Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sample Collection and Determination of Physical and Chemical Properties
2.2. Spectra Measurements
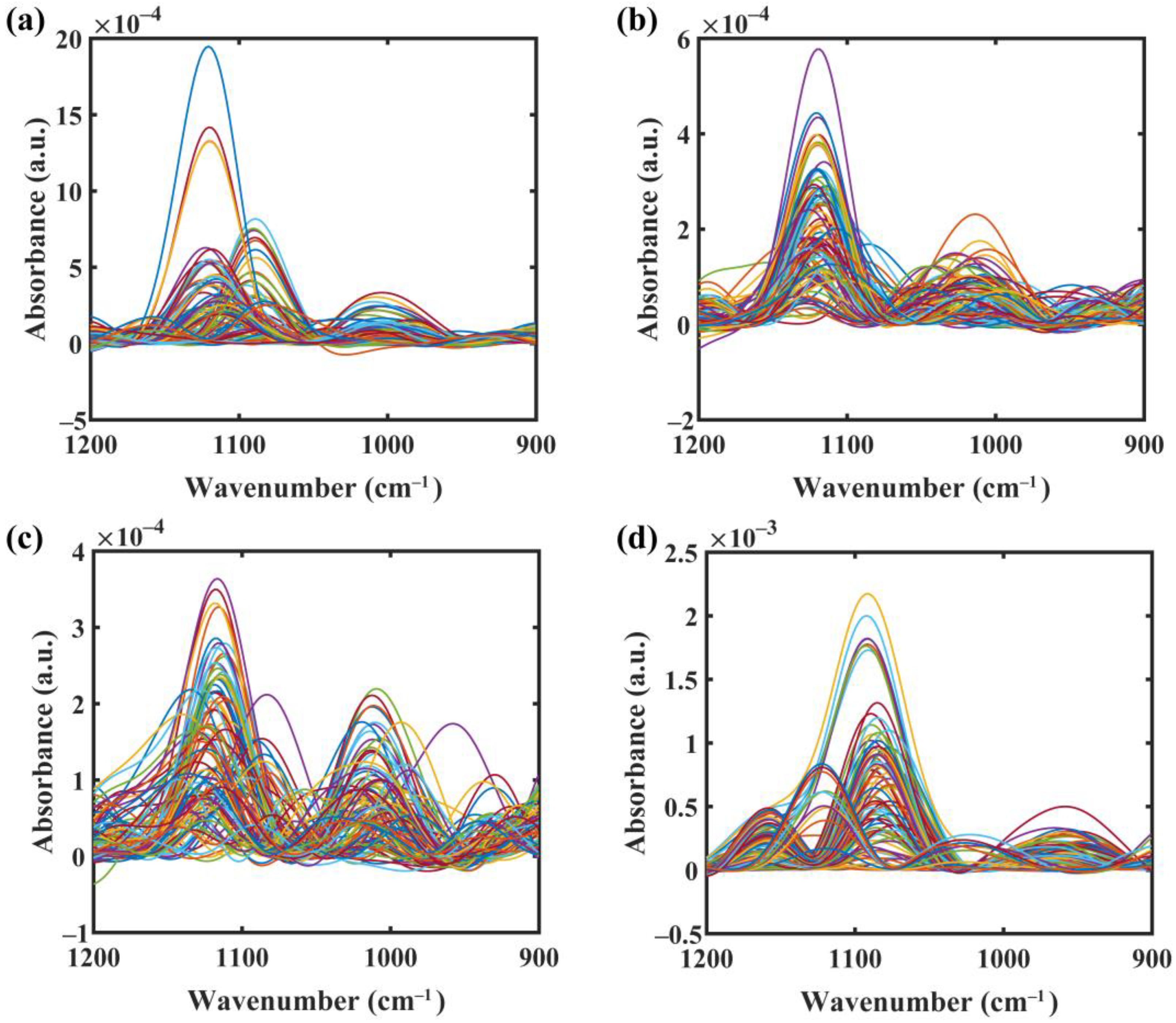
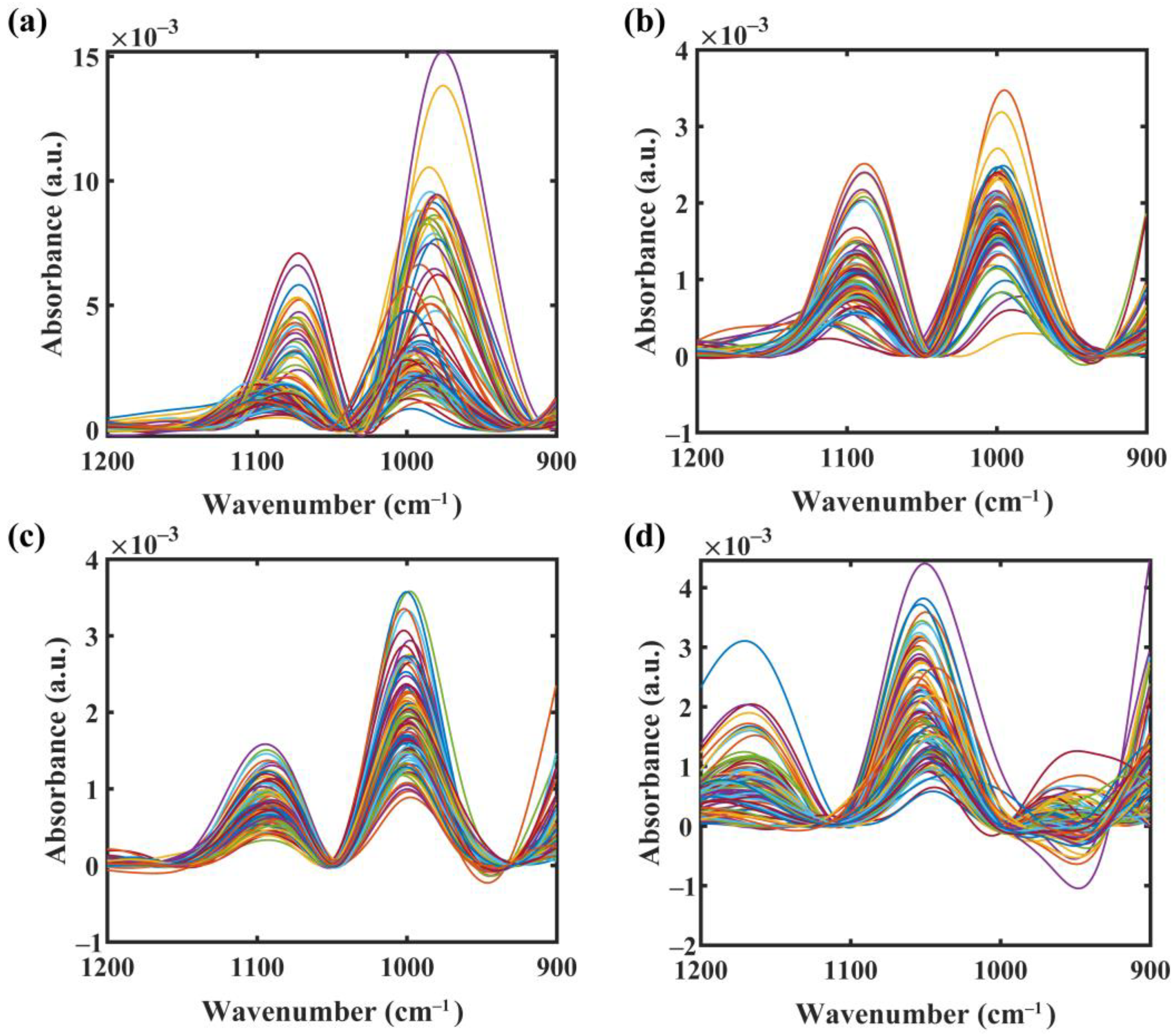
2.3. Preprocessing of Spectra
2.4. Prediction Models
2.5. Model Evaluation
3. Results and Discussion
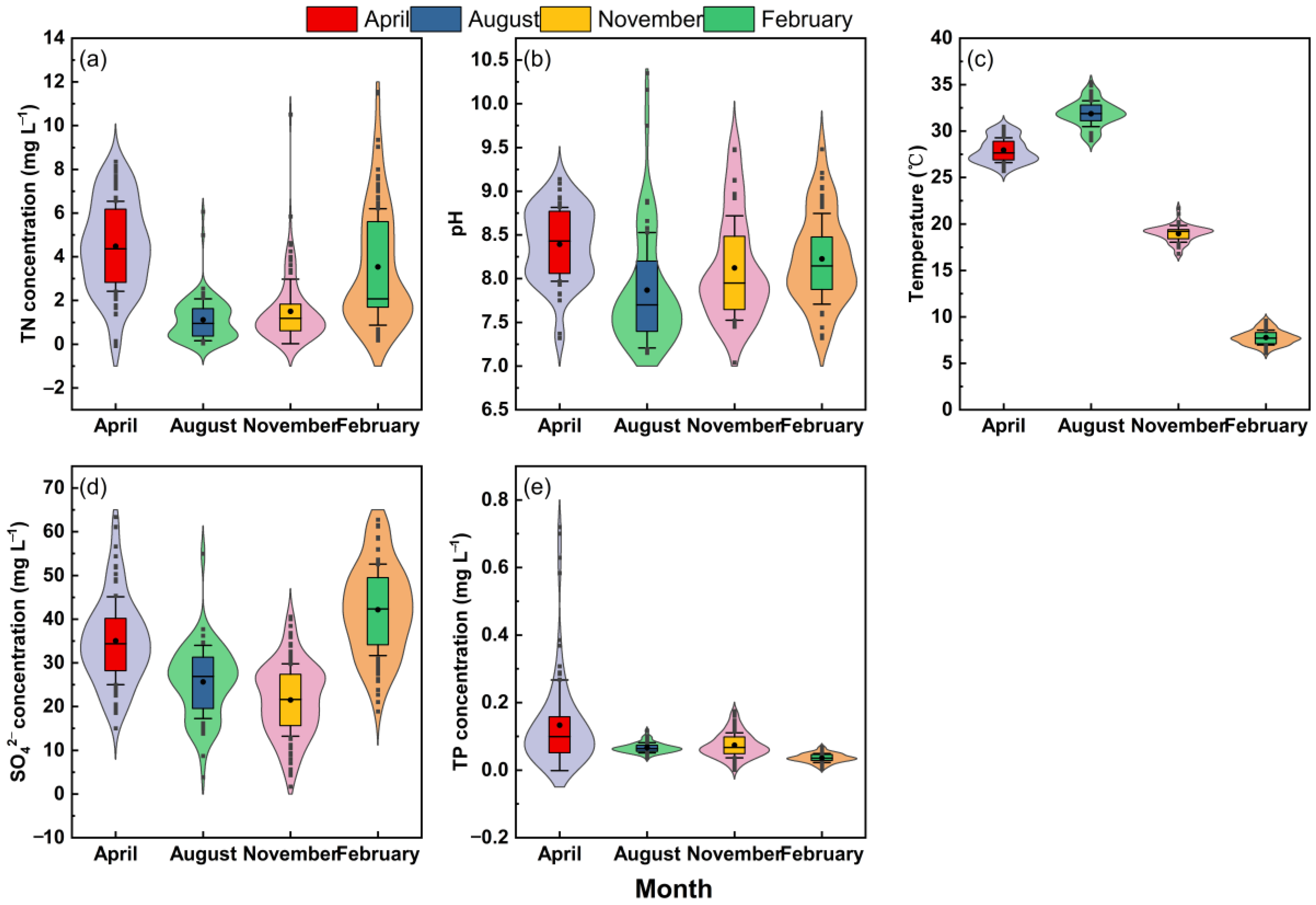
3.1. Properties and Characteristics of Natural Water Samples
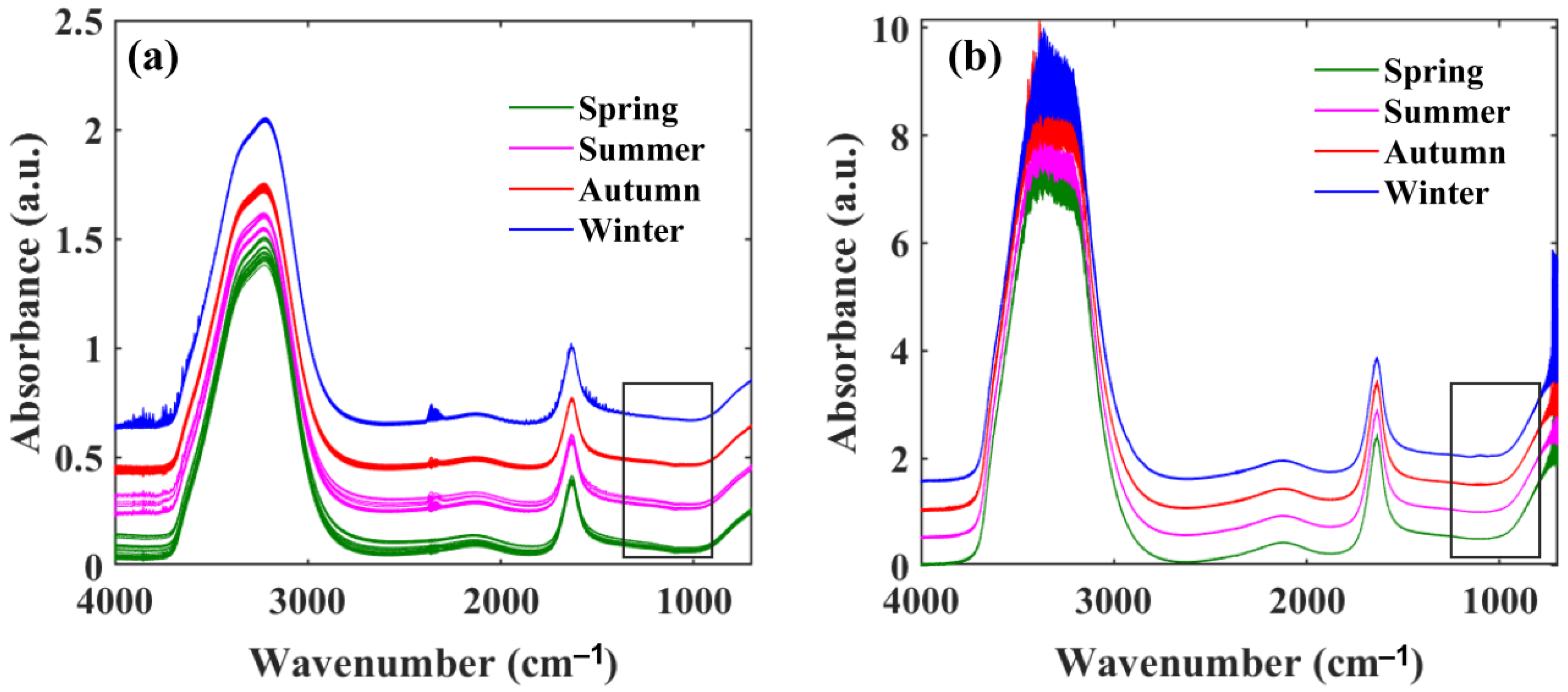
3.2. FTIR-ATR Spectra of Natural Water Samples
3.3. PCA Analysis
3.4. Prediction of TP in Water Based on Si-ATR and ZnSe-ATR
3.5. Effect of Season Variation on TP Prediction
3.6. Application of SA-PLS Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Sheng, S.; Mao, S.; Wu, X.; Li, Z.; Tao, W.; Jenkinson, I.R. Highly sensitive and selective fluorescent detection of phosphate in water environment by a functionalized coordination polymer. Water Res. 2019, 163, 114883. [Google Scholar] [CrossRef] [PubMed]
- Boeykens, S.P.; Piol, M.N.; Samudio Legal, L.; Saralegui, A.B.; Vázquez, C. Eutrophication decrease: Phosphate adsorption processes in presence of nitrates. J. Environ. Manag. 2017, 203, 888–895. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Shen, X.; Bai, L.; Jiang, H. Particle size-related vertical redistribution of phosphorus (P)-inactivating materials induced by resuspension shaped P immobilization in lake sediment profile. Water Res. 2022, 213, 118150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, L.; Deng, C. Changes in net anthropogenic nitrogen and phosphorus inputs in the Yangtze River Economic Belt, China (1999–2018). Ecol. Indic. 2022, 145, 109674. [Google Scholar] [CrossRef]
