Abstract
Mercury (Hg) is a naturally occurring element and has been released through human activities over an extended period. The major source is the steel industry, especially sinter plants. During a sintering process, high amounts of dust and gaseous emission are produced. These gases contain high loads of SOx and NOX as well as toxic pollutants, such as heavy metals like Hg. These toxic pollutants are removed by adsorbing to solids, collected as by-products and deposited as hazardous waste. The by-products contain a high amount of salt, resulting in a high water solubility. In this study, to ultimately reduce the waste amount in landfills, leachates of the by-products have been produced. The dissolved Hg concentration and its distribution across different charges were determined. Hg concentrations between 3793 and 12,566 µg L−1 were measured in the leachates. The objective was to lower the Hg concentration in leachates by chemical precipitation with sodium sulfide (Na2S) or an organic sulfide followed by filtration. Both reagents precipitate Hg with removal rates of up to 99.6% for the organic sulfide and 99.9% for Na2S, respectively. The dose of the precipitator as well as the initial Hg concentration affected the removal rate. In addition to Hg, other relevant heavy metals have to be included in the calculation of the amount of precipitator as well. Between relevant heavy metals including Hg and sulfide, the ratio should be more than 1.5. The novelty of this study is the measurement and treatment of Hg in wastewater with a high ionic strength. The high salt concentrations did not influence the efficiency of the removal methods. An adjustment of the precipitator dose for each sample is necessary, because an overdose potentially leads to the re-dissolving of Hg. It could be shown that the emission limit of 0.005 mg L−1 could be reached especially by precipitation with Na2S.
1. Introduction
Mercury (Hg) is a global pollutant with a high toxicity for humans and ecosystem health. Hg is a naturally occurring element but also is emitted into the ecosystem by humans over a long time. Generally, Hg is present in the environment in three different chemical fractions: elemental Hg, inorganic Hg and organic Hg [1]. Predominant in the atmosphere is the gaseous elemental Hg, which can undergo a long-range transport and atmospheric deposition into the aquatic systems [2]. Under reducing conditions, bacteria can form the highly toxic methylmercury, which biomagnifies in aquatic and terrestrial food chains, resulting in exposure to humans and wildlife throughout the world [3,4,5]. In EU freshwaters, mainly Hg, besides a few other ubiquitous priority pollutants, is responsible for the failure of the good chemical status of the water bodies in many member states [6]. Thermal industrial processes including steel production and especially sinter plants are significant emitters of elemental Hg [2,7]. Sintering means the agglomeration of iron ore in combination with other elements, which is then used in blast furnaces. This leads to the emission of high amounts of flue gas, accounting for approximately 40% of the total waste gas volume in the iron and steel industry. Due to the high temperatures, some volatile heavy metals, like Hg, are released from the feed material to the exhaust gas stream. The flue gases also contain dust, COX, NOX, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), polychlorinated (PCDDs) as well as polybrominated dibenzodioxins (PBDDs) and furans (PCDFs), acid gases (SOX, HF, HCl, etc.), alkali metals, organic carbon and other pollutants. For the removal of the above-mentioned components from the flue gas of sinter plants, a very efficient dry gas cleaning system, such as the MEROS (Maximized Emission Reduction of Sintering) process, can be used. It is a dry-type gas cleaning process, using carbon-based adsorbents and either hydrated lime or sodium bicarbonate as absorbent [8]. The absorbent materials used are hazardous waste in secure landfills. Due to a high concentration of soluble salts, the material is not stable and has to be stabilized to prevent the release of Hg and other elements into the environment [9]. By leaching the by-product with water as a pretreatment before landfill disposal, the soluble Hg is transferred to the water phase and is removed from the waste [10]. Afterwards, the leached water phase has to be treated to remove Hg and other dissolved heavy metals to fulfill the emission limits of surface water [8,11,12]. In Austria, the limitation value for Hg in surface water in accordance with the General Wastewater Emission Regulation is 10 µg L−1 (unfiltered) [13]. The Austrian Quality Target Ordinances for Chemistry of surface water prescribes a maximal allowed Hg concentration of 0.07 µg L−1 (filtered with a pore size of 0.45 µm) [14].
Conventional techniques for the removal of heavy metals from industrial wastewater include chemical precipitation, adsorption, floatation, ion exchange, coagulation/flocculation and electrochemical processes [15]. Chemical precipitation is one of the most mature and cost-effective methods, including hydroxide precipitation, carbonate precipitation and sulfide precipitation. Metal sulfide precipitation is a crucial treatment in industrial wastewater as well as extractive metallurgy [16,17]. Sulfide sources such as solid FeS, CaS and Na2S, aqueous NaHS, NH4S and gaseous H2S are the most commonly used reagents [18]. To control the sulfide concentration in the solution and to prevent an overdosage on an industrial scale, thioacetamide (CH3CSNH2) is mainly applied as an addition to the precipitator [19,20]. Many studies have proven the feasibility of sulfide precipitation for heavy metals in synthetic solutions [15,16,17,18,19,20,21,22,23,24,25,26]. Most of the studies do not address Hg or their application in high salt-containing wastewater with a high ionic strength and other precipitable heavy metals at high pH values of 8–8.5.
