Sewage Vertical Infiltration Introduced Polygenic Multipollutants into Groundwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Pollution Incident and Field Monitoring
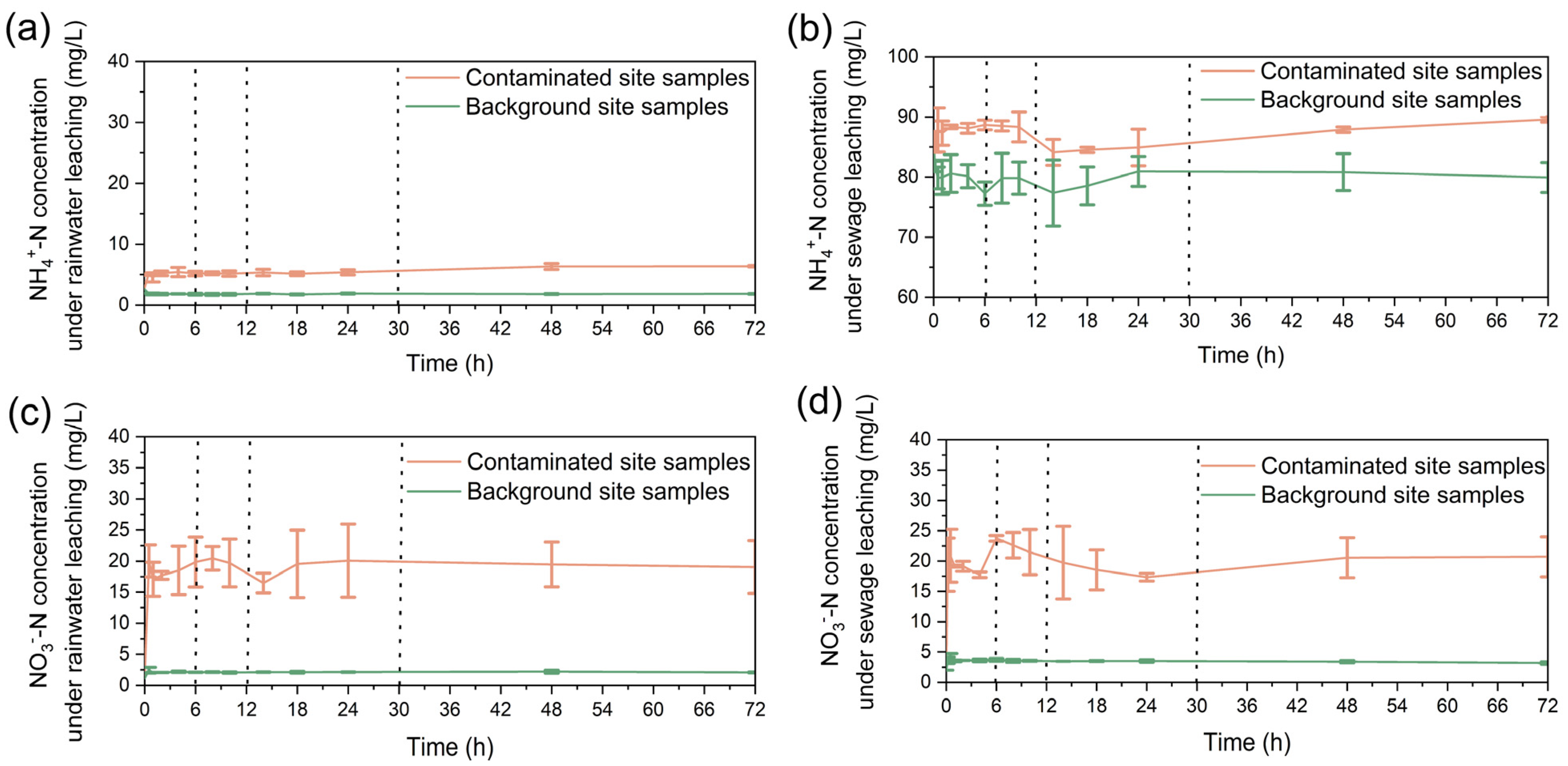
2.3. Laboratory Experiment
2.3.1. Sample Collection and Determination
2.3.2. Experimental Procedure
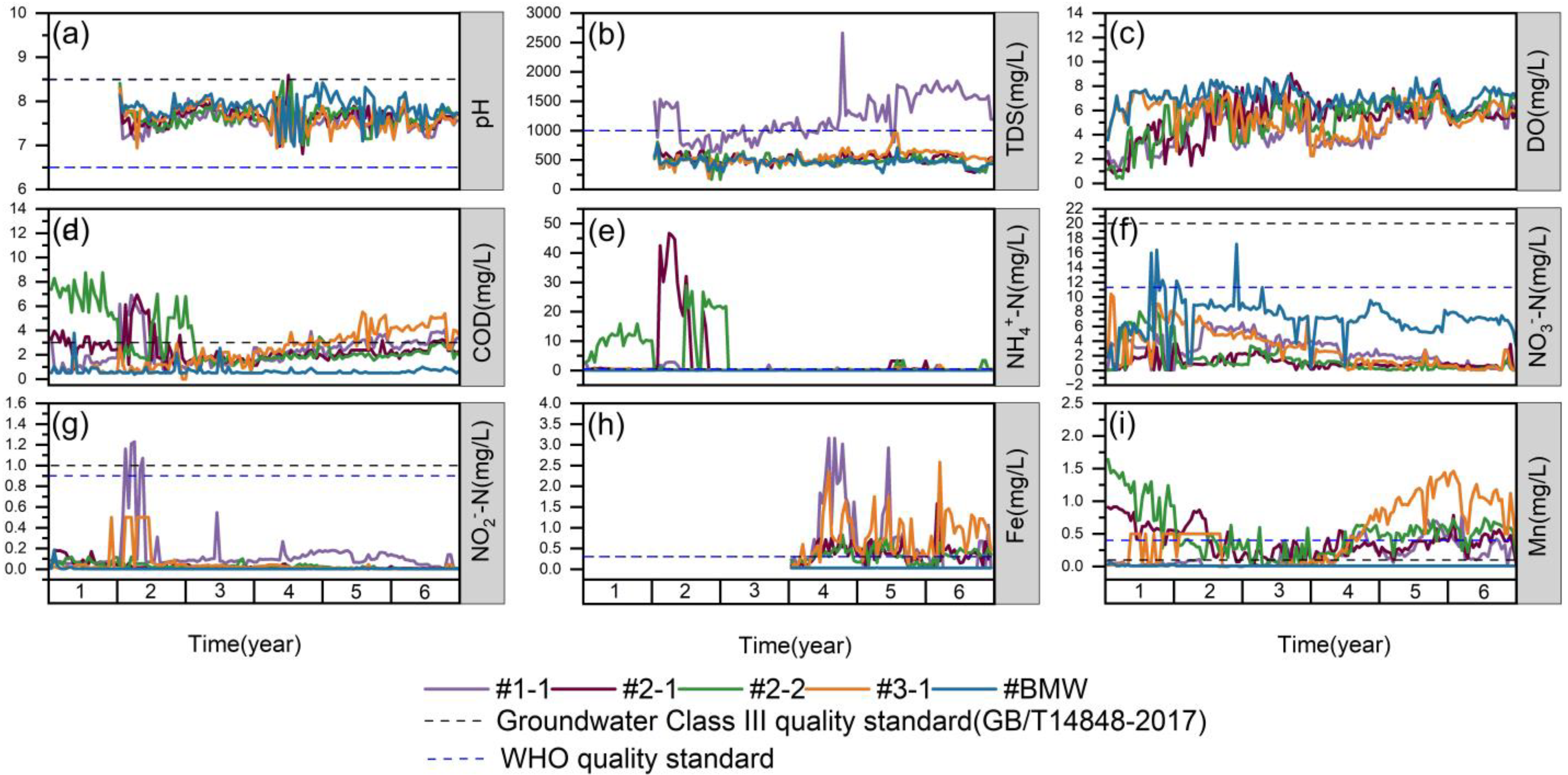
3. Results
3.1. Variations in the Concentrations of Nitrogen
3.2. Variations in the Concentrations of pH/Ca/Mg
3.3. Variations in the Concentrations of Mn/Fe
4. Discussion
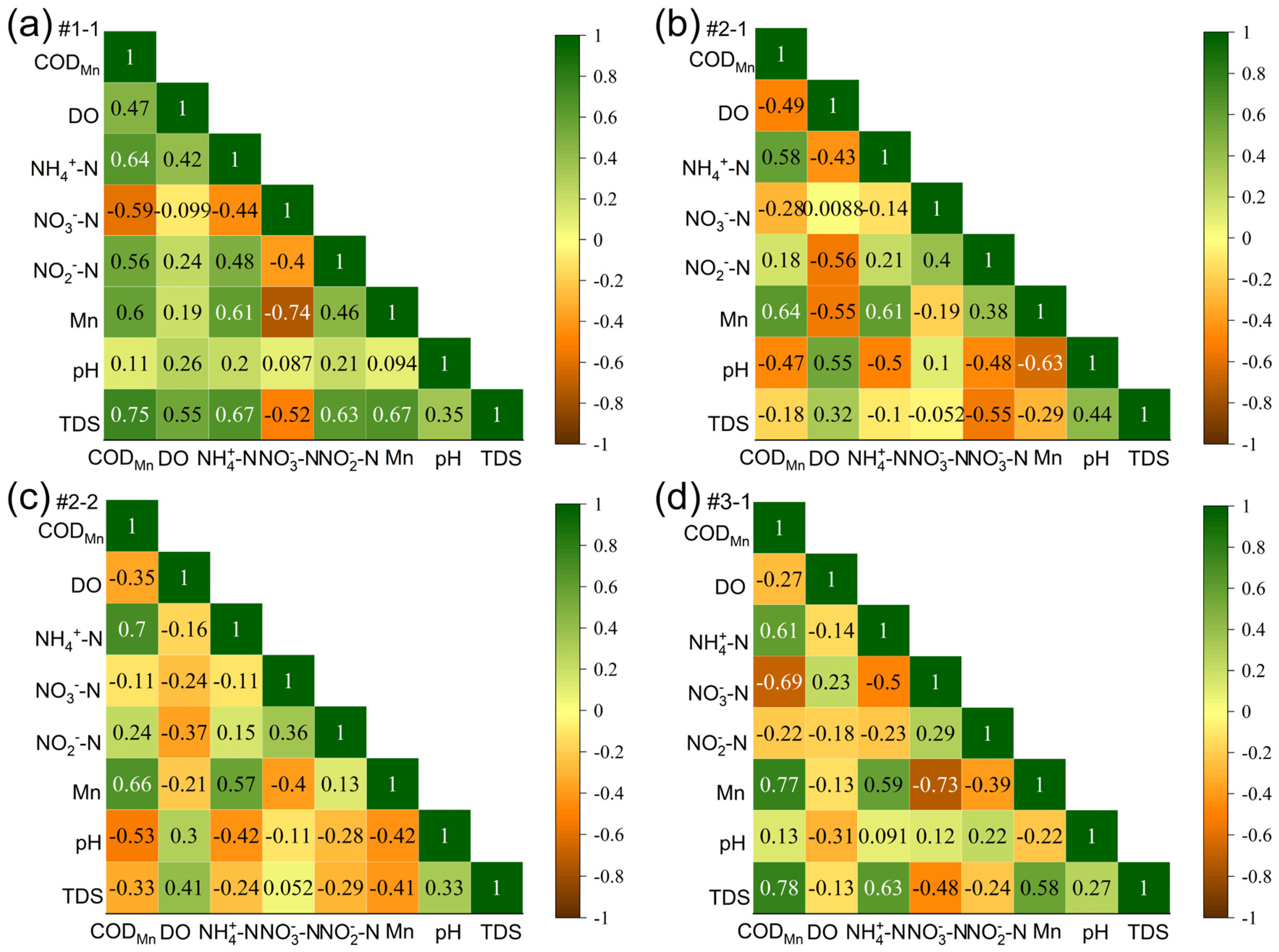
4.1. Enrichment of the Secondary NO3− in Oxidation Zone
4.2. Acid Environment Controlled by Rapid Reduction
4.3. Response of Mn Mineral Release to Acid Condition
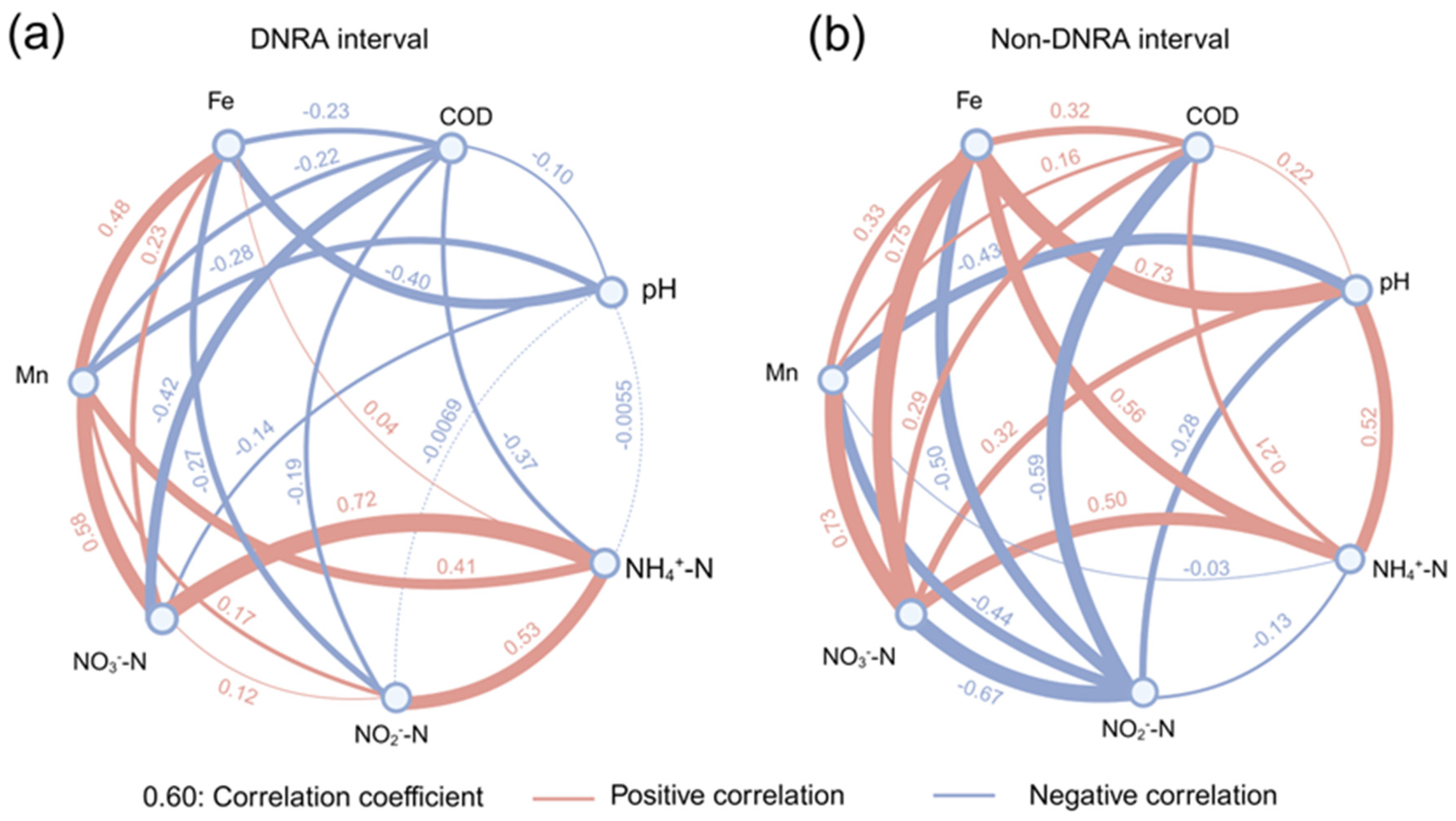
4.4. Active DNRA in High Carbon Load and Mn2+ Condition
4.5. Novelty, Future Work and Practical Implication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kabir, M.M.; Akter, S.; Ahmed, F.T.; Mohinuzzaman, M.; Didar-ul-Alam, M.; Mostofa, K.M.G.; Islam, A.R.M.T.; Niloy, N.M. Salinity-induced fluorescent dissolved organic matter influence co-contamination, quality and risk to human health of tube well water, southeast coastal Bangladesh. Chemosphere 2021, 275, 130053. [Google Scholar] [CrossRef] [PubMed]
