Abstract
Recently, water has become a resource that generates controversy due to shortage alarms and high consumption by production companies. Making good use of water has become the main objective of government institutions. The food industry generates a large amount of wastewater, concentrating the largest number of contaminants originated in its processes. Wastewater from the food industry is characterized by having a large amount of organic matter, especially fats and oils, as well as suspended solids. The objective of this research is to carry out a characterization of effluents generated in a wastewater treatment plant in the food sector based on Mexican and international regulations to determine whether it is reusable. This article addresses Mexico’s lag in the reuse of treated wastewater in the face of the water crisis, highlighting the urgency of adopting these practices to mitigate water scarcity. For the development of this investigation, samples were collected at the discharge point produced by the company’s effluents, followed by an evaluation of their physical, chemical, and biological parameters, and finally, it was determined that the effluent follows the regulatory standards for discharge into the city sewer but outside the range for reuse in productive processes or irrigation of green areas.
1. Introduction
In 2022, Mexico registered 217,477 food industry businesses, contributing to 55% of national exports, 12% of industrial output, 27% of employment, and 4% of the country’s Gross Domestic Product (GDP) [1]. As one of Mexico’s largest industrial sectors, the food industry generates vast quantities of wastewater, which, if inadequately treated, can degrade water quality, disrupt aquatic ecosystems, and pose serious public health risks. This wastewater is particularly concerning due to its high content of organic matter, nutrients, and contaminants like fats, oils, and greases (FOGs), which contribute significantly to water pollution. The increasing demand for food production and processing has exacerbated these issues, underscoring the need for more effective wastewater treatment solutions to address this pressing environmental challenge.
Recent advancements in wastewater treatment for the food industry have focused on improving the removal of organic matter and nutrients. Traditional methods like activated sludge processes are cost-effective and efficient in reducing BOD and COD but struggle with the complex composition of food industry effluents, often leaving pollutants that hinder compliance with strict discharge standards. The activated sludge process relies on aerobic microbial cultures and includes aeration to maintain homogeneity, followed by separation of sludge from water, with some biomass recirculated [2]. Cai et al. [3] explored using activated-sludge incineration ash as a Fenton-like catalyst to enhance pollutant degradation. However, challenges persist, particularly in consistently meeting reuse standards, especially given the presence of heavy metals in wastewater, which pose significant environmental and health risks. Gaytan et al. [4] developed a lab-scale treatment system, later scaled to a pilot level, to treat high organic load wastewater from sugar mills in Veracruz, Mexico. The system achieved 46–60% COD removal in the first stage using a Rotating Biological Contactor (RBA) and up to 95% COD removal in the second stage with a hydroponic cell planted with Canna indica L., Spathiphyllum wallisii, and Alpinia purpurata. The system also effectively eliminated Salmonella, fecal coliforms, and helminth eggs. Martinez et al. [5] evaluated spinach growth using different wastewater treatments, finding that treated wastewater from an urban forest improved root length by 13.1% and leaf area by 16.2% compared to untreated water, making it a viable option for irrigation despite untreated water’s toxic effects. Ríos et al. [6] conducted an electrocoagulation study using an Imhoff reactor, achieving significant reductions in COD (69%), turbidity (81.3%), total solids (61.3%), suspended solids (96.4%), and dissolved solids (55%) by varying current intensity and hydraulic retention time (HRT). Ramírez et al. [7] analyzed sludge from wastewater plants, finding it suitable for agricultural and forestry use but not for urban applications, as the compost did not meet specific Mexican standards. Robles et al. [8] developed an IoT-based system for a wastewater-treatment plant, optimizing the process for reusing treated water for irrigation, with a capacity of 8000 L in 8 h, compliant with Mexican standards and accessible for color-blind users.
The research presented in this paper is crucial for addressing the limitations of current wastewater treatment practices in Mexico’s food industry, with a focus on meeting both discharge and reuse standards. A careful and complete characterization of the wastewater intended to be treated is essential to ensure the success of the wastewater treatment plant. The failure of the greatest part of the treatment plants is due to a poor characterization of the water since it prevents correctly selecting treatments and applying appropriate criteria for design [9].
Wastewater reuse is becoming an innovative and crucial practice in Mexico, especially in the context of water scarcity exacerbated by historic droughts. In 2022, more than 70% of the Mexican territory suffered some degree of drought, severely affecting the availability of drinking water. Mexico’s lag in the reuse of treated wastewater is a significant challenge in the face of the growing water crisis. While countries such as Spain and France implemented these practices more than three decades ago, and the United States has made considerable progress since the early 2000s, Mexico is only just beginning to explore its potential. In Europe, water reuse in agricultural irrigation, urban parks, and industrial processes has been a strategic solution to address water scarcity, supported by robust regulatory frameworks and advanced technologies. In contrast, Mexico has been slow to adopt these strategies, due to limitations in infrastructure, insufficient investment in treatment plants, and a lack of public policies focused on water reuse. This situation has prompted recent research highlighting the feasibility of using treated wastewater not only for the irrigation of parks and gardens but also in industrial processing systems, thereby reducing the demand for drinking water. Through different types of treatment, the quality of recycled water for these purposes can be guaranteed, minimizing environmental and health risks, and significantly contributing to water sustainability in the country.
