cd, and Ni in the water from the collection areas was a reference to the fact that they are not found in the water of the northern part of the Mexican Caribbean, so their presence in the tissue suggests that they were absorbed during transport throughout the Caribbean. This confirms the sorption capacity of sargasso [15,37], which carries these metals along its course. On the other hand, the presence of Al and Zn detected in the tissue in 2020 but not in 2021 and in concentrations that did not refer to arrival through water discharges may be important for the development of microorganisms as a result of trace metals.
The presence of metals in the sargasso leachate varied depending on the collection site and generation process (per se or percolation). The mobility of metals is significant, since the leachate-generating degradation process allows them to be presented in a solution that allows their infiltration or displacement to other matrices, such as infiltration into the geological environment (sand or rock pores).
The concentration of B varied depending on the generation process; in the leachate generated per se, there was a lower concentration of the metal at the AL site (0.54 ± 0.4 mg/L), while in the leachate generated through percolation, the AL site presented the highest concentration (2.32 ± 0.04 mg/L) with respect to the other sites. For the rest of the metals, the results obtained showed a clear difference in the concentration of metals depending on the type of leachate, as well as differences between the study sites. Regarding the species, it was observed that the highest concentration of As in S. natans was in the leachate generated per se at the AL site (13.67 ± 0.4 mg/L).
Although sargassum tissue is the main concern in terms of the presence of metals, the results indicate that the sorption ability of sargassum was developed prior to its arrival on the coasts of the Mexican Caribbean, since the water not only presented metals that characterized the marine origin of the water column but also confirmed the high biosorptive capacity of brown algae (Phaeophyceae) for metals such as Cu, Cd, Ni, Mn, Co, Cr, and Zn, which is well known [38,39,40]. Thus, for example, the dry biomass of species of the genera Ascophyllum spp. and Sargassum spp. can concentrate lead and cadmium from highly diluted solutions and can accumulate more than 30% of their dry weight in metals [40]. More recently, these variations were confirmed, in addition to the sorptive preference for Ace [27,29,41].
The results obtained showed the presence of metals in the leachate generated both per se and through percolation. As, Cd, and Cu were prevalent in all samples, among which the first stood out in higher concentrations at all sites, with minimum values of 6.65 mg/L and maximum values of 13.13 mg/L. In general, the leachate collected in the final disposal sites of municipal solid waste (MSW) has a concentration in the range of 0.01–0.04 mg/L [42], while in the leachate of organic waste compost, a value of 0.024 mg/L was recorded [43].
This indicates that the leaching of MSW contains less As than that presented by Sargassum spp., which denotes that the concentration of metals in macroalgae will be reflected in the mobility of these metals in the generation of leachate.
A study performed in 2022 on sargasso collected in the Mexican Caribbean recorded As concentrations of 175 mg/Kg in S. fluitans and 210 mg/Kg in S. natans [27], while in a study on a Sargassum spp. consortium collected on beaches of Riviera Maya detected As values of 29 to 65.7 mg/Kg [34]. Therefore, in the degradation of sargassum, leaching of this metal can be found. Some authors mentioned that alkaline pH conditions in leachate cause the dilution of As in the form of an oxy-anion (arsenite and arsenate) [43,44]. According to these studies, the amount of As in the leachate generated from all samples was explained due to the alkaline conditions in which the leachate that was generated per se was found. Here, a major role is played by pH in metal biosorption, as it is known to influence the protonation of functional groups in biomass [45,46,47].
The difference in As values between sites may have been related to the volume of leachate generated among Sargassum spp., since, as mentioned above, the AL site presented greater leachate generation. This was related to the greater biosorption and dragging of As than that of the other study sites. Fang et al. [46] indicated that metal leaching occurs mainly at the beginning of leachate generation. This shows that the volume of leachate generated influences the mobility of elements present in sargasso.
In the case of the species, Cd was the metal that showed the most significant difference (p < 0.05), as S. natans showed a value of 0.1 ± 0.05 mg/L, compared with S. fluitans, which showed a value of 0.16 ± 0.03 mg/L, indicating that S. fluitans was the main source of Cd production in the generation of leachate per se. However, Cu was not detected at the AL site in any of the samples, and in the case of S. natans, it was only detected at the PL site. Davis et al. [48] reported that S. fluitans tissue had higher values of Cd (0.57 ± 0.02 mg/Kg) and Cu (4.47 ± 0.20 mg/Kg), while S. natans had Cd values of 0.40 ± 0.02 mg/Kg and Cu values of 2.78 mg/Kg. The results of this study agreed with the concentrations of these metals in the leachate by showing a direct relationship with the concentrations of the metals in tissue and their subsequent leaching in both the consortium samples and the species samples.
