Hydrochemical Evolution Process and Mechanism of Groundwater in the Hutuo River Alluvial Fan, North China
Abstract
1. Introduction
2. Materials and Methods
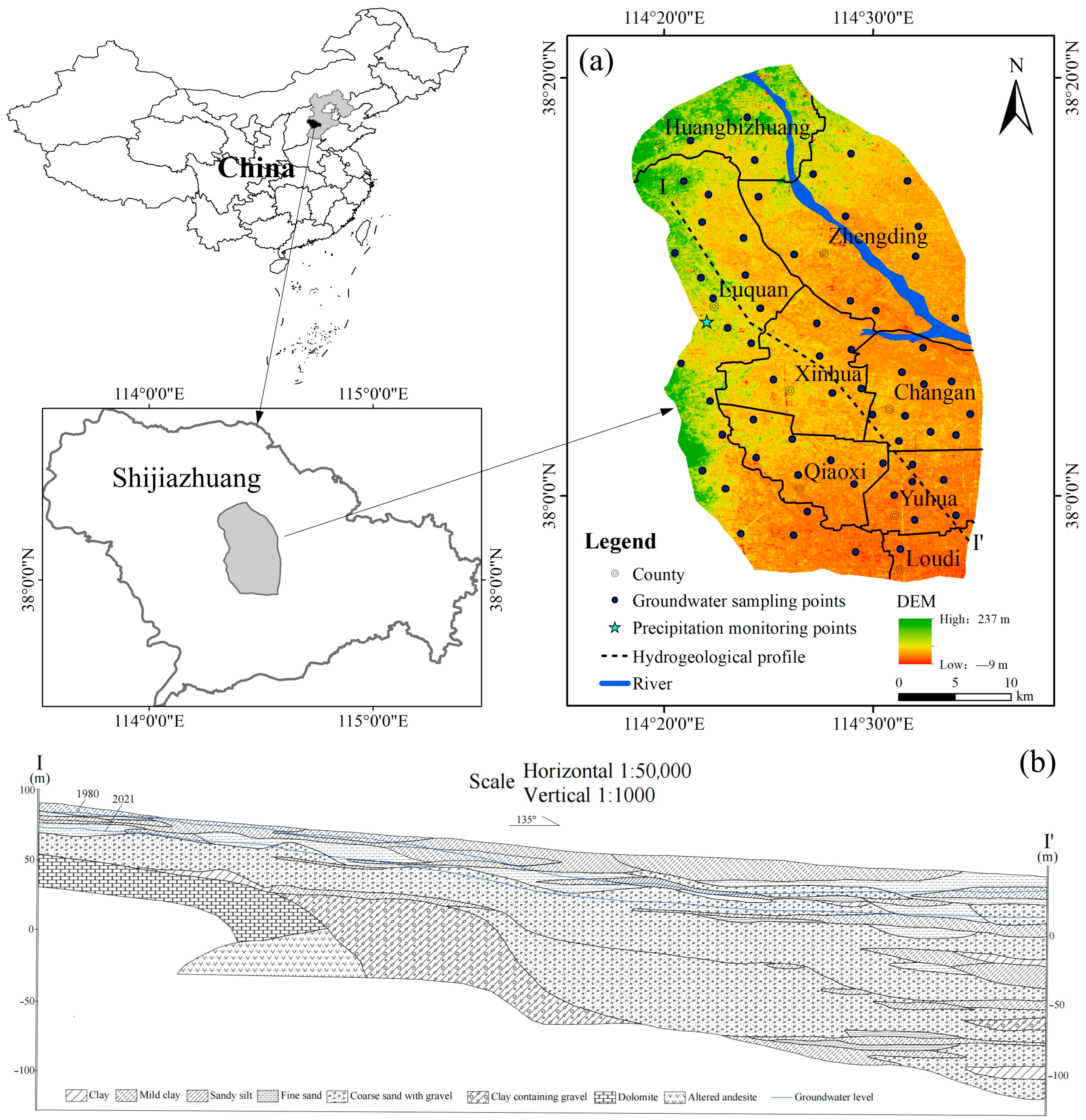
2.1. Study Area
2.2. Data Source
2.3. Research Method
3. Results
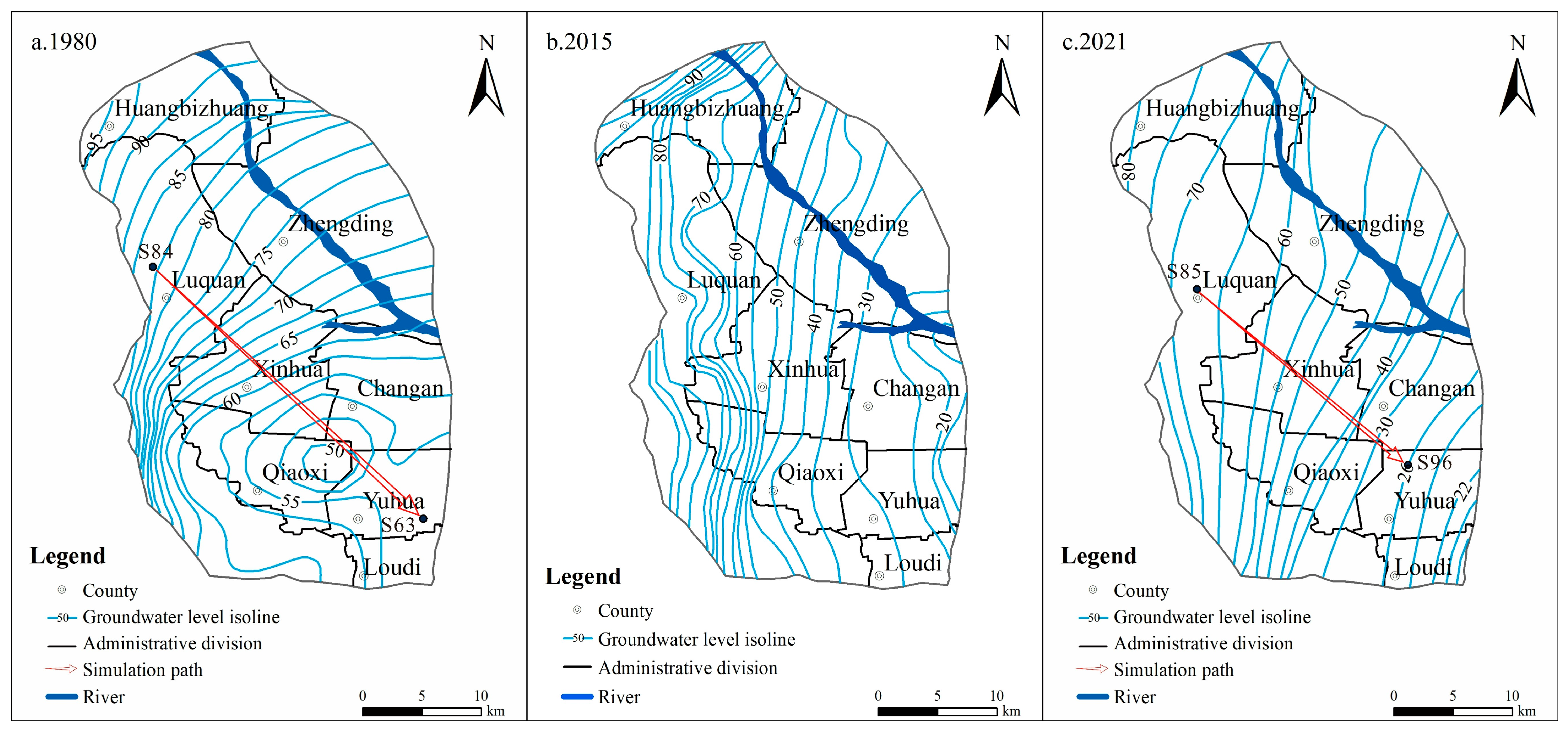
3.1. Characteristics of Groundwater Level Evolution
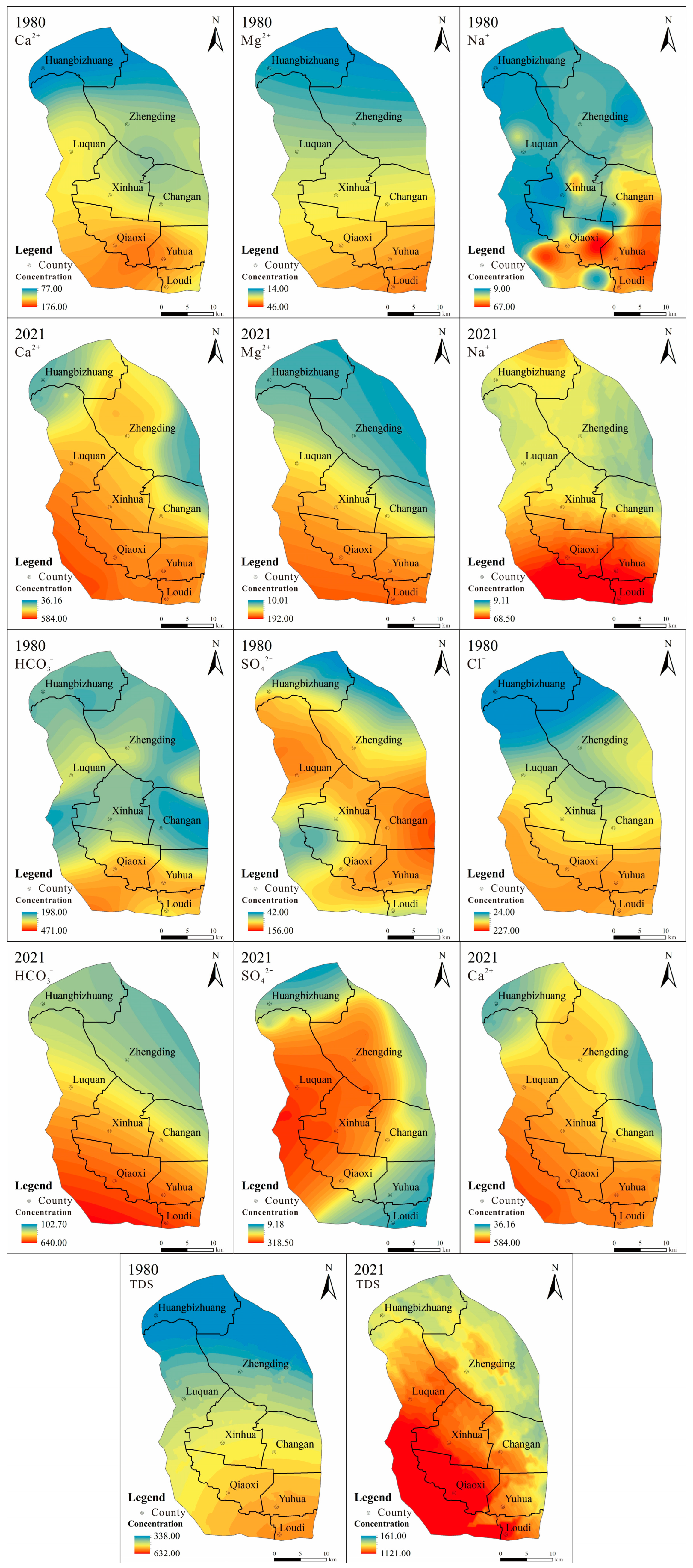
3.2. Distribution of Hydrochemical Characteristics
3.2.1. Temporal Distribution
