Study on Influencing Factors and Chemical Kinetics in the High-Concentration Simultaneous Nitrification and Denitrification (SND) Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Water Quality
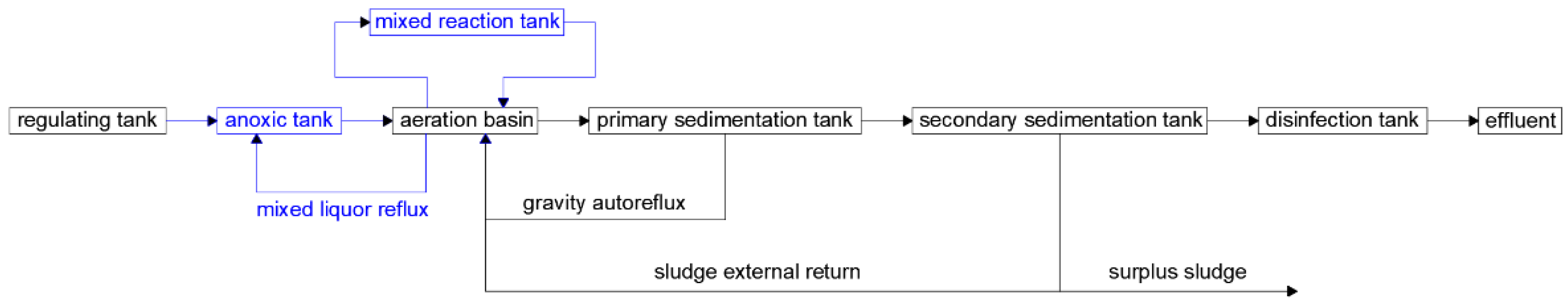
2.2. Test Equipment
2.3. Testing Instruments
2.4. Test Reagents
2.5. Analytical Items and Test Methods
3. Results and Discussion
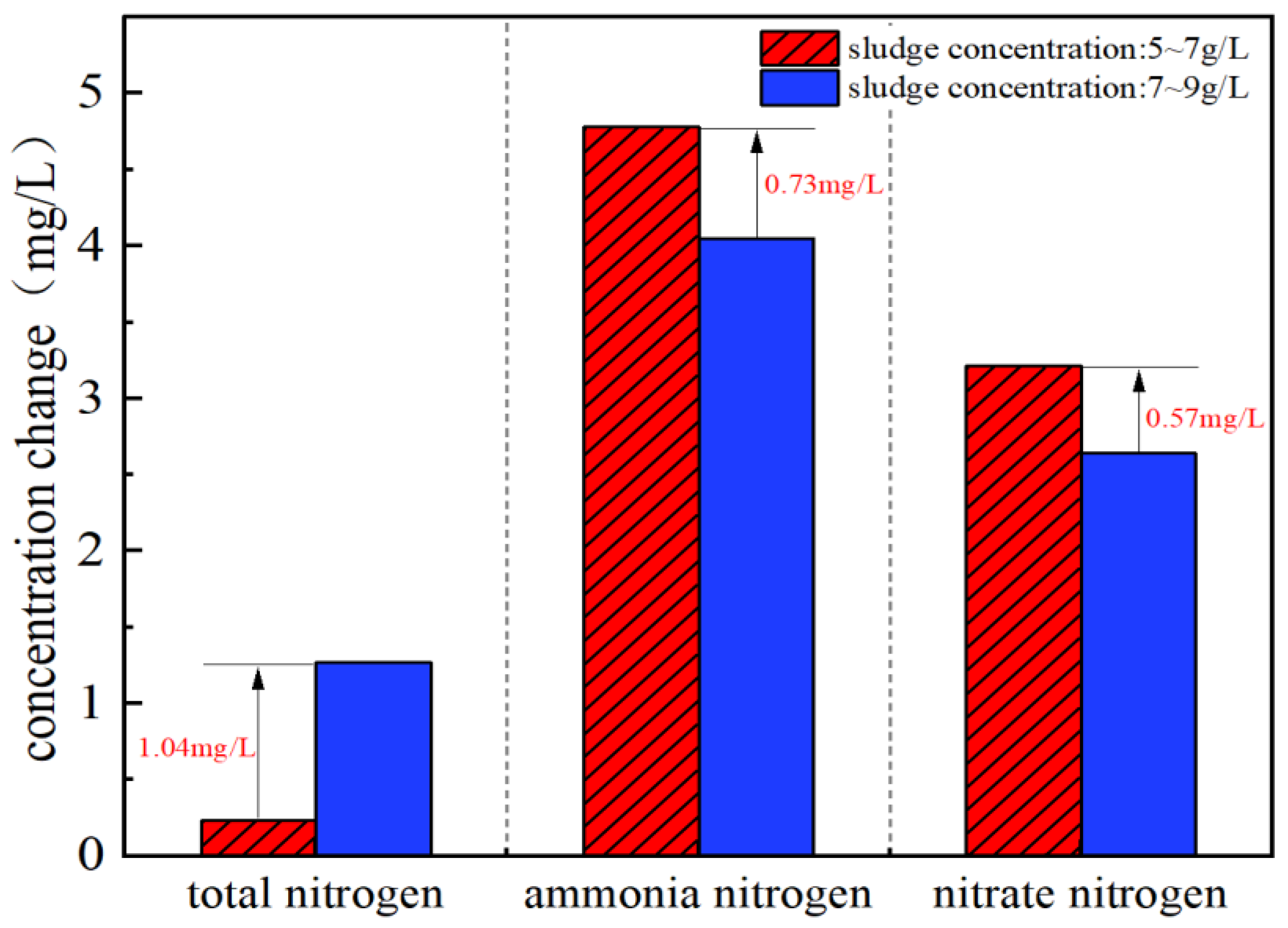
3.1. Effect of Sludge Concentration on SND
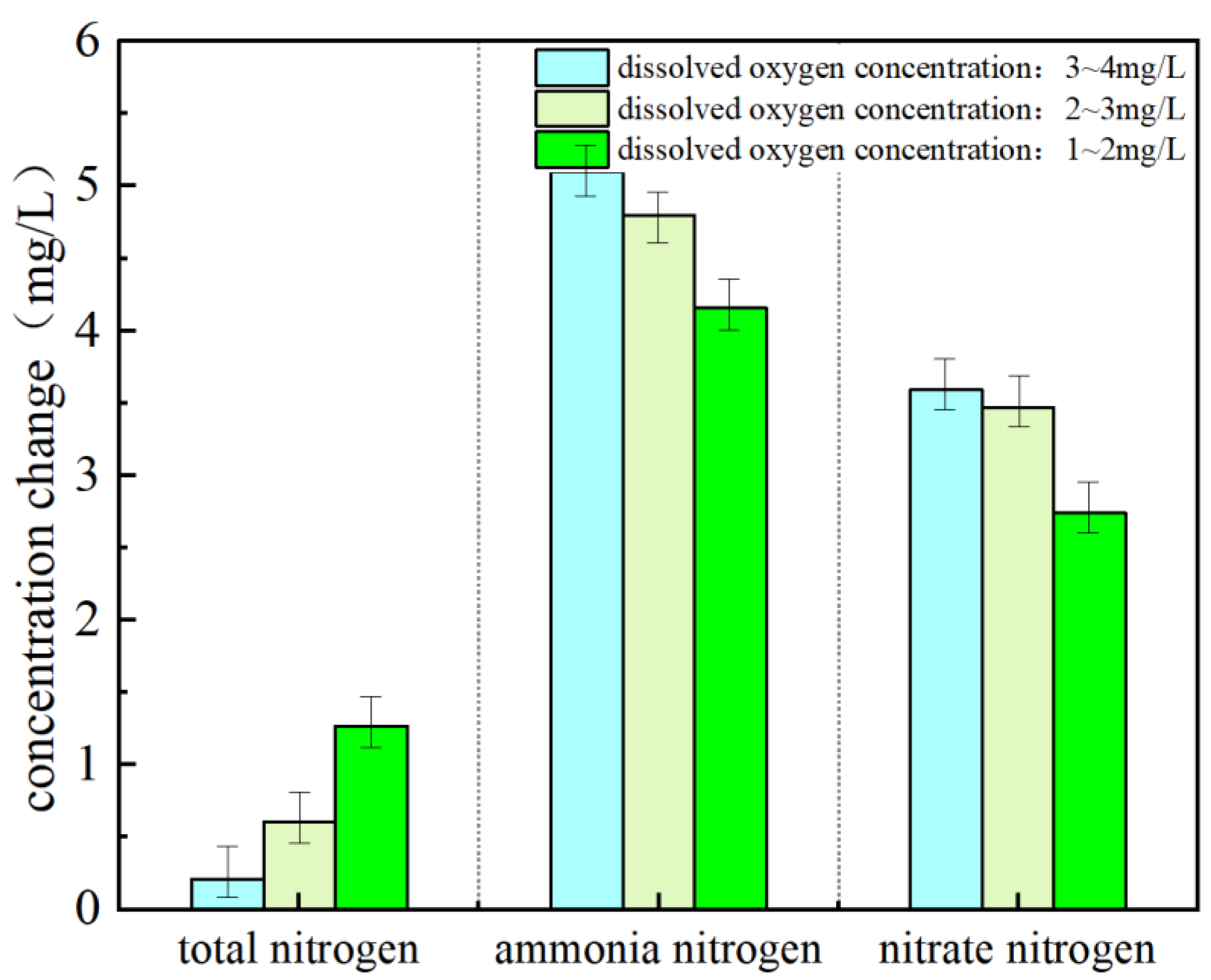
3.2. Effect of DO Concentration on SND
3.3. Analysis of Sludge Concentration and Sludge Settling Performance
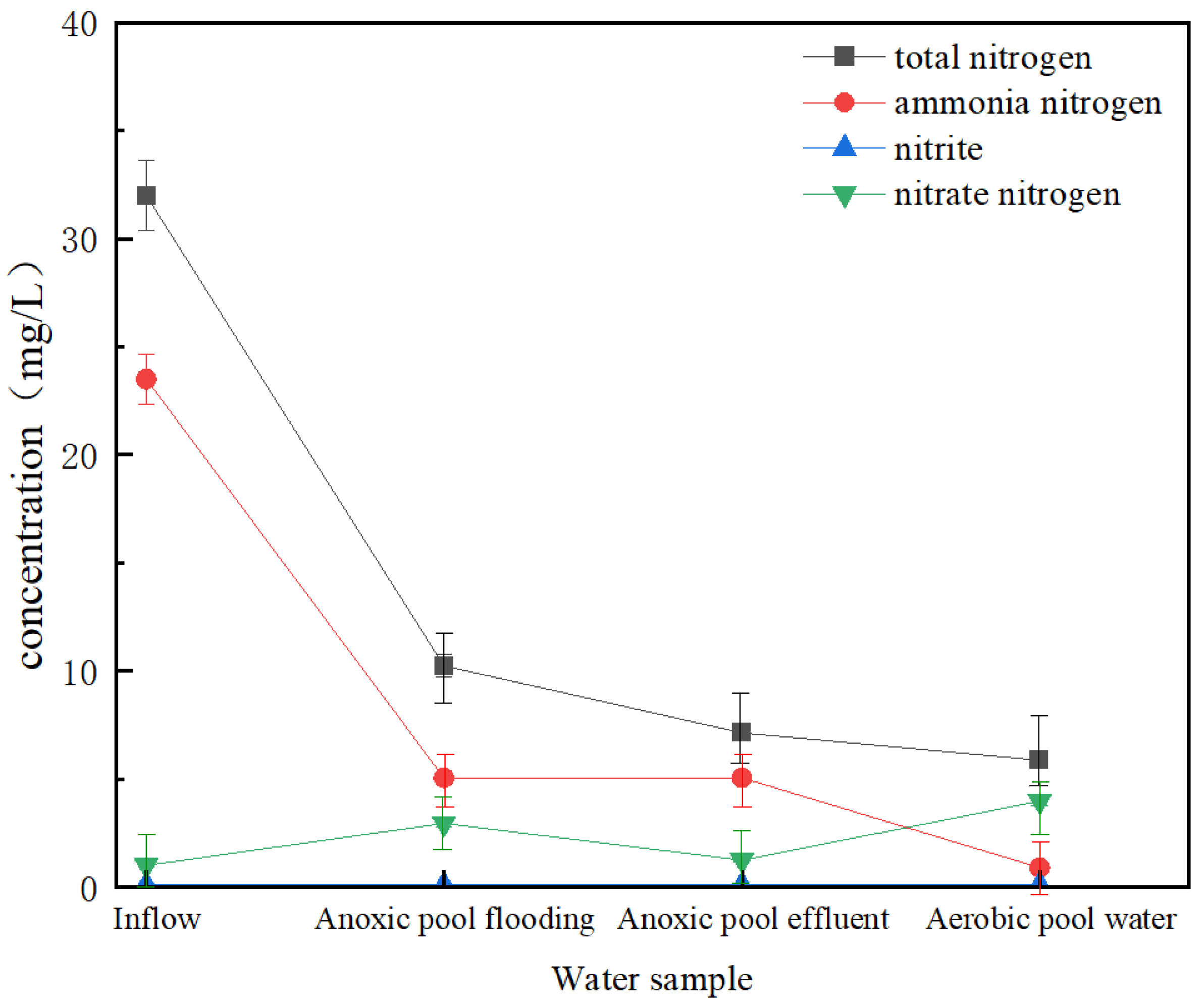
3.4. SND Mechanism Analysis
3.5. SND Reaction Kinetics of High-Concentration Activated Sludge Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shreve, M.J.; Brennan, R.A. Trace organic contaminant removal in six full-scale integrated fixed-film activated sludge (IFAS) systems treating municipal wastewater. Water Res. 2019, 151, 318–331. [Google Scholar] [CrossRef]
- Shi, X.; Liu, W.; Nie, Z.; Li, Q.; Liang, X.; Zhao, W.; Qu, H.; Bian, D. Seasonal effects on pilot-scale high-concentration activated sludge systems in cold regions. J. Water Process Eng. 2023, 52, 103575. [Google Scholar] [CrossRef]
- Shao, Z.G.; Zhang, S.F.; Yang, J.; Han, X.Y. Pilot-plant of multiple-stage high concentrated activated sludge system for P and N removal. Water Wastewater Eng. 2006, 7, 35–38. [Google Scholar] [CrossRef]
- Purba, L.D.A.; Ibiyeye, H.T.; Yuzir, A.; Mohamad, S. Various applications of aerobic granular sludge: A review. Environ. Technol. Innov. 2020, 20, 101045. [Google Scholar] [CrossRef]
- Duran, C.; Fayolle, Y.; Pechaud, Y.; Cockx, A.; Gillot, S.; Iwamoto, K.; Zamyadi, A.; Abdullah, N. Impact of suspended solids on the activated sludge non-newtonian behaviour and on oxygen transfer in a bubble column. Chem. Eng. Sci. 2016, 141, 154–165. [Google Scholar] [CrossRef]
