1. Introduction
Phytoplankton plays a vital role in the biogeochemical cycles of aquatic ecosystems, such as the elemental cycles of carbon (C), nitrogen (N), phosphorus (P), and iron (Fe) [
1]. Phytoplankton cells absorb these resources and use them in their activities [
2]. Then, the elements are introduced into the environment in the form of organic matter. Some of this organic matter (particles or dissolved) is taken up, absorbed, and discharged by the next trophic level. And others precipitate to the bottom of the river and enter nature through microbial action and water exchange [
3]. Therefore, phytoplankton absorb these nutrients and allocate them to their key activities. It will produce changes in its own element composition, food networks, and surrounding environmental elements. In the interaction process between the exchange of substances and life activities in this ecosystem, the balance of various chemical elements in phytoplankton will form a unique stoichiometric ratio [
4]. Ecological stoichiometry is an important means to study the balance of life elements and characteristics [
5].
Redfield proved that the elemental composition of surface plankton was generally consistent with the proportion of dissolved nutrients in the ocean, and determined the Redfield mole ratio of 106C:16N:1P at the beginning of the 20th century [
6]. This led to a hypothesis that the marine phytoplankton had evolved to have the ability to change their elemental levels to resemble those of the water environment in which they grew [
7]. However, the C:N:P ratios of different heterotrophic and autotrophic aquatic organisms are now known to not necessarily conform the to ratio of Redfield. Because the unique C:N:P stoichiometric ratio of biology was generated by the interaction of nutrient elements and physiological control, the N:P ratios of the same species of phytoplankton in different environments and different phytoplankton in the same environment are different. Mountainous rivers differ significantly from marine environments in factors like water flow, temperature, light, and nutrient sources. These differences may cause riverine phytoplankton to deviate from the Redfield ratio. Additionally, the diverse species and seasonal variations in rivers further necessitate adjustments to the Redfield model. The optimal N:P ratio of phytoplankton was between 8.2:1 and 45.0:1 according to statistics, and the conclusion proposed by Redfield was more like the average N:P ratio [
8]. Furthermore, phytoplankton C:N:P was higher than the Redifield ratio in Xiamen Bay and it may be limited by P due to imbalanced C:N:P input to seawater [
9]. Although carbon, nitrogen, and phosphorus were traditionally emphasized for their roles in primary production and nutrient cycling, sulfur, hydrogen, and iron also played crucial roles in the physiology of phytoplankton and the functioning of ecosystems. Sulfur is vital for synthesizing amino acids and proteins, and its availability can influence the overall metabolic processes in phytoplankton. Hydrogen is a key element in energy transfer, participating in the formation of organic molecules through photosynthesis and respiration. Iron is essential for photosynthesis and serves as a cofactor in various enzymatic reactions, particularly in cold regions where its availability may be limited due to low solubility in cold water. In cold areas, the availability and cycling of these elements are especially critical because of extreme environmental conditions, which can alter the biochemical processes in phytoplankton. Therefore, understanding the stoichiometric ratios of these elements in phytoplankton is crucial for a comprehensive understanding of nutrient dynamics and ecosystem functioning in cold region aquatic environments. With the development of analytical testing techniques, new results have been produced on the ecological stoichiometric characteristics of not only aquatic ecosystems but also various ecosystems under the global patter [
5,
10].
While much research has been conducted on phytoplankton stoichiometry in marine and temperate freshwater systems, there was a relative lack of studies focusing on cold mountain rivers. Unique environmental conditions such as extreme temperatures, seasonal ice closures, and limited growing seasons in cold regions create a challenging environment for primary producers such as phytoplankton. Environmental changes significantly affect the ecological dynamics of phytoplankton, leading to adaptive changes in phytoplankton stoichiometric ratios. Mountain rivers are dynamic ecosystems characterized by rapid water flow, fluctuating nutrient levels, and changing light conditions. These factors affect nutrient availability and phytoplankton growth capacity. Understanding how phytoplankton in these rivers respond to environmental variables can provide insights into broader biogeochemical cycles and potential downstream impacts on coastal ecosystems.
