Adsorbents Produced from Olive Mill Waste and Modified to Perform Phenolic Compound Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Waste Material Activation and Preparation
2.2. Analytical Characterization Techniques
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Prepared Materials Characterization
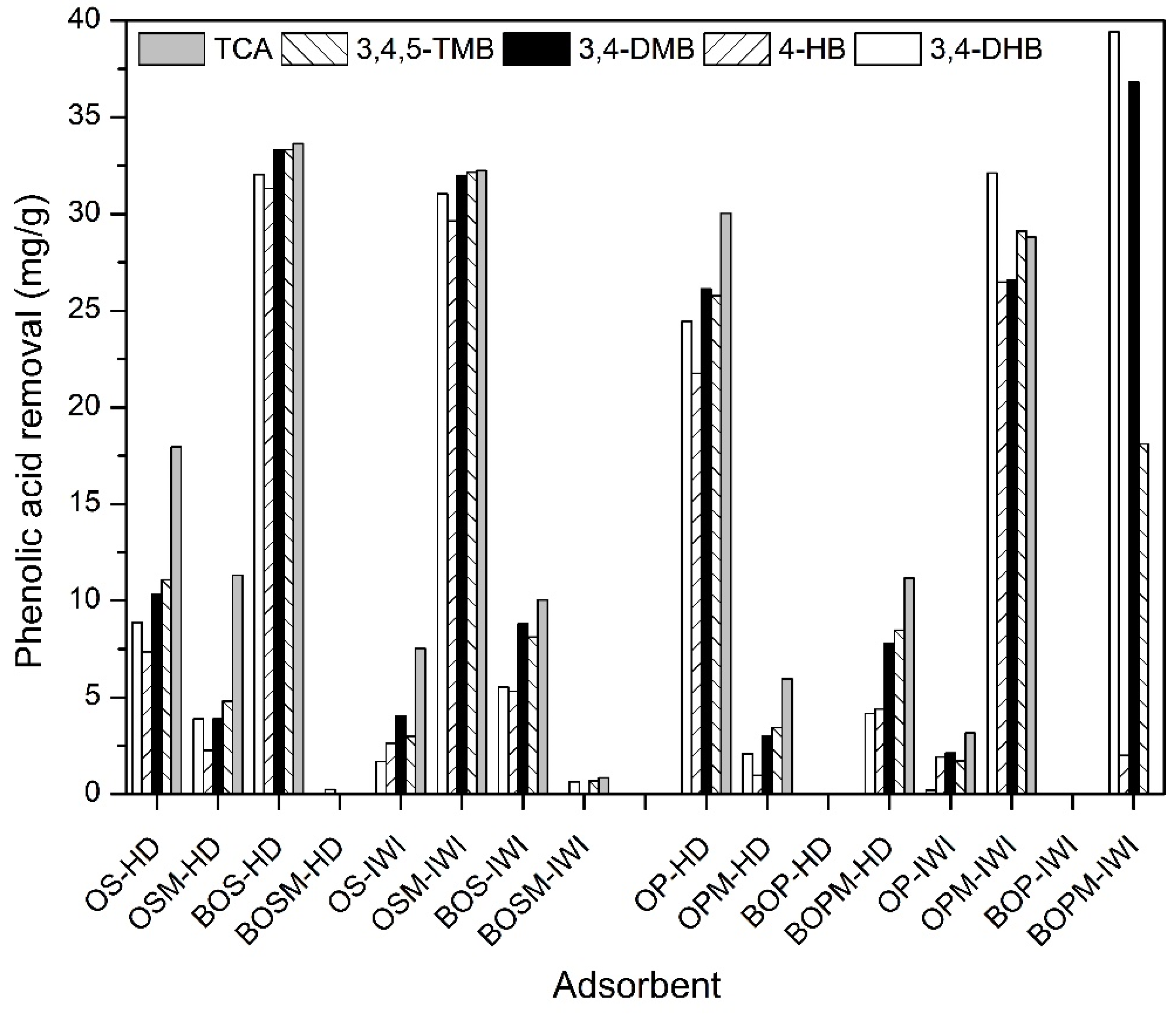
3.2. Phenolic Acid Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The World of Olive Oil. Available online: https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed on 20 January 2024).