- Layer, M.; Villodres, M.G.; Hernandez, A.; Reynaert, E.; Morgenroth, E.; Derlon, N. Limited simultaneous nitrification-denitrification (SND) in aerobic granular sludge systems treating municipal wastewater: Mechanisms and practical implications. Water Res. X 2020, 7, 100048. [Google Scholar] [CrossRef]
- Chang, M.; Wang, Y.; Pan, Y.; Zhang, K.; Lyu, L.; Wang, M.; Zhu, T. Nitrogen removal from wastewater via simultaneous nitrification and denitrification using a biological folded non-aerated filter. Bioresour. Technol. 2019, 289, 121696. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Chen, Y.Y.; Xu, T.; Cui, Z.W.; Zheng, Z.W. Efficient nitrogen removal by simultaneous heterotrophic nitrifying-aerobic denitrifying bacterium in a purification tank bioreactor amended with two-stage dissolved oxygen control. Bioresour. Technol. 2019, 281, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; He, X.; He, L.; Fan, X.; Shu, B.; Zhou, J.; He, Q. Overlooked pathways of endogenous simultaneous nitrification and denitrification in anaerobic/aerobic/anoxic sequencing batch reactors with organic supplementation. Water Res. 2023, 230, 119493. [Google Scholar] [CrossRef]
- Jetten, M.S.M. The microbial nitrogen cycle. Environ. Microbiol. 2008, 10, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Shao, Z.; Chai, H.; Ji, F.; He, Q. Functional microorganisms and enzymes related nitrogen cycle in the biofilm performing simultaneous nitrification and denitrification. Bioresour. Technol. 2020, 314, 123697. [Google Scholar] [CrossRef]
- Chai, H.; Xiang, Y.; Chen, R.; Shao, Z.; Gu, L.; Li, L.; He, Q. Enhanced simultaneous nitrification and denitrification in treating low carbon-to-nitrogen ratio wastewater: Treatment performance and nitrogen removal pathway. Bioresour. Technol. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, T.Z.; Zhou, K.; Wang, H.N.; Guo, S.J.; Shao, D.W.; Bian, D.J.; Wang, Y.; Zhu, S.Y. Enhanced treatment of low carbon-to nitrogen ratio domestic wastewater using a continuous anaerobic-anoxic-aerobic(A~2/O) process at high MLSS condition. Northeast Norm. Univ. (Nat. Sci. Ed.) 2017, 49, 163–168. [Google Scholar] [CrossRef]
