A Novel Screening Method of Surfactants for Promoting the Static Imbibition of Shale
Abstract
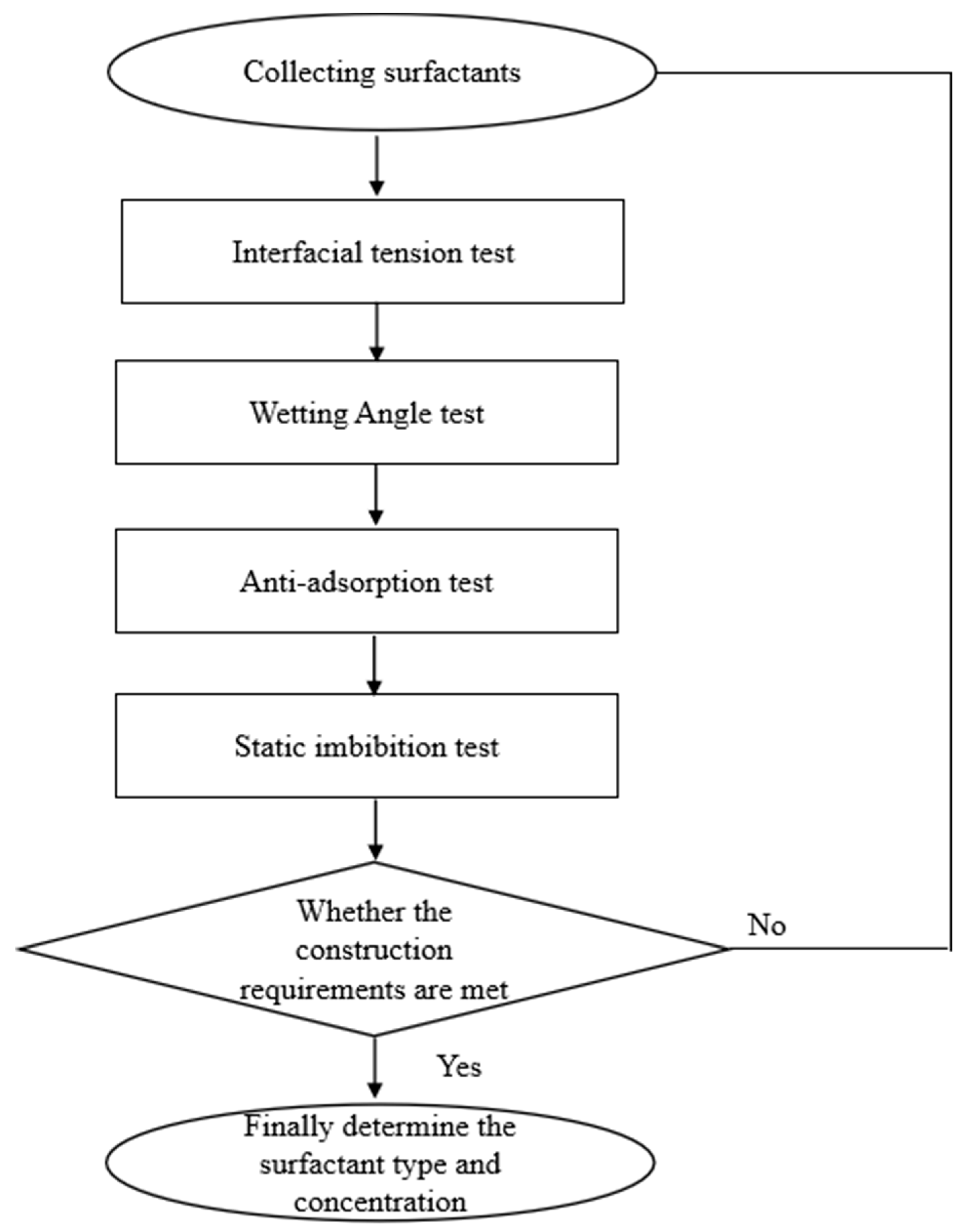
:1. Introduction
2. Materials and Methods
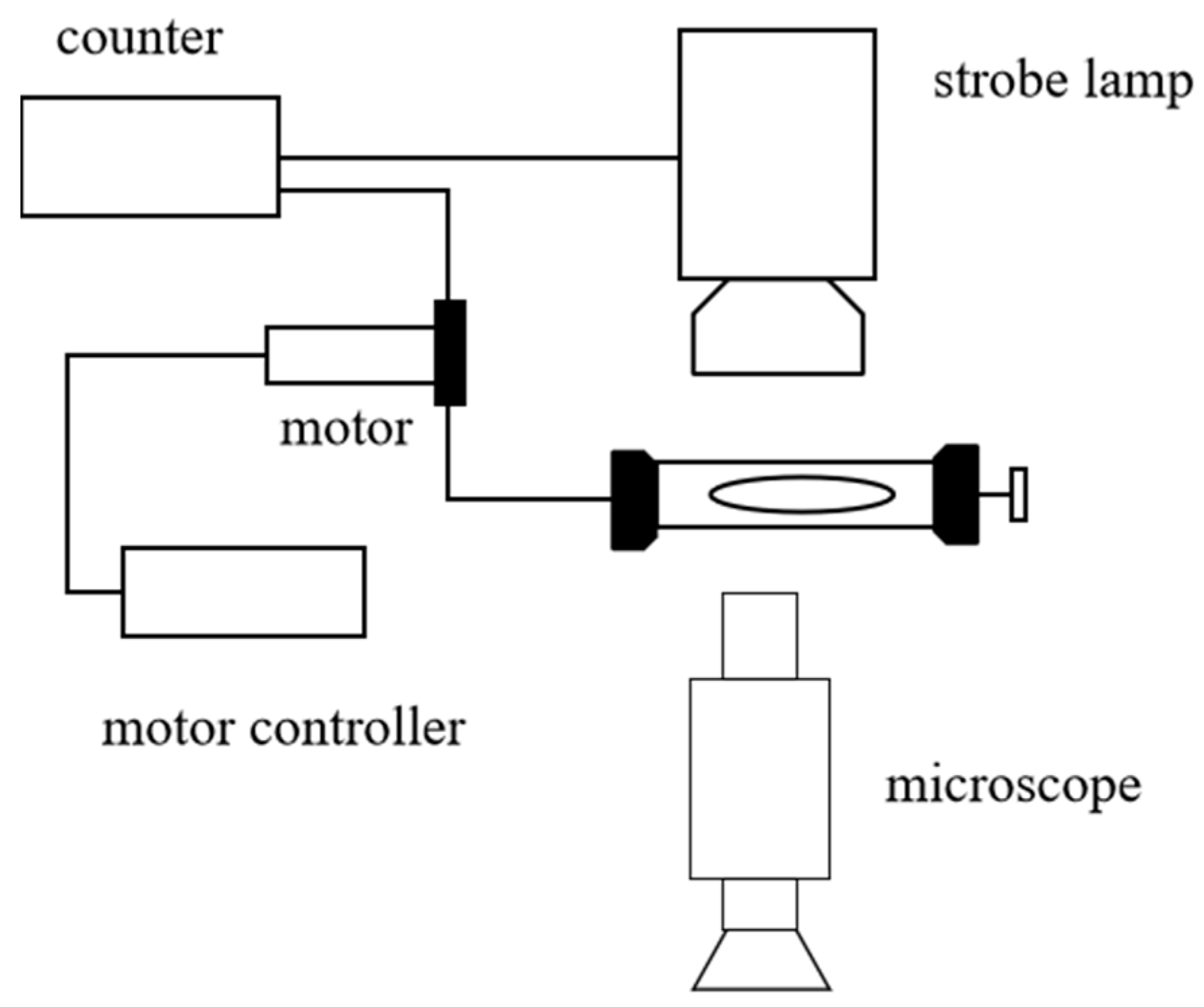
2.1. Experimental Apparatus and Materials
2.2. Experimental Steps and Methods
2.2.1. Interfacial Tension Experiment
2.2.2. Contact Angle Experiment
2.2.3. Anti-Adsorption Experiment
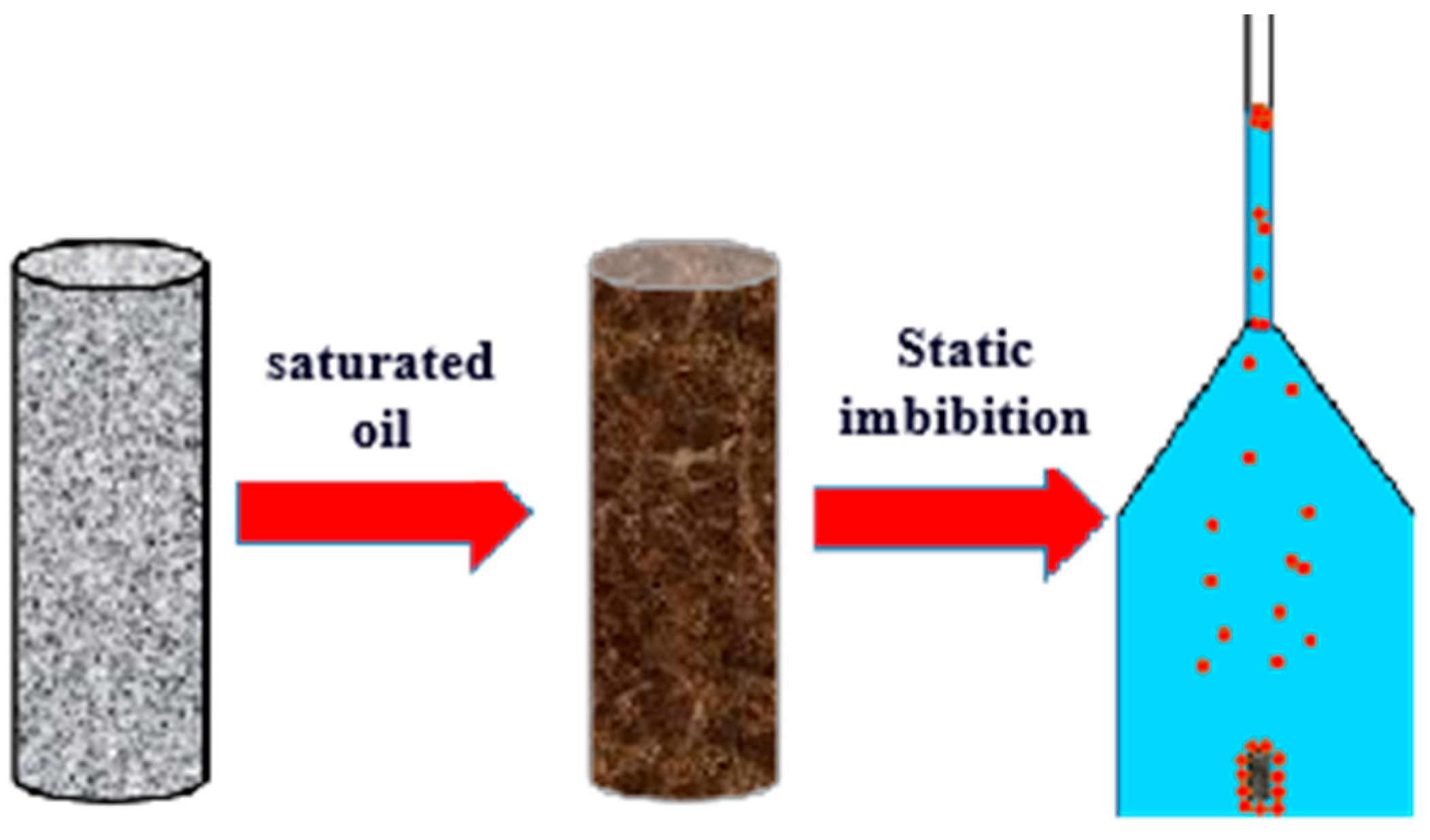
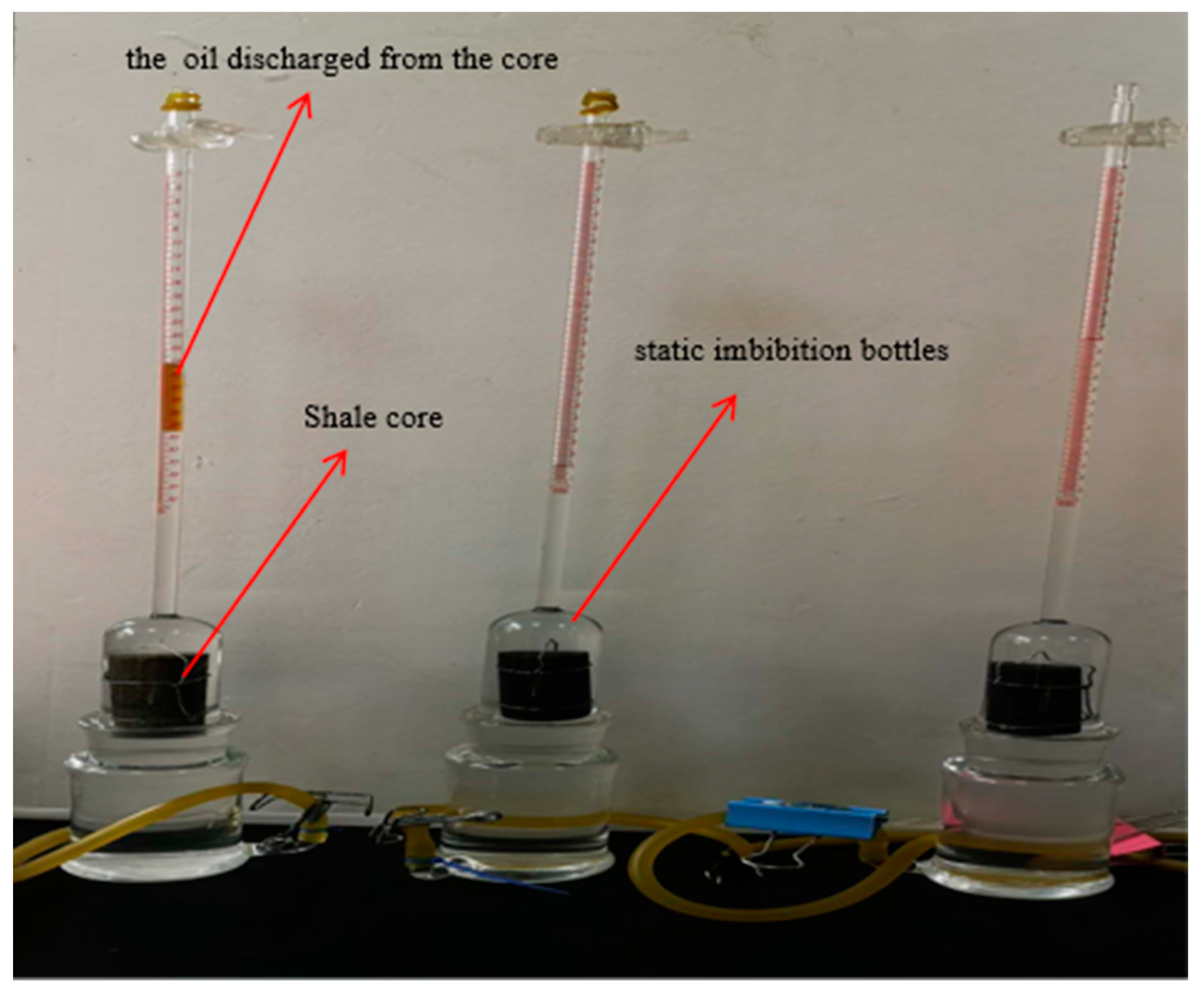
2.2.4. Static Imbibition Experiment
3. Results and Discussion
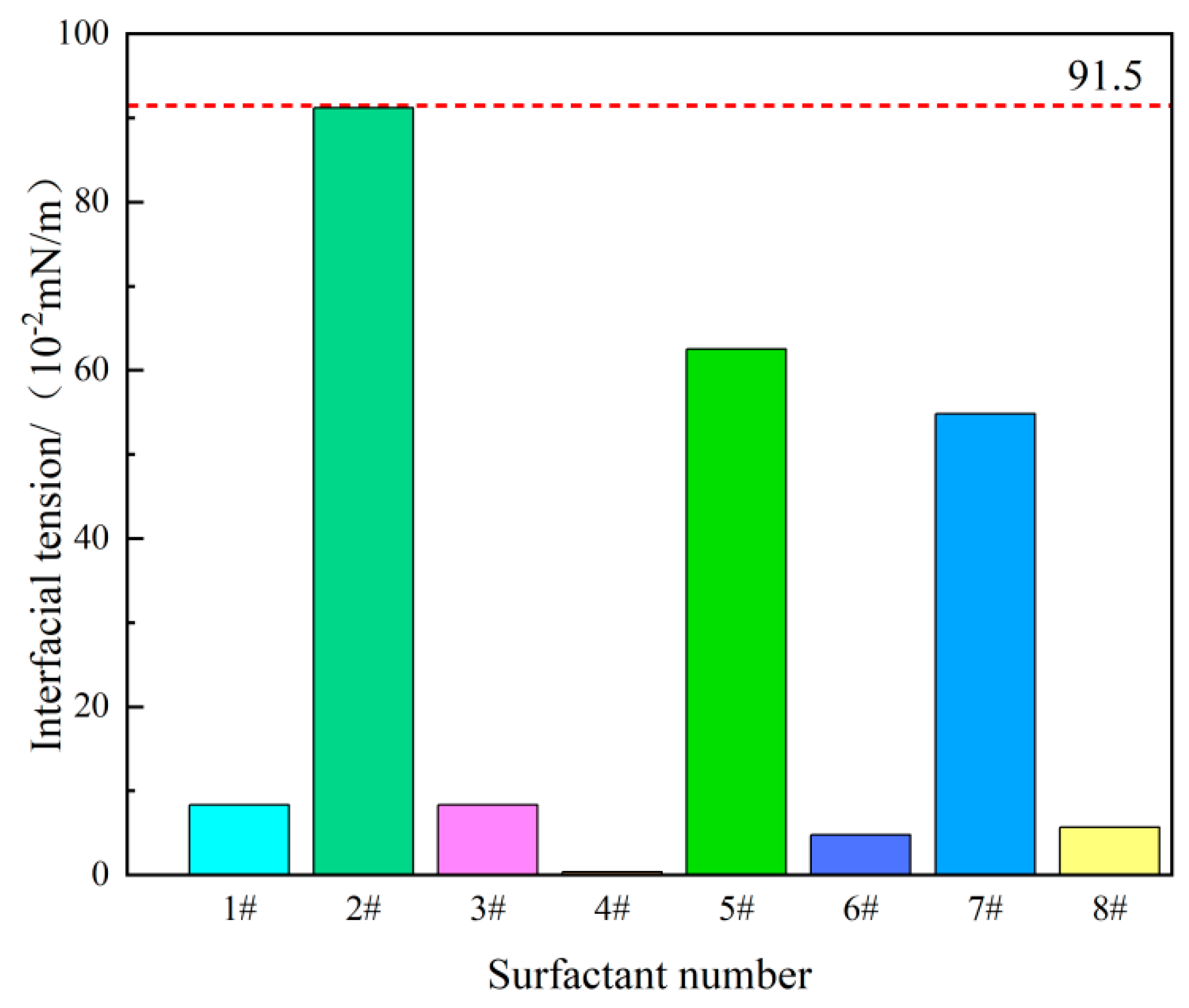
3.1. Experimental Results of Interfacial Tension
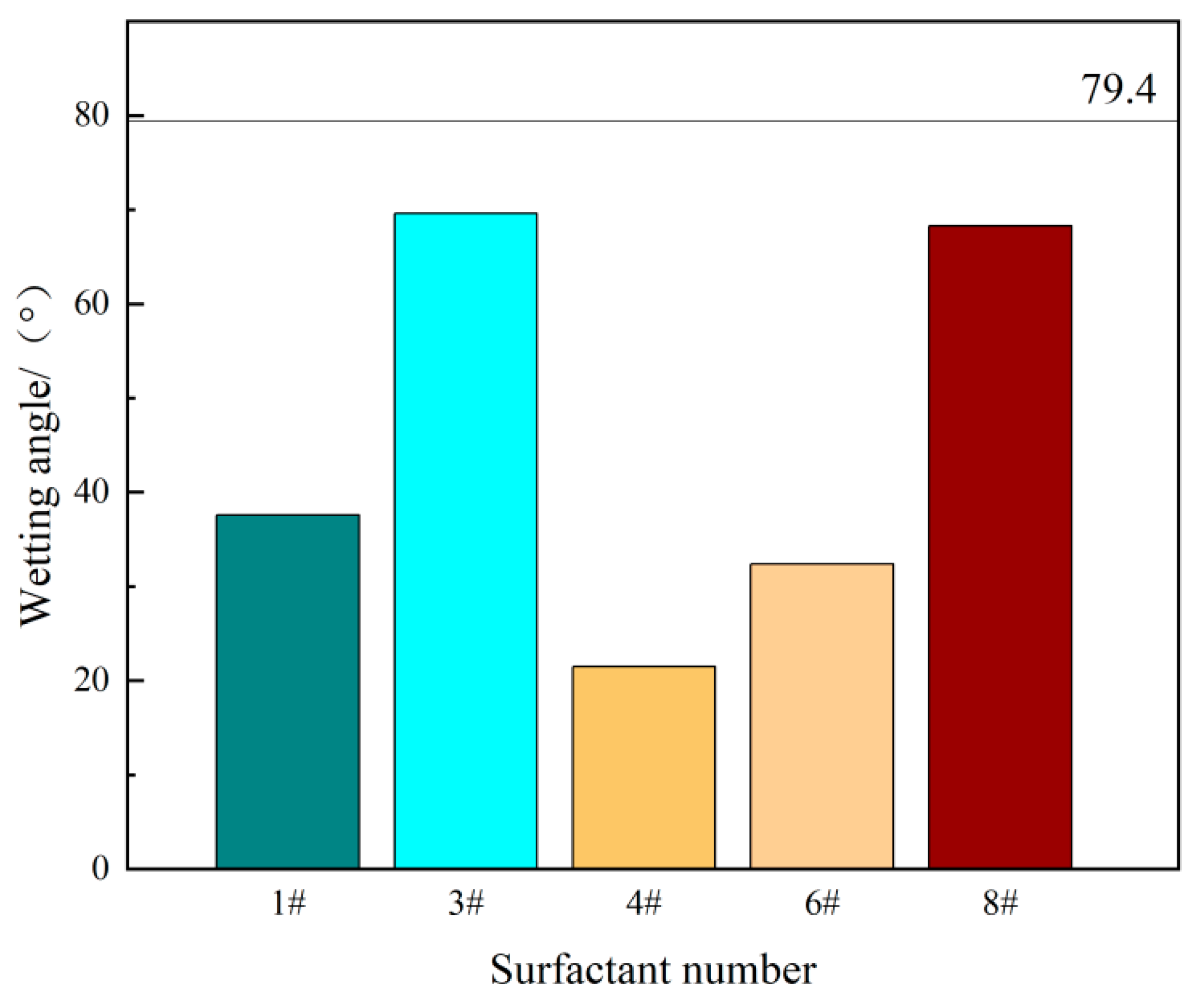
3.2. Contact Angle Experimental Results
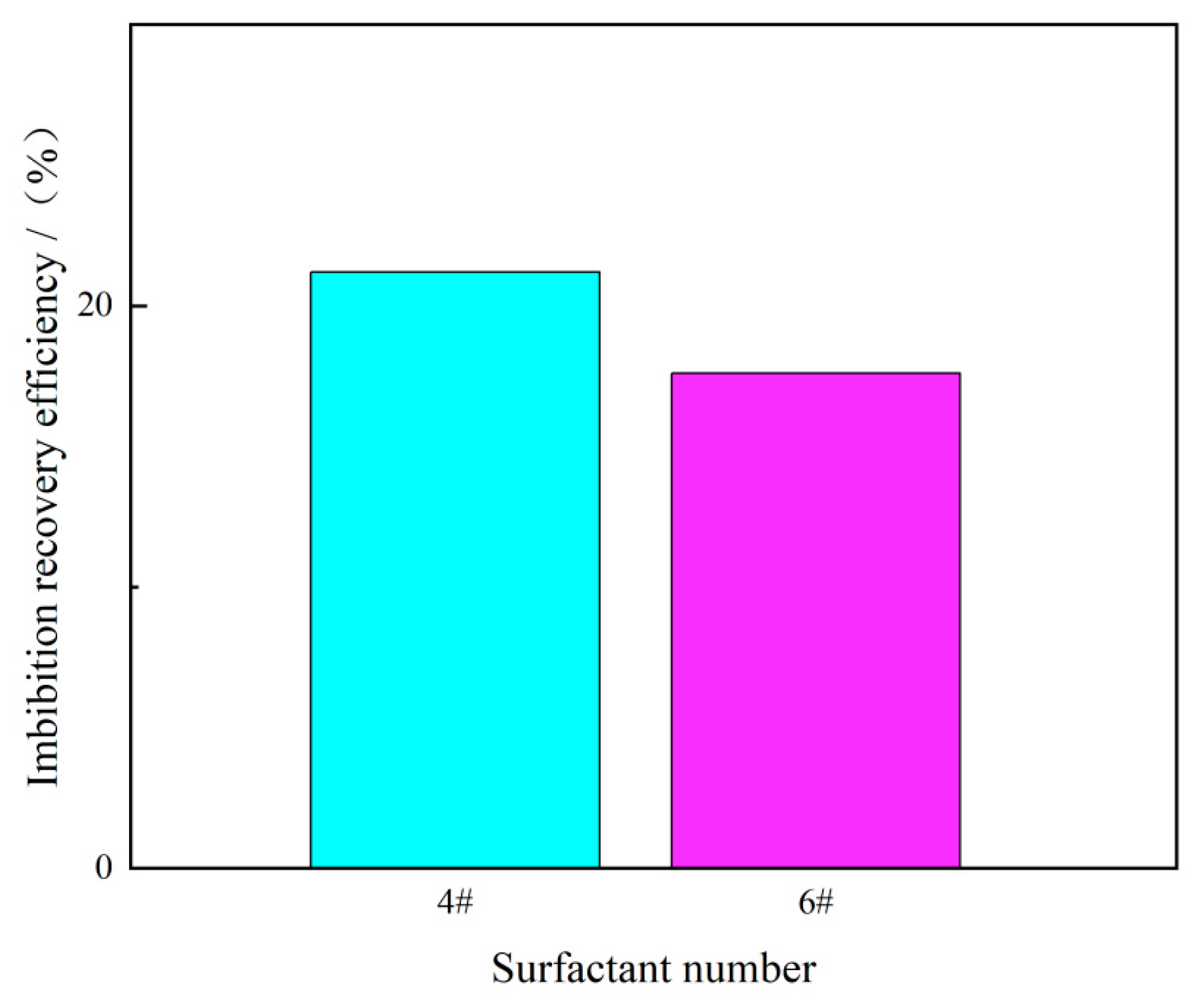
3.3. Anti-Adsorption Experimental Results
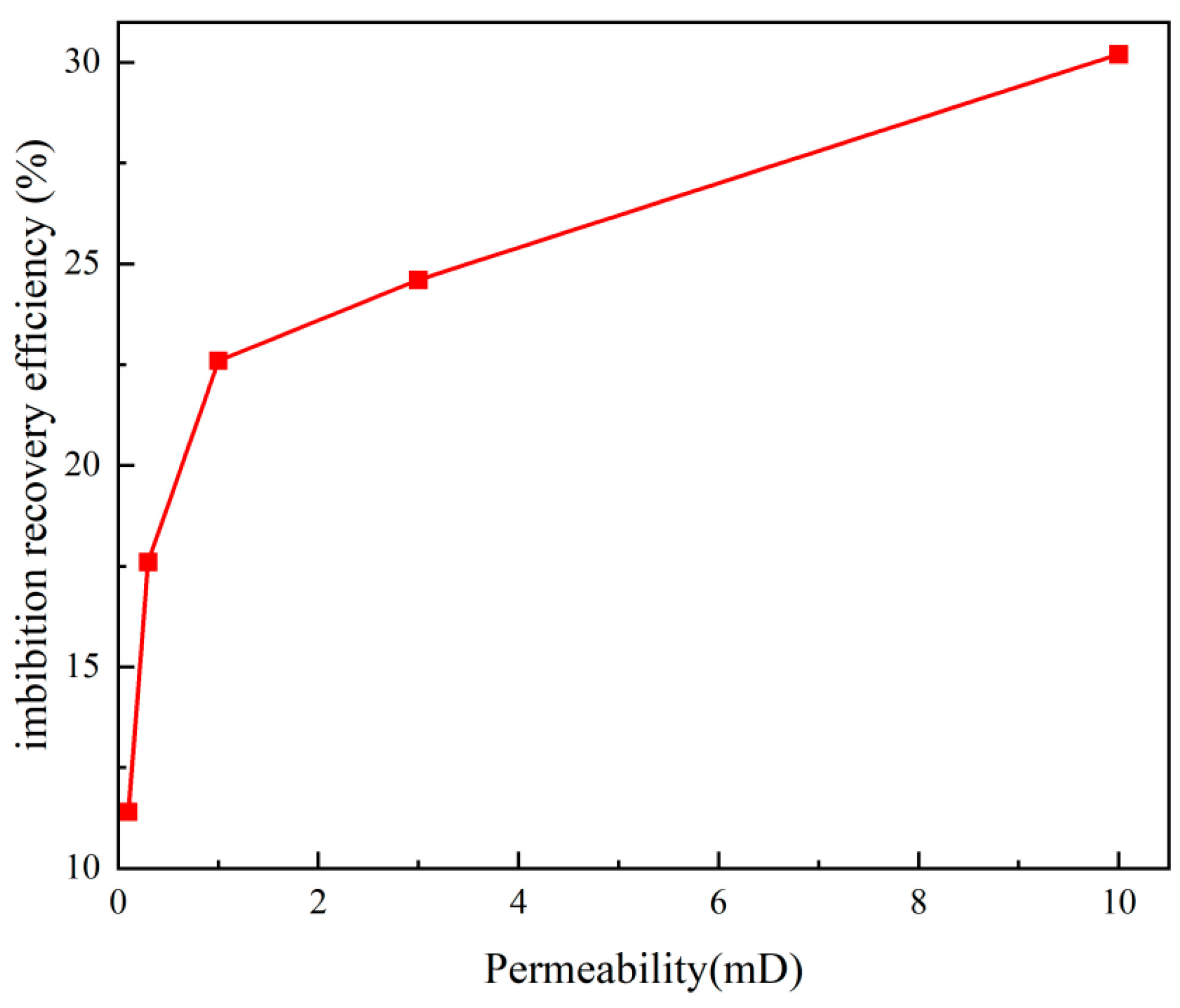
3.4. Experimental Results of Static Imbibition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.Y.; Pu, X.Y.; Zhang, L.H. Beneficial development of shale gas in China: Theoretical logic, practical logic and prospect. Nat. Gas Ind. 2023, 43, 177–183. [Google Scholar]
- Xu, Y.; Lun, Z.; Pan, Z. Occurrence space and state of shale oil: A review. J. Pet. Sci. Eng. 2022, 211, 110183. [Google Scholar] [CrossRef]
- Sun, X.; Yao, D.; Qu, J. A novel transient hole cleaning algorithm for horizontal wells based on drift-flux model. Geoenergy Sci. Eng. 2024, 233, 212517. [Google Scholar] [CrossRef]