- Osafo, N.O.-A.; Jan, J.; Porcal, P.; Borovec, J. Contrasting catchment soil pH and Fe concentrations influence DOM distribution and nutrient dynamics in freshwater systems. Sci. Total Environ. 2023, 858, 159988. [Google Scholar] [CrossRef]
- Jang, C.-S.; Chen, J.-S.; Lin, Y.-B.; Liu, C.-W. Characterizing hydrochemical properties of springs in Taiwan based on their geological origins. Environ. Monit. Assess. 2012, 184, 63–75. [Google Scholar] [CrossRef]
- Yang, F.; Yue, S.; Wu, X.; Zhang, C.; Li, D.; Zhu, R. Effects of flood inundation on biogeochemical processes in groundwater during riverbank filtration. J. Hydrol. 2023, 617, 129101. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Ahmad, S.; Gao, L.; Wang, Z.; El-Baz, A.; Ni, S.-Q. Energy-efficient and carbon neutral anammox-based nitrogen removal by coupling with nitrate reduction pathways: A review. Sci. Total Environ. 2023, 889, 164213. [Google Scholar] [CrossRef] [PubMed]
- Abiriga, D.; Vestgarden, L.S.; Klempe, H. Long-term redox conditions in a landfill-leachate-contaminated groundwater. Sci. Total Environ. 2021, 755, 143725. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.O.; Ali, S.S.; Al-Ansari, N.A.; Knutsson, S. Hydrogeochemical Evaluation of Groundwater and Its Suitability for Domestic Uses in Halabja Saidsadiq Basin, Iraq. Water 2019, 11, 690. [Google Scholar] [CrossRef]