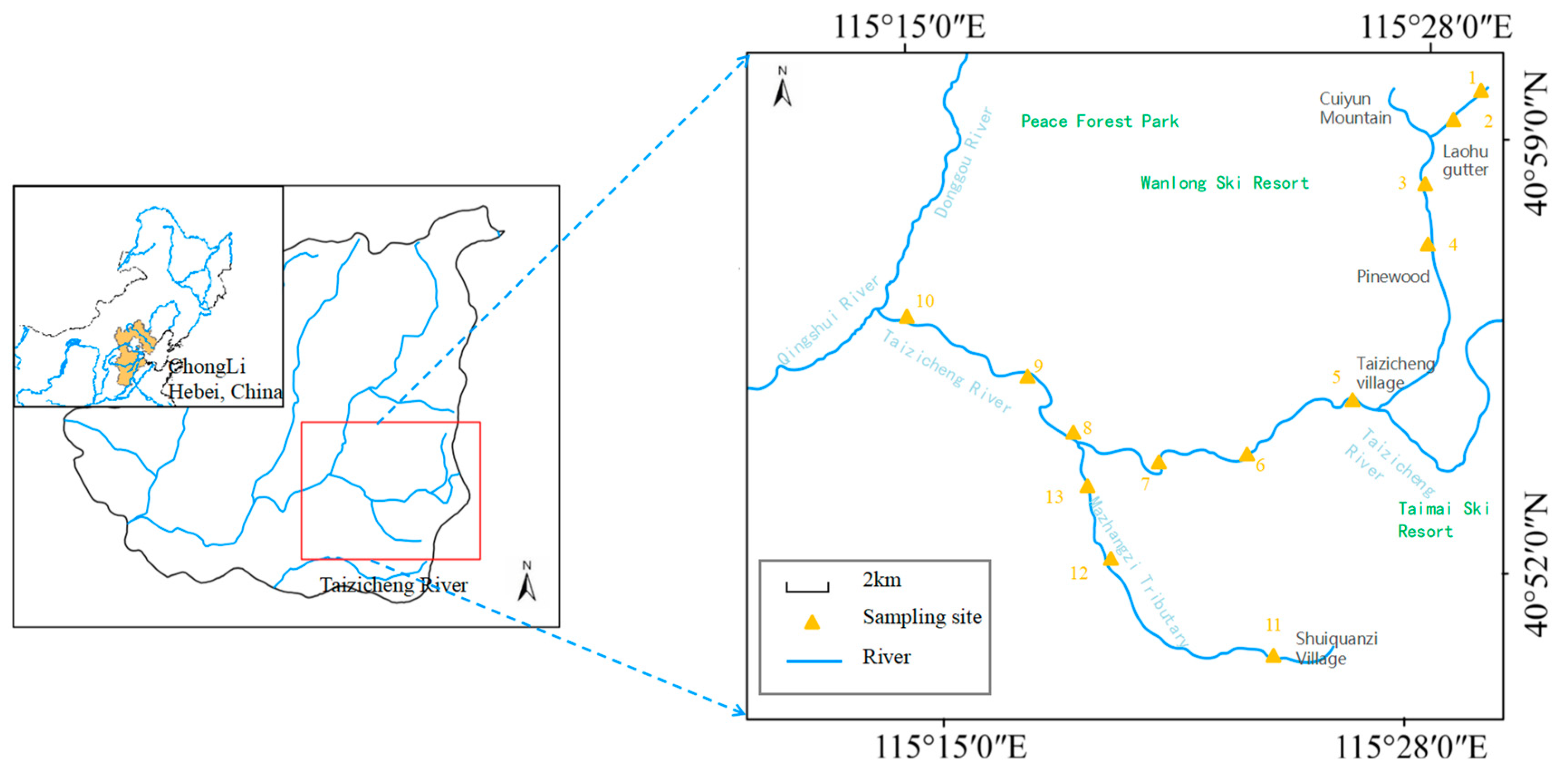
Mountain river ecosystems exhibit distinct characteristics, including a high altitude gradient, fast flow velocity, a scarcity of fish, and low temperature. These unique features make it an ideal object of study. In the current study, we aimed to investigate the spatial and temporal distribution and stoichiometric characteristics of carbon (C), nitrogen (N), and phosphorus (P) elements, as well as sulfur (S), hydrogen (H), and iron (Fe) of phytoplankton in the Taizicheng River ecosystem. Our objective was to better understand the characteristics of the ecological stoichiometric and element balance relationship of river organisms in cold mountain areas. Specifically, we focused on the following aspects: (1) to explore the effects of various elements on the nutrient cycle mechanism; (2) to reveal the stoichiometric characteristics of multi-element balance of phytoplankton in mountain rivers.