In this context, our research group is positioned as a pioneer in Mexico in the research and application of alternatives for the reuse of wastewater within the food industry, an area with strict quality and safety requirements. We consider this article to be a major novelty, as it introduces innovative practices that not only address the current problem but also promote a paradigm shift towards more sustainable water management in critical industrial sectors.
The authors have not identified other studies that have supported the comparison of international standards with Mexican standards regarding the topic addressed here. Therefore, this research provides a supported comparison that will serve as a basis for other studies, making it possible to increase the number of successful cases and focus on the sustainability of food-producing organizations. It is worth mentioning that reusing 100% of the water from the production plant in the case study addressed here implies a reduction in daily consumption of approximately 164,160 L, just over eight water pipes.
This study aims to characterize wastewater from a food company to determine its compliance with applicable regulations and assess its potential for reuse in the company’s processes, such as cooling towers or green areas. Six effluent samples were collected and combined into a composite sample to measure key parameters (TSS, COD, pH, conductivity, total nitrogen, and total phosphorus) and compare the results with established standards.
Additionally, the study compares these Mexican wastewater standards with international benchmarks, such as ISO standards, to identify gaps and opportunities for improving local practices. The insights gained from this investigation could benefit both the environment and the industry by encouraging the adoption of more sustainable wastewater management practices, ultimately enhancing water resource conservation and reducing pollution.
From this point on, the document is organized into five sections. Section 1 presents an introduction, a review of the literature on the characterizations carried out on effluents and advances in water treatment, as well as a discussion on various wastewater treatments. Section 2 exposes the methodology. Section 3 presents the results of the effluent characterization. Section 4 offers a discussion. Finally, Section 5 contains the conclusions.
Water-Polluting Elements
The most significant polluting element in domestic water and urban wastewater is organic matter because it is the cause of the depletion of oxygen. Table 1 shows the main products of decomposition of organic matter, mainly made up of CHONS (carbon, hydrogen, oxygen, nitrogen, and sulfur), constituting proteins (remains of animal and vegetable origin), carbohydrates (remains of plant origin), oils and fats (cooking and industry residues) and surfactants (detergents) [10].

Table 1.
Main products of the decomposition of organic matter.
A fundamental parameter in aquatic ecosystems is dissolved oxygen, which must be maintained above 4 mg/L to ensure the survival of higher organisms. This parameter is widely used as an indicator of pollution or, more broadly, the health of water bodies. For effective aerobic wastewater treatment, it is essential to maintain a minimum concentration of 1 mg/L [11].
Biochemical oxygen demand (BOD) and chemical oxygen demand (COD) are indirect measures of the organic matter in water. BOD is determined by the oxygen consumption of microorganisms over five days at 20 °C (BOD5), reflecting the biodegradable organic content [9]. For domestic wastewater, the BOD5 is typically about 75% of the ultimate BOD (BODu). COD, on the other hand, uses a chemical oxidant in an acidic medium to measure organic matter, providing results in about 3 h with greater accuracy than BOD. COD is often preferred due to its speed and lower error margin [12].
pH is crucial for controlling biological processes in wastewater treatment, with optimal microbial activity occurring between 6.5 and 8.5 units [13]. Nitrogen, essential for protein synthesis, exists in various forms like organic nitrogen, ammoniacal nitrogen, and nitrates, and is vital for the growth of algae and bacteria in purification. However, ammoniacal nitrogen levels above 1500 mg/L can inhibit treatment microorganisms [14]. Similarly, phosphorus is necessary for plant and microbial growth, but excessive levels can lead to eutrophication in water bodies [15]. Wastewater discharges provide organic matter that fungi and bacteria decompose, with bacteria thriving at a pH of 6.5 to 7.5. While some bacteria, like Escherichia coli, indicate fecal contamination, fungi dominate in industrial wastewater due to their resilience in low-pH and nutrient-poor conditions [14]. Protozoa, particularly ciliates, consume bacteria and organic matter, enhancing the microbiological quality of effluent plants [15]. However, filamentous bacteria like actinomycetes, notably Nocardia, can cause foam- and sludge-sedimentation issues in reactors, reducing treatment efficiency [9].
2. Method
The effluent from a wastewater-treatment plant with aerobic treatment, belonging to a food company in Celaya Guanajuato, was analyzed. Specific samples of the effluent were taken as established in the book of standard methods for the examination of water and wastewater [16].
2.1. Description of the Operation of the Food Company’s Treatment Plant
The treatment plant has been configured with a sequence of processes and unit operations, capable of removing both organic and inorganic contaminants (mainly solids) present in wastewater. This plant is mainly based on a two-stage process: a physical–chemical treatment and a biological treatment. These processes are complemented by a filtration stage with silica sand and a disinfection stage with sodium hypochlorite to increase treatment efficiency. The total capacity of the system is 1.9 L per second (L/s), receiving the flow fractions according to the following Table 2.

