Are Harmful Algal Blooms Increasing in the Great Lakes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Satellite Data Acquisition and Processing
2.3. Bloom Classification
2.4. Surface HAB Scum Classification
2.5. Mean Annual Extent Calculation
2.6. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapra, S.C.; Robertson, A. Great Lakes Eutrophication: The Effect of Point Source Control of Total Phosphorus. Science 1977, 196, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, J.C.; Bertram, P. Evidence for the Restoration of the Lake Erie Ecosystem. BioScience 1991, 41, 216–223. [Google Scholar] [CrossRef]
- De Stasio, B.; Schrimpf, M.; Beranek, A.; Daniels, W. Increased Chlorophyll a, phytoplankton abundance, and cyanobacteria occurrence following invasion of Green Bay, Lake Michigan by dreissenid mussels. Aquat. Invasions 2008, 3, 21–27. [Google Scholar] [CrossRef]
- Scavia, D.; Allan, J.D.; Arend, K.K.; Bartell, S.; Beletsky, D.; Bosch, N.S.; Brandt, S.B.; Briland, R.D.; Daloğlu, I.; DePinto, J.V.; et al. Assessing and addressing the re-eutrophication of Lake Erie: Central basin hypoxia. J. Great Lakes Res. 2014, 40, 226–246. [Google Scholar] [CrossRef]
- Baker, D.; Confesor, R.; Ewing, D.; Johnson, L.; Kramer, J.; Merryfield, B. Phosphorus loading to Lake Erie from the Maumee, Sandusky and Cuyahoga rivers: The importance of bioavailability. J. Great Lakes Res. 2014, 40, 502–517. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Liebig, J.R.; Carmichael, W.W.; Agy, M.A.; Johengen, T.H.; Fahnenstiel, G.L.; Nalepa, T.F. Zebra mussel (Dreissena Polymorpha) Sel. Filtr. Promot. Toxic Microcystis Bloom. Saginaw Bay (Lake Huron) Lake Erie. Can. J. Fish. Aquat. Sci. 2001, 58, 1208–1221. [Google Scholar] [CrossRef]
- Barbiero, R.P.; Tuchman, M.L. Changes in the crustacean communities of Lakes Michigan, Huron, and Erie following the invasion of the predatory cladoceran Bythotrephes Longimanus. Can. J. Fish. Aquat. Sci. 2004, 61, 2111–2125. [Google Scholar] [CrossRef]
- Hecky, R.E.; Smith, R.E.; Barton, D.R.; Guildford, S.J.; Taylor, W.D.; Charlton, M.N.; Howell, T. The nearshore phosphorus shunt: A consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 2004, 61, 1285–1293. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Mehler, K.; Hinchey, E.K.; Wick, M.; Bakowska, M.; Mrozinska, N. Rapid assessment of Dreissena Popul. Lake Erie Using Underw. Videogr. Hydrobiol. 2021, 848, 2421–2436. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Karatayev, V.A.; Burlakova, L.E.; Mehler, K.; Rowe, M.D.; Elgin, A.K.; Nalepa, T.F. Lake morphometry determines Dreissena invasion dynamics. Biol. Invasions 2021, 23, 2489–2514. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Sterner, R.W.; Reinl, K.L.; Lafrancois, B.M.; Brovold, S.; Miller, T.R. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol. Oceanogr. 2020, 65, 2984–2998. [Google Scholar] [CrossRef]
- Reinl, K.L.; Sterner, R.W.; Lafrancois, B.M.; Brovold, S. Fluvial seeding of cyanobacterial blooms in oligotrophic Lake Superior. Harmful Algae 2020, 100, 101941. [Google Scholar] [CrossRef] [PubMed]
- Boegehold, A.G.; Burtner, A.M.; Camilleri, A.C.; Carter, G.; DenUyl, P.; Fanslow, D.; Semenyuk, D.F.; Godwin, C.M.; Gossiaux, D.; Johengen, T.H.; et al. Routine monitoring of western Lake Erie to track water quality changes associated with cyanobacterial harmful algal blooms. Earth Syst. Sci. Data 2023, 15, 3853–3868. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada and the U.S. Environmental Protection Agency. Lake Superior 2020–2024 Lakewide Action & Management Plan. Cat No. En164-52/2022E-PDF. EPA 905-R22-002. 2022. Available online: https://binational.net/wp-content/uploads/2022/09/Lake-Superior-LAMP-2020-2024.pdf (accessed on 1 September 2023).
