Effects of Freeze−Thaw Cycles on Available Nitrogen Content in Soils of Different Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Processing
2.3. Experimental Design
2.4. Determination Method
2.5. Data Analysis
3. Results
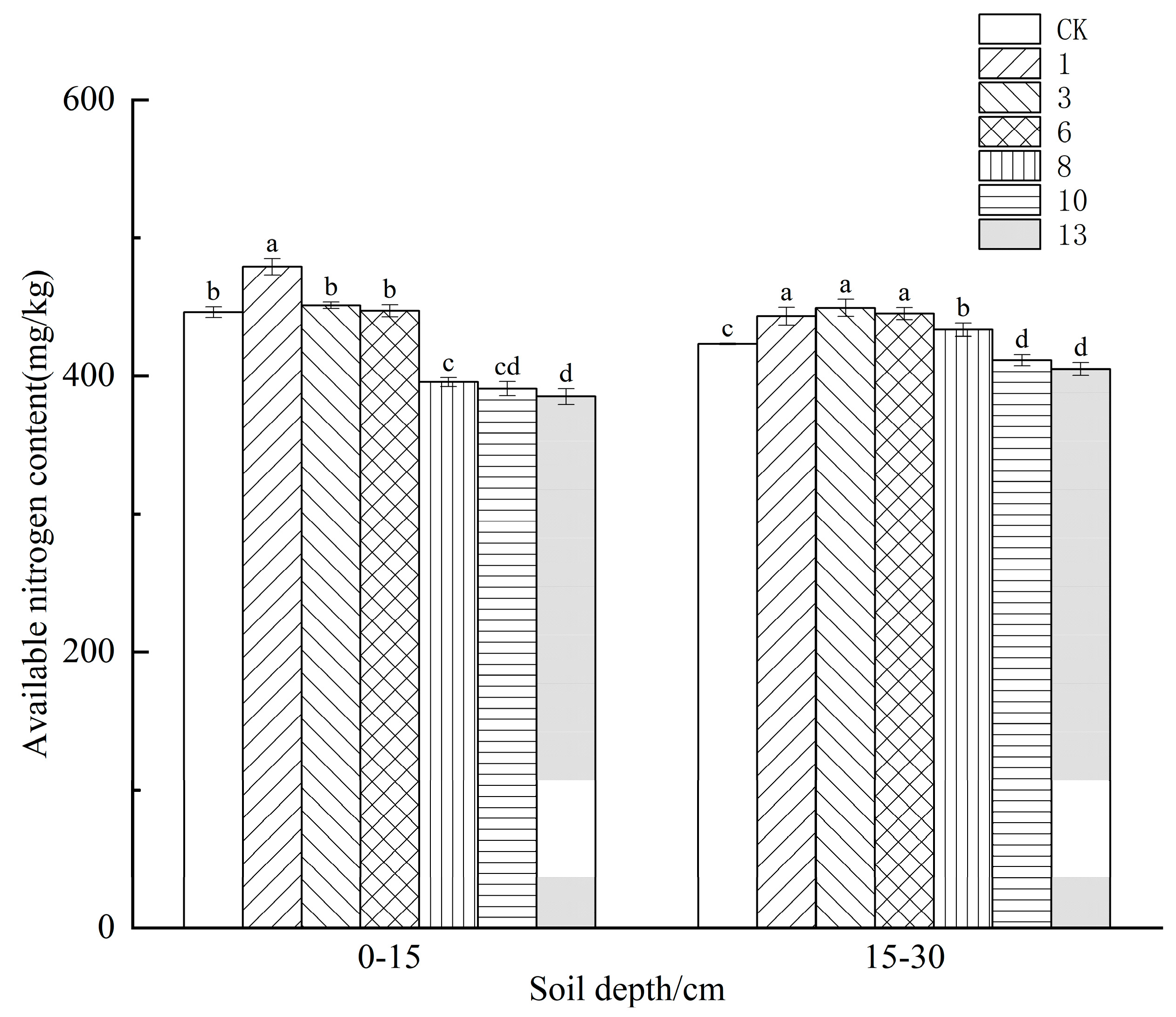
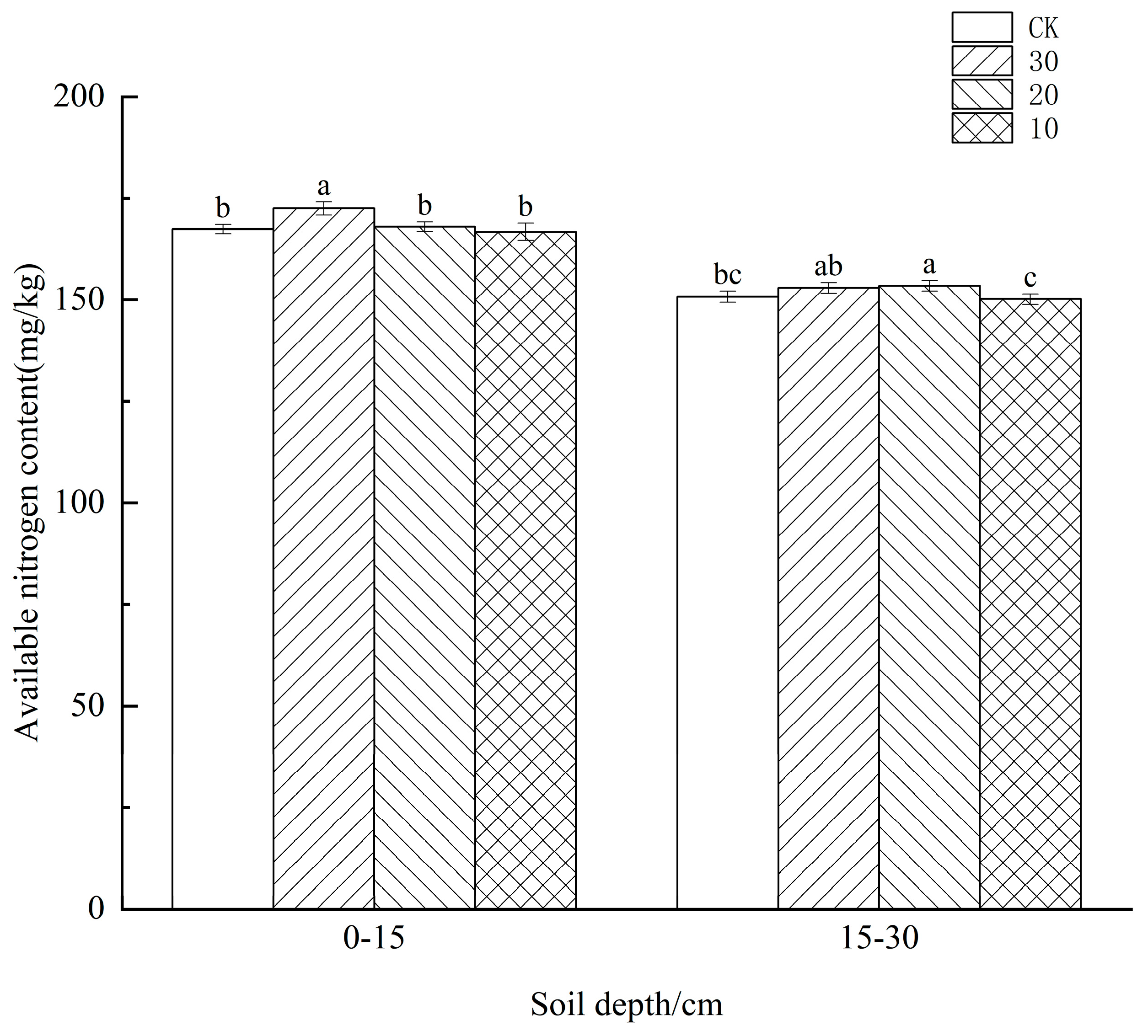
3.1. Effect of Freeze−Thaw Frequency on Available Nitrogen Content in the Soil of Different Crops
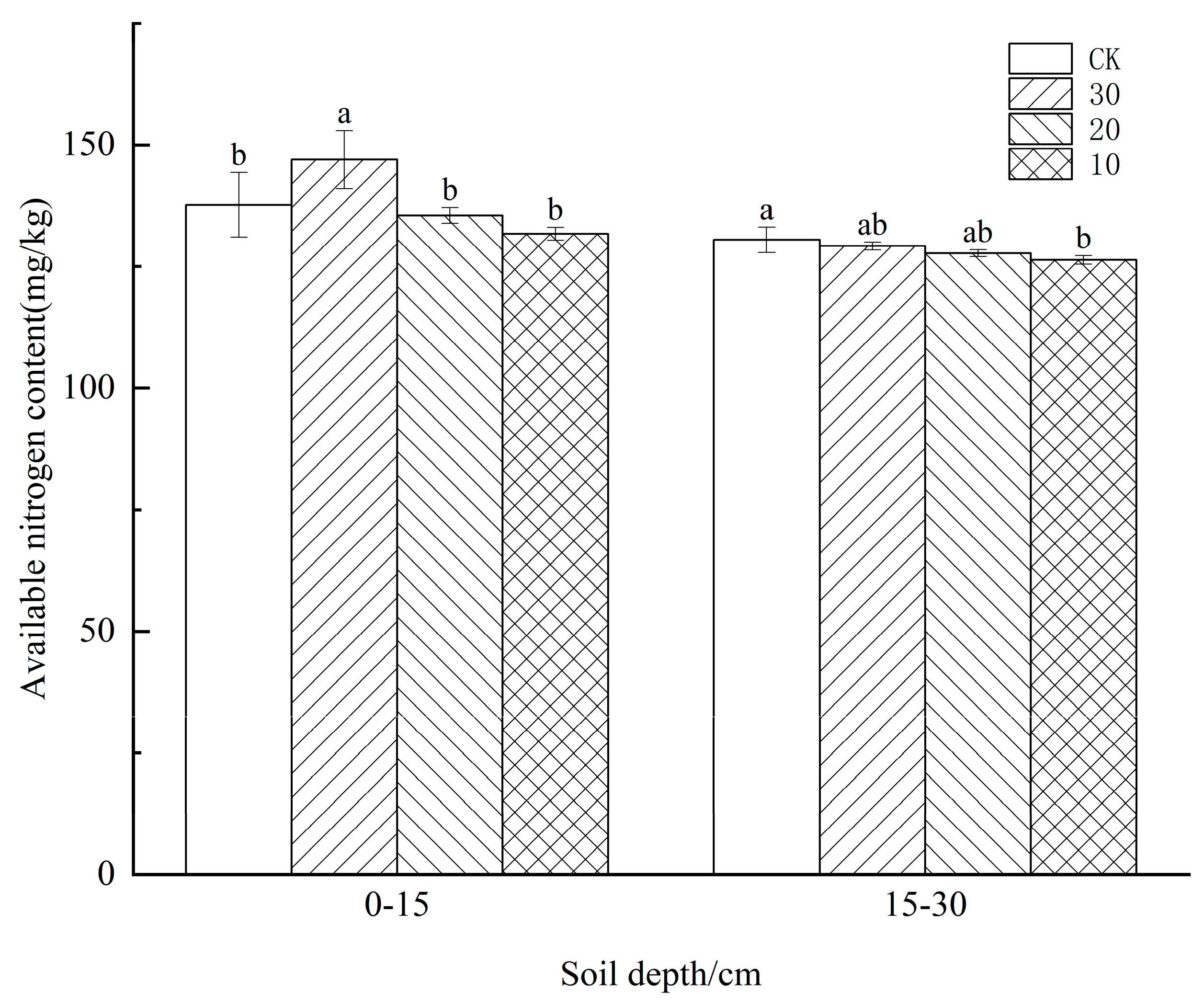
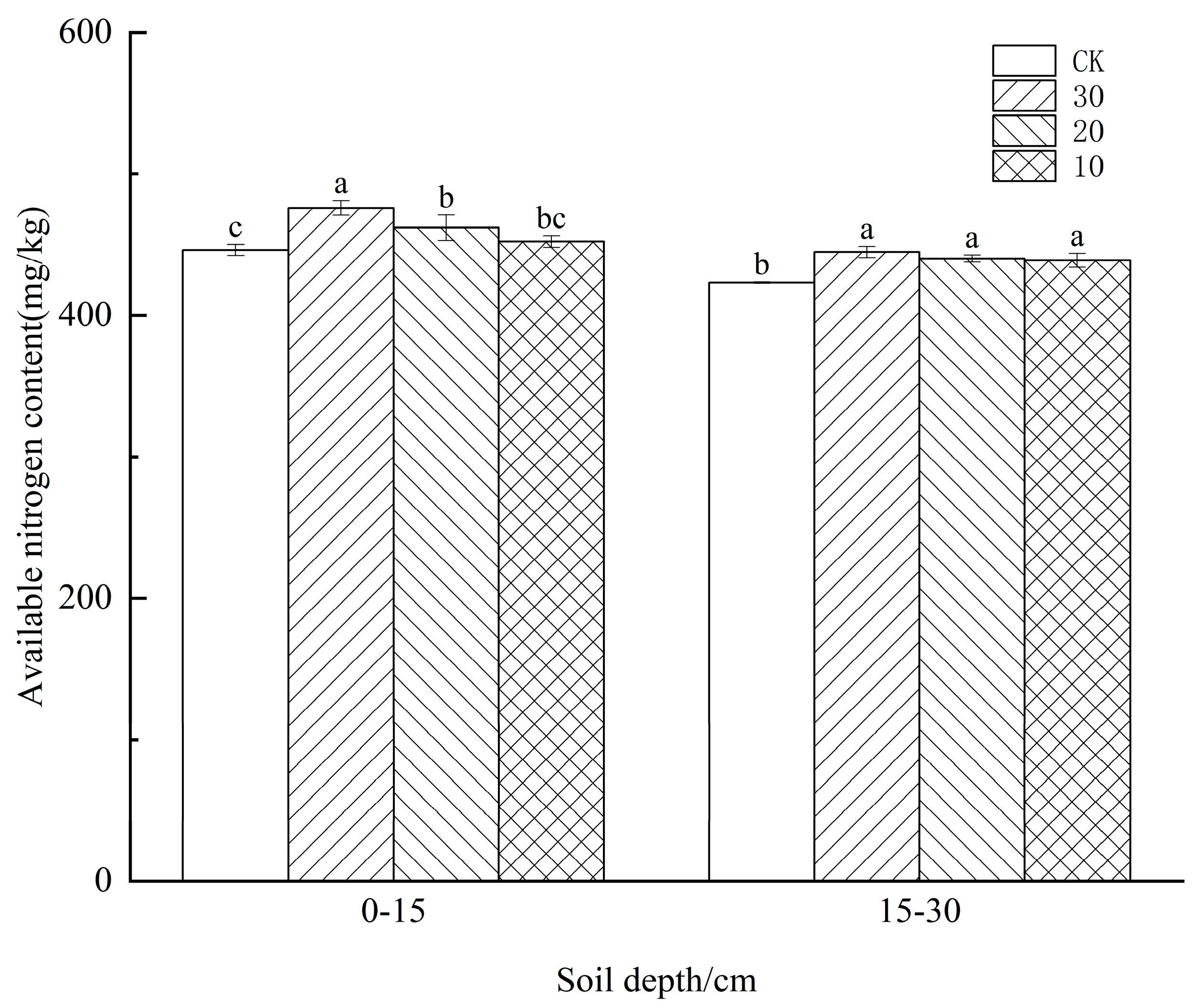
3.2. Effect of Freeze−Thaw Temperature Difference on Available Nitrogen Content in the Soil of Different Crops
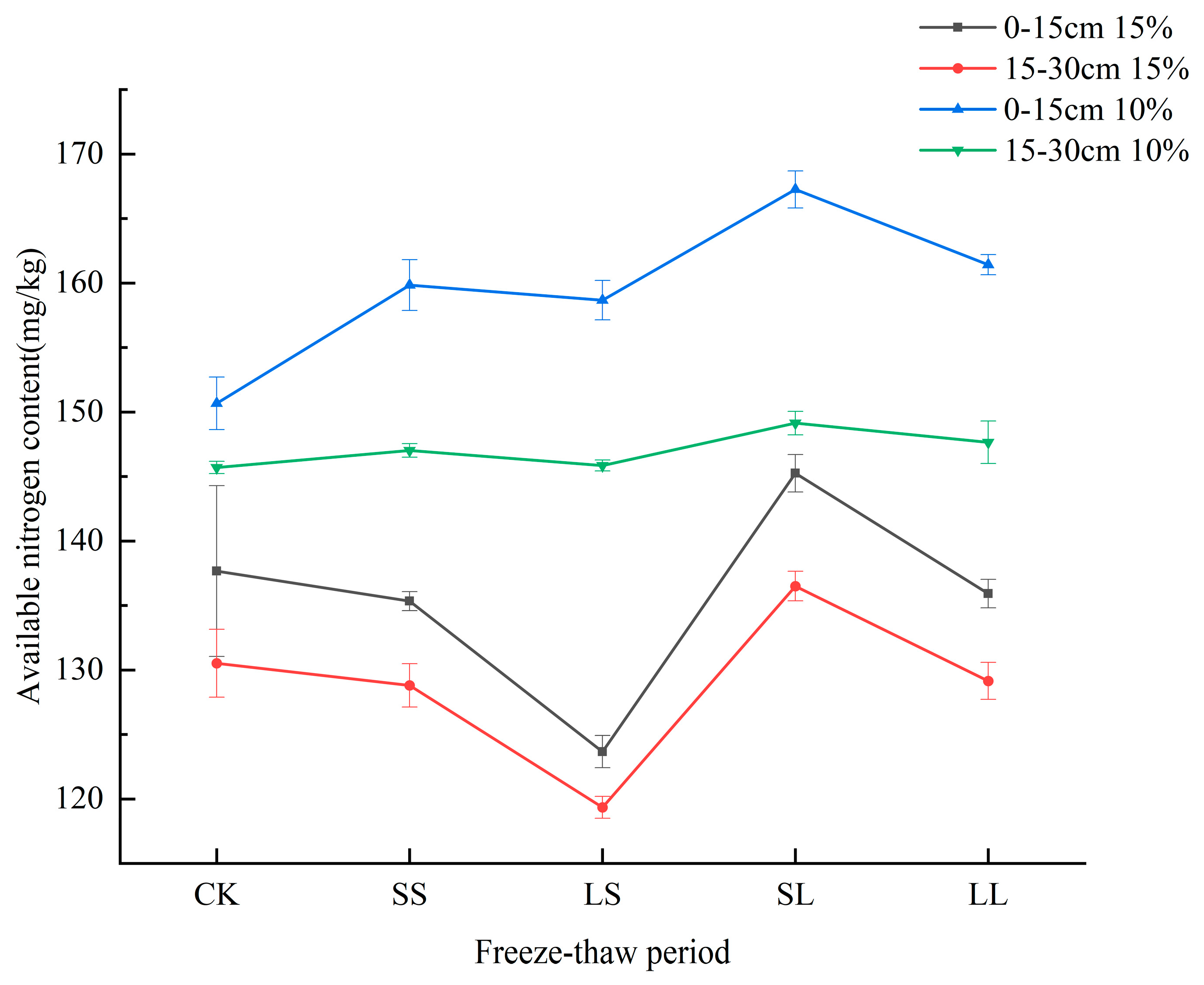
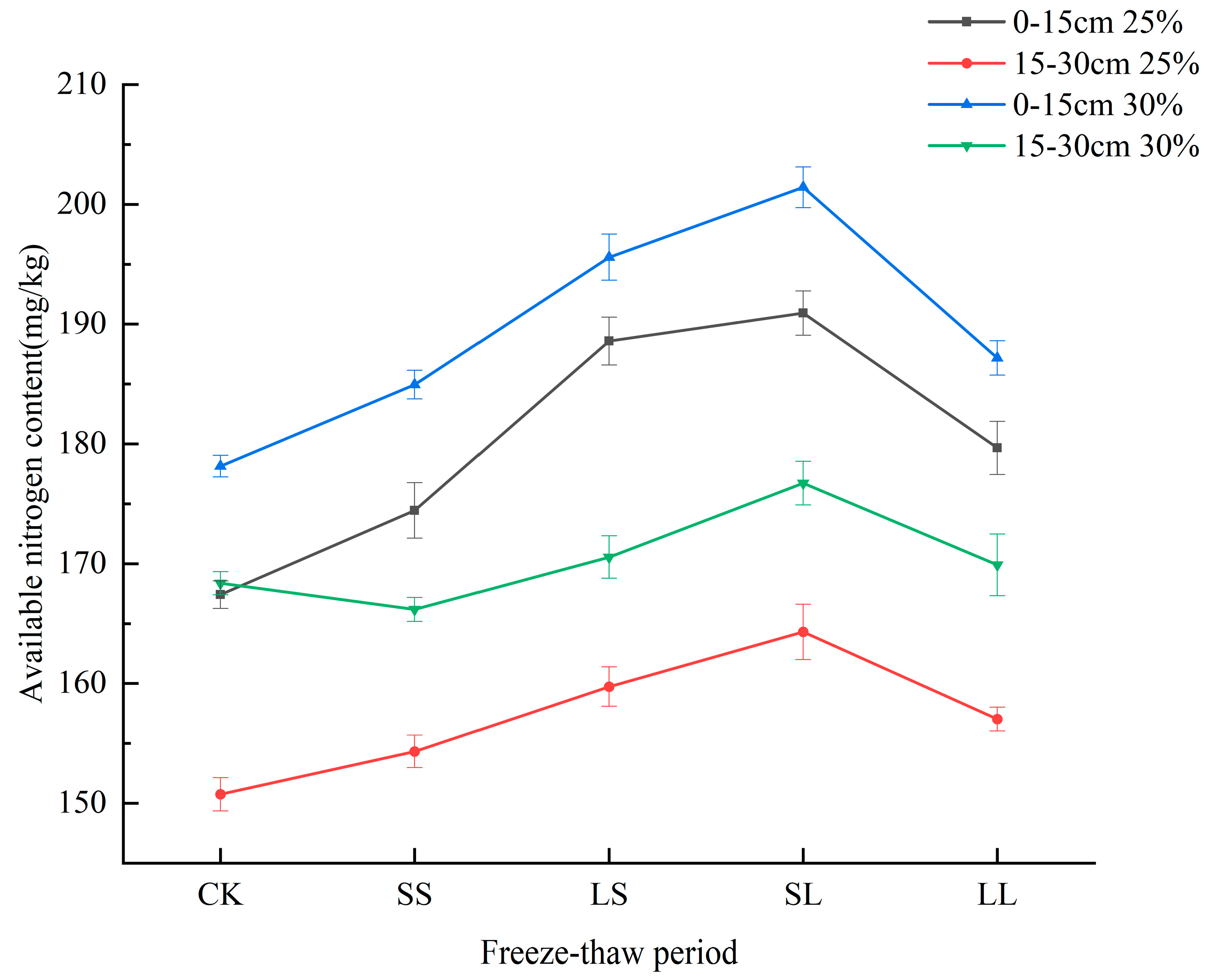
3.3. Effects of Freeze−Thaw Periods on Available Nitrogen Content in the Soil of Different Crops
4. Discussion
4.1. Effect of Freeze−Thaw Frequency on Available Nitrogen Content in the Soil of Different Crops
4.2. Effect of Freeze−Thaw Temperature Difference on Available Nitrogen Content in the Soil of Different Crops
4.3. Effects of Freeze−Thaw Periods on Soil Available Nitrogen Content of Different Crops
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Yang, X.; Wang, C.; Hao, X.; Hong, J.; Lin, X. Dynamics of available and enzymatically hydrolysable soil phosphorus fractions during repeated freeze-thaw cycles. Geoderma 2019, 345, 1–4. [Google Scholar] [CrossRef]