- Determan, R.T.; White, J.D.; McKenna, L.W. Quantile regression illuminates the successes and shortcomings of long-term eutrophication remediation efforts in an urban river system. Water Res. 2021, 202, 117434. [Google Scholar] [CrossRef]
- Chung, M.G.; Frank, K.A.; Pokhrel, Y.; Dietz, T.; Liu, J. Natural infrastructure in sustaining global urban freshwater ecosystem services. Nat. Sustain. 2021, 4, 1068–1075. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Xia, R.; Li, W.; Zhang, Y.; Zhang, K.; Tong, S.; Jia, R.; Hu, Q.; Wang, L.; et al. Phosphorus—The main limiting factor in riverine ecosystems in China. Sci. Total Environ. 2023, 870, 161613. [Google Scholar] [CrossRef]
- Liu, D.; Duan, H.; Yu, S.; Shen, M.; Xue, K. Human-induced eutrophication dominates the bio-optical compositions of suspended particles in shallow lakes: Implications for remote sensing. Sci. Total Environ. 2019, 667, 112–123. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhang, L.F.; Cen, Y.; Wang, S.; Zhang, Y.; Huang, Y.; Sultan, M.; Tong, Q.X. Prediction of Total Phosphorus Concentration in Macrophytic Lakes Using Chlorophyll-Sensitive Bands: A Case Study of Lake Baiyangdian. Remote Sens. 2022, 14, 3077. [Google Scholar] [CrossRef]
- Cozzolino, D.; Curtin, C. The use of attenuated total reflectance as tool to monitor the time course of fermentation in wild ferments. Food Control 2012, 26, 241–246. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An In Situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef]
