Abstract
Defluoridation of water was evaluated using a copper–magnesium (Cu-Mg) coated sand (CMCS) as a sustainable adsorbent containing binary metal oxides. The CMCS sorbent coating contained mostly amorphous copper and magnesium oxides in the Cu-Mg coating on the crystalline sand surface. Pseudo-second-order kinetics was observed where most fluoride was removed rapidly within an hour. Favorable adsorption occurred according to the Langmuir and Freundlich adsorption equations, while physisorption occurred according to the Dubinin–Radushkevich (D-R) adsorption equation. The adsorption capacity of the CMCS sorbent based on sorbent surface was similar to various other adsorbents with larger adsorbent surface areas, likely due to the efficacy of the Cu-Mg coating despite the CMCS sorbent’s much smaller surface area. Fluoride was adsorbed effectively from pH 3 to pH 11 through adsorption of anionic fluoride onto the CMCS sorbent’s protonated surface with a pHPZC of 10.5, indicative of electrostatic attraction as the main adsorption mechanism. The CMCS sorbent’s re-coating was conducive to successful recycling and reuse of the CMCS sorbent as a sustainable adsorbent for water defluoridation.
1. Introduction
Human consumption of fluoride contaminated water may lead to serious human health issues such as bone weakness, diseases of the skeleton, and Alzheimer’s disease [1,2]. Groundwater ingestion is the most common conduit and source of fluoride contamination in the body among numerous media such as air, soil, cosmetics, food, water, and more. Groundwater is a major source of drinking water in the world for more than 2.5 billion people, with a 30% projected increase in world demand for groundwater by the year 2050 [3,4]. According to the International Program on Chemical Safety [5], the United States receives 38% of its drinking water from subsurface water sources, while more than 50% of the supply of drinking water worldwide is from subsurface water sources. Metal, glass, semiconductor, phosphate, and aluminum processing industries—as well as coal plants and phosphate fertilizer applications—all have an impact on water fluoride concentrations of up to 1000 mg/L. According to the U.S. EPA [6], the western and southern states in the US have the highest fluoride levels, where fluoride levels ranged from 7.4 mg/L in Arizona to 15.9 mg/L in Idaho. The US Public Health Service (PHS) recommends a potable water fluoride level of 0.7 mg/L [7] while the U.S. EPA has a requirement for maximum contaminant limit of 4.0 mg/L fluoride in drinking water [8], whereas the World Health Organization (WHO) recommends a maximum drinking water fluoride level of 1.5 mg/L [9]. The fluoride limits in drinking water vary by location and region due to climate, where they may be greater in hotter areas. These recommendations can also be impacted by daily water consumption, eating habits, lifestyle, age, and potential fluoride sources [10].
Fluoride removal processes differ according to the polluted area’s proximity to natural water sources, financial constraints, the availability of competent labor or technicians, weather conditions, and population size. Each method has advantages and disadvantages, as well as optimal conditions to consider. Ion exchange defluoridation can remove up to 95% of fluoride. However, resin regeneration produces a large amount of rich waste and is costly due to resin manufacturing, pretreatment, pH adjustment, and disposal. Coagulation–precipitation is another defluoridation procedure. However, it necessitates continual computations and measurement of water chemical dosages. The reverse osmosis (RO) membrane technique removes more than 90% of fluoride from water, but it also eliminates important minerals for human growth and metabolism by removing all ions [10,11,12]. The adsorption technique has been widely explored and applied in recent years as a low-cost, high-quality water production method, which seems to be a preferred method for removal of fluoride from water among the methods discussed previously for several reasons, including the ability to use a wide range of materials as adsorbent depending on accessibility and budget, the great ability to remove fluoride in low to high concentrations in water, and the design, installation, and maintenance are typically low cost and do not require professional labor. There is the possibility of regenerating and reusing the exhausted adsorbent, and the treated water usually does not show signs of discoloration, unpleasant taste, or odor [10,12].
Various types of adsorbent materials, such as metal oxides/hydroxides (single, binary, and ternary metal oxides/hydroxides sorbents), biosorbents, geomaterials, carbonaceous adsorbents, and by-product materials, have been explored for removal of fluoride from water thus far [13,14,15,16,17]. Metal oxides/hydroxides are desirable alternative adsorbents for water defluoridation through selectivity and efficiency for fluoride removal, particularly in large-scale applications [16]. The majority of metal oxides/hydroxides research has focused on alumina and aluminum because of their cheap cost and minimal environmental impact. However, these adsorbents have certain drawbacks, such as slow adsorption rates in addition to lesser adsorption of fluoride at more alkaline solution pH (7–10) [18,19,20,21,22,23]. Therefore, polyvalent metals like magnesium, calcium, copper, zirconium, lanthanum, and cerium—which provide higher protonation potential and, consequently, better fluoride adsorption—have been recently considered [24,25]. Various polyvalent metal combinations have been explored and published, notably employing oxides of lanthanum), cerium, and zirconium, although all these polyvalent metals are often expensive and scarce [26,27,28,29].
Magnesium is a desirable coating element that can be used among various polyvalent metals because of its limited water solubility, non-toxicity, and application in a wide pH range due to its significant positive surface charge [30,31]. Copper is an element with excellent antibacterial activity and has been designated by the US EPA as an effective agent for antimicrobial activity in water treatment systems [32]; its high natural abundance makes it a promising option for testing as a fluoride adsorbent. To evaluate the fluoride removal performance of a metal-oxide-based adsorbent without the use of rare elements, a binary copper–magnesium-coated sand (CMCS) was fabricated for the removal of fluoride from water. Sand was selected to be the base material mainly because of its availability, low cost, and suitability as filtration media in continuous flow water treatment systems. Although adsorbents combining aluminum and copper [33], or magnesium with other elements such as aluminum [34], have been investigated, there has been no study in the past 20 years focusing on the binary combination of copper and magnesium specifically for fluoride removal; a novel binary metal-oxide-based adsorbent such as the CMCS sorbent has not as yet been researched and reported [14,15,17,35]. This study investigated the effectiveness of the CMCS sorbent for removal of fluoride from water, considering variables such as adsorbent dosage, pH and ionic strength, and adsorption kinetics. Furthermore, this study explored the reuse and the recycling of the copper–magnesium-coated sand as a sustainable adsorbent for defluoridation of water.