- Ioannou-Ttofa, L.; Michael-Kordatou, I.; Fattas, S.C.; Eusebio, A.; Ribeiro, B.; Rusan, M.; Americano, A.R.B.; Zuraiqi, S.; Waismand, M.; Linder, C.; et al. Treatment efficiency and economic feasibility of biological oxidation, membrane filtration and separation processes, and advanced oxidation for the purification and valorization of olive mill wastewater. Water Res. 2017, 114, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lu, M.; Zhang, T.; Zhang, Z.; Jin, Q.; Chang, M.; Wang, X. Evaluation of the antioxidant properties of micronutrients in different vegetable oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900079. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Medouni-Haroune, L.; Zaidi, F.; Medouni-Adrar, S.; Kecha, M. Olive pomace: From an olive mill waste to a resource, an overview of the new treatments. J. Crit. Rev. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Castro-Silva, S.; Martins, R.C. Olive oil extraction industry wastewater treatment by coagulation and Fenton’s process. J. Water Proc. Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A review of waste management options in olive oil production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Sampaio, M.A.; Gonçalves, M.R.; Marques, I.P. Anaerobic digestion challenge of raw olive mill wastewater. Bioresour. Technol. 2011, 102, 10810–10818. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Nunes, P.; Castro-Silva, S.; Quinta-Ferreira, R.M. From a lab test to industrial application: Scale-up of Fenton process for real olive mill wastewater treatment. J. Chem. Technol. Biotechnol. 2017, 92, 1336–1344. [Google Scholar] [CrossRef]
- Bovina, S.; Frascari, D.; Ragini, A.; Avolio, F.; Scarcella, G.; Pinelli, D. Development of a continuous-flow anaerobic co-digestion process of olive mill wastewater and municipal sewage sludge. J. Chem. Technol. Biotechnol. 2021, 96, 532–543. [Google Scholar] [CrossRef]
