Abstract
This study reports, for the first time, on the assessment of a multistage sequential system composed of coagulation–flocculation with different electro-Fenton-based configurations, followed by neutralization (N), for the treatment of raw textile wastewater heavily contaminated with acid black 194 dye and other pollutants. Electrochemical peroxidation (ECP-N), electro-Fenton (EF-N) and peroxi-coagulation (PC-N) were tested at laboratory scale and compared in terms of their efficiency for the removal of organic matter and color, current efficiency and energetic parameter, operating cost and environmental sustainability using life cycle analysis conducted in large-scale virtual reactors. The three electro-Fenton-based systems complied with current environmental standards (color removal > 87%, COD < 400 mg/L, among others) requiring different electrolysis times: ECP-N (52 min) < PC-N (120 min) < EF-N (160 min); energy consumptions: ECP-N (2.27 kWh/m3) < PC-N (4.28 kWh/m3) < EF-N (33.2 kWh/m3); operational costs: ECP-N (2.63 USD/m3) < EF-N (6.65 USD/m3) < PC-N (6.98 USD/m3); among others. Electricity (for ECP-N and EF-N) and reagents (for ECP-N and PC-N) were found as main environmental hotspots. ECP-N presented the lowest carbon footprint of 10.3 kg CO2-Eq/FU (<PC-N (26.3 kg CO2-Eq/FU) < EF-N (38.0 kg CO2-Eq/FU), had lower incidence in all the impact categories analyzed (ReCiPe-2016 at midpoint level) and can be considered technically, economically and environmentally sustainable for large-scale applications.
1. Introduction
The textile manufacturing industry has become one of the largest contributors to global water pollution, responsible for approximately 20% of worldwide clean water contamination, primarily due to its dyeing and finishing processes [1]. That generated by the textile industry (TI) contains a variety of harmful components, such as dyes, heavy metals, bases, starches and acids, which pose a significant threat to human and environmental health [2,3]. In response to these concerns, many countries have established strict effluent discharge standards for textile plants. Thus, to comply with current legislation, the TI requires effective, economical and environmentally friendly treatment methods [4]. Recently, advanced oxidation processes (AOPs) have been the subject of study, including ozonation [5], ultrasound [6], photochemical oxidation [7], photocatalytic oxidation [8] and Fenton [9]. Another alternative consists of the use of electrochemical methods, specifically electrochemical AOPs, EAOPs, i.e., electro-oxidation [10] and electro-Fenton [11]. The latter is considered clean, green and highly efficient for the treatment of refractory wastewater (such as textile effluents) due to the in situ generation of the extremely reactive hydroxyl radicals, •OH, as seen in Equation (1), at near ambient temperature and pressure, that non-selectively oxidize organic compounds.
The electro-Fenton process is a technique that involves the in situ generation of one or both Fenton reagents (Fe2+, H2O2) in an electrochemical cell, which requires the application of electric current. Within the electro-Fenton process, the following variants can be found [12]:
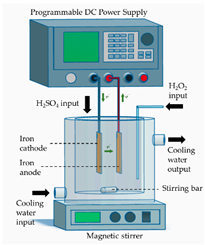
- Electrochemical peroxidation (ECP), in which H2O2 is externally added to the effluent (ex situ) while ferrous ions (Fe2+) are electro-generated from the anodic dissolution of sacrificial iron anode (in situ);
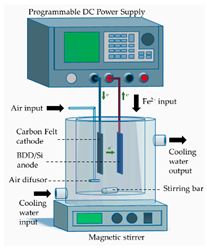
- Classical homogeneous electro-Fenton (EF), which involves the cathodic electro-generation of H2O2 (in situ) while Fe2+ ions are externally added to the effluent (ex situ);
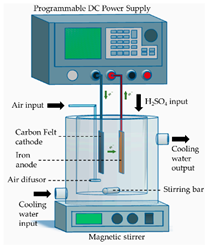
- Peroxi-coagulation (PC), in which both Fe2+ ions and H2O2 are electro-generated using a sacrificial anode and a carbonaceous cathode, respectively. Here, an Fe3+-saturated solution is produced, while the excess of Fe3+ precipitates as Fe(OH)3.
Table 1 resumes main reactions, requirements, advantages, implementation considerations and challenges for industrial applications of ECP, EF and PC processes.

Table 1.
Summary of the main reactions, requirements, advantages, implementation considerations and challenges for industrial applications of ECP, EF and PC processes.
Numerous studies have focused on identifying the optimal operating conditions for each of the three above-mentioned methods to achieve maximum mineralization and/or detoxification of colored effluents [12,13,14,15,16,17,18,19]. Nevertheless, only a few of them have addressed industrial wastewater [20,21,22,23,24,25,26], and even fewer have additionally reported on the operating costs [27,28]. Recently, Ghanbari and Moradi [29] presented a comparative study of EF, ECP and PC for the decolorization and COD removal of industrial textile wastewater, concluding that ECP was the most effective process. Alternatively, novel approaches built on the blending of techniques, like coagulation–flocculation (CF) and electro-Fenton, have been suggested to enhance the overall efficiency of the treatment system [30]. Nevertheless, further research on the potential environmental impacts that the treatment process may generate on the ecosystem is needed. This information can be crucial for decision making on treatment process selection. In this context, life cycle analysis (LCA) can offer valuable insights into the environmental implications associated with the implementation of a treatment process [31]. The environmental impacts can be calculated by considering all inventory elements (for example, from cradle-to-gate) using robust databases [32].
This study offers, for the first time, a detailed assessment of a multistage system composed of a sequential combination of coagulation–flocculation with different electro-Fenton-based configurations, followed by neutralization (N), for the treatment of raw textile wastewater (ITWW) heavily contaminated with acid black 194 dye (AB194) and other pollutants. CF was used as the initial phase of its management due to its remarkable capacity for decolorization and elimination of colloids and suspended solids. Within the scope of this study, three sequential processes, ECP-N, EF-N and PC-N, were compared in terms of their efficiency for the removal of organic matter and residual color, current efficiency and energetic parameters, operating costs and environmental sustainability using an LCA approach conducted in large-scale virtual reactors. The comparison of these three processes aims to evaluate their potential from a technical, economic and environmental point of view. This can allow us to conclude which alternative has greater advantages for its future large-scale application due to lower operating costs, operation times and environmental footprints. Initially, the effectiveness of ECP-N, EF-N and PC-N was assessed based on their ability to reduce chemical oxygen demand and color concentration. This in order to identify operating conditions that allow to meet the Colombian regulations for industrial textile wastewater discharge with a relatively low cost. Subsequently, the environmental impact of each process was thoroughly evaluated, identifying their advantages and drawbacks in terms of environmental implications and offering a solution that balances environmental compliance with economic considerations. This provides feedback on the most sustainable process for future applications on a large scale in textile wastewater treatment facilities. This comprehensive approach aimed to assess the overall performance of the system from environmental, economic and technical perspectives, with the goal of identifying potential strategies to mitigate additional burdens on the environment. Additionally, a closer examination of the feasibility of the most suitable alternative for wastewater treatment is provided. The long-term goal is to foster sustainable industrial growth and encourage innovation (within Sustainable Development Goal, SDG–9), aiming to find innovative solutions to minimize the environmental impact of the textile industry (within SDG–12).
2. Materials and Methods
2.1. Sampling, Preservation and Management of Raw Textile Industrial Wastewater
The industrial wastewater, the focus of this investigation, was collected from a textile facility situated in the central area of Colombia (South America). A typical sample of the raw effluent (ITWW) originated from the dyeing process with acid black 194 dye (AB194). Throughout this study, four samplings were carried out, monthly, directly from the homogenization tank with a total capacity of 2.5 m3, as described in detail previously [33]. The sampling, conservation, storage and management of ITWW were carried out in accordance with the guidelines and protocols established by the Colombian Institute of Hydrology, Meteorology and Environmental Studies [34,35,36,37].
Before the implementation of the sequential processes (ECP-N, EF-N and PC-N), the ITWW was pre-treated using coagulation–flocculation assisted by slaked lime (CF), under the following operating conditions: Alum: [Al2(SO4)3·14H2O] = 16.09 g/L and slaked lime [Ca(OH)2] = 5.16 g/L, previously optimized [33]. CF experiments were performed on a laboratory scale in accordance with the ASTM D2035-19 standard [38], using a jar testing apparatus (MaquinLab Electronic®, MDJf6, Bogotá, Colombia) with six equal compartments, each with a capacity of 2 L. Following the completion of each jar test, the treated effluent was filtered using a medium retention glass fiber filter (1.5 µm, grade 1, Ahlstrom®, 09471 Bärenstein, Germany). The supernatant obtained from each jar was subsequently analyzed at least in duplicate.
2.2. Chemicals
All chemicals were used as received, without any further purification. For CF treatment, aluminum sulfate tetradecahydrate (Al2(SO4)3.14H2O, Alum, 98 wt. %, Protokimica, Medellín, Colombia) and calcium hydroxide (Ca(OH)2, slaked lime, 95 wt. %, BDH, Bogotá, Colombia) were applied as a coagulant and auxiliary agent, respectively. For all EF-based configurations, anhydrous sodium sulfate (Na2SO4, extra pure grade, Duksan, Republic of Korea) was used as the supporting electrolyte. Sulfuric acid (H2SO4, 95–97%, 1.84 g/cm3, Merck KGaA, Darmstadt, Germany) was employed to adjust the pH to 3.0 and maintain it in the range of 2.8–3.4 during electrolysis, to clean the iron electrodes and to clean and activate the BDD/Si anode. Sodium hydroxide (NaOH, ≥98%, Carlo Erba, Val-de-Reuil, France) or calcium hydroxide (Ca(OH)2, 97 wt. %, BDH, Bogotá, Colombia) were used as neutralizing agents. For EF, ferrous sulfate heptahydrate (FeSO4.7H2O, extra pure grade, Duksan, Republic of Korea) was employed as the source of Fe2+ ions. For ECP, hydrogen peroxide (H2O2, 30%, Bioquigen, Bogotá, Colombia) was used as the source of hydroxyl radials. In the case of EF and PC, compressed air (99.9%, Praxair, Manizales, Colombia) was used to saturate the treated effluent with oxygen, thus facilitating the electrochemical generation of H2O2. All aqueous solutions were freshly prepared using ultrapure water obtained from a Milli-Q system (Billerica, MA, USA), which had a conductivity of less than 0.055 μS/cm.
2.3. Materials
All materials used for the construction of the electrochemical cells, including their suppliers, are detailed in Table 2. A stainless-steel sheet (submerged area of 24 cm2, type 316 stainless steel, Bronces & Láminas S.A.S, Manizales, Colombia) was used as the cathode in the auxiliary electrochemical device for cleaning and activation of the BDD/Si anode.

Table 2.
Main characteristics of sequential processes (ECP-N, EF-N and PC-N) implemented for the treatment of ITWW-CF.