2. Materials and Methods
2.1. Chemicals
Copper (II) chloride dihydrate (99% purity), magnesium chloride hexahydrate (99% purity), sodium fluoride, sodium bicarbonate, sodium sulfate, hydrochloric acid, and sodium hydroxide were obtained from Fisher Scientific (Fairlawn, NJ, USA), and were all ACS grade or equivalent. The quartz silica sand had a particle size of 0.250 mm (50–70 US mesh) and was obtained from Sigma-Aldrich (St. Louis, MO, USA). Deionized (DI) water was produced in the laboratory with a resistance exceeding 18 MΩ. A 1000 mg/L stock solution of fluoride was prepared in the laboratory by adding sodium fluoride (NaF) to DI water; the initial stock solution of fluoride was diluted in DI water to prepare serial dilutions of the fluoride stock solution. The serial dilutions of the stock solution to prepare fluoride solutions were as follows: 3:100 (30 mg/L), 1:40 (25 mg/L), 1:50 (20 mg/L), 3:200 (15 mg/L), 3:250 (12 mg/L), 1:100 (10 mg/L), 7:1000 (7 mg/L), 1:200 (5 mg/L), 3:1000 (3 mg/L).
2.2. Preparation of Coated Sand Sorbent
To determine the optimal ratio(s) using different combinations of copper and magnesium for fabrication of the coated sand sorbent, various coating mixtures were prepared using 1 M magnesium solution and 1 M copper solution, with volume percentages ranging from 25% to 75% of 100 mL total volume for the coating solution. A mass of 203.3 g of MgCl2·6H2O was used to prepare 1 L of 1 M magnesium solution, and a mass of 170.5 g of CuCl2·2H2O was used to prepare 1 L of 1 M copper solution. Preliminary tests indicated that a 50–50% mixture of 1 M copper solution and 1 M magnesium solution (total volume of 100 mL coating solution) was found to be most effective for removal of fluoride. The percentages of removal of fluoride was 59%, 56%, 98%, and 96% for coated sand sorbent with coating solution containing 100% Mg, 25% Cu–75% Mg, 50%Cu–50% Mg, and 100% Cu, respectively. The 50% Cu–50% Mg coating solution, which had the largest fluoride removal efficiency, was therefore selected for further investigation in this study. For the CMCS sorbent preparation, a mixture of 50 mL of 1 M copper solution and 50 mL of 1 M magnesium solution was added to 40 g of silica sand; the sand-coating solution mixture was mixed in a beaker placed on a mixing plate. After 24 h of mixing, the wet coated sand was separated from the coating solution by decanting the residual coating solution; the coating solution was then placed in a polyethylene bottle for future use. A drying oven was used to dry the wet coated sand at 110 °C. After 24 h of oven drying, a furnace was used to calcine the dried coated sand at 220 °C. After 24 h, the calcined coated sand was removed from the furnace and then placed in polyethylene bottles after cooling down to room temperature for future use. The CMCS sorbent preparation procedure is shown in Figure S1.
2.3. Characterization of Coated Sand
The microstructure of the CMCS sorbent was investigated using a JEOL JEM-3010 (JEOL, Tokyo, Japan) 300 kV Transmission Electron Microscope (TEM). A TOPCON ABT-150S (Tokyo, Japan). Scanning Electron Microscope (SEM) was used to examine the surface morphology of the CMCS sorbent. Electron Dispersive X-ray Spectroscopy (EDX) was employed for elemental analysis of the CMCS sorbent surface. X-ray diffraction (XRD) using an XRD-Bruker D8 Discover System (Billerica, MA, USA) was carried out to determine the crystallinity of the coated sand surface. An Accelerated Surface Area and Porosimetry system, ASAP 2010 (Micromeritics Instrument Corporation, Norcross, GA, USA) was employed to determine the Brunauer–Emmett–Teller (BET) specific surface area of the coated sand sorbent.
2.4. Adsorption Experiments
The adsorption of fluoride onto the CMCS sorbent was investigated using batch adsorption experiments through evaluation of adsorption equilibrium and adsorption kinetics. The study also examined the effect of adsorbent dosage, adsorption pH, and common ions present in natural waters on the removal of fluoride by the CMCS sorbent. For each batch adsorption experiment, 50 mL of a fluoride solution and one gram of the CMCS sorbent were placed in polypropylene bottles, and the bottles were shaken using a rotating tumbler. After mixing for 24 h at 20 rpm, the sorbent–solution mixtures were centrifuged at 9000 rpm for 10 min to separate the solution from the CMCS sorbent. The concentration of fluoride in solution was determined using a fluoride electrode following Standard Method 4500-F [36]. The removal efficiency of fluoride and adsorption (uptake) of fluoride by the CMCS sorbent were determined using Equations (1) and (2), respectively:
where C0 (mg/L) and Ce (mg/L) were the initial and final (equilibrium) fluoride concentrations, respectively. The mass of the CMCS sorbent was M (kg), and the volume of the fluoride solution was V (L), while qe (mg/kg) was the fluoride adsorption (uptake) of the CMCS sorbent.
2.5. Zeta Potential Analysis
Experiments were performed by mixing 0.5 g of the CMCS sorbent with a volume of 1000 mL solution of 1 mM NaCl with and without fluoride. A Zeta-Meter system 3.0 (Zeta Meter Inc., Staunton, VA, USA) was employed to carry out the zeta potential analysis of the CMCS sorbent within a pH range of 5 to 11. A 0.1 M solution of HCl and a 0.1 M solution of NaOH were used to adjust the initial solution pH of the samples. All zeta potential experiments were carried out in triplicate.
2.6. Effect of pH on Fluoride Adsorption
The removal of fluoride by the CMCS sorbent was investigated as a function of solution pH. The effect of pH on adsorption was evaluated using a pH range of 3 to 11 for the initial solution pH. The initial solution pH of each sample was adjusted using a 0.1 M NaOH solution and a 0.1 M HCl solution. The samples were mixed using a rotating tumbler at 20 rpm. After 24 h of mixing, the final fluoride concentration in solution and the final solution pH were determined for each sample. All pH study experiments were performed in triplicates.
