1. Introduction
Urban drinking water quality can be influenced by various factors, including environmental pollution at water sources, aging water supply infrastructure, and secondary contamination [
1]. Currently, metal pollution has become a significant global challenge for drinking water quality [
2]. Sources of metal contamination in drinking water can be attributed to natural processes such as mineral weathering or human activities, including mining, agriculture, industrial production, and waste management [
3]. A study by Kunfeng Zhang’s team [
4] surveyed eight heavy metals (beryllium (Be), antimony (Sb), barium (Ba), molybdenum (Mo), nickel (Ni), vanadium (V), cobalt (Co), and titanium (Ti)) in the inlet and outlet waters of 146 water treatment plants in China. They found that traditional and conventional water treatment processes such as coagulation, sedimentation, and chlorine disinfection might be inadequate for removing heavy metal ions from source water, with an average removal rate of only 16.30–95.64%.
Many countries and regions have started to focus on and study metal contamination in drinking water, including the Haryana region of India [
5,
6], Malaysia [
7], Kurdistan Region [
8], Pakistan [
9], Nigeria [
10], and Iran [
11]. These studies are not limited to traditional heavy metals such as arsenic (As), lead (Pb), and cadmium (Cd), but have also expanded to other potentially harmful metal elements such as barium, molybdenum, and antimony. Research methods have evolved from single-pollutant detection to simultaneous multi-element analysis and risk assessment, allowing for more comprehensive and precise monitoring of drinking water quality [