The processes of generation of leachate per se and through percolation denote differences in the mobility and concentration of metals. In the leachate generated per se, a greater quantity and variety of metals were obtained both in the composite sample and the species samples (As, B, Cd, Al, Cu, and Ba), while in the leachate generated through percolation, Cu and Ba were not detected in the consortium and S. natans samples. In the S. fluitans samples, only Cu was detected at AL, and only Ba was detected DB. Fang et al. [44] and Stevens et al. [49] mentioned that, under alkaline conditions, dissolved organic matter forms complexes with metal cations, resulting in their mobilization in the leachate; this mainly applies to Cu and Cd.
This is consistent with the differences in the metals found in the leachate generated per se and through percolation, since, at the beginning of the degradation of sargasso, soluble organic matter is dissolved in water; it can form bonds with metals and, thus, influence their mobilization in leachate generated per se. That is, leaching per se allows the mobility of metals through desorption, while in percolation, the metals are dragged; this represents the potential impact that leachate can have on the aquatic environment, whether on the coast or inland (groundwater).
Fang et al. and Stevens et al. [44,49] mentioned that, under alkaline conditions, dissolved organic matter forms complexes with metal cations, resulting in their mobilization in leachate; this mainly applies to Cu and Cd. This is consistent with the differences in the metals found in the leachate generated per se and through percolation, since, at the beginning of the degradation of sargasso, soluble organic matter is dissolved in water; it can form bonds with metals and, thus, influence their mobilization in leachate generated per se.
In the case of urban solid waste, soil/liquid interaction causes an increase in dissolution in the mineral phase, leading to a greater release of metals [43], suggesting that the metals that were concentrated in the Sargassum spp. tissue were carried away by the water added to the samples in the experiments. On the other hand, Oyesiku et al. [50] indicated that the oxidation of sulfate to sulfide mobilizes metals that are associated with sulfate, and since the concentration of sulfate polysaccharides in sargasso (including alginate) is high [51], the gradual leaching of metals is to be expected.
In the case of aluminum (Al), it was found in a higher concentration in the leachate generated through percolation. Despite the different origin of the MSW studied, this metal was detected in concentrations similar to those in organic waste of terrestrial origin [52]. The presence of metals such as As, B, Cd, Al, Cu, B, and Ni in sargasso tissue was evidence that the tissue adsorbed these metals and integrated them into its structure, referring to the adsorption capacity of brown algae [38,39,40,53].
The variations in this detection at the collection sites stood out, that is, there was no constant concentration of each of these metals. It was highlighted that the samples of external flows (AL site) showed significantly higher concentrations in both the tissue and in the leachate, and sometimes, the beach collections were also higher than those detected in the pre- and post-barrier collections. This may have been related to the heterogeneity of the conditions of sargasso that had just run aground and sargasso that had already been on the beach for a longer time.
The presence of metals in sargasso has been documented, and variability was found in the concentrations of the metals under study and their spatial distribution with respect to the collection point [18,19,34,35,54]. Therefore, it is advisable to include an evaluation of the presence and mobility of these metals temporarily and to identify if there is a trend during the arrival cycle of sargasso in one year.
The detection of metals in leachate indicated their mobility through the processes of degradation; however, the variations in the metals released from sargasso are not the result of a desorption process but a process of depolymerization by a specific microorganism. Dominguez-Maldonado et al. [17] evaluated the relative abundance of genera in leachates according to changes in the microbiome. Thus, populations of Fischerella (cyanobacteria) and bacteria such as Acidiphilium and Thermoflum dominated the process of degradation over 30 and 90 days, and they were responsible for the depolymerization of Sargassum spp., and, as a consequence, the release of metals in the leachate generated through both the per se and percolation processes.
The results obtained for the concentrations of metals detected in the generated leachate, especially arsenic, far exceeded the values of NOM-001-SEMARNAT-2021, which establishes the maximum permissible limits of contaminants in wastewater discharges into waters and goods in Mexico as 0.2 mg/L as a monthly average, 0.3 mg/L as a daily average, and 0.4 mg/L as an instant value for Mexican marine water. Therefore, the control and management of leachate at the collection and final disposal sites are required.