3.2.2. Correlation Analysis between Groundwater Level and Hydrochemistry
3.2.3. Spatial Distribution
3.3. Sources of Hydrochemical Components
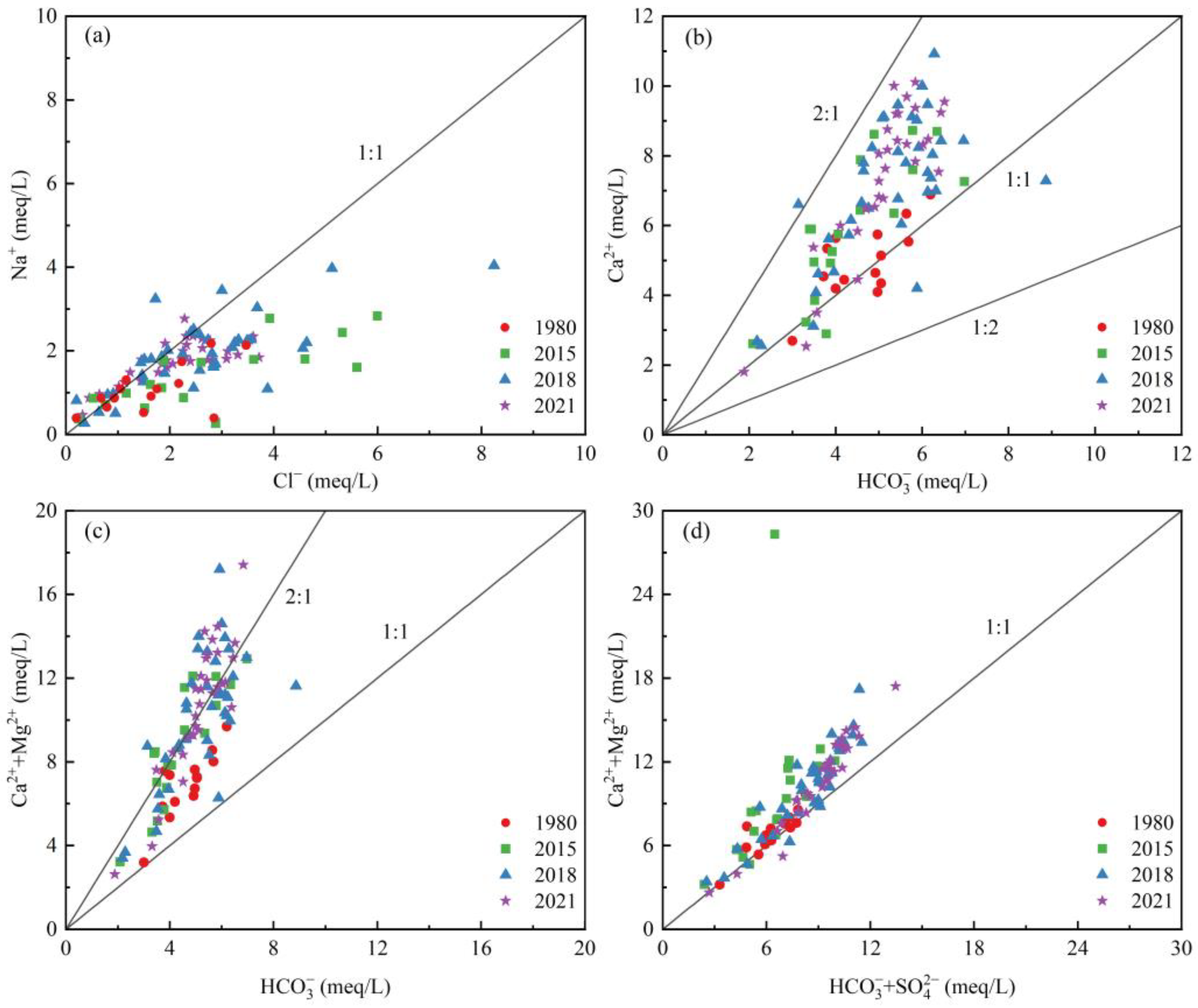
3.3.1. Dissolution
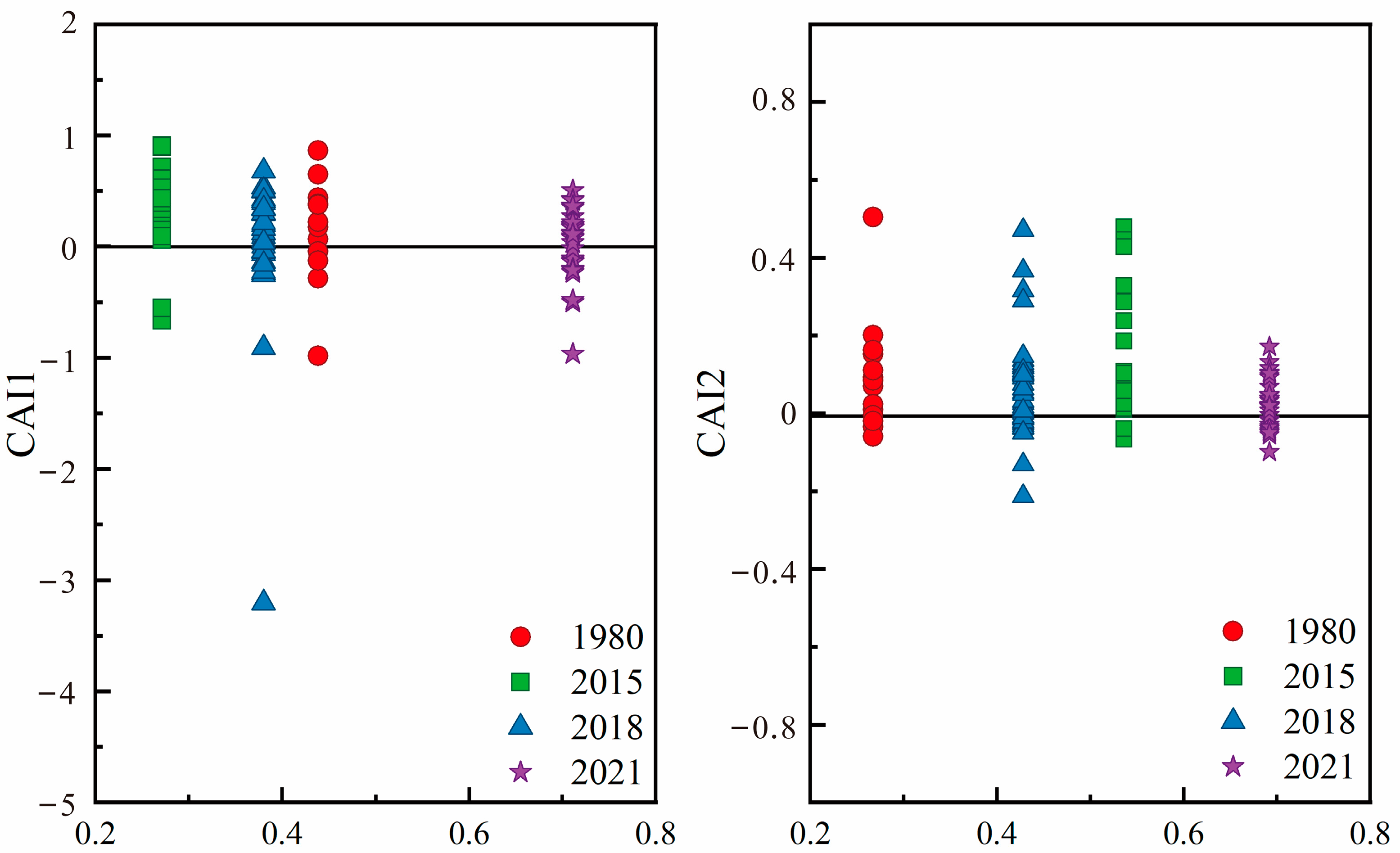
3.3.2. Ion Exchange
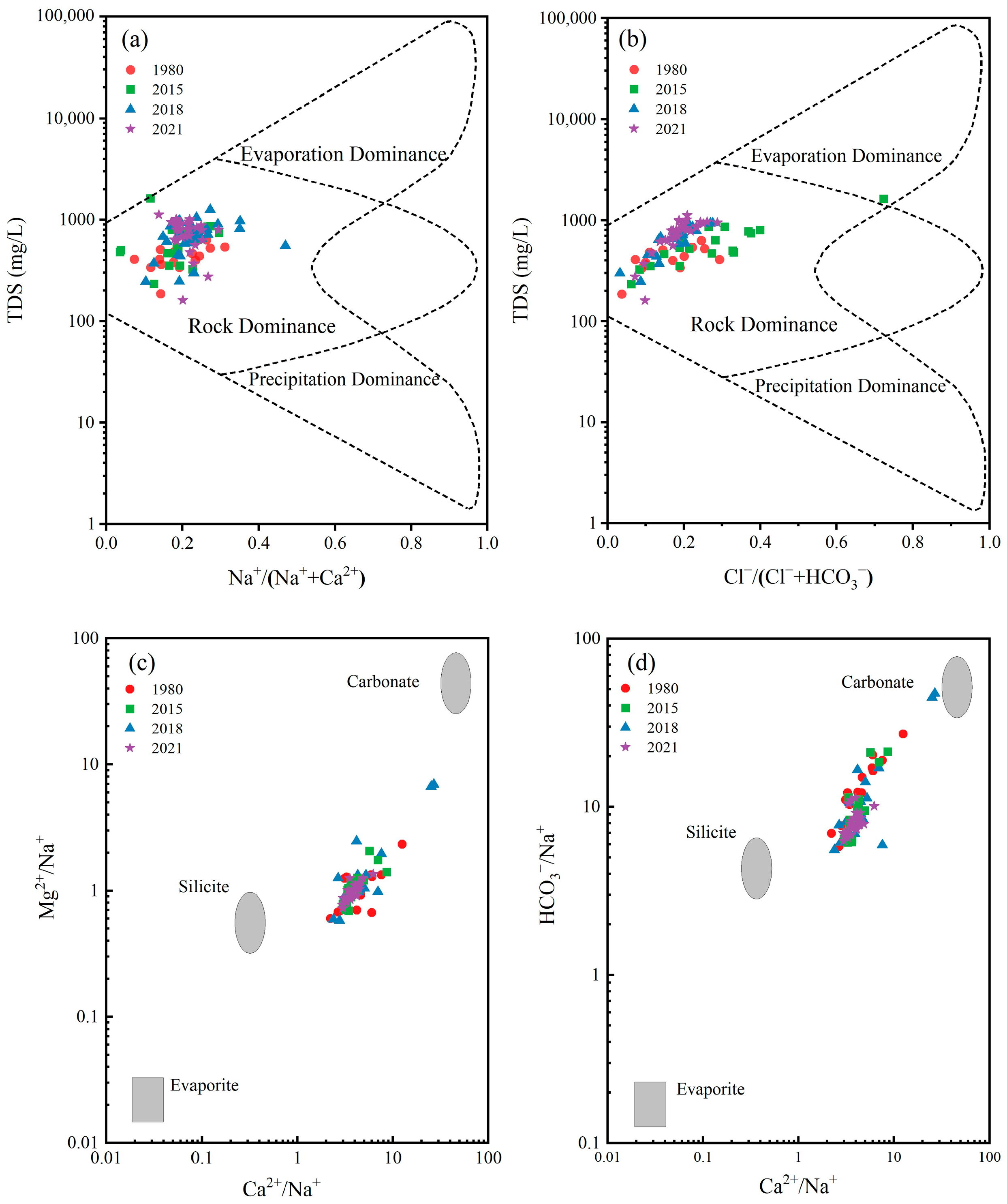
3.3.3. Ion Proportion Relation
3.4. Inverse Hydrogeochemical Simulation
4. Discussion
4.1. Influence of Groundwater Dynamic Field
4.2. Ion Sources and Hydrochemical Evolution
4.3. Human Activities
5. Conclusions
- (1)
