Surfactant-Modified Bolivian Natural Zeolite for the Adsorption of Cr (VI) from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Surfactant Modified Bolivian Zeolite
2.2. Characterization of the Adsorbents
2.3. Adsorption Test of Chromium
3. Results and Discussion
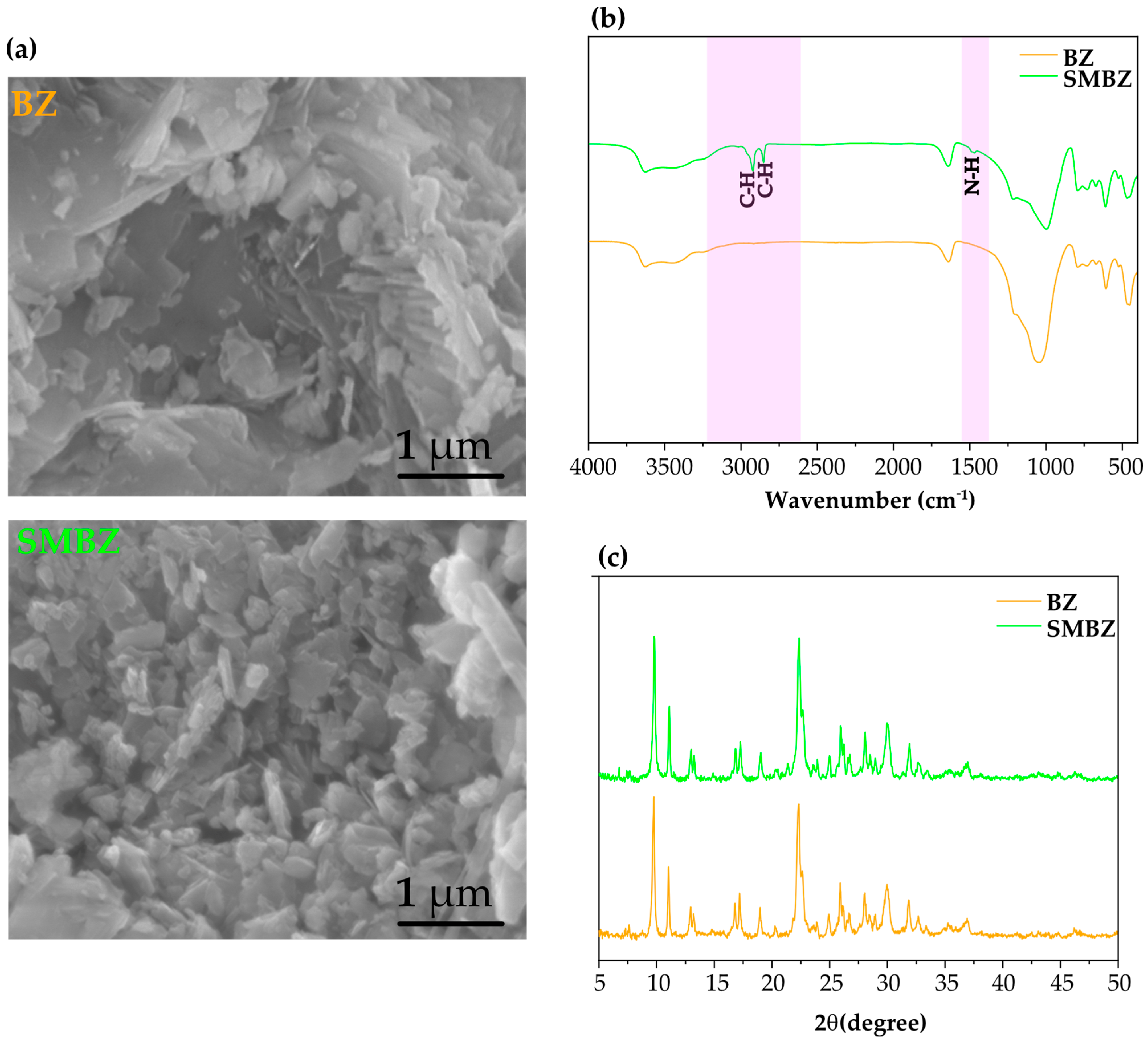
3.1. Structure and Properties of SMBZ
3.2. Adsorption Behavior of Chromium on SMBZ
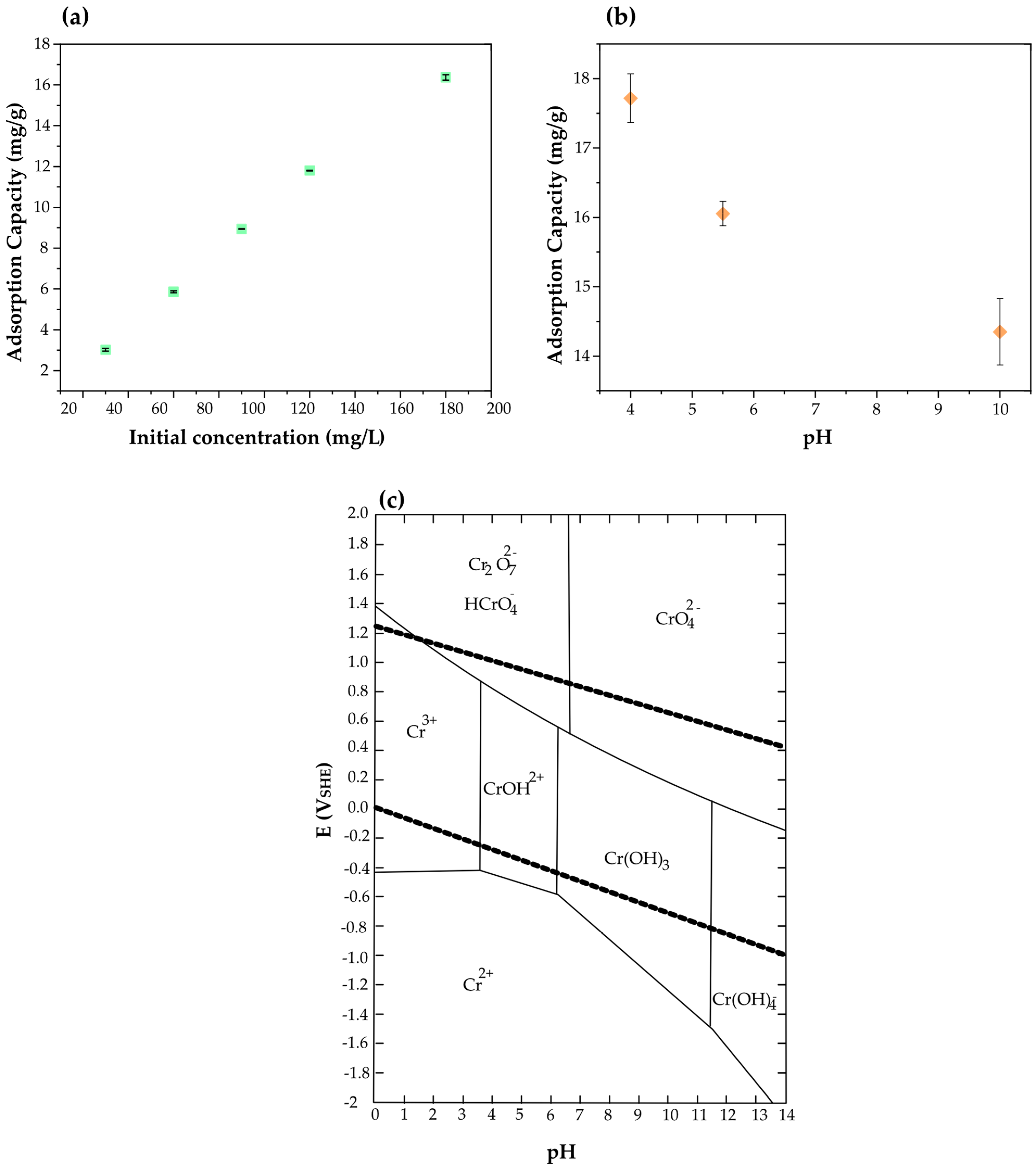
3.2.1. Effect of pH and Initial Concentration
3.2.2. Adsorption Modeling of Chromium on SMBZ
Isotherm Modeling
Kinetic Modeling
3.3. Thermodynamics
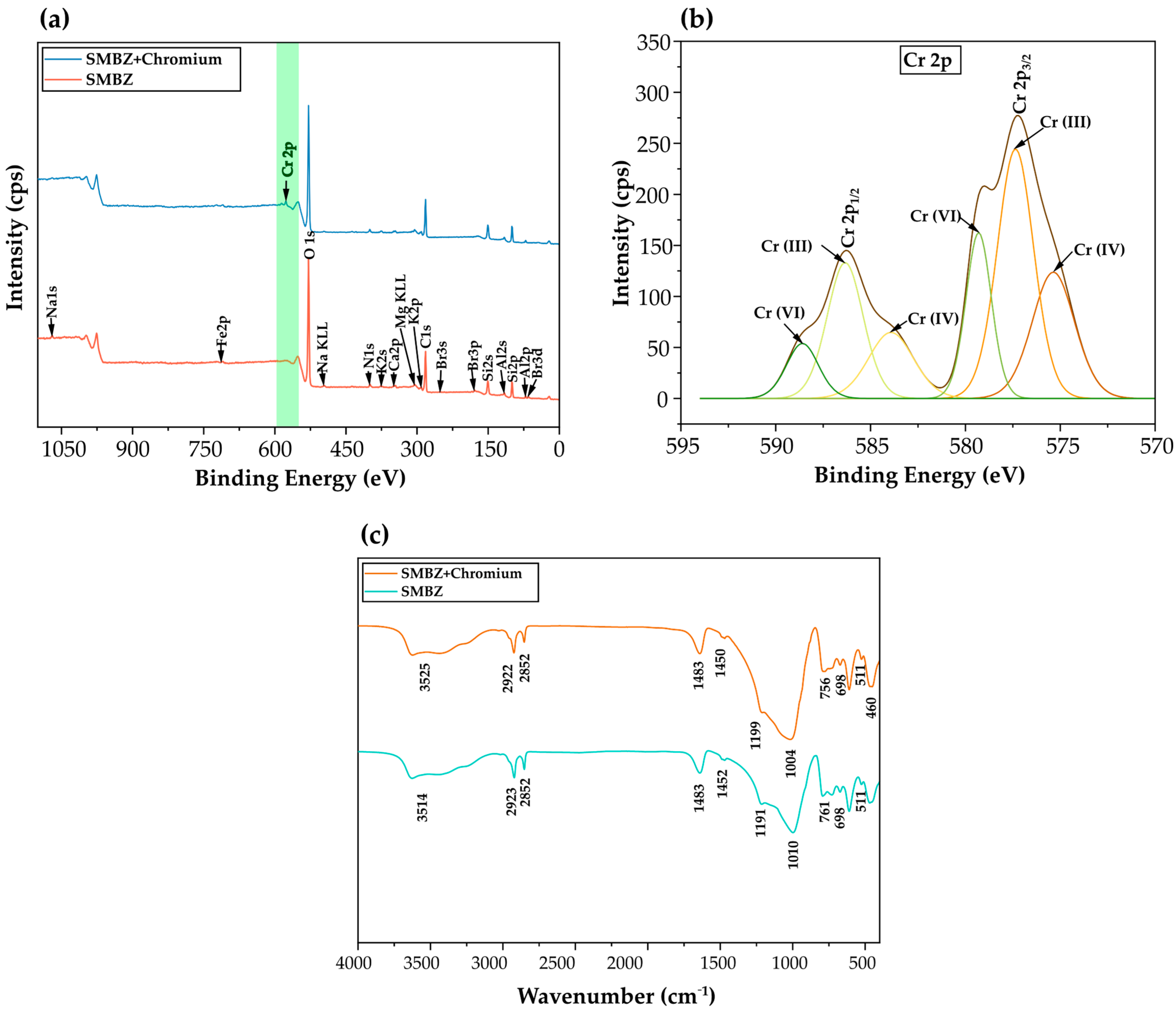
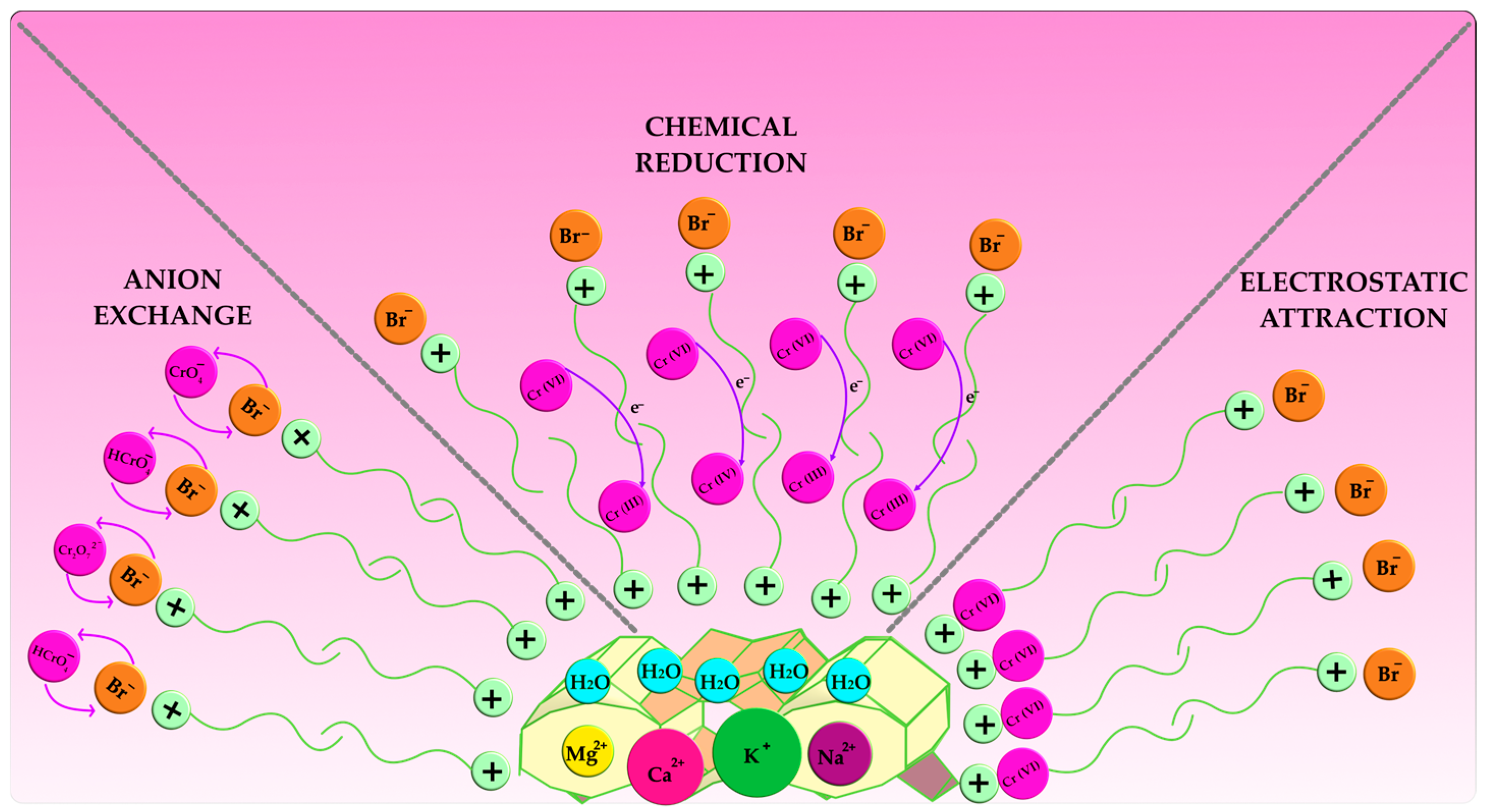
3.4. Mechanism Analysis of Cr (VI) Adsorption on SMBZ
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Fourth Edition Incorporating the First and Second Addenda Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2017; Volume 4, p. 641. [Google Scholar]
- Ogata, F.; Nagai, N.; Itami, R.; Nakamura, T.; Kawasaki, N. Potential of virgin and calcined wheat bran biomass for the removal of chromium(VI) ion from a synthetic aqueous solution. J. Environ. Chem. Eng. 2020, 8, 103710. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C.; Xu, B. Preparation of magnetic kaolin embedded chitosan beads for efficient removal of hexavalent chromium from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105438. [Google Scholar] [CrossRef]
- Younas, F.; Niazi, N.K.; Bibi, I.; Afzal, M.; Hussain, K.; Shahid, M.; Aslam, Z.; Bashir, S.; Hussain, M.M.; Bundschuh, J. Constructed wetlands as a sustainable technology for wastewater treatment with emphasis on chromium-rich tannery wastewater. J. Hazard. Mater. 2022, 422, 126926. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Bolaños-Benítez, V.; van Hullebusch, E.; Birck, J.-L.; Garnier, J.; Lens, P.N.L.; Tharaud, M.; Quantin, C.; Sivry, Y. Chromium mobility in ultramafic areas affected by mining activities in Barro Alto massif, Brazil: An isotopic study. Chem. Geol. 2021, 561, 120000. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Petersen, L.R.; Kjeldsen, P.; Jakobsen, R. Amendment of arsenic and chromium polluted soil from wood preservation by iron residues from water treatment. Chemosphere 2011, 84, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Filella, M. Lead and chromium in European road paints. Environ. Pollut. 2023, 316, 120492. [Google Scholar] [CrossRef]