5. Conclusions
In this study, the presence of metals in tissue indicates that the sorption process occurred before entering the waters of the Mexican Caribbean, and its variability depended not only on the distance at which it was collected from the coast but also on the proportion of species upon arrival, as mentioned in recent studies.
This was the first study to focus on how many metals were released from sargassum and which ones could appear in the leachate generated through two processes: (a) leachate generated by fresh sargassum through biological activity and (b) that generated through the percolation of rainwater in sargassum that was accumulated and dehydrated by solar radiation.
This study indicates that, of the 10 metals evaluated in the tissue, 4 to 8 of them could be mobilized in the leachate, regardless of the generation process, collection distance (site), and fresh or residual state. These include As, Fe, and Al, which represent a potential impact on coastal systems and infiltration into shallow water table areas.
Moreover, the results indicate that there was significant mobility of the metals present in the tissue to the aqueous phase of leachate, and their concentration also varied spatially, which seemed to be related to the proportion of species in Sargassum spp.; therefore, the proportion of S. natans and S. fluitans in sargassum influences how many metals mobilize into leachate.
Of the metals that were clearly detected in Sargassum spp. tissue, arsenic, as well as aluminum and cadmium, maintained its presence in the leachate generated either per se or through the percolation process. This implies that the management of sargassum must involve the contemplation of the generation of leachate that maintains the risk of metal infiltration if it is deposited in areas that are vulnerable to infiltration, with the potential to affect aquatic ecosystems, become deposited in sediments, or reach the water table and modify the conditions of the groundwater, so it is important to evaluate the impact that the mobility of these metals may generate in the medium and long term.
The fact that the previously absorbed metals in the sargassum were mobilized represents a potential impact on the environment, especially for groundwater, due to the high intrinsic permeability of the Yucatan Peninsula. This highlights the importance of monitoring of groundwater in accumulation zones and deposit sites, since the infiltration of metals through leachate can represent a risk to the health of aquatic and human ecosystems, as groundwater is the main source of water resources.
As stakeholders adapt strategies and regulations for the control or potential use of sargassum, leachate control and disposition must be included, since leachate mobility is the vector of the release of the sorbed metals into soil and groundwater, which will affect the health of aquatic ecosystems, water distribution, and, potentially, human health. Further studies that include the possible sources of the sorbed metals need to be performed to understand the possible factors that contribute to their release.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16192719/s1.
Author Contributions
Conceptualization, R.M.L.-B.; Data curation, E.O.-M. and G.G.L.; Formal analysis, R.C.-V., G.A.-G., and D.O.-C.; Investigation, J.C.R.-G.; Methodology, G.A.-G. and R.T.-T.; Resources, R.M.L.-B.; Software, D.O.-C.; Validation, J.E.B.-G., R.T.-T. and G.G.L.; Writing—original draft, R.M.L.-B.; Writing—review and editing, E.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Sectorial Fund for Research and Development in Naval Sciences of the National Council of Science and Technology (CONHACYT), the Navy Minister (SEMAR) as project no. 305292, and through the Master’s scholarship number 753155.
Data Availability Statement
Data is available in a publicly accessible repository that does not issue DOIs LINK: http://cicy.repositorioinstitucional.mx/jspui/handle/1003/2252 (accessed on 16 September 2024).
Acknowledgments
The authors wish to thank Jorge Peniche for the technical assistance.
Conflicts of Interest
The authors declare no competing interests. The study was carried out with permit nos. INAPESCA PPF/DGOPA-053/21 and CNANP CNANP-00-007.