- Vogel, C.; Sekine, R.; Steckenmesser, D.; Lombi, E.; Herzel, H.; Zuin, L.; Wang, D.; Félix, R.; Adam, C. Combining diffusive gradients in thin films (DGT) and spectroscopic techniques for the determination of phosphorus species in soils. Anal. Chim. Acta 2019, 1057, 80–87. [Google Scholar] [CrossRef]
- Auer, B.M.; Skinner, J.L. IR and Raman spectra of liquid water: Theory and interpretation. J. Chem. Phys. 2008, 128, 224511. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared spectroscopy coupled with chemometrics: A tool for the analysis of intact food systems and the exploration of their molecular structure-quality relationships—A review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xu, X.; Chen, G.; Zhou, J.; Ma, F.; Du, C. Rapid detection of phosphorus in water using silicon attenuated total reflectance infrared spectroscopy coupled with the algorithms of deconvolution and partial least squares regression. Sens. Actuators B Chem. 2023, 380, 133372. [Google Scholar] [CrossRef]
- Karabudak, E.; Kas, R.; Ogieglo, W.; Rafieian, D.; Schlautmann, S.; Lammertink, R.G.; Gardeniers, H.J.; Mul, G. Disposable attenuated total reflection-infrared crystals from silicon wafer: A versatile approach to surface infrared spectroscopy. Anal. Chem. 2013, 85, 33–38. [Google Scholar] [CrossRef]
- Wang, N.; Xie, L.Y.; Zuo, Y.; Wang, S.W. Determination of total phosphorus concentration in water by using visible-near-infrared spectroscopy with machine learning algorithm. Environ. Sci. Pollut. Res. 2023, 30, 58243–58252. [Google Scholar] [CrossRef]
- Shi, Z.; Chow, C.W.K.; Fabris, R.; Liu, J.; Sawade, E.; Jin, B. Determination of coagulant dosages for process control using online UV-vis spectra of raw water. J. Water Process Eng. 2022, 45, 102526. [Google Scholar] [CrossRef]