- Gao, M.; Yang, M.; Lu, Y.; Levin, V. Mechanical characterization of uniaxial compression associated with lamination angles in shale. Adv. Geo-Energy Res. 2024, 13, 56–68. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Liu, Y. Mechanism of low chemical agent adsorption by high pressure for hydraulic fracturing-assisted oil displacement technology: A study of molecular dynamics combined with laboratory experiments. Langmuir 2023, 39, 16628–16636. [Google Scholar] [CrossRef]
- Zhu, H.J.; Huang, C.; Ju, Y.W.; Bu, H.L. Multi-scale multidimensional characterization of clay-hosted pore networks of shale using FIBSEM, TEM, and X-raymicro-tomography: Implications for methane storage and migration. Appl. Clay Sci. 2021, 213, 106239. [Google Scholar] [CrossRef]
- An, J.; Tang, M.R.; Cao, Z.X. Transformation of development model of horizontal wells in ultra-low permeability and low-pressure reservoirs. Lithol. Reserv. 2019, 31, 134–140. [Google Scholar]
- Zhao, H.; Kang, W.; Yang, H.; Zhang, H.; Zhu, T.; Wang, F.; Li, X.; Zhou, B.; Sarsenbekuly, B.; Aidarova, S.; et al. Imbibition enhancing oil recovery mechanism of the two surfactants. Phys. Fluids 2020, 32, 047103. [Google Scholar] [CrossRef]
- Habibi, A.; Esparza, Y.; Boluk, Y.; Dehghanpour, H. Enhancing imbibition oil recovery from tight rocks by mixing nonionic surfactants. Energy Fuels 2020, 34, 12301–12313. [Google Scholar] [CrossRef]
- Aronofsky, J.S.; Masse, L.; Natanson, S.G. A model for the mechanism of oil recovery from the porous matrix due to water invasion in fractured reservoirs. Trans. AIME 1958, 213, 17–19. [Google Scholar] [CrossRef]
- Graham, J.W.; Richardson, J.G. Theory and application of imbibition phenomena in recovery of oil. J. Pet. Technol. 1959, 11, 65–69. [Google Scholar] [CrossRef]
- Mattax, C.C.; Calvin, C.; Kyte, J.R. Imbibition oil recovery from fractured, water-drive reservoir. Soc. Pet. Eng. J. 1962, 2, 177–184. [Google Scholar] [CrossRef]