12]. However, although existing studies cover major cities and basins, most are still focused on specific regions or types of metals, lacking comprehensiveness and universality. Many areas still lack data on the metal contamination status of drinking water. Furthermore, a lack of long-term monitoring data is a problem, limiting the accurate assessment of trends in metal pollution and long-term effects in drinking water [
13].
Studies indicate that elements such as lithium (Li) and barium (Ba) also pose certain health risks in addition to the known health hazards posed by heavy metals such as arsenic and lead. Long-term exposure to lithium and barium may lead to kidney damage [
14]. The extensive and systematic detection of metal elements in drinking water, along with an assessment of their potential health risks, is crucial for ensuring water safety and providing early warnings of metal health hazards [
15].
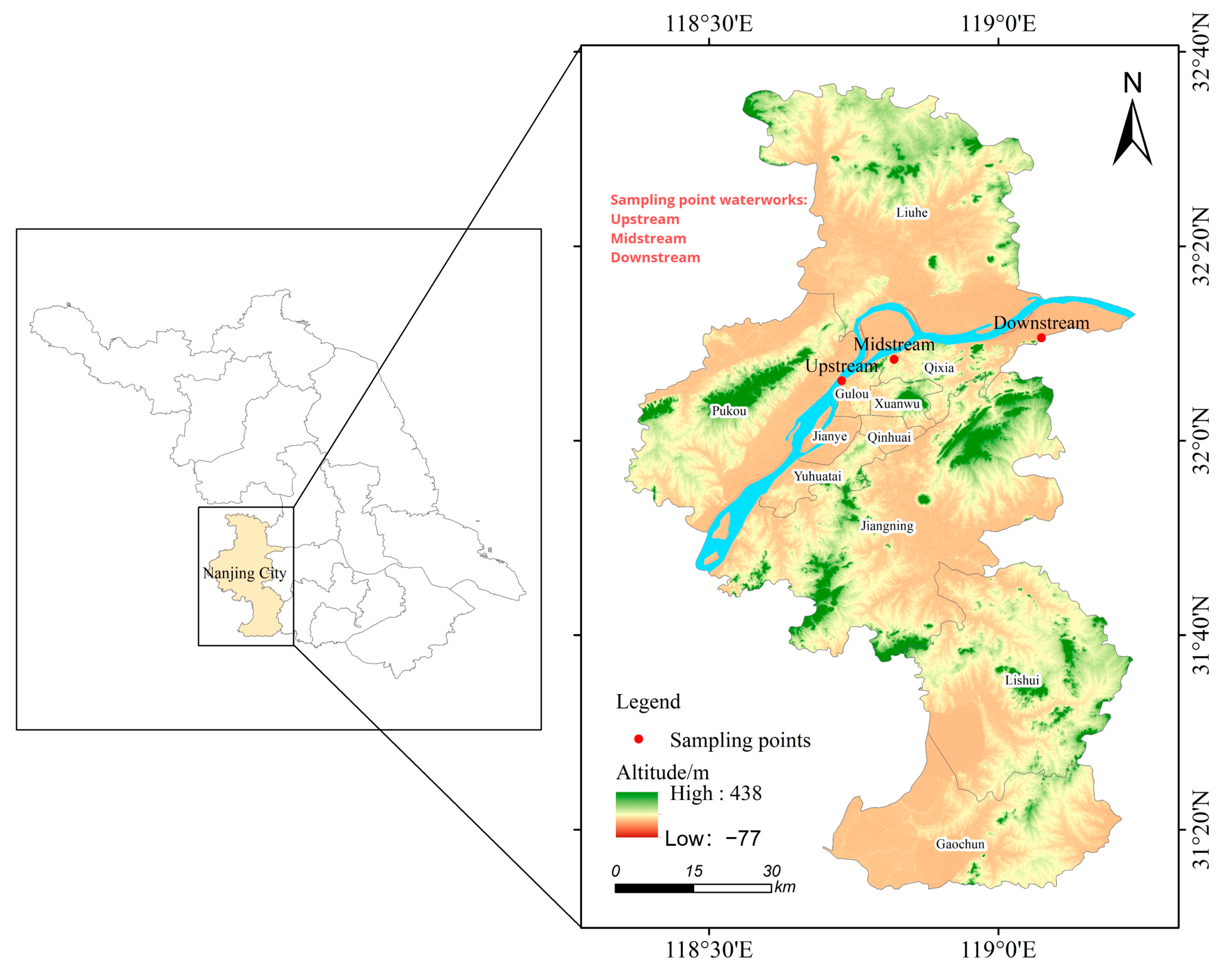
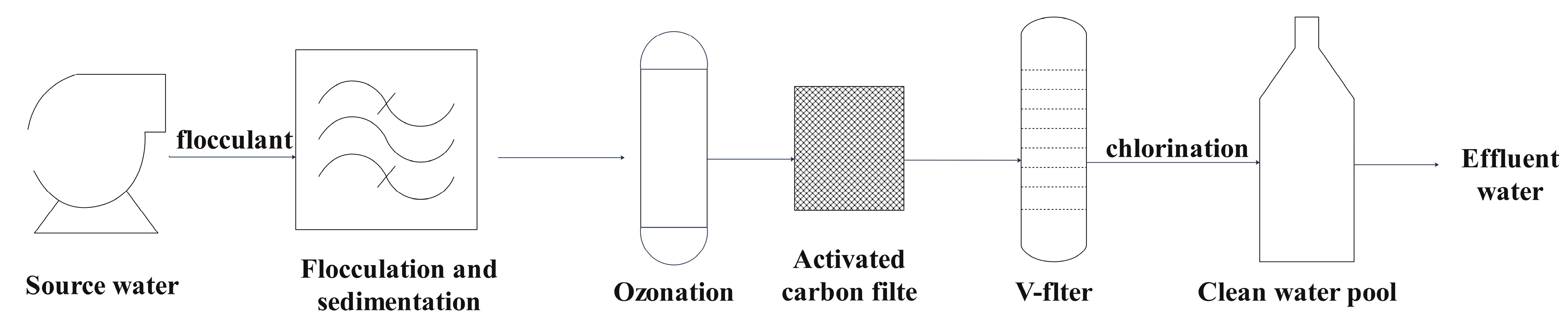
This study uses Nanjing City as an example to supplement research on the presence of metals and metalloids in advanced water treatment plants and their health risks to different populations. Water samples were collected from three different water sources along the Nanjing section of the Yangtze River and from advanced treatment process plants, including source water, sedimentation effluent, ozone-activated carbon-V-type filter effluent, and finished water. A comprehensive analysis of 33 metals and metalloids was conducted in different seasons and from different water sources and plants. The health risks of these metals in drinking water for different populations were assessed using the Environmental Health Risk Assessment Model of the United States Environmental Protection Agency (USEPA). The aim is to provide data for the assessment of drinking water monitoring and safety.
