Abstract
Freshwater is a limited resource that is needed by all living things. However, the available amount of it cannot counterbalance the explosion of the human population in recent years. This condition is worsened because of the contamination of many bodies of water by industrialization and urbanization. Nanomaterials offer an alternative sustainable solution due to their unique size-dependent properties, i.e., high specific surface area and discontinuous properties. These advantages can be utilized to reuse wastewater to become a sustainable water source for drinking water. Many recent studies have proven that nanotechnologies in the forms of nano-adsorbents, nanomembranes, and nano-catalysts have high performances in water contaminants removal. This review provides a comprehensive discussion around these nanotechnologies from the mechanism, applications, efficacy, advantages, disadvantages, and challenges in applications for producing drinking water including by wastewater reusing. Each nanotechnology reviewed here has been proven to perform effectively for water contaminants removal in laboratory scale. An initial study is also performed in this review to analyze the sustainability of nanotechnology for producing drinking water. In spite of the great efficacy, nanotechnologies utilization in commercial scales is still limited which requires further studies.
1. Introduction
Water is the source of living things that covers 70% of the earth’s surface, but water scarcity has become a real danger affecting more than 2.4 billion people of the world’s population today [1]. The explosion of the population in recent years has taken the world to the global water crisis. The available amount of freshwater on earth cannot counterbalance the growth of the human population [2]. Thus, the global water crisis has threatened the life of more than 800 million people in the world [1]. In 2022, around 2.2 billion people did not have access to a safely managed drinking water service, which is defined as the water available when needed and free from contamination [1]. Global water withdrawal increased from around 1700 km3/year in 1960 to almost 4000 km3/year in 2010 and by the end of the century, it was projected to reach 6000 km3/year [3]. Following this trend, more than half of the world’s population will face water-stressed conditions caused by an insufficient amount of water by 2025.
As only 3% of water is freshwater, including lakes, ponds, rivers, streams, springs, wetlands, and frozen glaciers, there are not plenty of usable sources [4]. Moreover, the increasing economic activities in the past years have resulted in cumulative contaminated water. Pollution occurs when external objects and the amounts of chemicals, both natural and unnatural, in an aquatic ecosystem accumulate or increase. There is enough freshwater on the planet for now, but it is distributed unevenly and too much of it mundanely wasted, contaminated, and unsustainably handled; hence, the present condition will insinuate the phenomenon if it is not being alleviated immediately [5].
Clean freshwater is needed for human and industrial activities, especially for food and drink production. It was reported that 80% of the world’s untreated wastewater is recklessly discharged to the body of water, back to the rivers, lakes, and oceans [6]. As a result, waterborne diseases are threatening the lives of citizens, such as diarrhea, dysentery [7], arsenicosis [8], polio [9], trachoma [10], typhoid fever [11], schistosomiasis [12], cholera [13], lead poisoning [14], etc. For example, extremely contaminated water in Woburn, Massachusetts between 1969 and 1979 was responsible for leukemia and birth defects of 12 children in the suburban city. The water in wells G and H which were used as the Woburn municipal water supply was contaminated by the spill from chemical barrels because they were connected to the Aberjona River in a historically industrial area. Ten years after the residents complained, the Massachusetts Department of Public Health announced that the wells were contaminated with tetrachloroethylene/perchloroethylene (PERC), trichloroethylene (TCE), chloroform, 1,2-dichloroethene, and inorganic arsenic by 20.8 µg/L, 267.4 µg/L, 11.8 µg/L, 53 µg/L, 10 µg/L, respectively [15].
One of the major causes of waterborne diseases is low drinking water quality. The limited access to clean drinking water has affected around 2.3 billion people across the world with water-related diseases [16]. In 2020, it is estimated that nearly 2 billion people globally still drank fecal-contaminated water [17]. Therefore, the Sustainable Development Goal (SDG) target 6.1 from the United Nations aims for universal and equitable access to safe and affordable drinking water by 2030. In order to achieve this target, some actions need to be taken to guarantee the availability of freshwater including sustainable water management by wastewater treatment as also emphasized by SDG target 6.3 [18].
As a universal solvent, water easily dissolves various contaminants and contains more substances than any other liquid element on earth. The classification of water pollution falls into six categories, i.e., groundwater, surface water, ocean water, point source, nonpoint source, and transboundary [19]. Groundwater comes from the rain that is absorbed deeply into the earth, filling the cracks and porous spaces. It gets polluted when the contaminant leaks from landfills [20] and septic systems [21]. Surface water pollution sources are rivers, lakes, and oceans that get contaminated. A point source is when contamination comes from a single source; for example, wastewater, sewage, oil spills, and illegal dumping. For example, water contamination in Nansi Lake, China, was caused by several industries along the shore of the lake, such as the mining and washing of coal, manufacture of raw chemical materials and chemical products, papermaking industry, and food processing industry [22]. The nonpoint source refers to contamination from diffusion; for example, debris blown from uncontained landfills. Trans-boundary is pollution as a result of a significant disaster in a country into waters in other places; for example, oil spills as happened in Montara and sparked debates between Australia and Indonesia [23].
In recent years, nanomaterials have attracted many people and industries to treat wastewater in order to gain clean freshwater ready for reuse. Nanomaterials have unique size-dependent properties related to their high specific surface area (fast dissolution, high reactivity, strong sorption) and discontinuous properties (such as super-paramagnetism, localized surface plasmon resonance, and quantum confinement effect) [24] which overcome several difficulties and drawbacks in applying conventional water treatment technologies. Treatment effectiveness of contaminants can be increased with the application of nanomaterials, thanks to the increased selectivity and reactivity, as well as the ability to undergo reactions that are not possible with conventional materials. The treatment process can thus be made simpler, with a reduction in energy, time, and cost [25]. Thus, nanotechnology is perceived as a sustainable way to manage water resources for providing freshwater for the population [26].
This review paper will focus on various nanotechnology applications for drinking water production from different water resources, including reusing contaminated wastewater. Based on the data available, an initial sustainability evaluation of nanotechnology available for water treatment will be performed in this review paper. The possibility of nanotechnologies for potable water production at a large scale will also be assessed based on the advantages, drawbacks, and challenges in the current development stage.
2. Limited Drinking Water Sources and Efforts to Reuse Wastewater
Conventionally, drinking water is produced from groundwater or surface freshwater (rivers or lakes) that undergoes several treatments to satisfy the quality for drinking [27]. The World Health Organization and United Nations Children’s Fund (WHO/UNICEF) Joint Monitoring Program for Water Supply, Sanitation and Hygiene has defined five levels of drinking water services, i.e., [28]:
- Safely managed: drinking water comes from an improved source that is available when needed and free from fecal and priority chemical contamination;
- Basic: drinking water is sourced from an improved source with a collection time no more than 30 min for a round trip;
- Limited: drinking water is sourced from an improved source with a collection time more than 30 min for a round trip;
- Unimproved: drinking water comes from an unprotected dug well or unprotected spring;
- Surface water: drinking water is sourced directly from a river, dam, lake, pond, stream, canal or irrigation canal.