References
- Durand, L.; Sundberg, J.; Rodríguez-Martínez, R.E. Seaweed blooms in paradise: Ecological reflexivity, governance and the Sargassum crisis in the Mexican Caribbean. Ocean Coast. Res. 2024, 72, e24014. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Magalhães, C.A.; Taniguchi, S.; Siqueira, S.G.L.; Jacobucci, G.B.; Leite, F.P.P.; Bícego, M.C. Evaluation of macroalgae and amphipods as bioindicators of petroleum hydrocarbons input into the marine environment. Mar. Pollut. Bull. 2019, 145, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, R.; Muñiz-Castillo, A.I.; Euán-Avila, J.I.; Hernández-Núñez, H.; Valdés-Lozano, D.S.; Collí-Dulá, R.C.; Arias-González, J.E. Assessing temporal dynamics on pelagic Sargassum influx and its relationship with water quality parameters in the Mexican Caribbean. Reg. Stud. Mar. Sci. 2021, 48, 102005. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Jordán-Dahlgren, E. Pelagic Sargassum cleanup cost in Mexico. Ocean Coast. Man. 2023, 237, 106542. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Machado, C.B.; Almela, V.D.; Oxenford, H.A.; Jayson-Quashigah, P.N.; Tonon, T.; Dash, J. Innovative spectral characterisation of beached pelagic sargassum towards remote estimation of biochemical and phenotypic properties. Sci. Total Environ. 2024, 914, 169789. [Google Scholar] [CrossRef]
- SEMARNAT. Lineamientos Técnicos y de Gestión para la Atención de la Contingencia Ocasionada por Sargazo en el Caribe Mexicano y el Golfo de México. Gobierno de Mexico Home Page. Available online: https://www.gob.mx/semarnat/documentos/lineamientos-tecnicos-y-de-gestion-para-la-atencion-de-la-contingencia-ocasionada-por-sargazo-en-el-caribe-mexicano-y-el-golfo-de-mexico (accessed on 13 June 2021).
- Adhikari, B.; Dahal, K.R.; Khanal, S.N. A review of factors affecting the composition of municipal solid waste landfill leachate. Int. J. Eng. Sci. Innov. Tech. 2014, 3, 273–281. [Google Scholar]
- Aziz Abdul, H.; Mohd. Zahari, M.; Bashir, M.; Hung, Y.T. Chapter 13: Groundwater Contamination at Landfill Site. In Handbook of Environment and Waste Management, Land and Groundwater Pollution Control; Hung., Y.T., Wang, L., Shammas, N., Eds.; World Scientific Publishing Company: Singapore, 2014; Volume 2, pp. 781–799. [Google Scholar] [CrossRef]
- Devault, D.A.; Pierre, R.; Marfaing, H.; Dolique, F.; Lopez, P.J. Sargassum contamination and consequences for downstream uses: A review. J. Appl. Phycol. 2021, 33, 567–602. [Google Scholar] [CrossRef]
- Yang, R.; Ren, J.; Chang, X.; Yang, K. Seepage Model of Heterogeneous Municipal Solid Waste Landfill and Application under Process of Waste Accumulation. Water 2023, 15, 4115. [Google Scholar] [CrossRef]
- Antonio-Martínez, F.; Henaut, Y.; Vega-Zepeda, A.; Cerón-Flores, A.I.; Raigoza-Figueras, R.; Cetz-Navarro, N.P.; Espinoza-Avalos, J. Leachate effects of pelagic Sargassum spp. on larval swimming behavior of the coral Acropora palmata. Sci. Rep. 2020, 10, 3910. [Google Scholar] [CrossRef]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Lam, S.S.; Athapattu, B.C.L.; Vithanage, M. Progress and prospects in mitigation of landfill leachate pollution: Risk, pollution potential, treatment and challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef]
- Zhao, Z.-F.; Zhong, Z.-H.; Wang, X.; Li, J.-L.; Tong, S.-Y.; Zhang, J.-H.; Liu, Z.-Y.; Qin, S. Effects of desiccation and rehydration on carbon fixation and DOC release in Sargassum thunbergii. Aquat. Bot. 2022, 179, 103516. [Google Scholar] [CrossRef]
- Azcorra-May, K.J.; Olguin-Maciel, E.; Dominguez-Maldonado, J.; Toledano-Thompson, T.; Leal-Bautista, R.M.; Alzate-Gaviria, L.; Tapia-Tussell, R. Sargassum biorefineries: Potential opportunities towards shifting from wastes to products. Biomass Convers. Biorefin. 2023, 14, 1837–1845. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Hernández-Vargas, G.; Iqbal, H.M.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- Mohammad, A.; Singh, D.N.; Podlasek, A.; Osinski, P.; Koda, E. Leachate characteristics: Potential indicators for monitoring various phases of municipal solid waste decomposition in a bioreactor landfill. J. Environ. Manag. 2022, 309, 114683. [Google Scholar] [CrossRef]