2.4. Electrochemical Experimental Setup
The sequential processes (ECP-N, EF-N and PC-N), implemented for the treatment of the supernatant of the CF process (ITWW-CF), were carried out in a laboratory-scale electrochemical cell, as detailed in Table 2. Before each experimental run, all electrodes underwent a cleaning and/or activation procedure. The iron electrodes (anode and cathode) were first sanded with 1000 grit sandpaper and thoroughly washed with water. Subsequently, they were immersed in a 30% w/w H2SO4 for 10 min, washed with distilled water and finally dried at 105 °C for 1 h. Cleaning and activation of the BDD/Si anode involved the assembly of a single-compartment auxiliary electrochemical cell consisting of a BDD/Si anode and a stainless-steel cathode, arranged in a parallel configuration and operating in a monopolar mode. Next, electrolysis of 0.2 L of freshly prepared 1 M H2SO4 was carried out for 30 min, without stirring, under a current density of 30 mA/cm2, resulting in an electrical current (I) of 720 mA. Once the electrolysis was completed, the electrodes were removed from the cell and the BDD/Si electrode was immersed in deionized water for 15 min in order to ensure the removal of any residual traces of the H2SO4 and finally dried at room temperature. Once the anode was used in the EF process, it was washed with water and next immersed again in deionized water for at least 30 min. Before performing a new experimental run, the cleaning and activation protocol was repeated to ensure maximum electrode performance. The PAN-Based Soft Graphite Felt was also cleansed before and after use. Before use, a new sheet of felt was washed to remove any impurities. To perform this, several ultrasonic cleaning cycles were carried out, each lasting at least 10 min, in deionized water. These cleaning cycles were continued until the conductivity of the cleaning water reached a level of less than 10 µS/cm. Once this step was completed, the sheet of felt was kept submerged in deionized water until required for use. After use in EF or PC process, a sheet of felt was also cleaned. At first, it was immersed in deionized water for at least 24 h. Next, it was subjected to several ultrasonic cleaning cycles with deionized water, as described above.
Prior to electrolysis, in all EF-based configurations, the initial pH of the effluent was adjusted to 3.0 using a 5 M aqueous solution of H2SO4, under continuous stirring. At this pH value, the Fenton reaction is expected to generate the maximum amount of •OH radicals and the Fe2+ ion concentration reaches its peak [12,13,14]. The effluent conductivity was subsequently adjusted to 5.80 mS/cm by adding anhydrous Na2SO4 (1.55 ± 0.39 g/L) due to its strong ionic properties and minimal interference in aqueous solutions. Furthermore, in the case of EF and PC, the ITWW-CF was saturated with O2 using compressed air for 15 min at a flow rate of 1.9 L/min to maximize the production rate of H2O2. In the case of EF, 1 min prior to the electrolysis, and under continuous stirring, the corresponding amount of Fe2+ ions was added to the treated effluent using a freshly prepared aqueous Fe2+ stock solution with a concentration of 41.65 mM. The application of an electrical current to the electrochemical cell initiated the process of electrolysis under conditions of continuous stirring. During ECP, H2O2 was continuously added to the reaction medium using a peristaltic pump (Lead Fluid Technology Co., BT101L, Xiangtan, China) with a dosing rate of 27.827 μL/min. The dosage of H2O2 began 1 min before the application of electric current. During EF and PC, compressed air was also bubbled at a rate of 1.9 L/min. In all EF-based configurations, the pH of the effluent was monitored during the electrolysis process. In order to maintain it within the range of 2.8 to 3.4, in the case of ECP and PC, it was regulated every 10 min using 5 M H2SO4. This was in order to prevent the precipitation of iron oxyhydroxides. It is important to note that the application of a peristaltic pump for H2O2 addition to the reaction medium caused less significant changes in the pH of the treated effluent during the electrolysis. Therefore, a lower amount of H2SO4 was required to maintain its pH within the desired range and in turn a lower amount of sludge was formed. Additionally, the cell voltage was monitored every 5 min using an external voltmeter in order to determine the cell energy consumption (kWh/m³). Experiments were performed under different operating conditions (Table 2). ECP ran at different concentrations of H2O2 and electrical currents (as a source of Fe2+ ions). The EF process operated at different concentrations of Fe2+ ions and electrical currents (as a source of H2O2). PC involved the application of different electrical currents (as a source of both H2O2 and Fe2+). Once the established electrolysis time was reached, the physicochemical parameters of the treated effluent were quantified. Following the removal of the electrodes, the reactor content was neutralized to achieve a pH in the range of 6.6–8.8. This was in order to comply with the Colombian regulations governing the discharge of industrial effluents, specifically with respect to the pH parameter [39]. The neutralization process was carried out by gradually adding the neutralizing agent under constant stirring until the target pH was attained. In the case of PC, an aqueous solution of NaOH (16 M) was used, whereas for EF and ECP, a Ca(OH)2 suspension was employed. The total volume of H2SO4 used for the acidification (prior to and during the electrolysis) and/or alkali for the neutralization was less than 5 mL. Following the completion of the neutralization process, the entire cell content, excluding the extracted aliquots, was filtered using a medium retention glass fiber filter, 1.5 μm, grade 1 (Whatman®), and the resulting supernatant was analyzed. All experiments were conducted at least in triplicate.
2.5. Operating Conditions of Electro-Fenton-Based Processes
2.5.1. Electrochemical Peroxidation (ECP)
- The first series of experiments was performed to establish the effect of electric current on the ECP efficiency. The specific operating conditions (e.g., Fenton reagent concentration) were defined based on the following considerations:
- (i)
- The theoretical dose of H2O2 necessary to oxidize the organic matter that contributes to the COD of the sample, assuming that 1 g/L of O2 oxidizes 1 g/L of COD. This was determined from the stoichiometry of the decomposition of H2O2 into water and oxygen (Equation (10)).Therefore, to generate 1332.8 mg/L of oxygen required to oxidize the organic matter present in the CF supernatant (corresponding to its COD), a minimum of 2832 mg/L of H2O2 would be needed.
- (ii)
- The relative mass proportion of organic load and H2O2 (COD/H2O2) in the range of 1.14 ≤ COD/H2O2 ≤ 1.68 based on bibliographic review [29,40,41,42,43]. Thus, for an intermediate value of COD/H2O2 = 1.40 and COD of 1332.8 mg/L (CF-supernatant), the concentration of H2O2 was set at 951.7 mg/L (27.98 mM).
- (iii)
- The relative molar ratio of Fenton reagents: H2O2/Fe2+ = 6.0, 3.0, 1.5 and 1.0, as reported in the open literature [29,40,41,42,43].
Thus, based on Faraday’s Law and considering H2O2/Fe2+ molar ratios, the electric current (Equation (11)) was determined in the range of 0.05–0.3 A (corresponding to the concentration of Fe2+ ions in the range of 4.66–27.98 mM, during 1 h of electrolysis).
where I: electric current, A; [Ci]: total concentration of added i (Fe2+ or H2O2), mol/m3; V: ITWW volume, m3; F: Faraday constant, 96,485.3 A s/mol; z: valence electrons; t: treatment time, s; and H2O2/Fe2+: molar ratio of Fenton reagents.
- 2.
- The second series of experiments was carried out to determine the effect of H2O2 concentration on the ECP efficiency. The specific operating conditions (e.g., Fenton reagent concentration) were defined based on the following considerations:
- (i)
- A fixed electric current value (of 0.2 A) selected from the first series of experiments. Thus, during 60 min of electrolysis, the current of 0.2 A provides 18.66 mM Fe2+ ions.
- (ii)
- The relative molar ratio of Fenton reagents: H2O2/Fe2+ = 4.0, 3.0, 1.5 and 1.0, according to the literature [29,40,41,42,43] and results of the first series of experiments. Thus, for the fixed value of Fe2+ ion concentration of 18.66 mM, the concentration of H2O2 was established (74.62 mM, 55.97 mM, 27.98 mM and 18.66 mM).
2.5.2. Electro-Fenton (EF)
- The initial experiments were carried out to determine the impact of electrical current on the efficiency of the EF process. The specific operational parameters (e.g., Fenton reagent concentrations) were established based on the following considerations:
- (i)
- The generation of H2O2 was carried out by applying the same electric current (0.05–0.3 A) to the electrochemical cell as in the case of generation of Fe2+ ions in the ECP process, for comparative purposes.
- (ii)
- The concentration of Fe2+ of 1 mM was fixed for the first series of experiments. This corresponds to an intermediate Fe2+ concentration used in the EF process [12,40,41,42,43].
- Next, the effect of Fe2+ ion concentration on the EF efficiency was determined. The specific operational parameters were defined as follows:
- (i)
- A fixed electric current value (of 0.3 A) required for H2O2 generation selected from the first series of experiments.
- (ii)
- The concentration of Fe2+ ions was in the range of 1–6 mM, according to the open literature [12,40,41,42,43].
2.5.3. Peroxi-Coagulation (PC)
A series of experiments was carried out to establish the effect of electric current on the efficiency of PC. The specific operating conditions were established considering the generation of both H2O2 and Fe2+ by applying the same electric current (0.05–0.3 A) to the electrochemical cell as in the case of generation of Fe2+ ions in the ECP process, and/or H2O2 generation in EF, for comparative purposes.
2.6. Analytical Methods
The physicochemical parameters of ITWW and ITWW-CF were selected primarily in accordance with the current environmental legislation in Colombia [39], to assess the status of the ITWW under study and establish the contaminant removal efficiency achieved in each stage of the sequential processes. They were determined following the standards methods [44]. All quantifications were performed at least in duplicate to confirm the reproducibility of the results. For the determination of COD, a 20 mL aliquot was taken. To ensure the reliability of the measurements, potential interferences were initially eliminated according to the procedure described by Do et al. [45], which is based on the H2O2 decomposition reaction catalyzed by manganese oxide. To determine the amount of sludge generated, the entire volume (excluding the extracted aliquots) was filtered using a medium retention glass fiber filter, 1.5 μm, grade 1 (Whatman®). The solids generated were dried at 103–105 °C until their constant weight was obtained (Method 2540-D [44]) and quantified.
2.7. Treatment Efficiency and Economic Evaluation
The efficiency of sequential processes (ECP-N, EF-N and PC-N) was evaluated based on the percentages of contaminant removal (%RX, where X: Color, COD, TOC, BOD5) and operating costs. The latter were assessed for the EAOP stage (OpCEAOP-N) and for the sequential process CF-EAOP-N (TOpCsequential), in accordance with the expressions provided in Table 2. The COD and TOC values were also used to evaluate the carbon oxidation state of the ITWW, ITWW-CF and CF-EAOP-N effluents, as described in Equation (12) [46,47].
The carbon oxidation state (COS) can range from −4, which represents the most reduced carbon state (in the form of CH4), to +4, which represents the most oxidized carbon state (in the form of CO2) [48,49]. For most organic compounds, this interval takes values between −2.2 and +3 [50].
2.8. Environmental Impact Assessment
This study considered a cradle-to-gate approach to assess the environmental sustainability of different EF-based processes through life cycle analysis (LCA). LCA was conducted in compliance with the guidelines and requirements specified in the ISO 14040 and 14044 international standards for environmental management [51,52]. The analysis comprised four primary activities: (1) goal and scope definition, (2) inventory analysis, (3) life cycle impact assessment and (4) interpretation of the results [53].
