Nitrogen Removal from Polluted Water by an Integrated Constructed Wetland-Microbial Electrolysis Cell System
Abstract
1. Introduction
2. Materials and Methods
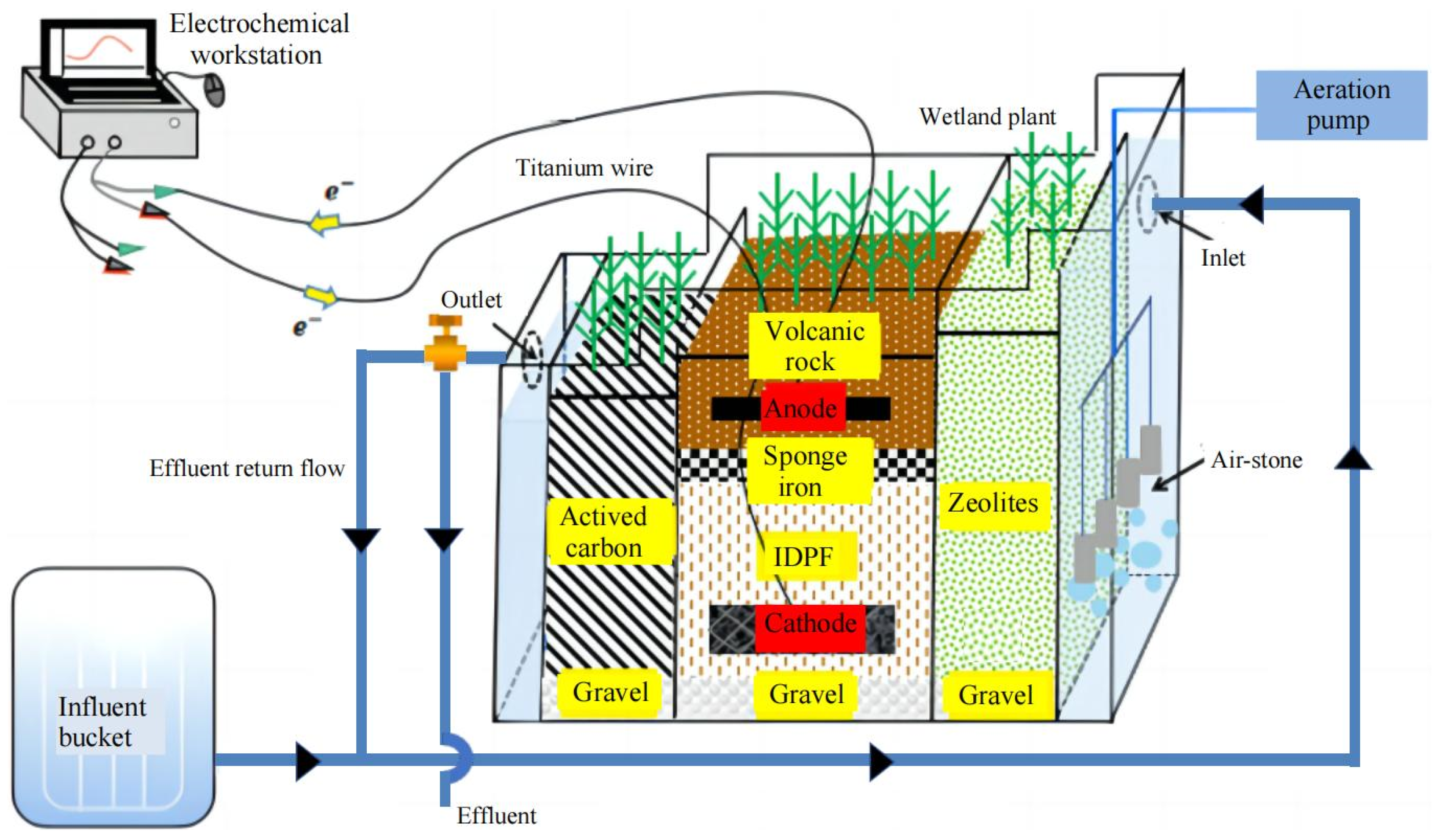
2.1. Construction and Start-Up of Experimental Systems
2.2. Experiment Operation
2.3. Sampling, Physical and Chemical Analysis
2.4. High-Throughput Sequencing and Microbial Informatics Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Bio-Carriers and Electrode Surface Morphology
3.2. Effect of Operating Conditions on the Pollutant Removal Performance in the System
3.2.1. Effect of Pollutant Loading on the Pollutant Removal Performance
3.2.2. Effect of HLR on the Pollutant Removal Performance
3.2.3. Effect of Aeration on the Removal Performance
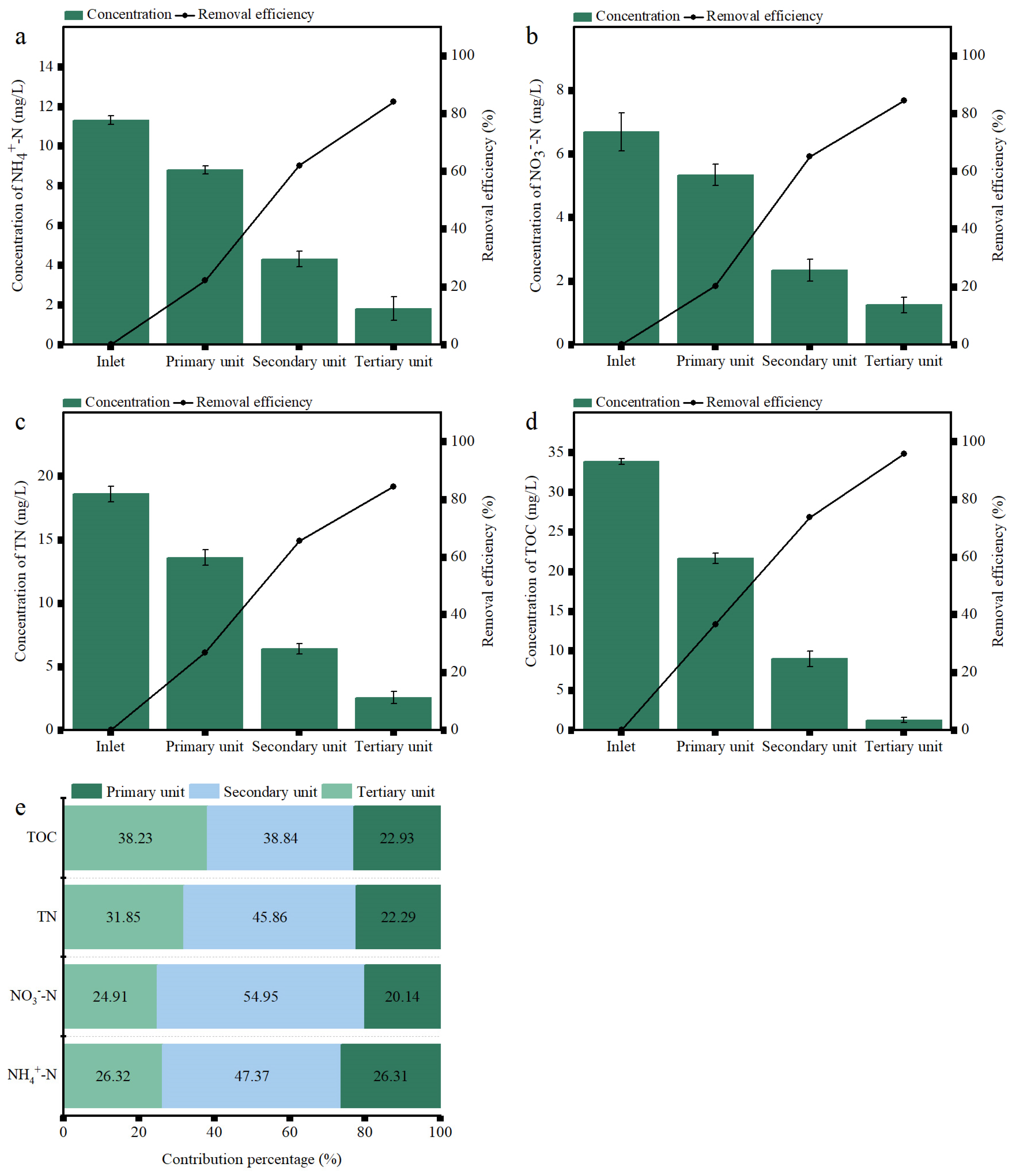
3.3. Contribution to the Removal Performance by Each Unit
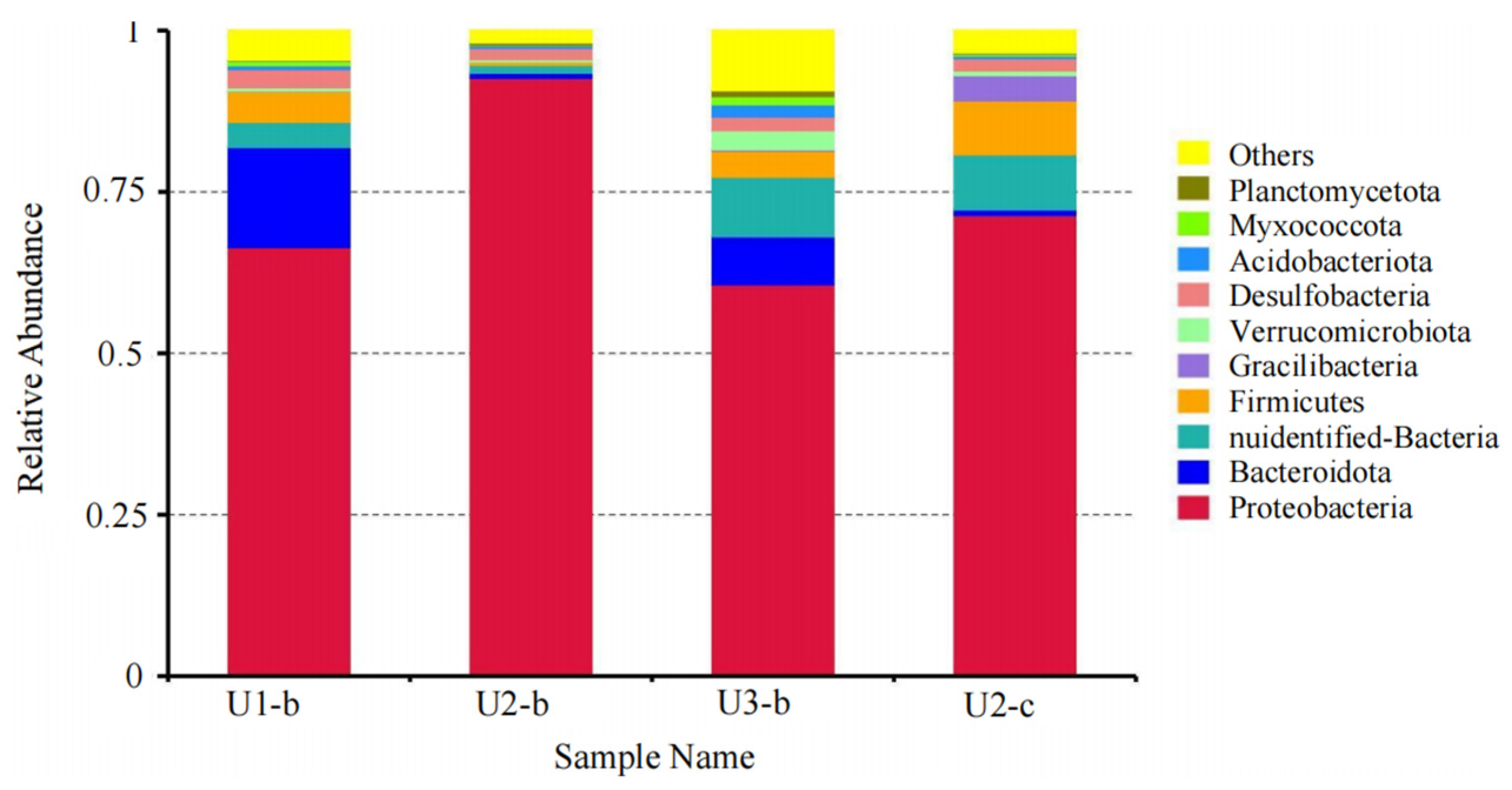
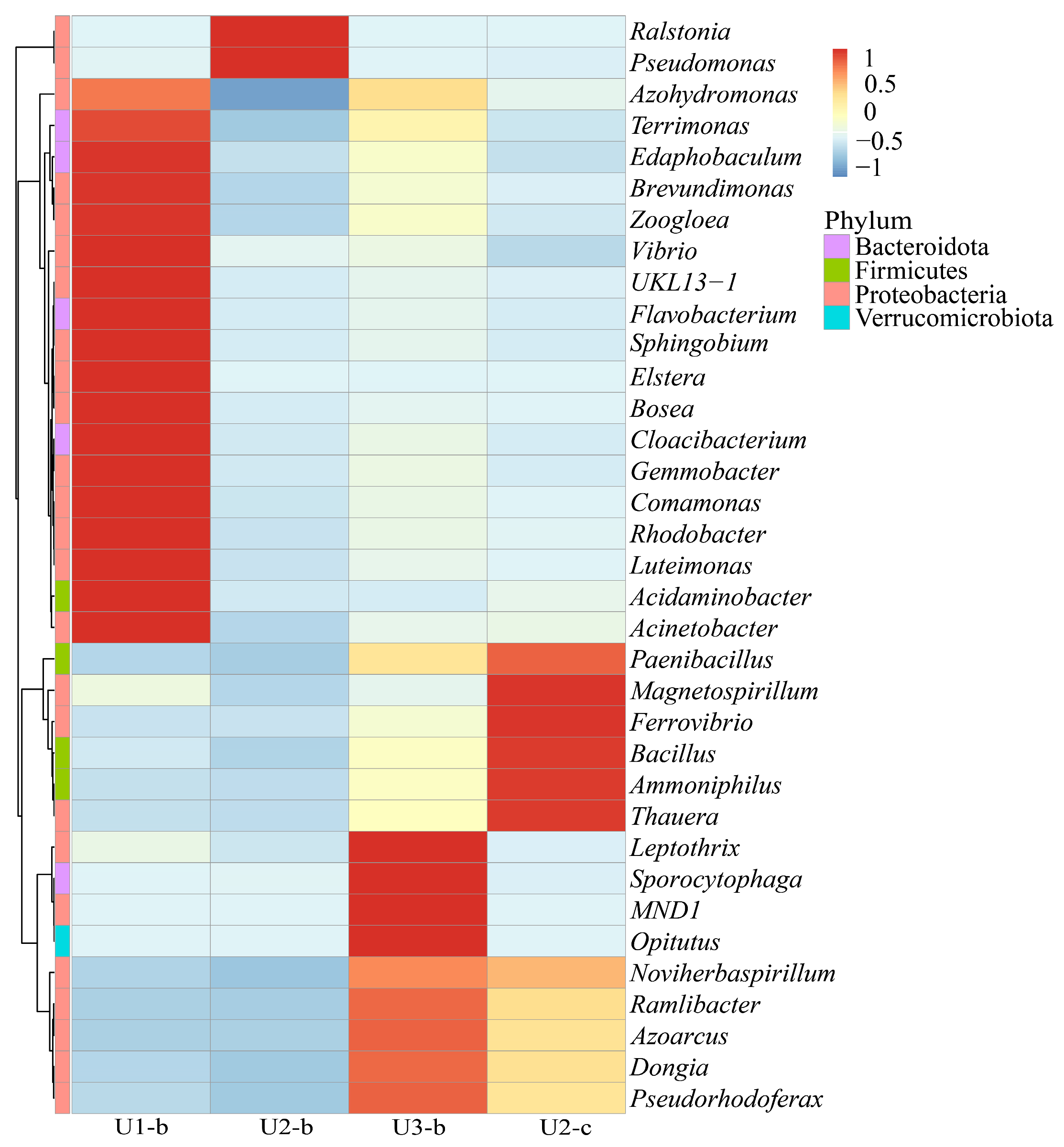
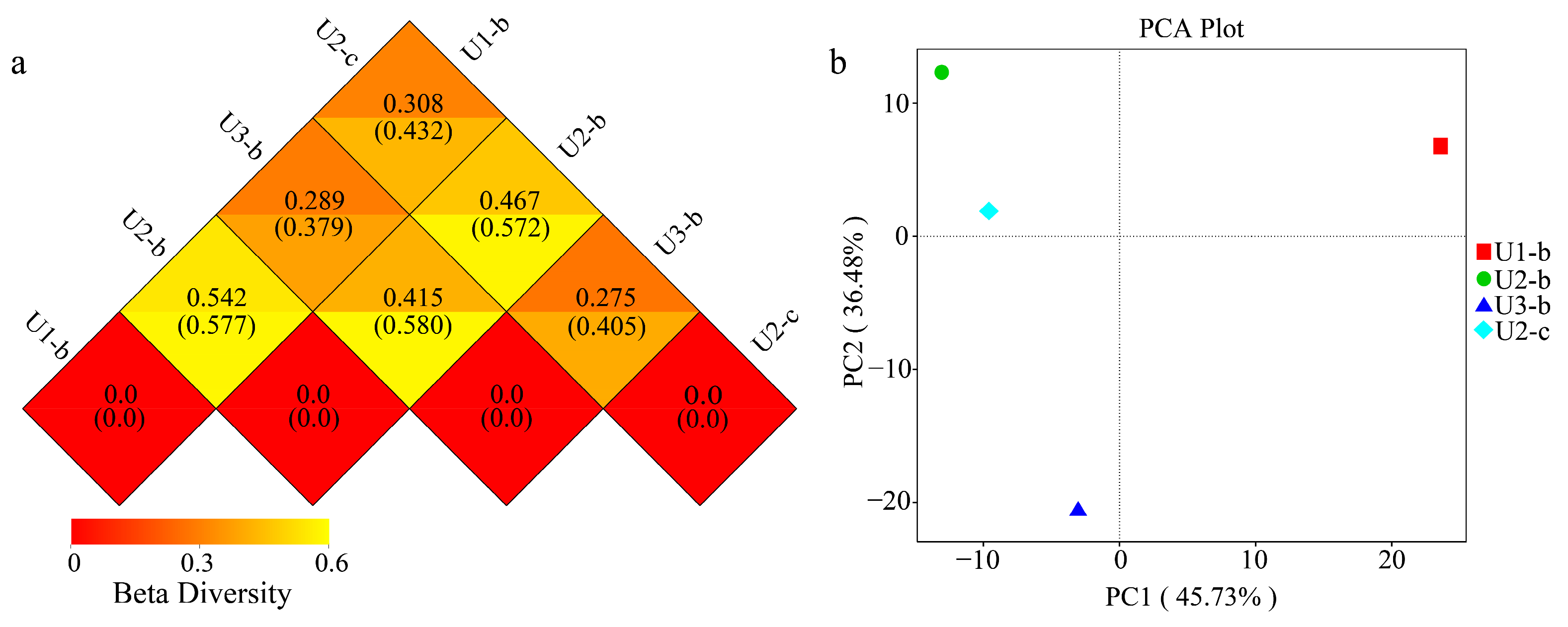
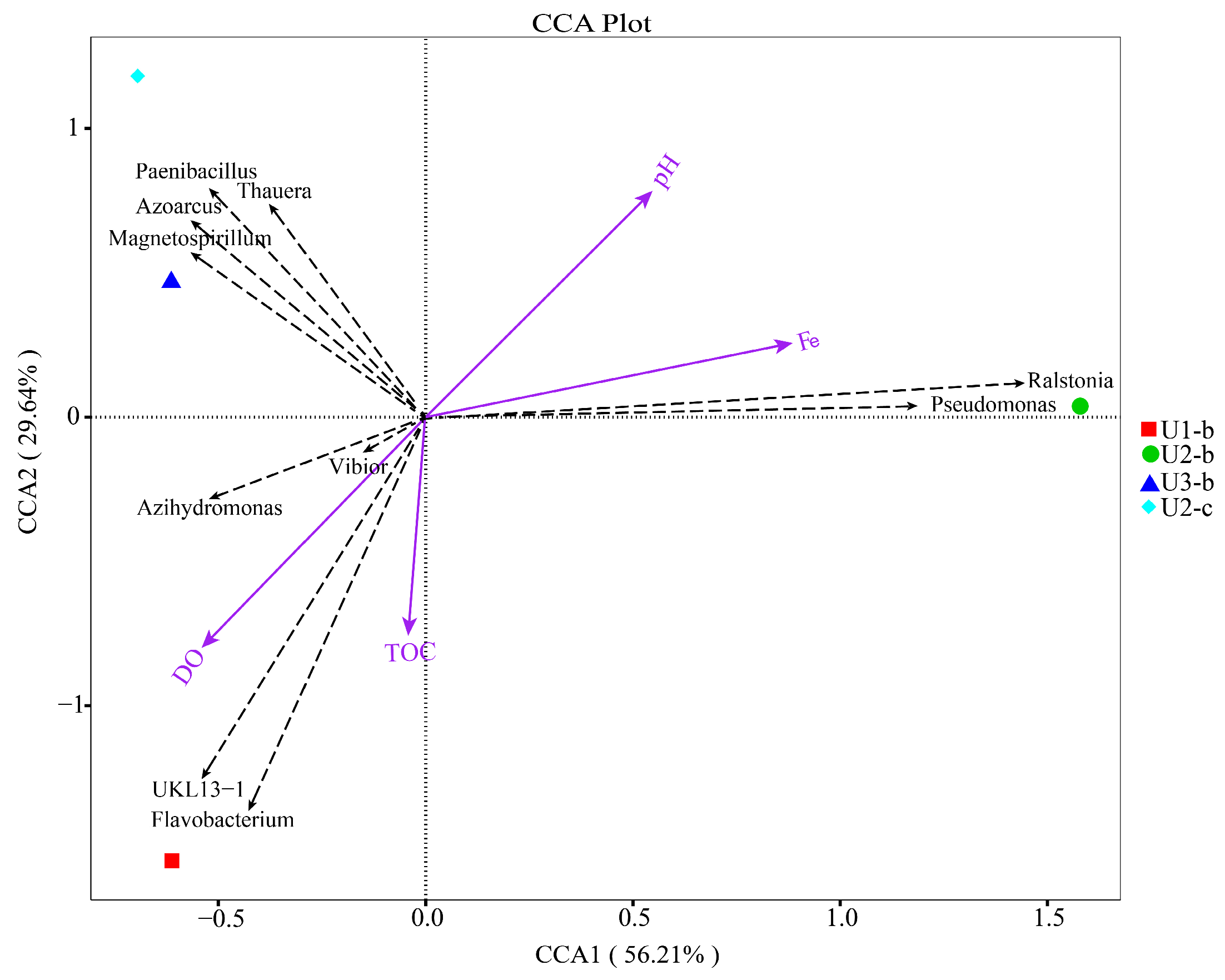
3.4. Structure, Diversity, Impact Factors and Function of Microbial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pu, Y.; Li, Y.; Zhu, L.; Cheng, Y.; Nuamah, L.A.; Zhang, H.; Chen, H.; Du, G.; Wang, L.; Song, C. Long-term assessment on performance and seasonal optimal operation of a full-scale integrated multiple constructed wetland-pond system. Sci. Total Environ. 2023, 862, 161219. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A. Decentralized constructed wetlands for domestic wastewater treatment in developing countries: Field-scale case studies, overall performance and removal mechanisms. J. Water Process Eng. 2024, 57, 104710. [Google Scholar] [CrossRef]
- Wu, Y.; Han, R.; Yang, X.; Zhang, Y.; Zhang, R. Long-term performance of an integrated constructed wetland for advanced treatment of mixed wastewater. Ecol. Eng. 2017, 99, 91–98. [Google Scholar] [CrossRef]