- The ion concentrations gradually increased along the groundwater flow path and displayed a pattern of lower levels in the northwest and higher levels in the southeast. The spatial distribution of major ion concentrations in 1980 indicated a diverse spread. In 2021, the spatial distribution of major ion concentrations indicated a more uniform distribution. From 1980 to 2021, the concentrations of major ions increased.
- (2)
- In 1980, the dominant cation in the groundwater was Ca2+, and the dominant anion was HCO3−, where the hydrochemical type was primarily the HCO3—Ca type. From 1980 to 2015, the concentrations of Cl− and SO42− increased, which rendered the hydrochemical type more complex and resulted in the formation of HCO3·Cl—Ca, HCO3—Ca·Mg, and HCO3·SO4—Ca types. Following artificial management, the groundwater level rose, which led to an increase in the concentrations of SO42− and Mg2+. The implementation of artificial governance in 2015 resulted in the emergence of HCO3·SO4—Ca·Mg-type water. By 2021, this type of groundwater had become the predominant hydrochemical type.
- (3)
- The groundwater flowed from northwest to southeast. The western region was an area of rising groundwater levels, while the eastern region experienced declining groundwater levels. Changes in the groundwater level and ion concentrations were strongly quantitatively correlated and exhibited spatial similarity. The evolution of the groundwater chemical components was predominantly influenced by fluctuations in the groundwater levels.