- Wang, H.; Lu, K.; Shen, C.; Song, X.; Hu, B.; Liu, G. Human health risk assessment of groundwater nitrate at a two geomorphic units transition zone in northern China. J. Environ. Sci. 2021, 110, 38–47. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Liu, H.; Wu, C.; Bi, W.; Yuan, Y.; Liu, X. Simultaneous Fe(III) reduction and ammonia oxidation process in Anammox sludge. J. Environ. Sci. 2018, 64, 42–50. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, Y.; Lu, H.; Du, Q.; Xia, X.; Teng, Y.; Zuo, R.; Wang, J. Interactions between anthropogenic pollutants (biodegradable organic nitrogen and ammonia) and the primary hydrogeochemical component Mn in groundwater: Evidence from three polluted sites. Sci. Total Environ. 2022, 808, 152162. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Y.; Jia, Y.; Lian, X.; Feng, F.; Shang, C.; Zang, Y.; Xi, B. Anthropogenic perturbation enhances the release of geogenic Mn to groundwater: Evidence from hydrogeochemical characteristics. Sci. Total Environ. 2023, 891, 164450. [Google Scholar] [CrossRef]
- Kelley, C.J.; Keller, C.K.; Evans, R.D.; Orr, C.H.; Smith, J.L.; Harlow, B.A. Nitrate–nitrogen and oxygen isotope ratios for identification of nitrate sources and dominant nitrogen cycle processes in a tile-drained dryland agricultural field. Soil Biol. Biochem. 2013, 57, 731–738. [Google Scholar] [CrossRef]
- Xiu, W.; Yu, X.; Guo, H.; Yuan, W.; Ke, T.; Liu, G.; Tao, J.; Hou, W.; Dong, H. Facilitated arsenic immobilization by biogenic ferrihydrite-goethite biphasic Fe(III) minerals (Fh-Gt Bio-bi-minerals). Chemosphere 2019, 225, 755–764. [Google Scholar] [CrossRef]
- Lei, X.; Cui, G.; Sun, H.; Hou, S.; Deng, H.; Li, B.; Yang, Z.; Xu, Q.; Huo, X.; Cai, J. How do earthworms affect the pathway of sludge bio-stabilization via vermicomposting? Sci. Total Environ. 2024, 916, 170411. [Google Scholar] [CrossRef]
- Morrissy, J.G.; Currell, M.J.; Reichman, S.M.; Surapaneni, A.; Megharaj, M.; Crosbie, N.D.; Hirth, D.; Aquilina, S.; Rajendram, W.; Ball, A.S. Nitrogen contamination and bioremediation in groundwater and the environment: A review. Earth-Sci. Rev. 2021, 222, 103816. [Google Scholar] [CrossRef]
- Roșca, O.M.; Dippong, T.; Marian, M.; Mihali, C.; Mihalescu, L.; Hoaghia, M.; Jelea, M. Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains, Romania. Environ. Res. 2020, 182, 109136. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, H.; Zhang, P.; Zhao, L.; Ding, K.; Yuan, S. Attenuation of Fe(III)-reducing bacteria during table fluctuation of groundwater containing Fe2+. Sci. Total Environ. 