2.8.1. Goal and Scope Definition
The environmental performance of the sequential processes (ECP-N, EF-N and PC-N) for the treatment of ITWW-CF effluent was also evaluated and compared through LCA. For this purpose, the specific operating conditions were determined experimentally. These allowed compliance with current Colombian environmental legislation regarding residual COD content (<400 mg/L), among others [39]. For comparison purposes, the functional unit (FU) of the ITWW-CF effluent, to be treated by sequential processes, was 1 m3. For the design of large-scale electrochemical devices, the required quantities of chemicals, the specific operating conditions and the energy inputs necessary for each treatment process were determined experimentally. Additional decision making was based on our previous studies and relevant academic sources [54]. For each EF-based configuration, a circular concrete tank equipped with the corresponding electrodes was assessed. To induce the electric field inside the electrochemical reactor, a DC power source was connected to the electrodes, with a monopolar configuration arranged in parallel, under galvanostatic operation mode. For EF and PC, oxygen was additionally supplied from the bottom of the reactor through a diffused aeration system. Once the electrolysis was completed and the electrodes were removed, the content of the reactor was neutralized by adding caustic soda or slake lime. The sludge generated was then settled and disposed of. All tanks within the system were equipped with a motor to facilitate the stirring of the effluent during both the electrolysis and neutralization stages. The system boundaries under study include the following stages (Figure 1):
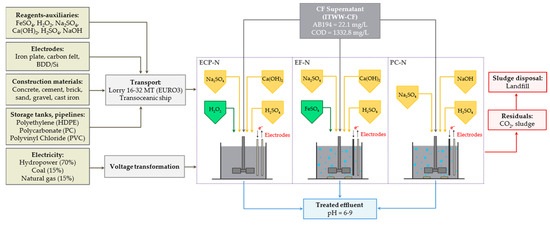
Figure 1.
System boundaries used for the life cycle assessment (LCA) of sequential processes ECP-N, EF-N and PC-N.
- Production of reagents and materials necessary to carry out the treatment (Na2SO4: electrical conductivity adjustment; H2SO4: pH adjustment; H2O2 and FeSO4: Fenton’s reagents; NaOH or slaked lime: neutralization; and electrodes).
- Production of materials for the construction of electrochemical reactors and their corresponding infrastructure (reaction tank: concrete, cement, brick, sand, gravel, cast iron; chemical storage tanks: high-density polyethylene (HDPE); roof: polycarbonate (PC); pipelines: polyvinyl chloride (PVC)).
- Generation of solid and gaseous residuals (disposal of sludge generated as well as CO2 emissions into the atmosphere, a product of mineralization through sequential processes). The generated sludge was considered inert waste and was subsequently designated for disposal in a landfill. The CO2 emissions into the atmosphere were determined based on the efficiency of the mineralization of the organic matter, in terms of TOC, through sequential oxidative processes.
- Transportation logistics. The majority of the reagents and construction materials were sourced from local providers in close proximity to the treatment plant, minimizing the need for long-distance transportation. The materials and electrodes that were manufactured outside the country were transported by ship to the port of Buenaventura, located at latitude 3° 52′ 48.36″ N and longitude 77° 01′ 52.18″ W, and then transported by road in EURO3 vehicles (16–32 metric ton) to the city of Manizales, as shown in Table 3. The final disposal of the sludge generated was carried out within the metropolitan area.
 Table 3. Life cycle inventory (LCI) per FU for the main activities of sequential processes (ECP-N, EF-N and PC-N).
Table 3. Life cycle inventory (LCI) per FU for the main activities of sequential processes (ECP-N, EF-N and PC-N). - Electricity consumption (for pumping, stirring, aeration, electrochemical device operation) and voltage transformation from high to medium tension. The electricity supply was derived from two main sources: hydroelectric plants (70%) and thermal plants operating with coal (15%) and natural gas (15%) [55]. The energy consumption was calculated as presented in the footer of Table 2.
Furthermore, the following assumptions were considered:
- The treatment plant was situated within the municipal boundaries of Manizales (South America, Colombia, latitude = 5° 4′12.99″ N, longitude = 75° 30′49.74″ W).
- A lifespan of 20 years was estimated, with the FU device operating reliably for 330 days per year and performing one task per day.
- Losses of materials and reagents of 10% and 8%, respectively, were assumed, considering the total amount required by the FU, as commonly observed in construction projects.
- Electrode useful lifetime was considered as follows: carbon felt cathode (six months), BDD/Si anode (ten years), sacrificial iron anodes (one month, up to 70% weight loss), iron cathode (ten years).
- Sequential processes operated at ambient temperature.
2.8.2. Life Cycle Inventory (LCI)
The information used in this work for the construction of the life cycle inventory (LCI), as shown in Table 3, originated from (i) the specific operating conditions of each EF-based process that allowed compliance with the current Colombian environmental legislation regarding to the residual COD content (<400 mg/L), among other parameters; (ii) the additional information available in scientific reports; and (iii) the expert knowledge of professionals in specific fields. Reliable LCI data sets on reagents, materials, transportation logistics, electricity and sludge management were obtained from the Ecoinvent v3.3 database [56].
2.8.3. Life Cycle Impact Assessment (LCIA)
The life cycle impact assessment (LCIA) of the sequential processes (ECP-N, EF-N and PC-N) was evaluated using the ReCiPe-2016 v1.1 method. For this, OpenLCA 1.11 software (GreenDelta©, Berlin, Germany) was used. This method offers a comprehensive and simultaneous assessment of different environmental impacts across numerous categories, providing a broad understanding of environmental burdens. For impact evaluation, the ReCiPe-2016 method uses both midpoint and endpoint approaches. The midpoint analysis measures (e.g., kg CO2 equivalents) the specific environmental mechanisms or stressors that contribute to environmental impacts. In this context, the direct emissions or releases of substances and their potential effects on various (18) environmental categories were evaluated. These midpoint indicators offer detailed information about the specific environmental stressors caused by activities throughout the life cycle, assisting in identifying areas where mitigation efforts may be most effective. Conversely, the endpoint analysis groups these multiple midpoint impacts into three categories: damage to human health (HH, unit: year, represents the years that are lost or that a person is disabled due to a disease or accident), damage to ecosystem quality (ED, unit: species per year, represents the local species loss integrated over the time) and damage to resource availability (RA, unit: USD, represents the extra costs involved for mineral and fossil resource extraction in the future) [57]. ReCiPe-2016 v1.1 considers three perspectives—individualistic, hierarchical and egalitarian—to account for various sources of uncertainty and alternative scenarios. In this study, the hierarchical perspective, which is based on scientific consensus on the time frame and plausibility of impact mechanisms, was employed [57].
2.8.4. Life Cycle Interpretation
The last phase of the LCA process involved the analysis of the LCI and LCIA results to discuss the sustainability of the evaluated treatment processes and to identify the main environmental hotspots that need to be addressed for future large-scale applications. This was in order to guide towards more sustainable practices. Additionally, a local sensitivity analysis was performed, for the most promising process, to determine the effect of varying the input data of the most significant parameters (one-at-a-time approach) on the LCA results. The sensitivity ratio (SR) was calculated as follows (Equation (13)).
3. Results
3.1. ITWW and ITWW-CF Characterization
Table 4 summarizes principal characteristics of industrial textile wastewater (ITWW) originating from the dyeing stage with acid black 194 dye (AB194) in a textile manufacturing company. The standard methods used for their determination are also included [44]. AB194 (CAS number 61931-02-0, molecular formula: C40H20N6O14S2CrNa3, molecular weight: 993.71 g/mol, characteristic wavelength: λmax = 575 nm) belongs to the family of dyes called Mordant Black and is commonly used to color different types of fabrics (leather, wool, polyamide, silk–wool blends and non-woven microfabrics). For comparative purposes, Table 4 also includes the Colombian emission limits established for the textile industry [39]. Note that ITWW has an intense black color, mainly due to the presence of AB194, among other contaminants (TSS, oil and grease, phenols, hydrocarbons). High values of COD, TOC and BOD5 imply a high content of organic matter with a low oxidation degree (COS ≈ 0.17).

Table 4.
The main characteristics of the raw ITWW and the supernatant of the coagulation–flocculation treatment (ITWW-CF), objects of this study. Also included are the standard methods used for their determination [44] as well as the maximum permissible discharge limits to surface water bodies (*) for effluents from the textile industry according to current environmental regulations [39]. The values reported for ITWW are average values of four samplings carried out monthly. The values reported for ITWW-CF are average values of the supernatant of the CF process and correspond to approx. 20 L of treated ITWW, specifically 10 jars of 2 L each.
Pre-treatment of ITWW using CF was efficient for the removal of colloids, suspended solids and much of the color (Table 4). However, the physicochemical parameters of the CF supernatant did not meet the maximum permissible limits specified in the regulations for discharge into surface water bodies and public sewage systems [39] nor the requirements for the reuse of the treated effluent [58]. Furthermore, it remained colored and contained carbonaceous compounds with still low oxidation degree (COS ≈ 2.43), probably recalcitrant and resistant to degradation. Thus, in order to satisfy the requirements of environmental legislation, EF-based processes (ECP-N, EF-N, PC-N) were implemented sequentially.
3.2. Electrochemical Peroxidation (ECP)
A key factor that determines the performance of the electrochemical reactions in the ECP process is the concentration of H2O2 and Fe2+ ions, the latter depending on the applied electrical current. Their intervals, tested in this study, were set as described in Section 2.5.1. (Materials and Methods). The effect of electric current on the residual COD fraction, color and treatment costs is presented in Figure 2a.
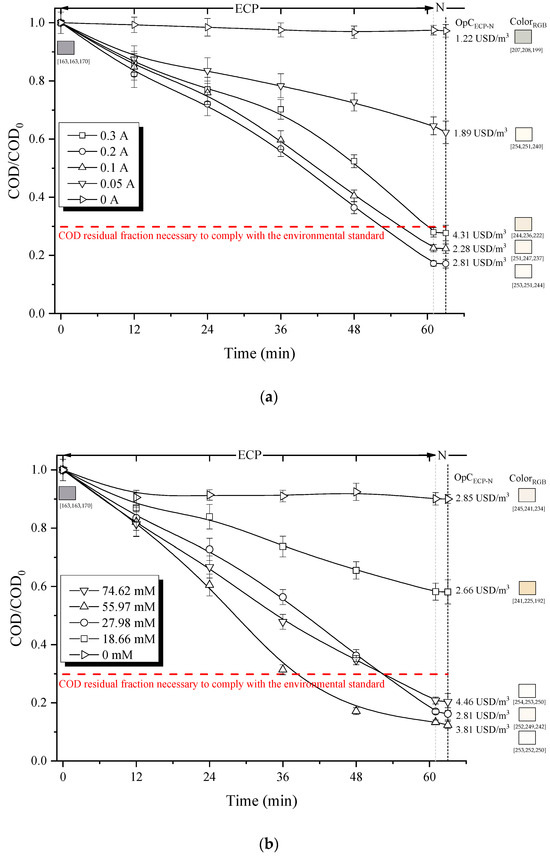
Figure 2.