- (4)
- In 1980s, the groundwater hydrochemical composition was primarily controlled by the dissolution of albite, dolomite, halite, and quartz; reverse cation exchange; and groundwater exploitation. Since 2015, the hydrochemical composition has been mainly influenced by the dissolution of albite, calcite, and quartz; positive cation exchange; river–groundwater mixing; and industrial activities, with an increasing intensity of both water–rock interactions and human activities.
- (5)
- The water–rock interactions predominantly involved cation exchange and the dissolution of silicate and carbonate rocks, while human activities were mainly influenced by industrial activities.
- (6)
- The hydrochemical evolution and formation mechanisms of the Hutuo River alluvial fan in 1980 and post-2015 were analyzed in this study. However, the hydrochemical evolution of groundwater between 1980 and 2015 was not analyzed. Future research could address this gap and extend the findings of this study to explore further aspects, such as the response mechanisms of groundwater chemical evolution. This study found that the concentrations of SO42- and Mg2+ in the groundwater increased following the artificial groundwater governance. This phenomenon was also observed in Hengshui City, Hebei Province, China, where similar governance practices were implemented [8]. Investigating the reasons for this occurrence will be a direction for future research.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zekri, S. (Ed.) Water Policies in MENA Countries; Global Issues in Water Policy; Springer International Publishing: Cham, Switzerland, 2020; Volume 23, ISBN 978-3-030-29273-7. [Google Scholar]
- Chatterjee, R.; Jain, A.K.; Chandra, S.; Tomar, V.; Parchure, P.K.; Ahmed, S. Mapping and management of aquifers suffering from over-exploitation of groundwater resources in Baswa-Bandikui watershed, Rajasthan, India. Environ. Earth Sci. 2018, 77, 157. [Google Scholar] [CrossRef]
- Jasechko, S.; Seybold, H.; Perrone, D.; Fan, Y.; Shamsudduha, M.; Taylor, R.G.; Fallatah, O.; Kirchner, J.W. Rapid groundwater decline and some cases of recovery in aquifers globally. Nature 2024, 625, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Nazanin, S. Temporal Analysis of Land Subsidence and Groundwater Depletion Using the DInSAR and Kriging Methods: A Case Study and Insights. J. Hydrol. Eng. 2024, 29, 04024011. [Google Scholar]
- Lu, C.; Wu, C.; Sun, Q.; Wu, X.; Yan, L.; Qin, T. Seasonal river–lake-groundwater coupling simulation and groundwater overexploitation and ecological environment assessment in the Aiding Lake Basin, NW China. J. Hydrol. 2024, 632, 130896. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Partani, S.; Araghi, A.; Barati, R.; Farnoush, H.; Torabi Haghighi, A. Iran’s Groundwater Hydrochemistry. Earth Space Sci. 2021, 8, e2021EA001793. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Zhu, Y.; Liu, Y.; Li, Y.; Cao, S.; Hao, Q.; Liu, S.; Kong, X.; Han, Z.; et al. Evolution of groundwater hydrochemical characteristics and formation mechanism during groundwater recharge: A case study in the Hutuo River alluvial–pluvial fan, North China Plain. Sci. Total Environ. 2024, 915, 170159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, B.; Xu, T.; Xia, F. Hydrochemical evolution characteristics and mechanism of groundwater funnel areas under artificial governance in Hengshui City, North China. Ecol. Indic. 2023, 148, 110059. [Google Scholar] [CrossRef]
- Alfarrah, N.; Berhane, G.; Bakundukize, C.; Walraevens, K. Degradation of groundwater quality in coastal aquifer of Sabratah area, NW Libya. Environ. Earth Sci. 2017, 76, 664. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C.; Pan, S.; Shi, L. Analysis on the spatiotemporal evolutions of groundwater hydrochemistry and water quality caused by over-extraction and seawater intrusion in eastern coastal China. Front. Earth Sci. 2024, 12, 1391235. [Google Scholar] [CrossRef]
- Fuster, R.; Escobar-Avaria, C.; Silva-Urrutia, K.; Moya-Jofré, H.; Palacios-Quezada, A.K. Local institutional adaptation to groundwater overexploitation challenges: Case study from Copiapó aquifer, Chile. Water Int. 2024, 49, 369–376. [Google Scholar] [CrossRef]