3. Results
3.1. Metal Levels in Source Water and Finished Water from the Nanjing Section of the Yangtze River Basin
The PCA revealed no significant differences in the total concentrations of metals/metalloids among the upstream, midstream, and downstream regions in either the source or finished water (
Figures S1 and S2). However, specific metals/metalloids showed notable variations (
Table 2 and
Figure S5).
The concentrations of various metals in the source water were analyzed across upstream, midstream, and downstream water treatment plants, after which statistical significance tests were conducted. The results indicated significant differences for certain metals of different locations. The Li, Ti, and V concentrations were higher in the upstream region, with Li showing the greatest fluctuation, reaching 12.267 μg/L. As and Se also had higher concentrations upstream, particularly the former, with a concentration of 3.305 μg/L. The statistical analysis revealed that As concentrations were significantly higher in the upstream and downstream regions compared to midstream (p = 0.038), while Se concentrations were significantly lower in the midstream compared to the upstream and downstream regions (p = 0.001).
The following metals and their respective concentrations were significantly higher in the midstream compared to the upstream and downstream regions: Al (p = 0.026), with a median of 37.748 μg/L; Ni (p = 0.013), with a median of 0.687 μg/L; and Zn (p = 0.003), with a median of 13.622 μg/L. The concentration differences of Mn among the three locations were also significant (p = 0.037). These data indicate significant differences in the concentrations of certain metals of different water treatment plant locations, particularly As, Se, Al, Mn, Ni, and Zn.
The upstream region showed higher concentrations of Li, Ti, and V in finished water (
Table 3 and
Figure S6), with Li exhibiting the greatest fluctuation, reaching 12.211 μg/L. As and Se also had higher concentrations upstream, particularly the former, with a concentration of 1.390 μg/L. A statistical analysis showed that As concentrations were significantly higher in the upstream and downstream regions compared to midstream (
p = 0.001), while Se concentrations were significantly higher in the midstream compared to the upstream and downstream regions (
p = 0.023). Al, Cu, and Zn concentrations were significantly higher in the midstream compared to the upstream and downstream regions (
p = 0.001 for Al and Cu;
p = 0.002 for Zn), with median values of 136.339 μg/L, 2.136 μg/L, and 41.759 μg/L, respectively. Ba concentrations were also higher in the midstream region (
p = 0.048). The following metals and their respective concentrations were significantly higher downstream compared to the upstream and midstream regions: Mo (
p = 0.001), with a median of 5.764 μg/L; Mg (
p = 0.001), with a median of 10.864 μg/L; and Ca (
p = 0.041), with a median of 62.171 μg/L.
Despite the lack of significant differences in the total concentrations of metals, the significant concentration variations of highly toxic metals such as As, Pb, and Cd in different regions indicate the need to optimize water treatment processes for different areas and establish continuous monitoring mechanisms to ensure water quality safety.