4. Discussion
Although the application of ecological stoichiometry began with plankton, the research of ecological stoichiometry in China has been more concentrated on soil, wetland, forest, grasslands, meadow, etc., and there are few studies on aquatic ecosystems, especially on river plankton [
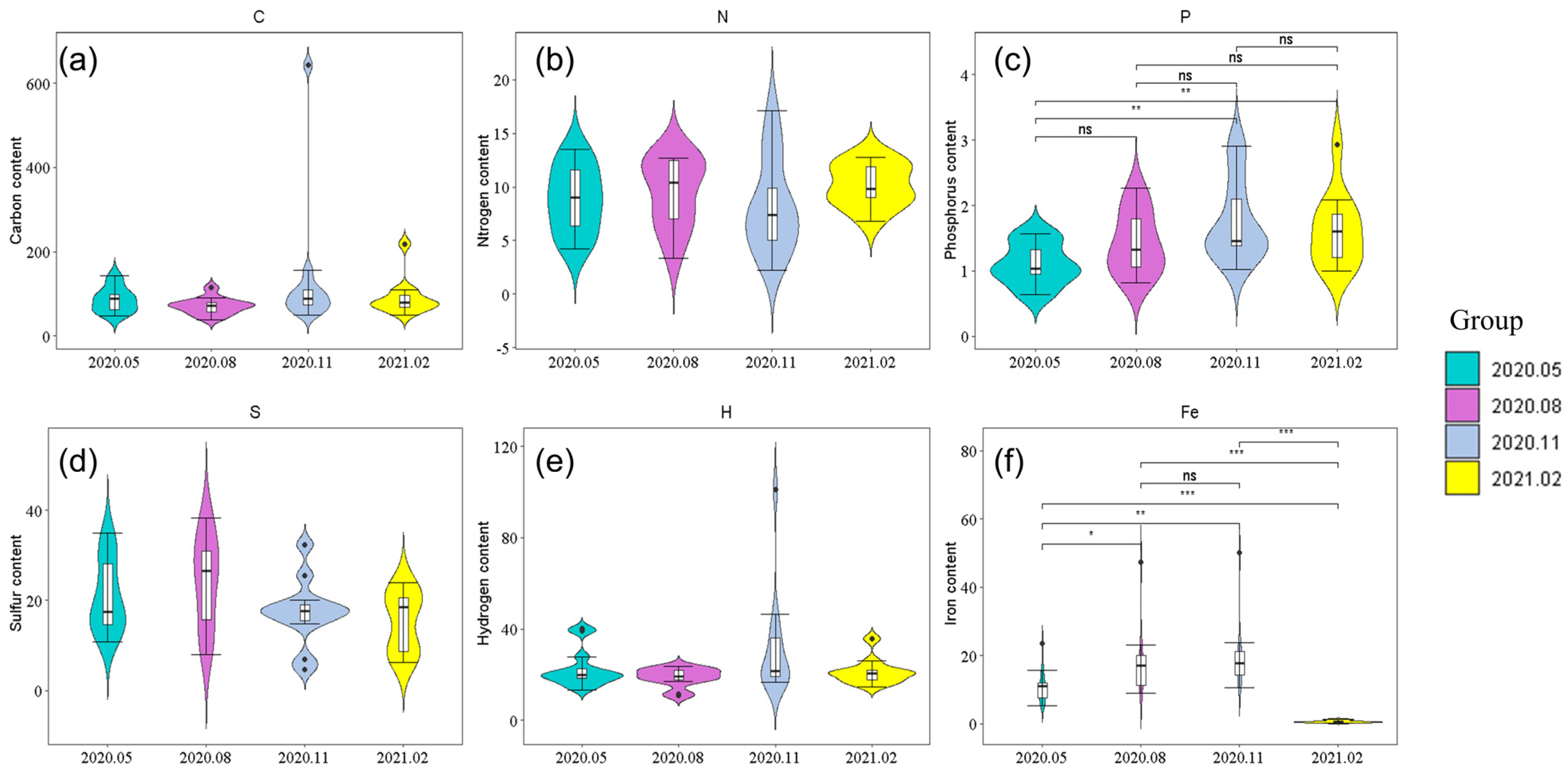
12]. Based on the classical Redfield ratio and the previous research results, our study analyzed the distribution pattern of elements C, N, P, S, H, and Fe of phytoplankton in the Taizicheng River, which is a mountain river in a cold area. Element C is a structural element of phytoplankton, and mainly participates in the synthesis of carbohydrates, with the highest content and the highest proportion, but its coefficient of variation was higher than that of N and P [
13]. The coefficient of variation of N and P was about 37%, while that of C was 40.31%. The low content of N and P was relatively stable, which may be due to the fact that the input of nutrient elements N and P in the Taizicheng River was small and stable, and phytoplankton was limited by the supply of elements, absorbing only N and P elements for its use, resulting in its stable content. Phytoplankton can utilize dissolved CO