- Chatterjee, R.S.; Singha, S.; Aggarwal, A.; Sharma, V.; Pranjal, P.; Karunakalage, A.; Jain, P.K.; Nagar, A.; Mitra, D.S.; Kumar, D.; et al. Reconnaissance to characterisation of land subsidence due to groundwater overdraft and oil extraction in and around Mehsana City, Gujarat, India by long-term hybrid differential interferometric SAR technique. J. Hydrol. 2023, 627, 130441. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Zhang, Y. Spatio-temporal analysis of groundwater chemistry, quality and potential human health risks in the Pinggu basin of North China Plain: Evidence from high-resolution monitoring dataset of 2015–2017. Sci. Total Environ. 2021, 800, 149568. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Gao, Z.; Chen, Q.; Wu, G.; Li, F. Hydrochemical characteristics and water quality assessment of groundwater in the Yishu River basin. Acta Geophys. 2020, 68, 877–889. [Google Scholar] [CrossRef]
- Carrión-Mero, P.; Montalván, F.J.; Morante-Carballo, F.; Heredia, J.; Elorza, F.J.; Solórzano, J.; Aguilera, H. Hydrochemical and Isotopic Characterization of the Waters of the Manglaralto River Basin (Ecuador) to Contribute to the Management of the Coastal Aquifer. Water 2021, 13, 537. [Google Scholar] [CrossRef]
- Kay, M.; Dimitrakopoulos, R. Integrated Interpolation Methods for Geophysical Data: Applications to Mineral Exploration. Nat. Resour. Res. 2000, 9, 53–64. [Google Scholar]
- Manish, K.; Rajesh, K.; Kumar, S.C.; Alok, K. Identification of Playa Lakes and tracking their evolution pathways using geochemical models in the Great Indian Thar desert. Sci. Total Environ. 2024, 912, 169250. [Google Scholar]
- Piantadosi, J.; Howlett, P.; Boland, J. Matching the grade correlation coefficient using a copula with maximum disorder. J. Ind. Manag. Optim. 2007, 3, 305–312. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.P.; Kamal, V.; Sen, R.; Mukherjee, S. Assessment of hydrogeochemistry and the quality of groundwater in 24-Parganas districts, West Bengal. Environ. Earth Sci. 2015, 73, 375–386. [Google Scholar] [CrossRef]
- Baumann, G.O.; Vital, M.; Glok-Galli, M.; Grondona, S.; Massone, H.; Martinez, D.E. Hydrogeochemical modeling and dedolomitization processes in the Patagonian Boulders and Patagonia Formation in the eastern Patagonia, Argentina. Environ. Earth Sci. 2019, 78, 572. [Google Scholar] [CrossRef]
- Shuaibu, A.; Kalin, R.M.; Phoenix, V.; Banda, L.C.; Lawal, I.M. Hydrogeochemistry and Water Quality Index for Groundwater Sustainability in the Komadugu-Yobe Basin, Sahel Region. Water 2024, 16, 601. [Google Scholar] [CrossRef]
- Saghravani, S.R.; Yusoff, I.; Vadiati, M.; Alias, Y.; Sracek, O.; Bhattacharya, P. Multi-isotopic and hydrochemical evidence of water resources evolution and recharge estimation in the tropical coastal aquifer. Groundw. Sustain. Dev. 2024, 24, 101065. [Google Scholar] [CrossRef]
- Olea-Olea, S.; Silva-Aguilera, R.A.; Alcocer, J.; Escolero, O.; Morales-Casique, E.; Florez-Peñaloza, J.R.; Almora-Fonseca, K.A.; Oseguera, L.A. Water–Rock Interaction Processes in Groundwater and Flows in a Maar Lake in Central Mexico. Water 2024, 16, 715. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, A.; Gao, S.; Zhao, D. Both natural and anthropogenic factors control surface water and groundwater chemistry and quality in the Ningtiaota coalfield of Ordos Basin, Northwestern China. Environ. Sci. Pollut. R. 2023, 30, 67227–67249. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, W.; Qiu, Y.; Chen, S.; Wang, D.; Luo, Y.; Qu, S.; Gao, R.; Xue, B.; Wang, G.; et al. Identifying the spatio-seasonal pattern of hydrochemical evolution and surface water-groundwater interaction in a large urban river basin, Northwest China. Sci. Total Environ. 2024, 944, 173989. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Zhang, Z.; Fei, Y.; Li, Y.; Zhang, F.; Chen, J.; Qian, Y. Assessment of deep groundwater over-exploitation in the North China Plain. Geosci. Front. 2011, 2, 593–598. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Q.; Liu, Y.; Zhu, J.; Chai, H.; Chen, K. Chemical composition of groundwater and its controlling factors in the Liuzhuang coal mine, northern Anhui Province, China. Water Supply 2023, 23, 4937–4956. [Google Scholar] [CrossRef]