2.7. Effect of Co-Existing Ions on Fluoride Adsorption
The investigation focused on the impact of several ions commonly found in natural waters on the adsorption and removal of fluoride by the CMCS sorbent. Solutions containing individual co-existing ions calcium (Ca2+), bicarbonate (HCO3−), and sulfate (SO42−) were prepared for the following concentrations: 0.5, 1, and 2 mM for each ion, and 5 mM only for bicarbonate. The initial concentration of fluoride was 5 mg/L in all solutions containing individual ions. The collective influence of the three co-existing ions on adsorption of fluoride was also explored by combining 1 mM (40 mg/L) calcium, 2.5 mM (152 mg/L) bicarbonate, and 1 mM (96 mg/L) sulfate in the same solution referred to as the synthetic solution. In addition, the adsorption of fluoride from two natural water sources was evaluated using municipal tap water for Chicago, Illinois (obtained from Lake Michigan) and a groundwater from North-Central Illinois. The synthetic solution, the Chicago tap water, and the real groundwater samples were all spiked with 5 mg/L of fluoride to evaluate the removal of fluoride. The synthetic solution had a pH of 7.99, a TDS of 340 mg/L, a total hardness of 100 mg/L as CaCO3, and an alkalinity of 125 mg/L as CaCO3. The Chicago tap water had a pH of 7.86, a TDS of 171 mg/L, a total hardness of 140 mg/L as CaCO3, an alkalinity of 103 mg/L as CaCO3, and a background fluoride concentration of 0.9 mg/L. The groundwater sample had a pH of 7.91, a TDS of 1220 mg/L, a total hardness of 796 mg/L as CaCO3, an alkalinity of 150 mg/L as CaCO3, and a background fluoride concentration of 0.3 mg/L.
2.8. Adsorbent Recycling and Re-Use Experiments
The CMCS sorbent was recycled through the re-coating of the used sorbent. Before and after the re-coating of the CMCS sorbent, three sequential batch adsorption tests were carried out in triplicates. For the first adsorption cycle, a volume of 250 mL of a 5 mg/L fluoride solution was mixed with 5 g of fresh CMCS sorbent at 20 rpm in a rotating tumbler. After 24 h of mixing, the sorbent-solution mixture was centrifuged to separate the CMCS sorbent from the solution. For the second adsorption cycle, 250 mL of fresh 5 mg/L fluoride solution was added to the once-used 5 g of the CMCS sorbent obtained from the first batch adsorption test. The same procedure was repeated for the third adsorption cycle. After performing three sequential adsorption cycles, the re-coating of the exhausted CMCS sorbent was carried out using the spent coating solution initially used for preparation of the fresh CMCS sorbent (Section 2.2). The re-coated CMCS sorbent was dried and calcined according to the procedure for preparation of the coated sand sorbent described in Section 2.2. The re-coated CMCS sorbent was then reused for the removal of fluoride in three sequential adsorption cycles similar to the procedure carried out for the fresh CMCS sorbent.
3. Results
3.1. Characterization of CMCS Sorbent
Figure 1 shows the XRD patterns for the CMCS sorbent and the sand. The XRD spectra revealed that there were no peaks associated with crystalline oxides of magnesium or copper. The observed peaks were due to crystalline silicon dioxide of the silica sand. This observation showed that the coating (magnesium and copper) may have formed amorphously on the surface of the crystalline silica sand. Figure S2 shows an SEM image for the CMCS sorbent with a particle size of about 250 microns, while the observed coating clusters ranged from 5 to 20 microns. Figure 2 shows the SEM-EDX spectra for the CMCS sorbent. The SEM-EDX results indicate that the surface coating contained copper and magnesium, while the silica sand (sorbent base material) contained silicon and oxygen on the sand surface. The average weight percentages of copper and magnesium on the CMCS sorbent surface were 8.75% and 4.36%, respectively. The loading of sand with copper–magnesium oxides was estimated to be 10.95% for copper oxide (CuO) and 7.23% for magnesium oxide (MgO), for a total of 18.18% copper–magnesium oxides.
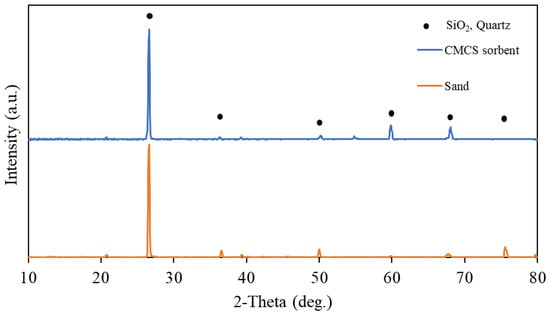
Figure 1.
XRD patterns for the CMCS sorbent and sand.
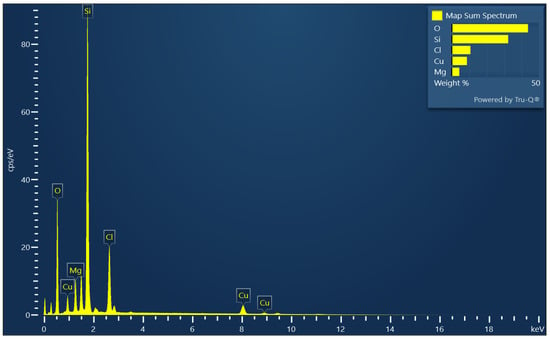
Figure 2.
EDX spectra for the CMCS sorbent.
Figure S3 shows the mapping images for the distribution of copper and magnesium on the surface of the CMCS sorbent. The mapping images indicate comparable distribution of copper and magnesium on the CMCS sorbent surface, where the major distribution of magnesium was mapped in similar fashion with the distribution of copper. Both copper and magnesium appeared in two main clusters, in a large cluster on the left side of the images and in another large cluster toward the right side of the images while there was more magnesium present in the upper portion of the right cluster. The mapping images show that while copper was present in the upper center sector, there was little magnesium present in the same sector. Besides the two main clusters for both copper and magnesium, the mapping results also indicate a wider distribution of copper than magnesium on the CMCS sorbent surface.
