Evaluating the Mechanisms and Efficiency of Johkasou Systems for Decentralized Domestic Effluent Treatment: A Review
Abstract
1. Introduction
2. Mechanism of Pollutant Removal in Johkasou
2.1. Organic Matter Removal Mechanism
2.1.1. Organic Matter Removal Pathway
2.1.2. Microbial Analysis in Organic Matter Removal
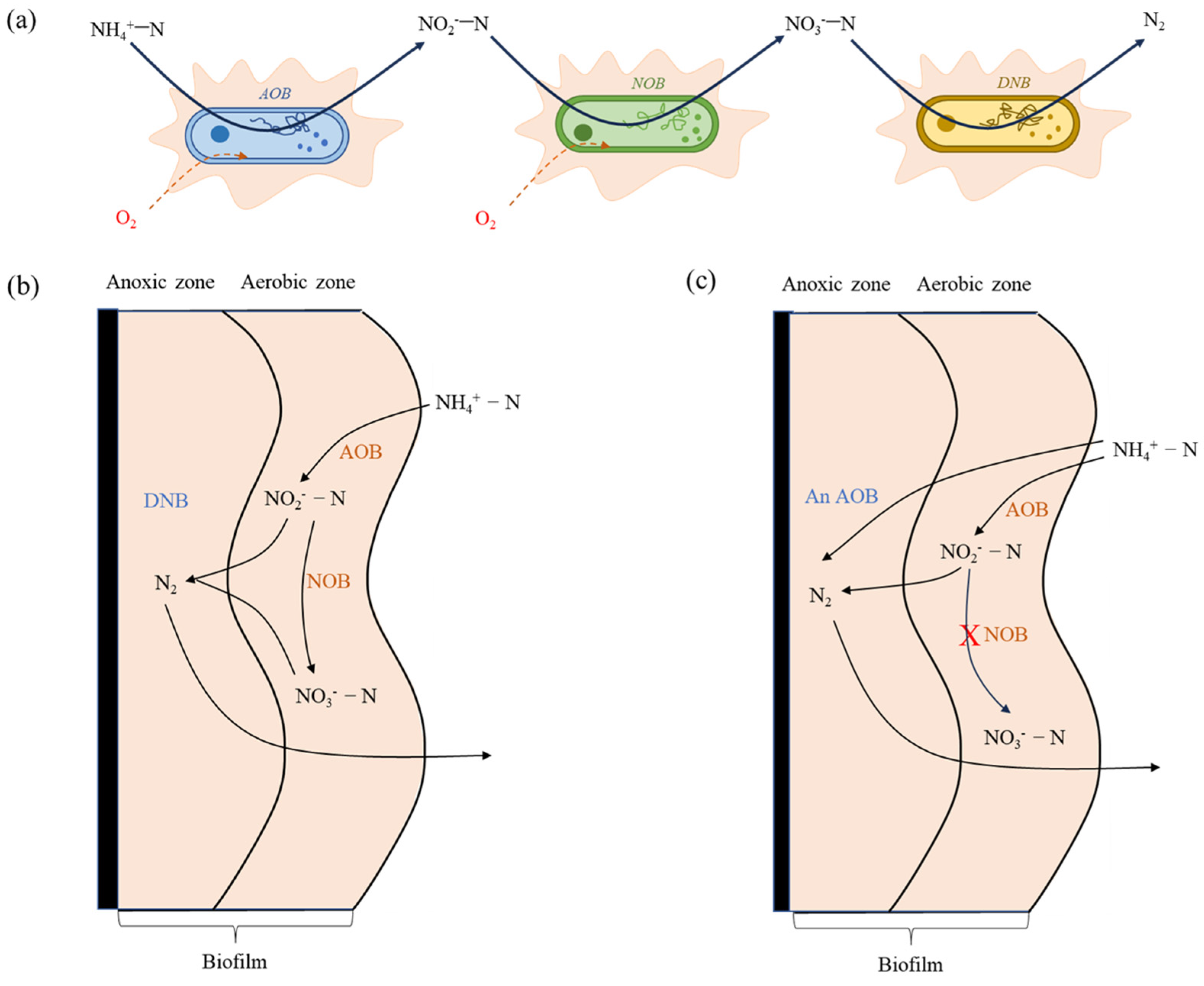
2.2. Nitrogen Removal Mechanism
2.2.1. Nitrogen Removal Pathway
→ 1.02 N2 + 0.26 NO3−-N + 0.066 CH2O0.5N0.15 + 2.03 H2O
2.2.2. Microbial Analysis in Denitrification
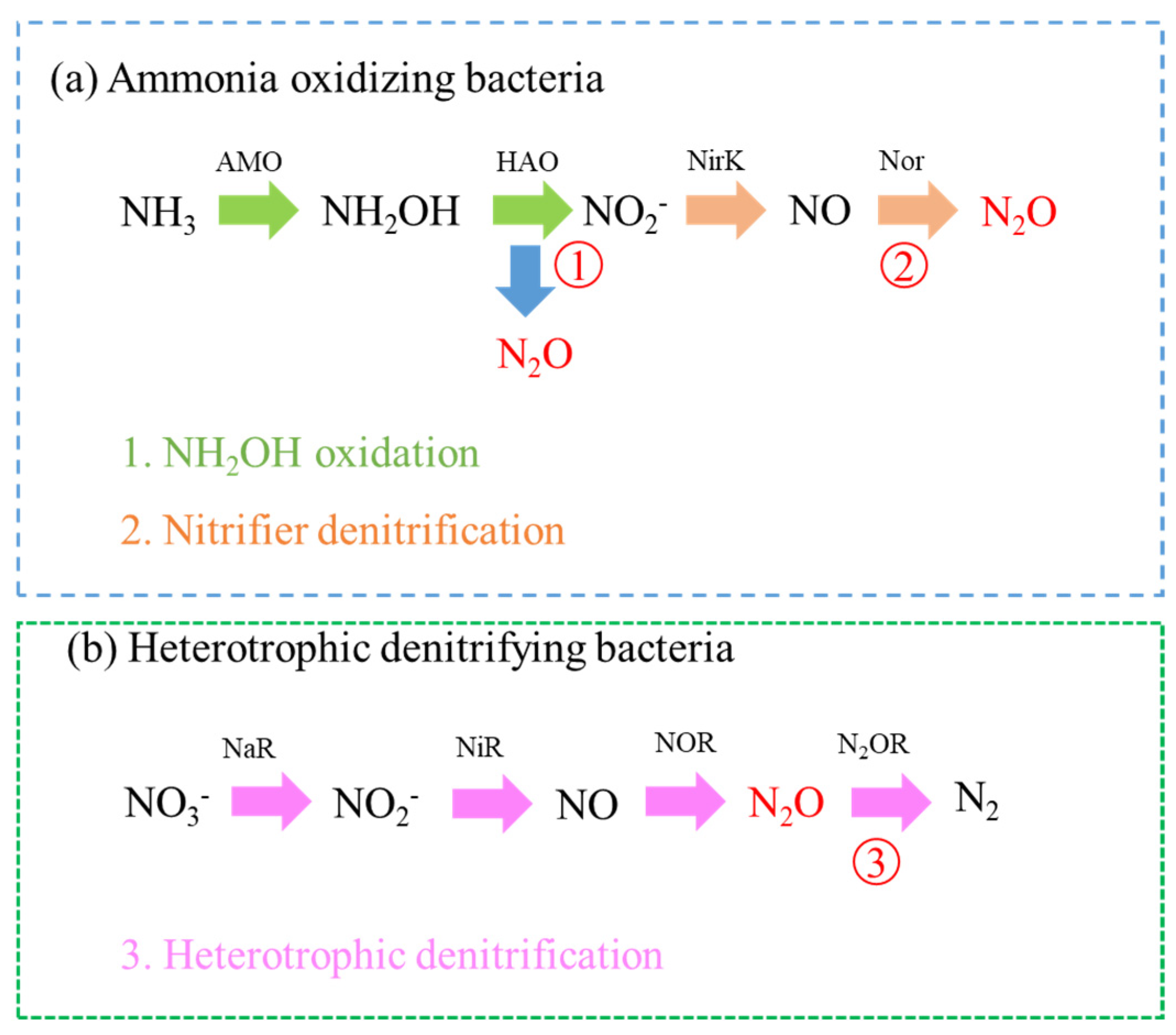
2.2.3. N2O Accumulation Problem
2.3. Phosphorus Removal Mechanism
2.3.1. Phosphorus Removal Pathway
2.3.2. Microbial Analysis in Phosphorus Removal
2.4. Comparison of Johkasou with Other Wastewater Treatment Processes
3. Influencing Factors of Nitrogen and Phosphorus Removal in the Johkasou Process
3.1. Filler
3.2. Effects of Operation Parameters
3.2.1. pH
3.2.2. Temperature
3.2.3. Dissolved Oxygen
3.2.4. Hydraulic Retention Time
3.2.5. Carbon Sources
4. Application of Johkasou in Domestic Sewage
4.1. Application of Conventional Johkasou
4.2. Application of Innovative Johkasou
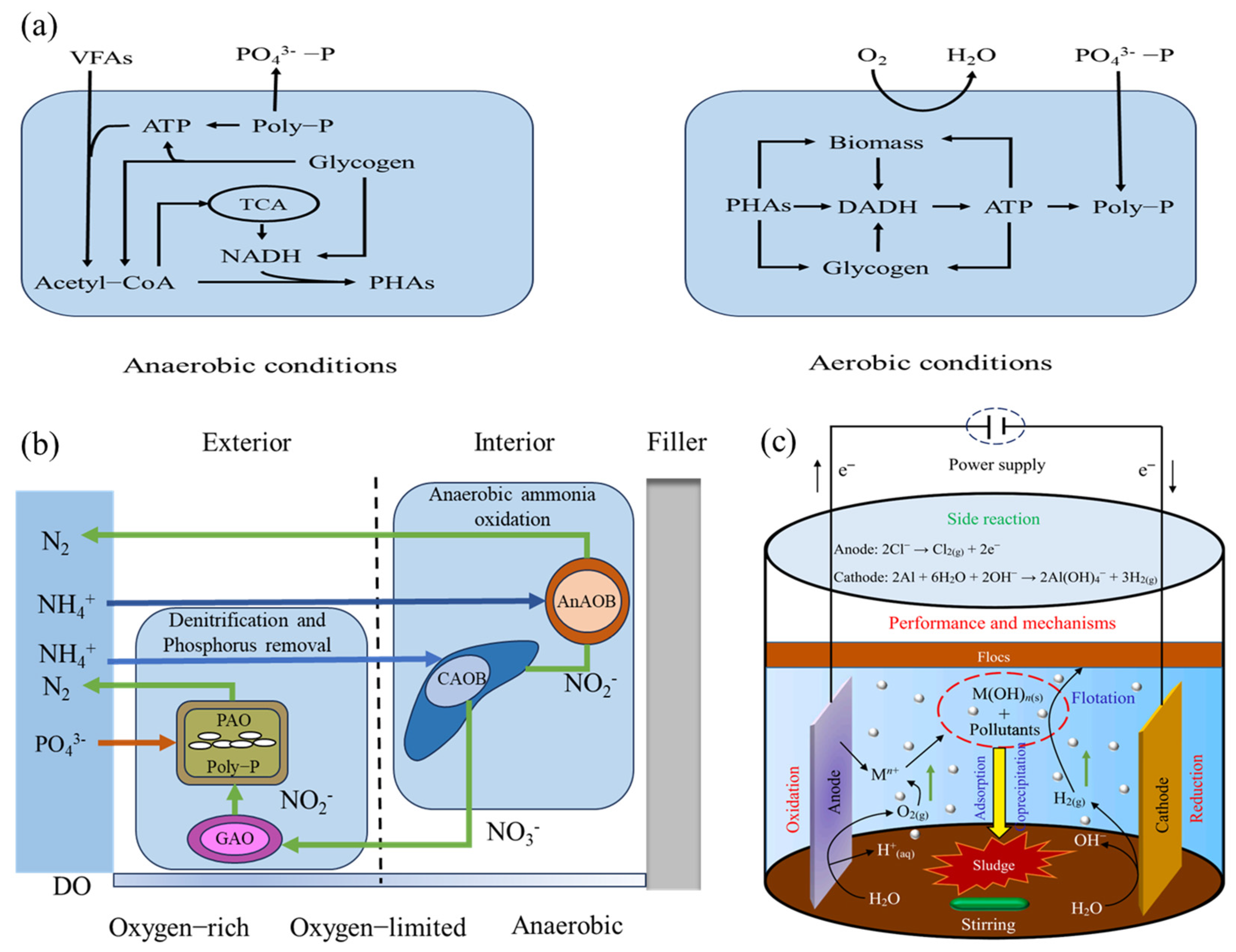
4.2.1. Combination of Johkasou and Ecological Treatment
4.2.2. Combination of Johkasou and Electrolysis
5. Conclusions
6. Prospects
- (1)
- Metabolomics analysis. This approach could provide detailed insights into the denitrification and phosphorus removal processes at the molecular level, elucidating microbial growth and metabolic pathways. Currently, metabolomics analysis in Johkasou systems is limited, and expanding this research could reveal critical functional genes and pathways involved in nutrient removal;
- (2)