3.2. Seasonal Distribution Characteristics of Metals
The PCA (
Figures S3 and S4) revealed seasonal differences in the concentrations of metals/metalloids in the source water and finished water. Specifically, the concentrations of certain metals/metalloids varied significantly across different seasons in the source water (
Table 4,
Figure 3 and
Figure S6). In the spring, concentrations of Li, Ti, and Ga were higher, with Li showing the greatest fluctuation, reaching up to 9.765 μg/L. Statistical analysis showed that As concentrations in spring were significantly higher than in other seasons (
p = 0.001), reaching 2.354 μg/L, as were Rb concentrations (
p = 0.001). In winter, Sr and Ca concentrations were significantly higher than in other seasons, with Sr reaching 266.769 μg/L and Ca reaching 59.610 μg/L (
p = 0.002 and 0.001, respectively). Additionally, Tl and Pb concentrations were significantly higher in winter (
p-values of 0.001 and 0.001, respectively). In autumn, V and Zn concentrations were significantly higher than in other seasons, with V reaching 2.142 μg/L and Zn reaching 32.093 μg/L (
p = 0.001 and 0.001, respectively). Al concentrations were also higher in autumn, reaching 26.171 μg/L (
p = 0.002). In summer, Co and Cd concentrations were significantly higher than in other seasons, with Co reaching 0.465 μg/L and Cd reaching 0.726 μg/L (
p-values of 0.007 and 0.001, respectively). Additionally, B concentrations were lower in summer, at 28.115 μg/L (
p = 0.009).
The concentrations of certain metals/metalloids in finished water also varied significantly across different seasons (
Table 5,
Figure 3 and
Figure S6). The statistical analysis showed that concentrations of Li, Ti, and Ga were higher in the spring (
p = 0.001), with Li showing the greatest fluctuation, reaching up to 9.778 μg/L, whilst Ti and Ga concentrations reached 25.367 μg/L and 12.323 μg/L, respectively. The same analysis showed that V, Co, and Rb concentrations were significantly higher in summer than in other seasons (
p = 0.001), with V reaching 2.347 μg/L, Co reaching 0.099 μg/L, and Rb reaching 3.191 μg/L. It was also found that Sr, Zr, and Al concentrations were significantly higher in autumn than in other seasons (
p = 0.001), with Sr reaching 264.888 μg/L, Zr reaching 0.488 μg/L, and Al reaching 93.276 μg/L. Finally, the analysis showed that Tl, Hg, and Ca concentrations were significantly higher in winter than in other seasons (
p = 0.001), with Tl reaching 0.062 μg/L, Hg reaching 0.386 μg/L, and Ca reaching 90.536 μg/L. Additionally, Cd concentrations in summer were significantly higher than in other seasons, reaching 0.605 μg/L, while in winter, the concentration was even higher, reaching 0.781 μg/L (
p = 0.001). Pb concentrations in summer were also significantly higher, at 0.020 μg/L.
Combined with
Figure 3, these results show that metal concentrations were significantly higher in winter and spring than in the other seasons, suggesting that these two seasons have the most significant differences in water quality and may pose a greater threat to health risks, thus requiring special attention and optimization of water treatment processes to ensure safe water quality.
3.3. Metal Removal Efficiency
The analysis of metal removal from raw water showed that the removal percentages of various metallic elements ranged from −144.22% to 100.00% (
Table S7). Among them, the concentrations of the following four macroelements did not vary much: Na, Ca, K, and Mg. Trace elements such as Pb, Mn, Zn, Cu, and Co showed high removal rates of more than 30%. In addition, elements such as Bi, Ti, V, Sb, Cd, Tl, Se, Sr, and Li had lower removal rates ranging from 10% to 30%. Elements with removal rates below 10% included Sr, K, Na, and Mg.
In terms of negative removal rates, Al, Be, Cr, Mg, and Li had very low removal rates, ranging from −144.22% to−15.96%. In addition, Sn and Mo had removal rates ranging from −63.61% to −50.00%. Elements such as B, Ba, and Zr showed slight increases in removal rates, with negative removal rates ranging from −0.55% to 1.66%.