- Carmichael, W. A world overview-One-hundred-twenty-seven years of research on toxic cyanobacteria-Where do we go from here? In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; pp. 105–125. [Google Scholar] [CrossRef]
- Favot, E.J.; Holeton, C.; DeSellas, A.M.; Paterson, A.M. Cyanobacterial blooms in Ontario, Canada: Continued increase in reports through the 21st century. Lake Reserv. Manag. 2023, 39, 1–20. [Google Scholar] [CrossRef]
- Benesh, K.; Lafrancois, B.; Reinl, K. 2023 Lake Superior Bloom Bulletin; Lake Superior National Estuarine Research Reserve, 2024. Available online: http://digital.library.wisc.edu/1793/85297 (accessed on 1 July 2024).
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Sayers, M.; Fahnenstiel, G.L.; Shuchman, R.A.; Whitley, M. Cyanobacteria blooms in three eutrophic basins of the Great Lakes: A comparative analysis using satellite remote sensing. Int. J. Remote Sens. 2016, 37, 4148–4171. [Google Scholar] [CrossRef]
- Sayers, M.J.; Grimm, A.G.; Shuchman, R.A.; Bosse, K.R.; Fahnenstiel, G.L.; Ruberg, S.A.; Leshkevich, G.A. Satellite monitoring of harmful algal blooms in the Western Basin of Lake Erie: A 20-year time-series. J. Great Lakes Res. 2019, 45, 508–521. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Wynne, T.T.; Baker, D.B.; Fahnenstiel, G.L. Interannual Variability of Cyanobacterial Blooms in Lake Erie. PLoS ONE 2012, 7, e42444. [Google Scholar] [CrossRef] [PubMed]
- Wynne, T.; Stumpf, R. Spatial and Temporal Patterns in the Seasonal Distribution of Toxic Cyanobacteria in Western Lake Erie from 2002–2014. Toxins 2015, 7, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, R.P.; Johnson, L.T.; Wynne, T.T.; Baker, D.B. Forecasting annual cyanobacterial bloom biomass to inform management decisions in Lake Erie. J. Great Lakes Res. 2016, 42, 1174–1183. [Google Scholar] [CrossRef]
- Ho, J.C.; Stumpf, R.P.; Bridgeman, T.B.; Michalak, A.M. Using Landsat to extend the historical record of lacustrine phytoplankton blooms: A Lake Erie case study. Remote Sens. Environ. 2017, 191, 273–285. [Google Scholar] [CrossRef]
- Maccoux, M.J.; Dove, A.; Backus, S.M.; Dolan, D.M. Total and soluble reactive phosphorus loadings to Lake Erie. J. Great Lakes Res. 2016, 42, 1151–1165. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Litaker, R.W.; Hood, R.R. Cyanobacterial bloom phenology in Saginaw Bay from MODIS and a comparative look with western Lake Erie. Harmful Algae 2021, 103, 101999. [Google Scholar] [CrossRef] [PubMed]
- Binding, C.E.; Stumpf, R.P.; Shuchman, R.A.; Sayers, M.J. Advances in Remote Sensing of Great Lakes Algal Blooms. In Contaminants of the Great Lakes; Springer International Publishing: Cham, Switzerland, 2020; pp. 217–232. [Google Scholar] [CrossRef]
- Bertani, I.; Steger, C.E.; Obenour, D.R.; Fahnenstiel, G.L.; Bridgeman, T.B.; Johengen, T.H.; Sayers, M.J.; Shuchman, R.A.; Scavia, D. Tracking cyanobacteria blooms: Do different monitoring approaches tell the same story? Sci. Total Environ. 2017, 575, 294–308. [Google Scholar] [CrossRef]
- Ju, J.; Roy, D.P. The availability of cloud-free Landsat ETM+ data over the conterminous United States and globally. Remote Sens. Environ. 2008, 112, 1196–1211. [Google Scholar] [CrossRef]
- Bailey, S.W.; Franz, B.A.; Werdell, P.J. Estimation of near-infrared water-leaving reflectance for satellite ocean color data processing. Opt. Express 2010, 18, 7521. [Google Scholar] [CrossRef]