- Al-Bsoul, A.; Al-Shannag, M.; Tawalbeh, M.; Al-Taani, A.A.; Lafi, W.K.; Al-Othman, A.; Alsheyab, M. Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci. Total Environ. 2020, 700, 134576. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.; Quina, M.M.J.; Martins, R.C.; Gomes, J. Olive mill wastewater treatment strategies to obtain quality water for irrigation: A review. Sci. Total Environ. 2024, 931, 72676. [Google Scholar] [CrossRef] [PubMed]
- Mata-Sánchez, J.; Pérez-Jiménez, J.A.; Díaz-Villanueva, M.J.; Serrano, A.; Núñez-Sánchez, N.; López-Giménez, F.J. Corrosive properties prediction from olive byproducts solid biofuel by near infrared spectroscopy. Energy Fuels 2014, 28, 5136–5143. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef]
- Duman, A.K.; Özgen, G.Ö.; Üçtuğ, F.G. Environmental life cycle assessment of olive pomace utilization in Turkey. Sustain. Prod. Consum. 2020, 22, 126–137. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842–124852. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Abid, N.; Masmoudi, M.A.; Megdiche, M.; Barakat, A.; Ellouze, M.; Chamkha, M.; Ksibi, M.; Sayadi, S. Biochar from olive mill solid waste as an eco-friendly adsorbent for the removal of polyphenols from olive mill wastewater. Chem. Eng. Res. Des. 2022, 181, 384–398. [Google Scholar] [CrossRef]
- Rocha, K.O.; Brandão, F.; Mendes, C.; Carvalho, M.G.V.S.; Mazierski, P.; Zaleska-Medynska, A.; Gomes, J.; Martins, R.C.; Domingues, E. Olive mill waste bio-based catalyst application in advanced oxidation processes for wastewater treatment. Catal. Today 2024, 432, 114618. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Mutjé, P.; Boufi, S. Olive stones flour as reinforcement in polypropylene composites: A step forward in the valorization of the solid waste from the olive oil industry. Ind. Crops Prod. 2015, 72, 183–191. [Google Scholar] [CrossRef]
- Akbas, Y.A.; Yuan, S. Development, and characterization of non-treated and chemically modified olive pomace biosorbents to remove Ce(III) ions from aqueous solutions. J. Radioanal. Nucl. Chem. 2020, 323, 763–772. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Elia, I.; Petalas, A.V.; Halvadakis, C.P. Removal of total phenols from olive-mill wastewater using an agricultural by-product, olive pomace. J. Hazard Mater. 2008, 160, 408–413. [Google Scholar] [CrossRef]