Further analysis was conducted on the metal removal efficiencies at different stages of treatment in the Nanjing Municipal Water Plant, including post-precipitation output water, ozone-activated carbon-V filter output water, and post-chlorination output water (
Table S6). During the post-precipitation stage, Zn, As, Cd, Co, Cr, Se, Cu, and Mo exhibited high removal efficiencies. Conversely, Al, Mn, and Ni showed increased concentrations, indicating negative removal efficiencies, whereas Sn, Sb, Cs, Hg, Tl, and Be had low or near-zero removal efficiencies.
In the ozone-activated carbon-V filter stage, high removal rates were observed for Pb, Al, Mn, Ni, Co, Sn, and Cu. However, Cd, Mo, Se, and Zn had negative removal efficiencies, indicating an increase in concentrations during this stage. Be, B, Sb, Cs, Hg, Tl, and V showed unchanged or lower removal performances.
An effective removal of Li, V, Ga, As, Sb, Ba, and Mo was observed during the chlorination treatment (finished water) stage. At the same time, concentrations of Cr, Cu, Mn, Ni, and Zn increased, while Co, Rb, Sn, Cs, Hg, Tl, Be, Cd, and B had low or negative removal efficiencies.
Overall, Pb, Cu, Co, and Mn showed high removal efficiencies across multiple treatment stages, while Al, Ni, and Zn exhibited increases in some stages. Additionally, Tl, Be, and Sn generally had low or indistinct removal efficiencies throughout the water treatment process.
3.4. Metal Inter-Correlation Analysis
We identified positive correlations (r > 0.7) among the studied elements through a Spearman correlation analysis of the concentrations of metals/metalloids in source water and finished water.
In the source water, multiple groups of metals/metalloids exhibited positive correlations, potentially indicating shared pollution sources or similar geological or environmental influences. Specifically, the positively correlated metal/metalloid pairs included the following (
Figure 4): Sr with Cr and Hg; B with Ti and Mo; Fe with Cu, Pb, and Zr; Ti with B and Ga; Li with Hg; Ga with Pb; Mo with B; Cu with Fe; Cr with Sr; Pb with Fe, Ga, and Zr; Zr with Fe and Pb; and Hg with Sr and Li.
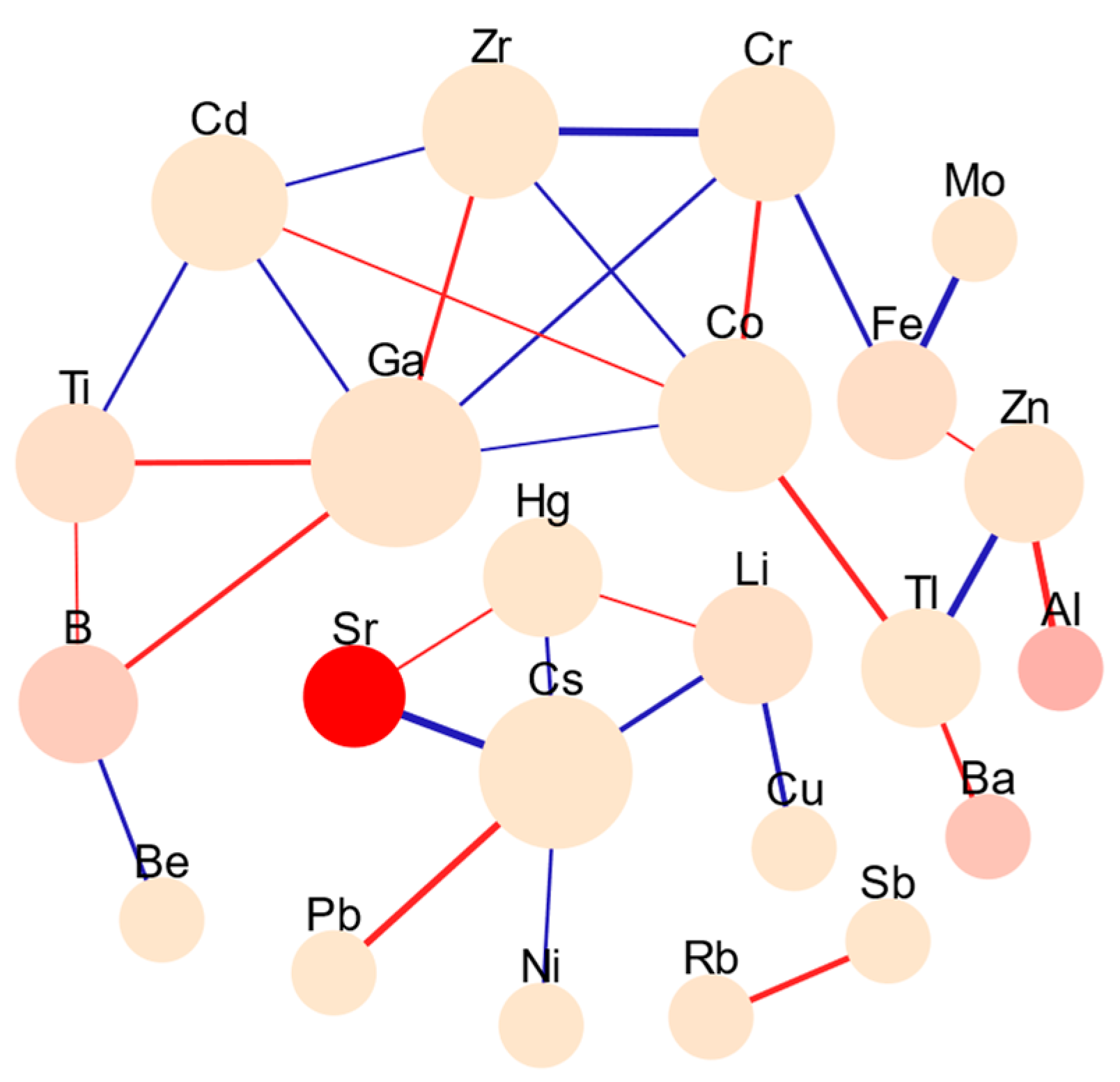