- Lesht, B.M.; Barbiero, R.P.; Warren, G.J. A band-ratio algorithm for retrieving open-lake chlorophyll values from satellite observations of the Great Lakes. J. Great Lakes Res. 2013, 39, 138–152. [Google Scholar] [CrossRef]
- Mouw, C.B.; Chen, H.; McKinley, G.A.; Effler, S.; O’Donnell, D.; Perkins, M.G.; Strait, C. Evaluation and optimization of bio-optical inversion algorithms for remote sensing of Lake Superior’s optical properties. J. Geophys. Res. Ocean. 2013, 118, 1696–1714. [Google Scholar] [CrossRef]
- Shuchman, R.A.; Leshkevich, G.; Sayers, M.J.; Johengen, T.H.; Brooks, C.N.; Pozdnyakov, D. An algorithm to retrieve chlorophyll, dissolved organic carbon, and suspended minerals from Great Lakes satellite data. J. Great Lakes Res. 2013, 39, 14–33. [Google Scholar] [CrossRef]
- Lesht, B.M.; Barbiero, R.P.; Warren, G.J. Verification of a simple band ratio algorithm for retrieving Great Lakes open water surface chlorophyll concentrations from satellite observations. J. Great Lakes Res. 2016, 42, 448–454. [Google Scholar] [CrossRef]
- Fahnenstiel, G.L.; Sayers, M.J.; Shuchman, R.A.; Yousef, F.; Pothoven, S.A. Lake-wide phytoplankton production and abundance in the Upper Great Lakes: 2010–2013. J. Great Lakes Res. 2016, 42, 619–629. [Google Scholar] [CrossRef]
- Bosse, K.R.; Sayers, M.J.; Shuchman, R.A.; Lekki, J.; Tokars, R. Measuring the Impact of the COVID-19 Shutdown on Great Lakes Water Quality Using Remote Sensing. Front. Mar. Sci. 2021, 8, 673989. [Google Scholar] [CrossRef]
- De Stasio, B.; Schrimpf, M.; Cornwell, B. Phytoplankton Communities in Green Bay, Lake Michigan after Invasion by Dreissenid Mussels: Increased Dominance by Cyanobacteria. Diversity 2014, 6, 681–704. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Pokrzywinski, K.L.; Litaker, R.W.; De Stasio, B.T.; Hood, R.R. Cyanobacterial Bloom Phenology in Green Bay Using MERIS Satellite Data and Comparisons with Western Lake Erie and Saginaw Bay. Water 2022, 14, 2636. [Google Scholar] [CrossRef]
- Cael, B.B.; Bisson, K.; Boss, E.; Dutkiewicz, S.; Henson, S. Global climate-change trends detected in indicators of ocean ecology. Nature 2023, 619, 551–554. [Google Scholar] [CrossRef]
- Cochrane, D.; Orcutt, G.H. Application of Least Squares Regression to Relationships Containing Auto-Correlated Error Terms. J. Am. Stat. Assoc. 1949, 44, 32–61. [Google Scholar] [CrossRef]
- Millie, D.F.; Fahnenstiel, G.L.; Weckman, G.R.; Klarer, D.M.; Dyble, J.; Vanderploeg, H.A.; Fishman, D.B. An “Enviro-Informatic” Assessment of Saginaw Bay (Lake Huron, USA) Phytoplankton: Data-Driven Characterization and Modeling of Microcystis (Cyanophyta): Characterization Modeling Saginaw Bay Phytoplankton. J. Phycol. 2011, 47, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Stow, C.A.; Reckhow, K.H.; DeMarchi, C.; Johengen, T.H. Phosphorus load estimation in the Saginaw River, MI using a Bayesian hierarchical/multilevel model. Water Res. 2010, 44, 3270–3282. [Google Scholar] [CrossRef] [PubMed]
- Stow, C.A.; Dyble, J.; Kashian, D.R.; Johengen, T.H.; Winslow, K.P.; Peacor, S.D.; Francoeur, S.N.; Burtner, A.M.; Palladino, D.; Morehead, N.; et al. Phosphorus targets and eutrophication objectives in Saginaw Bay: A 35 year assessment. J. Great Lakes Res. 2014, 40, 4–10. [Google Scholar] [CrossRef]