- Akar, T.; Tosun, I.; Kaynak, Z.; Ozkara, E.; Yeni, O.; Sahin, E.N.; Akar, S.T. An attractive agro-industrial by-product in environmental cleanup: Bye biosorption potential of untreated olive pomace. J. Hazard Mater. 2009, 166, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Tomul, A.; Arslan, Y.; Kabak, B.; Trak, D.; Kendüzler, E.; Lima, E.C.; Tran, H.N. Peanut shells-derived biochars prepared from different carbonization processes: Comparison of characterization and mechanism of naproxen adsorption in water. Sci. Total Environ. 2020, 726, 137828. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Adsorption 2024, 30, 167–183. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Lan, B.; Huang, R.; Li, L.; Yan, H.; Liao, G.; Wang, X.; Zhang, Q. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- Esteves, B.M.; Morales-Torres, S. Maldonado-Hódar, F.J.; Madeira, L.M. Integration of olive stones in the production of Fe/AC-catalysts for the CWPO treatment of synthetic and real olive mill wastewater. Chem. Eng. J. 2021, 411, 128451. [Google Scholar] [CrossRef]
- Frainetti, A.J.; Klinghoffer, N.B. Recent experimental advances on the utilization of biochar as a tar reforming catalyst: A review. Int. J. Hydrogen Energy 2023, 48, 8022–8044. [Google Scholar] [CrossRef]
- Gustafsson, J.; Mikkola, P.; Jokinen, M.; Rosenholm, J.B. The influence of pH and NaCl on the zeta potential and rheology of anatase dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2000, 175, 349–359. [Google Scholar] [CrossRef]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and Theoretical Interpretation of Points of Zero Charge/Potential of BSA Protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef]