- Microbial diversity analysis. Understanding the diversity of microbial communities, particularly phosphorus-accumulating organisms (PAOs), is crucial for optimizing phosphorus removal. While there is extensive research on the diversity of denitrifying bacteria, the diversity of PAOs in Johkasou systems remains underexplored. Future studies should prioritize identifying key microbial species involved in phosphorus removal;
- (3)
- N2O accumulation. While many studies report N2O accumulation, Johkasou systems effectively reduce N2O production through the anammox pathway. However, N2O emissions have been rarely addressed in Johkasou’s research, highlighting a need for future studies to investigate this issue;
- (4)
- Influencing actors. Carbon source availability is a primary constraint on nitrogen and phosphorus removal efficacy in Johkasou systems. While there is extensive research on the C/N ratio, studies on the C/P ratio are relatively lacking. Future research should focus more on the C/P ratio’s impact on removal processes;
- (5)
- Phosphorus Removal Enhancement. The limited and unstable phosphorus removal efficiency in Johkasou systems often necessitates combining them with advanced treatment processes. Combining Johkasou systems with electrolysis has proven to be an effective and cost-efficient method for enhancing phosphorus removal, particularly in real-world domestic wastewater treatment. This approach represents a promising area for future research;
- (6)
- The removal of sulfate from domestic wastewater is an important but under-addressed issue in Johkasou research. Future studies should focus on developing strategies to enhance sulfate removal in Johkasou systems, further improving their ability to meet environmental health standards.