- Song, L.; Zang, S.; Lin, L.; Lu, B.; Jiao, Y.; Sun, C.; Wang, H. The interaction between vegetation types and intensities of freeze-thaw cycles during the autumn freezing affected in-situ soil N2O emissions in the permafrost peatlands of the Great Hinggan Mountains, Northeastern China. Atmos. Environ. X 2022, 14, 100175. [Google Scholar] [CrossRef]
- Edwards, L.M. The effect of alternate freezing and thawing on aggregate stability and aggregate size distribution of some Prince Edward Island soils. J. Soil Sci. 1991, 42, 193–204. [Google Scholar] [CrossRef]
- Kang, K.H.; Shin, H.S.; Park, H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water Res. 2002, 36, 4023–4032. [Google Scholar] [CrossRef]
- Wood, P.M. Autotrophic and heterotrophic mechanisms for ammonia oxidation. Soil Use Manag. 1990, 6, 78–79. [Google Scholar] [CrossRef]
- Perfect, E.; Williams, P.J. Thermally induced water migration in frozen soils. Cold Reg. Sci. Technol. 1980, 3, 101–109. [Google Scholar] [CrossRef]
- Schadt, C.W.; Martin, A.P.; Lipson, D.A.; Schmidt, S.K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 2003, 301, 1359–1361. [Google Scholar] [CrossRef]
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, X.; Qu, F.; Xiao, Z.; Zhang, X.; Zhang, S. Responses of soil total phosphorus to freeze and thaw cycles in a Mollisol watershed. Geoderma 2020, 376, 114571. [Google Scholar] [CrossRef]
