The Relationship between Ribosomal RNA Operon Copy Number and Ecological Characteristics of Activated Sludge Microbial Communities across China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample 16S rRNA Gene High-Throughput Sequencing
2.3. Data Processing and Analysis Methods
3. Results and Discussion
3.1. Geographical Distributions and Influencing Factors of Average rrn Copy Number of Microbial Communities
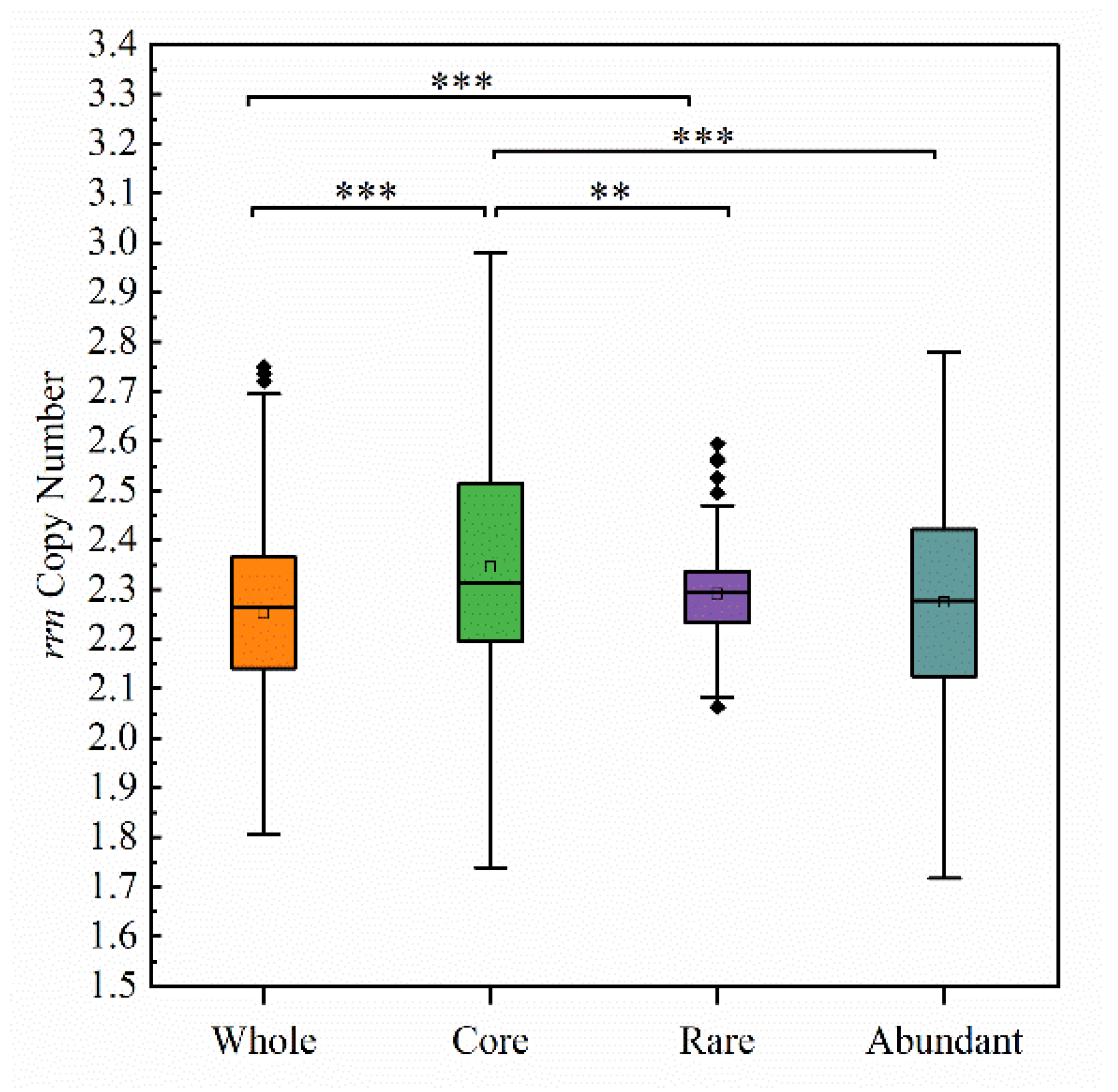
3.1.1. Geographical Distributions of Average rrn Copy Number
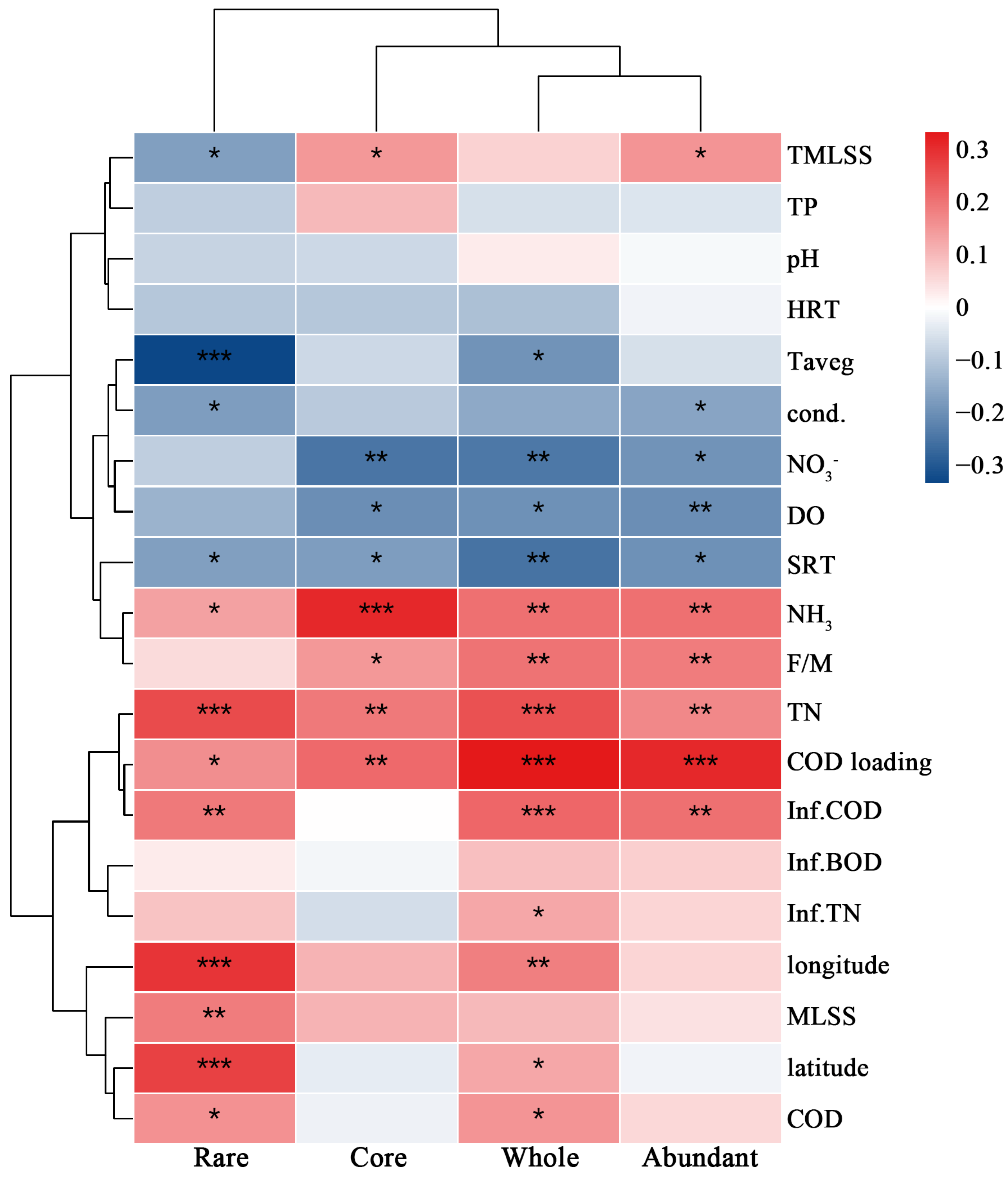
3.1.2. Influencing Factors of Average rrn Copy Number
3.2. The Relationship of Community Average rrn Copy Number to Biodiversity and Assembly Mechanism
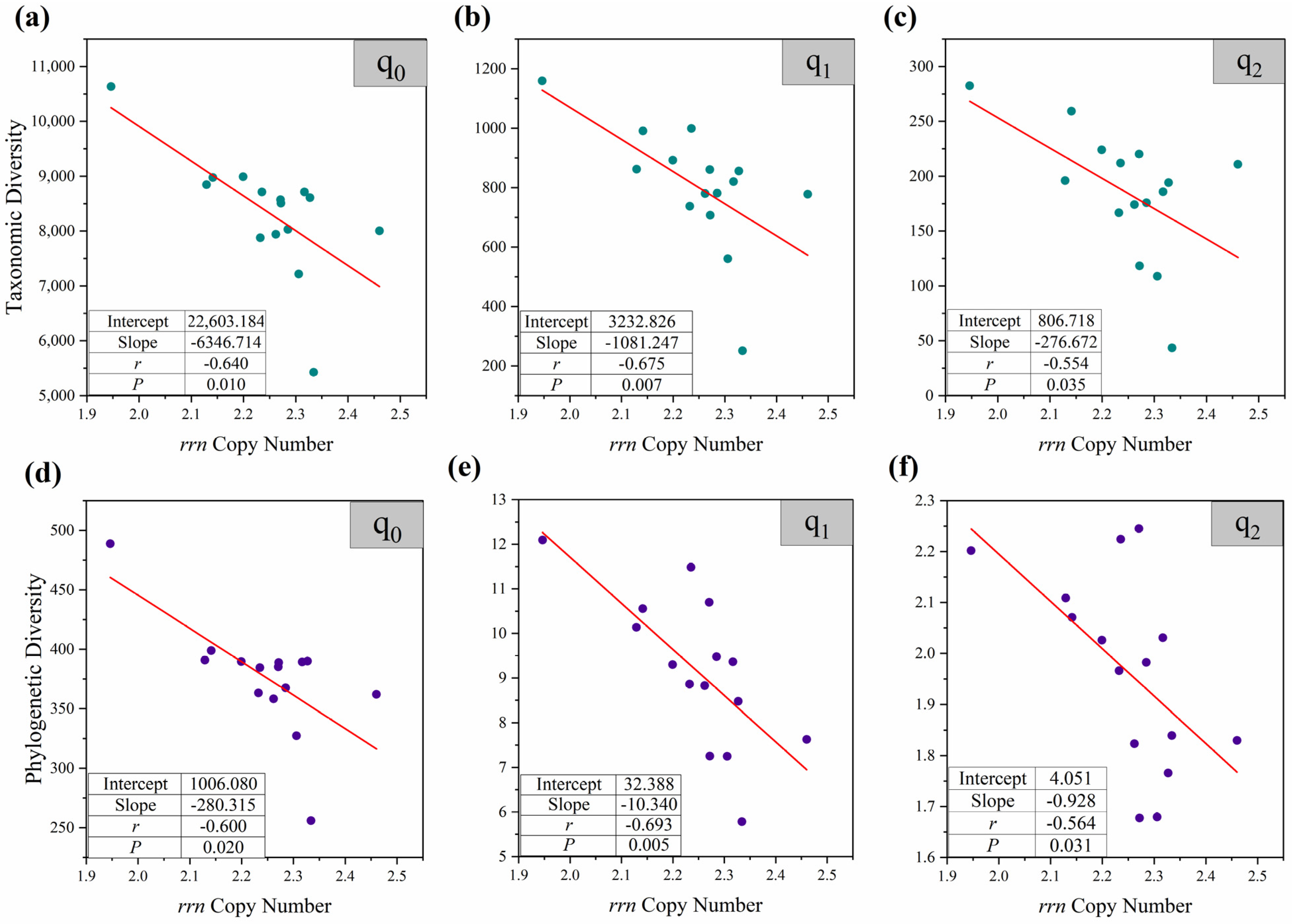
3.2.1. The Response of Community Diversity to Average rrn Copy Number
3.2.2. The Relationship between the Assembly Mechanism and Average rrn Copy Number
3.3. Study on the Relationship between Average rrn Copy Number and Community Microbial Function
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ning, D.; Van Nostrand, J.D.; Sun, C.; Yang, Y.; Zhou, J.; Wen, X. Biogeography and assembly of microbial communities in wastewater treatment plants in China. Environ. Sci. Technol. 2020, 54, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Begmatov, S.; Dorofeev, A.G.; Kadnikov, V.V.; Beletsky, A.V.; Pimenov, N.V.; Ravin, N.V.; Mardanov, A.V. The structure of microbial communities of activated sludge of large-scale wastewater treatment plants in the city of Moscow. Sci. Rep. 2022, 12, 3458. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, W.; Lin, H.-m.; Chen, T.; Yang, T.; Zhang, B.; Wen, X. Ecological and functional differences of abundant and rare sub-communities in wastewater treatment plants across China. Environ. Res. 2024, 243, 117749. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, S.; Rice, D.T.F.; Batinovic, S.; Nittami, T.; Seviour, R.J. The community compositions of three nitrogen removal wastewater treatment plants of different configurations in Victoria, Australia, over a 12-month operational period. Appl. Microbiol. Biotechnol. 2020, 104, 9839–9852. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, B.; Ning, D.; Zhang, Y.; Dai, T.; Wu, L.; Li, T.; Liu, W.; Zhou, J.; Wen, X. Seasonal dynamics of the microbial community in two full-scale wastewater treatment plants: Diversity, composition, phylogenetic group based assembly and co-occurrence pattern. Water Res. 2021, 200, 117295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wen, X. How exogenous influent communities and environmental conditions affect activated sludge communities in the membrane bioreactor of a wastewater treatment plant. Sci. Total Environ. 2019, 692, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ning, D.; Van Nostrand, J.D.; Sun, C.; Yang, Y.; Zhou, J.; Wen, X. The call for regional design code from the regional discrepancy of microbial communities in activated sludge. Environ. Pollut. 2021, 273, 116487. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Sun, Y.; Li, B.; Feng, Z.; Wu, G. The r/K selection theory and its application in biological wastewater treatment processes. Sci. Total Environ. 2022, 824, 153836. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Gibson, C.; Frigon, D. Wastewater microbial community structure and functional traits change over short timescales. Sci. Total Environ. 2019, 662, 779–785. [Google Scholar] [CrossRef]