2019, 694, 133660. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Han, Y.; Xia, X.; Li, X.; Lu, H.; Teng, Y.; Wang, J. Anthropogenic Organic Pollutants in Groundwater Increase Releases of Fe and Mn from Aquifer Sediments: Impacts of Pollution Degree, Mineral Content, and pH. Water 2021, 13, 1920. [Google Scholar] [CrossRef]
- Su, X.; Zheng, Z.; Chen, Y.; Wan, Y.; Lyu, H.; Dong, W. Effects of carbon load on nitrate reduction during riverbank filtration: Field monitoring and batch experiment. Sci. Total Environ. 2022, 845, 157198. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, Q.; Huang, G.; Liu, C.; Zhang, Y. Elevated manganese concentrations in shallow groundwater of various aquifers in a rapidly urbanized delta, south China. Sci. Total Environ. 2020, 701, 134777. [Google Scholar] [CrossRef]
- Cao, X.; He, W.; Fan, M.; He, W.; Shi, Y.; An, T.; Chen, X.; Zhang, Z.; Liu, F.; Zhao, Y.; et al. Novel insights into source apportionment of dissolved organic matter in aquifer affected by anthropogenic groundwater recharge: Applicability of end-member mixing analysis based optical indices. Sci. Total Environ. 2023, 863, 160885. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chang, F.; Zhang, Y.; Duan, L.; Liu, Q.; Li, H.; Hu, G.; Zhang, X.; Gao, Y.; Zhang, H. Arsenic migration at the sediment-water interface of anthropogenically polluted Lake Yangzong, Southwest China. Sci. Total Environ. 2023, 879, 163205. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shi, J.; Zheng, S.; Guo, X.; Wang, J.; Zhai, Y.; Teng, Y.; Zuo, R. Compartmentalization transformation of Fe2+, Mn2+ and NH4+ in groundwater: A comparative research containing experimental medium and functional genera profiling derived from experiment data. J. Water Process Eng. 2024, 58, 104794. [Google Scholar] [CrossRef]
- Duan, L.; Song, J.; Yin, M.; Yuan, H.; Li, X.; Zhang, Y.; Yin, X. Dynamics of arsenic and its interaction with Fe and S at the sediment-water interface of the seasonal hypoxic Changjiang Estuary. Sci. Total Environ. 2021, 769, 145269. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, X.; Chen, Y.; Li, J.; Zhai, Y. Flood irrigation increases the release of phosphorus from aquifer sediments into groundwater. J. Contam. Hydrol. 2024, 261, 104297. [Google Scholar] [CrossRef] [PubMed]
- GB/T 36197-2018[S/OL]; Soil Quality – Guidance on Sampling Techniques. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Standards Press of China: Beijing, China, 2018.