- Domínguez-Maldonado, J.A.; Solís-Pereira, S.E.; Valle-Gough, R.E.; Álvarez, A.A.M.; Olguín-Maciel, E.; Alzate-Gaviria, L.; Tapia-Tussell, R. Microbial communities present in Sargassum spp. leachates from the Mexican Caribbean which are involved in their degradation in the environment, a tool to tackle the problem. Environ. Sci. Pollut. Res. 2024, 31, 19904–19916. [Google Scholar] [CrossRef]
- Ortega-Flores, P.A.; Serviere-Zaragoza, E.; De Anda-Montañez, J.A.; Freile-Pelegrín, Y.; Robledo, D.; Méndez-Rodríguez, L.C. Trace elements in pelagic Sargassum species in the Mexican Caribbean: Identification of key variables affecting arsenic accumulation in S. fluitans. Sci. Total Environ. 2022, 806, 150657. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, 8667. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Leal-Bautista, R.M.; Alzate-Gaviria, L.; Domínguez-Maldonado, J.; Tapia-Tussell, R. Environmental impact of Sargassum spp. landings: An evaluation of leachate released from natural decomposition at Mexican Caribbean coast. Environ. Sci. Pollut. Res. 2022, 29, 91071–91080. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Beddows, P.A.; Bouchot, G.G.; Metcalfe, T.L.; Li, H.; Van Lavieren, H. Contaminants in the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico. Environ. Pollut. 2011, 159, 991–997. [Google Scholar] [CrossRef]
- Leal-Bautista, R.M.; Rodriguez-Garcia, J.C.; Acosta-González, G.; Chablé-Villacis, R.; Tapia-Tussell, R.; Bautista-García, J.E.; Olguìn-Maciel, E.; Alzate-Gaviria, L.; González-López, G. Assessment of Leachate Generated by Sargassum spp. in the Mexican Caribe: Part 1 Spatial Variations. Water 2024, 16, 1251. [Google Scholar] [CrossRef]
- CONAGUA. Estadisticas del Agua en Mexico, Atlas del Agua en México 2010. Comision Nacional del Agua Home page. Available online: http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/ATLAS2015 (accessed on 17 October 2020).
- CONAGUA. Estadisticas del Agua en Mexico, Atlas del Agua en México 2018. Comision Nacional del Agua Home page. Available online: http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/ATLAS2019 (accessed on 17 October 2020).
- NMX-AA-008-SCFI-2016. Análisis de Agua. Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba (Cancela a la NMXAA-008-SCFI-2011). Secretaría de Economía. Unidad de Normatividad, Competitividad y Competencia. Dirección General de Normas. Diario Oficial de la Federación Home Page. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5452147&fecha=09/09/2016#gsc.tab=0 (accessed on 13 July 2020).
- NMX-AA-093-SCFI-2018. Análisis de Agua-Medición de la Conductividad Eléctrica en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba (Cancela a la NMX-AA-093-SCFI-2000). Secretaría de Economía. Unidad de Normatividad, Competitividad y Competencia. Dirección General de Normas. Diario Oficial de la Federación Home Page. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5529045&fecha=26/06/2018#gsc.tab=0 (accessed on 13 July 2020).
- Cipolloni, O.A.; Gigault, J.; Dassié, É.P.; Baudrimont, M.; Gourves, P.Y.; Amaral-Zettler, L.; Pascal, P.Y. Metals and metalloids concentrations in three genotypes of pelagic Sargassum from the Atlantic Ocean Basin-scale. Mar. Pollut. Bull. 2022, 178, 113564. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, Ed.; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- NMX-AA-099-SCFI-2021. Análisis de Agua-Medición de Nitrógeno de Nitritos en Aguas Naturales, Residuales, Residuales Tratadas y Marinas. Método de Prueba (Cancela a la NMX-AA-099-SCFI-2006). Secretaría de Economía. Unidad de Normatividad, Competitividad y Competencia. Dirección General de Normas. Diario Oficial de la Federación Home Page. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5629556&fecha=13/09/2021#gsc.tab=0 (accessed on 14 July 2020).
- Method 350.1: Nitrogen, Ammonia (Colorimetric, Automated Phenate). Revision 2.0. Environmental Sampling and Analytical Methods (ESAM) Program. United States Environmental Protection Agency Home Page. Available online: https://www.epa.gov/esam/epa-method-3501-determination-ammonia-nitrogen-semi-automated-colorimetry (accessed on 11 August 2020).