- Wu, L.; Wen, C.; Qin, Y.; Yin, H.; Tu, Q.; Van Nostrand, J.D.; Yuan, T.; Yuan, M.; Deng, Y.; Zhou, J. Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol. 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.V.; Guerrero, L.D.; Orellana, E.; Figuerola, E.L.; Erijman, L. Time series genome-centric analysis unveils bacterial response to operational disturbance in activated sludge. MSystems 2019, 4, e00169-19. [Google Scholar] [CrossRef] [PubMed]
- Klappenbach, J.A.; Dunbar, J.M.; Schmidt, T.M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 2000, 66, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Nuccio, E.; Herman, D.J.; Rijkers, R.; Estera, K.; Li, J.; Da Rocha, U.N.; He, Z.; Pett-Ridge, J.; Brodie, E.L.; et al. Successional trajectories of rhizosphere bacterial communities over consecutive seasons. MBio 2015, 6, e00746-15. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Chang. 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Dai, T.; Zhao, Y.; Ning, D.; Huang, B.; Mu, Q.; Yang, Y.; Wen, D. Dynamics of coastal bacterial community average ribosomal RNA operon copy number reflect its response and sensitivity to ammonium and phosphate. Environ. Pollut. 2020, 260, 113971. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, W.; Dong, Z.; Yang, Z.; Zhao, J.; Guo, X. Distinct mediating patterns between metal filtering and species coexistence of rare and abundant subcommunities in heavily polluted river sediments. Environ. Int. 2023, 172, 107747. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, S.; Deng, Y.; Wang, S.; Fan, H.; Li, X.; Bai, Z.; Zhuang, X. Distinct functions and assembly mechanisms of soil abundant and rare bacterial taxa under increasing pyrene stresses. Front. Microbiol. 2021, 12, 689762. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 126673. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Roller, B.R.K.; Stoddard, S.F.; Schmidt, T.M. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat. Microbiol. 