- James, S.N.; Vijayanandan, A. Anoxic-Aerobic-Anoxic sequencing batch reactor for enhanced nitrogen removal. Bioresour. Technol. 2022, 363, 127892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.J.; Duan, Y.J.; Wu, G.L.; Xiong, Y.H.; Luo, W.S.; Li, T.F.; Zhang, X.B. Study of high concentration activated sludge method for municipal wastewater with insufficient carbonsource. Water Wastewater Eng. 2009, 35, 41–46. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Lu, L.; Wang, J.; Huang, G.; Chen, F.; Zuo, J.-E. Non-uniform dissolved oxygen distribution and high sludge concentration enhance simultaneous nitrification and denitrification in a novel air-lifting reactor for municipal wastewater treatment: A pilot-scale study. Bioresour. Technol. 2023, 384, 129306. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Geng, M.; Gao, S.; Yin, X.; Tian, J. In-depth insight into improvement of simultaneous nitrification and denitrification/biofouling control by increasing sludge concentration in membrane reactor: Performance, microbial assembly and metagenomic analysis. Bioresour. Technol. 2024, 393, 130013. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, S.; Liu, Q.; Zhang, M.; Liu, Y.; Wen, Y.; Chen, Z.; Zhang, Y.; Yang, Q. Improved performance of simultaneous nitrification and denitrification via nitrite in an oxygen-limited SBR by alternating the DO. Bioresour. Technol. 2019, 275, 153–162. [Google Scholar] [CrossRef]
- Jin, Y.; Ding, J.; Zhan, W.; Du, J.; Wang, G.; Pang, J.; Ren, N.; Yang, S. Effect of dissolved oxygen concentration on performance and mechanism of simultaneous nitrification and denitrification in integrated fixed-film activated sludge sequencing batch reactors. Bioresour. Technol. 2023, 387, 129616. [Google Scholar] [CrossRef]