Effect of (a) electric current (H2O2 concentration = 27.98 mM) and (b) H2O2 concentration (electric current = 0.20 A) on the degradation capacity of organic matter (residual COD fraction), decolorization (RGB scale) and total operation costs for ECP-N treatment. (Note: RGB color system constructs all the colors from the combination of the red, green and blue colors. Each parameter (red, green and blue) defines the intensity of the color with a value in the range of 0 to 255. For example, RGB (255, 0, 0) is displayed as red because red is set to its highest value (255), and the other two (green and blue) are set to 0).
By increasing the electric current up to 0.2 A, a significant decrease in the residual fraction of COD was observed, achieving an elimination of 83% (COD/COD0 = 0.17) after 60 min of treatment, which allows compliance with environmental standards. The ECP process utilizes electric current as the source of Fe2+ ions, which act as a catalyst for the decomposition of H2O2. This decomposition generates hydroxyl radicals (Equation (1)) and hydroperoxyl radicals (Equation (14)).
Thus, at higher currents, the concentration of Fe2+ ions increase and more •OH radicals are formed. Moreover, higher amounts of Fe(OH)n flocs are produced, which in turn can absorb organic matter. Interestingly, as the electrical current increases further, up to 0.3 A, the COD removal efficiency actually drops, to around 72%. This suggests that the system reached its maximum oxidation capacity at approximately 0.2 A. This pattern is common for persistent organic pollutants, as the decomposition of COD and TOC is greatly boosted by higher electrical currents, but only up to a point at which reaction kinetics become limiting [59,60]. Nevertheless, excessive concentration of Fe2+ ions in the solution promotes the hydroxyl radical scavenging reactions, Equations (15)–(17), reducing the overall oxidative capacity of ECP.
It is worth noting that the use of hydrogen peroxide alone (Figure 2a, 0 A) results in a COD removal efficiency of only 1.8%, since H2O2 has a lower oxidative potential (E0 = 1.77 V) compared to that of hydroxyl radical (E0 = 2.80 V). On the other hand, color removal varied between 94% and 84% for electric currents ranging from 0.05 A to 0.30 A, respectively. Note also that boosting the electrical current leads to an increase in operational expenses, attributed to amplified energy usage and greater sludge production. Consequently, evaluating the elimination of COD and dye concentration (87% vs. 69% for 0.2 A and 0.1 A, respectively) along with operational costs, an electric current of 0.2 A was chosen to assess the impact of H2O2 concentration (in the range of 18.66 mM–74.62 mM), as described in Section 2.5.1. (Materials and Methods).
The effect of H2O2 concentration on COD removal efficiency, color and treatment costs is illustrated in Figure 2b. The residual fraction of COD progressively decreases to 0.13 (for a total added H2O2 concentration of 55.97 mM). Note that the residual fraction below 0.3 allows compliance with environmental regulations. It is evident that a higher concentration of H2O2 allows for the generation of a greater quantity of •OH radicals. These highly reactive species induce the destruction of organic compounds and color within the system. Nevertheless, the COD removal decreased when 74.62 mM of H2O2 was used. This phenomenon can be mostly attributed to the self-decomposition of H2O2 (Equation (18)) and/or the decomposition of H2O2 catalyzed by hydroxyl radicals (Equations (19) and (20)) [61].
Tests performed without hydrogen peroxide resulted in an 8% decrease in the remaining COD fraction. This was due to the generation of ferrous ions through the dilution of the anode, as described in Equation (2). However, these particular operating conditions exhibited limited efficacy in the elimination of both COD and coloration, as the effluent had already undergone a coagulation–flocculation (CF) treatment. This pre-treatment successfully removed organic molecules that became complexed due to a charge imbalance.
Similarly, increasing the H2O2 concentration (from 18.66 mM to 74.62 mM) improved the decolorization efficiency (from 78% to 93%). Higher concentrations of H2O2 result in the production of more hydroxyl radicals, which subsequently induce the destruction of organic compounds and the reduction in color. Interestingly, significant (approx. 85%) discoloration was observed in the test performed without the presence of hydrogen peroxide (Figure 2b). These operating conditions correspond to an electrocoagulation process that promotes the generation of Fe2+ and Fe3+ ions, which, during the neutralization stage, react with hydroxide ions (OH−) forming gelatinous solids (Fe(OH)2(s) and Fe(OH)3(s)). These, during the sedimentation process by sweep capture, can adsorb and/or trap the residual dye present in ITWW-CF, thus attenuating the coloration of the effluent, without necessarily having a greater impact on the removal of COD [29,62].
Increasing the total concentration of H2O2 used in the ECP process also leads to a rise in costs (Figure 2b). Similarly, the amount of sludge produced grows (from 3.81 g/L to 5.06 g/L) proportionally to the increase in the H2O2 concentration (from 18.66 M to 74.62 mM). This is likely due to the oxidation of organic matter and the subsequent formation of flocs when interacting with the ferrous ion in the solution.
After 60 min of electrolysis, the highest COD removal of 87% was achieved, at an electric current of 0.20 A and H2O2 concentration of 55.97 mM, resulting in 4.62 g/L of sludge generated and an operational cost of 3.81 USD/m3. Note that a slightly lower COD removal efficiency of 83% was also obtained at 0.20 A but using a smaller amount of H2O2 (27.98 mM). These two operating conditions allowed compliance with current environmental regulations [39] regarding COD parameter, among others, and offered practically colorless effluents. However, a different electrolysis time was required for this purpose (38.5 min using 55.97 mM H2O2 vs. 52.33 min using 27.98 mM H2O2). Thus, despite the slightly longer operating time, the use of a lower concentration of H2O2 was less expensive (2.63 USD/m3) and generated a lower amount of sludge (3.81 g/L). All this suggests that these latest operating conditions are the most efficient of those analyzed, with an energy consumption of 2.27 kWh/m3. Thus, the relative molar ratio of the Fenton reagents (H2O2/Fe2+) of 1.5 was the most beneficial for the treatment of ITWW-CF by ECP.
3.3. Electro-Fenton (EF)
Figure 3a illustrates the impacts of varying electrical current on organic load (COD) and color removals, as well as treatment cost, maintaining a fixed total Fe2+ concentration of 1 mM.
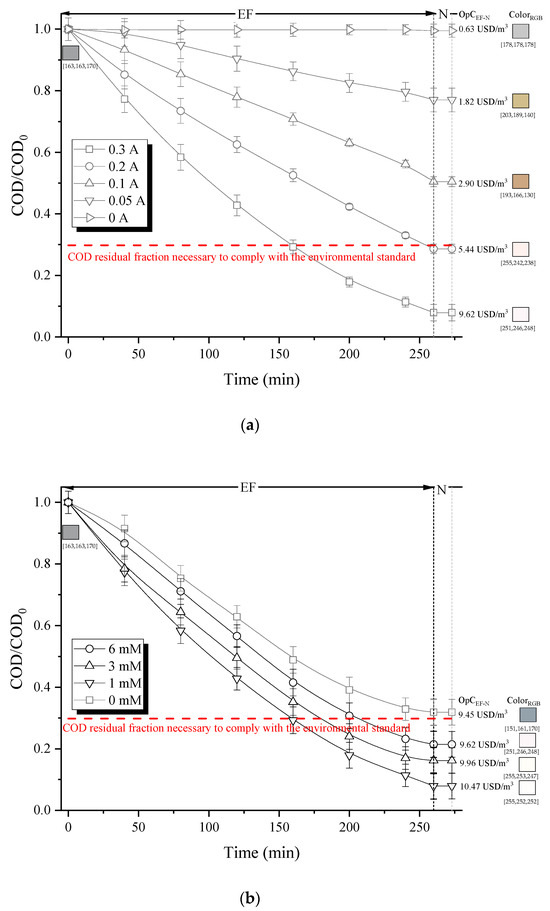
Figure 3.
Effect of (a) electric current (Fe2+ concentration = 1 mM) and (b) Fe2+ concentration (electric current = 0.30 A) on the degradation capacity of organic matter (residual COD fraction), decolorization (RGB scale) and total operation costs for EF treatment. (Note: RGB color system, constructs all the colors from the combination of the red, green and blue colors. Each parameter (red, green and blue) defines the intensity of the color with a value in the range of 0 to 255. For example, RGB (255, 0, 0) is displayed as red because red is set to its highest value (255), and the other two (green and blue) are set to 0).
As anticipated, increasing the electrical current from 0.05 to 0.30 A significantly enhances COD removal. EF degradation of organics is mainly ascribed to their oxidation with •OH in the bulk, produced homogeneously from Fenton’s reaction (Equation (1)), but also by the action of heterogeneously formed hydroxyl radical BDD(•OH) at the anode surface (Equation (21)) [12].
The oxidative action of BDD(•OH) is very efficient and can completely mineralize aromatics and unsaturated compounds such as carboxylic acids [12]. The low adsorption capacity of hydroxyl radicals on BDD runs their dimerization, leading to the formation of H2O2 (Equation (22)). Moreover, its high oxidation potential facilitates the generation of other weaker oxidants such as peroxydisulfate ions (Equation (23)), a strong oxidant (E0 = 2.1 V) but kinetically slow to destroy many organic contaminants.
Thus, the increasing electric current can accelerate all these processes. Note that the ITWW-CF contained a significant concentration of sulfate ions, due to both the intrinsic characteristics of the ITWW and the reagents used in the EF process (ferrous sulfate, sulfuric acid, supporting electrolyte (Na2SO4)). Thus, in the presence of ferrous ion, peroxodisulfate ion can be transformed into the sulfate-free radicals (SO4•-) [63] (Equation (24)), additionally contributing to the overall efficiency of the process [12].
At the given reaction conditions, an electrical current above 0.2 A allowed compliance with environmental regulations. This monitoring threshold was achieved after 160 min of treatment using 0.3 A, with an operational cost of 6.65 USD/m3. However, when the electrical current of 0.4 A was applied, no increase in COD removal was observed. This is probably due to the H2O2 decomposition on the anode surface (Equations (25) and (26)) [12].
Considerable decolorization was also observed for electrical currents exceeding 0.2 A, indicating increased H2O2 production and higher Fe2+ concentration regenerated at the cathode, among others (Equations (21) and (24)). This suggests enhanced •OH generation in the solution and the propagation of Fenton chain reactions.
Based on these findings, the effect of Fe2+ dosage, in the range of 1 mM to 6 mM, on COD removal at an electric current of 0.3 A was examined. The highest COD removal (92%) was obtained for Fe2+ concentration of 1 mM (Figure 3b). Higher Fe2+ concentrations led to a decrease in the degradation of organic pollutants. This can be attributed to excessive presence of ferrous ions, which tend to scavenge •OH radicals, as described in Equation (15). Note that in the absence of Fe2+ (0 mM, Figure 3b), the scope of the oxidative reaction was limited due to the lack of •OH in the solution and the restricted oxidation capacity of H2O2.
Hence, the following operational conditions were identified: 1 mM of Fe2+ and an electrical current of 0.3 A. Under these parameters, it is possible to comply with environmental regulations (COD < 400 mg/L) after 160 min of electrolysis, at an operating cost of 6.65 USD/m3 and energy consumption of 33.2 kWh/m3.
3.4. Peroxi-Coagulation (PC)
In the PC process, production of both Fenton’s reagents is carried out electrochemically. Accordingly, the electrical current significantly impacts the efficiency of wastewater treatment by this method. As depicted in Figure 4, the increase in electric current from 0.05 A to 0.3 A leads to greater COD removal.