In 2022, 73% of world population gained safely managed drinking water sources, while around 18% had access to basic drinking water sources. Only 4% had limited drinking water sources, another 4% had unimproved source, and 1% sourced their drinking water directly from surface water [28]. Although it seems that most of the world population has gained sustainable sources of drinking water, this condition can be worsened in the future as shown by water stress level. The condition of the water stress in the world in 2023 is depicted in Figure 1. The water-stressed condition starts when the available water in a country drops below 1700 m3/year/person or 4600 L/day/person. Water scarcity is reached at below 1000 m3/year/person or 2700 L/day/person [29]. There were 25 countries which were categorized as extremely high water-stressed from annually withdrawn water reported, as the higher percentage indicates the higher difficulty of obtaining water.
The most common way to ensure a stable freshwater source is desalination of seawater and runoff waters [30]. Recently, an unconventional yet interesting alternative, namely treated wastewater, has gained attention. Methods for the treatment and reuse of water from grey and industrial wastewater systems have been developed [31,32]. A San Fransisco Bay-based company, Silicon Valley Clean Water, has designed an effective wastewater treatment system that is claimed to be capable of removing 97% of organic materials, solid wastes, and pathogens, and is also beneficial for around 200,000 people in the surrounding area [33]. The benefit of this approach is enjoyed the most in the agriculture area, especially since the treated water has a high nutrient content [34].
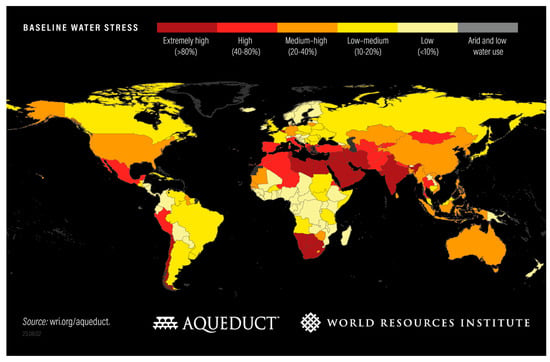
Figure 1.
Water-stressed level area all over the world [35].
Another successful story of reusing wastewater to obtain potable water for a large population is the NEWater project in Singapore. This plant utilized mainly domestic sewage to produce clean industrial and drinking water by a series of treatments, i.e., sedimentation, microfiltration, ultrafiltration, reverse osmosis, and ultraviolet disinfection [36]. This plant can recover around 73.5% of the wastewater feed, with a very high performance of water purification, including 93.4% turbidity removal, higher than 99% desalination rate, and 99.4% total organic carbon separation rate [37]. The produced potable water satisfies Singapore’s and the World Health Organization’s drinking water standards [37], which fulfills around 30% of Singapore’s water demand, and is expected to increase to 55% by 2060 with a capacity of producing 440 million gallons of clean water per day [36]. From a sustainability perspective, assessed by a life cycle assessment (LCA), NEWater potable water has much lower water depletion potential compared to the usual tap water, although produces around twice as much carbon dioxide per m3 water produced as carbon dioxide from conventional tap water production [38].
3. Drinking Water Sources Contamination
In the industrialization era, it is quite common that drinking water sources (groundwater and surface water) become contaminated by human activities. No contamination is usually observed in groundwater from deep and confined aquifers, but groundwater from shallow aquifers are prone to industrial wastes, agricultural chemicals seepages, and sanitation discharges [39] which cause chemical contamination and high bacteriological content in groundwater [40]. Surface water quality is affected by upstream anthropogenic activities, such as animal farming, agriculture, household activities, and industrial discharges, which may create a transboundary issue if a river flows through several countries [40].
In order to preserve the bodies of water, every government has set a regulation of wastewater requirement before being discharged. Although enforcement of the law has been maximized, still some non-compliant industries with wastewater composition exceeding the thresholds can be observed [41]. Rivers in heavily industrialized, irrigated, or populated areas around the world tend to have lower quality which sometimes occur more than half the year or even year-around [42]. In Europe, it has been observed that small receiving water bodies are sometimes not sufficient to dilute the amount of wastewater discharged, thus more than half of the rivers and streams are still in a low ecological status in spite of intense regulatory efforts [43].
Chemical contaminants in wastewater can be divided into two categories, i.e., organic and inorganic pollutants. Organic contaminants can come from various industries, such as agricultural (fertilizers and pesticides), oil and gas (hydrocarbons and phenols), pharmaceuticals [44], and even food industries such as sugarcane mills [45] and dairy [46]. Nowadays, a group of organic pollutants called emerging organic contaminants (EOCs) from pharmaceuticals, personal care products, and pesticides has received the public’s attention because of its harmful effects even in small quantities and its difficulties to be degraded [47]. Some of the common inorganic contaminants in wastewater are heavy metals (e.g., arsenic, chromium, mercury, lead, cadmium, chromium, and cobalt), halides, oxyanions, and radioactive materials. The main contributors for inorganic contaminants are iron and steel industries, thermal and nuclear power plants, mine and quarries, chemical industries, and pulp and paper industries [48]. Freshwater for drinking water also has to be free from biological contaminants, which include parasites, pathogenic bacteria (for example, Escherichia coli, Salmonella typhi, Staphylococcus aureus, Klebsiella sp., Shigella, and Vibrio cholera) [49], and viruses such as hepatitis viruses, rotavirus, astrovirus, and adenovirus [50].
Many countries have developed their own standards for safe drinking water quality. The World Health Organization also established general guidelines for drinking water quality with chemical and biological thresholds [39]. In order to fulfill these standards of drinking water quality, several treatment steps are needed to remove the contaminants from the water sources, especially if wastewater is used as the drinking water source.
4. Nanotechnology Applications for Drinking Water Treatment
Nanotechnology can be implemented in the drinking water treatment and reuse of wastewater as a drinking water source. The utilization of nanomaterials in water and wastewater treatment has attracted much attention, because of their unusual properties [51] and the importance of water as a limited resource [52,53,54]. This strategy is thus suitable for countries with increasing concerns over the rapid deterioration of water quality and access, as well as for countries with increasing demand for cleaner water.
The quality and cleanliness of drinking water, in particular, have become concerns, with arsenic and mercury as two principal toxic (metal) pollutants that cause many serious health problems. Some conventional techniques that are mainly applied for eliminating these heavy metals are adsorption, chemical oxidation, electrochemical and photocatalytic oxidations, chemical coagulation, filtration, ion exchange, reverse osmosis, and bioremediation [51,55]. Nanotechnology serves as an alternative way for those conventional technologies to treat contaminated water for drinking water supply due to several properties as listed in Table 1.

Table 1.
Prospects for engineered nanomaterials in wastewater treatment and reuse [34].
Several techniques are used to manufacture nanomaterials to obtain those properties. In general, the manufacturing approaches can be divided into two categories, namely top-down approach and bottom-up approach [65].