- Vega De Lille, M.I.; Hernández Cardona, M.A.; Tzakum Xicum, Y.A.; Giácoman-Vallejos, G.; Quintal-Franco, C.A. Hybrid constructed wetlands system for domestic wastewater treatment under tropical climate: Effect of recirculation strategies on nitrogen removal. Ecol. Eng. 2021, 166, 106243. [Google Scholar] [CrossRef]
- Shi, B.; Cheng, X.; Zhu, D.; Jiang, S.; Chen, H.; Zhou, Z.; Xie, J.; Jiang, Y.; Liu, C.; Guo, H. Impact analysis of hydraulic loading rate and antibiotics on hybrid constructed wetland systems: Insight into the response to decontamination performance and environmental-associated microbiota. Chemosphere 2024, 347, 140678. [Google Scholar] [CrossRef]
- Wu, Y.; Han, R.; Yang, X.; Fang, X.; Chen, X.; Yang, D.; Zhang, R. Correlating microbial community with physicochemical indices and structures of a full-scale integrated constructed wetland system. Appl. Microbiol. Biotechnol. 2016, 100, 6917–6926. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Yang, S.; Xu, Z.; Feng, C.; Zhao, F. Enhanced nitrogen removal driven by S/Fe2+ cycle in a novel hybrid constructed wetland. J. Clean. Prod. 2023, 426, 139113. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Liu, R.; Xia, H.; Zhang, Y. Enhanced removal performance and mechanism of NH4+/NO3− in Starch-FeS-biochar-amended vertical flow constructed wetlands under Pb stress. J. Water Process Eng. 2023, 55, 104170. [Google Scholar] [CrossRef]
- Hu, X.; Wan, X.; Tan, W.; Xie, H.; Zhuang, L.; Zhang, J.; Liang, S.; Hu, Z. More is better? Constructed wetlands filled with different amount of Fe oxides showed opposite phosphorus removal performance. J. Clean. Prod. 2021, 329, 129749. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Abbassi, R.; Garaniya, V.; Lewis, T. Denitrification in a low carbon environment of a constructed wetland incorporating a microbial electrolysis cell. J. Environ. Chem. Eng. 2018, 6, 5602–5607. [Google Scholar] [CrossRef]