- Yang, Z.M.; Zhu, W.Y.; Chen, Q. Imbibition mechanism and mathematical model of low permeability fractured sandstone reservoir. J. Jianghan Pet. Inst. 2001, 23, 25–33. [Google Scholar]
- Mason, G.; Fernø, M.A.; Haugen, A.; Morrow, N.R. Spontaneous counter-current imbibition outwards from a hemi-spherical depression. J. Pet. Sci. Eng. 2012, 90, 131–138. [Google Scholar] [CrossRef]
- Wu, R.T.; Yang, S.L.; Xie, J.Y. Experiment and mechanism of spontaneous imbibition of matrix core in tight oil-gas reservoirs. Pet. Geol. Recovery Effic. 2017, 24, 98–104. [Google Scholar]
- Ashraf, S.; Visavale, G.; Phirani, J. Spontaneous imbibition in randomly arranged interacting capillaries. Chem. Eng. Sci. 2018, 192, 218–234. [Google Scholar] [CrossRef]
- Liu, Q.; Song, R.; Liu, J.; Lei, Y.; Zhu, X. Pore-scale visualization and quantitative analysis of the spontaneous imbibition based on experiments and micro-CT technology in low-permeability mixed-wettability rock. Energy Sci. Eng. 2020, 8, 1840–1856. [Google Scholar] [CrossRef]
- Patel, K.R.; Mehta, M.N.; Patel, T.R. A mathematical model of imbibition phenomenon in heterogeneous porous media during secondary oil recovery process. Appl. Math. Model. 2013, 37, 2933–2942. [Google Scholar] [CrossRef]
- Andersen, P.Ø.; Evje, S.; Kleppe, H. A model for spontaneous imbibition as a mechanism for oil recovery in fractured reservoirs. Transp. Porous Media 2014, 101, 299–331. [Google Scholar] [CrossRef]
- Pathak, S.; Singh, T. A mathematical modelling of imbibition phenomenon in inclined homogenous porous media during oil recovery process. Perspect. Sci. 2016, 8, 183–186. [Google Scholar] [CrossRef]