In the finished water, significant positive correlations remained among certain metals/metalloids despite the treatment processes. The following strongly correlated pairs were included (
Figure 5): Sr with Hg; Ba with Tl; B with Ti, Ga; Fe with Zn; Ti with B, Ga; Rb with Sb; Co with Cd, Tl, and Cr; and Cs with Pb. This suggests that the treatment efficiencies of these metals/metalloids may produce co-exposure characteristics. In the correlation study of finished water, a negative correlation was found between Al and Hg, as well as Ba. This indicates that as the concentration of aluminum increases, the concentrations of mercury and barium decrease, suggesting that the added coagulants may specifically remove Hg and Ba. Additionally, Fe showed positive correlations with several metals, such as Cu, Zr, and Pb, indicating that these metals may have common exposure sources during the treatment process. This situation could stem from the corrosion and aging of metal pipes and construction materials used in the treatment process, leading to the leaching of these metals.
3.5. Carcinogenic Health Risk Evaluation
The health risks associated with the presence of As, Cd, and Cr
6+ in drinking water are detailed in
Table 6. The order is ranked by annual carcinogenic risk: As > Cd > Cr
6+. The USEPA stipulates that an acceptable range for carcinogenic risk to the population is between 10
−6 and 10
−4, and the International Commission on Radiological Protection (ICRP) recommends a maximum acceptable risk level of 5 × 10
−5. Our comparison analysis reveals that the annual risk values of the three carcinogenic heavy metals in drinking water exceed the ICRP risk level. The cumulative carcinogenic health risk for children surpasses the USEPA risk level, while the cumulative carcinogenic health risks are close to the threshold for adult men and women.
3.6. Non-Carcinogenic Health Risk Evaluation
The health risk assessment of metals in drinking water is presented in
Table 7. The cumulative annual health risks for metals in the finished water from the advanced treatment processes in Nanjing’s urban area are as follows: adult males (0.314), adult females (0.275), and children (0.667). The cumulative annual HI is less than 1, which is within the acceptable limits. The primary contributors to the metal health risks are As (30.82%), Tl (3.92%), and V (1.88%).