- Yu, B.; Liu, C.; Wang, S.; Wang, W.; Zhao, S.; Zhu, G. Applying constructed wetland-microbial electrochemical system to enhance NH4+ removal at low temperature. Sci. Total Environ. 2020, 724, 138017. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, M.A.; Maldonado, J.E.; González, M.; Silva, H.; Covarrubias, J.I. Evaluation of a red grape marc extract as a natural nitrification inhibitor and its effect on soil bacterial community. J. Soil Sci. Plant Nutr. 2023, 23, 2708–2722. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Sánchez, E.; Herrmann, C. Application of zeolites for biological treatment processes of solid wastes and wastewaters—A review. Bioresour. Technol. 2020, 301, 122808. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Ma, J.; Ma, H.; Cheng, H.; Weng, Z.; Kong, Z.; Shao, Z.; Yuan, Y.; Xu, Y.; Ni, Q.; et al. Enhanced nutrient removal of agricultural waste-pyrite bioretention system for stormwater pollution treatment. J. Clean. Prod. 2023, 395, 136457. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.S.A.; Altaf, M.; Hossain, I.; El Sayed, M.E.; Kallel, M.; El-Bahy, Z.M.; Rehman, A.U.; Najam, T.; Nazir, M.A. Activated carbon derived from biomass for wastewater treatment: Synthesis, application and future challenges. J. Anal. Appl. Pyrolysis 2024, 179, 106480. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Wei, J.; Kong, L.; Yang, B.; Wu, G.; Lei, L.; Li, Z. Electrolysis-integrated constructed wetland with pyrite filler for simultaneous enhanced phosphorus and nitrogen removal. Chem. Eng. J. 2023, 451, 138542. [Google Scholar] [CrossRef]
- Chi, Z.; Hou, L.; Li, H. Effects of pollution load and salinity shock on nitrogen removal and bacterial community in two-stage vertical flow constructed wetlands. Bioresour. Technol. 2021, 342, 126031. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J. Effect of primary treatment and organic loading on methane emissions from horizontal subsurface flow constructed wetlands treating urban wastewater. Ecol. Eng. 2015, 80, 79–84. [Google Scholar] [CrossRef]