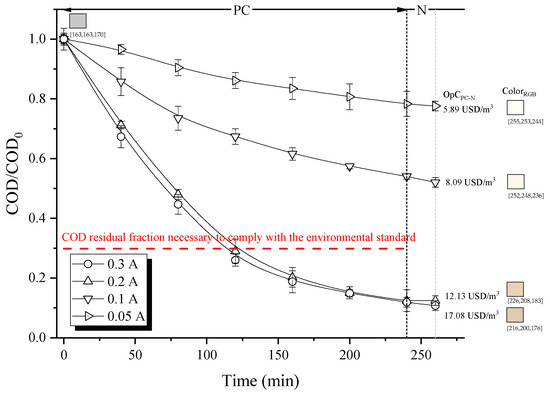
Figure 4.
Effect of electric current on the degradation capacity of organic matter (residual COD fraction), decolorization (RGB scale) and total operation costs for PC treatment. (Note: RGB color system, constructs all the colors from the combination of the red, green and blue colors. Each parameter (red, green and blue) defines the intensity of the color with a value in the range of 0 to 255. For example, RGB (255, 0, 0) is displayed as red because red is set to its highest value (255), and the other two (green and blue) are set to 0).
This is due to the simultaneous generation of H2O2 and Fe2+ at the cathode and anode, (Equations (7) and (9), respectively), as well as Fe3+ regeneration, (Equation (8)), resulting in a higher production of •OH, (Equation (1)), the primary oxidizing agent. Interestingly, no substantial differences in COD removal were observed between 0.2 A and 0.3 A, suggesting that the system reached its maximum oxidation capacity at around 0.2 A. Furthermore, the decolorization of the treated solution was more effective at currents lower than 0.3 A, compared to the value obtained at 0.2 A. This could be attributed to the increased formation of Fe2+, which, if not fully oxidized by H2O2, contributes negatively to the color of the solution (its precipitation as Fe(OH)3 is prevented by the continuous pH regulation using H2SO4). Therefore, operating the system at 0.2 A (yielding 37.31 mM of Fe2+) for 120 min allowed to meet environmental regulations at a cost of 6.98 USD/m3 (energy consumption of 4.28 kWh/m3), providing savings in energy consumption and amount of sludge generated.
3.5. Scope of EF-Based Configurations (ECP-N, EF-N, PC-N) Implemented for the Treatment of ITWW-CF
The selected operating conditions of the three EF-based configurations (Table 5), implemented sequentially for the treatment of the supernatant of the CF process, allowed compliance with the requirements of current environmental regulations regarding COD (<400 mg/L), among other parameters. In addition, they showed similar removal capacity for TOC, BOD5 and color. However, under this requirement (compliance with environmental regulations), the treatments presented significant differences (Table 5) with respect to (i) specific operating conditions and required materials; (ii) electrolysis times: ECP-N (52 min) < PC-N (120 min) < EF-N (160 min); (iii) energy consumptions: ECP-N (2.27 kWh/m3) < PC-N (4.28 kWh/m3) < EF-N (33.2 kWh/m3); (iv) average current efficiencies: ECP-N (358%) > PC-N (156%) > EF-N (78%); (v) solids generated: EF-N (0.82 g/L) < ECP-N (3.81 g/L) < PC-N (3.96 g/L); and (vi) operational costs: ECP-N (2.63 USD/m3) < EF-N (6.65 USD/m3) < PC-N (6.98 USD/m3). These findings allow to identify ECP-N as a promising treatment alternative. However, due to the significant differences mentioned, it is essential to have more elements for decision making before their optimization and future large-scale application.

Table 5.
Operating conditions and scope of sequential processes (ECP-N, EF-N, PC-N) implemented for the treatment of ITWW-CF.
Note that the total operational costs (TOpCsequential) of applied CF-EF-based configurations, followed by neutralization (N), required to comply with the environmental standard regarding COD, are in the range of 8.62–12.97 USD/m3 (Table 5). The values fall within the typical range reported by various textile companies across EU nations (0.3 USD/m3 and 27.3 USD/m3) [64]. However, these types of direct comparisons may not be fair since the cost of treatment is influenced by capital investment, operational expenses, regulatory compliance requirements, the volume and characteristics of the wastewater treated, the chosen treatment technology and local economic conditions.
Thus, to understand the overall environmental performance of studied EF-based configurations, to compare their environmental impacts, to identify key areas for their optimization and balance environmental compliance with economic considerations, life cycle analysis (LCA) was performed.
3.6. Life Cycle Analysis
3.6.1. Environmental Performance at Midpoint Level
Table 6 summarizes the results obtained in each of the impact categories considered in the ReCiPe-2016 method at the midpoint level for the three treatment processes studied. They indicate the environmental impact of the processes per unit of pollutant released or resources used.

Table 6.
Midpoint analysis results per FU for the electrochemical processes: ECP, EF and PC.
In comparison, the ECP process has the lowest relative impact (between 13.1 and 52.5%) in the 18 categories evaluated (Figure 5a). Of these, the carbon footprint (CFP or climate change) was significantly lower for the ECP (10.3 kg CO2-Eq/FU) compared to the EF (38.0 kg CO2-Eq/FU) and PC (26.3 kg CO2-Eq/FU) processes (Table 6). This performance can be mainly attributed to a shorter electrolysis time required and lower electricity consumption. The latter due to higher conductivity of iron electrodes, compared to those of EF and PC processes. Thus, for ECP, a lower voltage was registered during the electrolysis (2.59 V), implying lower energy consumption (2.27 kWh/m3) and lower operational costs (2.63 USD/m3). It is important to highlight that for the ECP process, the CFP value (in addition to the other impact categories) can even be lower if its operating conditions are optimized (i.e., reagent dosage, current density, treatment time).
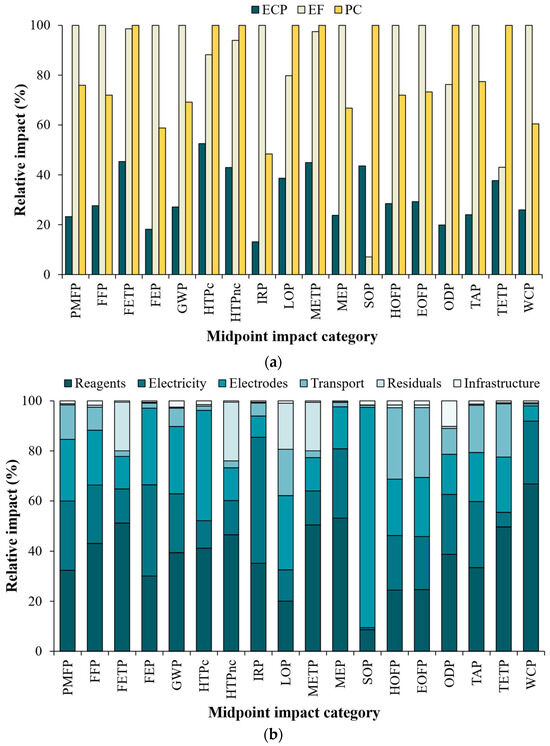
Figure 5.
Results of midpoint analysis according to the ReCiPe-2016 method. (a) Relative impact contribution of ECP, EF and PC processes and (b) the main activities of the ECP process.
A more detailed analysis of the ECP process showed that the consumption stages of reagents, electricity and electrodes (iron plates) contributed between 62 and 98% of the relative impact in all categories considered in the ReCiPe-2016 method (Figure 5b). For the LCA of this process, four reagents were considered: H2O2 (oxidizing agent and source of ●OH radicals), Na2SO4 (support electrolyte), H2SO4 (pH adjustment) and Ca(OH)2 (neutralization of the treated effluent). Of these reactants, H2O2 was the main contributor in this stage, being responsible for between 6.3 and 60% of global emissions. This agrees with the results of other studies reported in the open literature on AOPs based on the Fenton reaction, where H2O2 consumption is identified as an important environmental aspect [65,66,67]. Despite its non-toxic nature and being considered a green reagent (reaction by-products: H2O and O2), its industrial production is very demanding in environmental terms. This includes a hydrogenation and oxidation process of anthraquinone dissolved in a mixture of organic solvents, followed by a liquid–liquid extraction process of H2O2 and subsequent distillation to increase its purity [68,69]. These stages are characterized by high energy consumption (800–1600 kWh/ton H2O2, for heat and electricity supply), high temperatures (approx. 100 °C) and pressures (up to 10 bar), high water consumption (1–2 m3/ton H2O2 for the extraction process), waste generation, wastewater generation (COD up to 2425 mg O2/L; TOC up to 900 mg C/L) and polluting emissions (i.e., VOCs, CH4). In addition, all the risks associated with its handling, transportation and storage in high concentrations [69,70,71] must be considered when it is used in the wastewater treatment. These characteristics represent a limitation on the sustainability of this type of treatment process; so, low H2O2 consumption could improve their environmental performance.
For its part, electricity consumption in the ECP process had a global impact between 0.8 and 50.3% (Figure 5b). This is mainly associated with the requirement for voltage transformation (from high to medium voltage), through which the electricity produced must pass for subsequent use. Only in this stage (energy consumption), voltage transformation contributes between 20 and 99% of the impacts. Note that the voltage transformation process involves (1) use of non-renewable materials for its operation (i.e., porcelain, aluminum, copper, steel, insulating materials); (2) losses of sulfur hexafluoride (SF6), an insulating gas used in transformer equipment that has a high global warming potential (GWP-100 = 23,500); (3) emission of polluting gases, such as NOx and O3; and (4) electricity losses during distribution and transmission. Although losses do not represent more than 1% of the total electricity flowing through transformers, they are responsible for between 66 and 98% of the environmental impacts of electricity transmission and distribution [72,73]. LCA also highlights a higher incidence in the IRP category (50.3%). This is associated with the emission of radionuclides from, for example, the burning of coal for electricity production [57]. However, because in Colombia the majority of electricity comes from hydroelectric plants (approx. 70%), the contribution of coal-based electricity production does not have a significant influence in this category. In fact, voltage transformation almost entirely affects this category.
The third stage with the greatest impact on the environmental footprint of the ECP process is the material required for the electrodes, which had a contribution between 6.1 and 88% of the global relative impact (Figure 5b). This stage involves the extraction of iron and the subsequent production of steel plates. The steel industry is known worldwide as an activity with high energy demand, being the second energy consumer in the industrial sector. Within its stages, a series of environmental impacts that are difficult to mitigate occur, including the increase in CO2 emissions, as well as the release of polluting substances (i.e., PM10, SO2, NOx, dust, fluorine, lead, H2S, VOCs, phenol) and generation of wastewater and hazardous waste. It is estimated that for the year 2019, this industry had a discharge of 2.8 billion tons of CO2, which is related to the high CFP in the production of this material (3.78–4.4 kg CO2-Eq/kg of steel) [74]. The impact of the production of this material is particularly more noticeable in the SOP category (88%), which involves the extraction of mineral resources (Figure 5b). This is due to the high demand for materials required for steel production such as iron mineral (848 kg/ton steel), limestone (195 kg/ton steel), quicklime (921 kg/ton steel), fluorite (996 kg/ton steel), lump ore (153 kg/ton of steel), among others [74].