This study investigates the uptake of Hg from by-products of dry gas cleaning plants by the water phase (eluates) using leaching and how the Hg concentrations were distributed in the wastewater produced by different charges of dry gas cleaning dust. Additionally, two different precipitation agents, Na2S and an organic sulfide (NaS2CN(C2H5)2), for the removal of Hg from the leaching eluates were compared using calculated removal rates and discharge limits. Due to the high salt amounts and the presence of other precipitable heavy metals in the eluates, side reactions of the precipitators can occur, and the optimal dosing of the precipitator has to be found to reach an effective Hg removal and to avoid the re-dissolution of HgS. Two different calculation strategies of the precipitator dosing were applied. To investigate the efficiency of the Na2S treatment and to provide different handling options for the industry, Na2S was applied as a solid and as a prepared solution and compared to the Hg removal efficiency of an organic sulfide as solution. The mechanism of Hg removal in this study is the precipitation with S. Compared to the literature, Hg was always treated in synthetic solutions or wastewater without high ionic strength. In this study, high salt-containing wastewater samples were treated, and the results are compared with the literature.
2. Materials and Methods
2.1. Description of the Sinter Plant
Solid residues of different charges were collected from the dry gas cleaning system of a sinter plant. The following design parameters give an overview of the plant size. The sinter strand is 250 m2, the sinter capacity is 7920 t d−1 and the waste gas stream is approximately 600,000 Nm3 h−1. The designated desulfurization rate and by-product formation is between 60% and 70% and approx. 620 kg h−1, respectively.
2.2. Sampling
The samples were taken as grab samples from different charges in the period from 2019 to 2021. From sample A-D1, an amount of 120 kg was directly delivered from the production site to the university and stored in 4 different barrels in the technical lab of the SIG Institute of the University of Natural Resources and Life Sciences Vienna. The samples A-D2 to A-D4 were submitted in sizes of about 2 kg in plastic bottles.
2.3. Production of the Eluates and Their Quality
For the water-soluble phase of the samples, 1:10 (mass per volume) eluates were prepared. First, 100 g of dust was weighted in a volumetric flask and filled up to 1000 mL with deionized water. The mixtures were stirred for 120 min by magnetic stirrers at room temperature (20 °C). After sedimentation of the undissolved particles, the samples were decanted and filtered by membrane pressure filtration (cellulose nitrate, 47 mm diameter, pore size of 0.45 µm; 6 bar). These filtered 1:10 solutions in water are called eluates (e.g., A stands for the plant, Dn for the number of dust and E for the eluate: A-Dn-E). These eluates have an initial pH of 8 to 8.5 (20 °C); the quality of the eluates (ions and heavy metals) is shown in Table 1. In a screening, the ions were measured by ion chromatography and the heavy metals by ICP-MS, as described in Section 2.7. The Hg concentration in Table 2 is a calculated mean value of six Hg measurements (see Figure 1).

Table 1.
Anion and cation concentrations in the eluates of the dust samples used.

Table 2.
Heavy metals concentrations in the eluates of the dust samples used.
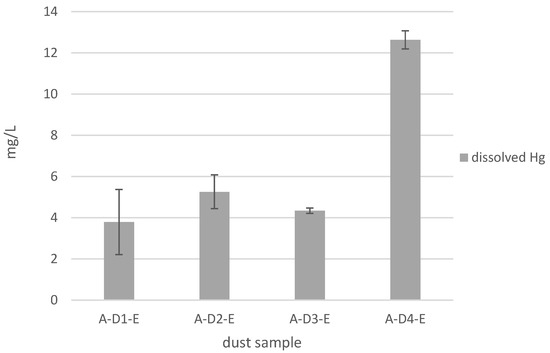
Figure 1.
Distribution of dissolved Hg in the eluates of the dry gas cleaning dusts of the sinter plant. Values given as mean values (6 samples) and standard deviation.
We found that 85% to 88% of dusts A-D1 to A-D3 and 92% of dust A-D4 are water soluble. K and Na made up the highest number of cations and Cl and SO4 made up the highest number of anions in the solutions. Although there are differences within the composition of the charges, the salts are in the same high-concentration ranges.