- Top-down approachThis approach is used to convert bulk materials into smaller particles within nanometer sizes to produce nanomaterials. The simplest method of the top-down technique is ball milling, whose principle is applying high energy to mechanically grind powder materials (such as metals and polymers) by frictions of balls inside a rotating drum [66]. Ball milling is suitable for high-capacity production, although it also has some drawbacks such as excessive energy requirements and the possibility of destroying crystal structures during processing [65]. Another method to produce nanomaterials through a top-down approach is by thermal evaporation. In this method, bulk materials are heated to a specific temperature to break chemical bonds [67]. The materials are then evaporated and deposited into a substrate to form a thin layer [65] by various methods, such as electrochemical and sputtering which involves high energy plasma or gas [68]. Laser ablation is a method to generate nanostructures using a pulsed laser to remove molecules from a substrate surface [69]. For producing carbon-based nanomaterials, the arc discharge method can be used. This technique generates high temperature plasma by electricity to allow sublimation of carbon in the cathode. The carbon vapors are subsequently aggregated and deposited onto the anode to form carbon nanostructures [70].
- Bottom-up approachThis approach is the opposite of the top-down approach, where nanomaterials are grown from atoms and molecules to nano-sized particles. The most common technique for the bottom-up method is the sol-gel procedure, where nanomaterial precursors are hydrolyzed to form a colloidal structure with suspended solid particles called sol [67]. The suspended solids are then condensed through an ageing process to form a gel structure, which afterwards can be separated from the liquid by various drying methods, such as thermal, supercritical, or freeze-drying [65]. Another common method to produce nanomaterials by the bottom-up approach is by chemical vapor deposition/CVD. This method utilizes a high temperature to perform heterogeneous chemical reactions of reactant gases on a heated surface. The reactions then form a continuous thin film [71]. The requirement of special apparatus and formation of highly toxic gases as by-products are disadvantages of the CVD method [67]. Frequently used chemical reactions in CVD with an example for each reaction are [72]:Thermal decomposition: SiH4(g) → Si(s) + 2 H2(g)Reduction reaction: 2 BCl3(g) + 3 H2(g) → 2 B(s) + 6 HCl(g)Exchange reaction: SnCl4(g) + O2(g) → SnO2(s) + 2 Cl2(g)Coupled reaction: 2 AlCl3(g) + 3 CO2(g) + 3 H2(g) → Al2O3(s) + 3 CO(g) + 6 HCl(g)The co-precipitation method is another bottom-up technique to manufacture nanomaterials. In this method, a precipitation chemical reaction is performed in a solution with the addition of precipitation agents drop-by-drop, thus producing nano-sized particles. Precipitates can then be aged to obtain bigger particles, and separated from the solution with the centrifugation or filtration process [67]. Nanomaterials can also be synthesized with the hydrothermal or pyrolysis method. The solution precursor is fed into a reactor with a high temperature and high pressure to produce nanomaterials through several chemical reactions [65,67].
5. Available Nanotechnologies for Drinking Water Treatment
Among studies conducted by researchers, nanotechnologies that show the most advanced results in water treatments are (a) nano-adsorbents; (b) nanomembranes; and (c) nano-photocatalysts. The application of nanomaterials as sensors for monitoring water quality is also discussed in this chapter.
5.1. Nano-Adsorbents
The nano-adsorbents method takes advantage of contaminant molecules’ ability to adhere to the surface of nanomaterials. The solids whose surfaces are attracted into are called adsorbents, while the adsorbed material is called adsorbate. Several examples of nano-adsorbents that have been used and studied for water treatment are explained below.
5.1.1. Carbon Nano Tubes
Carbon Nano Tubes or CNTs (as shown in Figure 2) have been deeply studied in the last ten years because of their particular properties in optical, electronic, vibrational, mechanical, and thermal properties [73]. Because of some other exceptional properties, for example a large surface area, uncomplicated chemical or physical modification, and capability of removing organic and also inorganic pollutants, carbon-based nanomaterials have some potencies as alternatives for water treatment [74]. Chemical modification was reportedly performed by adding functional groups of –COOH, –NH2, or –OH on the surface of carbon nanotubes to improve adsorption capacity.
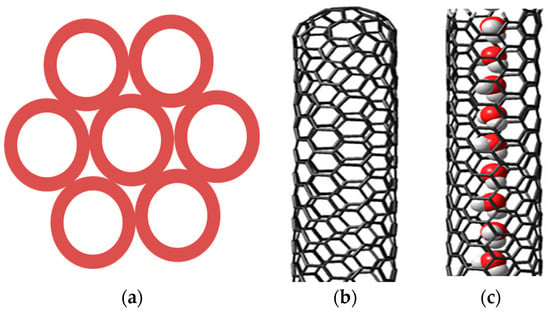
Figure 2.
(a) A bundle of open-ended single-walled carbon nanotubes (SWNTs); (b) a sealed and empty single-walled carbon nanotube; and (c) a nanotube filled with water molecules [75].
CNTs exhibit antimicrobial properties due to oxidative stress in bacteria, causing the cell membranes to disrupt. There are no toxic side products produced although chemical oxidation may happen, thus making it advantageous for the disinfection of water [76]. Adsorption-based technology has a potential for point-of-use water treatment such as desalination. Nevertheless, the capacity for salt adsorption is limited. For this challenge, plasma-treated ultralong CNTs with an ultrahigh specific adsorption capacity has been developed successfully [77].
CNTs have proved their specialty and effectiveness in removing contaminants in water and reusability in some applications at large municipal water and desalination plants. Plata et al. [78] from Duke University stated that using high-purity carbon nanotubes will lead to a 15-fold yield improvement, a 50 percent reduction in energy costs, and an order of magnitude reduction in the volume of hazardous byproduct formation. Table 2 shows some applications of CNT materials to adsorb various contaminants in water.

Table 2.
Adsorption performances of CNT-based nanomaterials for various pollutants.
5.1.2. Graphene Oxide
Graphene oxide (GO) is a carbon-based nanomaterial that has an effective ability in removing heavy metals from the wastewater. It is produced from the oxidation of graphene and contains various oxygen groups such as hydroxyl, carboxyl, epoxide, and carbonyl functional groups. It was reported that fluoride ions can be captured with a capacity of 35.6 mg/g at pH 7.0 and 25 °C [89]. Another application of GO is to adsorb the presence of humic acid in an aqueous solution with a capacity of 190 mg/g by following the Langmuir isotherm. Gao et al. [90] also reported the use of GO for the adsorption of some types of antibiotics, i.e., tetracycline, oxytetracycline, and doxycycline, with estimated maximum adsorption capacities of 313, 212, and 398 mg/g for the three substances, respectively. Table 3 presents some GO materials that have been investigated.

Table 3.
Graphene Oxide Materials and Adsorption Performances.
5.1.3. Polymeric Nano-Adsorbents
Polymeric nano-adsorbents are repetitively branched molecules with the capabilities of removing organics and heavy metal. It consists of the interior part of hydrophobic shells to absorb organic compounds and the outer part which can be modified to adsorb specific heavy metals. Another type of polymeric nano-adsorbent is Molecular Imprinted Polymers (MIPs) with a rebinding mechanism to effectively absorb dye. Polymeric matrix β-cyclodextrin and chitosan were imprinted and tested to have negative Gibbs energy. The process is endothermic with a high preference for dye molecules [107]. Biodegradable, biocompatible, and nontoxic bio-adsorbents such as chitosan are preferred in the future. A chitosan and hydroxyapatite nano-composite has been studied to adsorb norfloxacin, a type of antibiotic, from municipal wastewater and showed a good performance with a maximum adsorption capacity of 625 mg/g [108]. A study with nano-adsorbent created from polypyrrole-polyethyleneimine for Pb2+ ion elimination from wastewater also showed good results with a maximum adsorption capacity of 75.60 mg/g [109].