Table 2.
System capacity.
Table 3 shows the concentrations of the most relevant parameters present in the wastewater of the food company discharged to the WWTP.

Table 3.
Tributary concentrations.
The wastewater treatment plant of the food company has been designed to achieve an effluent quality that meets the concentrations of contaminants established in [17], which establishes the maximum permissible limits of contaminants in the wastewater discharges to the urban or municipal sewage systems which are mentioned in Table 4.

Table 4.
Maximum permissible limits of contaminants in wastewater discharges.
The operation sequence of the WWTP is shown in Figure 1.
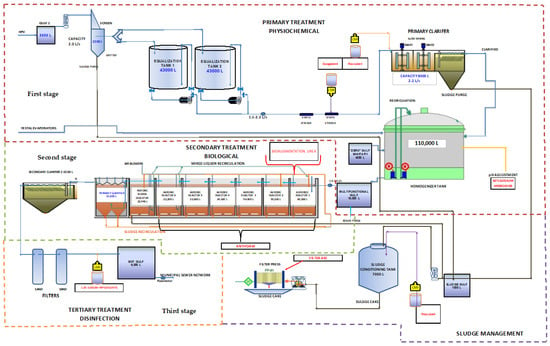
Figure 1.
Methodological flowchart.
The first stage of treatment (represented in red dotted line in Figure 1) begins with the reception of the hydrolyzed protein vegetable (HPV) flow. The HPV flowing is received in the gulf 1 from where it is pumped to the wastewater treatment plant using a double diaphragm pneumatic pump. The water pumped is passed through a static screen where fine and coarse solids are removed. Subsequently, the water from the screen passes to a vertical sand trap where through a sludge purge, they are removed by gravity. From the vertical sand trap, the water is directed towards equalization tanks 1 or 2, depending on the treatment stage in which these tanks are. In these tanks, the water is homogenized to obtain stable conditions for its chemical treatment. The treatment is carried out by “batch”: while one tank is being treated, the other is receiving the residual water from the sand trap. In the next stage of the physical–chemical treatment, suspended solids are removed, including non-soluble organic matter.
Some of the stages considered important in the process are detailed below:
Coagulation: The water from the equalization tank being treated is pumped to the chemical treatment. During this stage, a coagulant (iron base) is dosed online and passed through static mixers for a correct reaction in the wastewater. The colloidal particles and suspended solids are destabilized, neutralizing their charge (coagulation) to be sedimented.
Flocculation: The coagulated water passes to the primary clarifier where an anionic flocculant is dosed and passes through slower agitation allowing the formation of “flocs”. The coagulated particles are agglomerated, forming particles of greater weight and size.
Clarification: the water passes to the clarifier tank, where we no longer have any type of agitation, and the flow is more laminar, allowing the flocs particles to settle at the bottom of the clarifier, and then the clarified water comes out due to overflow through the top of the tank to the next stage.
The water clarified in the physical–chemical treatment goes to the homogenization tank where a pH adjustment is made with 50% sodium hydroxide until neutralized, and a homogeneous residual water is maintained to be fed to the biological treatment under stable conditions. The brewer yeast discharge is also received in this tank, which normally brings a very low concentration of suspended solids and dissolved solids (conductivity) but a high concentration of organic matter.
The water from the homogenizing tank passes by gravity to the multifunctional gulf where the service water (sanitary) is also received, which is collected in the zero gulf and pumped to this gulf to mix with the water from the homogenizing tank and be fed to the biological treatment. Finally, the water from the multifunctional gulf is pumped to the biological treatment where it is put in contact with aerobic microorganisms that are responsible for degrading the organic matter present.
The second stage of the treatment (represented in green dotted line in Figure 1) begins with the reception of water from the multifunctional gulf to the biological reactors.
The biological treatment is composed of six aerobic reactors, a sludge biodigester, and two clarifiers. In aerobic reactors, specific microorganisms are constantly dosed, and air is supplied through blowers to oxygenate the water. The water from the multifunctional gulf passes through overflow through each one of the six aerobic reactors, having a total retention time in the system of 1.7 days, time in which microorganisms degrade organic matter and form solids of greater weight and size.
The water from the aerobic reactor 6 passes to the primary biological clarifier, where the heavier and larger solids are precipitated by gravity. The solids precipitated in the clarifier are removed by a pneumatic pump and sent back to the aerobic reactors since they contain most of the microorganisms in the system. The clarified water passes through overflow to the secondary biological clarifier which also helps in the removal of solids.
When the concentration of solids in the reactors requires it, a sludge purge is carried out in the system. The solids settled in the primary biological clarifier will be pumped to the sludge biodigester for deactivation and subsequent treatment (represented by the purple, dotted line in Figure 1).
The third stage of treatment (represented by the orange, dotted line in Figure 1) occurs with the reception of water from the secondary clarifier and its passage through the sand filters. The water from the secondary biological clarifier is passed through silica sand filters, where fine suspended solids are removed. The filtered water passes to the outlet gulf, where 1.2% sodium hypochlorite is dosed for disinfection and is pumped to the municipal sewer network.