2 and HCO
3− from the river water to meet their carbon demands, particularly given the global increase in atmospheric CO
2 concentrations [
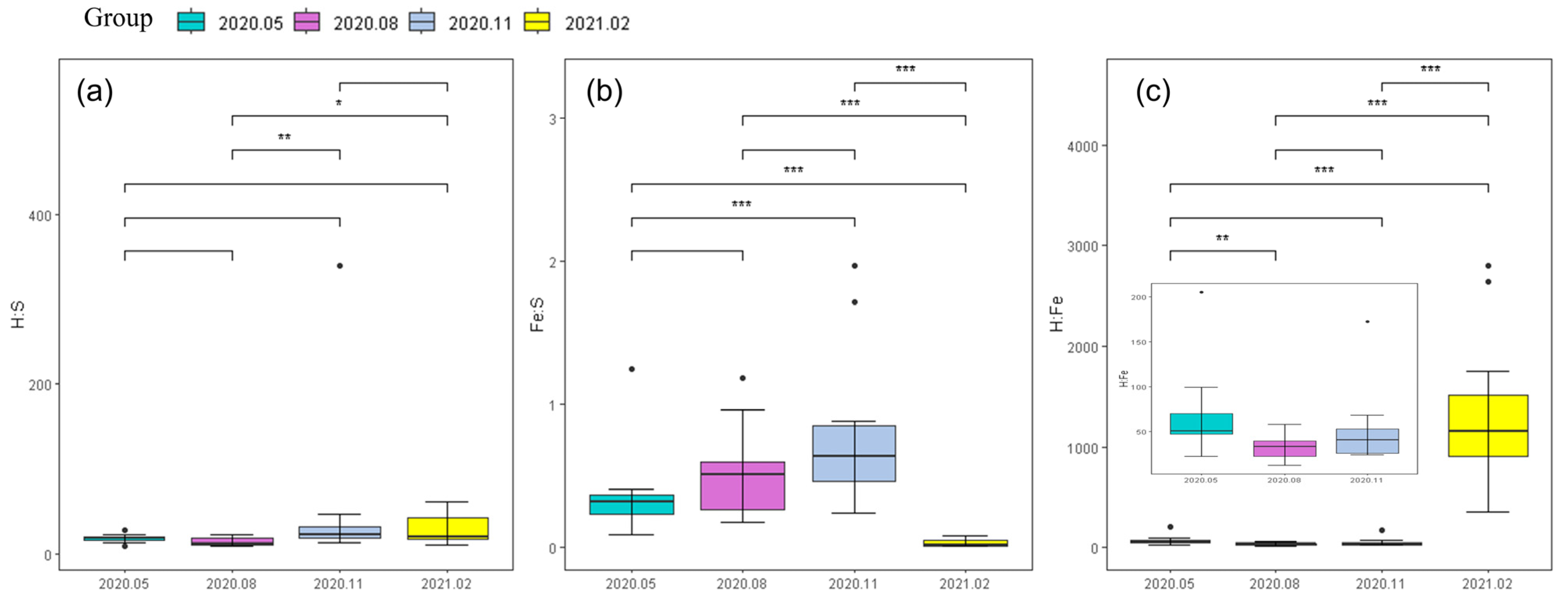
14]. According to its own growth stage, the absorption of element C was also different, resulting in a large fluctuation in its element C content and a large coefficient of variation. Although the content of element C in phytoplankton changed greatly, it was rarely limited by C, and more nutrient elements N and P limit the growth of phytoplankton. The coefficient of variation of S, H, and Fe elements was slightly larger than that of C, N, and P. As important nutrient elements of plankton, S and Fe were mainly involved in protein synthesis, while the latter were indispensable elements in plant photosynthesis, respiration, and other processes. The differences in plant life activities in different seasons form the temporal distribution characteristics of S and Fe. The absorption and action of hydrogen elements were related to water, with less content and relatively large fluctuations.