- Bang, W.H.; Jung, Y.; Park, J.W.; Lee, S.; Maeng, S.K. Effects of hydraulic loading rate and organic load on the performance of a pilot-scale hybrid VF-HF constructed wetland in treating secondary effluent. Chemosphere 2019, 218, 232–240. [Google Scholar] [CrossRef]
- Kan, P.; Zhang, N.; Zeng, B.; Yao, J.; Zhi, S.; Chen, H.; Yao, Z.; Yangyao, J.; Zhang, Z. Satellite taxa regulated the response of constructed wetlands microeukaryotic community to changing hydraulic loading rate. Sci. Total Environ. 2023, 863, 160742. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, D.; Kan, P.; Yangyao, J.; Yao, Z.; Zhu, D.Z.; Gan, H.; Zhu, B. Impact analysis of hydraulic loading rate on constructed wetland: Insight into the response of bulk substrate and root-associated microbiota. Water Res. 2022, 216, 118337. [Google Scholar] [CrossRef]
- Moussavi, G.; Jafari, S.J.; Yaghmaeian, K. Enhanced biological denitrification in the cyclic rotating bed reactor with catechol as carbon source. Bioresour. Technol. 2015, 189, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Feng, Y.; Lu, W.; Wang, L.; Li, H.; Teng, Y.; Bai, Y.; Qu, K.; Song, Y.; Cui, Z. Effect of hydraulic retention time on denitrification performance and microbial communities of solid-phase denitrifying reactors using polycaprolactone/corncob composite. Mar. Pollut. Bull. 2024, 205, 116559. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, Y.; Xiang, X.; Zhou, L.; Zhang, P.; Wang, Q.; Li, Y.; Wei, J.; Li, L.; Cheng, S. Study on the impact of hydraulic loading rate (HLR) on removal of nitrogen under low C/N condition by modular moving bed constructed wetland (MMB-CW) system. Environ. Technol. Innov. 2024, 34, 103579. [Google Scholar] [CrossRef]