Author Contributions
Funding
Conflicts of Interest
References
- Jia, X.; He, X.; Han, K.; Saykham, V. Research progress of sewage treatment technology in rural areas. Technol. Water Treat. 2018, 44, 22–26. [Google Scholar]
- Chen, P.Z.; Zhao, W.J.; Chen, D.K.; Huang, Z.P.; Zhang, C.X.; Zheng, X.Q. Research progress on integrated treatment technologies of rural domestic sewage: A review. Water 2022, 14, 2439. [Google Scholar] [CrossRef]
- Cai, R.; Ni, H.; Xi, H.; Li, J.; Wu, Z. Treatment of diepersed domestic sewage by johkasou with basalt fiber carrier media. Environ. Eng. 2022, 40, 146–152. (In Chinese) [Google Scholar]
- Li, X.L.; Bao, D.G.; Zhang, Y.Z.; Xu, W.Q.; Zhang, C.; Yang, H.Y.; Ru, Q.J.; Wang, Y.F.; Ma, H.; Zhu, E.R.; et al. Development and application of membrane aerated biofilm reactor (MABR)—A review. Water 2023, 15, 436. [Google Scholar] [CrossRef]
- Ali, A.E.; Salem, W.M.; Younes, S.M.; Kaid, M. Modeling climatic effect on physiochemical parameters and microorganisms of Stabilization Pond Performance. Heliyon 2020, 6, e04005. [Google Scholar] [CrossRef] [PubMed]
- Slimowitz, R. The great influenza: The epic story of the deadliest plague in history. Am. J. Health-Syst. Pharm. 2007, 64, 998. [Google Scholar] [CrossRef]
- Yang, X.M.; Morita, A.; Nakano, I.; Kushida, Y.; Ogawa, H. History and current situation of night soil treatment systems and decentralized wastewater treatment systems in Japan. Water Pract. Technol. 2010, 5, wpt2010096. [Google Scholar] [CrossRef]
- Chen, X.F.; Chao, L.Q.; Wan, Y.L.; Wang, X.Y.; Pu, X.C. Study of the characteristics of pollutants in rural domestic sewage and the optimal sewage treatment process: A Chengdu Plain case study. Water Sci. Technol. 2023, 87, 2373–2389. [Google Scholar] [CrossRef]
- Hu, X.; Luo, H.; Jing, Z.; Zhang, Z. Research progress of rural domestic sewage treatment technology. Appl. Chem. Ind. 2020, 49, 2871–2876. [Google Scholar]
- Gaulke, L.S. On-site wastewater treatment and reuses in Japan. Proc. Inst. Civ. Eng.-Water Manag. 2006, 159, 103–109. [Google Scholar] [CrossRef]
- Ohmori, H.; Yahashi, T.; Furukawa, Y.; Kawamura, K.; Yamamoto, Y. Treatment performance of newly developed johkasous with membrane separation. Water Sci. Technol. 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Itayama, T.; Kiji, M.; Suetsugu, A.; Tanaka, N.; Saito, T.; Iwami, N.; Mizuochi, M.; Inamori, Y. On site experiments of the slanted soil treatment systems for domestic gray water. Water Sci. Technol. 2006, 53, 193–201. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, C.; Zhang, K.; Rong, H. Influence of DO on simultaneous nitrogen and phosphorus removal in A/O process. China Water Wastewater 2011, 27, 80–82. [Google Scholar]
- Zhong, Y.; Zhu, G.; Ren, L.; Zhang, Z.; Li, A. Effect of influent conditions on the sewage treatment effect by small integrated devices. Technol. Water Treat. 2021, 47, 102–105. [Google Scholar]
- Liu, S.; Xue, L.; Yang, S.; Huang, Z. Application of biological contact oxidation purification tank in the ecological treatment of black and smelly river. Environ. Sci. Technol. 2018, 41, 115–120. [Google Scholar]
- Guo, H.; Zhao, Y.; Ye, J.; Ma, W. Pilot scale study on folk catering wastewater treatment by jogkasou. Technol. Water Treat. 2013, 39, 80–83. [Google Scholar]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. China Environmental Science Press: Beijing, China, 2002.