The heavy metal concentrations show a high variability between the samples of different charges with potentially high dissolved concentrations (>1000 µg L−1) of Pb, Cd, Cu, Zn, Mn, Cr, Tl and Mo and especially Hg.
2.4. Hg Removal Methods
Two different chemical agents were used for the removal of dissolved Hg. The dose of the precipitator was calculated either based on the molecular concentration of Hg including different equivalent concentrations between Hg and the precipitators or on the molar concentration of the heavy metals with a low water solubility in the sulfide form. As precipitator, Na2S as solid or in solution (1 g L−1) as well as an organic sulfide solution (30% NaS2CN(C2H5)2) were used. The theoretical dosage of each precipitator was calculated according to two strategies presented below.
2.5. Calculation Strategies of the Theoretical Precipitator Dosage and the Removal Rate
The theoretical dosage of precipitators was calculated according to two strategies.
Strategy 1: The calculation was based on the initial dissolved Hg concentration in the eluates. Theoretically, 1 mol of Hg2+ consumes 1 mol of S2− and produces 1 mol of HgS. The mass of the precipitator required by the theory was obtained according to the following Equation (1):
Strategy 2: The calculation was based on the initial concentration of precipitable heavy metals (Pb, Cd, Cu, Fe, Zn, Hg, Mn, Ni, Cr, Tl and Mo) in the eluates. For the calculation of the amount of precipitator, the following Equation (2) was used:
The dosage of reagents is indicated, e.g., n4, which means 4 times the theoretical mass of Na2S required to precipitate all Hg as well as other heavy metals in the eluate.
The removal rate (R) was calculated according to the following Equation (3):
In all equations, “m” represents the mass of substance with the unit mg; “c” represents the concentration in mg L−1; “M” represents the molar mass with the unit g mol−1; “V” represents the volume in mL; “n” is the ratio between the mole Hg and the mole precipitator; “C0” represents the initial concentration; and “CA” represents the final dissolved Hg concentration in µg L−1 after sulfide precipitation and filtration.
2.6. Treatments for the Removal of Hg
All removal experiments were carried out in laboratory scale under the same operational conditions: 20 °C, initial pH of the eluates 8 to 8.5, 120 mL sample volume, 20 min mixing time and 40 min of sedimentation. The solids were removed by pressure filtration (pore size 0.45 µm). Table 3 shows the calculated molar ratios for the experiments carried out during this research. For all experiments, the Hg concentrations in the untreated eluates were determined, too.

Table 3.
Overview of all treatments to reduce the Hg concentration in the eluates.
Run 1 to Run 3 tested the effect of the addition of different equivalents of Hg and solid sodium sulfide (Na2S water-free): 1:4, 1:6 and 1:10. The solid Na2S treatment applied in this research was based on the studies of AHM Veeken (2003) [27], Li et al. (2019) [22] and Prokkola (2020) [23]. The studies confirmed the feasibility of using sulfide precipitation to remove heavy metals in a multi-metals system from different wastewaters, especially Hg [28]. Run 4 to Run 11 were designed to study the Hg removal efficiency of sulfide precipitates by using solid Na2S and a Na2S solution (1 g L−1).
In Run 12 to 19, the efficiency of Hg removal by the treatment with solid Na2S compared to organic sulfide (NaS2CN(C2H5)2) solution was examined.
2.7. Analytical Methods
The dissolved fractions (eluate A-Dn-E) were analyzed for selected ions and dissolved heavy metals. The heavy metal components were determined by means of ionized plasma and detection by mass spectroscopy (ICP-MS, ELAN DRC-e, Perkin Elmer, Waltham, MA, USA) and then quantified according to DIN EN ISO 17294 [29]. The samples were acidified with 2% HNO3 suprapure, and for the Hg measurement, the samples were stabilized with 5 ppb Au solution additionally. Several dilutions of the samples were analyzed to cover the different concentration ranges and stay within the working range of the various ions and heavy metals, and the limits of quantification (LOQ) are listed in Table 4.

Table 4.
Limits of quantification for selected heavy metals.
Ions were separated by a liquid chromatograph (DIONEX ICS 3000, DIONEX Softron, Germering, Germany) equipped with an autosampler, suppressor and conductivity detector. For cation separation, a CS12A 250 mm × 2 mm + CG12A 50 mm × 2 mm column was used, and for anion separation, an AS15 250 mm × 2 mm + AG15 50 mm × 2 mm column was used. The eluent (mobile phase) for the cation determination was methane sulfonic acid solution, and for the anion determination, it was potassium hydroxide solution. All standards were prepared using stock standards (Merck, Darmstadt, Germany), and dilutions and solutions were prepared with deionized water. Several dilutions of the samples were analyzed to cover the different concentration ranges and stay within the working range of the various ions. The dilutions resulted in different limits of quantification listed in Table 5.