Redfield studied the elemental composition of marine plankton for the first time and proposed the elemental ratio of C
106H
263N
16P. According to the changes in hydrological environment and geological factors in the water ecosystem, there are differences in plankton stoichiometric ratio and biological composition [
12]. Anderson proposed a new element composition of plankton, C
106H
263N
16P, through actual measurement. Wollast added the study of the S element and obtained the molecular formula of C
106H
263N
16S
1.7P [
13]. Xu Delin measured the elements of C
113.14N
20.06P and C
105.86N
14.75P in the grass and algal lakes of Taihu Lake [
14]. Klausmeier calculated the N:P ratio between the phytoplankton growth explosion period and the relative plateau period, and found that the N:P ratio was higher in the competitive equilibrium period rather than the growth explosion period. The optimal N:P ratio of phytoplankton was between 8.2 and 45.0. It was suggested that the classical ratio N:P = 16, which was the average ratio of each species in different periods and environments. Walker and Asams showed that organic C, N, P, and S were strongly related, both among different soils and with soil depth, and that the organic N:P ratio falls with depth in New Zealand grassland soils [
15]. Kirkby et al. concluded that SOM of mineral soil has an approximately constant stoichiometry, and calculated a rounded stoichiometry of 52:5:1:1 (C:N:P:S) according to the data they collated for soils from 22 countries [
16]. Our results showed that the phytoplankton element ratio was C
156.00 N
15.41 S
1.54 H
51.17 Fe
5.10 P in the Taizicheng River, and there were higher C, N, P contents than the classical Redfield ratio, and the proportion of H share fell dramatically.
The shallow water and strong illumination of the Taizicheng River may be the reason for the high proportion of phytoplankton element C. Berger studied the stoichiometry of plankton at different depths in 65 lakes in central Europe, and proved the positive correlation between light and C:P ratio [
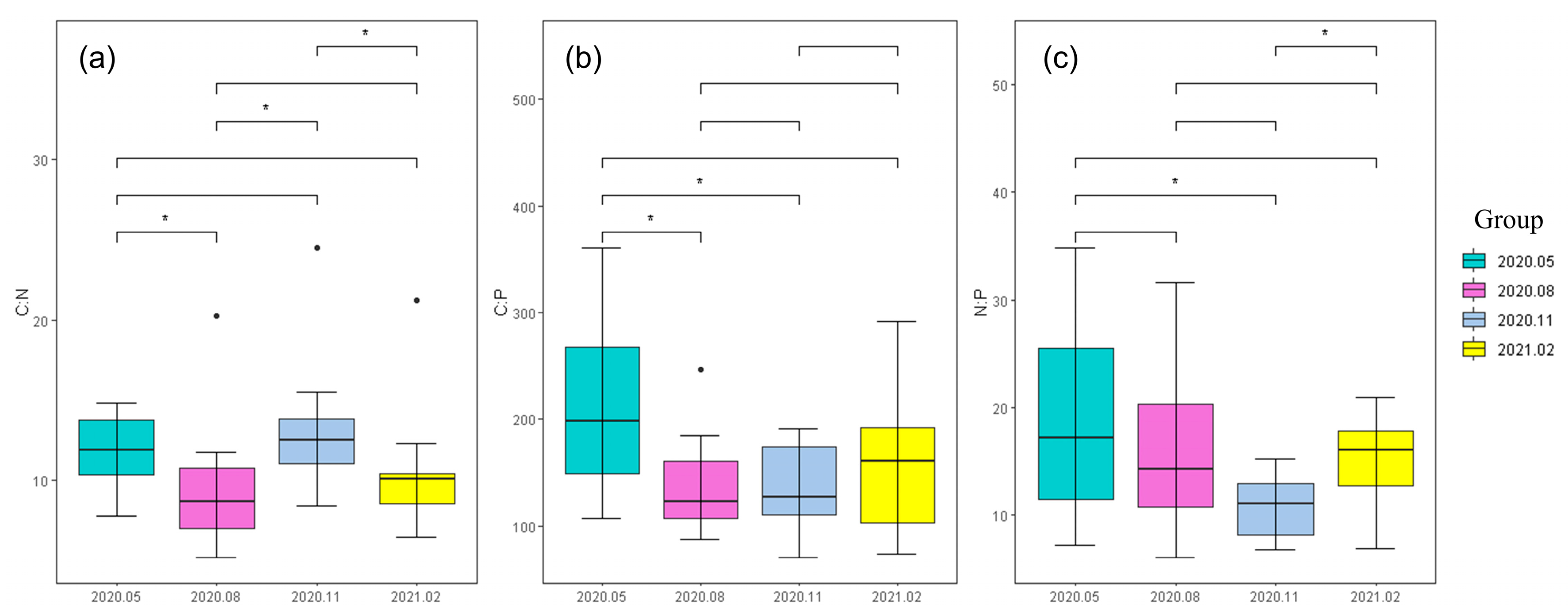
