Application of Marine Mollusk Shells (Meretrix lusoria) as Low-Cost Biosorbent for Removing Cd2+ and Pb2+ Ions from Aqueous Solution: Kinetic and Equilibrium Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scientific Hypothesis
2.3. Preparation of Adsorbents for Analysis
2.4. Biosorbent Characterization
2.5. Methods of Biosorption Experimentation
2.5.1. Effect of pH Value
2.5.2. Effect of Contact Time
2.5.3. Impact of Sorbent Dosage
2.5.4. Influence of Initial [M2+]
2.5.5. Effect of Temperature
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of Adsorbent
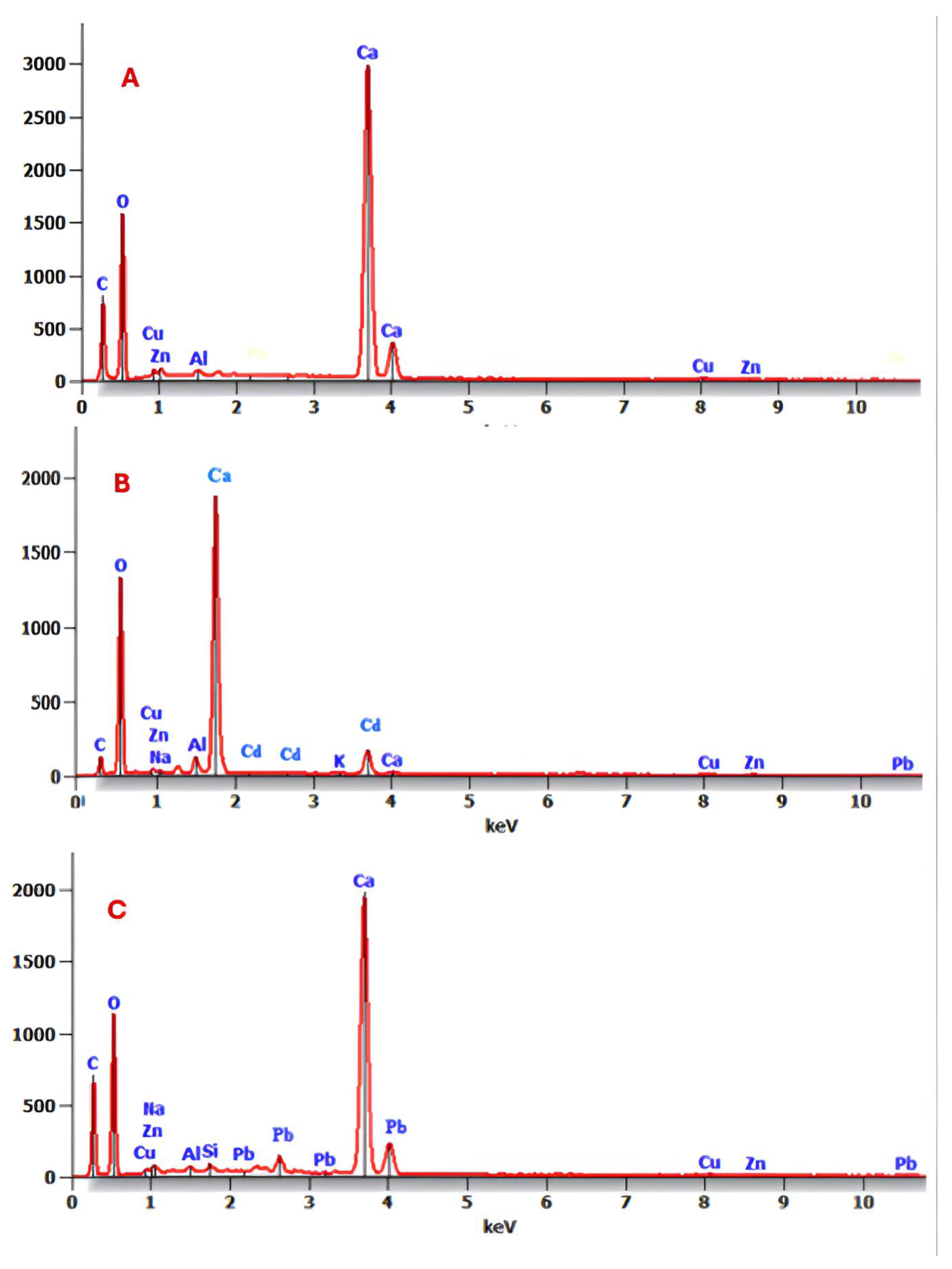
3.1.1. SEM Analysis
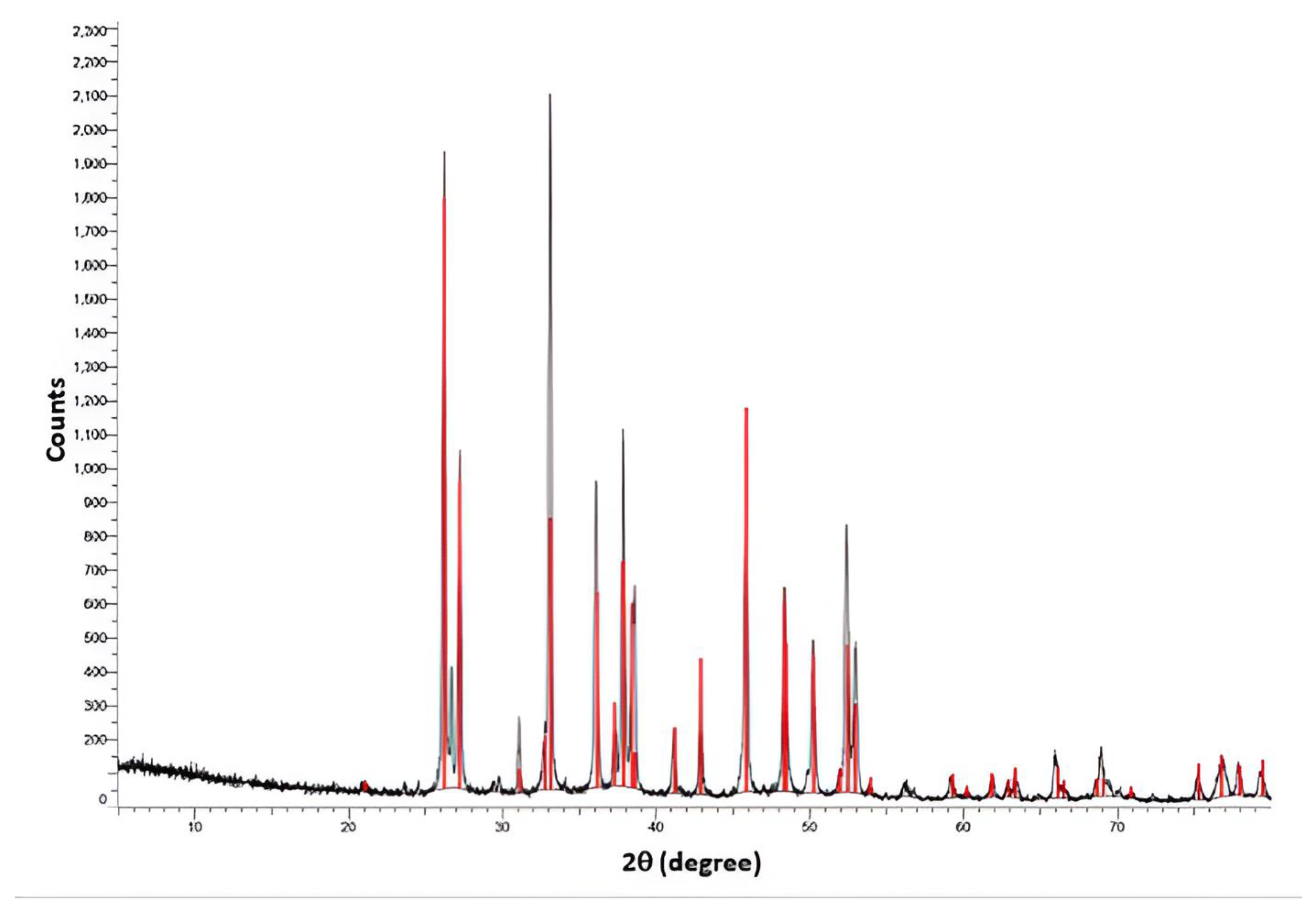
3.1.2. X-ray Diffraction (XRD)
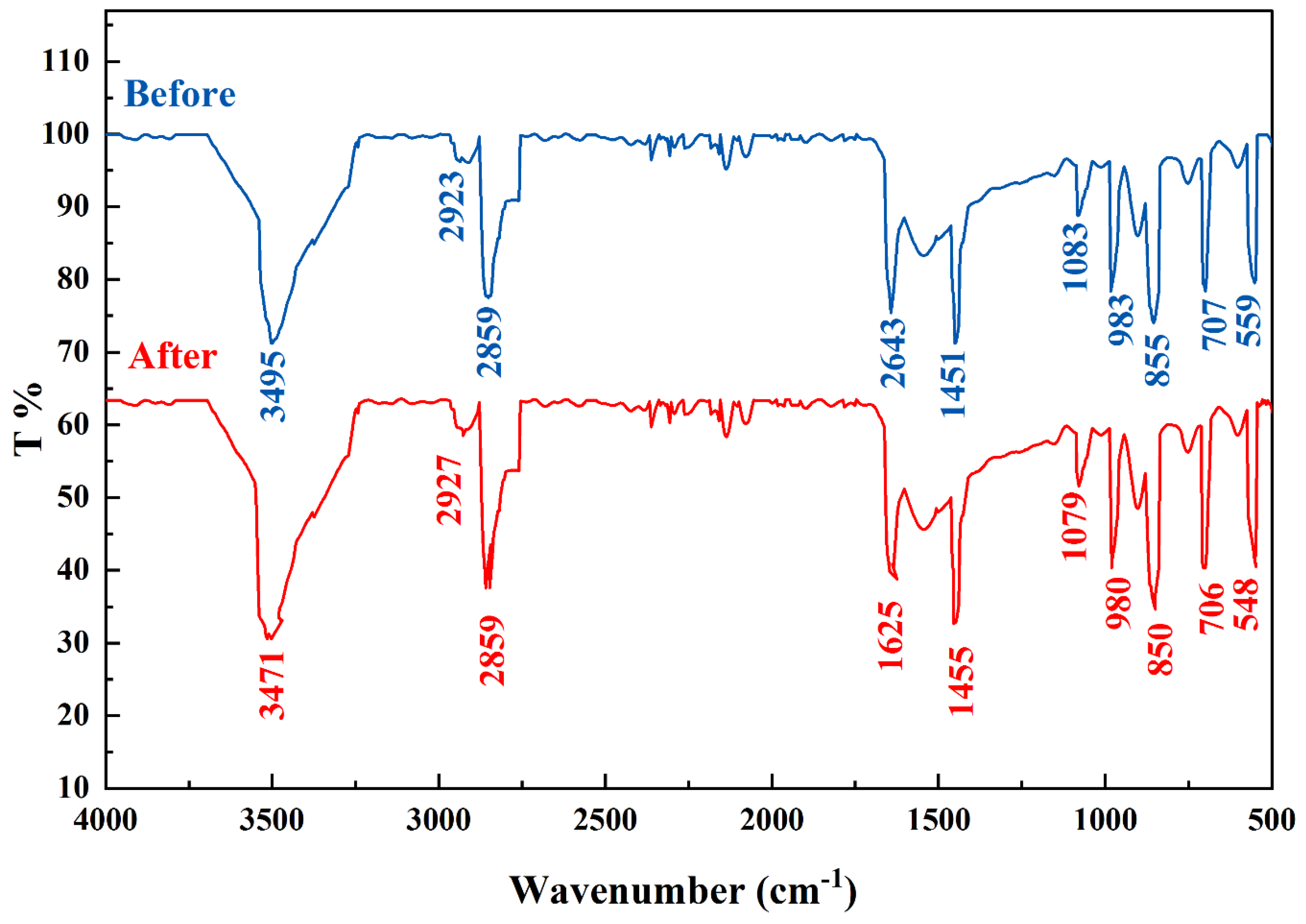
3.1.3. FTIR
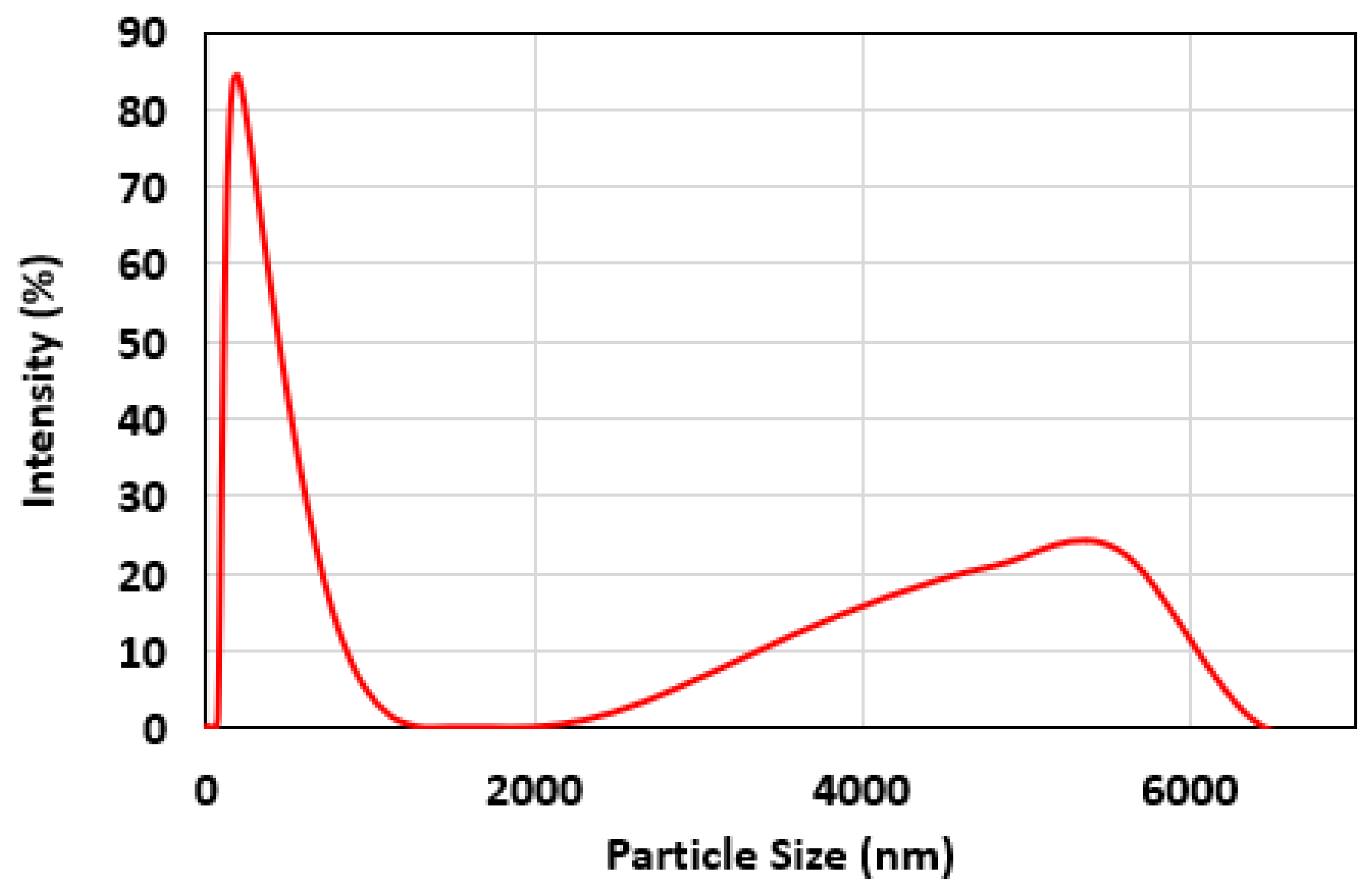
3.1.4. Particle Size
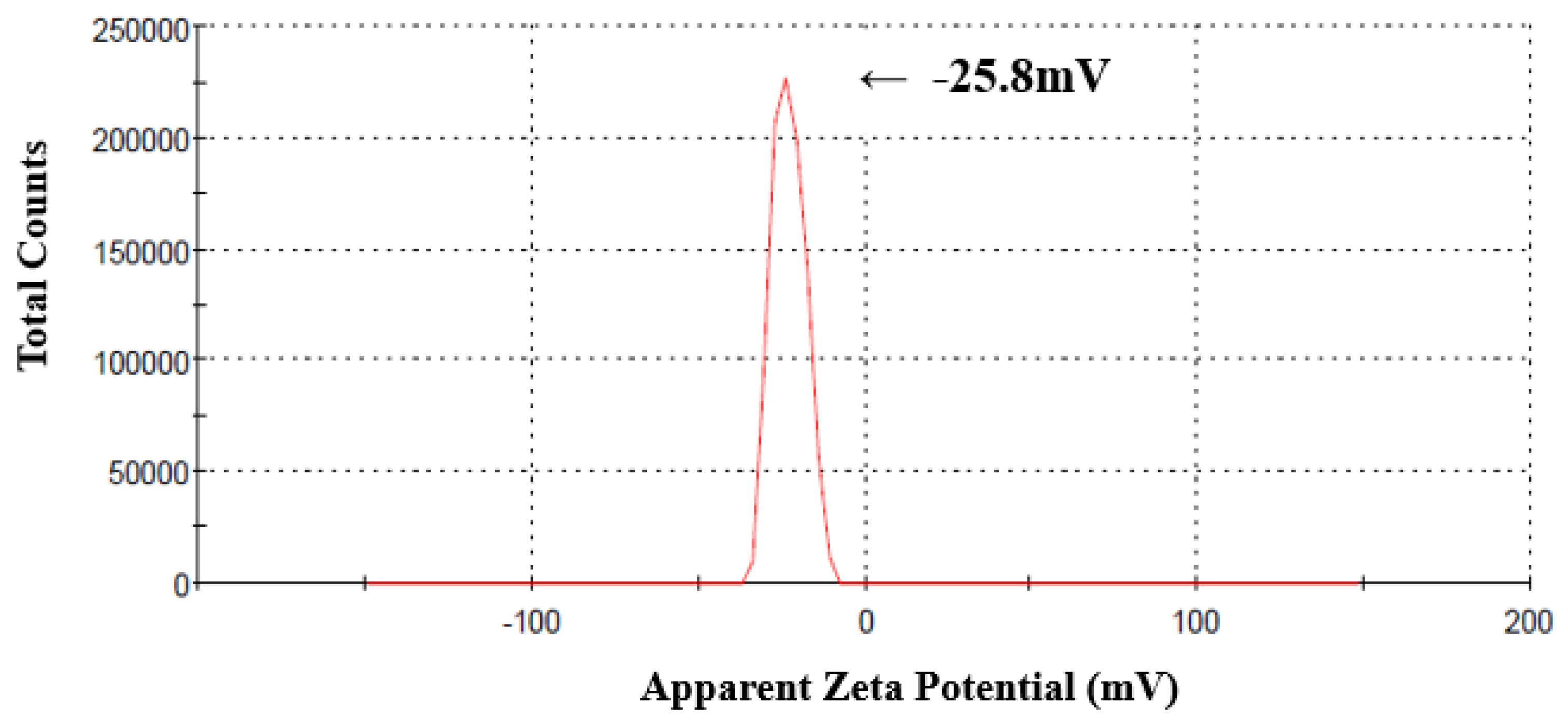
3.1.5. Zeta Potential
3.1.6. BET Analysis
3.2. Optimization of Biosorption Parameters
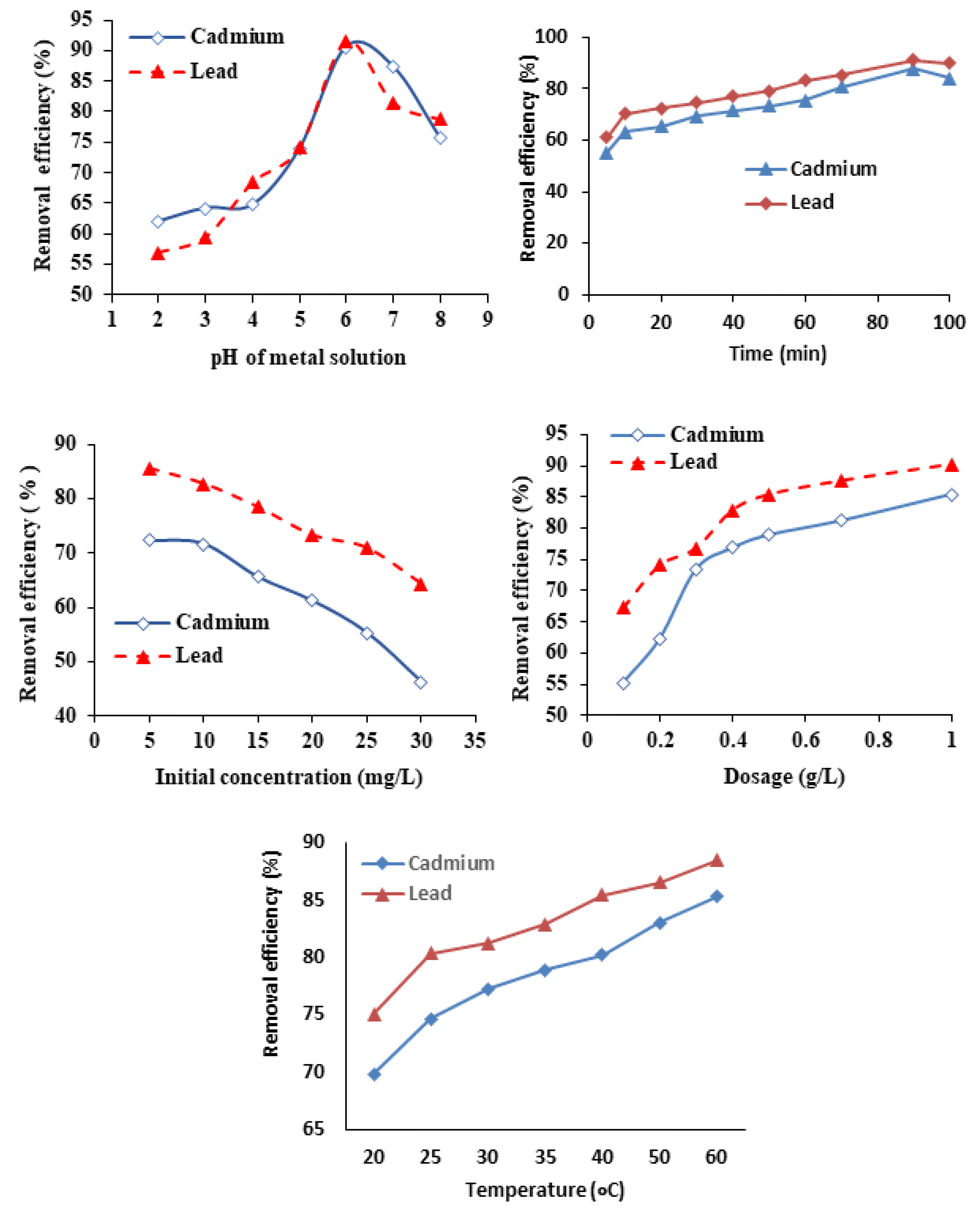
3.2.1. Effect of pH
3.2.2. Effect of Contact Time
3.2.3. Effect of Biosorbent Dose
3.2.4. Effect of Initial Concentration
3.2.5. Temperature Effect
3.3. Modelling of Biosorption
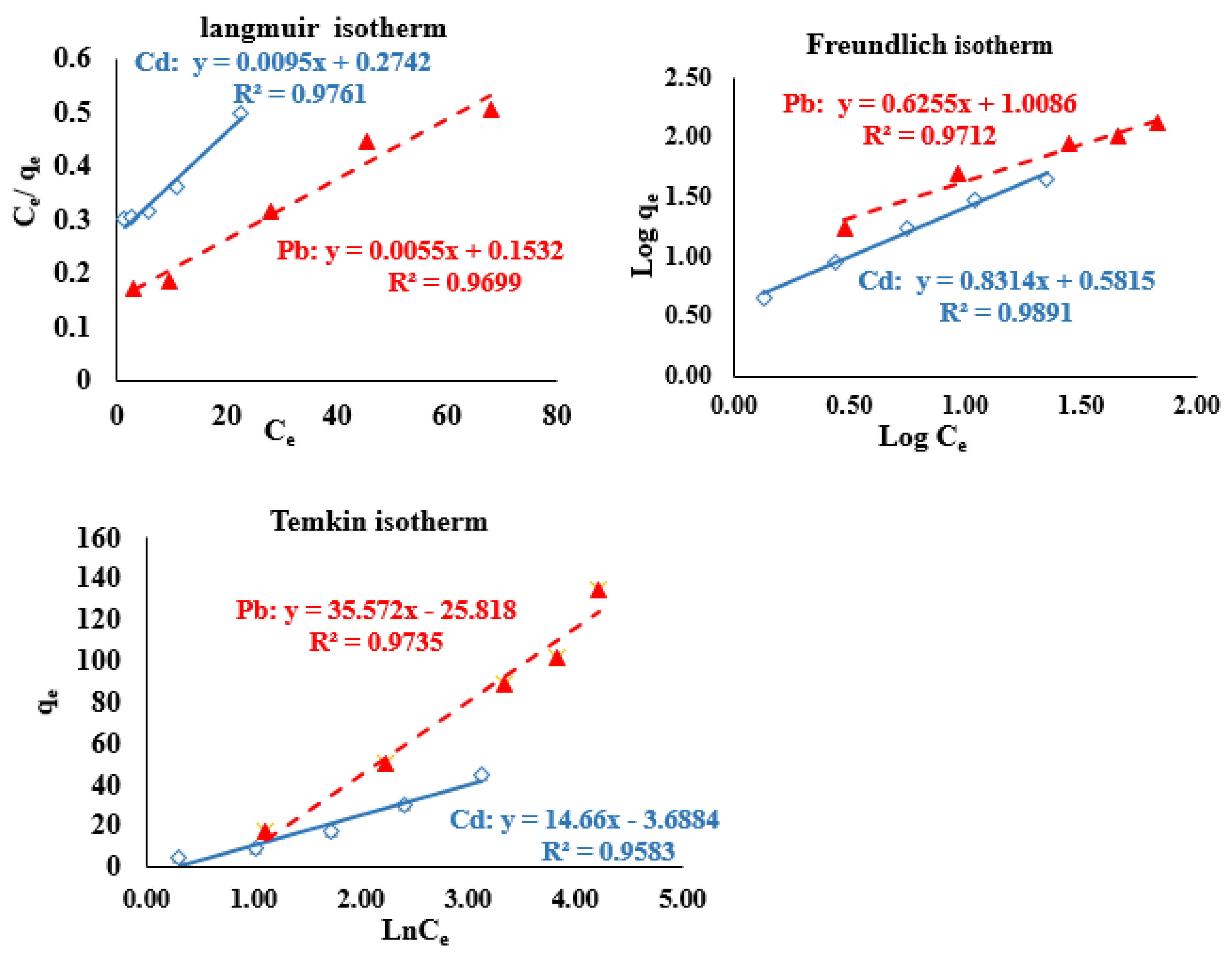
3.3.1. Biosorption Isotherm Models
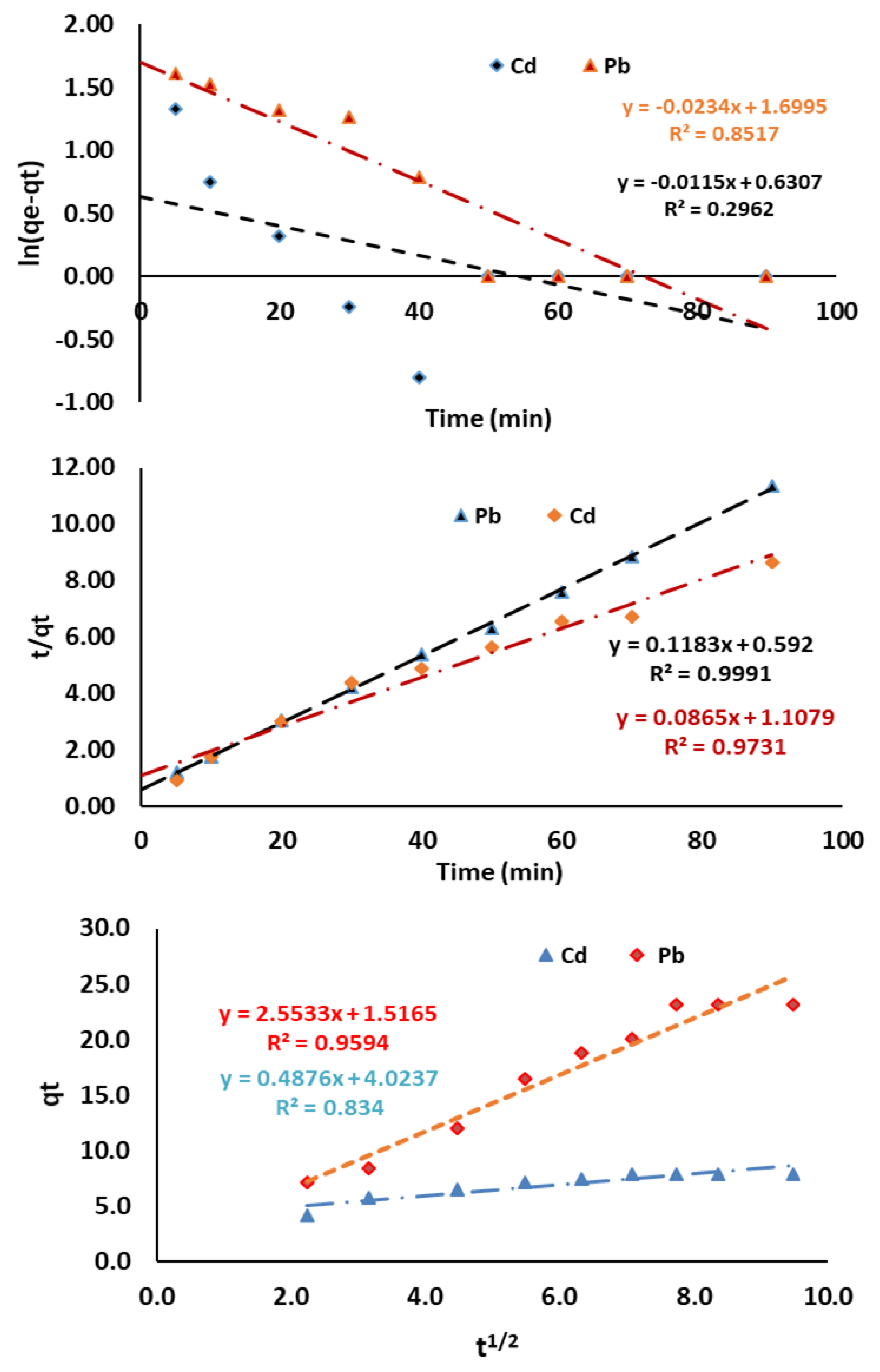
3.3.2. Kinetic Study
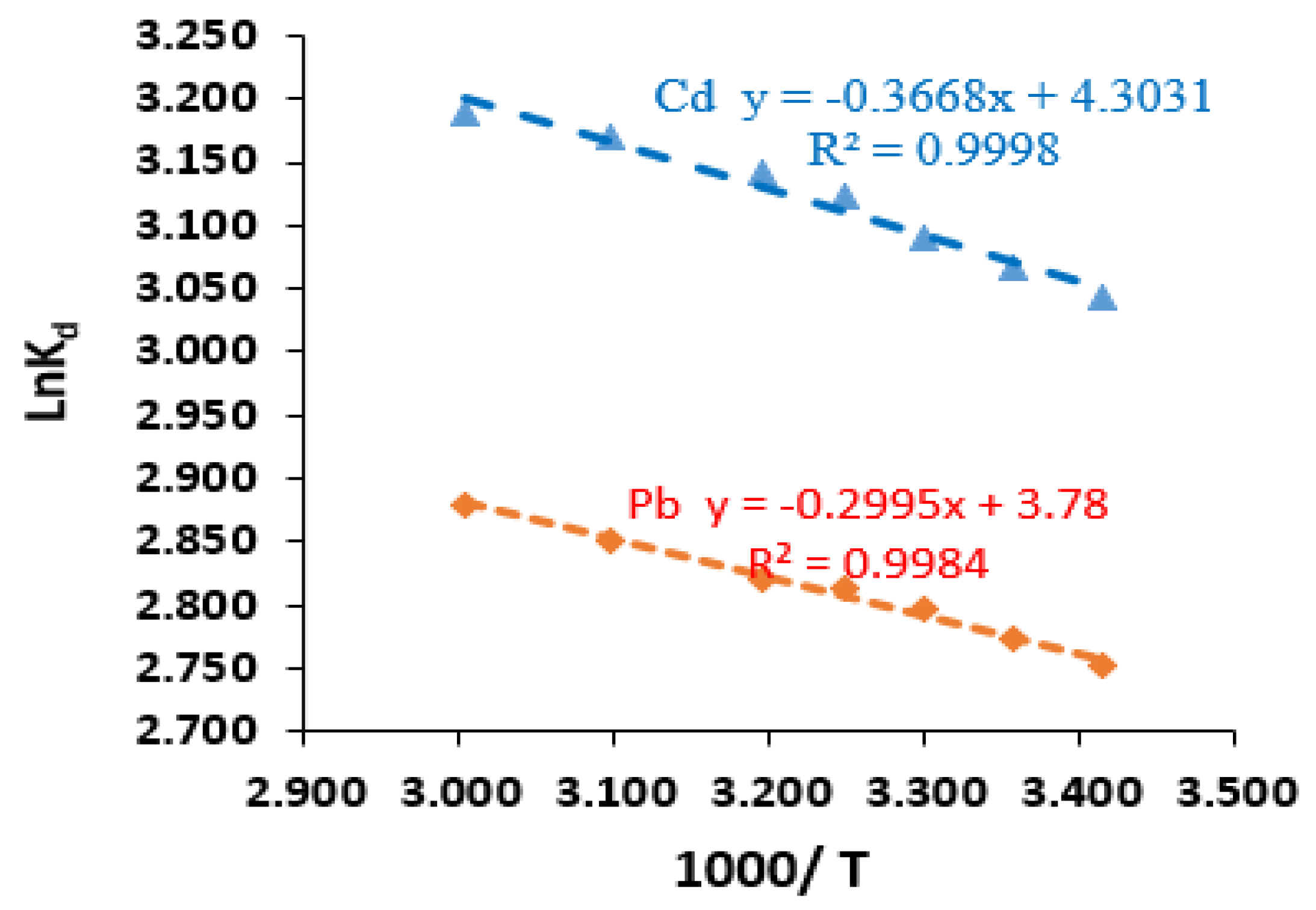
3.3.3. Thermodynamics of Biosorption
3.3.4. Application of the Green-Synthesized Adsorbents to Remove Cd2+ and Pb2+ from Real Water Samples
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Li, S.; Wang, Z.; Liang, Z.; Chen, J.; Liang, B. Contamination, potential mobility, and origins of lead in sediment cores from the Shima River, south China. Environ. Pollut. 2018, 242, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Yang, K.; Lu, W.; Cui, K.; Li, J.; Liang, Y.; Hou, G.; Zhao, X.; Li, H. An overview of heavy metal pollution in Chaohu Lake, China: Enrichment, distribution, speciation, and associated risk under natural and anthropogenic changes. Environ. Sci. Pollut. Res. 2019, 26, 29585–29596. [Google Scholar] [CrossRef]