5.1.4. Nano-Silicate
Silica-based nanomaterials, as shown in Figure 3, have nontoxic and superior surface properties. They are commonly found in sand. The size of nano-silicate materials is 10–20 nm and it has a specific surface area of 60–600 m2/g. It is effective in removing heavy metals. Nano-silicate could be modified by adding functional groups such as -NH2, -SH, or serve as the support for other nanocomposite materials. The addition of silica to polymeric membranes could increase the hydrophilicity of the membrane surface and reduce fouling. Nano-silicate can also be added to titania in photocatalytic agents to enhance activity or red-shift for energy saving [110]. An experimental study was performed to investigate the efficiency of using nano-silicate coupled with herbal additives such as turmeric, cinnamon, and saffron to treat the water in the swimming pool and resulted in a better quality of water [111]. Other studies regarding the application of nano-silicate for dyes and heavy metals removal from water are displayed in Table 4.
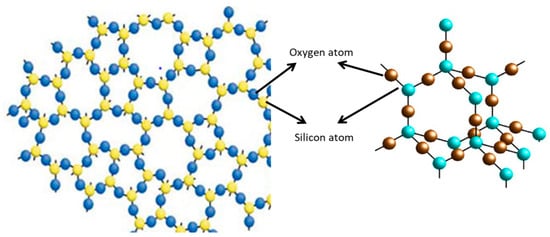
Figure 3.
Nano-silicate atoms and molecules of SiO2.

Table 4.
Application of nano-silicate for dyes and contaminants removal.
5.1.5. Other Nano-Adsorbents
Nano-silver and nano-titanium oxides have adsorbent properties to remove heavy metal and radionuclides contaminants. They have high specific surface areas, feature a short intraparticle diffusion distance, and are compressible without a significant reduction of surface area [24]. They have bactericidal and nontoxic properties although they have low durability, leading to high maintenance costs. They also act as anti-biofouling agents on the surfaces [24]. TiO2 needs to be activated by ultraviolet lamps while nano-silver does not need external energy to be activated.
Silver nanoparticles exhibited reactivity in reducing mercury with the removal capacity reaching 800 mg/g [118]. Gold nanoparticles could be used for removing mercury in the wastewater up to 4.065 g/g, with easy recovery when supported on aluminum [119,120]. Other frequent materials used as adsorbents are oxide of iron (Fe), manganese (Mn), silicon (Si), and tungsten (W). Metal oxides are low-cost materials and easily accommodated various needs. Iron-based metal oxides also have superparamagnetic properties that promote easy separation. Fe-La composite oxide with a size of 20–200 nm effectiveness to remove As(III) was investigated by Zhang et al. [121]. It showed a rapid kinetic process by achieving 80% of the adsorption capacity equilibrium in 240 min with a maximum adsorption capacity of 58.2 mg/g In neutral acidity. Tungsten oxide also shows efficient adsorption of organic dyes in water [122].
The presence of lead, copper, and arsenic in the water is hazardous and could lead to various diseases. Anatase nano-adsorbent has the capability to remove all of these contaminants. It was produced by a sol-gel technique followed by calcination at 400 °C. The amount of contaminant adsorbed was constant along with the dynamic change in pH. The sorption kinetic trend corresponded to the pseudo-second-order model. The optimum capacities were reached at 31.25 mg/g, 23.74 mg/g, and 16.98 mg/g for Pb(II), Cu(II), and As(III), respectively [123].
Nano zero-valent iron (nZVI) in suspension also displayed a beneficial capacity in water remediation. Zero-valent metal nanoparticles are highly reactive reducing agents used in groundwater treatment contaminated by hydrocarbons or heavy metal [73,124,125,126]. In recent years, various zero-valent nanoparticles have been extensively investigated; for example, nano-sized zero-valent zinc was observed to have effectively removed dioxin contaminants [127]. Zero-valent iron has received significant attention for its performance in the removal of heavy metals such as Hg(II), Cr(VI), Cu(II), Ni(II), Cd(II), etc. [128,129,130]. The nanoparticles consisting of zero-valent iron coated by ferric oxide have been proven to be able to remove polychlorinated biphenyls, chlorinated solvents, and heavy metals [131]. All of these nano-adsorbent applications for heavy metal and dye contaminants are listed in Table 5.

Table 5.
Adsorption Capacity and Performance of Various Nanomaterial Adsorbents.
5.2. Nanomembranes
Nanomembranes are very thin selective permeable membranes, with less than 100 nanometers in thickness, that utilize the filtration principle to remove contaminants present in the water [184]. It is a pressure-driven process to separate particles less than 0.5–1 nm in size [24]. The desired properties of nanomembranes are molecular sieve, hydrophilicity, antimicrobial, nontoxic materials, self-cleaning, high mechanical, and chemical stability [185]. The critical point of nanomembranes is the membrane material, whose types are nanocomposite, nanofiber, self-assembling, and aquaporin-based membranes.
5.2.1. Nanocomposite Membranes
Nanocomposite membranes are nanomembranes prepared by adding nanoparticle materials as filler into macroscopic membrane matrix materials [186]. Metal oxide materials are widely used as the filling materials for nanocomposite membranes; for example Al2O3, TiO2, and zeolite. The usage of metal oxide is purposely to supply hydrophilicity and reduce fouling. To add antimicrobial properties, nano-silver and CNTs are commonly used. Alumina [187], silica [188], zeolite [189], and TiO2 [190] addition to polymeric ultrafiltration membranes enhance the material hydrophilicity, water permeability, or fouling resistance.
Nano-zeolite is the most commonly used dopant in thin film nanocomposite (TFN) membranes. It was found to increase the membrane permeability, negative charge, and thickness of the polyamide active layer [191]. A novel-type TFN was developed by incorporating nanomaterials into the active layer via doping in casting solutions or surface modification. The addition of ordered mesoporous carbons as nanofillers into TFN membranes created semipermeable membranes with a selective layer on the upper surface that is usually applied for reverse osmosis [192].
CNT-embedded membranes were fabricated by depositing CNTs onto the ceramic membrane through a chemical vapor. The integrated ceramic and CNTs displayed complete rejection of oil with a flux rate of 36 L/(h.m2.bar) and also showed resistance of organic fouling [193]. Nano-silver grafted to polymeric membranes was proven to prevent biofouling due to the antimicrobial nature in nano-silver [194,195]. The combination of TiO2 nanoparticles into a thin-film composite layer led to the increasing resistance of salts while maintaining permeability. The addition of aminosilanized TiO2 nanoparticles into the polyamide skin layer was able to reduce surface energy, thus improving NaCl resistance to 54% [196].
A novel thin-film S-layer protein applied on a metallic micro-sieve using the layer-to-layer process was being developed by Gehrke in 2014 [197] with high selectivity of heavy metals removal. There are vast opportunities to advance the membrane using bio nanocomposite materials such as polylactic acid, cellulose, acetate, etc. The use of natural polymer will allow the materials to degrade without endangering the environment.