Table 5.
Limits of quantification of selected ions.
3. Results and Discussion
3.1. Dissolved Hg Distribution within the Eluates
Over a time period of one year, six eluates per dust were produced, and the Hg concentrations were measured directly after the production. Figure 1 shows the distribution of dissolved Hg in the eluates of samples A-D1-E to A-D4-E.
The eluates contain Hg concentrations between 3.7 and 12.6 mg L−1. Differences can be determined between and within the charges of the plant (for A-D1-E, between 1.4 and 4.7 mg L−1 (Table 6) and the standard deviation in Figure 1) that were taken over a time period of one year. The mean value and standard deviation for all samples were calculated with six values. Especially the sample A-D4-E contains a high concentration of dissolved Hg (12.6 mg L−1). The Hg concentrations in all eluates exceed the emission limit value of 0.005 mg L−1 and have to be reduced. Although the dusts were sampled in small amounts (sample A-D1, A-D2 and A-D4), the results show inhomogeneities and high standard deviations within the dust samples.

Table 6.
List of all experimental runs with related sample used, precipitation agent and applied ratio, the initial Hg concentration of the sample as well as the reached Hg concentration after the treatment and the calculated removal rate.
The variation in the Hg concentrations can be explained by differences in the raw materials, especially the lignite coal and the inhomogeneity in the dusts caused during formation [10]. The Hg amount in the lignite coal depends on the origin; many authors have presented data between 0.01 and 1.5 mg kg−1 lignite coal depending on the country of origin [30,31,32]. The literature presents studies where leaching is used to remove Hg from contaminated soil. But there is a lack of studies where leaching is used as a Hg removal method of the specific by-products of dry gas cleaning plants. Leaching as pretreatment before depositing avoids the necessity of removing the Hg from the contaminated soil [33,34,35].
3.2. Treatments for Removal of Hg
Table 6 shows the results of the treatment experiments for the Hg removal with Na2S and organic sulfide solution. The results of Run 1 to Run 3 indicate the effect of different Na2S concentrations on the Hg removal. The dosage of the two precipitators in Run 1 to 3 was calculated according to Strategy 1 and in Run 4 to 19 according to Strategy 2; for a better comparison, both calculated ratios are given in Table 6.
Generally, higher ratios resulted in better removal rates of Hg with Na2S. The addition of solid Na2S resulted in mostly lower final concentrations than the addition of a prepared Na2S solution. By comparing the final Hg concentrations after Na2S treatment to that after organic sulfide addition, the values are always lower after the Na2S treatment. This indicates that treatments with solid Na2S are more effective. In practical applications, treating with a Na2S solution increases the total amount of water volume by approximately 0.7% to 2%. Given that a substantial amount of water is already required to dissolve the dust, this would result in a waste of water resources. The use of solid Na2S gave slightly better results, but the technical feasibility for the application and dosage for treatment needs to be evaluated. Unfortunately, a high removal rate does not result in fulfilling the different national emission standards. Even if the removal rate of the same dust sample reached >99%, the methods have to be adjusted and optimized to reach the limited emission values of 0.005 or 0.01 mg L−1 for the discharge into a receiving water body. Therefore, the removal rate is not sufficient to assess the appropriateness of the removal methods. Although a higher Hg removal with Na2S could be reached, for the application at an industrial scale, the use of organic sulfide solution is recommended, because under acidic conditions, toxic H2S gas is released from Na2S. As the treatment with organic sulfide obtained removal rates over 87%, with an optimization and adjustment, the limitation values could probably be reached, too. Although the focus was on the precipitation of Hg, other water-insoluble sulfides of heavy metals in the eluates need to be considered. Therefore, the resulting final concentrations of Hg in relation to the applied sulfide ratios according to the two calculation strategies are shown in Figure 2.
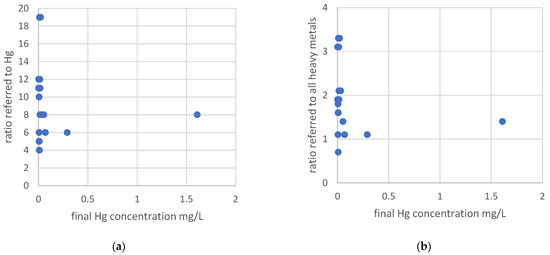
Figure 2.
(a) Relation between the final dissolved Hg concentrations and the ratio referred to the initial Hg concentration; (b) relation between the final dissolved Hg concentrations and the ratio referred to the initial concentrations of precipitable heavy metals. Y-axis has different scales.