- Sarker, M.S.; Quadir, Q.F.; Zakir, H.M.; Nazneen, T.; Rahman, A. Evaluation of commonly used fertilizers, fish and poultry feeds as potential sources of heavy metals contamination in food. Asian-Australas. J. Food Saf. Secur. 2017, 1, 74–81. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, L.; Li, D.L.; Ling, F.; Wang, G.X. Developmental toxicity in rare minnow (Gobiocypris rarus) embryos exposed to Cu, Zn and Cd. Ecotoxicol. Environ. Saf. 2014, 104, 269–277. [Google Scholar] [CrossRef]
- Liu, Z.; He, C.; Chen, M.; Yang, S.; Li, J.; Lin, Y.; Deng, Y.; Li, N.; Guo, Y.; Yu, P.; et al. The effects of lead and aluminum exposure on congenital heart disease and the mechanism of oxidative stress. Reprod. Toxicol. 2018, 81, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Zhao, D.; Chen, X.; Zhang, C.; Zheng, J.; Liu, X. Zinc deficiency induces abnormal development of the myocardium by promoting SENP5 overexpression. PLoS ONE 2020, 15, e0242606. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology–a review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of heavy metals on early development, growth and reproduction of fish—A review. Toxicol. Rep. 2022, 9, 858–868. [Google Scholar] [CrossRef]
- Emon, J.F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and bioremediation of heavy metals in fishes—A review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareema, M.A.; Mahmoudd, M.S.; Olabi, A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef]
- Mariappan, R.; Vairamuthu, R.; Ganapathy, A. Use of chemically activated cotton nut shell carbon for the removal of fluoride contaminated drinking water: Kinetics evaluation. Chin. J. Chem. Eng. 2015, 23, 710–721. [Google Scholar] [CrossRef]
- Ho, K.Y.; Mckay, G.; Yeung, K.L. Selective adsorbents from ordered mesoporous silica. Langmuir 2003, 19, 3019–3024. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorization possibilities of exhausted biosorbent loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Ketwong, C.; Trisupakitti, S.; Nausri, C.; Senajuk, W. Removal of heavy metal from synthetic waste water using calcined golden apple snail shells. Naresuan Univ. J. Sci. Technol. (NUJST) 2018, 26, 61–70. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Tamjidi, S. The role of bentonite clay and bentonite clay@ MnFe2O4 composite and their physico-chemical properties on the removal of Cr (III) and Cr (VI) from aqueous media. Environ. Sci. Pollut. Res. 2020, 27, 14044–14057. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.Y. Taguchi optimization for cd (II) removal from aqueous solutions using oyster shell powders. Desalin. Water Treat. 2016, 57, 20430–20438. [Google Scholar] [CrossRef]
- Mosher, S.; Cope, G.W.; Weber, F.X.; Shea, D.; Kwak, T.J. Effects of lead on Na+, K+-ATPase and hemolymph ion concentrations in the freshwater mussel Elliptio complanata. Environ. Toxicol. 2012, 27, 268–276. [Google Scholar] [CrossRef]
- Adewuyi, A.P.; Franklin, S.O.; Ibrahim, K.A. Utilization of mollusc shells for concrete production for sustainable environment. Int. J. Sci. Eng. Res. 2015, 6, 201–208. [Google Scholar]
- Yoon, G.-L.; Kim, B.-T.; Kim, B.-O.; Han, S.-H. Chemical–mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Kwon, H.-B.; Lee, C.-W.; Jun, B.-S.; Yun, J.-D.; Weon, S.-Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Ortiz Olivares, R.D.; Okoye, P.U.; Ituna-Yudonago, J.F.; Njoku, C.N.; Hameed, B.H.; Song, W.; Li, S.; Longoria, A.; Sebastian, P.J. Valorization of biodiesel byproduct glycerol to glycerol carbonate using highly reusable apatite-like catalyst derived from waste Gastropoda Mollusca. Biomass Convers. Biorefinery 2023, 13, 619–631. [Google Scholar] [CrossRef]
- Yamakawa, A.Y.; Yamaguchi, M.; Imai, H. Genetic Relationships among Species of Meretrix (Mollusca: Veneridae) in the Western Pacific Ocean1. Pac. Sci. 2008, 62, 385–394. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Xu, F. Taxonomic study on Meretrix (bivalvia, veneridae) from China seas. Acta Zootaxonomica Sin. Dongwu Fenlei Xuebao 2012, 37, 473–479. [Google Scholar]
- Thangaraj, S.; Purushothaman, Y.; Palani, D.; Velmurugan, K.; Dilip, K.J.; Gopal, D.; Thangavel, B.; Ramalingam, K. Assessment of trace metal contamination in the marine sediment, seawater, and bivalves of Parangipettai, southeast coast of India. Mar. Pollut. Bull. 2019, 149, 110499. [Google Scholar] [CrossRef]
- Dodd, J.R. Paleoecological Implications of the Mineralogy, Structure, and Strontium and Magnesium Contents of Shells of the West Coast Species of the Genus Mytilus. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 1961. [Google Scholar] [CrossRef]