5.2.2. Nanofiber Membranes
Nanofiber membranes are manufactured from fibrous materials within 1 to 100 nm diameter [198]. Ultrafine fibers for nanofiber membranes are synthesized by electrospinning polymers, ceramics, or metals [199]. It is adequate to remove micro-sized particles without noticeable fouling [200]. Distinct nanomaterials can be doped effortlessly into the spinning solutions to impregnate the nanofiber [201]. An example of a nanofiber membrane commercial product is Nanoceram©, a small diameter fiber with a high surface area (300–600 m2/g). A study investigated the addition of 10, 20, and 40% of tetramethyl orthosilicate to polyvinylidene fluoride membrane via electrospinning and thermal treatment. The membranes showed enhanced mechanical properties and hydrophobicity, and effectively separated organic solvents with high flux efficiency [202]. Further work is needed to develop composite nanofiber membranes and bio-nanofiber membranes with higher porosity, higher permeate efficiency, and the ability to disinfect.
5.2.3. Self-Assembling Membranes
The self-assembling membrane, also known as the block co-polymer-based membrane, is a membrane produced by a technique that utilizes different properties of the functional parts (blocks) of the monomer. The main block of the co-polymer serves as the matrix, while the other blocks will form the membrane’s pores with a homogenous size [203]. This method produces high pore density which induces high water permeability at around 1000–3000 L/(m2·h·bar) with molecular weight cut-off at around 70 kDa [204]. High-density cylindrical nanopores are formed to fit the micro/nanofluidic devices [197].
A study to produce a selective membrane for Pd(II) recovery from electroplating wastewater was performed by Ma et al. [205]. By assembling a triblock co-polymer namely poly(4-vinylpyridine)-b-polysulfone-b-poly (4-vinylpyridine), the researchers successfully created a membrane with excellent Pd(II) rejection from the wastewater, reaching up to 96.8%. The 4-vinylpyridine block of the co-polymers enhances hydrophilicity of the membrane with high water flux. It also provides high adsorption capacity for the Pd(II) ions at around 103 mg/g. The aforementioned membrane showed high selectivity towards Pd(II) compared to other cations in the wastewater, e.g., Cu(II) and Ni(II).
5.2.4. Aquaporin-Based Membranes
Aquaporin membranes are membranes with a protein channel that allows water flux across the cell membranes. They are also known as biologically inspired membranes. The high selectivity and water permeability properties have made it an interesting approach to improve polymeric membranes. It is used in low-pressure desalination although the drawback would lie in the durability of the membranes. Aquaporin-Z made from Escherichia coli impregnated into amphiphilic tri-block polymer vesicles showed the full rejection of glucose, glycerol, salt, and urea [206]. The lipid bilayer has also been used in commercial nanofiltration membranes with certain capabilities. The first commercial membrane incorporated with aquaporins is Aquaporin Inside from Denmark which could withstand pressure up to 10 bar and a water flux rate up to 100 L/(h·m2). Further study to improve the stabilization process of surface imprinting and polymer embedding is necessary in order to be applied in larger industries.
5.3. Nano-Photocatalysts
Photocatalytic oxidation is a process to remove trace contaminants and microbial pathogens from water by oxidation with the aid of light energy and catalysts. It can be used as a pretreatment method in promoting biodegradability for hazardous and non-biodegradable contaminants and a polishing step for recalcitrant organic compounds [207,208]. Due to its high availability, low toxicity, cost efficiency, and well-known material properties, nano-sized TiO2 is frequently utilized as a photocatalyst. TiO2 is irradiated by ultraviolet light with a wavelength in the range of 200–400 nm, which will cause electrons to be excited and move into the conduction band as result. Due to photonic excitation, electron-hole (e− and h+) pairs are created, leading to an oxidative-reductive reaction chain [24]. The example of chain reactions of organic pollutants using photocatalysts are [209]:
Energy + Photocatalyst → e− + h+
e− + O2 →HO2• → H+ + O2−•
h+ + H2O → HO• + H+
O2-• + H+ → HOO•
HOO• + HOO• →H2O2 + O2
e− + H2O2 → HO• + OH−
O2−• + H2O2 → HO• + OH− + O2
h+ + OH− →HO•
HO• + pollutants → H2O + CO2
HOO• + pollutants → H2O + CO2
With this oxidation-reduction reaction chain, nano-photocatalysts can degrade various contaminants in water, including heavy metals, organic pollutants, bacteria, and viruses [210]. This property is proven by an experiment using a nano-photocatalyst produced from composites of K4Nb6O17 nanosheets with g-C3N4, iron nitride (Fe3N), and Fe2O3 which was used to degrade a pesticide compound (acetamiprid) and U87-MG cancer cells. This nano-photocatalyst efficiently degrades acetamiprid with 76% removal even after being used for five repeated cycles. It also had sufficient efficacy to eradicate the cancer cells under visible light [211]. Table 6 summarizes some other research utilizing nanomaterials as photocatalysts to degrade pollutants from wastewater.

Table 6.
Photocatalyst nanomaterial performances on pollutant degradation from wastewater.
5.4. Nanomaterials for Water Quality Sensors
The monitoring of water quality is challenging due to the low concentration of pollutants, as well as the variability and complexity of the matrix. Nanotechnology-enabled sensors are promising tools for widespread and inexpensive monitoring of drinking water pollutants [219]. A nano-sensor consists of (1) a nanomaterial, (2) a recognition element, and (3) a mechanism for signal transduction. Sensors respond to physical stimuli induced by chemical and biological substances and convert them into electronic signals that are conveyed to the reading devices [51]. The specificity is achieved either by detecting an intrinsic signal from the analyte or by employing highly specific recognition elements that ideally bind only to a given target. The use of nanomaterials in sensors increases the surface zones, detection limit, speed, and overall sensitivity, thanks to the big surface-to-volume ratio.
Since the promising reports of the use of a nanowire [220] and quantum dot [221], nanotechnology-enabled sensing has gained increasing attention from the scientific community. However, as suffered by many nanotechnology products, only a few sensor devices can be found in the market [219]. Some examples of utilizations of nanotechnology-enabled water quality sensors are presented in Table 7.

Table 7.
Documented utilizations of nanomaterial for water quality sensor [219].
6. Advantages and Disadvantages of Nanotechnologies for Drinking Water Treatment
The previous chapter has summarized the benefits of several nanotechnologies for contaminants removal to produce drinking water. Despite the high performance of those technologies, there are still some disadvantages that prevent nanotechnologies to be used commercially. The advantages and disadvantages of the nanotechnologies in comparison with conventional water treatment technologies are listed in Table 8.

Table 8.
Advantages and disadvantages of each nanotechnology reviewed in this publication [208,222,223,224,225].
7. Initial Sustainability Analysis on Nanotechnology
Nanotechnology is still in the research and development phase and still far from maturity. Nonetheless, nanotechnology started to gain the trust of the public as a potential technology to support the sustainability of human systems. In order to provide civilization with the nanotechnology for sustainable drinking water, a sustainability analysis must be performed with the adequacy of data. A life cycle assessment (LCA) of nanotechnology has been conducted by many authors and researchers to evaluate its sustainability. In spite of many various analyses, there are still some challenges in general for comparing the result with equal criteria. Following the model of sustainability, the evaluation of sustainability is down to three domains: economy, environment, and society (Figure 4).