- Balabin, R.M.; Lomakina, E.I. Support vector machine regression (SVR/LS-SVM)—An alternative to neural networks (ANN) for analytical chemistry? Comparison of nonlinear methods on near infrared (NIR) spectroscopy data. Analyst 2011, 136, 1703–1712. [Google Scholar] [CrossRef]
- Kim, S.; Seo, Y.; Malik, A.; Kim, S.; Heddam, S.; Yaseen, Z.M.; Kisi, O.; Singh, V.P. Quantification of river total phosphorus using integrative artificial intelligence models. Ecol. Indic. 2023, 153, 110437. [Google Scholar] [CrossRef]
- Ma, F.; Du, C.W.; Zhou, J.M. A self-adaptive model for the prediction of soil organic matter using mid-infrared photoacoustic spectroscopy. Soil Sci. Soc. Am. J. 2016, 80, 238–246. [Google Scholar] [CrossRef]
- Xu, X.; Du, C.; Ma, F.; Shen, Y.; Zhang, Y.; Zhou, J. Modified self-adaptive model for improving the prediction accuracy of soil organic matter by laser-induced breakdown spectroscopy. Soil Sci. Soc. Am. J. 2020, 84, 1995–2009. [Google Scholar] [CrossRef]
- Hu, M.; Ma, F.; Li, Z.; Xu, X.; Du, C. Sensing of soil organic matter using laser-induced breakdown spectroscopy coupled with optimized self-adaptive calibration strategy. Sensors 2022, 22, 1488. [Google Scholar] [CrossRef]
- Li, Z.; Zhan, D.J.; Wang, J.J.; Huang, J.; Xu, Q.S.; Zhang, Z.M.; Zheng, Y.B.; Liang, Y.Z.; Wang, H. Morphological weighted penalized least squares for background correction. Analyst 2013, 138, 4483–4492. [Google Scholar] [CrossRef]
- Gan, F.; Wu, K.; Ma, F.; Du, C. In Situ Determination of Nitrate in Water Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy Coupled with Deconvolution Algorithm. Molecules 2020, 25, 5838. [Google Scholar] [CrossRef]