- Lu, C.; Xiu, W.; Guo, H.; Lian, G.; Yang, B.; Zhang, T.; Bi, E.; Shi, Z. Multi-Isotope Based Identification and Quantification of Oxygen Consuming Processes in Uranium Hosting Aquifers with CO2 + O2 In Situ Leaching. Water Resour. Res. 2023, 59, e2022WR033980. [Google Scholar] [CrossRef]
- Lu, C.; Xiu, W.; Yang, B.; Lian, G.; Zhang, T.; Bi, E.; Guo, H. Characteristics of Dissolved Organic Matter in Uranium Hosting Aquifers and Potential Molecular Transformation During Neutral In Situ Leaching. JGR Biogeosciences 2024, 129, e2023JG007851. [Google Scholar] [CrossRef]
- Lu, C.; Xiu, W.; Yang, B.; Zhang, H.; Lian, G.; Zhang, T.; Bi, E.; Guo, H. Natural Attenuation of Groundwater Uranium in Post-Neutral-Mining Sites Evidenced from Multiple Isotopes and Dissolved Organic Matter. Environ. Sci. Technol. 2024, 58, 12674–12684. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848-2017[S/OL]; Groundwater Quality Standard. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China. Standards Press of China: Beijing, China, 2017.
- World Health Organization. Guidelines for Drinking-Water Quality. World Health Organ. 2011, 216, 303–304. [Google Scholar]
- Xia, Q.; He, J.; He, B.; Chu, Y.; Li, W.; Sun, J.; Wen, D. Effect and genesis of soil nitrogen loading and hydrogeological conditions on the distribution of shallow groundwater nitrogen pollution in the North China Plain. Water Res. 2023, 243, 120346. [Google Scholar] [CrossRef]
- Chen, Y.; Su, X.; Wan, Y.; Lyu, H.; Dong, W.; Shi, Y.; Zhang, Y. Quantifying the effect of the nitrogen biogeochemical processes on the distribution of ammonium in the riverbank filtration system. Environ. Res. 2023, 216, 114358. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Du, R.; Cao, S.; Berry, M.; Peng, Y. Tracing and utilizing nitrogen loss in wastewater treatment: The trade-off between performance improvement, energy saving, and carbon footprint reduction. J. Environ. Manag. 2024, 349, 119525. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhou, Q.; Li, Y.; Wu, W. Application of manganese oxides in wastewater treatment: Biogeochemical Mn cycling driven by bacteria. Chemosphere 2023, 336, 139219. [Google Scholar] [CrossRef] [PubMed]
- Abedi Koupai, J.; Fatahizadeh, M.; Mosaddeghi, M.R. Effect of pore water pH on mechanical properties of clay soil. Bull. Eng. Geol. Environ. 2020, 79, 1461–1469. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Xing, S.; Huang, F.-Y.; Xiu, W.; Rensing, C.; Zhao, Y.; Guo, H. Metabolic coupling of arsenic, carbon, nitrogen, and sulfur in high arsenic geothermal groundwater: Evidence from molecular mechanisms to community ecology. Water Res. 2024, 249, 120953. [Google Scholar] [CrossRef] [PubMed]
- Nortjé, G.P.; Laker, M.C. Factors That Determine the Sorption of Mineral Elements in Soils and Their Impact on Soil and Water Pollution. Minerals 2021, 11, 821. [Google Scholar] [CrossRef]
- McMahon, P.B.; Belitz, K.; Reddy, J.E.; Johnson, T.D. Elevated Manganese Concentrations in United States Groundwater, Role of Land Surface–Soil–Aquifer Connections. Environ. Sci. Technol. 2019, 53, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Moore, O.C.; Xiu, W.; Guo, H.; Polya, D.A.; Van Dongen, B.E.; Lloyd, J.R. The role of electron donors in arsenic-release by redox-transformation of iron oxide minerals—A review. Chem. Geol. 2023, 619, 121322. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Z.; Fu, Y.; Zhao, L.; Ren, Y.; Nan, T.; Guo, H. Prediction of arsenic and fluoride in groundwater of the North China Plain using enhanced stacking ensemble learning. Water Res. 2024, 259, 121848. [Google Scholar] [CrossRef]