Figure 3 shows the TEM micrographs of the CMCS sorbent. The results from TEM analysis indicate that the coating on the CMCS sorbent surface exhibited a mainly amorphous structure with mainly non-crystalline oxides of copper and magnesium. The TEM analysis also identified a crystalline region of the surface coating, which was likely associated with quartz given its lattice spacing of 0.49 nm, closely resembling the lattice parameters for quartz (a = b = 4.91 Å, c = 5.4 Å); the probability of these crystals being related to magnesium and copper was low, as the MgO lattice parameter is 4.21 Å, ruling out magnesium, while copper oxides can be complex due to copper’s multiple valences. The CMCS sorbent had a BET specific surface area of 1.055 m2/g.
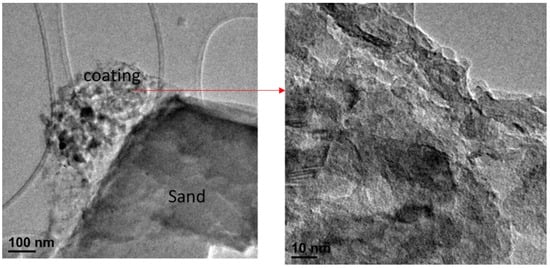
Figure 3.
CMCS sorbent TEM images.
The TEM, SEM/EDX, and XRD results demonstrate that the CMCS sorbent coating had a mostly amorphous structure while containing non-crystalline oxides of copper and magnesium, whereas the silica sand base material was highly crystalline silica.
3.2. Adsorption Kinetics Experiments
Figure 4 shows the removal of fluoride from water as function of time using the CMCS sorbent. The results from Figure 4 show the rapid removal of fluoride by the CMCS sorbent, exceeding 87% removal of fluoride within the initial 30 min of contact time. Thereafter the fluoride removal continued to increase, surpassing 90% removal within the first 2 h of contact time. The solution pH values ranged from 5.45 to 5.96.
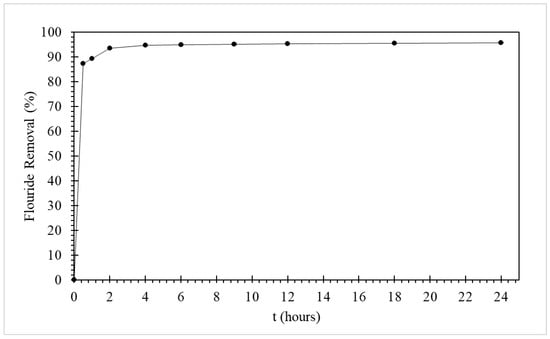
Figure 4.
Removal of fluoride with time using 20 g/L of CMCS sorbent for an initial fluoride concentration of 5 mg/L.
The adsorption kinetics for fluoride adsorption onto the CMCS sorbent were determined using the fluoride removal data shown in Figure 4. The pseudo-first-order adsorption kinetics model [37] and the pseudo second-order adsorption kinetics model [38] were used to evaluate the fluoride adsorption kinetics. The fluoride removal data were fitted to the linearized equations for the pseudo-first-order and the pseudo-second-order adsorption kinetics models as shown in Equation (3) and Equation (4), respectively:
The fluoride adsorption or uptake (mg/kg) is qe at equilibrium and is qt at time t, while the first-order adsorption rate constant is k1 (1/min), and the second-order rate constant is k2 (g/mg·min). Figure S4 shows the plots for the fit of adsorption kinetics data to the linearized pseudo-first-order and pseudo-second-order adsorption kinetics equations. Table 1 shows the results obtained from the plots in Figure S4, indicating that fluoride adsorption kinetics followed pseudo-second-order kinetics with a R2 value of 1.0; the k2 value was 0.1148 (g/mg-min), and the calculated qe value was 270.3 mg/kg, closely matching the experimental value of 270.5 mg/kg for qe.

Table 1.
Fluoride adsorption kinetics parameters.
The fluoride adsorption kinetics data were also analyzed to determine the adsorption rate controlling process using the intraparticle diffusion equation proposed by Weber and Morris [39]:
where kid (mg/g·min½) is the intraparticle diffusion constant and the C value is indicative of boundary layer effect. Figure S5A shows a plot of qt versus t1/2 where a non-zero value of C was obtained (Table 1). The results for the non-zero value of C indicate that adsorption of fluoride was not solely controlled by intraparticle diffusion.
Figure S5B shows a relatively flat second segment of the plot (for t1/2 range from 5 to 38), whereas the first segment of the plot in Figure S5B showed a steep slope (for t1/2 from 0 to 5). The steep slope of the plot’s first segment indicates that the fluoride adsorption rate was mainly controlled by external mass transfer, while the less-steep slope of the plot’s second segment indicates that intraparticle diffusion contributed to the fluoride adsorption process to a lesser extent.
3.3. Adsorption Equilibrium Experiments
The adsorption of fluoride was evaluated as function of adsorbent dosage through the application of different masses of the CMCS sorbent from a dosage of 2 g/L to a dosage of 48 g/L for a 5 mg/L fluoride solution. The effect of adsorbent dosage on fluoride removal is shown in Figure 5, where the fluoride removal percentage increased from 69% to 97% for a 2 g/L to 12 g/L increase in CMCS sorbent dosage, while higher removal of fluoride was observed in small increments for CMCS sorbent dosages greater than 12 g/L. The solution pH values ranged from 5.69 to 6.35.
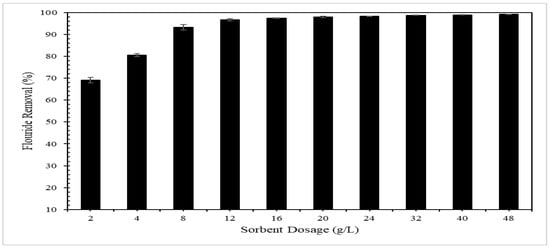
Figure 5.
Fluoride removal as function of CMCS sorbent dosage for 5 mg/L fluoride solution.
The removal of fluoride as a function of fluoride initial concentration is shown in Figure 6 using a 12 g/L dosage of CMCS sorbent. For an initial fluoride concentration from 3 mg/L to 10 mg/L, the CMCS sorbent removed more than 90% of fluoride from solution. For an initial fluoride concentration from 10 mg/L to 30 mg/L, the CMCS sorbent removed less fluoride from the solution (about 92% down to about 70%).