- Shao, Y.Q.; Du, C.W.; Zhou, J.M.; Ma, F.; Zhu, Y.; Yang, K.; Tian, C. Quantitative analysis of different nitrogen isotope labelled nitrates in paddy soil using mid-infrared attenuated total reflectance spectroscopy. Anal. Methods 2017, 9, 5388–5394. [Google Scholar] [CrossRef]
- Cozzolino, D.; Smyth, H.E.; Gishen, M. Feasibility study on the use of visible and near-infrared spectroscopy together with chemometrics to discriminate between commercial white wines of different varietal origins. J. Agric. Food Chem. 2003, 51, 7703–7708. [Google Scholar] [CrossRef] [PubMed]
- Rossel, R.A.V. ParLeS: Software for chemometric analysis of spectroscopic data. Chemometr. Intell. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Wold, S.; Martens, H.; Wold, H. The Multivariate Calibration Problem in Chemistry Solved by the PLS Method; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Rossel, R.A.V.; Behrens, T. Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 2010, 158, 46–54. [Google Scholar]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; van Wesemael, B. Measuring soil organic carbon in croplands at regional scale using airborne imaging spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, C.J. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 27. [Google Scholar] [CrossRef]
- Huang, G.; Chen, L. Enhanced random search based incremental extreme learning machine. Neurocomputing 2008, 71, 3460–3468. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV–vis–NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, S.; Sheng, H.; Liu, X.; Hua, H.; Liu, X.; Zhang, Y. Human perturbation of the global phosphorus cycle: Changes and consequences. Environ. Sci. Technol. 