- Ruiz-Pico, Á.; Pérez-Cuenca, Á.; Serrano-Agila, R.; Maza-Criollo, D.; Leiva-Piedra, J.; Salazar-Campos, J. Hydrochemical characterization of groundwater in the Loja Basin (Ecuador). Appl. Geochem. 2019, 104, 1–9. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Gao, X.; Khattak, S.A.; Ali, L.; Li, C.; Ahmad, A.; Khan, S.; Rinklebe, J.; Ahmad, P. Hydrogeochemical characteristics, stable isotopes, positive matrix factorization, source apportionment, and health risk of high fluoride groundwater in semiarid region. J. Hazard. Mater. 2024, 469, 134023. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Jinlong, Z.; Yunfei, C.; Xing, W.; Yanyan, Z.; Ying, S. Identifying the factors controlling surface water and groundwater chemical characteristics and irrigation suitability in the Yarkant River Basin, northwest China. Environ. Res. 2023, 223, 115452. [Google Scholar]


| Item | Date | Minimum | Maximum | Mean | Median | Coefficient of Variation |
|---|---|---|---|---|---|---|
| Ca2+ | 1980 | 77.00 | 176.00 | 110.36 | 107.00 | 0.23 |
| 2015 | 52.36 | 398.79 | 132.77 | 118.20 | 0.55 | |
| 2018 | 51.30 | 248.50 | 144.20 | 149.15 | 0.31 | |
| 2021 | 36.16 | 584.00 | 152.82 | 151.10 | 0.51 | |
| Mg2+ | 1980 | 14.00 | 46.00 | 26.39 | 25.00 | 0.28 |
| 2015 | 7.33 | 102.22 | 35.70 | 33.30 | 0.57 | |
| 2018 | 8.75 | 59.30 | 36.29 | 35.61 | 0.35 | |
| 2021 | 10.01 | 192.00 | 41.13 | 40.02 | 0.27 | |
| Na+ | 1980 | 9.00 | 67.00 | 26.15 | 25.00 | 0.50 |
| 2015 | 4.40 | 63.80 | 30.56 | 26.20 | 0.60 | |
| 2018 | 6.24 | 92.94 | 43.92 | 43.21 | 0.46 | |
| 2021 | 9.11 | 68.50 | 39.71 | 39.68 | 0.35 | |
| Cl− | 1980 | 24.00 | 227.00 | 73.79 | 62.00 | 0.71 |
| 2015 | 8.44 | 212.53 | 94.84 | 80.23 | 0.65 | |
| 2018 | 7.09 | 292.13 | 91.03 | 89.15 | 0.59 | |
| 2021 | 11.20 | 246.00 | 86.84 | 80.94 | 0.67 | |
| SO42− | 1980 | 42.00 | 156.00 | 85.33 | 83.00 | 0.33 |
| 2015 | 15.40 | 200.70 | 101.03 | 98.55 | 0.45 | |
| 2018 | 17.00 | 261.60 | 154.65 | 158.25 | 0.44 | |
| 2021 | 9.18 | 318.50 | 164.29 | 162.10 | 0.44 | |
| HCO3− | 1980 | 198.00 | 471.00 | 293.94 | 300.00 | 0.18 |
| 2015 | 127.02 | 425.63 | 269.29 | 247.68 | 0.27 | |
| 2018 | 132.84 | 540.63 | 316.08 | 331.93 | 0.26 | |
| 2021 | 102.70 | 640.00 | 313.99 | 417.00 | 0.25 | |
| NO3− | 1980 | \ | \ | \ | \ | \ |
| 2015 | 1.56 | 103.63 | 41.68 | 33.57 | 0.65 | |
| 2018 | 0.68 | 148.81 | 37.36 | 20.65 | 1.05 | |
| 2021 | 0.81 | 44.47 | 16.65 | 16.33 | 0.61 | |
| TDSs | 1980 | 186.00 | 632.00 | 428.27 | 408.00 | 0.25 |
| 2015 | 233.38 | 1629.07 | 628.73 | 536.53 | 0.48 | |
| 2018 | 245.54 | 1255.12 | 718.75 | 727.23 | 0.31 | |
| 2021 | 161.00 | 1121.00 | 761.26 | 797.00 | 0.29 |
| Mineral Phase | 1980 | 2021 |
|---|---|---|
| Simulation path | S84→S63 | S85→S96 |
| Albite | +0.0007420 | +0.001706 |
| Anorthite | \ | \ |
| Calcite | −0.2318 | +1.105 |
| Dolomite | +0.4754 | −0.1372 |
| Gypsum | −0.2640 | −0.7201 |
| Halite | +2.225 | −0.4434 |
| Quartz | +0.07118 | +0.04011 |
| CaX2 | +0.6152 | −0.7278 |
| NaX | −1.230 | +1.456 |
| CO2 (g) | +1.464 | +1.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, J.; Yan, B.; Fan, C.; Tuo, Y.; Ma, M. Hydrochemical Evolution Process and Mechanism of Groundwater in the Hutuo River Alluvial Fan, North China. Water 2024, 16, 2229. https://doi.org/10.3390/w16162229
Gai J, Yan B, Fan C, Tuo Y, Ma M. Hydrochemical Evolution Process and Mechanism of Groundwater in the Hutuo River Alluvial Fan, North China. Water. 2024; 16(16):2229. https://doi.org/10.3390/w16162229
Chicago/Turabian StyleGai, Junbai, Baizhong Yan, Chengbo Fan, Yapeng Tuo, and Miaomiao Ma. 2024. "Hydrochemical Evolution Process and Mechanism of Groundwater in the Hutuo River Alluvial Fan, North China" Water 16, no. 16: 2229. https://doi.org/10.3390/w16162229
APA StyleGai, J., Yan, B., Fan, C., Tuo, Y., & Ma, M. (2024). Hydrochemical Evolution Process and Mechanism of Groundwater in the Hutuo River Alluvial Fan, North China. Water, 16(16), 2229. https://doi.org/10.3390/w16162229