- Lagergren, S. Zurtheorie der sogenannten adsorption gelosterstoffe. K. Sven. Vetenskapsakademiens-Kadem. 1898, 24, 1–39. [Google Scholar]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Seczkowska, M.; Deryło-Marczewska, A. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: Effect of temperature. Adsorption 2016, 22, 777–790. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G.M. Pseudo-second order model for sorption process. Proc. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1918, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]
- Masood, M.S.; Zidan, A.A.; El Zokm, G.M.; Elsamra, R.M.; Okbah, M.A. Humic acid and nano-zeolite NaX as low cost and eco-friendly adsorbents for removal of Pb (II) and Cd (II) from water: Characterization, kinetics, isotherms and thermodynamic studies. Biomass Convers. Biorefinery 2024, 14, 3615–3632. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. A brief review of aragonite precipitated calcium carbonate (PCC) synthesis methods and its applications. Korean Chem. Eng. Res. 2017, 55, 443–455. [Google Scholar] [CrossRef]
- Rao, H.J. Characterization Studies on Adsorption of Lead and Cadmium Using Activated Carbon Prepared from Waste Tyres. Nat. Environ. Pollut. Technol. 2021, 20, 561–568. [Google Scholar] [CrossRef]
- Pokroy, B.; Fieramosca, J.S.; Von Dreele, R.B.; Fitch, A.N.; Caspi, E.N.; Zolotoyabko, E. Atomic structure of biogenic aragonite. Chem. Mater. 2007, 19, 3244–3251. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, Y.W. A study of the correlation between organic matrices and nanocomposite materials in oyster shell formation. Biomaterials 2000, 21, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Sawant, S.G. Identification of CaCO3 polymorphs of shellfish by FTIR spectroscopy and evaluation of metals adsorption by powdered exoskeleton shell. Indian J. Geo Mar. Sci. 2022, 51, 304–309. [Google Scholar]
- Poduska, K.M.; Regev, L.; Boaretto, E.; Addadi, L.; Weiner, S.; Kronik, L.; Curtarolo, S. Decoupling local disorder and optical effects in infrared spectra: Differentiating between calcites with different origins. Adv. Mater. 2011, 23, 550–554. [Google Scholar] [CrossRef]