2018, 52, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Goyette, J.O.; Bennett, E.M.; Maranger, R. Low buffering capacity and slow recovery of anthropogenic phosphorus pollution in watersheds. Nat. Geosci. 2018, 11, 921–925. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Tracing riverine sulfate source in an agricultural watershed: Constraints from stable isotopes. Environ. Pollut. 2021, 288, 117740. [Google Scholar] [CrossRef]
- Hong, B.; Swaney, D.P.; Mörth, C.M.; Smedberg, E.; Hägg, H.E.; Humborg, C.; Howarth, R.W.; Bouraoui, F. Evaluating regional variation of net anthropogenic nitrogen and phosphorus inputs (NANI/NAPI), major drivers, nutrient retention pattern and management implications in the multinational areas of Baltic Sea basin. Ecol. Model. 2012, 227, 117–135. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Zhang, Y.; Shen, H.; Yao, M.; Dahlgren, R.A.; Chen, D. Long-term (1980–2015) changes in net anthropogenic phosphorus inputs and riverine phosphorus export in the Yangtze River basin. Water Res. 2020, 177, 115779. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhang, M. Regime of fluvial phosphorus constituted by sediment. Front. Environ. Sci. 2023, 11, 1093413. [Google Scholar] [CrossRef]
- Eiriksdottir, E.S.; Oelkers, E.H.; Hardardottir, J.; Gislason, S.R. The impact of damming on riverine fluxes to the ocean: A case study from Eastern Iceland. Water Res. 2017, 113, 124–138. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Zhu, J.Z.; Gao, Z.M.; Li, S.J.; Zhu, Q.Z.; Wang, H.L.; Huang, Q. Spatial dynamics and risk assessment of phosphorus in the river sediment continuum (Qinhuai River basin, China). Environ. Sci. Pollut. Res. 2023, 31, 2198–2213. [Google Scholar] [CrossRef]