- Sriwiriyarat, T.; Ungkurarate, W.; Fongsatitkul, P.; Chinwetkitvanich, S. Effects of dissolved oxygen on biological nitrogen removal in integrated fixed film activated sludge (IFAS) wastewater treatment process. J. Environ. Sci. Health Part A 2008, 43, 518–527. [Google Scholar] [CrossRef]
- Di Capua, F.; Iannacone, F.; Sabba, F.; Esposito, G. Simultaneous nitrification–denitrification in biofilm systems for wastewater treatment: Key factors, potential routes, and engineered applications. Bioresour. Technol. 2022, 361, 127702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.F.; Tay, J.H. Improved stability of aerobic granules by selecting slow-growing nitrifying bacteria. J. Biotechnol. 2004, 108, 161–169. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; El-Din, M.G. Heterotrophic nitrification and aerobic denitrification process: Promising but a long way to go in the wastewater treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, Q. Performance and microbial characterization of aerobic granular sludge in a sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal with varying C/N ratios. Bioprocess Biosyst. Eng. 2020, 43, 663–672. [Google Scholar] [CrossRef]
- Xavier, J.A.; Santos, S.G.S.; Monteiro, J.P.; Silva, T.; Costa, R.H.R.; Vilar, V.J.P. Towards a better understanding concerning carbon to nitrogen ratio and the carbon source in aerobic granular sludge. J. Environ. Chem. Eng. 2023, 11, 110457. [Google Scholar] [CrossRef]
- Wen, J.; LeChevallier, M.W.; Tao, W. Nitrification kinetics and microbial communities of activated sludge as a full-scale membrane bioreactor plant transitioned to low dissolved oxygen operation. J. Clean. Prod. 2020, 252, 119872. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Christensen, P.B.; Revsbech, N.P.; Sørensen, J. Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol. 1990, 19, 63–72. [Google Scholar] [CrossRef]