- Verma, V.K.; Tewari, S.; Rai, J.P.N. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef]
- Darjito, M.; Khunur, M.; Purwonugroho, D.; Yuliantari, M.A.; Sari, Z.R.; Suryaningrat, L.H.A.K.; Prananto, Y.P. Adsorption of M (II)(M = Mn, Cu, Zn) in various pH and contact time using chitosan-silica prepared by sol-gel method. Rasayan J. Chem. 2019, 12, 1485–1492. [Google Scholar] [CrossRef]
- Chowdhury, S.; Das, P. Mechanistic, kinetic, and thermodynamic evaluation of adsorption of hazardous malachite green onto conch shell powder. Sep. Sci. Technol. 2011, 46, 1966–1976. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Sun, S.; Wang, Y.; Xu, L. Preparation of PVA/waste oyster shell powder composite as an efficient adsorbent of heavy metals from wastewater. Heliyon 2022, 8, e11938. [Google Scholar] [CrossRef]
- Huang, H.; Jia, Q.; Jing, W.; Dahms, H.; Wang, L. Screening strains for microbial biosorption technology of cadmium. Chemosphere 2020, 251, 126428. [Google Scholar] [CrossRef]
- Liu, Z.-R.; Zhou, S.-Q. Adsorption of copper and nickel on Na-bentonite. Process Saf. Environ. Prot. 2010, 88, 62–66. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lanjwani, M.F.; Khuhawar, M.Y.; Khuhawar, T.M.J.; Lanjwani, A.H.; Memon, S.Q.; Soomro, W.A.; Rind, I.K. Photocatalytic degradation of eriochrome black T dye by ZnO nanoparticles using multivariate factorial, kinetics and isotherm models. J. Clust. Sci. 2023, 34, 1121–1132. [Google Scholar] [CrossRef]
- El-Enein, S.A.; Okbah, M.A.; Hussain, S.G.; Soliman, N.F.; Ghounam, H.H. Adsorption of selected metals ions in solution using nano-bentonite particles: Isotherms and kinetics. Environ. Process. 2020, 7, 463–477. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H.; Saad, Z. Synthetic nax zeolite as a very efficient heavy metals sorbent in batch and dynamic conditions. Colloids Interfaces 2018, 2, 22. [Google Scholar] [CrossRef]
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Elsamra, R.M.; Masoud, M.S.; Zidan, A.A.; Zokm, G.M.E.; Okbah, M.A. Green synthesis of nanostructured zinc oxide by Ocimum tenuiflorum extract: Characterization, adsorption modeling, cytotoxic screening, and metal ions adsorption applications. Biomass Convers. Biorefinery 2023, 14, 16843–16856. [Google Scholar] [CrossRef]