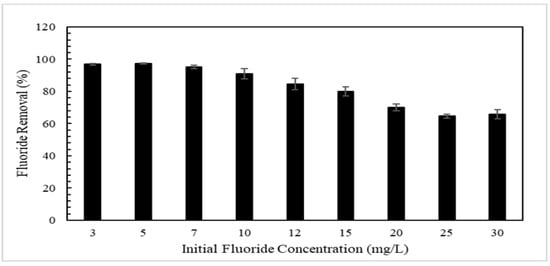
Figure 6.
Fluoride removal as function of initial fluoride concentration using 12 g/L dosage of CMCS sorbent.
Figure 7 shows the adsorption equilibrium isotherm data obtained from adsorption isotherm experiments.
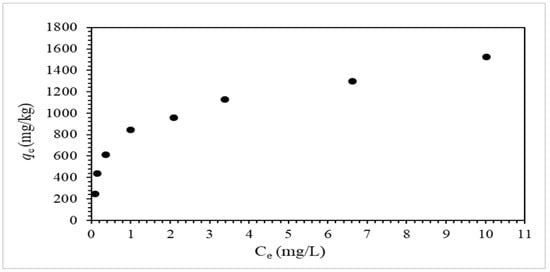
Figure 7.
Adsorption equilibrium isotherm data (3 mg/L to 25 mg/L fluoride solutions, 12 g/L sorbent dosage).
The linearized forms of the Langmuir adsorption equation, the Freundlich adsorption equation, and the Dubinin–Radushkevich (D-R) adsorption equation are shown in Table 2 (Equations (6)–(11)); the adsorption isotherm data from Figure 7 were fitted to the equations in Table 2. The adsorption process is described by the Langmuir adsorption model as a monolayer over the homogeneous surface of the adsorbent with uniform adsorption energy on active sites. The Langmuir adsorption model parameters (Equation (6)) are the adsorption capacity (qm) and the adsorption (binding) strength (KL). The RL value for Langmuir adsorption model (Equation (7)) is the separation factor, which represents the adsorption favorability; the adsorption is favorable for an RL value of 0 < RL < 1.

Table 2.
Linearized adsorption equations used for equilibrium adsorption of fluoride.
The Freundlich adsorption model describes a heterogeneous surface of the adsorbent with multilayer adsorption. The Freundlich adsorption equation (Equation (8)) parameter 1/n represents the adsorption (binding) strength and the adsorption parameter KF represents the adsorption capacity of the adsorbent; favorable adsorption is designated by 1/n values less than 1.0. The mean free energy of adsorption (E) may be determined from the Dubinin–Radushkevich (D-R) adsorption equation (Equation (9)). For calculation of ε (Equation (10)), R is the universal gas constant (8.314 J/mol K) and T is the temperature (degrees K). The value of E is indicative of physical adsorption for E less than 8 kJ/mol while it is indicative of chemisorption for E between 8 and 16 kJ/mol.
The adsorption parameter obtained for the Langmuir adsorption equation, the Freundlich adsorption equation and the D-R adsorption equation are shown in Table 3. The results from Table 3 show that the CMCS sorbent adsorbed fluoride according to both the Langmuir equation (R2 = 0.9844) and the Freundlich equation (R2 = 0.9404). The results indicate that fluoride adsorbed homogeneously onto the CMCS sorbent surface active sites with similar energy while fluoride also adsorbed onto active adsorption sites with dissimilar energy levels; the combination of active site energy levels was likely due to the presence of amorphous magnesium oxides and copper oxides in the surface coating of the CMCS sorbent. The results from Table 3 show a qm value of 1667 mg/kg and a KL value of 1.2 L/mg, while showing a KF value of 739 and a 1/n value of 0.342. The adsorption of fluoride was highly favorable according to the value of 0.342 for 1/n and the value of 0.143 for RL; a mean free energy (E) of 3.5 kJ/mol was indicative of physical adsorption of fluoride.

Table 3.
Adsorption equilibrium parameters.
The surface-normalized adsorption capacity (mg/m2) of an adsorbent may be reported based on the surface area of the adsorbent by dividing the qm value (mg/kg) by the BET surface area of the adsorbent (m2/g). For the adsorption of fluoride by the CMCS sorbent, a surface-normalized adsorption capacity of 1580 µg/m2 was determined by dividing the qm value (1667 mg/kg) by the BET area (1.055 m2/g). The surface-normalized adsorption capacity of the CMCS sorbent and several other metal oxide adsorbents are shown in Table 4, indicating that adsorption capacity of the CMCS sorbent per unit surface area was either greater than or comparable to those of other single or binary metal oxide adsorbents, which was likely attributed to the effectiveness of the Cu-Mg coating present on an appreciably smaller surface area of the CMCS sorbent. A comparison of the Langmuir adsorption parameter KL is also shown in Table 4, where the KL value of 1.2 L/mg obtained for the CMCS sorbent was comparable to or greater than the KL values reported for several other metal oxide adsorbents, indicative of stronger binding of fluoride with the CMCS sorbent than with most of the other binary metal adsorbents. The CMCS sorbent’s KL value of 1.2 L/mg was also greater than an aluminum-coated sand sorbent’s KL value of 0.33 L/mg, which used the same base material of sand [43]. The ΔG° for adsorption of fluoride onto the CMCS sorbent was estimated to be about −7.8 kJ/mol at 25 °C based on the equation for ΔG° = −RT(lnKL); the negative value of ΔG° indicates that the adsorption process was spontaneous.

Table 4.
Comparison of surface normalized adsorption capacities.
3.4. Surface Charge Analysis of Adsorbent
The results for the surface charge analysis of the CMCS sorbent are presented in Figure 8, showing the zeta potential values for the CMCS sorbent in a solution containing 5 mg/L fluoride and in a solution without fluoride. Figure 8 shows that the CMCS sorbent surface was positively charged for pH less than 10.5 while the CMCS sorbent surface was negatively charged for pH greater than 10.5, with a pHPZC value attained at 10.5. Other metal oxide adsorbents containing magnesium or copper, such as magnesia-amended silicon dioxide granules [44], zinc–magnesium–aluminum ternary metal oxide [48], aluminum–copper oxide nanoparticles supported on steel slag waste [46], and cupric oxide nanoparticles [49] had pHPZC values of 10, 10.5, 8.74, and 8.6, respectively. In the presence of fluoride in solution, the CMCS sorbent exhibited a significant shift in the pHPZC from 10.5 to 9.6, indicating the inner sphere complexation of fluoride onto the CMCS sorbent; the adsorption of the negatively charged fluoride onto the CMCS sorbent resulted in a more negatively charged surface of the CMCS sorbent.