- Method 352.1: Nitrogen, Nitrate (Colorimetric, Brucine) by Spectrophotometer. Environmental Sampling and Analytical Methods (ESAM) Program. United States Environmental Protection Agency Home Page. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_352-1_1971.pdf (accessed on 12 August 2020).
- 600/4-79-020. Methods for Chemical Analysis of Water and Wastes. Environmental Sampling and Analytical Methods (ESAM) Program. United States Environmental Protection Agency Home Page. Available online: https://www.wbdg.org/FFC/EPA/EPACRIT/epa600_4_79_020.pdf (accessed on 13 August 2020).
- SW-846 Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. Environmental Sampling and Analytical Methods (ESAM) Program. United States Environmental Protection Agency Home Page. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3052-microwave-assisted-acid-digestion-siliceous-and-organically-based (accessed on 18 August 2020).
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Devault, D.A.; Massat, F.; Lambourdière, J.; Maridakis, C.; Dupuy, L.; Péné-Annette, A.; Dolique, F. Micropollutant content of Sargassum drifted ashore: Arsenic and chlordecone threat assessment and management recommendations for the Caribbean. Environ. Sci. Pollut. Res. 2022, 29, 66315–66334. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Riley, J.P. The bromide/chlorinity and sulphate/chlorinity ratio in sea water. Deep-Sea Res. Ocean. Abstr. 1966, 13, 699–705. [Google Scholar] [CrossRef]
- González Fernández, L.A.; Navarro Frómeta, A.E.; Carranza Álvarez, C.; Flores Ramírez, R.; Díaz Flores, P.E.; Castillo Ramos, V.; Sánchez Polo, M.; Carrasco Marín, F.; Medellín Castillo, N.A. Valorization of Sargassum Biomass as Potential Material for the Remediation of Heavy-Metals-Contaminated Waters. Int. J. Environ. Res. Public Health 2023, 20, 2559. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. J. Chem. Eng. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2021, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Gobert, T.; Gautier, A.; Connan, S.; Rouget, M.L.; Thibaut, T.; Stiger-Pouvreau, V.; Waeles, M. Trace metal content from holopelagic Sargassum spp. sampled in the tropical North Atlantic Ocean: Emphasis on spatial variation of arsenic and phosphorus. Chemosphere 2022, 308, 136186. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Roy, D.; Azaïs, A.; Benkaraache, S.; Drogui, P.; Tyagi, R.D. Composting leachate: Characterization, treatment, and future perspectives. Rev. Environ. Sci. Biotechnol. 2018, 17, 323–349. [Google Scholar] [CrossRef]
- Abramov, S.; He, J.; Wimmer, D.; Lemloh, M.L.; Muehe, E.M.; Gann, B.; Roehm, E.; Kirchhof, R.; Babechuk, M.; Schoenberg, R.; et al. Heavy metal mobility and valuable contents of processed municipal solid waste incineration residues from Southwestern Germany. Waste Manag. 2018, 79, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Huang, L.; Wang, J.; He, G.; Reible, D. Environmental assessment of heavy metal transport and transformation in the Hangzhou Bay, China. J. Hazard. Mater. 2016, 302, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Ankit; Bauddh, K.; Korstad, J. Phycoremediation: Use of Algae to Sequester Heavy Metals. Hydrobiology 2022, 1, 288–303. [Google Scholar] [CrossRef]
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Stevens, B.N.; Betts, A.R.; Miller, B.W.; Scheckel, K.G.; Anderson, R.H.; Bradham, K.D.; Casteel, S.W.; Thomas, D.J.; Basta, N.T. Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice. Soil Syst. 2018, 2, 27. [Google Scholar] [CrossRef]
- Oyesiku, O.O.; Egunyomi, A.O.O.O.A.E. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr. J. Biotechnol. 2014, 1, 288–303. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Piña, J.J.; Balbín, A.I.; Pérez-Cordovés, A.I. La Contaminación por Metales Pesados en Sargazos Procedentes de la Costa sur en la Península de Guanahacabibes,¿ Aún no es Preocupante? Rev. Cuba. Química 2010, 22, 83–88. [Google Scholar]
- NOM-001-SEMARNAT-2021. Establece los Límites Máximos Permisibles de Contaminantes en las Descargas de Aguas Residuales en Aguas y Bienes Nacionales. Unidad de Normatividad, Competitividad y Competencia. Dirección General de Normas. Diario Oficial de la Federación Home Page. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022 (accessed on 13 September 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).