- Brooks, P.D.; Williams, M.W.; Schmidt, S.K. Inorganic Nitrogen and Microbial Biomass Dynamics before and during Spring Snowmelt. Biogeochemistry 1998, 43, 1–15. [Google Scholar] [CrossRef]
- Elliott, A.C.; Henry, H. Freeze-thaw cycle amplitude and freezing rate effects on extractable nitrogen in a temperate old field soil. Biol. Fertil. Soils 2009, 45, 469–476. [Google Scholar] [CrossRef]
- Deelstra, J.; Kvaernø, S.H.; Granlund, K.; Sileika, A.S.; Gaigalis, K.; Kyllmar, K.; Vagstad, N. Runoff and nutrient losses during winter periods in cold climates--requirements to nutrient simulation models. J. Environ. Monit. JEM 2009, 11, 602–609. [Google Scholar] [CrossRef]
- Ouyang, W.; Xu, X.; Hao, Z.; Gao, X. Effects of soil moisture content on upland nitrogen loss. J. Hydrol. 2017, 546, 71–80. [Google Scholar] [CrossRef]
- Kämäri, M.; Huttunen, I.; Valkama, P.; Huttunen, M.; Korppoo, M.; Tattari, S.; Lotsari, E. Modelling inter- and intra-annual variation of riverine nitrogen/nitrate losses from snowmelt-affected basins under agricultural and mixed land use captured with high-frequency monitoring. Catena 2019, 176, 227–244. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, P.; Xu, G.; Wang, X.; Li, Z.; Cheng, S.; Huang, M. Effects of dynamic factors of erosion on soil nitrogen and phosphorus loss under freeze-thaw conditions. Geoderma 2021, 390, 114972. [Google Scholar] [CrossRef]