The other stages considered in the ECP process had variable contributions in the different impact categories: transportation (0.8–28.5%); residuals (0.1–23.5%); and infrastructure (0.4–10.2%). Generally, the transportation of materials and infrastructure does not have special importance in AOPs due to its low environmental impact during its life cycle [67,75]. On the other hand, the stage residuals (CO2 from mineralization and sludge disposal), which is rarely included in this type of study, resulted in a smaller environmental footprint. This stage had a significant contribution in the impact categories associated with toxicity, FETP (19.4%), HTPnc (23.5%) and METP (19.4%), due to the technology considered for waste disposal (landfill). In turn, the land occupation for this final disposal system negatively impacts the LOP category (18.4%) (Figure 5b).
Regarding the other two treatment processes evaluated, the EF process is seriously affected by electricity consumption, which contributes between 72.2 and 98.2% of the relative impact of the categories analyzed. The higher energy consumption of this process compared to the ECP is due to (1) higher current necessary for compliance with Colombian environmental legislation (I = 0.3 A); (2) longer operation time (160 min); (3) continuous aeration of wastewater; and (4) higher voltage to perform the electrolysis (8.30 V) due to the material of the electrodes (carbon felt and BDD/Si). On the other hand, in the PC process, although there is an increase in electricity consumption of up to two times compared to the ECP, the greatest impact is associated with the high consumption of reagents (H2SO4 and NaOH, mainly; relative impact: 13.2–74.5%) and the iron electrode (relative impact: 5.2–84.5%) for the supply of Fe2+ ions in the Fenton reaction.
3.6.2. Environmental Performance at Endpoint Level
The environmental analysis at the endpoint level involved three protection areas: human health (HH), ecosystem quality (ED) and resource availability (RA). These allow for evaluating the impact that the midpoint level categories have on the protection areas because these are related to each other [57]. Within this work, this analysis was carried out for the ECP process due to its better environmental and economic performance from midpoint analysis. The total impact generated in the protection areas, HH, ED and RA, by FU was 2.88 × 10−5 DALY, 4.94 × 10−8 species × year and USD 0.72, respectively. Figure 6 shows the contribution of the different categories at the midpoint level.
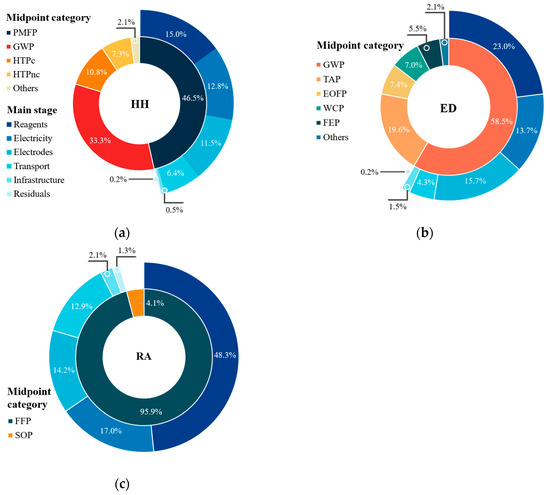
Figure 6.
Results of endpoint analysis for ECP process according to the ReCiPe-2016 method. Relative impact contribution of midpoint categories to (a) damage to human health, (b) damage to ecosystem quality and (c) damage to resource availability.
Note that the PMFP (46.5%) and GWP (33.3%) categories had the greatest contribution to the impact on HH (Figure 6a). The PMFP category implies the emission of PM2.5, which consists of a mixture of organic and inorganic polluting substances (i.e., SO2, NH3, NOx, dust, metals) discharged directly into the air. These come from different sources, such as transportation, industries, burning of fuels and waste, among others [76]. Due to the particle size, PM2.5 is easily absorbed into the respiratory system and subsequently into the bloodstream. This brings serious effects on human health, such as cardiopulmonary diseases, cancer and neonatal disorders [77]. On the other hand, the increase in the emission of greenhouse gases (i.e., CO2, CH4, N2O; contributors to the GWP category) and their concentration in the atmosphere can have different consequences (e.g., increase in the temperature of the planet, increase in cases of respiratory diseases, vector-borne diseases, deaths due to catastrophic events, among others [78]). Within the different activities considered in the LCA, and consistent with the results obtained at the midpoint level, the consumption of reagents, electricity and use of electrodes have the greatest contribution in the PMFP (84.6%) and GWP (89.7%) categories, as they are closely related to industrial processes with high environmental impact.
Regarding damage to the quality of ecosystems (ED), the contribution of the GWP (58.5%) and TAP (19.6%) categories stands out. This endpoint level category considers damage to terrestrial, freshwater and marine species. However, LCA showed that 94% of the potential impacts are linked to terrestrial ecosystems. As shown in Figure 6b, the consumption of reagents, electricity and use of electrodes also have the largest contribution to greenhouse gas emissions. The effects of climate change on ecosystems include droughts, forest fires, floods, water scarcity, rising ocean levels, loss of biodiversity, among others [79]. As for the TAP category, this implies changes in the acidity of the soil due to the atmospheric deposition of substances such as SO2, NOx, NH3, phosphates and sulfates, which are present in the different processes for the production of reagents, electricity and electrodes [53,80]. This change in soil acidity directly threatens its fertility, bringing negative consequences on plant diversity (i.e., low root and biomass production, low germination, reduction in photosynthetic activity).
Finally, the FFP category had the greatest contribution (95.9%) over the RA endpoint category, being mainly dominated by the consumption of reagents (50.4%) and electricity (17.8%) (Figure 6c). This contribution is mainly due to the need for fossil fuels and high energy consumption required in the production of H2O2.
3.6.3. Sensitivity Analysis of the ECP Process
To identify parameters or decisions (i.e., assumptions, variability in data, impact methods) that have great influence on the LCA results, a sensitivity analysis was carried out following a one-at-a-time approach: individually decreasing each parameter of interest by 20% (i.e., H2O2, electrodes or electricity), keeping the other parameters constant and analyzing the change in the results in the impact categories considered in the ReCiPe-2016 method [81,82]. The variation in the results was evaluated by calculating the sensitivity ratio (SR, Equation (13)) [83,84]. Here, an SR value equal to one implies that a 20% decrease in the parameter of interest leads to an equal reduction in the final result of the analyzed impact category.
As shown in Figure 7, a 20% decrease in the consumption of H2O2, electrodes or electricity had no significant variations (less than 10%, SR < 0.5) in the relative impact of most of the categories considered in the ReCiPe-2016 method. Specifically, in the CFP (GWP-100), changing the parameters of interest allowed their value to be reduced between 4 and 6% of the base scenario (10.3 kg CO2-Eq/FU) previously analyzed. This is of special interest in the environmental performance of the ECP process, since a large variation (increase or decrease) in these parameters will not lead to significant variations in CO2-Eq emissions. The decrease in H2O2 consumption allows to obtain SR values lower than 0.6, which indicates a reduction in emissions of less than 20% in all impact categories (Figure 7). The most sensitive categories (METP, FETP, MEP and WCP) presented a decrease in their relative impact between 8.5 and 12% (SR = 0.4–0.6). In other categories (i.e., SOP, LOP), registered decrease was less than 3% (SR = 0.06–0.15). The use of H2O2 is related to different environmental problems due to its highly demanding industrial process, contributing to impacts related to toxicity (METP, FETP) and especially in water consumption (WCP). In fact, in this study the WCP category is one of the most affected due to H2O2 consumption, contributing 60% to the relative impact of the ECP process.
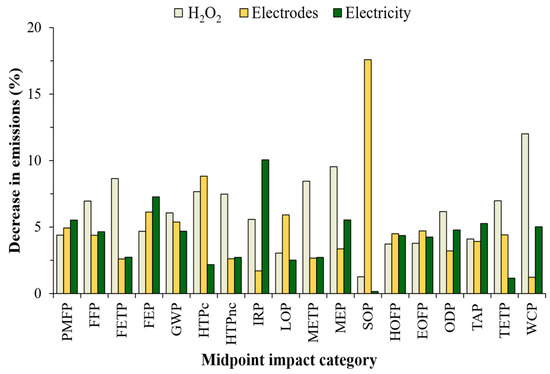
Figure 7.
Sensitivity analysis for the ECP process. Decrease in emissions caused by a reduction of 20% in the consumption of H2O2, electrodes or electricity.
On the other hand, when the ECP process has a 20% decrease in electrode consumption (mass lost due to electrolysis), the SOP category (highest sensitivity) presents an SR value of 0.88, allowing a variation of 17.6% in the relative impact (Figure 7). This category is associated with the extraction of mineral resources for use in different activities, causing their scarcity and generating effects at the midpoint and endpoint levels [57]. As mentioned previously, the production of the electrodes involves a large use of different resources, such as iron, limestone, quicklime, fluorite, lump, among others [74]. Specifically, this activity had the greatest contribution to the environmental footprint associated with the SOP category (88%). As shown in Figure 7, 13 of the 18 categories analyzed had SR values between 0.06 and 0.25, contributing a variation of less than 5% in the global relative impact. According to this analysis, reducing the current applied in the ECP process will have the advantage associated with lower consumption of iron anode, thus contributing to a large reduction in the impact of the SOP category. Finally, the IRP category (related to the emission of radionuclides) is seriously affected by electricity consumption (especially the voltage transformation process), resulting in the category with the highest sensitivity (SR = 0.5). This implies that decreasing this parameter by 20% leads to a 10.1% reduction in its relative impact. For most of the other impact categories, this variation does not imply significant contributions to the reduction in the environmental footprint (less than 5%) (Figure 7). The SR results suggest that LCA does not present a high sensitivity in most categories, which is due to the variations in 20% of the parameters of interest implying reductions of less than 10% in the relative impact, which constitutes an advantage for the environmental sustainability of the ECP process. However, the LCA results were sensitive in categories such as WCP, SOP and IRP, where small variations in the consumption of H2O2, electrodes or electricity, respectively, resulted in large changes in their environmental performance.
4. Conclusions
This study reported, for the first time, on the assessment of multistage system composed of a sequential combination of coagulation–flocculation with different electro-Fenton-based configurations, followed by neutralization, for the treatment of raw textile wastewater (ITWW) heavily contaminated with acid black 194 dye (AB194) and other pollutants.
The three EF-based systems (ECP-N, EF-N, PC-N) complied with current environmental standards under different (i) specific operating conditions and materials; (ii) electrolysis times: ECP-N (52 min) < PC-N (120 min) < EF-N (160 min); (iii) energy consumptions: ECP-N (2.27 kWh/m3) < PC-N (4.28 kWh/m3) < EF-N (33.2 kWh/m3)); (iv) average current efficiencies: ECP-N (358%) > PC-N (156%) > EF-N (78%); (v) solids generated: EF-N (0.82 g/L) < ECP-N (3.81 g/L) < PC-N (3.96 g/L); and (vi) operational costs: ECP-N (2.63 USD/m3) < EF-N (6.65 USD/m3) < PC-N (6.98 USD/m3).