- James, S.N.; Vijayanandan, A. Recent advances in simultaneous nitrification and denitrification for nitrogen and micropollutant removal: A review. Biodegradation 2023, 34, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, H.; Yan, G.; Yan, Q.; Kim, J.R. Limited dissolved oxygen facilitated nitrogen removal at biocathode during the hydrogenotrophic denitrification process using bioelectrochemical system. Bioresour. Technol. 2023, 372, 128662. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Wang, W.; Huang, X.; Kou, X.; Wu, S.; Shao, T. High-rate nitrogen removal in a continuous biofilter anammox reactor for treating low-concentration nitrogen wastewater at moderate temperature. Bioresour. Technol. 2021, 337, 125496. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ormeci, B.; Mishra, S.; Abid, H. Simultaneous partial Nitrification, ANAMMOX and denitrification (SNAD)–A review of critical operating parameters and reactor configurations. Chem. Eng. J. 2022, 433, 133677. [Google Scholar] [CrossRef]
- Chang, M.; Liang, B.; Zhang, K.; Wang, Y.; Jin, D.; Zhang, Q.; Hao, L.; Zhu, T. Simultaneous shortcut nitrification and denitrification in a hybrid membrane aerated biofilms reactor (H-MBfR) for nitrogen removal from low COD/N wastewater. Water Res. 2022, 211, 118027. [Google Scholar] [CrossRef]
- Huang, R.; Meng, T.; Liu, G.; Gao, S.; Tian, J. Simultaneous nitrification and denitrification in membrane bioreactor: Effect of dissolved oxygen. J. Environ. Manag. 2022, 323, 116183. [Google Scholar] [CrossRef]
- Li, B.; Bishop, P.L. Micro-profiles of activated sludge floc determined using microelectrodes. Water Res. 2004, 38, 1248–1258. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Jin, Q.; Zhang, M.; Wu, J. A novel feedforward control strategy for simultaneous nitrification and denitrification (SND) in aerobic granular sludge sequential batch reactor (AGS-SBR). J. Environ. Manag. 2020, 260, 110103. [Google Scholar] [CrossRef] [PubMed]