- Costa, D.; Pomeroy, J.W.; Brown, T.; Baulch, H.; Elliott, J.; Macrae, M. Advances in the simulation of nutrient dynamics in cold climate agricultural basins: Developing new nitrogen and phosphorus modules for the Cold Regions Hydrological Modelling Platform. J. Hydrol. 2021, 603, 126901. [Google Scholar] [CrossRef]
- Juan, Y.H.; Liu, Y.; Gong, L.; Sun, W.T. Response of nitrogen transformation properties to freezing-thawing cycles in farmland soils. Jiangsu Agric. Sci. 2019, 47, 282–285. [Google Scholar]
- Qi, Y.; Hu, Y. Spatial-temporal Variation and Driving Forces of Water Yield in Harbin City. Bull. Soil Water Conserv. 2023, 43, 294–303. [Google Scholar]
- Zhang, Y. Soil, Water and Plant Physicochemical Analysis Course, 1st ed.; China Forestry Publishing House: Beijing, China, 2011; pp. 59–61. [Google Scholar]
- Fitzhugh, R.D.; Driscoll, C.T.; Groffman, P.M.; Tierney, G.L.; Fahey, T.J.; Hardy, J.P. Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 2001, 56, 215–238. [Google Scholar] [CrossRef]
- Deluca, T.H.; Keeney, D.R.; Mccarty, G.W. Effect of freeze-thaw events on mineralization of soil nitrogen. Biol. Fertil. Soils 1992, 14, 116–120. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, W.; Gilliam, F.S.; Wang, Q.; Han, X. Effects of in situ freezing on soil net nitrogen mineralization and net nitrification in fertilized grassland of northern china. Grass Forage Sci. 2011, 66, 391–401. [Google Scholar] [CrossRef]