- Wang, C.; Hu, J.; Wang, Y.; Ma, Y.; Zeng, M.; Li, L. Study on nitrogen removal effect of non-circumfluence bio-filter purifying tank. J. Agro-Environ. Sci. 2018, 37, 316–322. [Google Scholar]
- Wang, R.; Wang, C.; Du, X. Study on compact household sewage purifying-tank with natural flowing process. Environ. Eng. 2007, 25, 21–24. [Google Scholar]
- Wang, C.; Yang, X.; Sakai, Y.; Lin, P.; Jia, Q. Study on impact resistance of sewage inflow in new purifying tank. Environ. Eng. 2014, 32, 59–63. [Google Scholar]
- Fajri, J.A.; Fujisawa, T.; Trianda, Y.; Ishiguro, Y.; Cui, G.Y.; Li, F.S.; Yamada, T. Effect of Aeration Rates on Removals of Organic Carbon and Nitrogen in Small Onsite Wastewater Treatment System (Johkasou). In Proceedings of the 3rd International Conference on Sustainable Infrastructure and Built Environment (SIBE), Bandung, Indonesia, 26–27 September 2017; Institut Teknologi Bandung, Faculty of Civil & Environmental Engineering: Bandung, Indonesia, 2017. [Google Scholar]
- Ebie, Y.; Kondo, T.; Kadoya, N.; Mouri, M.; Maruyama, O.; Noritake, S.; Inamori, Y.; Xu, K. Recovery oriented phosphorus adsorption process in decentralized advanced Johkasou. Water Sci. Technol. 2008, 57, 1977–1981. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, S.; Chen, Y.; Wang, W.; Qin, Y.; Li, Y.-Y. Evaluation of bioenergy production and material flow in treating Japanese concentrated Johkasou sludge using high-solid anaerobic membrane bioreactor based on one-year operation. Chem. Eng. J. 2023, 469, 143918. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Wen, X.; Xia, Y. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour. Technol. 2012, 117, 72–79. [Google Scholar] [CrossRef]
- Zhu, G.B.; Peng, Y.Z.; Wu, S.Y.; Wang, S.Y.; Xu, S.W. Simultaneous nitrification and denitrification in step feeding biological nitrogen removal process. J. Environ. Sci. 2007, 19, 1043–1048. [Google Scholar] [CrossRef]
- Xia, S.; Li, J.; Wang, R. Nitrogen removal performance and microbial community structure dynamics response to carbon nitrogen ratio in a compact suspended carrier biofilm reactor. Ecol. Eng. 2008, 32, 256–262. [Google Scholar] [CrossRef]
- Henze, M.; Van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008; Volume 112, 139. [Google Scholar]
- Veldman, B.A.; Spiering, W.; Doevendans, P.A.; Vervoort, G.; Kroon, A.A.; de Leeuw, P.W.; Smits, P. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J. Hypertens. 2002, 20, 2023–2027. [Google Scholar] [CrossRef]
- Pas-Schoonen, K.T.V.d.; Schalk-Otte, S.; Haaijer, S.; Schmid, M.; den Camp, H.O.; Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Complete conversion of nitrate into dinitrogen gas in co-cultures of denitrifying bacteria. Biochem. Soc. Trans. 2005, 33, 205–209. [Google Scholar] [CrossRef]
- Zhang, M.C.; Lawlor, P.G.; Wu, G.X.; Lynch, B.; Zhan, X.M. Partial nitrification and nutrient removal in intermittently aerated sequencing batch reactors treating separated digestate liquid after anaerobic digestion of pig manure. Bioprocess Biosyst. Eng. 2011, 34, 1049–1056. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Sun, Y.; Han, P.; Chen, Y.; Liu, Q.; Xu, G. Feasibility of simultaneous nitration and denitrification at low temperature. Ind. Water Treat. 2022, 42, 83–88. [Google Scholar]
- Zhao, W.; Chen, T.; Zhang, Q.; Peng, W.; Xie, J. Effect of dissolved oxygen on removal of nitrogen and phosphorus in Biolak/A2O process. Acta Sci. Circumstantiae 2014, 34, 2754–2758. [Google Scholar]
- Li, Y.Z.; He, Y.L.; Ohandja, D.G.; Ji, J.; Li, J.F.; Zhou, T. Simultaneous nitrification-denitrification achieved by an innovative internal-loop airlift MBR: Comparative study. Bioresour. Technol. 2008, 99, 5867–5872. [Google Scholar] [CrossRef]
- Helmer, C.; Kunst, S. Simultaneous nitrification/denitrification in an aerobic biofilm system. Water Sci. Technol. 1998, 37, 183–187. [Google Scholar] [CrossRef]
- Andrade do Canto, C.S.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M.; Foresti, E. Feasibility of nitrification/denitrification in a sequencing batch biofilm reactor with liquid circulation applied to post-treatment. Bioresour. Technol. 2008, 99, 644–654. [Google Scholar] [CrossRef]
- Hellinga, C.; Schellen, A.; Mulder, J.W.; van Loosdrecht, M.C.M.; Heijnen, J.J. The SHARON process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci. Technol. 1998, 37, 135–142. [Google Scholar] [CrossRef]
- Blackburne, R.; Yuan, Z.G.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef]
- Zhi, X.; Ding, F.; Peng, Y.; Ma, L. The study of shortcut nitrification-denitrification at normal temperature. Environ. Pollut. Control 2006, 28, 254–256. [Google Scholar]
- Date, Y.; Isaka, K.; Sumino, T.; Tsuneda, S.; Inamori, Y. Microbial community of anammox bacteria immobilized in polyethylene glycol gel carrier. Water Sci. Technol. 2008, 58, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Laureni, M.; Weissbrodt, D.G.; Szivak, I.; Robin, O.; Nielsen, J.L.; Morgenroth, E.; Joss, A. Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Water Res. 2015, 80, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Jaroszynski, L.W.; Cicek, N.; Sparling, R.; Oleszkiewicz, J.A. Importance of the operating pH in maintaining the stability of anoxic ammonium oxidation (anammox) activity in moving bed biofilm reactors. Bioresour. Technol. 2011, 102, 7051–7056. [Google Scholar] [CrossRef]
- Chen, C.J.; Sun, F.Q.; Zhang, H.Q.; Wang, J.F.; Shen, Y.L.; Liang, X.Q. Evaluation of COD effect on anammox process and microbial communities in the anaerobic baffled reactor (ABR). Bioresour. Technol. 2016, 216, 571–578. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Chen, Z.; Li, X.; Yi, X.; Niu, G.; He, J.; Lu, S.; Ke, Y.; Huang, M. Free nitrous acid prediction in ANAMMOX process using hybrid deep neural network model. J. Environ. Manag. 2023, 345, 118566. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K.; Kazama, F.; Sumino, T. Effects of nitrite inhibition on anaerobic ammonium oxidation. Appl. Microbiol. Biotechnol. 2010, 86, 359–365. [Google Scholar] [CrossRef]
- Lotti, T.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.Y.; Cao, S.B.; Miao, Y.Y.; Jia, F.X.; Du, R.; Peng, Y.Z. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.B.; Wang, S.Y.; Feng, X.J.; Fan, G.N.; Jetten, M.S.M.; Yin, C.Q. Anammox bacterial abundance, biodiversity and activity in a constructed wetland. Environ. Sci. Technol. 2011, 45, 9951–9958. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Mao, Y.J.; Shi, Y.P.; Quan, X. Start-up and bacterial community compositions of partial nitrification in moving bed biofilm reactor. Appl. Microbiol. Biotechnol. 2017, 101, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Analysis of denitrification and dephosphorization products and identification of dominant strains in combined biological purification tank. China Water Wastewater 2014, 30, 77–80. [Google Scholar]