- John, Y.; Langergraber, G.; Adyel, T.M.; David, E.V., Jr. Aeration intensity simulation in a saturated vertical up-flow constructed wetland. Sci. Total Environ. 2020, 708, 134793. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.Q.; Tay, J.H.; Ning, P. Operational strategies for nitrogen removal in granular sequencing batch reactor. J. Hazard. Mater. 2011, 189, 342–348. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shen, L.; Chen, F. DO diffusion profile in aerobic granule and its microbiological implications. Enzym. Microb. Technol. 2008, 43, 349–354. [Google Scholar] [CrossRef]
- Richardson, D.J.; Wehrfritz, J.M.; Keech, A.; Crossman, L.C.; Roldan, M.D.; Sears, H.J.; Butler, C.S.; Reilly, A.; Moir, J.W.; Berks, B.C.; et al. The diversity of redox proteins involved in bacterial heterotrophic nitrification and aerobic denitrification. Biochem. Soc. Trans. 1998, 26, 401–408. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, H.; Zhang, J.; Zhu, S.; Wang, Y.; Lei, Y.; Zhang, R.; Song, L. Simultaneous heterotrophic nitrification and aerobic denitrification (SND) for nitrogen removal: A review and future perspectives. Environ. Adv. 2022, 9, 100254. [Google Scholar] [CrossRef]





| Serial Number | Sports Event | Realm | Average Value |
|---|---|---|---|
| 1 | CODcr (mg/L) | 45.2–373 | 138 |
| 2 | BOD5 (mg/L) | 29.5–169 | 67.3 |
| 3 | NH3-N (mg/L) | 9.54–49.8 | 23.7 |
| 4 | TN (mg/L) | 12.9–87.8 | 36.1 |
| 2 | TP (mg/L) | 1.24–7.39 | 3.52 |
| Equipment Name | Equipment Model |
|---|---|
| PH meter | Hash HQ-40d Portable Monitor |
| dissolved oxygen concentration meter | Hash HQ-40d Portable Monitor |
| pump | SCN35.65.037.4 |
| stirrer | CMJB-0.25A |
| dosing pump | Solenoid pump |
| electromagnetic flow meter | HXLD-DN40 electromagnetic flow meter |
| electromagnetic flow meter | HXLD-DN15 electromagnetic flow meter |
| electrically heated drying oven | 101-1AB |
| muffle furnace | SX-4-10 |
| Reagent Name | Fineness |
|---|---|
| PAC | AR |
| CPAM | AR |
| potassium dichromate | AR |
| concentrated sulfuric acid | GR |
| ferrous sulfate | AR |