- van Bochove, E.; Jones, H.G.; Bertrand, N.; Prévost, D. Winter fluxes of greenhouses gases from snow-covered agricultural soil: Intraannual and interannual variations. Glob. Biogeochem. Cycles 2000, 14, 113–115. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Tsuruta, H.; Chen, G.; Yagi, K. N20 and NO production in various Chinese agricultural soils by nitrification. Soil Biol. Biochem. 2004, 36, 953–963. [Google Scholar] [CrossRef]
- Tian, X.H.; Li, S.X. Uptake capacity of several vegetable crops to nitrate and ammonium. J. Plant Nutr. Fertil. 2000, 6, 194–201. [Google Scholar]
- Zhou, W.M.; Chen, H.; Zhou, L.; Lewis, B.J.; Ye, Y.; Tian, J.; Li, G.; Dai, L. Effect of freezing-thawing on nitrogen mineralization in vegetation soils of four landscape zones of Changbai Mountain. Ann. For. Sci. 2011, 68, 943–951. [Google Scholar] [CrossRef]
- Yu, X.F.; Zou, Y.C.; Jiang, M.; Lu, X.G.; Wang, G.P. Response of soil constituents to freeze-thaw cycles in wetland soil solution. Soil Biol. Biochem. 2011, 43, 1308–1320. [Google Scholar] [CrossRef]
- Vaz, M.; Edwards, A.; Shand, C.; Cresser, M. Changes in the chemistry of soil solution and acetic-acid extractable P following different types of freeze/thaw episodes. Eur. J. Soil Sci. 1994, 45, 353–359. [Google Scholar] [CrossRef]