- Domingues, E.; Assunção, N.; Gomes, J.; Lopes, D.V.; Frade, J.R.; Quina, M.J.; Quinta-Ferreira, R.; Martins, R.C. Catalytic efficiency of red mud for the degradation of olive mill wastewater through heterogeneous Fenton’s process. Water 2019, 11, 1183. [Google Scholar] [CrossRef]
- Shan, X.; Guo, X.; Yin, Y.; Miao, Y.; Dong, H. Surface modification of graphene oxide by goethite with enhanced tylosin photocatalytic activity under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 420–427. [Google Scholar] [CrossRef]
- Anstey, A.; Vivekanandhan, S.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Oxidative acid treatment and characterization of new biocarbon from sustainable Miscanthus biomass. Sci. Total Environ. 2016, 550, 241–247. [Google Scholar] [CrossRef]
- Le, K.C.; Henriksson, J.; Bengtsson, P.-E. Polarization effects in Raman spectroscopy of light-absorbing carbon. J. Raman Spectr. 2021, 52, 1115–1122. [Google Scholar] [CrossRef]
- Ferrari, A.; Baski, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Plessis, M. Relationship between specific surface area and pore dimension of high porosity nanoporous silicon—Model and experiment. Phys. Status Solidi (a) 2007, 204, 2319–2328. [Google Scholar] [CrossRef]
- Lim, C.K.; Bay, H.H.; Noeh, C.H.; Aris, A.; Majid, Z.A.; Ibrahin, Z. Application of zeolite-activated carbon macrocomposite for the adsorption of acid orange 7: Isotherm. Kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2013, 20, 7243–7255. [Google Scholar] [CrossRef]
- Nawrocki, J.; Rigney, M.; McCormick, A.; Carr, P.W. Chemistry of zirconia and its use in chromatography. J. Chromatogr. A 1993, 657, 229–282. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Samsudin, M.H.; Yusoff, M.Z.M.; Zakaria, M.R.; Mokhtar, M.N.; Shirai, Y. Adsorption mechanism and effectiveness of phenol and tannic acid removal by biochar produced from oil palm frond using steam pyrolysis. Environ. Poll. 2021, 269, 116197. [Google Scholar] [CrossRef]