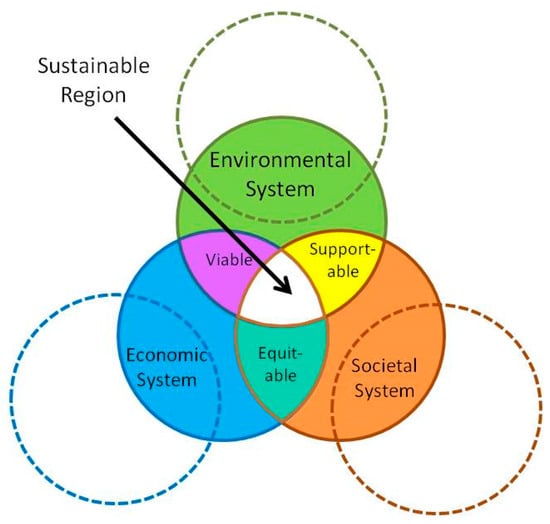
Figure 4.
Generally accepted sustainability model (modified with permission from [226]).
Mata et al. [227] has formulated some indicators to analyze the sustainability and some future challenges of the nanotechnology application in daily life. The application of those indicators in evaluating CNTs as a model for nanotechnology application in water treatment is shown in Table 9.

Table 9.
Initial sustainability analysis of nanotechnology application in water treatment [227].
By looking through the indicators, the lack of information needed to evaluate the system will become clear. There are a lot of examples that when a technology is made to please society, there is less effort in addressing its hazard meticulously including its sustainability. Therefore, by learning from mistakes, the approach for making efficient progress and appropriate nanomaterials for human wealth has to be conducted in a holistic way supported by adequate data and universal guidelines.
8. The Challenges
The main challenge in nanotechnology applications is the fact that very few nanomaterials have been produced commercially, despite many reports showing how promising they are [34]. The industrialization of nanotechnology applications in water treatment so far is limited by the high production cost [25]. It is common that new technologies face the reluctance of investors in funding the research and production of new products. Moreover, there is also a lack of government guidance and support (for example, regulations) for the implementation.
An example of challenges faced by nanotechnologies is reviewed by Lens [228] for the commercial viability of nanomembranes in Figure 5. It shows that nowadays, there are no types of nanomembrane with high potential performance enhancement which possess potential commercial viability due to high material costs and difficult manufacturing scalability. However, there might be changes in the future when biologically inspired membranes reach maturity, thus providing nanomembranes in the optimal (upper right) quadrant.
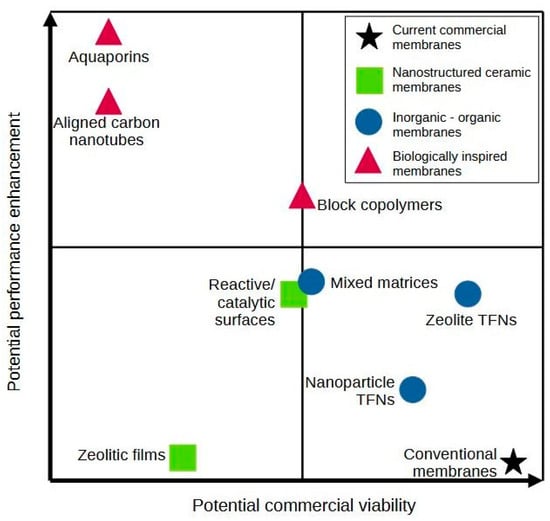
Figure 5.
Comparison of potential performance and commercial viability of various nanomembranes and conventional membranes technology [228].
8.1. Safety and Environmental Risks
Some questions remain regarding the safety and potential toxicity of nanomaterial utilization, as well as their life cycle in the environment, not only in the environmental remediation industry but additionally in daily life applications [25]. The knowledge gaps that must be addressed and clarified before wide application in the industry are still wide.
The main issue for the environmental risks is the useful properties of the nanomaterials themselves, i.e., small size, shape, reactivity, conductivity, and mobility. These properties make it easier for nanomaterials to be inhaled, ingested, or up-taken. A small size can be a tricky property when it comes to detection [51]. Small sizes make the deposit rate of nanomaterials in the air very low, while high mobility favors rapid mixture and results in higher spreading. They can enter the body via various routes [229]. Nanoparticles in the air may present the risk of photochemical conversion when exposed to the ultraviolet wavelength of daylight [230].
According to the European Chemicals Agency’s (ECHA) guidance on the registration, substances like nanomaterials can be differentiated into those that exist only in nano-forms and those that also exist in bulk (non-nano) forms. The various forms create further problems in detection and toxicity evaluations. Safety data generated for one form might not be adequate for the other forms. Moreover, differentiation between the forms or types of nanoparticles itself can be a difficult task. Studies using pure nanoparticles cannot be reliable for assessing the used particles [231]. Most toxicological studies were carried out by using laboratory culture media composed of proteins and other biological compounds. The results cannot be directly interpreted as representing real environmental conditions.
Studies on nanomaterials concentration in drinking water are still very few. Most studies discussed air-borne nanoparticles and their inhalation by living beings. The exposure of aquatic and terrestrial life to nanoparticles in water and soil is still not explored enough [231]. However, several early studies have indicated that some nanomaterials caused cellular and membrane damage to different aquatic organisms [232]. Engineered nanoparticles (ENPs) are far less commonly found than natural nanoparticles (NNPs) in drinking water. Nanoparticles could also come from corrosion of distribution pipes or in-home premise plumbing. They are incidentally released into the drinking water; thus, they are also referred to in the study as incidental nanoparticles (INPs). The three categories have similar elemental compositions or geometries [233,234]. Shape or composition is therefore not useful for the classification [235,236].
While the toxic effects have been widely studied for aquatic organisms and mammalian cells, they are still poorly understood when it comes to humans and other animals [25]. Environmental suitability of the materials with regards to the diversity of ecosystems should be the focus of research [237].
In general, the major preoccupation problems are as follows [238]:
- Limited public knowledge about the ecotoxicity and increasing exposure to nanomaterials.
- Absence of information regarding the risks of consumption of each nanomaterial.
- Insufficient regulatory controls that guarantee protection improvement and exploitation of nanomaterials.
- The hesitation and lack of commitment of the producers in communicating information about the risks to the customers.
To obtain a detailed risk assessment, characterization, and management, there are two important actions: hazard assessment and exposure assessment [239,240]. Some known environmental hazards of nanomaterials are listed in Table 10. In the laboratory, safe practices of handling nanomaterials can be ensured by using three different control approaches [229]:

Table 10.
Some known environmental effects of nanomaterials [51].
- Engineering control: ventilation, use of less toxic materials, specially designated storage cabinet.
- Administrative control: warning notices, chemical hazard labels.
- Personal protective equipment.