- Nielsen, C.; Groffman, P.; Hamburg, S.; Driscoll, C.; Fahey, T.; Hardy, J. Freezing effects on carbon and nitrogen cycling in northern hardwood forest soils. Soil Sci. Soc. Am. J. 2001, 65, 1723–1730. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Cook, A.; Lyter, S. Effects of experimental freezing on soil nitrogen dynamics in soils from a net nitrification gradient in a nitrogen saturated hardwood forest ecosystem. Can. J. For. Res. 2010, 40, 436–444. [Google Scholar] [CrossRef]
- Edwards, A.C.; Scalenghe, R.; Freppaz, M. Changes in the seasonal snow cover of alpine regions and its effect on soil processes: A review. Quat. Int. 2007, 162–163, 172–181. [Google Scholar] [CrossRef]
- Groffman, P.M.; Driscoll, C.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 2001, 56, 135–150. [Google Scholar] [CrossRef]
- Schimel, J.P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Burton, D.L.; Beauchamp, E.G. Profile nitrous oxide and carbon dioxide concentrations in a soil subject to freezing. Soil Sci. Soc. Am. J. 1994, 58, 115–122. [Google Scholar] [CrossRef]
- Chen, F.S.; Yu, K.; Gan, L.; Liu, Y.; Hu, X.F.; Ge, G. Effects of temperature, moisture and forest succession on nitrogen mineralization in hillside red soils in mid-subtropical region, China. Chin. J. Appl. Ecol. 2009, 20, 1529–1535. [Google Scholar]
- Christopher, S.; Shibata, H.; Ozawa, M.; Nakagawa, Y.; Mitchell, M. The effect of soil freezing on N cycling: Comparison of two headwater subcatchments with different vegetation and snowpack conditions in the northern hokkaido island of japan. Biogeochemistry 2008, 88, 15–30. [Google Scholar] [CrossRef]