- Loken, L.C.; Diebel, M.W.; Bonville, D.B.; Robertson, D.M.; Koltun, G.F.; Bertke, E.E.; Kula, S.P.; Komiskey, M.J. Phosphorus, Nitrogen, and Suspended-Sediment Loads Measured at the Great Lakes Restoration Initiative Tributary Monitoring Network: Water Years 2011–2020; USGS: Reston, VA, USA, 2023. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Binding, C.; Greenberg, T.; McCullough, G.; Watson, S.; Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Great Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Wilkinson, G.M.; Walter, J.A.; Buelo, C.D.; Pace, M.L. No evidence of widespread algal bloom intensification in hundreds of lakes. Front. Ecol. Environ. 2021, 20, 16–21. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. EOLakeWatch 2011 Algal Bloom Report-Lake Erie. 2019. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/eolakewatch/le/en/Lake-Erie-2011-Annual-BloomReport.pdf (accessed on 10 May 2024).
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J. Recent changes in cyanobacteria algal bloom magnitude in large lakes across the contiguous United States. Sci. Total Environ. 2023, 897, 165253. [Google Scholar] [CrossRef] [PubMed]
- Obenour, D.R.; Gronewold, A.D.; Stow, C.A.; Scavia, D. Using a Bayesian hierarchical model to improve Lake Erie cyanobacteria bloom forecasts. Water Resour. Res. 2014, 50, 7847–7860. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M. Phytoplankton blooms in Lake Erie impacted by both long-term and springtime phosphorus loading. J. Great Lakes Res. 2017, 43, 221–228. [Google Scholar] [CrossRef]
- Bocaniov, S.A.; Scavia, D.; Cappellen, P.V. Long-term phosphorus mass-balance of Lake Erie (Canada-USA) reveals a major contribution of in-lake phosphorus loading. Ecol. Inform. 2023, 77, 102131. [Google Scholar] [CrossRef]
- Scavia, D.; Wang, Y.C.; Obenour, D.R. Advancing freshwater ecological forecasts: Harmful algal blooms in Lake Erie. Sci. Total Environ. 2023, 856, 158959. [Google Scholar] [CrossRef]
- Conroy, J.D.; Kane, D.D.; Briland, R.D.; Culver, D.A. Systemic, early-season Microcystis Blooms in western Lake Erie and two of its major agricultural tributaries (Maumee and Sandusky rivers). J. Great Lakes Res. 2014, 40, 518–523. [Google Scholar] [CrossRef]
- Davis, T.W.; Watson, S.B.; Rozmarynowycz, M.J.; Ciborowski, J.J.H.; McKay, R.M.; Bullerjahn, G.S. Phylogenies of Microcystin-Producing Cyanobacteria in the Lower Laurentian Great Lakes Suggest Extensive Genetic Connectivity. PLoS ONE 2014, 9, e106093. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kanto, J.M.; Saxton, M.A.; DeBruyn, J.M.; Smith, J.L.; Marvin, C.H.; Krieger, K.A.; Sayler, G.S.; Boyer, G.L.; Wilhelm, S.W. The diversity and distribution of toxigenic Microcystis Spp. in present day and archived pelagic and sediment samples from Lake Erie. Harmful Algae 2009, 8, 385–394. [Google Scholar] [CrossRef]
- Millie, D.F.; Fahnenstiel, G.L.; Bressie, J.D.; Pigg, R.J.; Rediske, R.R.; Klarer, D.M.; Tester, P.A.; Litaker, R.W. Late-summer phytoplankton in western Lake Erie (Laurentian Great Lakes): Bloom distributions, toxicity, and environmental influences. Aquat. Ecol. 2009, 43, 915–934. [Google Scholar] [CrossRef]
- Kitchens, C.M.; Johengen, T.H.; Davis, T.W. Establishing spatial and temporal patterns in Microcystis Sediment Seed Stock Viability and Their Relationship to Subsequent Bloom Development in Western Lake Erie. PLoS ONE 2018, 13, e0206821. [Google Scholar] [CrossRef] [PubMed]
- Benesh, K.; Lafrancois, B.; Reinl, K.L.; Banerji, A. 2022 Lake Superior Bloom Bulletin; Lake Superior National Estuarine Research Reserve, 2023. Available online: http://digital.library.wisc.edu/1793/84423 (accessed on 24 August 2023).