The sludge generated in the WWTP (represented by the purple dotted line in Figure 1) is concentrated in the sludge-conditioning tank, and these correspond to the sludge generated in the vertical desander, physical–chemical clarifier, and the sludge biodigester of the aerobic biological treatment.
The sludge generated in the previous points is pumped to the sludge-conditioning tank using a pneumatic pump.
In this conditioning tank, the sludge is treated with calcium oxide and sodium hypochlorite for disinfection. A cationic flocculant is also added to dehydrate the sludge and facilitate its filtration in the filter press. A filter aid is used in the filter press. The sludge cake is taken to the container intended for disposal.
2.2. Determination of Samples Size
Six samplings were carried out on the effluents of the WWTP. The selection of six water samples was designed to provide a preliminary assessment of the wastewater characteristics at the specific food-processing facility being studied. These samples were strategically collected every four hours to complete a 24 h sampling period. This approach aimed to capture the variability in effluent quality throughout a typical operational day, considering potential fluctuations due to production processes. Furthermore, the selection of these six samples is based on the provisions of [17], which sets the number of samples according to the hours that the WWTP operates. In this case, the food company’s WWTP works 24 h; therefore, these samples must be taken with an interval of 3–4 h between each of them. While this sampling strategy offers valuable insights into the daily effluent characteristics of the facility, it may not fully represent the entire regional area or account for broader temporal variations.
The samples analyzed in this study represent a snapshot of the wastewater characteristics during a specific period, and as such, they do not capture seasonal variations or changes throughout the year.
The mandatory parameters according to the wastewater discharge and reuse regulation [17], and fecal coliforms were measured to obtain the levels of organic load present in the effluent.
2.3. Sample Taking
Sampling for the physicochemical analyses was carried out with previously washed, high-density, plastic bottles. The samples for microbiological determinations were placed in sterile glass bottles. Both were kept refrigerated during transport and were analyzed in the company’s laboratory. The analyzed parameters, their Mexican and international references, are shown in Table 5.

Table 5.
Physicochemical analysis and reference methods carried out on samples collected from wastewater.
Samples were taken in 2000 mL plastic containers, leaving 1/4 of the container empty. The samples were transported under refrigeration in coolers to the laboratory for their respective analysis. Once the samples arrived at the laboratory, the effluent was analyzed for macroscopic characterization; 1000 mL of the sample was poured into a 1000 mL test tube and allowed to rest for 20 min.
At that time, the following were observed:
- (a)
- Turbidity, observing the level of visibility through the test tube.
- (b)
- The number of flocs in suspension.
- (c)
- Sedimentability, given by the time in which the V30 level is reached (V30 = amount of sludge settled in 30 min measured in the field).
- (d)
- The smell of the sample.
The remaining sample was then mixed well in the 2000 mL container.
Once the sample was mixed, the analysis of the microscopic characteristics of the sludge continued by placing two drops on a slide and covering them with a coverslip, avoiding the formation of bubbles.
Subsequently, the sample was observed under a light microscope.
The following were observed:
- (a)
- The shape of the follicle, whether it is regular or irregular.
- (b)
- The size and structure, whether it is compact or open.
- (c)
- The puncture texture, to observe if it is strong or weak.
- (d)
- The floc coverage in the sample.
- (e)
- The amount of filaments in the floc as in dissolution.
- (f)
- The number of protozoa in the sample was quantified and they identified themselves.
Once the results of the effluent characterization were obtained through the index, they were compared with the results obtained from the water quality of the WWTP effluent with [17].
3. Results
The results obtained for each parameter are presented in Table 6. The results obtained comply with the provisions of [17], so it is observed that the WWTP treats the water in a successful manner. On the other hand, we can observe that the BOD and TSS values are out of specification for [38], which indicates that in its current state, it is not possible to use the water as converted water.

Table 6.
Results of the physical–chemical analysis.
The results of the effluent analyses show that the analyzed WWTP is functioning well, since according to the maximum permissible values for the mandatory parameters required by current Mexican legislation, none of the analyzed parameters are breached; however, fecal coliforms, although they are not a mandatory parameter, are an important point to take into account, since the result of these is high, 200 MPN/100 mL; this value shows the high fecal contamination present that can even be related with pathogens present in the water [41]. The pH value is 7.9, being suitable for the development of microorganisms [42].
These results coincide with Alpírez et al. [43], who demonstrated that activated sludge has a solids- and BOD-removal capacity of at least 70%, which demonstrates the importance of monitoring the quality of activated sludge.
Macroscopic and microscopic characteristics were observed in the effluent collected in the six samplings carried out. According to Fall [44], knowledge of biological activity is essential to evaluate the degradation of organic matter; this can be decreased by conditions that are not favorable in the system. The measurement of the bioactivity of the effluent can show the presence of sudden increases in the organic load or the entry of toxic elements [45].
The number of protozoa, bacteria, and larvae found in the effluent samples was quantified. The results are shown in Table 7. Figure 2 shows a microscopic view of some of the species found.