- Ballesteros, I.; Oliva, J.M.; Saez, F.; Ballesteros, M. Ethanol production from lignocellulosic byproducts of olive oil extraction. Appl. Biochem. Biotechnol. 2001, 91, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Marciniak, M.; Goscianska, J.; Pietrzak, R. Physicochemical characterization of ordered mesoporous carbons functionalized by wet oxidation. J. Mater. Sci. 2018, 53, 5997–6007. [Google Scholar] [CrossRef]
- Ashan, M.A.; Islam, M.T.; Hernandez, C.; Castro, E.; Katla, S.K.; Kim, H. Biomass conversion of saw dust to a functionalized carbonaceous materials for the removal of Tetracycline, Sulfamethoxazole and Bisphenol A from water. J. Environ. Chem. Eng. 2018, 6, 4329–4338. [Google Scholar]
- Teng, D.; Zhang, B.; Xu, G.; Wang, B.; Mao, K.; Wang, J.; Sun, J.; Feng, X.; Yang, Z.; Zhang, H. Efficient removal of Cd(II) from aqueous solution by pinecone biochar: Sorption performance and governing mechanisms. Environ. Pollut. 2020, 265, 115001. [Google Scholar] [CrossRef]
- Wie, J.; Tu, C.; Yuan, G.; Liu, Y.; Bi, D.; Xiao, L.; Lu, J.; Theng, B.K.G.; Wang, H.; Zhang, X. Assessing the effect of pyrolysis temperature on the molecular properties and copper sorption capacity of a halophyte biochar. Environ. Pollut. 2019, 251, 56–65. [Google Scholar]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops. Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Schaftenaar, G.; de Vlieg, J. Quantum mechanical polar surface area. J. Comput. Aided Mol. Des. 2012, 26, 311–318. [Google Scholar] [CrossRef]
- Lu, Q.; Sorial, G.A. Adsorption of phenolics on activated carbon––Impact of pore size and molecular oxygen. Chemosphere 2004, 55, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.D.; Abdel daiem, M.M.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Bautista-Toledo, I. Adsorption/bioadsorption of phthalic acid, an organic micropollutant present in landfill leachates, on activated carbons. J. Colloid Interface Sci. 2012, 369, 358–365. [Google Scholar] [CrossRef]
- Bouharat, D.; Yousfi, F.E.; Ellaghdach, A.; Souhail, B.D.; Alaoui, N.S. Physico-chemical characterization of olive-oil mill wastewaters of Ben Karrich area (Tetouan province, North of Morocco) and optimization study of their treatment using activated carbon. Mediterr. J. Chem. 2018, 7, 253–258. [Google Scholar] [CrossRef]