- Iyer, M.; Anand, U.; Thiruvenkataswamy, S.; Babu, H.W.S.; Narayanasamy, A.; Prajapati, V.K.; Tiwari, C.K.; Gopalakrishnan, A.V.; Bontempi, E.; Sonne, C.; et al. A review of chromium (Cr) epigenetic toxicity and health hazards. Sci. Total Environ. 2023, 882, 163483. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Vargas-Berrones, K.; Bernal-Jácome, L.; de León-Martínez, L.D.; Flores-Ramírez, R. Emerging pollutants (EPs) in Latin América: A critical review of under-studied EPs, case of study -Nonylphenol-. Sci. Total Environ. 2020, 726, 138493. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Zhou, X.H.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Guo, Z.W.; Guo, P.R.; Qian, W.; Kong, L.J.; et al. Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef]
- Xing, X.; Ren, X.; Alharbi, N.S.; Chen, C. Efficient adsorption and reduction of Cr(VI) from aqueous solution by Santa Barbara Amorphous-15 (SBA-15) supported Fe/Ni bimetallic nanoparticles. J. Colloid Interface Sci. 2023, 629, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, J.; Wang, H.; Bai, Y.; Liu, Y.; Bi, Y.; Zhang, H.; Chen, H.; Barnie, S.; Xie, H. Cr(VI) adsorption and reduction by magnetite-humic acid adsorption complexes under mildly acidic conditions: Synergistic/antagonistic mechanism and multi-step reaction model. Chem. Eng. J. 2023, 451, 138648. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, D.; Jiang, Y. Effect of the reduction–mineralization synergistic mechanism of Bacillus on the remediation of hexavalent chromium. Sci. Total Environ. 2021, 777, 146190. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, Q.; Hu, L.; Zhong, H.; He, Z. Bioreduction performances and mechanisms of Cr(VI) by Sporosarcina saromensis W5, a novel Cr(VI)-reducing facultative anaerobic bacteria. J. Hazard. Mater. 2021, 413, 125411. [Google Scholar] [CrossRef] [PubMed]
- Rezgui, S.; Ghazouani, M.; Bousselmi, L.; Akrout, H. Efficient treatment for tannery wastewater through sequential electro-Fenton and electrocoagulation processes. J. Environ. Chem. Eng. 2022, 10, 107424. [Google Scholar] [CrossRef]