- Handy, L.L. Determination of effective capillary pressures for porous media from imbibitiondata. Trans. AIME 1960, 219, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, C.J.; Liu, S.X. Experimental study and digital core simulation on dynamic imbibition mechanism of low permeability reservoir. Sci. Technol. Eng. 2021, 21, 1789–1794. [Google Scholar]
- Wang, X.; Sheng, J.J. Spontaneous imbibition analysis in shale reservoirs based on pore network modeling. J. Pet. Sci. Eng. 2018, 169, 663–672. [Google Scholar] [CrossRef]
- Kathel, P.; Mohanty, K.K. Dynamic surfactant-aided imbibition in fractured oil-wet carbonates. J. Pet. Sci. Eng. 2018, 170, 898–910. [Google Scholar] [CrossRef]
- Jadhunandan, P.P.; Morrow, N.R. Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv. Eng. 1995, 10, 40–46. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.L.; Xie, Y.G.; Ni, J.; Li, T. Imbibition and oil recovery mechanism of fracturing fluids in tight sandstone reservoirs. ACS Omega 2021, 6, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, E.; Mirzaei-Paiaman, A.; Masihi, M.; Harimi, B. Experimental study of some important factors on nonwetting phase recovery by cocurrent spontaneous imbibition. J. Nat. Gas Sci. Eng. 2015, 27, 1213–1228. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Wang, J. A critical review on fundamental mechanisms of spontaneous imbibition and the impact of boundary condition, fluid viscosity and wettability. Adv. Geo-Energy Res 2017, 1, 1–17. [Google Scholar] [CrossRef]