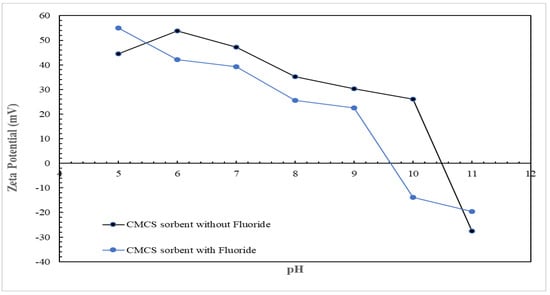
Figure 8.
CMCS sorbent surface charge analysis.
3.5. Effect of pH on Fluoride Adsorption
The removal of fluoride from water was affected by solution pH due to the changes in adsorbent characteristics based on the solution pH. Figure 9 shows the effect of solution pH on fluoride removal from water across a range of pH between pH 3 and pH 11. The CMCS sorbent removed greater than 90% of fluoride from water from pH 3 to pH 11, while the CMCS sorbent’s fluoride removal percentage decreased slightly from 98% at pH 10 to 91% at pH 11. The CMCS sorbent’s coating of magnesium and copper as multivalent metals resulted in significant protonation of the CMCS sorbent surface up to the pHPZC of 10.5, leading to an improved fluoride adsorption by the CMCS sorbent. At pH 11, the lesser adsorption of fluoride may be due to the negative surface charge of the CMCS sorbent above the pHPZC of 10.5 and an excess of hydroxide anions in higher solution pH. At higher solution pH, there could be competition between the fluoride anions (F−) and the hydroxide anions (OH−) for the available CMCS sorbent surface adsorption sites, even if a positive charge was retained on the CMCS sorbent surface [50]. The protonation of the CMCS sorbent surface at pH less than the pHPZC of 10.5 resulted in the positively charged surface of the CMCS sorbent at pH less than 10.5, while the CMCS sorbent surface became un-protonated at pH values higher than 10.5.
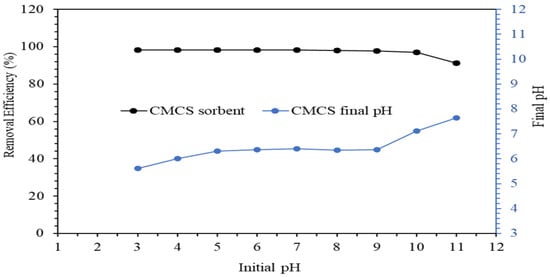
Figure 9.
Fluoride removal as function of solution pH using 20 g/L of CMCS sorbent and 5 mg/L fluoride solution.
3.6. Adsorption Mechanism
The hydroxide groups present on the surface of the CMCS sorbent served as the active adsorption sites for adsorption of fluoride. The un-protonated metal hydroxide groups on the surface of the CMCS sorbent “(Cu-Mg) OH” would become protonated in the presence of hydrogen ions, forming protonated hydroxide groups as follows:
Based on the surface charge analysis for the CMCS sorbent (Figure 8), the pHPZC of the CMCS sorbent was determined to be 10.5. The CMCS sorbent surface hydroxide groups would be protonated at pH below the pHPZC of 10.5, whereas the CMCS sorbent surface hydroxide groups would be un-protonated at pH above the pHPZC of 10.5. The possible interactions occurring between the fluoride anion and the protonated/un-protonated surfaces of the CMCS sorbent would be as follows:
3.7. Effect of Co-Existing Ions on Fluoride Adsorption
Natural waters contain a variety of cations and anions that can affect the adsorption of fluoride ions. Several ions commonly found in natural water were considered at varying concentrations. Figure 10 shows the effect of co-existing ions on fluoride removal. Fluoride removal was not affected by calcium but was slightly affected at high concentration sulfate, decreasing from 94% to 82% with increasing sulfate concentration from 0.5 mM to 2 mM. Fluoride removal was also affected at high bicarbonate concentration, decreasing from 87% to 74% with increasing concentration of bicarbonate from 2 mM to 5 mM. High bicarbonate concentrations resulted in production of hydroxide anions which would compete with fluoride anions for adsorption sites on the CMCS sorbent surface [51]. The decrease in fluoride removal due to sulfate may be attributed to the development of outer- and inner-sphere complexes on the CMCS sorbent surface [13,26]. Several studies using metal oxide adsorbents observed a decrease in fluoride adsorption due to bicarbonate and sulfate anions [21,26,33,34,44,51]. The removal of fluoride from the synthetic solution decreased slightly to 83% due to the combined effect of several ions on adsorption of fluoride. The CMCS sorbent was able to remove 88% fluoride from the Chicago municipal tap water and 68% fluoride from the groundwater sample. The results show the applicability and selectivity of the CMCS sorbent for removal of fluoride from natural waters with appreciable levels of ionic strength containing multiple common ions.
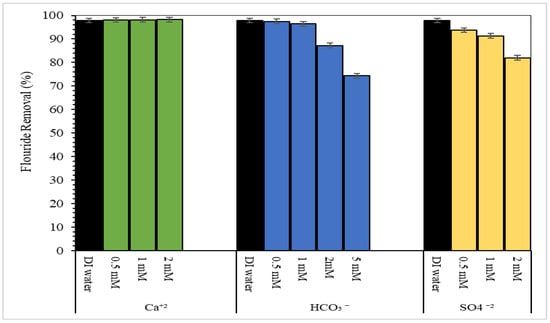
Figure 10.
Removal of fluoride as a function of co-existing ions using 20 g/L CMCS sorbent in 5 mg/L fluoride solution.