- Senol, A.; Hasdemir, İ.M.; Hasdemir, B.; Kurdaş, İ. Adsorptive removal of biophenols from olive mill wastewaters (OMW) by activated carbon: Mass transfer, equilibrium and kinetic studies. Asia-Pac. J. Chem. Eng. 2017, 12, 128–146. [Google Scholar] [CrossRef]
- Tang, C.; Ni, Z.; Xu, C.; Luo, Y.; Cai, X.; Gao, Q.; Fang, Y.; Zhong, G.; Qiu, R.; Zhang, S. Enhanced adsorption of organic pollutants using N-doped porous carbon derived from hemp stems: Insights into the mechanism. Sep. Purif. Technol. 2024, 333, 125878. [Google Scholar] [CrossRef]
- Solomakou, N.; Goula, A.M. Treatment of olive mill wastewater by adsorption of phenolic compounds. Rev. Environ. Sci. Biotechnol. 2021, 20, 839–863. [Google Scholar] [CrossRef]
- Wang, M.; Fu, M.; Li, J.; Niu, Y.; Zhang, Q.; Sun, Q. New insight into polystyrene ion exchange resin for efficient cesium sequestration: The synergistic role of confined zirconium phosphate nanocrystalline. Chin. Chem. Lett. 2024, 35, 108442. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Mandi, L.; Ouazzani, N. Removal of phenolic compounds from olive mill wastewater by adsorption onto wheat bran. Desalin. Water Treat. 2014, 52, 2875–2885. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; Gonzalez-Gutierrez, L.V.; Baldenegro-Perez, L.A. Biosorbents prepared from orange peels using instant controlled pressure drop for Cu (II) and phenol removal. Ind. Crops Prod. 2016, 84, 344–349. [Google Scholar] [CrossRef]
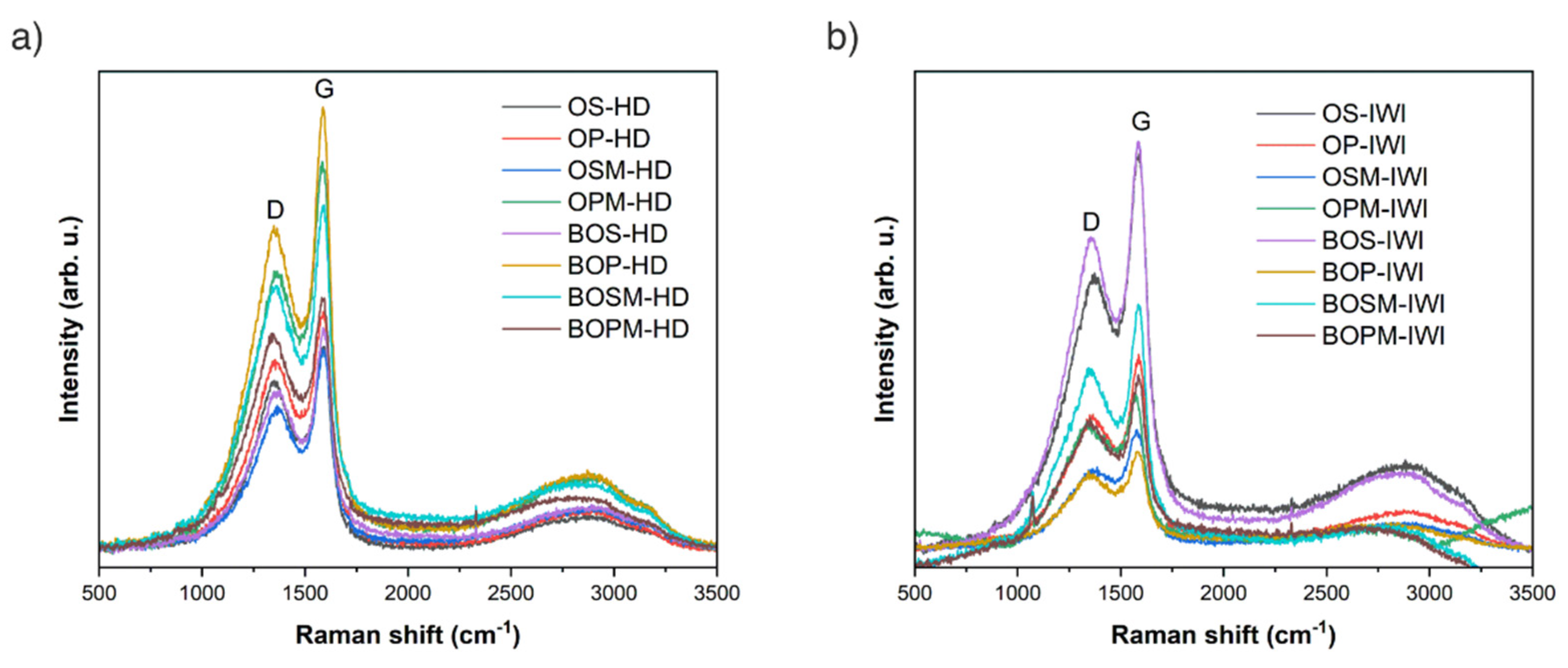
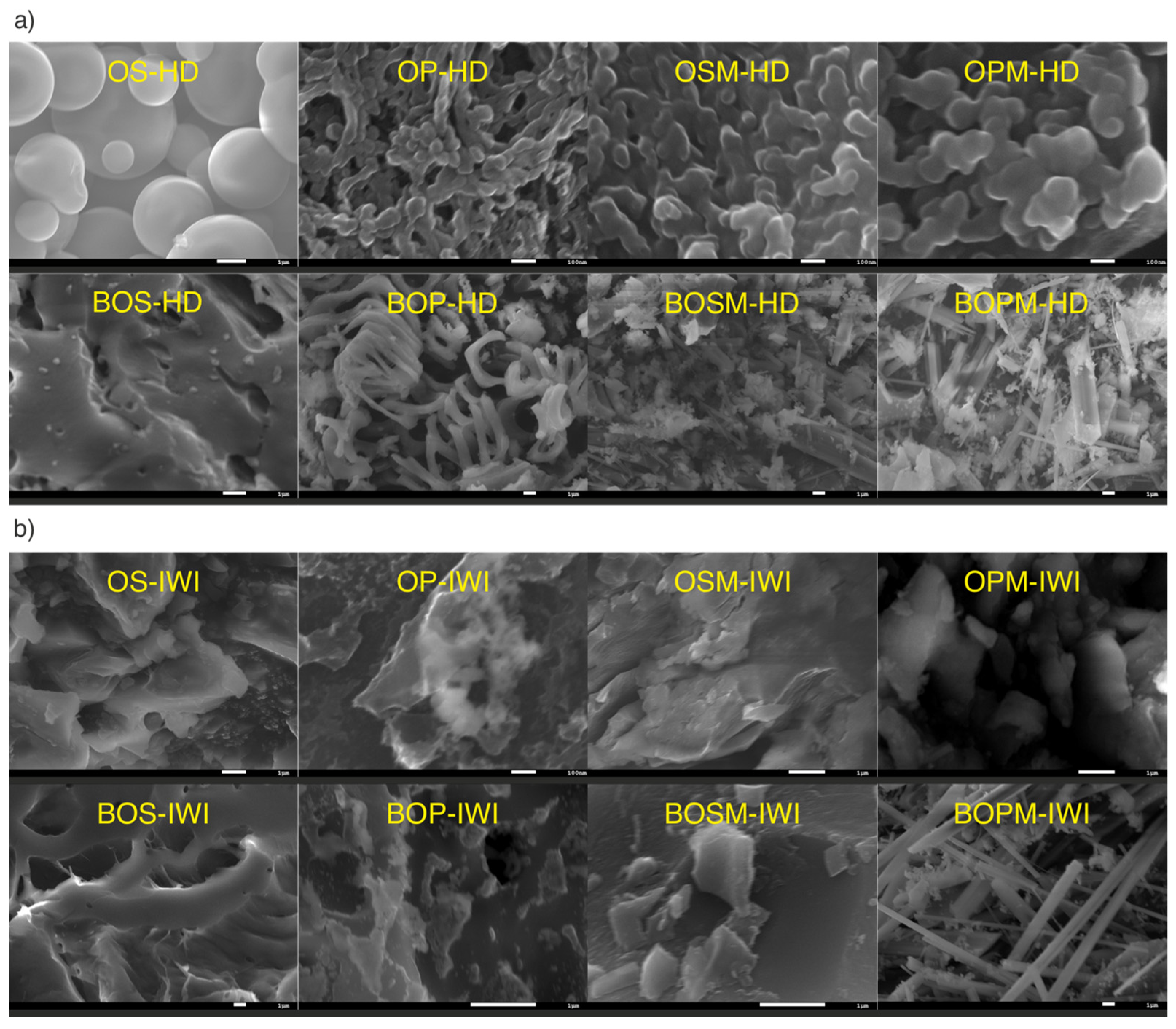
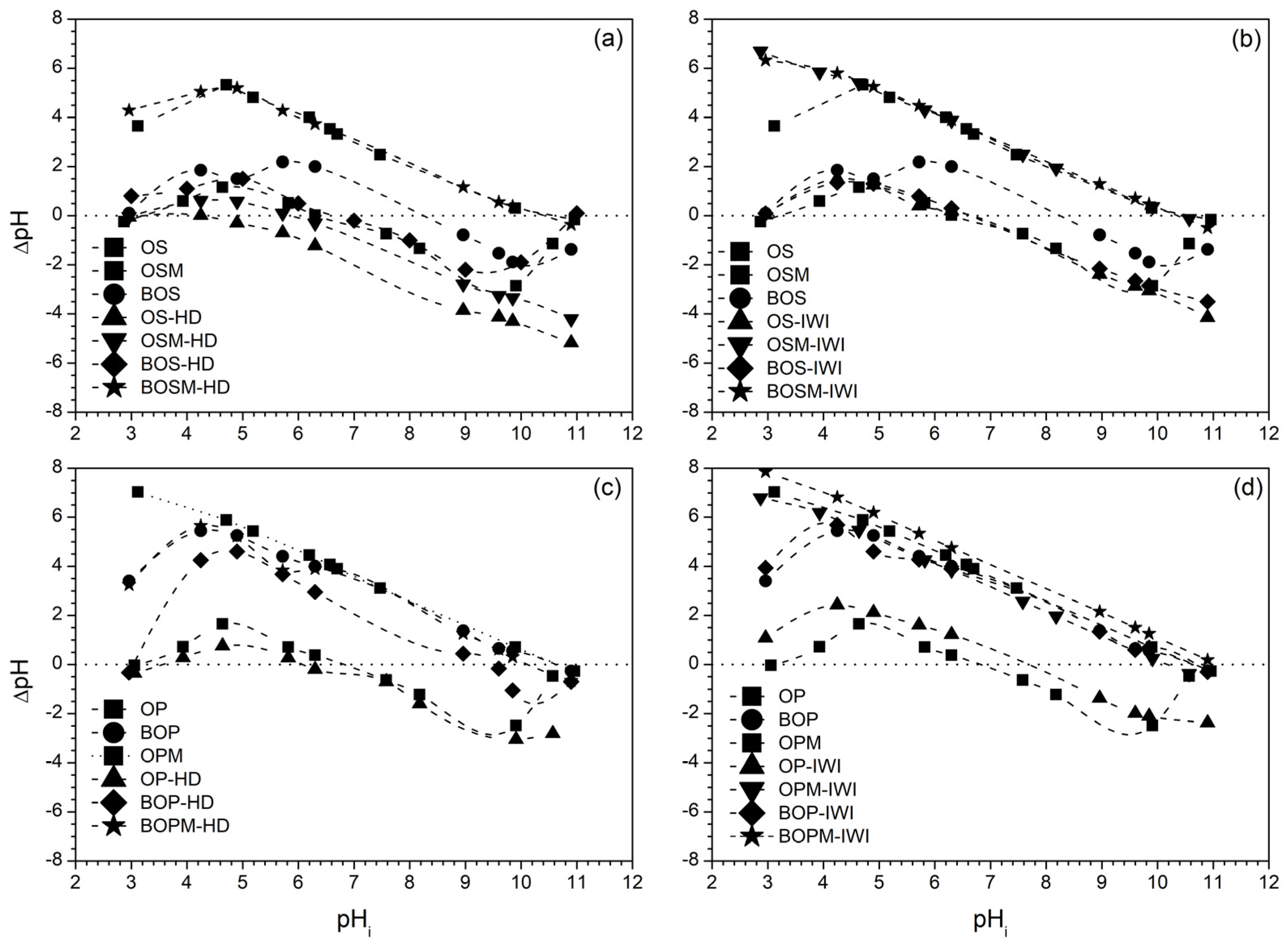
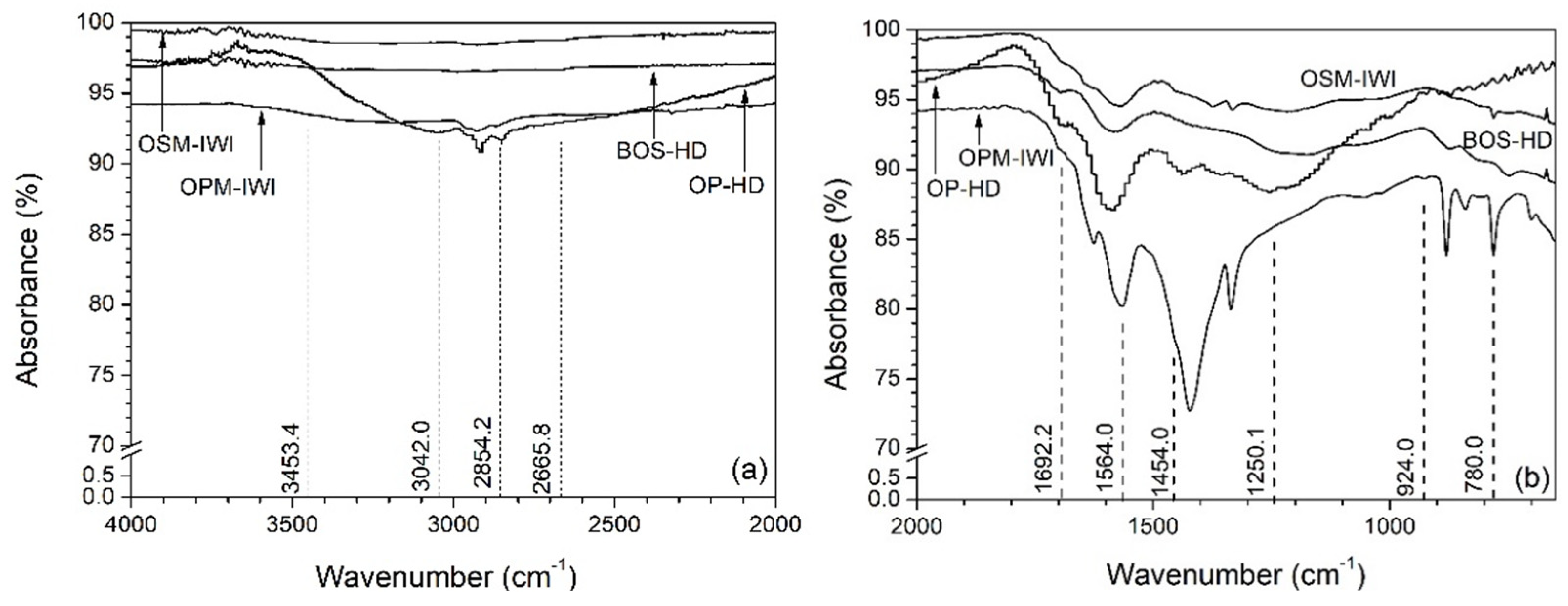
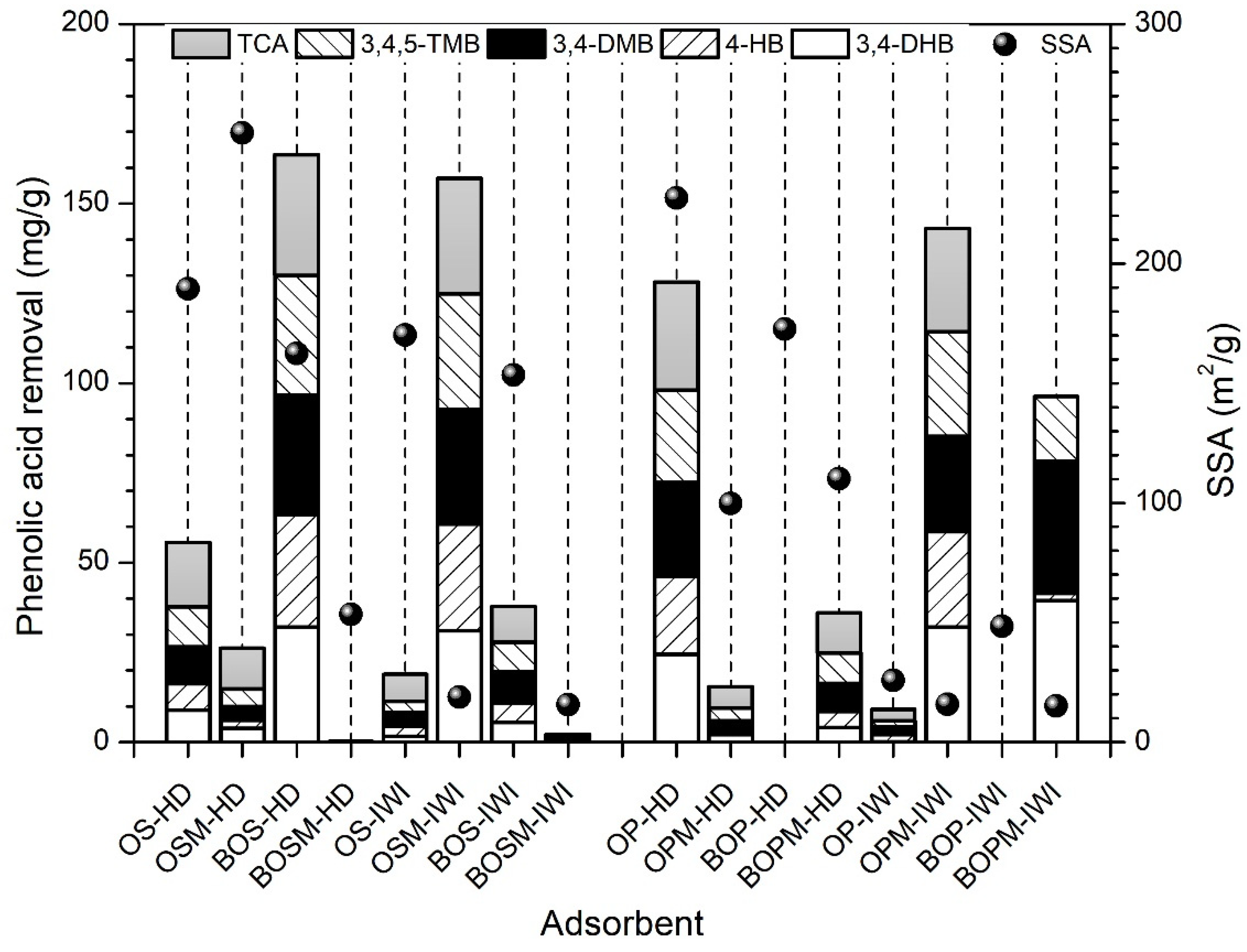
| Sample Label | pHzpc | BET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | ID/IG (Based on Raman) |
|---|---|---|---|---|---|
| OS-HD | 4.4 | 189.4 | 6.1 | 0.076 | 0.83 |
| OP-HD | 6.1 | 227.4 | 4.7 | 0.067 | 0.78 |