- Abd, A.S.; Elhafyan, E.; Siddiqui, A.R.; Alnoush, W.; Blunt, M.J. A review of the phenomenon of counter-current spontaneous imbibition: Analysis and data interpretation. J. Pet. Sci. Eng. 2019, 180, 456–470. [Google Scholar] [CrossRef]
- Tian, W.; Wu, K.; Gao, Y.; Chen, Z. A critical review of enhanced oil recovery by imbibition: Theory and practice. Energy Fuels 2021, 35, 5643–5670. [Google Scholar] [CrossRef]
- Ma, Q.; Zhu, W.; Bu, W.; Song, Z. Pore-scale imbibition comparisons between capillary and gravity forces reveal distinct drainage mechanisms and residual oil distributions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129981. [Google Scholar] [CrossRef]
- Fernø, M.A.; Grønsdal, R.; Asheim, J.; Nyheim, A. Use of sulfate for water based enhanced oil recovery during spontaneous imbibition in chalk. Energy Fuels 2011, 25, 1697–1706. [Google Scholar] [CrossRef]
- Sun, L.; Pu, W.F.; Xin, J.; Wu, Y.L. Influence of surfactant on high temperature imbibition of low permeability cores. J. China Univ. Pet. 2012, 36, 103–107. [Google Scholar]
- Chen, Z.S.; Wang, P.P.; Wu, Y.P.; Cheng, X.Y. The test feasibility of the second cycle of water swallowing spitting injection oil production of An 83 horizontal well. Petrochem. Ind. Appl. 2015, 34, 53–56. [Google Scholar]
- Xiang, Y.; Xiang, D.; Du, W.B. The application study of surfactants in water injection and intermittent production for a single oil well. Chem. Eng. Oil Gas 2003, 32, 102–103. [Google Scholar]
- Wei, F.L.; Yue, X.A.; Du, Z.J.H. The Influence of Surfactants on Suface Wettability and Spontaneous lmbibition of Water into Oil Wet Low Permeable Limestone Cores. Oilfield Chem. 2004, 21, 52–56. [Google Scholar]