| caustic soda | AR |
| anhydrous ethanol | AR |
| chlorine residual powder kit | DPD |
| total chlorine powder package | DPD |
| Water Quality Indicators | Detection Methods |
|---|---|
| COD | Potassium dichromate |
| NH4+-N | Nasher’s reagent photometric method |
| NO3-N | ultraviolet spectrophotometry |
| NO2-N | N-(1-Naphthyl)-ethylenediamine photometric method |
| TN | Alkaline potassium persulfate-ultraviolet spectrophotometry |
| TP | Molybdenum antimony spectrophotometry |
| PH, DO, temperature | Hash HQ-40d Portable Monitor |
| Sludge Testing Program | Measurement Methods |
|---|---|
| MLVSS | cauterization |
| MLSS | gravimetric method |
| SV% | sedimentation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Liu, Y.; Zhang, Q.; Yan, Y.; He, H.; Wang, Y.; Yang, X.; Li, J.; Huang, W.; Xu, J.; et al. Study on Influencing Factors and Chemical Kinetics in the High-Concentration Simultaneous Nitrification and Denitrification (SND) Process. Water 2024, 16, 2334. https://doi.org/10.3390/w16162334
Luo B, Liu Y, Zhang Q, Yan Y, He H, Wang Y, Yang X, Li J, Huang W, Xu J, et al. Study on Influencing Factors and Chemical Kinetics in the High-Concentration Simultaneous Nitrification and Denitrification (SND) Process. Water. 2024; 16(16):2334. https://doi.org/10.3390/w16162334
Chicago/Turabian StyleLuo, Benfu, Yuhang Liu, Qiang Zhang, Yujing Yan, Haixing He, Yin Wang, Xi Yang, Jinyin Li, Weiwei Huang, Jiaran Xu, and et al. 2024. "Study on Influencing Factors and Chemical Kinetics in the High-Concentration Simultaneous Nitrification and Denitrification (SND) Process" Water 16, no. 16: 2334. https://doi.org/10.3390/w16162334
APA StyleLuo, B., Liu, Y., Zhang, Q., Yan, Y., He, H., Wang, Y., Yang, X., Li, J., Huang, W., Xu, J., & Huang, W. (2024). Study on Influencing Factors and Chemical Kinetics in the High-Concentration Simultaneous Nitrification and Denitrification (SND) Process. Water, 16(16), 2334. https://doi.org/10.3390/w16162334