- Taha, A.; Shreadah, M.A.; Ahmed, A.; Heiba, H.F. Multi-component adsorption of Pb (II), Cd (II), and Ni (II) onto Egyptian Na-activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J. Environ. Chem. Eng. 2016, 4, 1166–1180. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El Zokm, G.M.; Farag, A.E.; Abdelwahab, M.S. Assessment of heat-inactivated marine Aspergillus flavus as a novel biosorbent for removal of Cd (II), Hg (II), and Pb (II) from water. Environ. Sci. Pollut. Res. 2017, 24, 18218–18228. [Google Scholar] [CrossRef]
- El Nemr, A.; Khaled, A.; Abdelwahab, O.; El-Sikaily, A. Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J. Hazard. Mater. 2008, 152, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- El Nemr, A.; Aboughaly, R.M.; El Sikaily, A.; Ragab, S.; Masoud, M.S.; Ramadan, M.S. Microporous Nano-activated carbon type I derived from orange peel and its application for Cr (VI) removal from aquatic environment. Biomass Convers. Biorefinery 2020, 12, 5125–5143. [Google Scholar] [CrossRef]
- Doke, K.M.; Khan, E.M. Adsorption thermodynamics to clean up wastewater; critical review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 25–44. [Google Scholar] [CrossRef]
- Ebisike, K.; Elvis, O.A.; Kanayo, A.K.; Jeremiah, A.O. Thermodynamic study of the adsorption of Cd2+ and Ni2+ onto chitosan–Silica hybrid aerogel from aqueous solution. Results Chem. 2023, 5, 100730. [Google Scholar] [CrossRef]


| Biosorption Models | Equations | Parameters |
|---|---|---|
| Kinetics | ||
| Pseudo-first-order model [32,33] | qe and qt are the values of amount biosorbed/unit mass at any time t at equilibrium condition. k1: equilibrium rate constant for pseudo-first-order biosorption. | |
| Pseudo-second-order model [34,35] | k2: equilibrium rate constant for pseudo-second-order biosorption. | |
| Intraparticle diffusion model [36] | qt: capacity of biosorption at any time t. kid: rate constant of intraparticle diffusion (mg/g min1/2), C: the film thickness. | |
| Isotherm | ||