- Lu, J.; Fan, R.; Wu, H.; Zhang, W.; Li, J.; Zhang, X.; Sun, H.; Liu, D. Simultaneous removal of Cr(VI) and Cu(II) from acid wastewater by electrocoagulation using sacrificial metal anodes. J. Mol. Liq. 2022, 359, 119276. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari Rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Mohammed, K.; Sahu, O. Recovery of chromium from tannery industry waste water by membrane separation technology: Health and engineering aspects. Sci. Afr. 2019, 4, e00096. [Google Scholar] [CrossRef]
- Roa, K.; Boulett, A.; Oyarce, E.; Sánchez, J. Removal of Cr(VI) by ultrafiltration enhanced by a cellulose-based soluble polymer. J. Water Process Eng. 2023, 51, 103478. [Google Scholar] [CrossRef]
- Ahmed, N.; Mir, F.Q. Chromium(VI) removal using micellar enhanced microfiltration (MEMF) from an aqueous solution: Fouling analysis and use of ANN for predicting permeate flux. J. Water Process Eng. 2021, 44, 102438. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Perspectives on green fabrication and sustainable utilization of adsorption materials for wastewater treatment. Chem. Eng. Res. Des. 2022, 187, 541–548. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, A.K.; Senthil Kumar, P.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Kudarova, A.; Guney, A.; Kinayat, N.; Tauanov, Z. Efficient mercury removal from water by using modified natural zeolites and comparison to commercial adsorbents. Sustain. Chem. Pharm. 2023, 32, 101017. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Yin, H.; Meng, L.; Jin, W.; Wang, F.; Xu, J.; Al-Tabbaa, A. Application of zeolites in permeable reactive barriers (PRBs) for in-situ groundwater remediation: A critical review. Chemosphere 2022, 308, 136290. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, B.; Belkacemi, H.; Brahmi-Ingrachen, D.; Braham, L.A.; Muhr, L. Study of nickel adsorption on NaCl-modified natural zeolite using response surface methodology and kinetics modeling. Groundw. Sustain. Dev. 2022, 17, 100757. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Song, W.; Shi, T.; Yang, D.; Ye, J.; Zhou, Y.; Feng, Y. Pretreatment effects on the sorption of Cr(VI) onto surfactant-modified zeolite: Mechanism analysis. J. Environ. Manag. 2015, 162, 96–101. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Dou, Y.; Xue, Y.; Ji, Y.; Tang, Y.; Hu, M. Removal difference of Cr(VI) by modified zeolites coated with MgAl and ZnAl-layered double hydroxides: Efficiency, factors and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126583. [Google Scholar] [CrossRef]
- Hailu, S.L.; Nair, B.U.; Redi-Abshiro, M.; DIaz, I.; Tessema, M. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng. 2017, 5, 3319–3329. [Google Scholar] [CrossRef]
- De Gennaro, B. Chapter 3. Surface Modification of Zeolites for Environmental Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Karnjanakom, S.; Maneechakr, P. Adsorption behaviors and capacities of Cr(VI) onto environmentally activated carbon modified by cationic (HDTMA and DDAB) surfactants. J. Mol. Struct. 2019, 1186, 80–90. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Cao, X.; Guang, L.; Zhang, D.; Li, M.; Meng, N.; Yin, J.; Yan, B. Chromium (VI) removal from water using cetylpyridinium chloride (CPC)-modified montmorillonite. Purif. Technol. 2020, 241, 116732. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Zhang, H.; Hao, C.; Zhao, P. Efficient removal of cationic and anionic dyes by surfactant modified Fe3O4 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127680. [Google Scholar] [CrossRef]
- Belachew, N.; Hinsene, H. Preparation of cationic surfactant-modified kaolin for enhanced adsorption of hexavalent chromium from aqueous solution. Appl. Water Sci. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Ghosh, R.; Sahu, A.; Pushpavanam, S. Removal of trace hexavalent chromium from aqueous solutions by ion foam fractionation. J. Hazard. Mater. 2019, 367, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Woo, H.; Lee, G.; Park, J. Removal of chromate from water using surfactant modified Pohang clinoptilolite and Haruna chabazite. Desalination 2010, 257, 102–109. [Google Scholar] [CrossRef]