4. Discussion
Since 2021, 15 municipal water plants in Nanjing have been using advanced water treatment technologies to meet stricter environmental standards and improve public health. This study collected raw and finished water samples from three water plants (upstream, midstream, and downstream) between 2019 and 2021. A total of 20 metals, including As, Cd, Cr, Pb, Hg, Se, Al, Fe, Mn, Cu, Zn, Sb, Ba, Be, B, Mo, Ni, Ag, Tl, and Na, were tested in the finished water samples. Additionally, 10 metals, including Cd, Cr, Hg, Mn, Pb, As, Fe, Cu, Se, and Zn, were tested in the raw water samples.
One-way ANOVA revealed no significant differences in metal concentrations among the three regions (
Table S8). From 2019 to 2021, the concentrations of certain metals in source water exhibited seasonal variations, including Hg, Mn, As, Fe, Cd, Zn, and Cu (
Table S10).
Table S11 compares the metal removal rates of conventional and advanced treatment processes. The advanced process achieved similar rates for lead and copper and outperformed the conventional process for arsenic removal.
Advanced processes have similar removal rates for Pb and Cu, and they outperform traditional processes in removing As. However, advanced processes have lower removal rates for Fe, Mn, Cd, Cr, Hg, Se, and Zn.
In advanced treatment processes, the addition of ozone oxidation and activated carbon adsorption does not significantly improve the efficiency of metal removal. Muthaian Jaya Rajan found that, although activated carbon can adsorb some metal ions, its removal efficiency in practical applications is limited by water chemistry conditions, leading to constraints in the effectiveness of activated carbon in treating these metals [
17]. Additionally, this study found a positive correlation between Fe and Mn during the water treatment process, indicating competitive adsorption between these metals. The advanced treatment processes showed reduced removal efficiency for Fe and Mn, likely due to their competition for adsorption sites on activated carbon, which diminishes the overall removal effectiveness [
18].
Variations in metal concentrations are influenced by a variety of factors. Changes in metal concentrations are influenced by a variety of factors, mainly including geographic features, human activities, and seasonal variations. In the upstream region, the mouth of the Chili River, characterized by mudflats, reeds, construction sites, and residential buildings, shows higher concentrations of lithium, titanium, vanadium, arsenic, and selenium. This is likely due to construction activities and residential runoff introducing pollutants into the water body. In the midstream region, near the Nanjing Yangtze River Bridge wetland park and residential areas [
19], higher concentrations of aluminum, manganese, nickel, and zinc are observed, attributed to natural soil erosion, industrial discharges, and residential runoff. Downstream, at the estuaries of the Rowazikou and Jiuxiang Rivers, which are surrounded by villages, farmlands, and industrial parks, elevated levels of molybdenum, magnesium, and calcium are noted, likely stemming from agricultural runoff and industrial activities. Seasonal variations are also an important factor affecting metal concentrations in the downstream region, in addition to the high likelihood of sewage discharge from nearby industrial areas. Studies indicate that industrial activities in autumn may lead to increased metal concentrations, whereas in winter, low temperatures may cause metal ions to deposit, settling at the bottom of water bodies or adhering to suspended particles and thereby leading to increased concentrations [
20]. Additionally, rainfall [
21] and surface water runoff can also affect the distribution and movement of metals in water bodies. Overall, controlling and managing metal pollution in water bodies requires a comprehensive consideration of geographical, climatic, and human factors.
This study indicates significant positive correlations among various metals in both source water and finished water, which may stem from the same pollution sources or similar geochemical characteristics. For example, the correlation between chromium and nickel might arise from their common use in industrial processes [
22]. Similarly, the high correlation between iron and copper could reflect their similar geochemical behaviors in water, such as forming complexes with organic or inorganic substances [
23].