- Chen, H.L.; Lucas, L.R.; Nogaret, L.A.D.; Yang, H.D. Laboratory monitoring of surfactant imbibition with computerized tomography. SPE Reserv. Eval. Eng. 2001, 4, 16–25. [Google Scholar] [CrossRef]
- Li, T.; Li, X.F.; Yang, Z. Laboratory study on fracturing fluid system with imbibition oil displacement effect. Petro Chem. Ind. Technol. 2018, 25, 307–308. [Google Scholar]
- Wu, X.M.; Chen, Y.N. Feasibility experimental investigation on the flowback liquid of a clear fracturing fluid with high performance for displacement of reservoir oil. Sci. Technol. Eng. 2017, 17, 245–250. [Google Scholar]
- Han, D.; Peng, Y.Q.; Guo, S.P. Imbibition behavior of surfactant in water-wet sandstone and its effects on recovery efficiency. J. China Univ. Pet. 2009, 33, 142–147. [Google Scholar]
- Hou, B.F.; Ye-Wang, F.W.; Huang, Y. Study of spontaneous imbibition of water by oil-wet sandstone cores using different surfactants. J. Dispers. Sci. Technol. 2015, 36, 1264–1273. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Wang, X. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. [Google Scholar] [CrossRef]
- Standnes, D.C.; Austad, T. Wettability alteration in carbonates: Low-cost ammonium surfactants based on bio-derivatives from the coconut palm as active chemicals to change the wettability form oil-wet to water-wet conditions. Colloids Surf. A Physicochem. Eng. Asp. 2003, 218, 161–173. [Google Scholar] [CrossRef]
- Fan, H.B.; Xue, X.J.; Li, K.; Zhou, X.Q. Development and application of flooding surfactant fracturing fluid. Chem. Eng. Oili Gas 2019, 48, 74–79. [Google Scholar]
- Zhang, Z.S. Multifunction surfactant oil-displacing fracturing fluid system suitable for tight sandstone reservoirs. Pet. Geol. Oilfield Dev. Daqing 2020, 39, 169–173. [Google Scholar]