- Zohary, T.; Robarts, R.D. Hyperscums and the population dynamics of Microcystis Aeruginosa. J. Plankton Res. 1990, 12, 423–432. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Vonk, M.; Los, H.F.J.; van der Molen, D.T.; Mooij, W.M. Fuzzy Modeling of Cyanobacterial Surface Waterblooms: Validation with Noaa-Avhrr Satellite Images. Ecol. Appl. 2003, 13, 1456–1472. [Google Scholar] [CrossRef]
- Paerl, H.W.; Ustach, J.F. Blue-green algal scums: An explanation for their occurrence during freshwater blooms1. Limnol. Oceanogr. 1982, 27, 212–217. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Dyble, J. Characterizing a cyanobacterial bloom in Western Lake Erie using satellite imagery and meteorological data. Limnol. Oceanogr. 2010, 55, 2025–2036. [Google Scholar] [CrossRef]
- Rowe, M.D.; Anderson, E.J.; Wynne, T.T.; Stumpf, R.P.; Fanslow, D.L.; Kijanka, K.; Vanderploeg, H.A.; Strickler, J.R.; Davis, T.W. Vertical distribution of buoyant Microcystis Bloom. A Lagrangian Part. Track. Model Short-Term Forecast. Lake Erie. J. Geophys. Res. Ocean. 2016, 121, 5296–5314. [Google Scholar] [CrossRef]
- Bosse, K.R.; Sayers, M.J.; Shuchman, R.A.; Fahnenstiel, G.L.; Ruberg, S.A.; Fanslow, D.L.; Stuart, D.G.; Johengen, T.H.; Burtner, A.M. Spatial-temporal variability of in situ cyanobacteria vertical structure in Western Lake Erie: Implications for remote sensing observations. J. Great Lakes Res. 2019, 45, 480–489. [Google Scholar] [CrossRef]
- Den Uyl, P.A.; Harrison, S.B.; Godwin, C.M.; Rowe, M.D.; Strickler, J.R.; Vanderploeg, H.A. Comparative analysis of Microcystis Buoyancy in western Lake Erie and Saginaw Bay of Lake Huron. Harmful Algae 2021, 108, 102102. [Google Scholar] [CrossRef] [PubMed]
- EPA; Environment and Climate Change Canada. State of the Great Lakes 2022 Technical Report. Cat No. En161-3/1E-PDF. EPA 905-R22-004. 2022. Available online: https://publications.gc.ca/collections/collection_2022/eccc/En161-3-1-2022-eng.pdf (accessed on 1 July 2024).