- Liu, T.; He, X.; Jia, G.; Xu, J.; Quan, X.; You, S. Simultaneous nitrification and denitrification process using novel surface-modified suspended carriers for the treatment of real domestic wastewater. Chemosphere 2020, 247, 125831. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xin, Y.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Characterization on the aerobic denitrification process of Bacillus strains. Biomass Bioenergy 2020, 140, 105677. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, F.; Zhang, R.; Zhao, X.; Wang, Y.; Wu, Y.; Liao, X.; Feng, Y.; Ma, J.; Lan, T. Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification bacterium Acinetobacter ZQ-A1 and community characteristics analysis of its application in pig farm wastewater. Environ. Sci. Pollut. Res. 2023, 30, 104029–104042. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, X.; He, Y.; Dong, S.; Zhao, K. Nitrogen removal capability and mechanism of a novel heterotrophic nitrification–aerobic denitrification bacterium Halomonas sp. DN3. Bioresour. Technol. 2023, 387, 129569. [Google Scholar] [CrossRef]
- Zeng, M.; Hu, J.; Wang, D.; Wang, H.; Wang, Y.; Wu, N.; Zhang, Z.; Wang, C. Improving a compact biofilm reactor to realize efficient nitrogen removal performance: Step-feed, intermittent aeration, and immobilization technique. Environ. Sci. Pollut. Res. 2018, 25, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Withers, E.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Use of untargeted metabolomics for assessing soil quality and microbial function. Soil Biol. Biochem. 2020, 143, 107758. [Google Scholar] [CrossRef]
- Tian, M.; Zhao, F.; Shen, X.; Chu, K.; Wang, J.; Chen, S.; Guo, Y.; Liu, H. The first metagenome of activated sludge from full-scale anaerobic/anoxic/oxic (A2O) nitrogen and phosphorus removal reactor using Illumina sequencing. J. Environ. Sci. 2015, 35, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Ekpeghere, K.I.; Ha, S.-Y.; Kim, B.-S.; Song, B.; Kim, J.-T.; Kim, H.-G.; Koh, S.-C. Full-scale biological treatment of tannery wastewater using the novel microbial consortium BM-S-1. J. Environ. Sci. Health Part A 2014, 49, 355–364. [Google Scholar] [CrossRef]
- Sul, W.-J.; Kim, I.-S.; Ekpeghere, K.I.; Song, B.; Kim, B.-S.; Kim, H.-G.; Kim, J.-T.; Koh, S.-C. Metagenomic insight of nitrogen metabolism in a tannery wastewater treatment plant bioaugmented with the microbial consortium BM-S-1. J. Environ. Sci. Health Part A 2016, 51, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Landman, W. Climate change 2007: The physical science basis. S. Afr. Geogr. J. 2010, 92, 86–87. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef]
- Pan, Y.; Ye, L.; Ni, B.-J.; Yuan, Z. Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res. 2012, 46, 4832–4840. [Google Scholar] [CrossRef]
- Massara, T.M.; Malamis, S.; Guisasola, A.; Baeza, J.A.; Noutsopoulos, C.; Katsou, E. A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci. Total Environ. 2017, 596–597, 106–123. [Google Scholar] [CrossRef]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Ni, B.J.; Pan, Y.T.; van den Akker, B.; Ye, L.; Yuan, Z.G. Full-scale modeling explaining large spatial variations of nitrous oxide fluxes in a step-feed plug-flow wastewater treatment reactor. Environ. Sci. Technol. 2015, 49, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; van der Star, W.R.L.; Wielders, H.A.; Mulder, J.W.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 2008, 42, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, F.; Guo, M.Z.; Zeng, M.; Liu, W.; Wang, Z.Q.; Wu, N.; Cao, J.G. A comprehensive literature mining and analysis of nitrous oxide emissions from different innovative mainstream anammox-based biological nitrogen removal processes. Sci. Total Environ. 2023, 904, 166295. [Google Scholar] [CrossRef] [PubMed]
- Mulkerrins, D.; Dobson, A.D.W.; Colleran, E. Parameters affecting biological phosphate removal from wastewaters. Environ. Int. 2004, 30, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.G.; Keller, J.; Blackall, L.L.; Reis, M.A.M. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Bio-Technol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Acevedo, B.; Oehmen, A.; Carvalho, G.; Seco, A.; Borrás, L.; Barat, R. Metabolic shift of polyphosphate-accumulating organisms with different levels of polyphosphate storage. Water Res. 2012, 46, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Zhou, T.; Xiang, Y.; Liu, S.; Shao, Z.; Liu, Y.; Ma, H.; He, Q.; Chai, H. Insights into simultaneous nitrogen and phosphorus removal in biofilm: The overlooked comammox Nitrospira and the positive role of glycogen-accumulating organisms. Sci. Total Environ. 2023, 887, 164130. [Google Scholar] [CrossRef]
- Hu, Q.; He, L.; Lan, R.; Feng, C.; Pei, X. Recent advances in phosphate removal from municipal wastewater by electrocoagulation process: A review. Sep. Purif. Technol. 2023, 308, 122944. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Zhang, S.-S.; Ma, W.-C.; Zhu, L.; Li, Y.-P.; Pan, Y.; Chen, L. Simultaneous phosphorus recovery from wastewater and sludge by a novel denitrifying phosphorus removal system. Bioresour. Technol. 2023, 384, 129284. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, J.; He, X.; He, L.; Lin, Z.; Shi, S.; Zhou, J. Simultaneous nitrogen and phosphorus removal from simulated digested piggery wastewater in a single-stage biofilm process coupling anammox and intracellular carbon metabolism. Bioresour. Technol. 2021, 333, 125152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Nie, E.; Li, J.H.; Yang, M.; Zhao, Y.J.; Luo, X.Z.; Zheng, Z. Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon. Environ. Sci. Pollut. Res. 2012, 19, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Feng, Y.; Feng, C. Phosphorus removal from effluent of Johkaso by electrocoagulation. Environ. Sci. Technol. 2008, 31, 103. (In Chinese) [Google Scholar]
- Wen, Q.; Wang, G.; Chen, Z.; Lu, B.; Shi, H. Effect analysis of biological phosphorus removal in polymeric aluminum-iron strengthened A2/O system. J. Harbin Inst. Technol. 2010, 42, 945–948. (In Chinese) [Google Scholar]
- Deng, L.; Dhar, B.R. Phosphorus recovery from wastewater via calcium phosphate precipitation: A critical review of methods, progress, and insights. Chemosphere 2023, 330, 138685. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, J.; Alavi Moghaddam, M.R.; Habibzadeh, S. The role of the current waveform in mitigating passivation and enhancing electrocoagulation performance: A critical review. Chemosphere 2023, 312, 137212. [Google Scholar] [CrossRef] [PubMed]