| OSM-HD | 5.8 | 254.6 | 4.3 | 0.065 | 0.72 |
| OPM-HD | 10.7 | 99.7 | 5.2 | 0.039 | 0.73 |
| BOS-HD | 6.6 | 162.5 | 4.5 | 0.049 | 0.71 |
| BOP-HD | 9.5 | 172.6 | 4.8 | 0.057 | 0.71 |
| BOSM-HD | 10.4 | 53.3 | 5.2 | 0.041 | 0.76 |
| BOPM-HD | 10.1 | 110.1 | 4.8 | 0.052 | 0.83 |
| OS-IWI | 6.6 | 170.1 | 4.8 | 0.045 | 0.66 |
| OP-IWI | 7.6 | 25.8 | 5.9 | 0.029 | 0.70 |
| OSM-IWI | 10.3 | 18.9 | 6.2 | 0.026 | 0.69 |
| OPM-IWI | 10.2 | 15.9 | 8.4 | 0.043 | 0.84 |
| BOS-IWI | 6.6 | 153.4 | 4.4 | 0.043 | 0.76 |
| BOP-IWI | 10.6 | 48.4 | 9.3 | 0.045 | 0.77 |
| BOSM-IWI | 10.3 | 15.6 | 6.1 | 0.029 | 0.74 |
| BOPM-IWI | 11.0 | 15.1 | 6.9 | 0.035 | 0.73 |
| OS | 6.3 | 17.5 | 5.8 | 0.034 | - |
| OP | 6.8 | 15.3 | 5.9 | 0.036 | - |
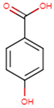
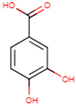
| TCA | 3,4,5-TMB | 3,4-DMB | 4-HB | 3,4-DHB |
|---|---|---|---|---|
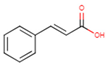 | 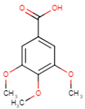 |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, K.d.O.; Brandão, F.; Mazierski, P.; Gomes, J.; Martins, R.C.; Domingues, E. Adsorbents Produced from Olive Mill Waste and Modified to Perform Phenolic Compound Removal. Water 2024, 16, 2379. https://doi.org/10.3390/w16172379
Rocha KdO, Brandão F, Mazierski P, Gomes J, Martins RC, Domingues E. Adsorbents Produced from Olive Mill Waste and Modified to Perform Phenolic Compound Removal. Water. 2024; 16(17):2379. https://doi.org/10.3390/w16172379
Chicago/Turabian StyleRocha, Kleper de Oliveira, Francisco Brandão, Pawel Mazierski, João Gomes, Rui C. Martins, and Eva Domingues. 2024. "Adsorbents Produced from Olive Mill Waste and Modified to Perform Phenolic Compound Removal" Water 16, no. 17: 2379. https://doi.org/10.3390/w16172379
APA StyleRocha, K. d. O., Brandão, F., Mazierski, P., Gomes, J., Martins, R. C., & Domingues, E. (2024). Adsorbents Produced from Olive Mill Waste and Modified to Perform Phenolic Compound Removal. Water, 16(17), 2379. https://doi.org/10.3390/w16172379