- Lovett, G.M.; Mitchell, M.J. Sugar maple and nitrogen cycling in the forests of eastern north america. Front. Ecol. Environ. 2004, 2, 81–88. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze-thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Sakib, S.; Gang, D.D. Nonpoint Source Pollution. Water Environ. Res. 2016, 88, 1594–1619. [Google Scholar] [CrossRef]
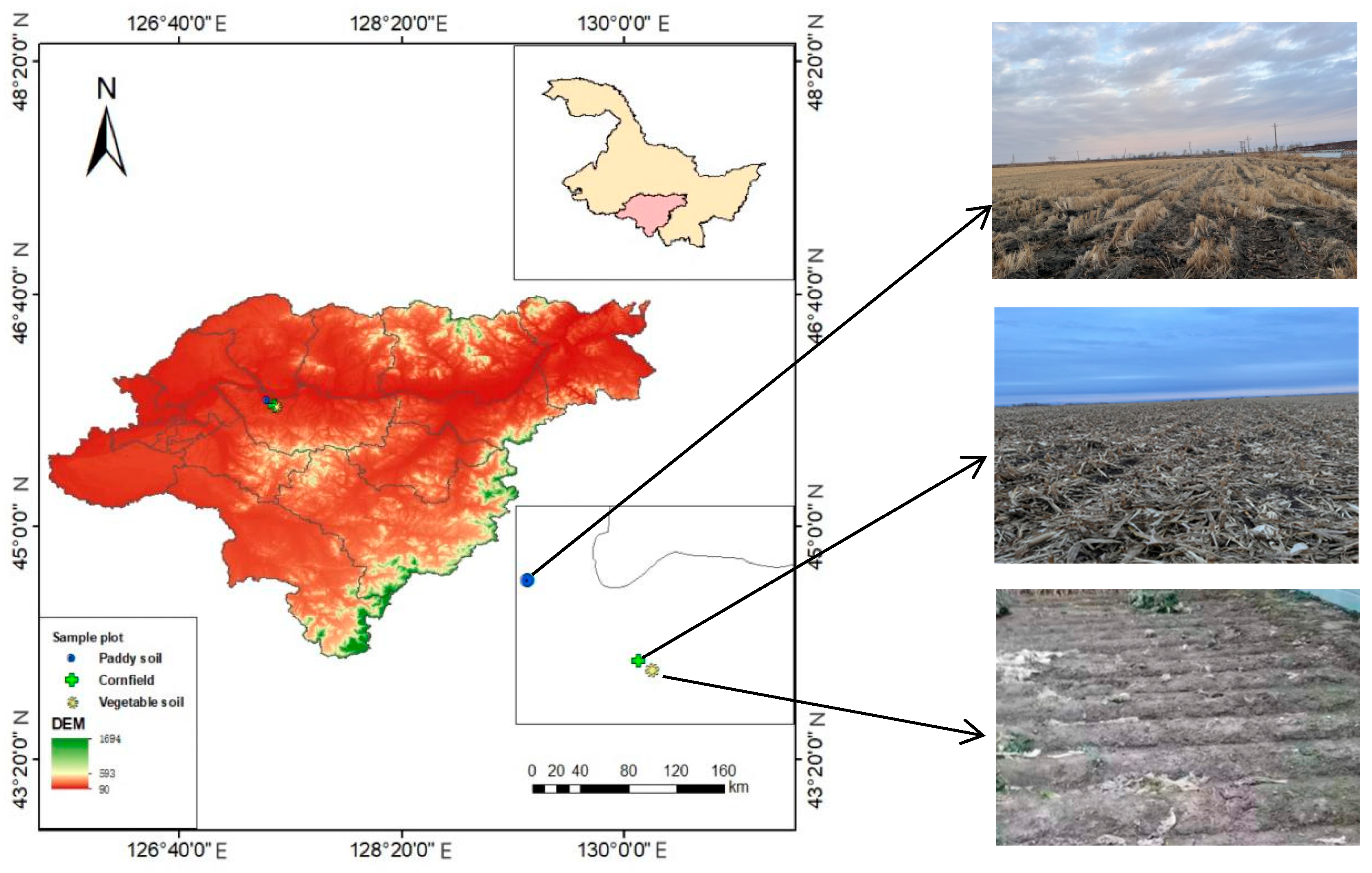



| Date | Plant Species Grown in Soil | Longitude | Latitude | Depth (cm) | Soil Density (g/cm3) | Soil Moisture Content (%) | Initial Available Nitrogen Content in Soil (mg/kg) |
|---|---|---|---|---|---|---|---|
| 4 November 2023 | Paddy | 127°19′5″ | 45°54′35″ | 0–15 | 1.54 | 26.15 | 138.02 |
| 15–30 | 1.67 | 29.07 | 130.94 | ||||
| Vegetable | 127°23′7″ | 45°51′48″ | 0–15 | 0.68 | 15.60 | 450.19 | |
| 15–30 | 0.73 | 17.13 | 424.07 | ||||
| Corn | 127°22′39″ | 45°52′4″ | 0–15 | 0.96 | 13.00 | 169.28 | |
| 15–30 | 1.02 | 15.09 | 149.83 |
| Processing Factors | Levels |
|---|---|
| Soil types | Corn soil, vegetable soil, paddy soil |
| Soil depths | 0–15 cm, 15–30 cm |
| Freeze−thaw times | Unfrozen and thawed CK |
| −20 °C freezing for 24 h, 15 °C thawing for 24 h | |
| 1 time, 3 times, 6 times, 8 times, 10 times, 13 times | |
| Freeze−thaw temperature differences | Unfrozen and thawed CK |