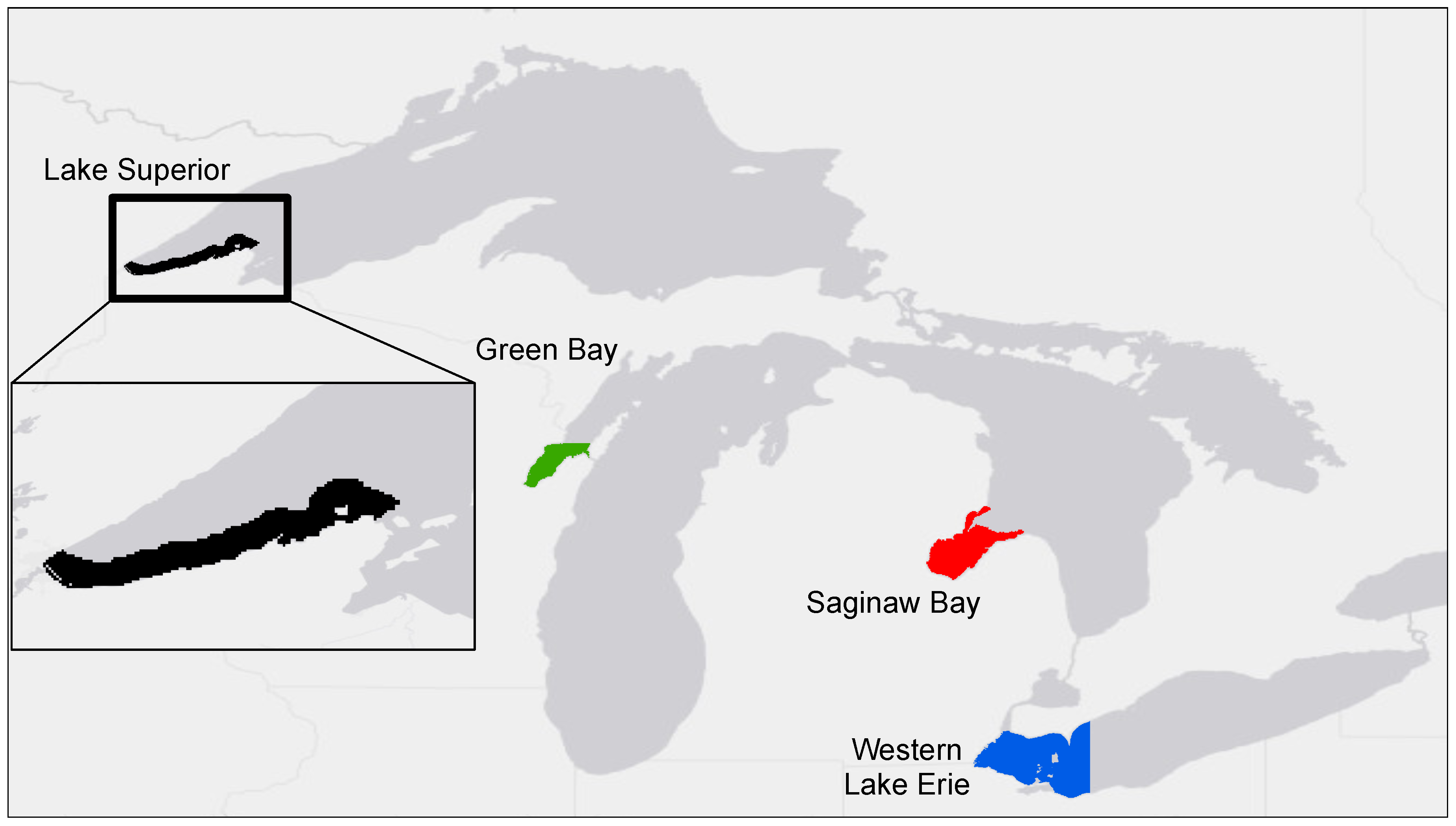

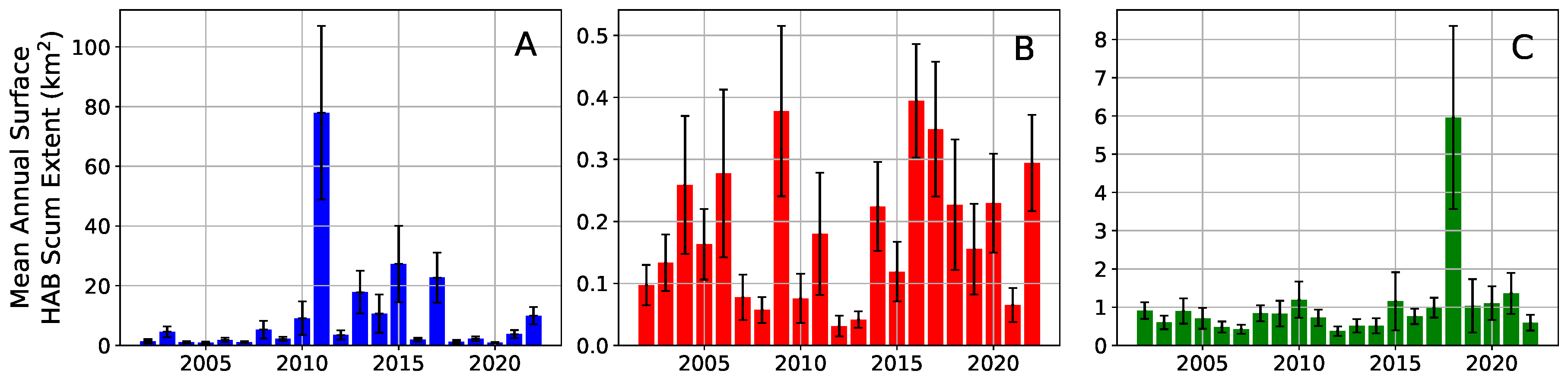

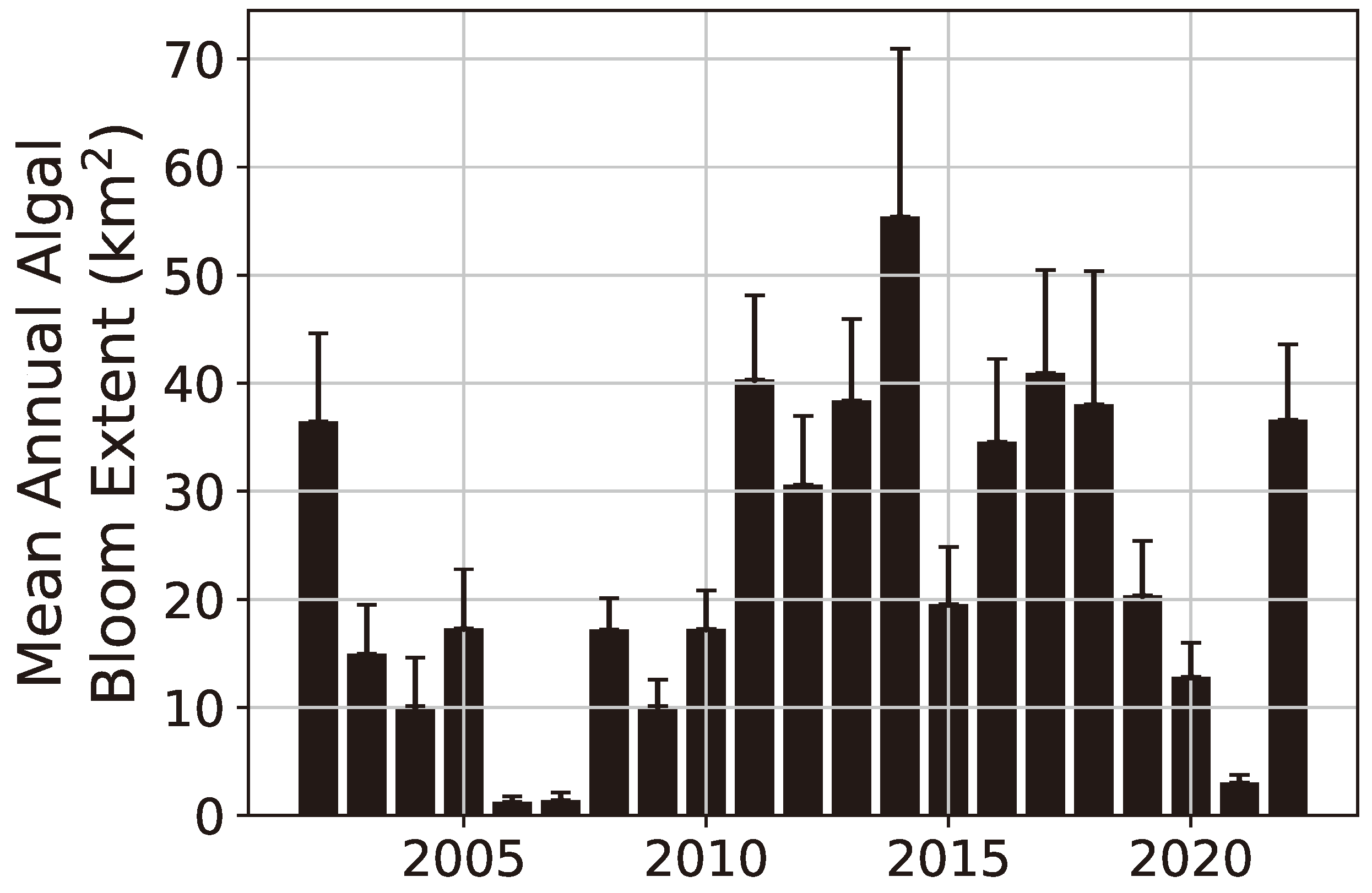
| Region | Min (km2) | Max (km2) | Mean (km2) | SEM (km2) | Slope/SNR |
|---|---|---|---|---|---|
| WLE | 147.5 (2009) | 1045.9 (2015) | 369.2 | 39.6 | 5.1/0.8 |
| Saginaw Bay | 7.0 (2017) | 91.7 (2006) | 27.4 | 4.3 | −2.1/3.9 |
| Green Bay | 2.8 (2009) | 31.5 (2018) | 10.3 | 1.7 | 0.1/0.5 |
| Lake Superior | 1.2 (2007) | 61.7 (2014) | 27.5 | 3.3 | 0.7/1.2 |
| Region | Min (km2) | Max (km2) | Mean (km2) | SEM (km2) | Slope/SNR |
|---|---|---|---|---|---|
| WLE | 0.87 (2005) | 77.9 (2011) | 9.9 | 3.8 | 0.25/0.4 |
| Saginaw Bay | 0.03 (2012) | 0.39 (2016) | 0.18 | 0.02 | 0.004/1.0 |