Table 7.
Microbiological results of wastewater.
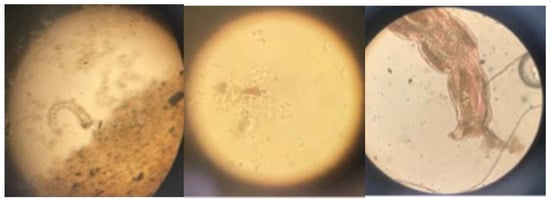
Figure 2.
Microscopic view of species in the effluent.
The presence of aerobic and anaerobic mesophilic microorganisms was observed; this type of flora is normal in transformation processes, due to the load residing in the fruit and machinery used in food production processes. It is worth mentioning that in the transformation process, there are no regulations regarding the microbial load because the manufactured product has a humidity of 9–12%, so the susceptibility to microbial deterioration is low. Additionally, there is a torrefaction process, in which a thermal process is carried out, reducing the remaining charge. The presence of coliforms in the analyzed sample was positive. The World Health Organization (WHO) suggests that there be zero coliform colonies per 100 mL of water in drinking water [46], so the effluent should not be used for other uses.
Crini and Lichtfouse [47] mention that low values of protozoa in plants indicate the existence of toxic substances such as heavy metals and cyanides; they are the most affected by this type of compounds, despite the low values detected in the sample; the presence of these elements is due to the corrosion and cleaning processes that the company carries out on its equipment.
The company’s production process involves the use of cooling towers and reactors. Cooling towers are used to dissipate heat from the industrial process; over time, the metal parts of the tower (such as pipes, heat exchangers, etc.) corrode due to the nature of the water and operating conditions. This corrosion releases metals such as iron, copper, or zinc into the cooling water. Reactors are used to carry out chemical reactions or processes such as sterilization or pasteurization. These reactors are built with metallic materials, such as stainless steel, due to their resistance to corrosion and their ability to withstand extreme operating conditions. The reactors are cleaned through washing processes that involve the use of water. This cleaning process carries with it dissolved metal debris or metal particles that are dislodged from the internal surfaces of the reactor due to corrosion or continuous operation under demanding conditions. The presence of these heavy metals in wastewater is a major concern due to toxicity and their accumulation in the environment, posing risks to human health and ecosystems.
Among the microorganisms that were identified in the WWTP, there is Paramecium, whose presence indicates low oxygenation in the biological process; however, these organisms also appear when the sample sludge begins to stabilize [48].
The presence of species such as Paramecium is associated with indicators of good purification processes [47], which agrees with the results obtained. Ciliated protozoa such as Vorticella and Opercularia were also identified, which are considered indicators of average water retention times in the plant, since when these colonies of microorganisms are formed, it is because the average retention time is high, which contributes to improving the quality of treatment [48]. Other species found, such as bacterivorous creepers, are indicators of good quality of effluents and lightly contaminated wastewater [49], also coinciding with [50], where they present high performance results in systems with a predominance of pedunculated ciliates, and directly relating performance and good purification conditions to the dominant species in the activated sludge.
It is important to mention that the composite sample used to obtain the values of the different parameters is completely representative of the discharge throughout the year, because the company has a stable production supported by certified suppliers where changes in raw materials do not affect the effluent quality, atypical variations in organic load, and nutrients in the influent rarely occur.
4. Discussion
In this study, the findings suggest that although the current treatment process is effective in protecting municipal wastewater systems, it is insufficient for producing an effluent that is versatile for other applications. This indicates a potential environmental and economic impact, as an untreated or partially treated effluent limits opportunities for water conservation and reuse within the industry. To address this, several recommendations are proposed. First, an optimization of the biological treatment processes could be pursued to reduce the levels of COD and TSS further. Specifically, the implementation of advanced biological treatment technologies, such as membrane bioreactors (MBR) or moving-bed biofilm reactors (MBBR), may enhance the removal efficiencies of these critical parameters. Additionally, the integration of advanced oxidation processes (AOPs), such as ozonation or Fenton’s reaction, could be considered to target the residual contaminants that remain after biological treatment.
The introduction of a tertiary treatment stage, including filtration, activated carbon adsorption, or ultraviolet (UV) disinfection, could further polish the effluent, making it more suitable for reuse in irrigation or industrial processes. These enhancements would not only align the effluent quality with reuse standards but also contribute to the sustainability objectives of the food industry by reducing freshwater consumption and minimizing environmental discharge impacts.
This study presents the following limitations: (1) the operations that generate the effluent within the system studied have not been considered, (2) drastic changes in the raw materials have not been considered, and (3) the effluent was considered without significant variations.
A year-long sampling strategy would be recommended for future studies, as this would allow for a better assessment of seasonal impacts on effluent quality. However, implementing such an extensive sampling campaign can be complicated due to significant economic and time constraints. These factors limit the feasibility of continuous sampling over an extended period in this current study.