17]. The succession of phytoplankton community structure can also lead to a change in stoichiometry. For example, diatom was the main contributor to C element composition in river plankton, and the higher the dominance of diatom in phytoplankton community structure, the greater the stoichiometric ratio of phytoplankton C element. Diatom was one of the main species of phytoplankton community in the Taizicheng River, and it was also the reason for the relatively high proportion of C element. The N:P ratio of phytoplankton in the Taizicheng River was 15.41, which basically accorded with the classical Redfield ratio. The content of P increased in November and February, and the content of N increased in February. Low temperatures would induce the plant to store nutrients to resist the adverse effects of winter on growth. In stoichiometric studies, the ratio of N:P was usually used to determine the limiting element. When N:P was less than 14, N was a restrictive nutrient element, and when N:P was greater than 16, P was the limiting nutrient element. Between 14 and 16, it was subject to the comprehensive limitation of N and P. Combined with the actual data, it can be concluded that the growth of phytoplankton in the Taizicheng River was restricted by N and P. Additionally, phosphorus was a limiting factor in river ecosystems, which may have important implications for predicting the effects of environmental changes, such as climate change or land use change, on ecosystem functioning.
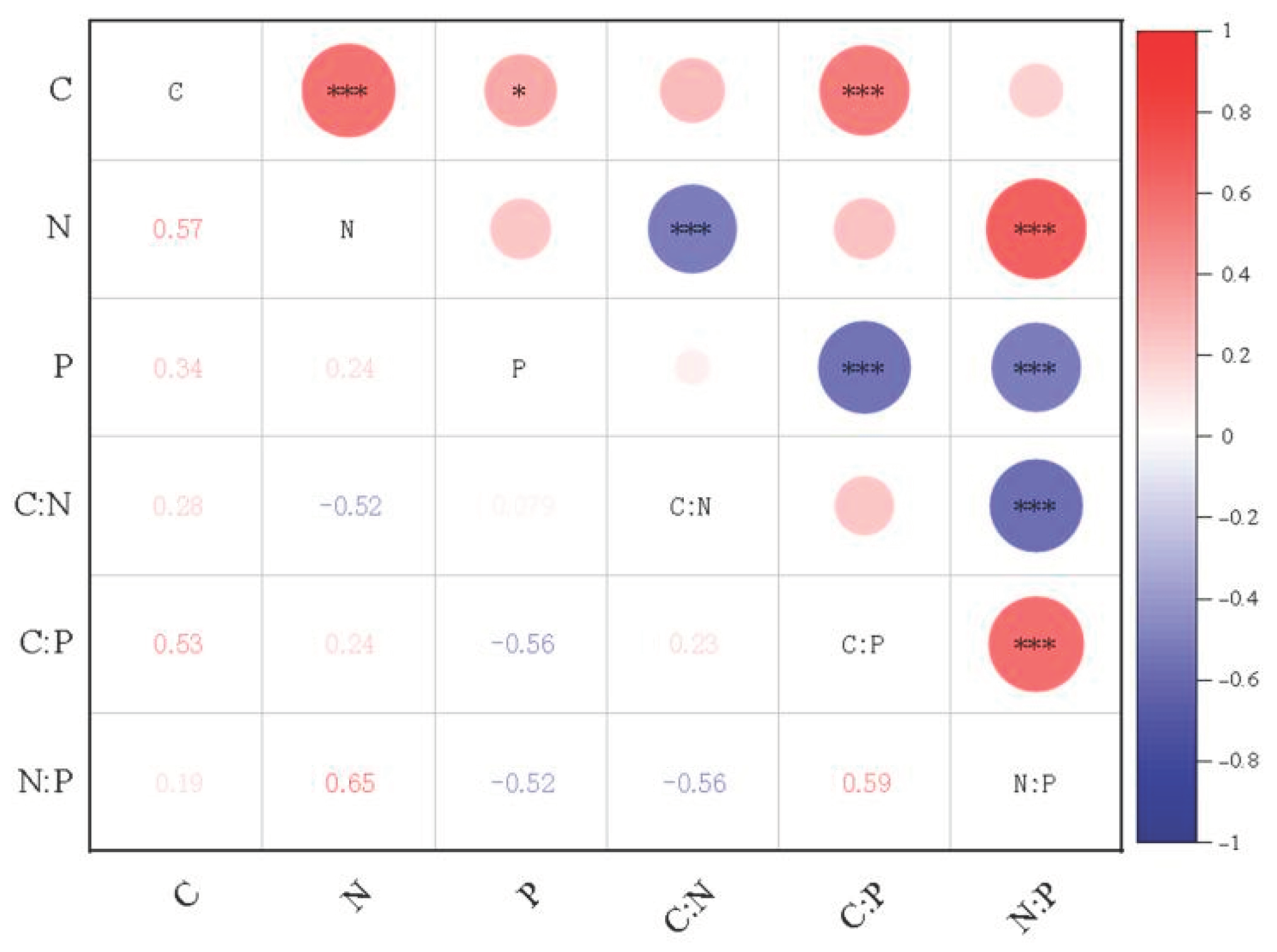
Phytoplankton elements were coupled with each other through their life activities. N was mainly used for plant protein production and P was mainly involved in the synthesis of nucleic acid. The storage of other elements relies on ion balance to adjust. The growth of plants needs to synthesize protein, which would inevitably increase the consumption of P element with the transcription of DNA to generate RNA, and this process significantly enhances the correlation between N and P. As a “skeleton element”, element C played a supporting role in the cell wall, and its content increased with the growth and development of plants. The contents of C, N, and P in plants were significantly correlated, while the correlation between S, H, and Fe was not significant. Only the elements S and N were significantly correlated at the level of 0.01, and both of them participated in protein synthesis. However, this did not prove that there was no interaction between elements. Some studies have shown that Fe in diatoms can affect the ratio of C:N:P by affecting their own growth rate and body size [