| Green Bay | 0.37 (2012) | 6.0 (2018) | 1.1 | 0.3 | 0.06/1.4 |
| WLE | Saginaw Bay | Green Bay | Lake Superior | |
|---|---|---|---|---|
| WLE | 1 | −0.24 (p = 0.3) | −0.18 (p = 0.42) | 0.25 (p = 0.28) |
| Saginaw Bay | 1 | −0.18 (p = 0.44) | −0.28 (p = 0.22) | |
| Green Bay | 1 | −0.05 (p = 0.84) | ||
| Lake Superior | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosse, K.R.; Fahnenstiel, G.L.; Buelo, C.D.; Pawlowski, M.B.; Scofield, A.E.; Hinchey, E.K.; Sayers, M.J. Are Harmful Algal Blooms Increasing in the Great Lakes? Water 2024, 16, 1944. https://doi.org/10.3390/w16141944
Bosse KR, Fahnenstiel GL, Buelo CD, Pawlowski MB, Scofield AE, Hinchey EK, Sayers MJ. Are Harmful Algal Blooms Increasing in the Great Lakes? Water. 2024; 16(14):1944. https://doi.org/10.3390/w16141944
Chicago/Turabian StyleBosse, Karl R., Gary L. Fahnenstiel, Cal D. Buelo, Matthew B. Pawlowski, Anne E. Scofield, Elizabeth K. Hinchey, and Michael J. Sayers. 2024. "Are Harmful Algal Blooms Increasing in the Great Lakes?" Water 16, no. 14: 1944. https://doi.org/10.3390/w16141944
APA StyleBosse, K. R., Fahnenstiel, G. L., Buelo, C. D., Pawlowski, M. B., Scofield, A. E., Hinchey, E. K., & Sayers, M. J. (2024). Are Harmful Algal Blooms Increasing in the Great Lakes? Water, 16(14), 1944. https://doi.org/10.3390/w16141944