Figure 2 demonstrates that the consideration of the concentrations of relevant heavy metals including Hg gives more consistent data, as they are also relevant because they react with sulfide, too. Figure 2 shows that ratios in which all heavy metals are below 1.5 do not result in sufficient low Hg concentrations. Therefore, the optimized dosage has to be calculated according to Strategy 2, referring to all heavy metals in ratio exceeding 1.5. In the study of Han et al. (2014), Hg was removed from synthetic wastewater by FeS particles [36]. The molar ratios used of Hg and FeS were n 1, n 2 and n 2.5. With the ratio n 1, more than 99% of Hg could be removed. The ratios n 2 and 2.5 showed removal rates of 96% to 97%. Summarizing, Han et al. found that a ratio of 1 resulted in a better Hg removal compared to the higher doses. Our study showed that the ratios referring to Hg are not consistent for an effective Hg removal. Our results are not in line with the study of Han et al., but the important difference is the composition of the wastewater used. For the experiments of Han et al. (2014) [36], synthetic solutions were used without any other substances that could react with sulfide. These results confirm the hypothesis that in real wastewater with a high ionic strength and other heavy metals, sulfide also precipitated with the heavy metals, and less of the total added sulfide is available for Hg [17,18,37]. The study of Chai et al. (2010) showed a Hg removal with Na2S from 48 to 0.12 mg L1 in a synthetic solution without any other sulfide reactants contained and a molar Hg to Na2S ratio of 1:16 at pH 9 [38]. These results are in line with the removal effort of our study. Hg can also form complexes with salts like Cl and the soluble HgCl2 complex, and due to that, not all Hg ions are available for the precipitation with sulfide [39,40]. Pb, Cd, Cu Fe and Zn react with sulfide too, and according to their low chemical solubility, they form insoluble sulfide precipitates and thereby can be removed from the wastewater by filtration or sedimentation afterwards [41]. It is an effective technique for the Hg removal but sensitive to overdosage due to the formation of soluble Hg–polysulfide complexes [37,39,42]. Sulfide precipitates are not amphoteric, so a high degree of metal removal in a shorter time over a wide pH range can be achieved [17,18].
A number of studies showed that Na2S is also a method used to remove other heavy metals (for example, Zn, Cu, and Pb). Table 7 provides measurements for different HMs for Run 1 to 3. For these experiments, relevant heavy metal concentrations were measured in the untreated eluates as well as after the treatment with solid Na2S in different ratios (Table 7 and Figure 3).

Table 7.
Dissolved concentrations of heavy metals in eluate A-D1-E before and after treatment with different concentrations of solid Na2S, and the ratios refer to all heavy metals.
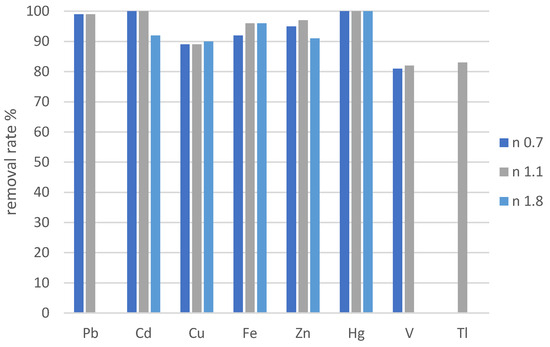
Figure 3.
Removal rates of selected heavy metals after treatment with solid Na2S in different concentrations in sample A-D1-E.
Table 7 and Figure 3 show that a higher ratio of solid Na2S does not necessarily lead to a higher removal of HM. A Na2S ratio of 1.1 resulted in the removal rates of Hg, Pb, Cd, and Zn being higher than 95%, while those of Fe and Cu were higher than 90% and V and that for Tl was over 80%. For Hg, the ratio (Strategy 2) should be more than 1.5, but the application of an excessive amount of Na2S would cause material waste and a secondary pollution due to the excess of sulfide [37]. Due to the high salt concentrations and the other present metals, it is expected that the precipitants do not only form insoluble complexes with Hg; the other metals are precipitated, too. For an efficient removal, the adjustment of the precipitator in the initial concentration of all precipitable heavy metals will be necessary. Due to the formation of the soluble Hg–polysulfide complexes, the Hg concentration can increase after the precipitation again, because this complex is not stable. The results show that also other heavy metals like Cu, Zn, Ni and Sn form soluble complexes with S, which supports the reported results in the literature [24,25,43]. The work of Fukuta et al. (2004) showed removal rates of 94.5% for Cu (pH 1.5), 75.9% for Zn (pH 2.5) and 65.9% for Ni (pH 5.5–6.0) [25]. A further study of Mahdi et al. (2012) showed that 90% of Cu, Ni and Zn could be reduced from a synthetic solution with Na2S in 30–60 min with the additional control of the sulfide concentration in the solution with thioacetamide (CH3CSNH2) as an addition to the precipitator to prevent the overdosage [19]. The work of Silvia et al. (2017) presents Cu, Zn and Ni removal over 90% with H2S gas [20]. The results of both studies are comparable with our study and show that the use of H2S is as efficient as Na2S for the removal of Cu, Zn and Ni. In another study, heavy oil fly ash with over 9.0% (weight per weight) of sulfur content has removed 99.99% of Cu from a synthetic solution under optimal operational conditions [26]. The removal treatments in all of the mentioned studies were applied to synthetic solutions, and different sulfide sources were used. The comparison of the results of the experiments of this study show that the removal rates are in the same range, although the wastewater used in our study had a high ionic strength. The concentrations of salts in the treated solution did not have an impact on the efficiency of heavy metal removal by sulfide precipitation from different sulfide sources, but as the comparison with the study of Han et al. showed, other heavy metals react with sulfide too and are removed as precipitates [36].