8.2. Regulations
The regulations about the use of nanomaterials are surprisingly still limited, although their industrialization has been running for years. The production and use of nanomaterials in the European Union (EU), for example, is regulated under Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) [241], which is the most comprehensive legislative provision for chemicals in the region [242]. Some EU countries, such as Denmark, France, Sweden, and Belgium, also have their regulations and schemes for nanotechnology-related products [242]. In the USA, the manufacturing of nanomaterials falls within the regulation of the Toxic Substances Control Act of the Environmental Protection Agency (EPA). Organization for Economic Cooperation and Development (OECD) via its Working Party on Manufactured Nanomaterials (WPMN) pays special attention to human health and environmental safety aspects of nanomaterials. It aims to ensure the safe use of nanotechnology through appropriate methods and strategies [243]. In the future, regulations regarding nanomaterials are needed with hazard studies as the basis for constructing the laws.
8.3. Synthesizing Cost
Manufacturing the cost of nanomaterials still pose a huge challenge for the wide application of nanotechnologies. GadelHak et al. [244] has compared several nano-adsorbents prices with conventional adsorbents prices, and found that generally nano-adsorbents cost higher than conventional ones. For example, the price of TiO2 nanoparticles vary from 10,000 to 16,000 USD/ton; the price of ZnO nanoparticles is around 20,000–285,000 USD/ton, the price of CNTs can range from 50,000 to 120 million USD/ton; the nZVI price is around 4.2 million USD/ton; and nano-silver prices can reach 37.5 million USD/ton. In comparison, commercial activated carbon that is commonly used for conventional water contaminants adsorption process costs around 500–395,000 USD/ton.
From an operating cost perspective, nanotechnologies sometimes can offer a cheaper option to conventional methods. Nano-TiO2 particles activated by UV light for treating textile wastewater was estimated to cost lower than the oxidation process with H2O2 or FeSO4, where nano-photocatalysis using nano-TiO2 cost around 0.77 USD/m3 of wastewater, while oxidation using H2O2 and FeSO4 cost 1.62 and 2.00 USD/m3, respectively [245]. Another study of textile wastewater treatment using nano-bimetallic iron/copper estimated a cost of 6.37 USD/m3, which is higher than treating using electrocoagulation and the ozonation process (5.8 USD/m3). However, a cost reduction is still possible if the iron/copper nano-adsorbents can be reused [246].
A suggested way to reduce the manufacturing cost of nanomaterials is by applying green nanoscience. This method uses environmentally friendly materials to produce nanomaterials so it is expected to reduce the overall cost of the treatment process and give a lower environmental footprint [34]. The lower overall production costs are certainly attractive for developing countries. However, green nanoscience often suffers from a lack of guiding scientific principles in the initial exploration stage. Moreover, there are only very few reported data that support the promising claims of green chemistry, especially the lower energy and material consumptions [231]. It also faces stricter regulatory obstacles compared to the conventional ones [247].
8.4. Other Challenges
The education of nanotechnology is an important area that is often overlooked. This may cause the problems of a lack of capable workers, which will decrease the progress of science and technology developments [248]. There is a growing need to prepare future generations, not only scientists, engineers, and technicians, but also policymakers, communicators, and regulators with good knowledge about the field [249]. The latter groups play a very important role in the commercialization of nanotechnology. The lack of availability is one of the current key factors that stall the dissemination of the developed technology.
9. Conclusions
The water scarcity problem has emerged and worsened in several areas in the past years and continues to threaten most of the human population in other areas. As all living beings’ lives are dependent on freshwater availability, technologies for achieving sustainable water resources management to solve the water scarcity problem need to be prioritized. Nanotechnology offers an effective alternative way to reuse and treat contaminated wastewater caused by human activities. Due to its small size, nanomaterials provide several advantages compared to conventional water treatment techniques, i.e., higher efficiency as a result of higher surface area, improved catalytic performance, and several additional properties such as antimicrobial, high conductivity, and self-assembly on surfaces. Nanotechnology effectiveness for treating contaminants in water, including emerging contaminants, has been studied in the past decades by many researchers, though only very few large-scale applications have been applied. At the moment, there is inadequate information to assess all aspects of sustainability for nanotechnology applications in water treatment; thus, this type of study is needed in the near future to accelerate the application of nanotechnology. Regulations are confirmed to be one of the current challenges for nanotechnology implementation in water resources management, since a comprehensive database for regulatory purposes is still limited. In the future, good collaboration between academia, business, and authorities is needed to accelerate the implementation of nanotechnology for water resources management so its benefit can be utilized for the benefit of mankind.
Author Contributions
Conceptualization, T.P.A.; methodology, A.I.; writing—original draft preparation, G.A.S. and A.M.; writing—review and editing, D.P., W.H.M.W.M. and I.K.; supervision, T.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations. Blueprint for Acceleration: Sustainable Development Goal 6 Synthesis Report on Water and Sanitation 2023; United Nations: New York, MY, USA, 2023; ISBN 9789210026444. [Google Scholar]
- du Plessis, A. Current and Future Water Scarcity and Stress. In Water as an Inescapable Risk; Springer Water; Springer: Cham, Switzerland, 2019; pp. 13–25. ISBN 978-3-030-03186-2. [Google Scholar]
- Wada, Y.; Bierkens, M.F.P. Sustainability of Global Water Use: Past Reconstruction and Future Projections. Environ. Res. Lett. 2014, 9, 104003. [Google Scholar] [CrossRef]
- Biswas, A.K. Water Availability and Use. In Water Resources of North America; Springer: Cham, Switzerland, 2003; pp. 163–174. [Google Scholar] [CrossRef]
- United Nations. Coping with Water Scarcity: Challenge of the Twenty-First Century; United Nations: New York, MY, USA, 2007; Volume 24. [Google Scholar]
- Corcoran, E.; Nellemann, C.; Baker, E.; Bos, R.; Osborn, D.; Savelli, H. Sick Water? The Central Role of Wastewater Management in Sustainable Development: A Rapid Response Assessment; United Nations Environment Programme: Nairobi, Kenya, 2010; Volume 30, ISBN 9788277010755. [Google Scholar]
- Ren, T.; Yuyan, J.; Huan, L.; Yingxin, P.; Dongmei, T.; Qiumei, D.; Zhong, Y. Investigation on an Outbreak of Bacillary Dysentery Due to Infection of Shigella Sonnei in a Town of Guangxi Province. Dialogues Health 2023, 2, 100072. [Google Scholar] [CrossRef]