According to LCA (ReCiPe-2016 method at the midpoint level), the ECP-N process exhibited the smallest environmental footprint in all the impact categories analyzed (relative impact from 13.1 to 52.5%). Reagents (mainly H2O2), electricity (mostly voltage transformation) and electrodes (largely their production process) contributed between 62 and 98% of the relative impact. The environmental impact of the EF-N process was primarily driven by electricity consumption, accounting for 72.2 to 98.2%, and that of PC by high consumption of reagents (particularly H2SO4 and NaOH, which contributed 13.2 to 74.5% of the impact). The lowest carbon footprint of 10.3 kg CO2-Eq/FU (CFP or climate change) was estimated for ECP-N (< PC-N (26.3 kg CO2-Eq/FU) < EF-N (38.0 kg CO2-Eq/FU).
According to the ReCiPe-2016 method at the endpoint level conducted for ECP-N, the PMFP (46.5%) and GWP (33.3%) categories had the largest contribution to the impact on human health; regarding the quality of the ecosystem, the GWP (58.5%) and TAP (19.6%) categories stood out; and the FFP category had the highest contribution (95.9%) over the resource availability.
The sensitivity analysis of ECP revealed that small variations in the consumption of H2O2, electrodes or electricity resulted in significant changes in categories such as WCP, SOP and IRP, which could affect its overall environmental performance.
The application of ECP-N, EF-N and PC-N processes was associated with a significant energy demand. Specifically, the electricity consumption required for the electrolysis, particularly in EF-N, has been identified as an environmental hotspot. To mitigate their environmental footprint before their scaling up for industrial applications, further research may be focused on two approaches: (1) Optimization of the operating conditions through the application of chemometric tools. This can help improve the energy consumption, reduce electrolysis time and decrease reactant usage; (2) usage of renewable energy sources, such as photovoltaic systems. This can lead to fewer polluting gas emissions and a shorter electricity generation chain, making these processes more environmentally friendly. By addressing these energy-related aspects, ECP-N, EF-N and PC-N can become a viable alternative for textile industrial wastewater treatment.
Within the scope of this work, the ECP-N process resulted as an interesting option with high potential for large-scale applications in terms of its technical, economic and environmental sustainability (easy operational implementation in existing treatment facilities; low operation time: 52 min; low-cost electrodes with wide local commercial availability; low operational cost of 2.63 USD/m3 and energy consumption of 2.27 kWh/m3; high efficiency in the removal of organic matter (<400 mg/L) and color (>87%); significant reduction in environmental footprint: relative impact reduction = 13.1–52.5%; and low carbon footprint: 10.3 kg CO2-Eq/FU).
Author Contributions
Conceptualization, I.D.-G. and M.-Á.G.-G.; methodology, I.D.-G. and M.-Á.G.-G.; software, L.-M.S.-S., J.-C.C.-S. and D.-O.S.-L.; validation, I.D.-G. and M.-Á.G.-G.; formal analysis, I.D.-G., L.-M.S.-S., J.-C.C.-S., D.-O.S.-L. and M.-Á.G.-G.; investigation, I.D.-G., L.-M.S.-S., J.-C.C.-S., D.-O.S.-L. and M.-Á.G.-G.; resources, I.D.-G. and M.-Á.G.-G.; writing—original draft preparation, I.D.-G., L.-M.S.-S. and M.-Á.G.-G.; writing—review and editing, I.D.-G., L.-M.S.-S., J.-C.C.-S., D.-O.S.-L. and M.-Á.G.-G.; visualization, I.D.-G., L.-M.S.-S., J.-C.C.-S. and M.-Á.G.-G.; supervision, I.D.-G. and M.-Á.G.-G.; project administration, I.D.-G. and M.-Á.G.-G.; funding acquisition, I.D.-G. and M.-Á.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Nacional de Colombia (Convocatoria para el Fortalecimiento de la Investigación, Creación, e Innovación Articulado con la Formación en la Universidad Nacional de Colombia 2020–2021: Proyectos: HERMES-51167, HERMES-51225) as well as MINCIENCIAS (Convocatoria 852–2019, Proyecto: 202010034716, Contrato: 172–2021, HERMES-46681).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panhwar, A.; Jatoi, A.S.; Mazari, S.A.; Kandhro, A.; Rashid, U.; Qaisar, S. Water Resources Contamination and Health Hazards by Textile Industry Effluent and Glance at Treatment Techniques: A Review. Waste Manag. Bull. 2024, 1, 158–163. [Google Scholar] [CrossRef]
- Prasetyo, H.; Norrdin, M.N.A.M.; Othman, M.H.D.; Jaafar, J.; Yoshioka, T.; Li, Z.; Rahman, M.A. Technologies for Treating Wastewater from Textile Industry: A Review. Mater Today Proc. 2022, 65, 3066–3072. [Google Scholar] [CrossRef]
- Castillo-Suárez, L.A.; Sierra-Sánchez, A.G.; Linares-Hernández, I. A Critical Review of Textile Industry Wastewater: Green Technologies for the Removal of Indigo Dyes. Int. J. Environ. Sci. Technol. 2023, 20, 10553–10590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Wu, C.H.; Ng, H.Y. Degradation of C.I. Reactive Red 2 (RR2) Using Ozone-Based Systems: Comparisons of Decolorization Efficiency and Power Consumption. J. Hazard. Mater. 2008, 152, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dede, O.T.; Aksu, Z.; Rehorek, A. Sonochemical Degradation of C.I. Reactive Orange 107. Environ. Eng. Sci. 2019, 36, 158–171. [Google Scholar] [CrossRef]
- Litter, M.I. Introduction to Photochemical Advanced Oxidation Processes for Water Treatment; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2. [Google Scholar]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of Water and Wastewater by Advanced Oxidation Processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Venny, S.G.; Ng, H.K. Current Status and Prospects of Fenton Oxidation for the Decontamination of Persistent Organic Pollutants (POPs) in Soils. Chem. Eng. J. 2012, 213, 295–317. [Google Scholar] [CrossRef]
- Liu, J.; Ren, N.; Qu, C.; Lu, S.; Xiang, Y.; Liang, D. Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process. Water 2022, 14, 3711. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical Oxidation Remediation of Real Wastewater Effluents—A Review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Kara, M.; El Manssouri, I.; Assouguem, A.; Almutairi, M.H.; Bayram, R.; Mohamed, H.R.H.; Peluso, I.; Eloutassi, N. Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts 2022, 12, 665. [Google Scholar] [CrossRef]
- Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. From Fenton and ORR 2e—Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process. Catalysts 2023, 13, 674. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods: A General Review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in Electro-Fenton Process for Water and Wastewater Treatment: An Overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-Fenton Process for Water and Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent Trends and Developments in Fenton Processes for Industrial Wastewater Treatment—A Critical Review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Luo, D.; Wang, L.; Wang, C.; Cao, Y.; Singh, L.; Ahmadzadeh, S.; He, Z. Current Status and Future Perspective in Electro-Fenton Techniques for Wastewater Treatment: A Bibliometric Review. Appl. Nanosci. 2023, 13, 5885–5902. [Google Scholar] [CrossRef]
- Wang, C.T.; Hu, J.L.; Chou, W.L.; Kuo, Y.M. Removal of Color from Real Dyeing Wastewater by Electro-Fenton Technology using a Three-Dimensional Graphite Cathode. J. Hazard. Mat. 2008, 152, 601–606. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; dos Santos, E.V.; Medeiros de Araújo, D.; Panizza, M. Applicability of Diamond Electrode/Anode to the Electrochemical Treatment of a Real Textile Effluent. J. Electroanal. Chem. 2012, 674, 103–107. [Google Scholar] [CrossRef]
- Sales Solano, A.M.; Costa de Araújo, C.K.; Vieira de Melo, J.; Peralta-Hernandez, J.M.; Ribeiro da Silva, D.; Martínez-Huitle, C.A. Decontamination of Real Textile Industrial Effluent by Strong Oxidant Species Electrogenerated on Diamond Electrode: Viability and Disadvantages of this Electrochemical Technology. Appl. Catal. B Environ. 2013, 130–131, 112–120. [Google Scholar] [CrossRef]
- Eslami, A.; Moradi, M.; Ghanbari, F.; Mehdipour, F. Decolorization and COD Removal from Real Textile Wastewater by Chemical and Electrochemical Fenton Processes: A Comparative Study. J. Environ. Health Sci. Eng. 2013, 11, 31. [Google Scholar] [CrossRef]
- Ramirez-Pereda, B.; Álvarez-Gallegos, A.; Bustos-Terrones, Y.A.; Silva-Martínez, S.; Hernández-Pérez, A. Effective Electro-Fenton Treatment for a Real Textile Effluent: A Case Study. J. Water Process Eng. 2020, 37, 101434. [Google Scholar] [CrossRef]
- Kuleyin, A.; Gök, A.; Akbal, F. Treatment of Textile Industry Wastewater by Electro-Fenton Process using Graphite Electrodes in Batch and Continuous Mod. J. Environ. Chem. Eng. 2021, 9, 104782. [Google Scholar] [CrossRef]
- Afanga, H.; Zazou, H.; Titchou, F.E.; Rakhila, Y.; Akbour, R.A.; Elmchaouri, A.; Ghanbaja, J.; Hamdani, M. Integrated ElectroChemical Processes for Textile Industry Wastewater Treatment: System Performances and Sludge Settling Characteristics. Sustain. Environ. Res. 2020, 30, 2. [Google Scholar] [CrossRef]
- Kaur, P.; Kushwaha, J.P.; Sangal, V.K. Transformation Products and Degradation Pathway of Textile Industry Wastewater Pollutants in Electro-Fenton Process. Chemosphere 2018, 207, 690–698. [Google Scholar] [CrossRef]
- Kaur, P.; Sangal, V.K.; Kushwaha, J.P. Parametric Study of Electro-Fenton Treatment for Real Textile Wastewater, Disposal Study and its Cost Analysis. Int. J. Environ. Sci. Technol. 2019, 16, 801–810. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.A. Comparative study of Electrocoagulation, Electrochemical Fenton, Electro-Fenton and Peroxi-Coagulation for Decolorization of Real Textile Wastewater: Electrical Energy Consumption and Biodegradability. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Yazici Guvenc, S.; Can-Güven, E.; Çifçi, D.İ.; Varank, G. Biodegradability Enhancement and Sequential Treatment of Real Chemical Industry Wastewater by Chemical Coagulation and Electro-Fenton Processes. Chem. Eng. Process.-Process Intensif. 2023, 194, 109598. [Google Scholar] [CrossRef]
- Moutik, B.; Summerscales, J.; Graham-Jones, J.; Pemberton, R. Life Cycle Assessment Research Trends and Implications: A Bibliometric Analysis. Sustainability 2023, 15, 13408. [Google Scholar] [CrossRef]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of Techno-Economic Analysis and Life Cycle Assessment for Sustainable Process Design—A Review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Quintero-Arias, J.D.; Gómez-García, M.Á.; Dobrosz-Gómez, I. The scope of alum coagulation-flocculation assisted by slaked lime for the treatment of industrial wastewater containing highly concentrated Acid Black 194 dye. Optimization, molecular weight distribution and toxicity analysis. Results Eng. 2024; under revision. [Google Scholar]
- NTC-ISO 5667-2:1995; Gestión Ambiental. Calidad del Agua. Muestreo. Técnicas Generales de Muestreo. El Instituto Colombiano de Normas Técnicas y Certificación: Bogotá, Colombia, 1995. Available online: https://www.academia.edu/33146907/NORMA_T%C3%89CNICA_NTC_ISO_COLOMBIANA_5667_2 (accessed on 6 July 2024).