- Fitch, A.; Balderas-Hernandez, P.; Ibanez, J.G. Electrochemical technologies combined with physical, biological, and chemical processes for the treatment of pollutants and wastes: A review. J. Environ. Chem. Eng. 2022, 10, 107810. [Google Scholar] [CrossRef]
- Devlin, T.R.; Kowalski, M.S.; Pagaduan, E.; Zhang, X.G.; Wei, V.; Oleszkiewicz, J.A. Electrocoagulation of wastewater using aluminum, iron, and magnesium electrodes. J. Hazard. Mater. 2019, 368, 862–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, X.; Luo, J.; Li, H.; How, S.-W.; Wu, D.; He, J.; Cheng, Z.; Gao, Y.; Lu, H. A review of the phosphorus removal of polyphosphate-accumulating organisms in natural and engineered systems. Sci. Total Environ. 2023, 912, 169103. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Ge, Y.; Zhao, L.; Long, S.; Zhang, R. Interaction between common antibiotics and a Shewanella strain isolated from an enhanced biological phosphorus removal activated sludge system. Bioresour. Technol. 2016, 222, 114–122. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Gu, A.Z.; McMahon, K.D. Progress toward understanding the distribution of Accumulibacter among full-scale enhanced biological phosphorus removal systems. Microb. Ecol. 2008, 55, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bassin, J.P.; Kleerebezem, R.; Dezotti, M.; van Loosdrecht, M.C.M. Simultaneous nitrogen and phosphate removal in aerobic granular sludge reactors operated at different temperatures. Water Res. 2012, 46, 3805–3816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Bi, X.J.; Peng, Y.Z.; Bai, M. Research advances of the phosphorus-accumulating organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Yu, J.; Qiu, C.; Wang, Y.; Sun, L. Review on metabolic pathway and characters of PAO. Ind. Water Treat. 2013, 33, 4. (In Chinese) [Google Scholar]
- Huang, R.; Pan, H.; Zheng, X.; Fan, C.; Si, W.; Bao, D.; Gao, S.; Tian, J. Effect of membrane pore size on membrane fouling of corundum ceramic membrane in MBR. Int. J. Environ. Res. Public Health 2023, 20, 4558. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Chen, Y.Y.; Xu, T.; Cui, Z.W.; Zheng, Z.W. Efficient nitrogen removal by simultaneous heterotrophic nitrifying-aerobic denitrifying bacterium in a purification tank bioreactor amended with two-stage dissolved oxygen control. Bioresour. Technol. 2019, 281, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A review of constructed wetland on type, treatment and technology of wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Cheng, P.-H.; Genthe, B.; Steyn, M. The environmental feasibility of low-cost algae-based sewage treatment as a climate change adaption measure in rural areas of SADC countries. J. Appl. Phycol. 2019, 31, 355–363. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Wu, J.; Cheng, R.; Shi, L.; Zheng, X.; Zhang, Z. Identify driving forces of MBR applications in China. Sci. Total Environ. 2019, 647, 627–638. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, R.; Husein, M.; Yang, S.; Wei, H.; Yang, Q. Study on the wastewater treatment performance by modified composite filler biofilm reactor. Technol. Water Treat. 2021, 47, 110. (In Chinese) [Google Scholar]
- Zhao, Y.; Liu, D.; Huang, W.; Yang, Y.; Ji, M.; Nghiem, L.D.; Trinh, Q.T.; Tran, N.H. Insights into biofilm carriers for biological wastewater treatment processes: Current state-of-the-art, challenges, and opportunities. Bioresour. Technol. 2019, 288, 121619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, X.; Wei, Q.; Wang, Y.; Zha, Y.; Mo, H.; Wang, M.; Yu, Y.; Qi, L.; Liu, G.; et al. Performance comparison and microbial community structure analysis of three fixed fillers in pilot IFAS system. Chin. J. Environ. Eng. 2019, 13, 1338–1349. (In Chinese) [Google Scholar]
- You, X.; Ding, X.; Huang, Y.; Pan, Y. Study on performance optimization of suspended biological fillers under different loads. Technol. Water Treat. 2020, 46, 102–105. (In Chinese) [Google Scholar]
- Wang, C.; Liu, N.; Jia, Q.; Liu, J.; Wang, Z. Influence of bio-filter bed on domestic sewage treatment in purifying tank. Technol. Water Treat. 2009, 35, 73. (In Chinese) [Google Scholar] [CrossRef]
- Murshid, S.; Antonysamy, A.; Dhakshinamoorthy, G.; Jayaseelan, A.; Pugazhendhi, A. A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci. Total Environ. 2023, 892, 164796. [Google Scholar] [CrossRef]
- Xu, G.; Hu, J.; Huang, Z.; Zhan, D.; Li, C.; Chen, J. Study on Rural Sanitary Sewage Treatment in Compound Purifying Tank. In Proceedings of the 2nd International Conference on Energy and Environmental Protection (ICEEP 2013), Guilin, China, 19–21 April 2013; p. 2599. [Google Scholar]
- Jiang, C.; Xu, S.; Wang, R.; Feng, S.; Zhou, S.; Wu, S.; Zeng, X.; Wu, S.; Bai, Z.; Zhuang, G.; et al. Achieving efficient nitrogen removal from real sewage via nitrite pathway in a continuous nitrogen removal process by combining free nitrous acid sludge treatment and DO control. Water Res. 2019, 161, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Jeison, D.; Chamy, R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Res. 2003, 37, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Zhang, L.F.; Su, J.F.; Ali, A.; Huang, T.L.; Chen, C.L.; Yang, W.S. A biofilm reactor based on slow-release carbon source effectively improved the continuous denitrification capacity of slightly polluted surface water at low carbon to nitrogen ratio. J. Environ. Chem. Eng. 2023, 11, 109552. [Google Scholar] [CrossRef]
- Hu, M.Y.; Luo, T.L.; Li, Q.L.; Xie, Y.F.; Liu, G.; Wang, L.J.; Peijnenburg, W. Remediation of low C/N wastewater by iron-carbon micro-electrolysis coupled with biological denitrification: Performance, mechanisms, and application. J. Water Process Eng. 2022, 48, 102899. [Google Scholar] [CrossRef]
- Hu, X.; Li, W.; Liu, J.; Zhao, Y.; Sun, T.; Sun, J. Influence of pH on denitrifying phosphorus removal using nitrite as electron acceptor. J. Cent. South Univ. Sci. Technol. 2013, 44, 2144–2149. (In Chinese) [Google Scholar]
- Nguyen, P.Y.; Marques, R.; Wang, H.M.; Reis, M.A.M.; Carvalho, G.; Oehmen, A. The impact of pH on the anaerobic and aerobic metabolism of Tetrasphaera-enriched polyphosphate accumulating organisms. Water Res. X 2023, 19, 100177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gan, C.-J.; Zhou, J. Effect of Environment Factors on Phosphorus Removal Efficiency of Phosphate Reduction System. In Proceedings of the International Conference on Civil Engineering and Building Materials (CEBM), Kunming, China, 29–31 July 2011; p. 2797. [Google Scholar]