- Brunet, D.; Barthès, B.G.; Chotte, J.L.; Feller, C. Determination of carbon and nitrogen contents in Alfisols, Oxisols and Ultisols from Africa and Brazil using NIRS analysis: Effects of sample grinding and set heterogeneity. Geoderma 2007, 139, 106–117. [Google Scholar] [CrossRef]
- Mutuo, P.K.; Shepherd, K.D.; Albrecht, A.; Cadisch, G. Prediction of carbon mineralization rates from different soil physical fractions using diffuse reflectance spectroscopy. Soil Biol. Biochem. 2006, 38, 1658–1664. [Google Scholar] [CrossRef]
- Moron, A.; Cozzolino, D. Determination of potentially mineralizable nitrogen and nitrogen in particulate organic matter fractions in soil by visible and near-infrared reflectance spectroscopy. J. Agric. Sci. 2004, 142, 335–343. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Li, H.D.; Liang, Y.Z.; Xu, Q.S. Support vector machines and its applications in chemistry. Chemometrics Intell. Lab. Syst. 2009, 95, 188–198. [Google Scholar] [CrossRef]
- Huang, G.B.; Zhou, H.M.; Ding, X.J.; Zhang, R. Extreme learning machine for regression and multiclass classification. IEEE Trans. Syst. Man Cybern. Part B-Cybern. 2012, 42, 513–529. [Google Scholar] [CrossRef]


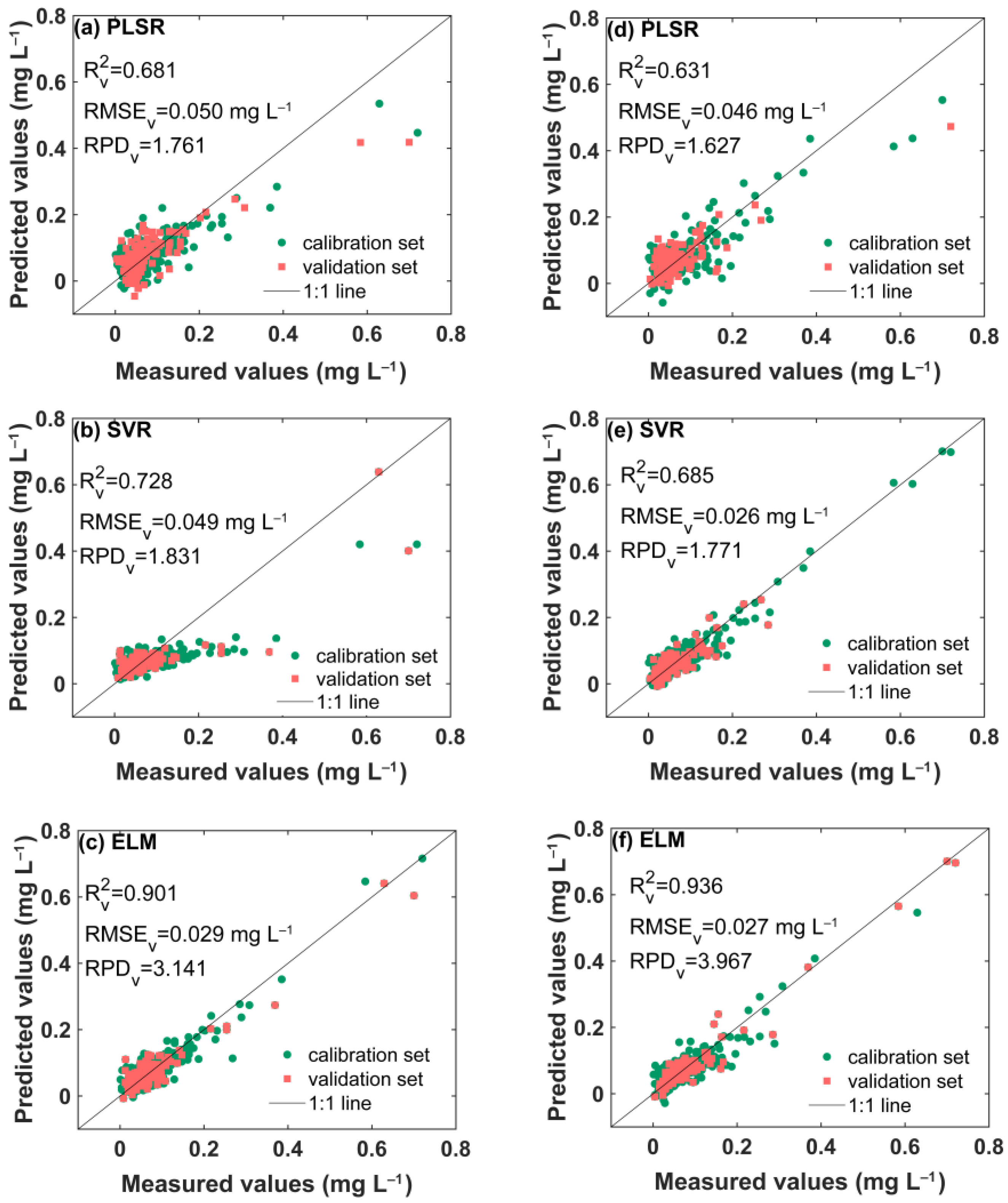
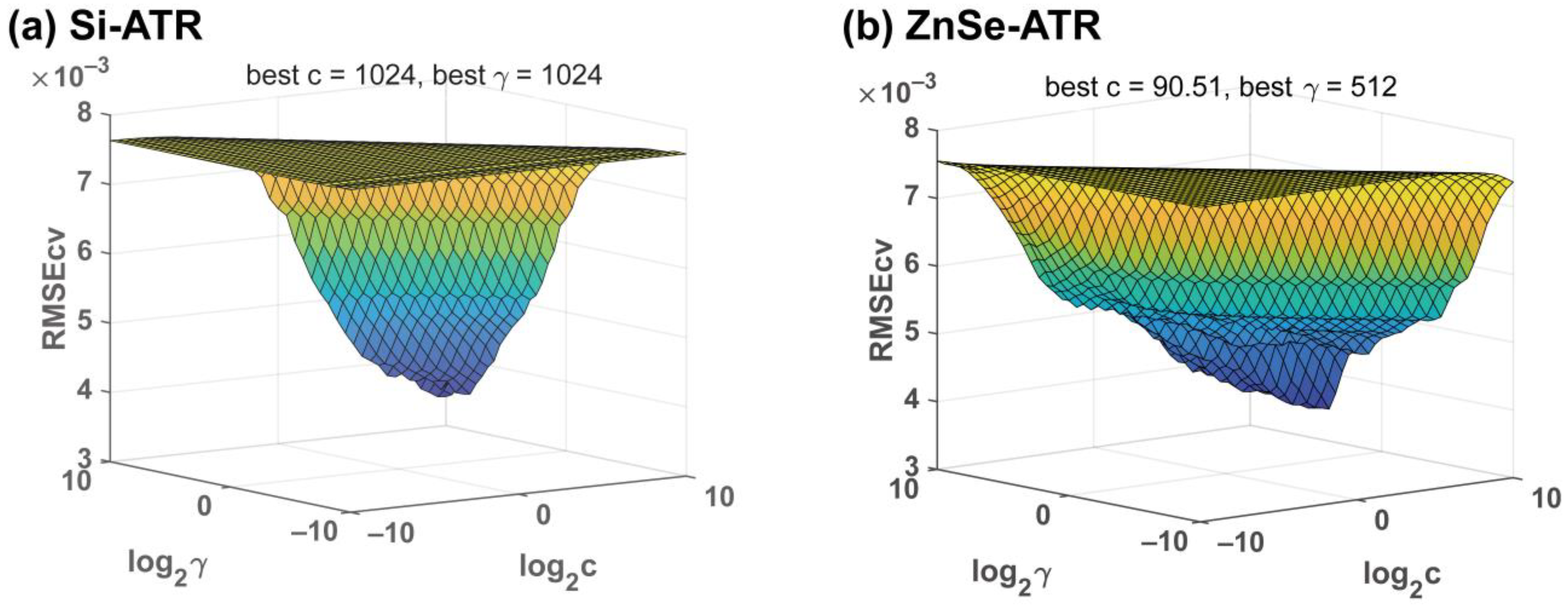
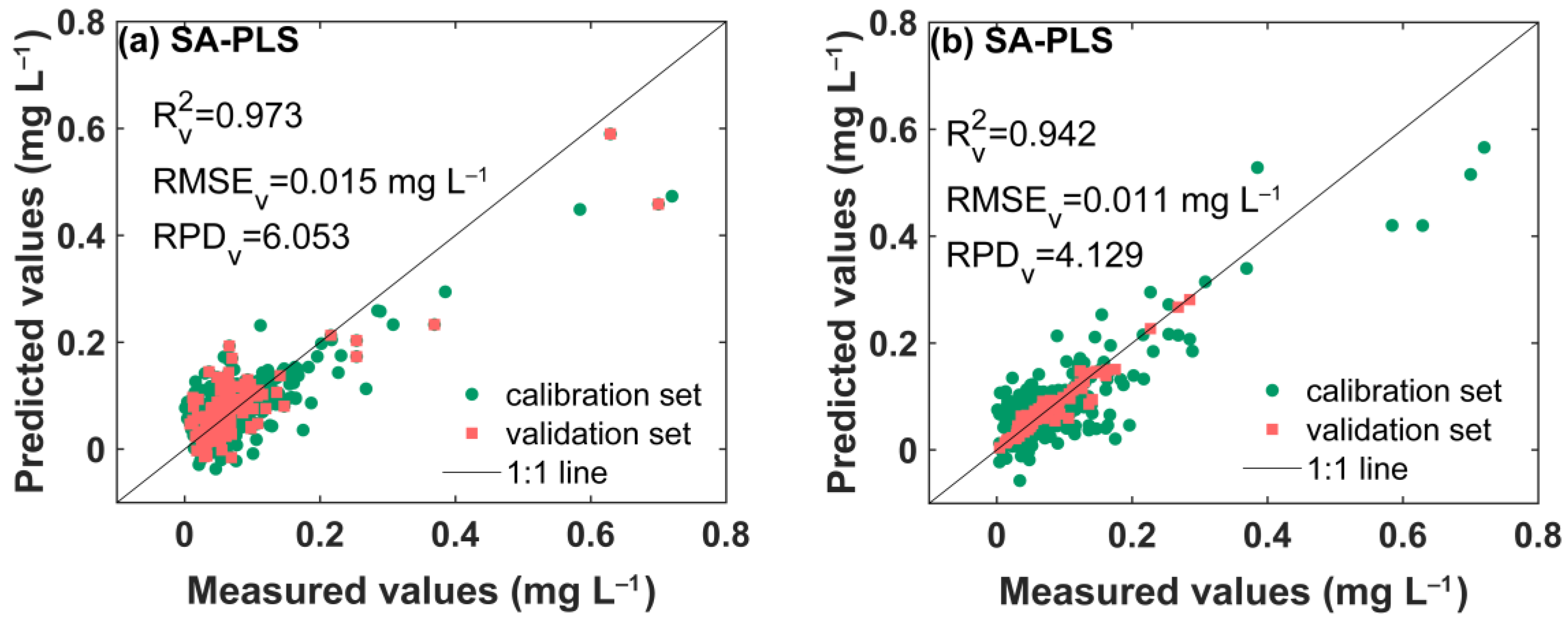

| ATR Accessory | Pretreatment | RV2 | RMSEV (mg L−1) | RPDV |
|---|---|---|---|---|
| Si | Original data | 0.423 | 0.052 | 1.316 |
| Base | 0.681 | 0.050 | 1.761 | |
| Base + MC | 0.664 | 0.053 | 1.721 | |
| Base + FD | 0.662 | 0.055 | 1.696 | |
| Base + SD | 0.641 | 0.053 | 1.669 | |
| ZnSe | Original data | 0.267 | 0.049 | 1.127 |
| Base | 0.631 | 0.046 | 1.627 | |