18,
19]. Boyd and Geider proved that iron in algae can promote some enzyme activities during the synthesis of proteins and nucleic acids and the absorption of CO
2 in the atmosphere, thus affecting the stoichiometric characteristics of their own C, N, and P [
20].
5. Conclusions
In this paper, the ecological stoichiometric characteristics of C, N, P, S, H, and Fe elements in river phytoplankton in cold mountain areas were studied, and the results were compared with the Redfield ratio and previous research results. We know that the phytoplankton elements of the Taizicheng River were C156.00N15.41S1.54H51.17Fe5.10P, fluctuating seasonally. Compared with the value of Redfield and previous studies, element C accounted for a higher proportion, element H accounted for a lower proportion, element Fe content changed greatly, and the remaining elements fluctuated within a small range. Further, we know that the growth of phytoplankton in the Taizicheng River was limited by both N and P elements according to the theory of chemometrics restrictive elements. In addition, the contents and ratios of elements C, N, and P of phytoplankton in the Taizicheng River were coupled through life activities, which had a strong correlation and had a great influence on the growth of phytoplankton. However, the S, H, and Fe elements were relatively independent and had no significant correlation. The elements and their proportions in river bodies and zooplankton need to be further explored in future studies. Overall, our results represent a considerable advancement in understanding the distribution patterns of phytoplankton stoichiometry and the impact of seasonal changes on phytoplankton stoichiometry in cold temperate area. It paves the way to better understand how these ecosystems work and for further guidance and a better methodology of maintaining the balance and stability of the freshwater ecological environment.