- Chen, H.; Chang, S. Impact of temperatures on microbial community structures of sewage sludge biological hydrolysis. Bioresour. Technol. 2017, 245, 502–510. [Google Scholar] [CrossRef]
- McAteer, P.G.; Christine Trego, A.; Thorn, C.; Mahony, T.; Abram, F.; O’Flaherty, V. Reactor configuration influences microbial community structure during high-rate, low-temperature anaerobic treatment of dairy wastewater. Bioresour. Technol. 2020, 307, 123221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Gujer, W. Nitrification and me—A subjective review. Water Res. 2010, 44, 1–19. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Chen, W.; Ma, F.; Liu, H.; Tian, Y. The regulation and control strategies of a sequencing batch reactor for simultaneous nitrification and denitrification at different temperatures. Bioresour. Technol. 2013, 133, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, S.; Zhang, W.; Li, Y. Effect of temperature on partial nitrification in biofilm reactor under high dissolved oxygen. Technol. Water Treat. 2020, 46, 104–108. (In Chinese) [Google Scholar]
- Gong, Y.; Peng, Y. Effect of temperature variation on short-cut biological nitrogen removal and nitrous oxide release. Technol. Water Treat. 2020, 46, 110. (In Chinese) [Google Scholar]
- Yang, Q.; Shen, N.; Lee, Z.M.-P.; Xu, G.; Cao, Y.; Kwok, B.; Lay, W.; Liu, Y.; Zhou, Y. Simultaneous nitrification, denitrification and phosphorus removal (SNDPR) in a full-scale water reclamation plant located in warm climate. Water Sci. Technol. 2016, 74, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fan, K.; Zhou, J.; Gong, B.; Meng, H.; He, X.; Zhou, J. Nitrogen and phosphorous removal performance and microbial community of modified SBBR at low temperature. China Water Wastewater 2022, 38, 82–87. (In Chinese) [Google Scholar]
- Fang, M.; Wang, C.; Wang, L. Screening of denitrifying polyphosphate-accumulating organisms and their biological characteristics. J. Harbin Eng. Univ. 2007, 28, 631–635. [Google Scholar]
- Wu, L.; Wang, J.; Liu, X. Enhanced nitrogen removal under low-temperature and high-load conditions by optimization of the operating modes and control parameters in the CAST system for municipal wastewater. Desalination Water Treat. 2015, 53, 1683–1698. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Chu, Z.; Zhang, J. Effect of temperature downshifts on a bench-scale hybrid A/O system: Process performance and microbial community dynamics. Chemosphere 2016, 153, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Li, X.; Xu, G.R.; Yu, H.R. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci. Total Environ. 2018, 643, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Y.; Wang, K.; Zhao, Y. Characteristics of microbial community, nitrogen and phosphorus removal in separated compartments of combined packing biological aerated filter. Acta Sci. Circumstantiae 2015, 35, 152–160. (In Chinese) [Google Scholar]
- Wang, X.J.; Xia, S.Q.; Chen, L.; Zhao, J.F.; Renault, N.J.; Chovelon, J.M. Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochem. 2006, 41, 824–828. [Google Scholar] [CrossRef]
- Tokutomi, T. Operation of a nitrite-type airlift reactor at low DO concentration. Water Sci. Technol. 2004, 49, 81–88. [Google Scholar] [CrossRef]
- Li, S.; Fei, X.; Chi, Y.; Jiao, X.; Wang, L. Integrated temperature and DO effect on the lab scale A2O process: Performance, kinetics and microbial community. Int. Biodeterior. Biodegrad. 2018, 133, 170–179. [Google Scholar] [CrossRef]
- Ju, Y.K.; Wang, H.L.; Zhang, Q.; Xing, J.L. Effect of Dissolved Oxygen on Nitrogen and Phosphorus Removal Rate in Biolak Process. In Proceedings of the International Conference on Materials, Transportation and Environmental Engineering (CMTEE 2013), Taichung, Taiwan, 21–23 August 2013; p. 1629. [Google Scholar]
- Fang, Q.; Zhang, C.; Zhang, K.; Rong, H. Effect of dissolved oxygen on denitrifying phosphorus removal bacteria. China Water Wastewater 2008, 24, 35–39. (In Chinese) [Google Scholar]
- Zaman, M.; Kim, M.; Nakhla, G. Simultaneous partial nitrification and denitrifying phosphorus removal (PNDPR) in a sequencing batch reactor process operated at low DO and high SRT for carbon and energy reduction. Chem. Eng. J. 2021, 425, 131881. [Google Scholar] [CrossRef]
- Ha, J.H.; Ong, S.K.; Surampalli, R.; Song, J. Temperature effects on nitrification in polishing biological aerated filters (BAFs). Environ. Technol. 2010, 31, 671–680. [Google Scholar] [CrossRef]
- Kawan, J.A.; Suja’, F.; Pramanik, S.K.; Yusof, A.; Abdul Rahman, R.; Abu Hasan, H. Effect of hydraulic retention time on the performance of a compact moving bed biofilm reactor for effluent polishing of treated sewage. Water 2022, 14, 81. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, X.; Hou, R.; Chen, Y. Effect of HRT on nitrogen and phosphorus removal in integrated domestic sewage treatment device. Technol. Water Treat. 2018, 44, 95. (In Chinese) [Google Scholar]
- Guan, Y.; Ning, T.; Zhang, L. Effects of HRT and media on nitrogen and phosphorus removal in an integrated biofilm reactor. J. Tsinghua Univ. (Sci. Technol.) 2009, 49, 359–363. (In Chinese) [Google Scholar]
- Hou, F.; Zhang, T.; Peng, Y.Z.; Cao, X.X.; Pang, H.T.; Shao, Y.Q.; Lu, X.C.; Yuan, J.; Chen, X.; Zhang, J. Partial anammox achieved in full scale biofilm process for typical domestic wastewater treatment. Front. Environ. Sci. Eng. 2022, 16, 33. [Google Scholar] [CrossRef]
- Song, K.G.; Cho, J.; Ahn, K.H. Effects of internal recycling time mode and hydraulic retention time on biological nitrogen and phosphorus removal in a sequencing anoxic/anaerobic membrane bioreactor process. Bioprocess Biosyst. Eng. 2009, 32, 135–142. [Google Scholar] [CrossRef]
- Brown, P.; Ong, S.K.; Lee, Y.W. Influence of anoxic and anaerobic hydraulic retention time on biological nitrogen and phosphorus removal in a membrane bioreactor. Desalination 2011, 270, 227–232. [Google Scholar] [CrossRef]
- Wu, C.Y.; Peng, Y.Z.; Li, X.L.; Wang, S.Y. Effect of carbon source on biological nitrogen and phosphorus removal in an anaerobic-anoxic-oxic (A2O) process. J. Environ. Eng.-ASCE 2010, 136, 1248–1254. [Google Scholar] [CrossRef]
- Xie, J.; Guo, Y.; Li, Y. The role of external carbon sources at each stage of an A2O process for simultaneously removing nitrogen and phosphorus. Environ. Prog. Sustain. Energy 2018, 37, 2010–2015. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, L.; Zuo, J.; Wang, L.; Mao, K.; Mei, L. Pilot study on high efficient wastewater treatment device based on A/O process. Technol. Water Treat. 2022, 48, 131–135. (In Chinese) [Google Scholar]