3.3. Cost Analysis of the Hg Removal Process
The amount of precipitator used in all of the experimental Run s is projected to the necessary amount for an industrial-scale plant, and the costs are calculated for each precipitator. The gas cleaning plant produces 620 kg by-product per hour. By dissolving this amount in a 1:10 dilution in water, approximately 6200 L eluate is produced per hour. The average price of Na2S with a purity +90% is 7440€ kg−1 (Merck), and for organic sulfide (NaS2CN(C2H5)2) with a purity +90%, it is 396 € kg−1 (Merck). Table 8 shows the costs for each Run.

Table 8.
Statement of costs for the Hg removal for 1 h.
The cost analysis shows that the use of Na2S is more expensive than the organic sulfide. In Run s 13 and 17, low final Hg concentrations of 18.2 µg L−1 and 15.9 µg L−1 Hg were reached. These results conform the use of organic sulfide for the industrial application.
4. Conclusions
The eluate produced from dry gas cleaning dusts in this study contains 85% to 92% of salts, and therefore the reaction might not be easily predicted from chemical reaction constants. Measurement of different eluates, produced separately from the same dust samples at different times, resulted in a high standard deviation of the dissolved Hg concentrations, and this was also true within single samples. High differences were also observed between different sample charges of the considered plant. Therefore, intensive investigations of the relevant heavy metals in the eluates are required for the optimized dosage of sulfide precipitators.
All dissolved Hg concentrations in the eluates exceeded the Austrian emission standard for surface water of 0.010 µg L−1. Due to this fact, the Hg concentration has to be reduced before discharging the wastewater into surface water.
The treatment with Na2S and organic sulfide solution showed high Hg removal rates between87.5% and 99.99%, depending on the type of precipitator and its dosage as well as the initial Hg concentrations. Sulfide does not only react with Hg; there are also other relevant heavy metals that have to be considered in the calculation of the optimal amount of precipitator; but an overdosing of the precipitator can re-dissolve Hg.
The testing of different ratios between heavy metals and the precipitator showed that a ratio of more than 1.5 (according to Strategy 2) is necessary to reduce Hg appropriately. With Na2S, a better Hg removal was reached compared to the organic sulfide solution, but for the application at an industrial scale, the use of organic sulfide solution is recommended because under acidic conditions, Na2S reacts to toxic H2S gas.
Na2S can be applied as a solid but also as prepared solution. The solution would increase the amount of water up to 2%, but as a solution, the adding of the precipitator in the wastewater and the homogenization would be easier at a technical scale. For the evaluation of the removal performance, the removal rate is not an appropriate parameter, but the final dissolved concentrations must be used. The precipitation experiments in this study showed that with Na2S treatment, it is possible to reach the effluent emission limit for Hg for different countries.
In addition to Hg, some other heavy metals precipitate with sulfide too and can be removed with Na2S from the wastewater produced.
The cost analysis confirms the use of org. sulfide, because it is less expensive, and with optimization, it can remove Hg efficiently as well.
Author Contributions
Conceptualization, M.F., C.H. and Y.Z.; data curation, C.H. and Y.Z.; funding acquisition, T.P.; writing—review and editing all authors, project administration, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FFG (Österreichische Forschungsfördergesellschaft) within the COMET Competence Centers for Excellent Technologies—COMET Centre (K1-Met), FFG Project No.: 869295. Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.
Acknowledgments
This study was elaborated within the COMET Competence Centers for Excellent Technologies—COMET Centre (K1-Met) and financially supported by the FFG (Österreichische Forschungsfördergesellschaft).