- Rivera, G.L.D.; Hernández, A.M.; Cabello, A.F.P.; Barragán, E.L.R.; Montes, A.L.; Escamilla, G.A.F.; Rangel, L.S.; Vazquez, S.I.S.; De Haro Del Río, D.A. Removal of chromate anions and immobilization using surfactant-modified zeolites. J. Water Process Eng. 2021, 39, 2020. [Google Scholar] [CrossRef]
- Warchoł, J.; Petrus, R. Modeling of heavy metal removal dynamics in clinoptilolite packed beds. Microporous Mesoporous Mater. 2006, 93, 29–39. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Jacobo-Azuara, A.; Diaz-Flores, E.; Guerrero-Coronado, R.M.; Mendoza-Barron, J.; Berber-Mendoza, M.S. Adsorption of chromium(VI) from an aqueous solution on a surfactant-modified zeolite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 35–41. [Google Scholar] [CrossRef]
- Thanos, A.G.; Katsou, E.; Malamis, S.; Psarras, K.; Pavlatou, E.A.; Haralambous, K.J. Evaluation of modified mineral performance for chromate sorption from aqueous solutions. Chem. Eng. J. 2012, 211–212, 77–88. [Google Scholar] [CrossRef]
- Velarde, L.; Nikjoo, D.; Escalera, E.; Akhtar, F. Bolivian natural zeolite as a low-cost adsorbent for the adsorption of cadmium: Isotherms and kinetics. Heliyon 2024, 10, e24006. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bowman, R.S. Counterion effects on the sorption of cationic surfactant and chromate on natural clinoptilolite. Environ. Sci. Technol. 1997, 31, 2407–2412. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Clarke, F.W.; Langmuir, B.I. Constitution of Solids and Liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Kelishami, A.R. Enhanced adsorption of congo red using cationic surfactant functionalized zeolite particles. Microchem. J. 2020, 153, 104281. [Google Scholar] [CrossRef]
- Solińska, A.; Bajda, T. Modified zeolite as a sorbent for removal of contaminants from wet flue gas desulphurization wastewater. Chemosphere 2022, 286, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Al-Ola, K.A.A.; Hamd, A.; Soliman, N.K.; Ahmed, S.A. Design, characterization, and adsorption properties of Padina gymnospora/zeolite nanocomposite for Congo red dye removal from wastewater. Sci. Rep. 2021, 11, 21058. [Google Scholar] [CrossRef]