3.8. Re-Coating and Reuse of the CMCS Sorbent
Figure 11 shows the results for the removal of fluoride from a 5 mg/L fluoride solution before and after the re-coating of the CMCS sorbent using three sequential adsorption cycles. The results show that before re-coating, 96%, 94%, and 81% fluoride removal was obtained for the fresh CMCS sorbent during the first, second, and third sequential adsorption cycles, respectively. After re-coating, 95%, 93%, and 80% fluoride removal were obtained for the once-re-coated CMCS sorbent during the first, second, and third sequential adsorption cycles, respectively. Furthermore, the twice re-coated CMCS sorbent using the same spent coating solution employed for the initial re-coating of the CMCS sorbent obtained 92%, 90%, and 63% fluoride removal during the first, second, and third sequential adsorption cycles, respectively. The findings underscore the successful reuse of the CMCS sorbent, as evidenced by its consistent performance across multiple adsorption cycles post-recoating. The potential for successive reuse and recycling of the CMCS sorbent hold promise for significant cost and energy savings; it eliminates the frequent application of fresh adsorbent while also reducing solid waste and the volume of wastewater produced during adsorbent regeneration as well as minimizing the use of chemical reagents involved in adsorbent regeneration. Ultimately, this approach would contribute to decreasing the environmental footprint associated with the CMCS sorbent reuse, conducive to a sustainable CMCS sorbent for defluoridation of water.
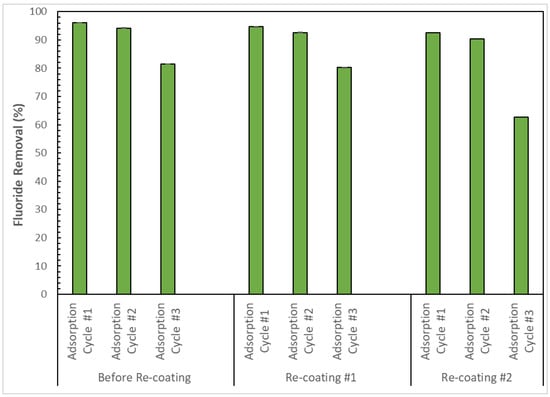
Figure 11.
Reuse of the CMCS sorbent through re-coating of the spent CMCS sorbent after three adsorption cycles using 20 g/L CMCS sorbent and 5 mg/L fluoride solution.
4. Conclusions
A hybrid binary metal oxide adsorbent containing a coating of two polyvalent metals of copper and magnesium on silica sand, namely a copper–magnesium-coated sand (CMCS) sorbent was developed for the removal of fluoride from water. The characterization of the CMCS sorbent surface was carried out using transmission electron microscopy, scanning electron microscopy and X-ray diffraction, showing that the magnesium and copper coating on the CMCS sorbent was predominantly amorphous. Adsorption equilibrium experiments demonstrated that fluoride adsorption using the CMCS sorbent was highly favorable based on adsorption parameters for the Langmuir and the Freundlich adsorption models. Physical adsorption of fluoride by the CMCS sorbent occurred according to the Dubinin–Radushkevish adsorption model. Rapid fluoride removal was obtained within 30 min of contact time, while pseudo-second-order adsorption kinetics were observed for the CMCS sorbent. Consistent fluoride removal efficiency of greater than 90% occurred from pH 3 to pH 11, while the CMCS sorbent’s pHPZC value was determined to be 10.5. The CMCS sorbent demonstrated promising selectivity for fluoride in natural waters, where fluoride removal decreased slightly at high concentrations of bicarbonate and sulfate. The CMCS sorbent was re-coated and recycled, underscoring the potential of the CMCS sorbent as a recyclable adsorbent for rapid defluoridation of water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16152178/s1, Figure S1. Procedure for preparation of CMCS sorbent; Figure S2. CMCS sorbent SEM image; Figure S3. SEM/EDX mapping images for CMCS sorbent; Figure S4. (A). Adsorption of fluoride by CMCS sorbent: pseudo-first-order kinetics. (B). Adsorption of fluoride by CMCS sorbent: pseudo-second-order kinetics; Figure S5. Adsorption of fluoride by CMCS sorbent: intraparticle diffusion; Figure S6. Adsorption mechanism for fluoride adsorption by CMCS sorbent.
Author Contributions
The conceptual design of the study was conducted by K.M. and A.P.K.; K.M. and J.W. carried out the material preparation, data collection, and analysis. K.M. wrote the first draft of the manuscript, A.P.K. edited the final manuscript, and the final manuscript was approved by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Illinois Chicago.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was conducted in the Department of Civil, Materials, and Environmental Engineering at the University of Illinois Chicago.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghosh, A.; Mukherjee, K.; Ghosh, S.K.; Saha, B. Sources and toxicity of fluoride in the environment. Res. Chem. Intermed. 2013, 39, 2881–2915. [Google Scholar] [CrossRef]
- Peterson, P.J. Assessment of exposure to chemical contaminants in water and food. Sci. Total Environ. 1995, 168, 123–129. [Google Scholar] [CrossRef] [PubMed]
- UNESCO World Water Assessment Program. The United Nations World Water Development Report 2021: Valuing Water; UNESCO: Paris, France, 2021; pp. 1–187. Available online: https://unesdoc.unesco.org/notice?id=p::usmarcdef_0000375724 (accessed on 9 October 2023).
- The United Nations Educational, Scientific and Cultural Organization. The United Nations World Water Development Report 2015; United Nations: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- WHO. International Program on Chemical Safety 1984 Fluorine and Fluorides; World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- US EPA. ALSA Tech, LLC, and Battelle. Design Manual: Removal of Fluoride from Drinking Water Supplies by Activated Alumina. (Report No. EPA/600/R-14/236); The Water Supply and Water Resources Division, National Risk Management Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2014.
- U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation. U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Fluoride in Drinking Water: A Scientific Review of EPA’s Standards; The National Academies Press: Washington, DC, USA, 2006.