- Tang, W.; Zou, C.; Peng, H.; Wang, Y. Influence of nanoparticles and surfactants on stability and rheological behavior of polymeric nanofluids and the potential applications in fracturing fluids. Energy Fuels 2016, 35, 8657–8671. [Google Scholar] [CrossRef]
- Baruah, A.; Pathak, A.K.; Ojha, K. Study on rheology and thermal stability of mixed (nonionic–anionic) surfactant based fracturing fluids. AIChE J. 2016, 62, 2177–2187. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H. High performance clean fracturing fluid using a new tricationic surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef]
- Pu, W.F.; Du, D.J.; Liu, R. Preparation and evaluation of supramolecular fracturing fluid of hydrophobically associative polymer and viscoelastic surfactant. J. Pet. Sci. Eng. 2018, 167, 568–576. [Google Scholar] [CrossRef]
- Wang, J.R.; Yang, S.L.; Cao, Y.J.; Wang, M.Y.; Yu, J.Y. Imbibition Mechanism of Tight Oil Cores and Experiments of Surfactants Enhancing Oil Recovery. Sci. Technol. Eng. 2020, 20, 1044–1050. [Google Scholar]





| Surfactant Types | Advantage | Disadvantage |
|---|---|---|
| Anionic surfactants | They have high interfacial activity, excellent temperature resistance, less formation adsorption, and a low price. | They have poor salt tolerance and are not resistant to divalent cations, including calcium and magnesium. |
| Nonionic surfactants | They have good salt resistance, good multivalent cation resistance, and many kinds of cations. | They have cloud points, poor stability, poor temperature resistance, high adsorption capacity, and a high price. |