5. Conclusions
The inspiration for this investigation is that the reuse of treated wastewater for irrigation or industrial processes could reduce the demand for fresh water, decreasing operating costs and minimizing the environmental footprint of the industry. Therefore, this study focused on the characterization of treated water, considering physical, chemical, and biological parameters. The authors emphasize COD and TSS because these parameters do not comply, unlike the rest of the parameters evaluated in this study.
The findings obtained in this research are consistent with previous studies that have identified challenges in meeting reuse standards with conventional wastewater treatment processes. For instance, studies by Matthew et al. [51] and Kesari et al. [52] reported similar difficulties in reducing COD and TSS to levels suitable for non-potable reuse applications. Ranjeet [53] suggests that this is typical of many industrial wastewater treatment systems that prioritize compliance with discharge standards overachieving higher quality for reuse.
It was determined that the effluent follows the regulatory standards for discharge into the city sewer but outside the range for reuse in productive processes or irrigation of green areas.
The potential risks and effects of using this treated wastewater as reclaimed water are multifaceted. Potential risks include the accumulation of contaminants, such as heavy metals and pathogenic microorganisms, which could pose environmental and health hazards if the water is used for purposes like irrigation or cooling towers without further treatment. In green areas, recovered water can introduce chemical contaminants or excess nutrients that alter soil health and plant growth; on the other hand, for use in cooling towers, treated water could contain residual pathogens or chemicals that could affect the efficiency of the system.
The present study reveals notable gaps between Mexican standards and ISO that highlight differing priorities and levels of international alignment in environmental regulations. Mexico has developed a comprehensive set of standards to address local environmental concerns, including parameters that lack ISO equivalents; this also underscores areas where Mexico’s standards may be more stringent or context-specific, reflecting unique national needs. However, the absence of ISO counterparts for many Mexican standards could indicate potential challenges in global harmonization, data comparability, and international collaboration. These gaps suggest that while Mexico’s standards are robust and tailored to local conditions, they might also create barriers to universal application and integration with global environmental frameworks, pointing to a need for greater alignment and dialog between national and international standard-setting bodies.
Author Contributions
Conceptualization, O.Y.-H. and A.J.R.-L.; formal analysis, Y.V.P.-P., E.A.R.-S., E.M.V.-G. and M.L.A.-E.; investigation, O.Y.-H., A.J.R.-L. and Y.V.P.-P.; methodology, O.Y.-H., A.J.R.-L. and Y.V.P.-P.; validation, O.Y.-H., A.J.R.-L. and Y.V.P.-P.; writing—original draft preparation, O.Y.-H. and A.J.R.-L.; writing—review and editing, O.Y.-H., A.J.R.-L., Y.V.P.-P., E.A.R.-S., E.M.V.-G. and M.L.A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The author would like to convey their heartfelt gratitude to the doctoral program in engineering sciences, Tecnológico Nacional de Mexico, Celaya, for providing research facilities to complete this research work. We would like to thank CONAHCYT for all the support provided for the development of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Secretariat of Economy of Mexico, Foreign Trade, Commercial Information (sp.). Available online: http://www.economia-snci.gob.mx/sic_php/pages/estadisticas/ (accessed on 5 March 2024).
- Reis, J.; Rodrigues, M.; Mendonca, A.; Silva, F.; Lara, L. Optimal controllers for the operation of activated sludge. Soc. Environ. Manag. Mag. 2024, 18, 14. Available online: https://link.gale.com/apps/doc/A781187439/IFME?u=anon~5ec6178b&sid=googleScholar&xid=700f71fa (accessed on 15 August 2024).
- Cai, M.; Li Yang, H.; Yang, C.; Zhou, Y.; WU, H. Activated Sludge Incineration Ash Derived Fenton-like Catalyst: Preparation and Its Degradation Performance of Methylene Blue. J. Inorg. Mater. 2024, 39, 1135–1142. [Google Scholar] [CrossRef]
- Gaytan, F.; Alvarado, A.; Vallejo, N.; Alvarado, A.; Sandoval, L. Hybrid treatment (Anaerobic Bioreactor-Constructed Wetland) for the sustainable management of wastewater from the sugar industry. Int. J. Sustain. Reg. Dev. 2021, 5, 451–452. Available online: http://www.rinderesu.com/index.php/rinderesu/article/view/101/105 (accessed on 15 August 2024).
- Martínez, A.; Simental, J.; González, L.; Méndez, A.; Leal, A.; Ramos, M.; Martínez, A. Effects of different residual effluents on the growth and development of Spinach (Spinacia oleracea L.), from Saltillo, Mexico. Agrar. Mag. 2022, 19, 70. [Google Scholar] [CrossRef]
- Ríos, J.; Cobos, J.; Estevez, L.; Casiano, N.; Hernández, E. Application of an Electrocoagulation Process in Continuous Mode in the Wastewater Treatment from a Sugar Mill, UAEH. 2022, p. 7780. Available online: https://www.uaeh.edu.mx/xv-encuentro-investigacion-aactym/memorias/docs/art21.pdf (accessed on 15 August 2024).