This research also finds that the advanced drinking water treatment processes still have limitations in terms of removing metal elements. Some metal concentrations in certain finished waters continue to increase despite the use of advanced treatment techniques. This could be due to the ozone being a strong oxidizer, which may also accelerate the corrosion of metal components in pipes and equipment despite effectively killing pathogens and removing certain organic matter, thus leading to the release of metal ions into the water [
24]. Biofilms might form in the water treatment system, especially in the activated carbon filters. The microbial activity in these biofilms or during backwashing could cause metal ions to dissolve or release into the water [
25]. Notably, aluminum levels increased in all three water plants, which was likely caused by the flocculants added during the treatment process, such as polyaluminum chloride [
26].
Strong positive correlations exist between Li and Hg, Ga and B, and Mo and B among the metals with negative removal efficiencies. This may be due to the interactions of metal elements in the environment, forming complex chemical substances such as compounds, complexes, precipitates, and colloids [
27,
28]. The properties of these substances can become varied due to the complexity of interactions between metals [
29]. This increases the complexity and cost of the technologies needed to remove these metals, which consequently adds to the difficulty of metal removal [
30,
31]. It is therefore necessary to consider the interactions between metals in order to more effectively remove metal pollutants from water.
Research has shown that metals in the environment can mutually influence each other’s distribution and concentration. The release of one metal, such as Cu or Pb, can lead to the displacement and increased mobility of others, such as Cd or Zn [
31,
32]. In this study, significant positive correlations were found among metals with higher health risk levels (HI > 10
−2), such as Ba and Tl and B and Mo [
33,
34]. These correlations suggest that high concentrations of one metal may drive increases in others, amplifying health risks. Therefore, environmental monitoring and management should consider the interactive effects of multiple metal contaminations and not only individual pollution levels.
It was found that the health risks of three carcinogenic metals to children exceeded USEPA standards according to risk assessment aspects, possibly due to children’s smaller size and physiological functions that have not fully matured, making them more sensitive and more susceptible to these metals/metalloids. The concentrations of metals are influenced by geographical location and seasonal changes, indicating complex and variable pollution sources. Metal pollution is a significant issue for the safety of public drinking water, especially in areas with frequent industrial activities. However, there is a possibility of the study results deviating from the true situation due to incomplete risk assessment parameters for metals, a lack of representativeness of water samples, limited sampling quantity, and the non-differentiation of Cr valence states in the study, where risk assessment was primarily for Cr6+, which could have led to an overestimation of the risk. In other words, this study may not fully reflect the actual conditions of the researched area. Therefore, the final results of this study could vary from reality, and further improvements are needed in future work.
5. Conclusions
The concentrations of total metals/metalloids did not vary regionally, but varied seasonally. There were significant differences in the concentrations of certain metallic elements between different water treatment plant sites, particularly As, Se, Al, Ba, Cu, Mo, Zn, Mg, and Ca. These findings are important for understanding the spatial distribution of metal pollution in water bodies. The concentrations of some metals/metalloids varied significantly from season to season in both raw and finished water, especially Li, Ti, V, Co, Ga, Rb, Sr, Zr, Al, Tl, Hg, Ca, As, and Cd. These findings are important for understanding the seasonal variations in metal contamination in water bodies. Advanced Nanjing water treatment processes have limited effectiveness in removing metals/metalloids. The risk assessment showed that the cumulative carcinogenic risks for adult males, adult females, and children were 9.56 × 10−5, 8.37 × 10−5, and 2.03 × 10−4, respectively, with the risks for children exceeding the acceptable levels established by the USEPA (1 × 10−6 to 1 × 10−4). Moreover, As was found to be the predominant carcinogenic element. The non-carcinogenic health risk assessment resulted in HI values of 0.314, 0.275, and 0.667 (HI < 1) for adult males, adult females, and children, respectively, with As, Tl, and V being the elements associated with the highest risk.