- Li, P.; Sabarathinam, C.; Elumalai, V. Groundwater pollution and its remediation for sustainable water management. Chemosphere 2023, 329, 138621. [Google Scholar] [CrossRef]
- Xu, F.; Li, P. Biogeochemical mechanisms of iron (Fe) and manganese (Mn) in groundwater and soil profiles in the Zhongning section of the Weining Plain (northwest China). Sci. Total Environ. 2024, 939, 173506. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yuan, Z.; Su, X. Spatiotemporal distribution patterns of iron, manganese, and arsenic within the river infiltration zone and the potential geochemical activity at key interfaces. Appl. Geochem. 2024, 172, 106123. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, G.; Wang, H.; Shrestha, S.; Xue, B.; Sun, W.; Yu, J. Macrozoobenthos variations in shallow connected lakes under the influence of intense hydrologic pulse changes. J. Hydrol. 2020, 584, 124755. [Google Scholar] [CrossRef]
- Dippong, T.; Mihali, C.; Năsui, D.; Berinde, Z.; Butean, C. Assessment of Water Physicochemical Parameters in the Strîmtori-Firiza Reservoir in Northwest Romania. Water Environ. Res. 2018, 90, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Deng, Y.; Ma, T.; Xu, Y.; Tao, Y.; Huang, Y.; Liu, R.; Wang, Y. Enrichment of Geogenic Ammonium in Quaternary Alluvial–Lacustrine Aquifer Systems: Evidence from Carbon Isotopes and DOM Characteristics. Environ. Sci. Technol. 2020, 54, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Song, X.; Lu, Y.; Liang, S.; Liu, G. Fluoride enrichment mechanisms and related health risks of groundwater in the transition zone of geomorphic units, northern China. Environ. Res. 2022, 212, 113588. [Google Scholar] [CrossRef] [PubMed]
- Champ, D.R.; Gulens, J.; Jackson, R.E. Oxidation–reduction sequences in ground water flow systems. Can. J. Earth Sci. 1979, 16, 12–23. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Environmental Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996. [Google Scholar]
- Li, Y.; Liu, Y.; Feng, L.; Zhang, L. A review: Manganese-driven bioprocess for simultaneous removal of nitrogen and organic contaminants from polluted waters. Chemosphere 2023, 314, 137655. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, Q.; Sheng, Y.; Chen, C.; Yu, G.; Kappler, A. Coupled iron cycling and organic matter transformation across redox interfaces. Nat. Rev. Earth Environ. 2023, 4, 659–673. [Google Scholar] [CrossRef]
- Hu, B.; Teng, Y.; Zhang, Y.; Zhu, C. Review: The projected hydrologic cycle under the scenario of 936 ppm CO2 in 2100. Hydrogeol. J. 2019, 27, 31–53. [Google Scholar] [CrossRef]
- Xin, J.; Liu, Y.; Chen, F.; Duan, Y.; Wei, G.; Zheng, X.; Li, M. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019, 165, 114977. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Han, Y.; Han, X.; Chen, Y.; Zhai, Y. Sewage Vertical Infiltration Introduced Polygenic Multipollutants into Groundwater. Water 2024, 16, 2305. https://doi.org/10.3390/w16162305
Dong Y, Han Y, Han X, Chen Y, Zhai Y. Sewage Vertical Infiltration Introduced Polygenic Multipollutants into Groundwater. Water. 2024; 16(16):2305. https://doi.org/10.3390/w16162305
Chicago/Turabian StyleDong, Yihan, Yifan Han, Xu Han, Yaoxuan Chen, and Yuanzheng Zhai. 2024. "Sewage Vertical Infiltration Introduced Polygenic Multipollutants into Groundwater" Water 16, no. 16: 2305. https://doi.org/10.3390/w16162305
APA StyleDong, Y., Han, Y., Han, X., Chen, Y., & Zhai, Y. (2024). Sewage Vertical Infiltration Introduced Polygenic Multipollutants into Groundwater. Water, 16(16), 2305. https://doi.org/10.3390/w16162305