Conflicts of Interest
Author Tobias Plattner was employed by the company Primetals Technologies Austria GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tuzen, M.; Sarı, A.; Mogaddam, M.R.A.; Kaya, S.; Katin, K.P.; Altunay, N. Synthesis of carbon modified with polymer of diethylenetriamine and trimesoyl chloride for the dual removal of Hg (II) and methyl mercury ([CH3Hg]+) from wastewater: Theoretical and experimental analyses. Mater. Chem. Phys. 2022, 277, 125501. [Google Scholar] [CrossRef]
- Ariya, P.A.; Peterson, K.A. Chemical transformation of gaseous elemental Hg in the atmosphere. In Dynamics of Mercury Pollution on Regional and Global Scales; Springer: Boston, MA, USA, 2005; pp. 261–294. [Google Scholar]
- Chen, C.Y.; Driscoll, C.T.; Eagles-Smith, C.A.; Eckley, C.S.; Gay, D.A.; Hsu-Kim, H.; Keane, S.E.; Kirk, J.L.; Mason, R.P.; Obrist, D.; et al. A critical time for mercury science to inform global policy. Environ. Sci. Technol. 2018, 52, 9556–9561. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Nakamura, M.; Murata, K. Mercury as a global pollutant and mercury exposure assessment and health effects. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2018, 73, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, P.; Fu, X.; Wang, X.; Zhang, H.; Lin, C.-J. Mercury pollution in China: Implications on the implementation of the Minamata Convention. Environ. Sci. Process. Impacts 2022, 24, 634–648. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Impact of Mercury on European Water Quality; European Environment Agency: Copenhagen, Denmark, 2018. [Google Scholar]
- Carpi, A. Mercury from combustion sources: A review of the chemical species emitted and their transport in the atmosphere. Water Air Soil Pollut. 1997, 98, 241–254. [Google Scholar] [CrossRef]
- Fleischanderl, A.; Steinparzer, T.; Plattner, T.; Neuhold, R.; Goetz, M. Green Solutions for Iron Ore Agglomeration Off-gas Treatment and By-Product Utilization. In Proceedings of the ESTAD 2023, Düsseldorf, Germany, 12–16 June 2023. [Google Scholar]
- Song, M.; Liu, J.; Xu, S. Characterization and solidification/stabilization of iron-ore sintering gas cleaning residue. J. Mater. Cycles Waste Manag. 2015, 17, 790–797. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zevenhoven, R.; Bhattacharya, P.; Sajwan, K.S.; Kikuchi, R. Mercury flow via coal and coal utilization by-products: A global perspective. Resour. Conserv. Recycl. 2008, 52, 571–591. [Google Scholar] [CrossRef]
- Hledik, C.; Goetz, M.; Ottner, F.; Fürhacker, M. MEROS Dust Quality of Different Plants and Its Potential Further Uses. Metals 2021, 11, 840. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Bundesministerium für Landwirtschaft, Regionen und Tourismus. Verordnung des Bundesministers für Land- und Forstwirtschaft über die Allgemeine Begrenzung von Abwasseremissionen in Fließgewässer und öffentliche Kanalisationen (Allgemeine Abwasseremissionsverordnung–AAEV); Bundesministerium für Landwirtschaft, Regionen und Tourismus: Vienna, Austria, 1996.
- Bundesgesetzblatt für die Republik Österreich. Verordnung des Bundesministers für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft über die Festlegung des Zielzustandes für Oberflächengewässer (Qualitätszielverordnung Chemie Oberflächengewässer–QZV Chemie OG); Bundesgesetzblatt für die Republik Österreich: Vienna, Austria, 2006.