- Yan, Z.; Han, X.; Wang, H.; Jin, Y.; Song, X. Influence of aeration modes and DO on simultaneous nitrification and denitrification in treatment of hypersaline high-strength nitrogen wastewater using sequencing batch biofilm reactor (SBBR). J. Environ. Manag. 2024, 359, 121075. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Zeng, A.; Cheng, B.; Song, Z.; Hu, Z. Effects of different aeration strategies and ammonia-nitrogen loads on nitrification performance and microbial community succession of mangrove constructed wetlands for saline wastewater treatment. Chemosphere 2023, 339, 139685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hao, Q.; Gou, Y.; Wang, X.; Chen, F.; He, Y.; Liang, Z.; Jiang, C. Changing the order and ratio of substrate filling reduced CH4 and N2O emissions from the aerated constructed wetlands. Sci. Total Environ. 2024, 941, 173740. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Q.; Ma, R.; Chen, X.; Xiong, Y.; Hu, J.; Zhang, G.; Jiang, C. Ferric-carbon micro-electrolysis and zeolite reduce CH4 and N2O emissions from the aerated constructed wetland. J. Clean. Prod. 2022, 342, 130946. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Su, J.; He, C.; Shi, J.; Yan, H.; Wei, H. Simultaneous removal of nitrate, lead, and tetracycline by a fixed−biofilm reactor assembled with kapok fiber and sponge iron: Comparative analysis of operating conditions and biotic community. Environ. Res. 2023, 219, 115163. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Feng, Z.-T.; Zhou, J.-M.; Ma, X.; Sun, Y.-J.; Liu, J.-Z.; Zhao, J.-Q.; Jin, R.-C. Roles of Fe(II), Fe(III) and Fe0 in denitrification and anammox process: Mechanisms, advances and perspectives. J. Water Process Eng. 2023, 53, 103746. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron robustly stimulates simultaneous nitrification and denitrification under aerobic conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chu, L.; Wang, Y.; Song, X.; Liu, Y.; Li, D.; Zhao, X. Microelectrolysis-integrated constructed wetland with sponge iron filler to simultaneously enhance nitrogen and phosphorus removal. Bioresour. Technol. 2023, 384, 129270. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Zeng, M.; Yang, X.; Qin, Y. Inhibition effect of Cu(II) on nitrogen removal in anammox-denitrification couple system. Sci. Total Environ. 2024, 941, 173723. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ma, H.; Liu, Y.; Ruan, Y.; Wei, C.; Song, J.; Wu, Y.; Han, R. Characterization of a novel marine aerobic denitrifier Vibrio spp. AD2 for efficient nitrate reduction without nitrite accumulation. Environ. Sci. Pollut. Res. 2021, 28, 30807–30820. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Zhang, G.; Ma, C.; Wang, H. Sulfur-manganese carbonate composite autotrophic denitrification: Nitrogen removal performance and biochemistry mechanism. Ecotoxicol. Environ. Saf. 2024, 272, 116048. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Sun, H.; Wang, W.; Yang, Y.; Qi, Z.; Liu, X. Ammonia assimilation: A double-edged sword influencing denitrification of Rhodobacter azotoformans and for nitrogen removal of aquaculture wastewater. Bioresour. Technol. 2022, 345, 126495. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Wang, D.; Lu, M.; Fan, Y.; Ji, J.; Wu, J. Combined effects of carbon source and C/N ratio on the partial denitrification performance: Nitrite accumulation, denitrification kinetic and microbial transition. J. Environ. Chem. Eng. 2024, 12, 113343. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Peng, F.; Zhang, A.; Pang, Q.; Lian, J.; Zhang, Y.; Yang, F.; Zhu, Y.; Ding, C.; et al. Intensified nitrogen removal in the tidal flow constructed wetland-microbial fuel cell: Insight into evaluation of denitrifying genes. J. Clean. Prod. 2020, 264, 121580. [Google Scholar] [CrossRef]