| Langmuir [37] | qe: the capacity of biosorption at equilibrium (mg/g), qmax: the maximum capacity of biosorption at single-layer coverage (mg/g). Ce: the [M2+] at equilibrium (mg/L), KL: the intensity of biosorption (L/mg). | |
| Freundlich [38] | Kf: biosorption capacity constant (mg/g), nf: Freundlich affinity constant, biosorption intensity of the solid biosorbent. | |
| Temkin and Pyzhev [39] | AT: the equilibrium binding constant (L/min), BT: constant correlated with the heat of biosorption process. |
| Before Biosorbent | Cd after Biosorbent | Pb after Biosorbent | ||||||
|---|---|---|---|---|---|---|---|---|
| Element | Mass % | Atom % | Element | Mass % | Atom % | Element | Mass % | Atom % |
| C | 6.23 ± 0.43 | 11.03 ± 0.25 | C | 2.34 ± 0.22 | 4.27 ± 0.20 | C | 6.11 ± 0.43 | 10.58 ± 0.42 |
| O | 45.11 ± 0.50 | 62.12 ± 0.12 | O | 44.35 ± 0.20 | 62.55 ± 0.32 | O | 42.31 ± 0.50 | 60.25 ± 0.08 |
| Ca | 44.25 ± 0.22 | 24.52 ± 0.51 | Ca | 40.09 ± 0.27 | 21.72 ± 0.26 | Ca | 39.25 ± 0.37 | 21.22 ± 0.47 |
| Al | 0.41 ± 0.13 | 0.35 ± 0.03 | Al | 0.32 ± 0.13 | 0.28 ± 0.10 | Al | 0.30 ± 0.22 | 0.25 ± 0.14 |
| Cd | 0.43 ± 0.38 | 0.19 ± 0.05 | Pb | 0.38 ± 0.25 | 0.14 ± 0.07 | |||
| Kinetic Models | Parameter | Pb2+ | Cd2+ |
|---|---|---|---|
| Langmuir | qmax (mg/g) KL (L/g) R2 | 98.039 23.667 0.9699 | 63.694 12.083 0.9761 |
| Freundlich | Kf (mg/g)/(L/mg)1/n n 1/n R2 | 2.0083 1.2475 0.8016 0.9712 | 1.8482 1.1490 0.8703 0.9891 |
| Temkin | AT (L/g) B (J/mol) R2 | 1.1488 10.689 0.9735 | 1.4082 10.128 0.9583 |
| Kinetics | Parameter | Pb2+ | Cd2+ |
|---|---|---|---|
| qe (experimental) (mg/g) | 9.16 | 7.91 | |
| Pseudo-first-order model | K1(min−1) qe (mg/g) R2 | −0.0234 5.4712 0.8517 | −0.0115 1.8789 0.2962 |
| Pseudo-second-order model | K2 (g/mg/min) qe (mg/g) R2 | 0.0236 11.5606 0.9991 | 0.0068 8.4530 0.9731 |
| Intraparticle diffusion model | C (mg/L) kdif (mg/g min1/2) R2 | 3.4747 0.7508 0.9594 | 4.0237 0.4876 0.8340 |
| Metals | T (°C) | T (K) | Kd | ∆G° | ∆H° | ∆S° | R |
|---|---|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (J/molK) | |||||
| Cd2+ | 20 | 293 | 20.997 | −7.415 | −0.3668 | 4.3031 | 0.9998 |
| 25 | 298 | 21.486 | −7.599 | ||||
| 30 | 303 | 21.990 | −7.787 | ||||
| 35 | 308 | 22.728 | −8.000 | ||||
| 40 | 313 | 23.163 | −8.179 | ||||