- Hallin, S.; Throback, I.N.; Dicksved, J.; Pell, M. Metabolic profiles and genetic diversity of denitrifying communities in activated sludge after addition of methanol or ethanol. Appl. Environ. Microbiol. 2006, 72, 5445–5452. [Google Scholar] [CrossRef]
- Li, G.C.; Chen, J.; Yang, T.; Sun, J.Q.; Yu, S.L. Denitrification with corncob as carbon source and biofilm carriers. Water Sci. Technol. 2012, 65, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.Q.; Li, J.X.; Wang, R.C.; Li, J.Y.; Zhang, Z.Q. Tracking composition and dynamics of nitrification and denitrification microbial community in a biofilm reactor by PCR-DGGE and combining FISH with flow cytometry. Biochem. Eng. J. 2010, 49, 370–378. [Google Scholar] [CrossRef]
- Iannacone, F.; Di Capua, F.; Granata, F.; Gargano, R.; Esposito, G. Shortcut nitrification-denitrification and biological phosphorus removal in acetate- and ethanol-fed moving bed biofilm reactors under microaerobic/ aerobic conditions. Bioresour. Technol. 2021, 330, 124958. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, W.; Zhang, W.; Hu, F.; Ye, J. Comprehensive treatment of rural domestic sewage in China. Strateg. Study CAE 2022, 24, 154–160. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.; Zhong, W.; Tang, Q. Application analysis of integrated AO+ packing in the treatment of domestic sewage in villages and towns. Technol. Water Treat. 2023, 49, 143–145. [Google Scholar]
- Bi, X.Q.; Guo, L.J. Treatment process of rural domestic sewage based on small purification tank technology. In Proceedings of the 2nd International Conference on Air Pollution and Environmental Engineering (APEE), Xi’an, China, 15–16 December 2019. [Google Scholar]
- Liu, J.; Wang, C.; Du, X.; Li, G.; Jia, Q. Influence of recirculation flow rate on domestic sewage treatment in purifying tank. China Water Wastewater 2011, 27, 98–101. (In Chinese) [Google Scholar]
- Zhang, Z. Biological purification tank/enhanced ecological floating rafts process for treatment of rural domestic sewage. China Water Wastewater 2009, 25, 8–11. (In Chinese) [Google Scholar]
- Huang, J.; Wu, J.; Wang, X.; Tang, Q.; Chen, S.; Zheng, H.; Tan, J. Pollutants removal efficiency and path of low polluted water treated by purification tank and analysis of root microbial community. J. Sichuan Univ. (Nat. Sci. Ed.) 2023, 60, 142–149. [Google Scholar]
- GB 3838-2002; Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Shang, G.; Huang, M.; Wu, L.; Dai, X.; Ruan, Y. The control test-study of biological purification tank on malodorous river water purification. China Environ. Sci. 2008, 28, 433–437. (In Chinese) [Google Scholar]
- Ding, R.N.; Li, Y.G.; Yu, X.; Peng, Y.M.; Zhang, Z.G.; Wei, L. Characteristics of rural agritainment sewage in Sichuan, China. Water Sci. Technol. 2019, 79, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Mishima, I.; Hama, M.; Tabata, Y.; Nakajima, J. Long-term investigation of phosphorus removal by iron electrocoagulation in small-scale wastewater treatment plants. Water Sci. Technol. 2018, 78, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Mishima, I.; Hama, M.; Tabata, Y.; Nakajima, J. Improvement of phosphorus removal by calcium addition in the iron electrocoagulation process. Water Sci. Technol. 2017, 76, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.F.; Liu, Y.Q.; Zhang, C.; Li, X.N. Study on influencing parameters and long-term operation of electrocoagulation phosphorus removal from small rural domestic sewage. Water Sci. Technol. 2023, 87, 1866–1878. [Google Scholar] [CrossRef]
- Mores, R.; Mello, P.D.; Zakrzevski, C.A.; Treichel, H.; Kunto, A.; Steffens, J.; Dallago, R.M. Reduction of soluble organic carbon and removal of total phosphorus and metals from swine wastewater by electrocoagulation. Braz. J. Chem. Eng. 2018, 35, 1231–1240. [Google Scholar] [CrossRef]

| Type | Processing Scale | Manufacturing Method |
|---|---|---|
| Small size | 5–50 people The average sewage volume is 10 m3/d | Mass production in the factory |
| Medium size | 51–500 people The average sewage volume is 10–100 m3/d | Installation of FRP on-site |
| Large size | More than 500 people The lowest sewage volume is 100 m3/d | On-site construction of reinforced concrete structures |
| Process | Advantages | Disadvantages | Removal Percentage of Parameters (%) | Reference | ||
|---|---|---|---|---|---|---|
| COD | TN | TP | ||||
| Johkasou | compact design, relatively low maintenance, Effective nitrogen removal | limited phosphorus removal, emission standards, and maintenance requirements are influenced by the region | 80–95 | 60–95 | 60–95 | [90] |
| Constructed wetlands | Low cost, easy to operate, with economic, ecological, and aesthetic value | Large land area, low loading capacity, aquatic plant vulnerability to pests and diseases | 60–85 | 60–80 | 40–90 | [91] |
| Oxidation ponds | Simple construction, low investment, landscaping function | Small treatment load, long hydraulic retention time, large floor space, odor, and mosquito breeding | 50–70 | 70–90 | 30–50 | [92] |
| MBR | Simpler process, more efficient treatment | Easy to cause membrane contamination, membrane clogging | 80–90 | 60–80 | 80–98 | [93] |
| Types | Representative | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fixed filler Suspended filler | Honeycomb filler, Spherical filler | Higher volume loading, higher biological activity | Uneven water and air distribution | [96] |
| Fixed filler Suspended filler | Soft filler, Semi-soft filler, Combined filler | Long service life, low cost | Easily accumulates sludge | [94] |
| Dispersed filler | Stacked filler, Suspension filler | Simple operation, high oxygen transfer efficiency | Higher cost | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cheng, S.; Chen, H. Evaluating the Mechanisms and Efficiency of Johkasou Systems for Decentralized Domestic Effluent Treatment: A Review. Water 2024, 16, 2266. https://doi.org/10.3390/w16162266
Wang X, Cheng S, Chen H. Evaluating the Mechanisms and Efficiency of Johkasou Systems for Decentralized Domestic Effluent Treatment: A Review. Water. 2024; 16(16):2266. https://doi.org/10.3390/w16162266
Chicago/Turabian StyleWang, Xu, Siyue Cheng, and Huilun Chen. 2024. "Evaluating the Mechanisms and Efficiency of Johkasou Systems for Decentralized Domestic Effluent Treatment: A Review" Water 16, no. 16: 2266. https://doi.org/10.3390/w16162266
APA StyleWang, X., Cheng, S., & Chen, H. (2024). Evaluating the Mechanisms and Efficiency of Johkasou Systems for Decentralized Domestic Effluent Treatment: A Review. Water, 16(16), 2266. https://doi.org/10.3390/w16162266