| Base + MC | 0.566 | 0.051 | 1.505 | |
| Base + FD | 0.683 | 0.053 | 1.744 | |
| Base + SD | 0.618 | 0.044 | 1.611 |
| ATR | Seasons | LV | Calibration Set (75 Samples) | Validation Set (25 Samples) | Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RMSECV | RC2 | RMSEC | RPDC | RV2 | RMSEV | RPDV | ||||
| Si | Spring | 6 | 0.052 | 0.897 | 0.044 | 3.116 | 0.863 | 0.049 | 2.401 | 0.007 |
| Summer | 8 | 0.010 | 0.707 | 0.008 | 1.847 | 0.745 | 0.008 | 1.904 | 0.000 | |
| Autumn | 9 | 0.020 | 0.774 | 0.017 | 2.102 | 0.728 | 0.022 | 1.836 | 0.002 | |
| Winter | 10 | 0.008 | 0.791 | 0.006 | 2.186 | 0.809 | 0.006 | 2.190 | 0.000 | |
| ZnSe | Spring | 6 | 0.059 | 0.810 | 0.052 | 2.297 | 0.763 | 0.078 | 2.053 | 0.000 |
| Summer | 13 | 0.009 | 0.761 | 0.007 | 2.047 | 0.743 | 0.008 | 1.918 | 0.000 | |
| Autumn | 9 | 0.026 | 0.693 | 0.022 | 1.804 | 0.707 | 0.017 | 1.838 | 0.000 | |
| Winter | 9 | 0.008 | 0.763 | 0.007 | 2.052 | 0.744 | 0.007 | 1.902 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Ma, F.; Zhou, J.; Du, C. Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches. Water 2024, 16, 2479. https://doi.org/10.3390/w16172479
Zheng S, Ma F, Zhou J, Du C. Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches. Water. 2024; 16(17):2479. https://doi.org/10.3390/w16172479
Chicago/Turabian StyleZheng, Shuailin, Fei Ma, Jianmin Zhou, and Changwen Du. 2024. "Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches" Water 16, no. 17: 2479. https://doi.org/10.3390/w16172479
APA StyleZheng, S., Ma, F., Zhou, J., & Du, C. (2024). Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches. Water, 16(17), 2479. https://doi.org/10.3390/w16172479