- World Health Organization. Guidelines for Drinking-Water Quality, Third Edition, Incorporating the First and Second Addenda. Volume 1: Recommendations; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride contamination, consequences and removal techniques in water: A review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Arora, M.; Maheshwari, R.C.; Jain, S.K.; Gupta, A. Use of membrane technique for potable water production. Desalination 2004, 170, 105–112. [Google Scholar] [CrossRef]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Tang, Y.; Guan, X.; Wang, J.; Gao, N.; McPhail, M.R.; Chusuei, C.C. Fluoride adsorption onto granular ferric hydroxide: Effects of ionic strength, pH, surface loading, and major co-existing anions. J. Hazard. Mater. 2009, 171, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, L.H.; Vences-Alvarez, E.; Flores-Arciniega, J.L.; Flores-Zuñiga, H.; Rangel-Mendez, J.R. Water Defluoridation with Special Emphasis on Adsorbents-Containing Metal Oxides and/or Hydroxides: A Review. Sep. Purif. Technol. 2015, 150, 292–307. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Chiou, H.-M. The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut. 2002, 133, 349–361. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- López Valdivieso, A.; Reyes Bahena, J.L.; Song, S.; Herrera Urbina, R. Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. J. Colloid Interface Sci. 2006, 298, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.P.; Deshpande, G.; Barve, P.P.; Rayalu, S.; Labhsetwar, N.K.; Malyshew, A.; Kulkarni, B.D. Adsorption of fluoride from aqueous solution by alumina of alkoxide nature: Batch and continuous operation. Desalination 2010, 264, 15–23. [Google Scholar] [CrossRef]
- Salifu, A.; Petrusevski, B.; Ghebremichael, K.L.; Modestus, L.; Buamah, R.; Aubry, C.; Amy, G.L. Aluminum (hydr)oxide coated pumice for fluoride removal from drinking water: Synthesis, equilibrium, kinetics and mechanism. Chem. Eng. J. 2013, 228, 63–74. [Google Scholar] [CrossRef]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of fluoride from water using aluminum coated sand: Kinetics, equilibrium, effect of pH, and coexisting ions. J. Geosci. Environ. Prot. 2022, 10, 224–241. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Defluoridation of drinking water using adsorption processes. J. Hazard. Mater. 2013, 248–249, 1–19. [Google Scholar] [CrossRef]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of fluoride from water using Al–Mg–Ca ternary metal oxide-coated sand. Water Supply 2023, 23, 4699–4713. [Google Scholar] [CrossRef]
- Dhillon, A.; Soni, S.K.; Kumar, D. Enhanced fluoride removal performance by Ce–Zn binary metal oxide: Adsorption characteristics and mechanism. J. Fluor. Chem. 2017, 199, 67–76. [Google Scholar] [CrossRef]
- Swaina, S.K.; Patnaik, T.; Patnaik, P.C.; Jha, U.; Dey, R.K. Development of new alginate entrapped Fe(III)-Zr(IV) binary mixed oxide for removal of fluoride from water bodies. Chem. Eng. J. 2013, 215–216, 763–771. [Google Scholar] [CrossRef]
- Thakre, D.; Jagtap, S.; Sakhare, N.; Labhsetwar, N.; Meshram, S.; Rayalu, S. Chitosan based mesoporous Ti-Al binary metal oxide supported beads for defluoridation of water. Chem. Eng. J. 2010, 158, 315–324. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, X.; Wu, P.; Zhou, Q.; Luo, X. Fluoride removal from aqueous solution by Al(III)-Zr(IV) binary oxide adsorbent. Appl. Surf. Sci. 2015, 357, 91–100. [Google Scholar] [CrossRef]
- Sundaram, C.S.; Viswanathan, N.; Meenakshi, S. Defluoridation of water using magnesia/chitosan composite. J. Hazard. Mater. 2009, 163, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Chen, S.; Drewes, J.E.; Helmreich, B. Characterization of granular matrix supported nano magnesium oxide as an adsorbent for defluoridation of groundwater. Chem. Eng. J. 2015, 281, 632–643. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency; Antimicrobial Copper Alloys Group. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/082012-00002-20101110.pdf (accessed on 28 January 2021).
- Bansiwal, A.; Pillewan, P.; Biniwale, R.B.; Rayalu, S.S. Copper oxide incorporated mesoporous alumina for defluoridation of drinking water. Microporous Mesoporous Mater. 2010, 129, 54–61. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Shukla, S.; Philip, L.; Nambi, I.M. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chem. Eng. J. 2008, 140, 183–192. [Google Scholar] [CrossRef]
- Mondal, P.; George, S. A review on adsorbents used for defluoridation of drinking water. Rev. Environ. Sci. Biotechnol. 2015, 14, 195–210. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Method 4500-F- Fluoride; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2018. [Google Scholar]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. Journal of the Sanitary Engineering Division. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of Fluoride from Water Using Aluminum-Coated Silica Adsorbents: Comparison of Silica Sand and Microcrystalline Silica. Separations 2024, 11, 125. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, N.; Liu, T.; Ma, C.; Jin, P.; Zhang, F.; Zhang, J.; Wang, X. Competitive adsorption behaviors of arsenite and fluoride onto manganese-aluminum binary adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 185–194. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, H.; Sun, B.; Deng, P.; Hou, S.; Yu, Y. Adsorption of fluoride from aqueous solution by magnesia-amended silicon dioxide granules. J. Chem. Technol. Biotechnol. 2009, 84, 1449–1455. [Google Scholar] [CrossRef]
- Guo, W.; Lin, H.; Zhu, H.; Mo, W.; Su, X.; Yang, J.; Ma, S.; Feng, J.; Lei, M. Efficient removal of fluorine by carbon fiber supported Mg-Fe binary metal oxide composite adsorbent and mechanism analysis based on DFT. Sep. Purif. Technol. 2024, 330, 125320. [Google Scholar] [CrossRef]
- Blanco-Flores, A.; Arteaga-Larios, N.; Pérez-García, V.; Martínez-Gutiérrez, J.; Ojeda-Escamilla, M.; Rodríguez-Torres, I. Efficient fluoride removal using Al-Cu oxide nanoparticles supported on steel slag industrial waste solid. Environ. Sci. Pollut. Res. 2018, 25, 6414–6428. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.C. Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc magnesium-aluminum ternary oxide microspheres. Chem. Eng. J. 2020, 380, 122459. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Balarak, D.; Hossein Panahi, A.; Kamani, H.; Hossein Mahvi, A. Fluoride removal from aqueous solutions by cupricoxide nanoparticles. Res. Rep. Fluoride 2016, 49 Pt 1, 233–244. [Google Scholar]
- Tripathy, S.S.; Bersillon, J.L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Removal of fluoride from drinking water using aluminum hydroxide coated activated carbon prepared from bark of Morinda tinctoria. Appl. Water Sci. 2017, 7, 2653–2665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).