- NTC-ISO 5667-3:2004; Calidad del agua: Directrices para la Preservación y Manejo de las Muestras. El Instituto Colombiano de Normas Técnicas y Certificación: Bogotá, Colombia, 2004. Available online: https://www.studocu.com/co/document/universidad-nacional-de-colombia/quimica-analitica/ntc-iso-5667-03-2004-directrices-para-la-preservacion-y-manejo-de-muestras/29728969 (accessed on 6 July 2024).
- NTC-ISO 5667-1:2010; Calidad del Agua. Muestreo. Parte 1: Directrices para el Diseño de Programas y Técnicas de Muestreo. El Instituto Colombiano de Normas Técnicas y Certificación: Bogotá, Colombia, 2010. Available online: https://tienda.icontec.org/gp-calidad-del-agua-muestreo-parte-1-directrices-para-el-diseno-de-programas-y-tecnicas-de-muestreo-ntc-iso5667-1-2010.html (accessed on 6 July 2024).
- IDEAM—Instituto de Hidrología, Meteorología y Estudios Ambientales. Toma y Preservación Demuestras. 2015. Available online: http://www.ideam.gov.co/documents/14691/38158/Toma_Muestras_AguasResiduales.pdf/f5baddf0-7d86-4598-bebd-0e123479d428 (accessed on 6 July 2024).
- ASTM D2035-19; Practice for Coagulation-Flocculation Jar Test of Water. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- MADS—Ministerio de Ambiente y Desarrollo Sostenible Resolución 0631 de 2015. 2015. Available online: https://www.minambiente.gov.co/documento-normativa/resolucion-631-de-2015/ (accessed on 6 July 2024).
- Kurt, U.; Apaydin, O.; Gonullu, M.T. Reduction of COD in Wastewater from an Organized Tannery Industrial Region by Electro-Fenton Process. J. Hazard. Mater. 2007, 143, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Shabanloo, A.; Fazlzadeh, M.; Poureshgh, Y. Investigation of Operational Parameters Influencing in Treatment of Dye from Water by Electro-Fenton Process. Desalination Water Treat. 2016, 57, 24387–24394. [Google Scholar] [CrossRef]
- Malakootian, M.; Asadi, M.; Mahvi, A.H. Evaluation of Electro-Fenton Process Performance for COD and Reactive Blue 19 Removal from Aqueous Solution. Iran. J. Health Environ. 2013, 5, 433–444. [Google Scholar]
- Suhan, M.B.K.; Shuchi, S.B.; Anis, A.; Haque, Z.; Islam, M.S. Comparative Degradation Study of Remazol Black B Dye Using Electro-Coagulation and Electro-Fenton Process: Kinetics and Cost Analysis. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100335. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Centennial Edition of American Public Health Association (APHA): Washington, DC, USA, 2017.
- Do, S.-H.; Batchelor, B.; Lee, H.-K.; Kong, S.-H. Hydrogen Peroxide Decomposition on Manganese Oxide (Pyrolusite): Kinetics, Intermediates, and Mechanism. Chemosphere 2009, 75, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.M.; Abdul Raman, A.A.; Asghar, A. A Review on Approaches for Addressing the Limitations of Fenton Oxidation for Recalcitrant Wastewater Treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- He, Y.Q. Experiment on Treating the Dying Wastewater with Blast-Furnace Ash and Fenton Reagent. Adv. Mater. Res. 2011, 295–297, 1120–1123. [Google Scholar] [CrossRef]
- Moradi, M.; Ghanbari, F. Application of Response Surface Method for Coagulation Process in Leachate Treatment as Pretreatment for Fenton Process: Biodegradability Improvement. J. Water Process Eng. 2014, 4, 67–73. [Google Scholar] [CrossRef]
- Masiello, C.A.; Gallagher, M.E.; Randerson, J.T.; Deco, R.M.; Chadwick, O.A. Evaluating Two Experimental Approaches for Measuring Ecosystem Carbon Oxidation State and Oxidative Ratio. J. Geophys. Res. 2008, 113, G03010. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. Internati onal Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://cdn.standards.iteh.ai/samples/37456/1fc9f64898c14240b034a77eccef42f3/ISO-14040-2006.pdf (accessed on 24 July 2024).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://cdn.standards.iteh.ai/samples/38498/17324bfe9ec44e27a2f84e1a8ac3ca26/ISO-14044-2006.pdf (accessed on 24 July 2024).
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA). A Guide to Best Practice; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Salazar-Sogamoso, L.M.; Gómez-García, M.Á.; Dobrosz-Gómez, I. Comparative life cycle assessment of sequential chemical and electrochemical processes for the treatment of industrial textile wastewater. J. Solid State Electrochem. 2024, 28. [Google Scholar] [CrossRef]
- UPME–Unidad de Planeación Minero Energética. Proyección de la Demanda de Energía Eléctrica, Potencia Máxima y Gas Natural 2023–2037. 2023. Available online: https://www1.upme.gov.co/DemandayEficiencia (accessed on 6 July 2024).
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Huijbregts, M.; Steinmann, Z.; Elshout, P.; Stam, G.; Verones, F.; Vieira, M.; Hollander, A.; Zijp, M.; van Zelm, M. ReCiPe 2016: A Harmonized Life Cycle Impact Assessment Method at Midpoint and Endpoint Level Report I: Characterization. 2017. Available online: https://rivm.openrepository.com/handle/10029/620793 (accessed on 24 July 2024).
- MADS—Ministerio de Ambiente y Desarrollo Sostenible. Por la Cual se Reglamenta el uso de las Aguas Residuales y se Adoptan Otras Disposiciones. 2021. Available online: https://www.minambiente.gov.co/documento-normativa/resolucion-1256-de-2021/ (accessed on 6 July 2024).
- Magdy, M.; Gar Alalm, M.; El-Etriby, H.K. Comparative Life Cycle Assessment of five chemical methods for removal of phenol and its transformation products. J. Clean. Prod. 2021, 291, 125923. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Moridi, A. Efficiency of Electro-Fenton Process in Removing Acid Red 18 Dye from Aqueous Solutions. Process Saf. Environ. Prot. 2017, 111, 138–147. [Google Scholar] [CrossRef]
- Abo-Farha, S.A. Comparative Study of Oxidation of Some Azo Dyes by Different Advanced Oxidation Processes: Fenton, Fenton-like, photo-Fenton and photo-Fenton-like. J. Am. Sci. 2010, 6, 128–142. [Google Scholar]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbæk, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- European Comission. Total Water Recycling in Textil Industry. D 2.6 Report on Wastewater Handling in the Textile Industry in Europe. 2017. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5d1a5037b&appId=PPGMS (accessed on 23 July 2024).
- Zou, M.; Wei, J.; Qian, Y.; Xu, Y.; Fang, Z.; Yang, X.; Wang, Z. Life cycle assessment of homogeneous Fenton process as pretreatment for refractory pharmaceutical wastewater. Front. Chem. Sci. Eng. 2024, 18, 49. [Google Scholar] [CrossRef]
- Ramírez-Díaz, R.C.; Prato-García, D. Can thermal intensification be considered a sustainable way for greening Fenton processes? J. Environ. Manag. 2021, 289, 112551. [Google Scholar] [CrossRef]
- Foteinis, S.; Monteagudo, J.M.; Durán, A.; Chatzisymeon, E. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci. Total Environ. 2018, 612, 605–612. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem.-Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef]
- European Commission, Joint Research Centre; Brinkmann, T.; Falcke, H.; Holbrook, S.; Sanalan, T.; Roth, J.; Delgado Sancho, L.; Clenahan, I.; Lopez Carretero, A.; Zerger Serge Roudier, B. Best Available Techniques (BAT) Reference Document for the Production of Large Volume Organic Chemicals. Publications Office. 2017. Available online: https://data.europa.eu/doi/10.2760/77304 (accessed on 6 July 2024).
- Lanza, M.R.V.; Cordeiro-Junior, P.J.M.; Kronka, M.S.; Goulart, L.A.; Veríssimo, N.C.; Mascaro, L.H.; dos Santos, M.C.; Bertazzoli, R. Catalysis of oxygen reduction reaction for H2O2 electrogeneration: The impact of different conductive carbon matrices and their physicochemical properties. J. Catal. 2020, 392, 56–68. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Arvesen, A.; Hauan, I.B.; Bolsøy, B.M.; Hertwich, E.G. Life cycle assessment of transport of electricity via different voltage levels: A case study for Nord-Trøndelag county in Norway. Appl. Energy 2015, 157, 144–151. [Google Scholar] [CrossRef]
- Jorge, R.S.; Hawkins, T.R.; Hertwich, E.G. Life cycle assessment of electricity transmission and distribution-part 2: Transformers and substation equipment. Int. J. Life Cycle Assess. 2012, 17, 184–191. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q.; Wang, C.; Li, Z.; Geng, Y.; Hong, J.; Chen, Y. Environmental sustainability challenges of China’s steel production: Impact-oriented water, carbon and fossil energy footprints assessment. Ecol. Indic. 2022, 136, 108660. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Bai, Q.; Liu, Y.; Wang, P.; Xue, S.; Yu, Q.; Li, Q. Road life-cycle carbon dioxide emissions and emission reduction technologies: A review. J. Traffic Transp. Eng. Engl. Ed. 2022, 9, 532–555. [Google Scholar] [CrossRef]
- UN Environment Program—UNEP. Pollution Action Note—Data You Need to Know. 2023. Available online: https://www.unep.org/interactives/air-pollution-note/ (accessed on 24 June 2024).
- Thangavel, P.; Park, D.; Lee, Y. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- World Health Organization—WHO. Climate Change. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 24 June 2024).
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Arias, P., Bustamante, M., Elgizouli, I., Flato, G., Howden, M., Méndez-Vallejo, C., Pereira, J.J., Pichs-Madruga, R., Rose, S.K., Saheb, Y., et al., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Azevedo, L.; van Zelm, R.; Hendriks, A.; Bobbink, R.; Huijbregts, M. Global assessment of the effects of terrestrial acidification on plant species richness. Environ. Pollut. 2013, 174, 10–15. [Google Scholar] [CrossRef]
- Groen, E.A.; Bokkers, E.A.M.; Heijungs, R.; de Boer, I.J.M. Methods for global sensitivity analysis in life cycle assessment. Int. J. Life Cycle Assess. 2017, 22, 1125–1137. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Rosenbaum, R.K.; Olsen, S.I. Life Cycle Assessment. Theory and Practice; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Clavreul, J.; Guyonnet, D.; Christensen, T.H. Quantifying uncertainty in LCA-modelling of waste management systems. Waste Manag. 2012, 32, 2482–2495. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Díaz, R.C.; Prato-Garcia, D.; Vasquez-Medrano, R. How Sustainable is the Biohydrogen Produced from Sugarcane Vinasse? An Approach Based on Life Cycle Assessment. Biomass Convers. Biorefin. 2023, 13, 14755–14775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).