| Anionic–nonionic amphoteric surfactants | They have good temperature resistance and salt tolerance, greatly reduce the chromatographic separation effect between anion and nonionic compounds, and have good compatibility. | They have less variety and a high price. |
| Polymeric surfactants | They have the role of decreasing interfacial tension and increasing viscosity, the adsorption capacity is small, and they have good temperature and salt resistance. | They have less variety and a high price. |
| Core Number | Length/cm | Diameter/cm | Permeability/mD |
|---|---|---|---|
| 1 | 5.0 | 2.5 | 1.01 |
| 2 | 5.0 | 2.5 | 1.05 |
| 3 | 5.0 | 2.5 | 1.02 |
| 4 | 5.0 | 2.5 | 1.01 |
| 5 | 5.0 | 2.5 | 0.1 |
| 6 | 5.0 | 2.5 | 0.3 |
| 7 | 5.0 | 2.5 | 3 |
| 8 | 5.0 | 2.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Yuan, Y.; Qu, J.; Chen, Y.; Sun, S.; He, Y. A Novel Screening Method of Surfactants for Promoting the Static Imbibition of Shale. Water 2024, 16, 2298. https://doi.org/10.3390/w16162298
Hou Z, Yuan Y, Qu J, Chen Y, Sun S, He Y. A Novel Screening Method of Surfactants for Promoting the Static Imbibition of Shale. Water. 2024; 16(16):2298. https://doi.org/10.3390/w16162298
Chicago/Turabian StyleHou, Zhaokai, Yuan Yuan, Jingyu Qu, Ye Chen, Shihui Sun, and Ying He. 2024. "A Novel Screening Method of Surfactants for Promoting the Static Imbibition of Shale" Water 16, no. 16: 2298. https://doi.org/10.3390/w16162298
APA StyleHou, Z., Yuan, Y., Qu, J., Chen, Y., Sun, S., & He, Y. (2024). A Novel Screening Method of Surfactants for Promoting the Static Imbibition of Shale. Water, 16(16), 2298. https://doi.org/10.3390/w16162298