- Valero, A.; Petrash, D.A.; Kuchenbuch, A.; Korth, B. Enriching electroactive microorganisms from ferruginous lake waters–Mind the sulfate reducers! Bioelectrochemistry 2024, 157, 108661. [Google Scholar] [CrossRef]
- Huang, J.; Ye, J.; Gao, W.; Liu, C.; Price, G.W.; Li, Y.; Wang, Y. Tea biochar-immobilized Ralstonia Bcul-1 increases nitrate nitrogen content and reduces the bioavailability of cadmium and chromium in a fertilized vegetable soil. Sci. Total Environ. 2023, 866, 161381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, X.; Cheng, X.; Huang, Z.; Dong, D.; Li, X. Enhanced denitrification of biodegradable polymers using Bacillus pumilus in aerobic denitrification bioreactors: Performance and mechanism. Bioresour. Technol. 2024, 394, 130240. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Jin, X.; Deng, S.; Guo, K.; Gao, Y.; Shi, X.; Xu, L.; Bai, X.; Shang, Y.; Jin, P.; et al. Oxygen sensing regulation mechanism of Thauera bacteria in simultaneous nitrogen and phosphorus removal process. J. Clean. Prod. 2024, 434, 140332. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, R.; Dong, H.; Yang, Z.; Chen, X. Behavior of nitrite removal and vivianite-based phosphorus recovery with biogenic bivalent iron mediated by iron-reducing bacteria: Competition or sequencing? J. Water Process Eng. 2024, 61, 105284. [Google Scholar] [CrossRef]
- Huang, R.; Meng, T.; Liu, G.; Gao, S.; Tian, J. Simultaneous nitrification and denitrification in membrane bioreactor: Effect of dissolved oxygen. J. Environ. Manag. 2022, 323, 116183. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, Z. Research on removal of fluoroquinolones in rural domestic wastewater by vertical flow constructed wetlands under different hydraulic loads. Chemosphere 2022, 303, 135100. [Google Scholar] [CrossRef]