| Freezing at −5 °C for 12 h, melting at 5 °C for 12 h; temperature difference 10 °C | |
| Freezing at −10 °C for 12 h, melting at 10 °C for 12 h; temperature difference 20 °C | |
| Freezing at −15 °C for 12 h, melting at 15 °C for 12 h; temperature difference 30 °C | |
| Freeze−thaw periods | Unfrozen and thawed CK |
| Freezing at −20 °C, melting at 15 °C | |
| SS, SL, LS, LL | |
| 0–12 h is short-term, 12 h–5 days is long-term | |
| Soil water content of corn soil is 10% and 15% | |
| Soil water content of vegetable soil is 15% and 20% | |
| Soil water content of paddy soil is 25% and 30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Chen, M.; Yuan, X.; Liu, Y. Effects of Freeze−Thaw Cycles on Available Nitrogen Content in Soils of Different Crops. Water 2024, 16, 2348. https://doi.org/10.3390/w16162348
Wang Q, Chen M, Yuan X, Liu Y. Effects of Freeze−Thaw Cycles on Available Nitrogen Content in Soils of Different Crops. Water. 2024; 16(16):2348. https://doi.org/10.3390/w16162348
Chicago/Turabian StyleWang, Qianfeng, Mo Chen, Xiaoyang Yuan, and Yuanyuan Liu. 2024. "Effects of Freeze−Thaw Cycles on Available Nitrogen Content in Soils of Different Crops" Water 16, no. 16: 2348. https://doi.org/10.3390/w16162348
APA StyleWang, Q., Chen, M., Yuan, X., & Liu, Y. (2024). Effects of Freeze−Thaw Cycles on Available Nitrogen Content in Soils of Different Crops. Water, 16(16), 2348. https://doi.org/10.3390/w16162348