| 50 | 323 | 23.690 | −8.513 | ||||
| 60 | 333 | 24.290 | −8.832 | ||||
| Pb2+ | 20 | 293 | 15.688 | −6.699 | −0.2995 | 3.7800 | 0.9984 |
| 25 | 298 | 16.002 | −6.863 | ||||
| 30 | 303 | 16.408 | −7.054 | ||||
| 35 | 308 | 16.660 | −7.196 | ||||
| 40 | 313 | 16.789 | −7.338 | ||||
| 50 | 323 | 17.301 | −7.653 | ||||
| 60 | 333 | 17.7886 | −7.973 |
| Hydrochemistry Properties | Major Constituents (mg/L) | Nutrient Salts (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Real samples | PSU | pH | DOM (mg/L) | Na+ | K+ | Ca2+ | Mg2+ | SO42 (g/L) | NO3 | NO2 | PO4 | SiO4 |
| Groundwater | 4.25 | 7.58 | 1.18 | 665 | 20 | 486 | 165 | 0.95 | 0.079 | 0.012 | 0.037 | 0.033 |
| Gulf of Aqaba, Saudi Arabia | 41.54 | 8.24 | 2.78 | 10786 | 305 | 742 | 2325 | 2.13 | 1.15 | 0.016 | 0.084 | 0.096 |
| Spiking | Cd2+ (%Removal) | Pb2+ (%Removal) | ||||
|---|---|---|---|---|---|---|
| Initial Metal (C0) | C0 = 10 µg/L | C0 = 20 µg/L | ||||
| Water sample | Run 1 | Run 2 | Run 3 | Run 1 | Run 2 | Run 3 |
| Groundwater | 93.81 | 91.75 | 90.44 | 90.86 | 90.87 | 91.77 |
| seawater (Gulf of Aqaba, Saudi Arabia) | 89.11 | 87.25 | 88.35 | 88.96 | 90. 28 | 91.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mur, B.A. Application of Marine Mollusk Shells (Meretrix lusoria) as Low-Cost Biosorbent for Removing Cd2+ and Pb2+ Ions from Aqueous Solution: Kinetic and Equilibrium Study. Water 2024, 16, 2615. https://doi.org/10.3390/w16182615
Al-Mur BA. Application of Marine Mollusk Shells (Meretrix lusoria) as Low-Cost Biosorbent for Removing Cd2+ and Pb2+ Ions from Aqueous Solution: Kinetic and Equilibrium Study. Water. 2024; 16(18):2615. https://doi.org/10.3390/w16182615
Chicago/Turabian StyleAl-Mur, Bandar A. 2024. "Application of Marine Mollusk Shells (Meretrix lusoria) as Low-Cost Biosorbent for Removing Cd2+ and Pb2+ Ions from Aqueous Solution: Kinetic and Equilibrium Study" Water 16, no. 18: 2615. https://doi.org/10.3390/w16182615
APA StyleAl-Mur, B. A. (2024). Application of Marine Mollusk Shells (Meretrix lusoria) as Low-Cost Biosorbent for Removing Cd2+ and Pb2+ Ions from Aqueous Solution: Kinetic and Equilibrium Study. Water, 16(18), 2615. https://doi.org/10.3390/w16182615