- Yang, P.; Yao, T.; Liu, X.; Zhang, A.; Zhang, J.; Pang, L. Efficient heterotrophic nitrification-aerobic denitrification by a novel bacterium Ralstonia pickettii J4: Isolation, identification, and application. Biochem. Eng. J. 2024, 210, 109417. [Google Scholar] [CrossRef]
- Chhe, C.; Uke, A.; Baramee, S.; Tachaapaikoon, C.; Pason, P.; Waeonukul, R.; Ratanakhanokchai, K.; Kosugi, A. Characterization of a thermophilic facultatively anaerobic bacterium Paenibacillus sp. strain DA-C8 that exhibits xylan degradation under anaerobic conditions. J. Biotechnol. 2021, 342, 64–71. [Google Scholar] [CrossRef]
- Chen, J.; Tang, X.; Wu, X.; Li, B.; Tang, X.; Lin, X.; Li, P.; Chen, H.; Huang, F.; Deng, X.; et al. Relating the carbon sources to denitrifying community in full-scale wastewater treatment plants. Chemosphere 2024, 361, 142329. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Li, K.; Yi, L.; Su, X.; Liu, C.; Rong, X.; Ran, H.; Wei, Y.; Wan, L.; Han, R.; et al. Nitrogen Removal from Polluted Water by an Integrated Constructed Wetland-Microbial Electrolysis Cell System. Water 2024, 16, 2368. https://doi.org/10.3390/w16172368
Zhang R, Li K, Yi L, Su X, Liu C, Rong X, Ran H, Wei Y, Wan L, Han R, et al. Nitrogen Removal from Polluted Water by an Integrated Constructed Wetland-Microbial Electrolysis Cell System. Water. 2024; 16(17):2368. https://doi.org/10.3390/w16172368
Chicago/Turabian StyleZhang, Ruina, Kexin Li, Longqiang Yi, Xin Su, Changyuan Liu, Xinyu Rong, Haoxin Ran, Yingjie Wei, Li Wan, Rui Han, and et al. 2024. "Nitrogen Removal from Polluted Water by an Integrated Constructed Wetland-Microbial Electrolysis Cell System" Water 16, no. 17: 2368. https://doi.org/10.3390/w16172368
APA StyleZhang, R., Li, K., Yi, L., Su, X., Liu, C., Rong, X., Ran, H., Wei, Y., Wan, L., Han, R., & Wu, Y. (2024). Nitrogen Removal from Polluted Water by an Integrated Constructed Wetland-Microbial Electrolysis Cell System. Water, 16(17), 2368. https://doi.org/10.3390/w16172368