2016, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, Y.; Chen, S.; Jason Shi, Z.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; et al. Microbial functional trait of rRNA operon copy numbers increases with organic levels in anaerobic digesters. ISME J. 2017, 11, 2874–2878. [Google Scholar] [CrossRef]
- Guo, B. Cellular Metabolic Markers and Growth Dynamics Definition of Functional Groups in Activated Sludge Wastewater Treatment Heterotrophic Population; McGill University: Montréal, QC, Canada, 2019. [Google Scholar]
- Haller, L.; Tonolla, M.; Zopfi, J.; Peduzzi, R.; Wildi, W.; Poté, J. Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res. 2011, 45, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmitz, B.W.; Caton, K.; Zhang, B.; Zabaleta, J.; Garai, J.; Taylor, C.M.; Romanchishina, T.; Gerba, C.P.; Pepper, I.L.; et al. Assessing the spatial and temporal variability of bacterial communities in two Bardenpho wastewater treatment systems via Illumina MiSeq sequencing. Sci. Total Environ. 2019, 657, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Vuono, D.C.; Benecke, J.; Henkel, J.; Navidi, W.C.; Cath, T.Y.; Munakata-Marr, J.; Spear, J.R.; Drewes, J.E. Disturbance and temporal partitioning of the activated sludge metacommunity. ISME J. 2015, 9, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Lin, S.; Ni, Y.; Yao, L.; Huang, S.; Zuo, T.; Wang, J.; Ni, W. Assembly of functional microbial communities in paddy soil with long-term application of pig manure under rice-rape cropping system. J. Environ. Manag. 2022, 305, 114374. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, D.R.; Knelman, J.E.; Ferrenberg, S.; Bilinski, T.; Melbourne, B.; Jiang, L.; Violle, C.; Darcy, J.L.; Prest, T.; Schmidt, S.K. Decreases in average bacterial community rRNA operon copy number during succession. ISME J. 2016, 10, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Van Der Gast, C.J.; Ager, D.; Lilley, A.K. Temporal scaling of bacterial taxa is influenced by both stochastic and deterministic ecological factors. Environ. Microbiol. 2008, 10, 1411–1418. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, C.; Lin, H.; Liu, W.; Qin, W.; Chen, T.; Yang, T.; Wen, X. Differences in distributions, assembly mechanisms, and putative interactions of AOB and NOB at a large spatial scale. Front. Environ. Sci. Eng. 2023, 17, 122. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, Y.; Ye, R.; Zhang, J.; Chen, Q.; Yang, T.; Chen, T.; Zhang, B. The Relationship between Ribosomal RNA Operon Copy Number and Ecological Characteristics of Activated Sludge Microbial Communities across China. Water 2024, 16, 2246. https://doi.org/10.3390/w16162246
Li J, Zhao Y, Ye R, Zhang J, Chen Q, Yang T, Chen T, Zhang B. The Relationship between Ribosomal RNA Operon Copy Number and Ecological Characteristics of Activated Sludge Microbial Communities across China. Water. 2024; 16(16):2246. https://doi.org/10.3390/w16162246
Chicago/Turabian StyleLi, Jiaying, Yunwei Zhao, Ruisi Ye, Jingyue Zhang, Qianhui Chen, Ting Yang, Tan Chen, and Bing Zhang. 2024. "The Relationship between Ribosomal RNA Operon Copy Number and Ecological Characteristics of Activated Sludge Microbial Communities across China" Water 16, no. 16: 2246. https://doi.org/10.3390/w16162246
APA StyleLi, J., Zhao, Y., Ye, R., Zhang, J., Chen, Q., Yang, T., Chen, T., & Zhang, B. (2024). The Relationship between Ribosomal RNA Operon Copy Number and Ecological Characteristics of Activated Sludge Microbial Communities across China. Water, 16(16), 2246. https://doi.org/10.3390/w16162246