- Dou, D.; Wei, D.; Guan, X.; Liang, Z.; Lan, L.; Lan, X.; Liu, P.; Mo, H.; Lan, P. Adsorption of copper (II) and cadmium (II) ions by in situ doped nano-calcium carbonate high-intensity chitin hydrogels. J. Hazard. Mater. 2022, 423, 127137. [Google Scholar] [CrossRef]
- Hamd, A.; Shaban, M.; AlMohamadi, H.; Dryaz, A.R.; Ahmed, S.A.; Abu Al-Ola, K.A.; Abd El-Mageed, H.R.; Soliman, N.K. Novel Wastewater Treatment by Using Newly Prepared Green Seaweed-Zeolite Nanocomposite. ACS Omega 2022, 7, 11044–11056. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Liu, Y.; Zhang, T. Green method to synthesize magnetic zeolite/chitosan composites and adsorption of hexavalent chromium from aqueous solutions. Int. J. Biol. Macromol. 2022, 194, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Dognani, G.; Hadi, P.; Ma, H.; Cabrera, F.C.; Job, A.E.; Agostini, D.L.S.; Hsiao, B.S. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Li, M.; Liu, X. Science of the Total Environment Cr(VI) removal from groundwater using double surfactant-modi fi ed nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Guiñón, J.L. Pourbaix diagrams for chromium in concentrated aqueous lithium bromide solutions at 25 °C. Corros. Sci. 2009, 51, 807–819. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Deng, L.; Fan, X.; Li, K.; Lu, H.; Li, W. Removal of heavy metal ion cobalt (II) from wastewater via adsorption method using microcrystalline cellulose–magnesium hydroxide. Int. J. Biol. Macromol. 2021, 189, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Gómez, N.; Macedo-Miranda, M.G.; Olguín, M.T. Chromium VI adsorption from sodium chromate and potassium dichromate aqueous systems by hexadecyltrimethylammonium-modified zeolite-rich tuff. Appl. Clay Sci. 2014, 95, 197–204. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, J.; Wu, D.; Gao, Z.; Sun, Y.; Luo, C. Selective Adsorption of Pb(II) and Cr(VI) by Surfactant-Modified and Unmodified Natural Zeolites: A Comparative Study on Kinetics, Equilibrium, and Mechanism. Water. Air. Soil Pollut. 2016, 227, 101. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, H.; Zhang, Q.; Jiang, G.; Cai, W.; Hu, W. Adsorption of cadmium (II) in wastewater by magnesium oxide modified biochar. Arab. J. Chem. 2022, 15, 104059. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Al-Ansari, T.; Ihsanullah, I.; Kochkodan, V.; Chatla, A.; Atieh, M.A.; Shanableh, A.; Laoui, T. Carbide-derived carbon as an extraordinary material for the removal of chromium from an aqueous solution. Chemosphere 2022, 307, 135953. [Google Scholar] [CrossRef] [PubMed]