- Ramirez, S.; Avila, L.; Gonzalez, J.; Rosas, J.; Umaña, N.; Hernandez, H. Physicochemical and microbiological characterization for the use of sludge of two wastewater treatment plants in Acapulco, Guerrero, Mexico. Explor. Intercamb. Y Relac. Entre El Diseño Y La. Tecnol. 2021, 6, 20–36. [Google Scholar] [CrossRef]
- Robles, F.; Becerra, M.; Avila, C.; Waybell, L.; Jasso, L.; Gonzalez, J.; Waybell, L. Intelligent Wastewater Treatment Plant with a System and Monitoring Method. Technol. Aware 2023, 65, 33–47. Available online: https://www.redalyc.org/journal/944/94475786004/94475786004.pdf (accessed on 15 August 2024).
- Metcalf, S.; Eddy, H.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; AECOM (Firm). Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGrawHill: New York, NY, USA, 2014; pp. 53–80. [Google Scholar]
- Arboleda, J. Theory and Practice of Water Purification, 3rd ed.; McGrawHill: Bogota, Colombia, 2000; pp. 143–145. [Google Scholar]
- Romero, J. Wastewater Treatment, Theory and Design Principles, 3rd ed.; Colombian School of Engineering: Bogota, Colombia, 2004; pp. 105–106. [Google Scholar]
- Lopez, C.; Buitron, G.; García, H.; Cervantes, F. Biological Wastewater Treatment: Principles, Modeling and Designs, 1st ed.; IWA publishing: Madrid, Spain, 2017; pp. 85–86. [Google Scholar]
- Reinoso, R. Wastewater Treatment Systems, 1st ed.; Spanish Academic Publisher: Madrid, Spain, 2012; pp. 40–42. [Google Scholar]
- Bustamante, R. Wastewater Treatment from Tannery Industry, 1st ed.; Spanish Academic Publisher: Madrid, Spain, 2011; pp. 71–72. [Google Scholar]
- Quispe, M.; Piñas, L.; Del Valle, J.; Aguirre, F. Technological Applications of Wastewater Treatment, 1st ed.; Nosótrica editions: Mexico City, Mexico, 2020; pp. 36–37. [Google Scholar]
- Ramalho, R. Wastewater Treatment, 1st ed.; Reverté Editorial: Mexico City, Mexico, 2003; pp. 23–25. [Google Scholar]
- NOM-002-ECOL-1996; Secretariat of Environment, Natural Resources and Fisheries. Maximum Permissible Limits of Contaminants in Wastewater Discharges into National Waters and Assets. Official Gazette of the Federation: Mexico City, Mexico, 1996.
- NMX-AA-030/2-SCFI-2011; Water Analysis-Measurement of Chemical Oxygen Demand in Natural, Wastewater and Treated Wastewater-Part 2: Test method (COD-COD). Ministry of Economy, Official Gazette of the Federation: Mexico City, Mexico, 2011.
- ISO 15705:2002; Water Quality-Determination of the Chemical Oxygen Demand Index (ST-COD)-Small-Scale Sealed-Tube Method. ISO: Geneva, Switzerland, 2002.
- NMX-AA-028-SCFI-2021; Water Analysis-Measurement of Biochemical Oxygen Demand in Natural, Wastewater and Treated Wastewater—Test Method. Ministry of Economy, Official Gazette of the Federation: Mexico City, Mexico, 2021.
- ISO 5815-1:2003; Water Quality-Determination of Biochemical Oxygen Demand after n Days (BODn)-Part 1: Dilution and Seeding Method with Allylthiourea Addition. ISO: Geneva, Switzerland, 2003.
- NMX-AA-034-SCFI-2015; Water Analysis-Measurement of Solids and Dissolved Salts in Natural, Wastewater and Treated Wastewater—Test Method. Ministry of Economy, Official Gazette of the Federation: Mexico City, Mexico, 2015.
- ISO 11923:1997; Water Quality-Determination of Suspended Solids by Filtration through Glass-Fibre Filters. ISO: Geneva, Switzerland, 1997.
- NMX-AA-005-SCFI-2013; Water Analysis-Measurement of Recoverable Fats and Oils in Natural, Wastewater and Treated Wastewater—Test Method (Cancels NMX-AA-005-SCFI-2000). Ministry of Economy, Official Gazette of the Federation: Mexico City, Mexico, 2013.
- NMX-AA-008-SCFI-2016; Environmental Protection-Soil Pollution-Biodegradable Polymers-Determination of Biodegradation of Plastic Materials under Compost Conditions by Carbon Dioxide Analysis—Test Method. General Directorate of Standards: Mexico City, Mexico, 2016.
- ISO 10523:2008; Water Quality-Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2008.
- NMX-AA-007-SCFI-2013; Environmental Protection-Soil Contamination-Solid Waste-Preparation of Solid Samples—Test Method. General Directorate of Standards: Mexico City, Mexico, 2013.
- NMX-AA-004-SCFI-2013; Environmental Protection-Soil contamination-Solid waste-Determination of moisture content in solid waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2013.