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Estay, H.; Barros, L.; Troncoso, E. Metal sulfide precipitation: Recent breakthroughs and future outlooks. Minerals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Irannajad, M.; Azadmehr, A.R. Selective Sulphide Precipitation of Heavy Metals from Acidic Polymetallic Aqueous Solution by Thioacetamide. Ind. Eng. Chem. Res. 2012, 51, 954–963. [Google Scholar] [CrossRef]
- Silva, P.M.; Raulino, G.S.; Vidal, C.B.; do Nascimento, R.F. Selective precipitation of Cu2+, Zn2+ and Ni2+ ions using H2S (g) produced by hydrolysis of thioacetamide as the precipitating agent. Desalination Water Treat. 2017, 95, 220–226. [Google Scholar] [CrossRef]
- Grau, J.; Akinc, M. Synthesis of nickel sulfide by homogeneous precipitation from acidic solutions of thioacetamide. J. Am. Ceram. Soc. 1996, 79, 1073–1082. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Long, J.; Zhang, P.; Chen, Y. Combined Fenton process and sulfide precipitation for removal of heavy metals from industrial wastewater: Bench and pilot scale studies focusing on in-depth thallium removal. Front. Environ. Sci. Eng. 2019, 13, 49. [Google Scholar] [CrossRef]
- Prokkola, H.; Nurmesniemi, E.-T.; Lassi, U. Removal of metals by sulphide precipitation using Na2S and HS−-solution. ChemEngineering 2020, 4, 51. [Google Scholar] [CrossRef]
- Tokuda, H.; Kuchar, D.; Mihara, N.; Kubota, M.; Matsuda, H.; Fukuta, T. Study on reaction kinetics and selective precipitation of Cu, Zn, Ni and Sn with H2S in single-metal and multi-metal systems. Chemosphere 2008, 73, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Ito, T.; Sawada, K.; Kojima, Y.; Matsuda, H.; Seto, F. Separation of Cu, Zn and Ni from plating solution by precipitation of metal sulfides. Kagaku Kogaku Ronbunshu 2004, 30, 227–232. [Google Scholar] [CrossRef]
- Rostamnezhad, N.; Kahforoushan, D.; Sahraei, E.; Ghanbarian, S.; Shabani, M. A method for the removal of Cu (II) from aqueous solutions by sulfide precipitation employing heavy oil fly ash. Desalination Water Treat. 2016, 57, 17593–17602. [Google Scholar] [CrossRef]
- Veeken, A.; De Vries, S.; Van der Mark, A.; Rulkens, W. Selective precipitation of heavy metals as controlled by a sulfide-selective electrode. Sep. Sci. Technol. 2003, 38, 1–19. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Riekkola-Vanhanen, M.-L.; Puhakka, J. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res. 2003, 37, 255–266. [Google Scholar] [CrossRef] [PubMed]
- ISO 17294-2:2023; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. Austrian Standards; ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/82245.html (accessed on 26 June 2024).
- Diehl, S.; Goldhaber, M.; Hatch, J. Modes of occurrence of mercury and other trace elements in coals from the warrior field, Black Warrior Basin, Northwestern Alabama. Int. J. Coal Geol. 2004, 59, 193–208. [Google Scholar] [CrossRef]
- Kolker, A.; Senior, C.L.; Quick, J.C. Mercury in coal and the impact of coal quality on mercury emissions from combustion systems. Appl. Geochem. 2006, 21, 1821–1836. [Google Scholar] [CrossRef]
- Park, J.Y.; Won, J.H.; Lee, T.G. Mercury analysis of various types of coal using acid extraction and pyrolysis methods. Energy Fuels 2006, 20, 2413–2416. [Google Scholar] [CrossRef]
- Reis, A.T.; Lopes, C.B.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Extraction of mercury water-soluble fraction from soils: An optimization study. Geoderma 2014, 213, 255–260. [Google Scholar] [CrossRef]
- Xu, J.; Kleja, D.B.; Biester, H.; Lagerkvist, A.; Kumpiene, J. Influence of particle size distribution, organic carbon, pH and chlorides on washing of mercury contaminated soil. Chemosphere 2014, 109, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Dong, K.; Wang, W.; Asselin, E. Leaching of mercury from contaminated solid waste: A mini-review. Miner. Process. Extr. Metall. Rev. 2020, 41, 187–197. [Google Scholar] [CrossRef]
- Han, D.S.; Orillano, M.; Khodary, A.; Duan, Y.; Batchelor, B.; Abdel-Wahab, A. Reactive iron sulfide (FeS)-supported ultrafiltration for removal of mercury (Hg (II)) from water. Water Res. 2014, 53, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Sedlak, D.L. Similarities between inorganic sulfide and the strong Hg (II)-complexing ligands in municipal wastewater effluent. Environ. Sci. Technol. 2005, 39, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.-y.; Wang, Q.-w.; Wang, Y.-y.; Li, Q.-z.; Yang, Z.-h.; Shu, Y.-d. Thermodynamic study on reaction path of Hg (II) with S (II) in solution. J. Cent. South Univ. Technol. 2010, 17, 289–294. [Google Scholar] [CrossRef]
- Jay, J.A.; Morel, F.M.; Hemond, H.F. Mercury speciation in the presence of polysulfides. Environ. Sci. Technol. 2000, 34, 2196–2200. [Google Scholar] [CrossRef]
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 1996, 30, 1835–1845. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1979–1980. [Google Scholar]
- Paquette, K.E.; Helz, G.R. Inorganic speciation of mercury in sulfidic waters: The importance of zero-valent sulfur. Environ. Sci. Technol. 1997, 31, 2148–2153. [Google Scholar] [CrossRef]
- Kondo, H.; Fujita, T.; Kuchar, D.; Fukuta, T.; Matsuda, H.; Kubota, M.; Yagishita, K. Separation of metal sulfides from plating wastewater containing Cu, Zn and Ni by selective sulfuration with hydrogen sulfide. J. Surf. Finish. Soc. Jpn. 2006, 57, 901–906. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).