- Wo, A.; Staszak, K.; Hubicki, Z. Effect of anionic surfactants on the heavy metal ions removal by adsorption onto ion exchangers-batch and column studies. J. Water Process Eng. 2023, 53, 103792. [Google Scholar] [CrossRef]
- Patra, C.; Gupta, R.; Bedadeep, D.; Narayanasamy, S. Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: A detail insight. Environ. Pollut. 2020, 266, 115102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Wang, S.; Fu, L.; Zhang, L. The one-step synthesis of a novel metal–organic frameworks for efficient and selective removal of Cr(VI) and Pb(II) from wastewater: Kinetics, thermodynamics and adsorption mechanisms. J. Colloid Interface Sci. 2023, 640, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Daradmare, S.; Xia, M.; Le, V.N.; Kim, J.; Park, B.J. Metal–organic frameworks/alginate composite beads as effective adsorbents for the removal of hexavalent chromium from aqueous solution. Chemosphere 2021, 270, 129487. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A. Ef fi cient adsorption of Cr(VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, M.; Abolghasemi, H.; Esmaeili, M.; Ehsani, A.; Badiei, A. Comprehensive batch and continuous methyl orange removal studies using surfactant modified chitosan-clinoptilolite composite. Purif. Technol. 2021, 267, 118601. [Google Scholar] [CrossRef]
- Islam, M.A.; Angove, M.J.; Morton, D.W. Recent innovative research on chromium (VI) adsorption mechanism. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100267. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr(VI) removal by amine cross-linking biosorbent. J. Colloid Interface Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, L.; Xu, S.; Chen, Y.; Liu, B.; Li, Z.; Jiang, C. Efficient removal of hexavalent chromium from water by an adsorption-reduction mechanism with sandwiched nanocomposites. RSC Adv. 2018, 8, 15087–15093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niu, W.; Sun, J.; Zhou, Q. Efficient removal of Cr(VI) from water by the uniform fiber ball loaded with polypyrrole: Static adsorption, dynamic adsorption and mechanism studies. Chemosphere 2020, 248, 126102. [Google Scholar] [CrossRef] [PubMed]

| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| (mg/g) | (L/mg) | R2 | (L/mg) | (mg/g) | R2 | |
| SMBZ | 17 | 0.85422 | 0.997 | 2.718 | 4.198 | 0.791 |
| Adsorbate | Adsorbent | (mg/g) | Reference |
|---|---|---|---|
| Cr (VI) | Mexican natural zeolite modified by HTDMA-Br | 5.07 | [42] |
| Korean and Japanese natural zeolite modified by HTDMA-Br | 3.55–8.83 | [39] | |
| Mexican natural zeolite modified by HTDMA-Br | 0.9–1.05 | [61] | |
| Chinese natural zeolite modified by CTMAB and CPB | 0.3–2 | [62] | |
| Mexican natural zeolite modified by HTDMA | 9.83 | [40] | |
| Bolivian natural zeolite | No-affinity towards Cr (VI) | This work | |
| Bolivian natural zeolite modified by HTDMA-Br | 17 | This work |
| Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (/min) | (mg/g) | R2 | (g/mg min) | (mg/g) | R2 | (mg/g min0.5) | R2 | ||
| 0.0506 | 1.58 | 0.768 | 0.076 | 16.33 | 1 | 0.118 | 14.9 | 0.799 | 16.17 |
| ∆H° (KJ/mol) | ∆S° (J/K mol) | ∆G° (KJ/mol) | ||
|---|---|---|---|---|
| 298 K | 313 K | 323 K | ||
| −123.57 | 415.49 | 306.60 | −8003.83 | −9659.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velarde, L.; Escalera, E.; Akhtar, F. Surfactant-Modified Bolivian Natural Zeolite for the Adsorption of Cr (VI) from Water. Water 2024, 16, 1954. https://doi.org/10.3390/w16141954
Velarde L, Escalera E, Akhtar F. Surfactant-Modified Bolivian Natural Zeolite for the Adsorption of Cr (VI) from Water. Water. 2024; 16(14):1954. https://doi.org/10.3390/w16141954
Chicago/Turabian StyleVelarde, Lisbania, Edwin Escalera, and Farid Akhtar. 2024. "Surfactant-Modified Bolivian Natural Zeolite for the Adsorption of Cr (VI) from Water" Water 16, no. 14: 1954. https://doi.org/10.3390/w16141954
APA StyleVelarde, L., Escalera, E., & Akhtar, F. (2024). Surfactant-Modified Bolivian Natural Zeolite for the Adsorption of Cr (VI) from Water. Water, 16(14), 1954. https://doi.org/10.3390/w16141954