- NMX-AA-058-SCFI-2001; Environmental Protection-Water Quality-Method for the Determination of Solids and Dissolved Salts, Suspended Solids and Settleable Solids in Natural, Wastewater and Treated Wastewater—Test Method. General Directorate of Standards: Mexico City, Mexico, 2001.
- NMX-AA-044-SCFI-2014; Environmental Protection-Soil Contamination-Waste-Determination of Moisture Content in Solid Waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2014.
- ISO 23913:2006; Water Quality-Sampling of Fish with Multi-Mesh Gillnets. International Organization for Standardization: Geneva, Switzerland, 2006.
- NMX-AA-026-SCFI-2010; Environmental Protection-Soil Pollution-Waste-Determination of the Leaching Ratio of Inorganic Constituents of Waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2010.
- ISO 5663:1984; Water Quality-Sampling of Surface Waters. International Organization for Standardization: Geneva, Switzerland, 1984.
- NMX-AA-039-SCFI-2001; Environmental Protection-Soil Pollution-Waste-Determination of Organic and Inorganic Carbon Content in Solid Waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2001.
- NMX-AA-006-SCFI-2010; Environmental Protection-Soil Contamination-Solid Waste-Determination of Heavy Metals in Waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2010.
- NMX-AA-093-SCFI-2018; Environmental Protection-Soil Pollution-Solid Waste-Determination of the Concentration of Heavy Metals by Atomic Absorption Spectrometry—Test Method. General Directorate of Standards: Mexico City, Mexico, 2018.
- NMX-AA-051-SCFI-2016; Environmental Protection-Soil Contamination-Determination of Heavy Metals in Solid Waste—Test Method by Atomic Absorption Spectrometry. General Directorate of Standards: Mexico City, Mexico, 2016.
- NOM-003-ECOL-1997; Permissible Limits of Concentration of Contaminants in Wastewater Discharged into Bodies of Water and on Public Roads. Secretariat of the Environment, Natural Resources and Fisheries: Mexico City, Mexico, 1997.
- NMX-AA-042-SCFI-2015; Environmental Protection-Soil Contamination-Solid Waste-Determination of the Content of Volatile Organic Compounds in Solid Waste—Test Method. General Directorate of Standards: Mexico City, Mexico, 2015.
- NMX-AA-113-SCFI-2012; Environmental Protection-Soil Pollution-Solid Waste-Determination of Organic Compounds in Solid Waste by Gas Chromatography—Test Method. General Directorate of Standards: Mexico City, Mexico, 2012.
- Sivaraja, R.; Nagarajan, K. Levels of indicator microorganisms (total and fecal coliforms) in surface waters of rivers Cauvery and Bhavani for circuitously predicting the pollution load and pathogenic risks. Int. J. PharmTech Res. 2014, 6, 455–461. Available online: https://sphinxsai.com/2014/PTVOL6/PT=07(455-461)AJ14.pdf (accessed on 11 March 2024).
- Eaton, A.; Clesceri, L.; Greenberg, A. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 125–126. [Google Scholar]
- Alpírez, J.; Avilés, K.; Castillo, H.; Pinzón, I.; Poveda, R.; Vallester, E. Evaluation of a biological system of laboratory scale activated sludge. Sci. Initiat. Mag. 2017, 3, 8. [Google Scholar] [CrossRef]
- Fall, C.; Cuenca, F.; Bâ, K.; Solís, C. Respirometry-based evaluation of the fate and possible effects of antifreeze on activated sludge. J. Environ. Manag. 2006, 80, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, G.; Sun, W. Measurement of temperature effects on oxygen uptake rate in activated sludge treatment. Rep. Mich. State Univ. Coll. Eng. 2007, 1–28. Available online: https://www.egr.msu.edu/~hashsham/courses/ene806/docs/OUR-Activated%20Sludge.pdf (accessed on 11 March 2024).
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 3rd ed.; World Health Organization (WHO): Geneva, Switzerland, 2006; Volume 1, Available online: https://www.who.int/es/publications/i/item/9789241549950 (accessed on 5 March 2024).
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. Environ. Chem. A Sustain. World 2018, 1, 1–2. [Google Scholar] [CrossRef]
- Dadi, D.; Mengistie, E.; Terefe, G.; Getahun, T.; Haddis, A.; Birke, W. Assessment of the effluent quality of wet coffee processing wastewater and its influence on downstream water quality. Ecohydrol. Hydrobiol. 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Sezerino, P.; Philippi, L.S.; Reginatto, V.; La-polli, F. Characterization of microfauna in an activated sludge sewage treatment plant: An instrument for evaluating and controlling the process. Eng. Sanit. Ambient. 2005, 10, 329–338. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Matthew, O.; Olusola, A.; Bilainu, O.; Peter, O. Challenges of wastewater generation and management in sub-Saharan Africa: A Review. Environ. Chall. 2023, 11, 85–97. [Google Scholar] [CrossRef]
- Kesari, K.; Soni, R.; Jamal, S. Wastewater Treatment and Reuse: A Review of Its Applications and Health Implications. Water Air Soil. Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Ranjeet, M.; Spandana, M.; Yash, M.; Naveen, M. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).