- Navarro-Espinoza, S.; Angulo-Molina, A.; Meza-Figueroa, D.; López-Cervantes, G.; Meza-Montenegro, M.; Armienta, A.; Soto-Puebla, D.; Silva-Campa, E.; Burgara-Estrella, A.; Álvarez-Bajo, O.; et al. Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model. Int. J. Environ. Res. Public Health 2021, 18, 805. [Google Scholar] [CrossRef]
- Mesfin, G.; Schluter, W.; Gebremariam, A.; Benti, D.; Bedada, T.; Beyene, B.; Yigzaw, A.; Taddess, Z.; Mbakuliyemo, N.; Babaniyi, O. Polio Outbreak Response in Ethiopia. East Afr. Med. J. 2008, 85, 222–231. [Google Scholar] [CrossRef][Green Version]
- Garn, J.V.; Boisson, S.; Willis, R.; Bakhtiari, A.; Al-Khatib, T.; Amer, K.; Batcho, W.; Courtright, P.; Dejene, M.; Goepogui, A.; et al. Sanitation and Water Supply Coverage Thresholds Associated with Active Trachoma: Modeling Cross-Sectional Data from 13 Countries. PLoS Negl. Trop. Dis. 2018, 12, e0006110. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Kazmi, S.U. Investigation of a Community Outbreak of Typhoid Fever Associated with Drinking Water. BMC Public Health 2009, 9, 476. [Google Scholar] [CrossRef]
- Kulinkina, A.V.; Kosinski, K.C.; Plummer, J.D.; Durant, J.L.; Bosompem, K.M.; Adjei, M.N.; Griffiths, J.K.; Gute, D.M.; Naumova, E.N. Indicators of Improved Water Access in the Context of Schistosomiasis Transmission in Rural Eastern Region, Ghana. Sci. Total Environ. 2017, 579, 1745–1755. [Google Scholar] [CrossRef]
- Von Nguyen, D.; Sreenivasan, N.; Lam, E.; Ayers, T.; Kargbo, D.; Dafae, F.; Jambai, A.; Alemu, W.; Kamara, A.; Islam, M.S.; et al. Cholera Epidemic Associated with Consumption of Unsafe Drinking Water and Street-Vended Water—Eastern Freetown, Sierra Leone, 2012. Am. J. Trop. Med. Hyg. 2014, 90, 518. [Google Scholar] [CrossRef]
- Santucci, R.J.; Scully, J.R. The Pervasive Threat of Lead (Pb) in Drinking Water: Unmasking and Pursuing Scientific Factors That Govern Lead Release. Proc. Natl. Acad. Sci. USA 2020, 117, 23211–23218. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, A.K. The Legacy of Woburn, Massachusetts and Trichloroethylene; University of Idaho: Moscow, ID, USA, 2000; pp. 1–23. [Google Scholar]
- Sauer, M.; Smith, S.; Clemens, B. Does It Pay to Invest in Potable Water in the Developing World? Relationships Between External Financing and Economic Development in Sustainable Community-Run Integrated Projects. J. Int. Dev. 2016, 28, 233–242. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund. State of the World’s Drinking Water: An Urgent Call to Action to Accelerate Progress on Ensuring Safe Drinking Water for All; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789280652901. [Google Scholar]
- Bhaduri, A.; Bogardi, J.; Siddiqi, A.; Voigt, H.; Vörösmarty, C.; Pahl-Wostl, C.; Bunn, S.E.; Shrivastava, P.; Lawford, R.; Foster, S.; et al. Achieving Sustainable Development Goals from a Water Perspective. Front. Environ. Sci. 2016, 4, 182047. [Google Scholar] [CrossRef]
- Denchak, M. Water Pollution Facts, Types, Causes and Effects of Water Pollution|NRDC. Available online: https://www.nrdc.org/stories/water-pollution-everything-you-need-know#categories (accessed on 28 October 2020).
- Koda, E.; Miszkowska, A.; Sieczka, A. Levels of Organic Pollution Indicators in Groundwater at the Old Landfill and Waste Management Site. Appl. Sci. 2017, 7, 638. [Google Scholar] [CrossRef]
- Schaider, L.A.; Ackerman, J.M.; Rudel, R.A. Septic Systems as Sources of Organic Wastewater Compounds in Domestic Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ. 2016, 547, 470–481. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, Y.; Guo, X.; Lu, S. Analysis of Point Source Pollution and Water Environmental Quality Variation Trends in the Nansi Lake Basin from 2002 to 2012. Environ. Sci. Pollut. Res. 2016, 23, 4886–4897. [Google Scholar] [CrossRef]
- Lyons, Y. Transboundary Pollution from Offshore Activities: A Study of the Montara Offshore Oil Spill. In Transboundary Pollution: Evolving Issues of International Law and Policy; Edward Elgar Publishing Ltd.: Northampton, MA, USA, 2015; pp. 162–189. ISBN 9781784715793. [Google Scholar]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in Nanotechnology for Water Treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Dermatas, D.; Mpouras, T.; Panagiotakis, I. Application of Nanotechnology for Waste Management: Challenges and Limitations. Waste Manag. Res. 2018, 36, 197–199. [Google Scholar] [CrossRef]
- Davarazar, M.; Kamali, M.; Lopes, I. Engineered Nanomaterials for (Waste)Water Treatment—A Scientometric Assessment and Sustainability Aspects. NanoImpact 2021, 22, 100316. [Google Scholar] [CrossRef]
- Schmoll, O. Protecting Groundwater for Health: Managing the Quality of Drinking-Water Sources. In Water Intelligence Online; World Health Organization: Geneva, Switzerland, 2013; Volume 12, p. 678. [Google Scholar] [CrossRef]
- WHO; UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene; World Health Organization: Geneva, Switzerland, 2023; ISBN 9789240030848. [Google Scholar]
- FAO. Coping with Water Scarcity An Action Framework for Agriculture and Food Security; Food and Agriculture Organization: Rome, Italy, 2012; Volume 79. [Google Scholar]
- Darre, N.C.; Toor, G.S. Desalination of Water: A Review. Curr. Pollut. Reports 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Birks, R.; Hills, S. Characterisation of Indicator Organisms and Pathogens in Domestic Greywater for Recycling. Environ. Monit. Assess. 2007, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater Reuse and Risk: Definition of Key Objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Silicon Valley Clean Water Silicon Valley Clean Water Facilities—Wastewater Treatment. Available online: https://svcw.org/what-we-do/facilities/wastewater-treatment/ (accessed on 21 October 2020).
- Madhura, L.; Singh, S.; Kanchi, S.; Sabela, M.; Bisetty, K. Inamuddin Nanotechnology-Based Water Quality Management for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 65–121. [Google Scholar] [CrossRef]
- Kuzma, S.; Saccoccia, L.; Chertock, M. 25 Countries Face Extremely High Water Stress|World Resources Institute. Available online: https://www.wri.org/insights/highest-water-stressed-countries (accessed on 14 April 2024).
- Lefebvre, O. Beyond NEWater: An Insight into Singapore’s Water Reuse Prospects. Curr. Opin. Environ. Sci. Health 2018, 2, 26–31. [Google Scholar] [CrossRef]
- Bai, Y.; Shan, F.; Zhu, Y.Y.; Xu, J.Y.; Wu, Y.S.; Luo, X.G.; Wu, Y.H.; Hu, H.Y.; Zhang, B.L. Long-Term Performance and Economic Evaluation of Full-Scale MF and RO Process—A Case Study of the Changi NEWater Project Phase 2 in Singapore. Water Cycle 2020, 1, 128–135. [Google Scholar] [CrossRef]
- Hsien, C.; Choong Low, J.S.; Chan Fuchen, S.; Han, T.W. Life Cycle Assessment of Water Supply in Singapore—A Water-Scarce Urban City with Multiple Water Sources. Resour. Conserv. Recycl. 2019, 151, 104476. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Houtman, C.J. Emerging Contaminants in Surface Waters and Their Relevance for the Production of Drinking Water in Europe. J. Integr. Environ. Sci. 2010, 7, 271–295. [Google Scholar] [CrossRef]
- Nguyen, M.D. Compliance of Paper-Making Plants with Regulations on Wastewater Management in Bac Ninh Province, Vietnam. Environ. Dev. Sustain. 2011, 13, 35–50. [Google Scholar] [CrossRef]
- Jones, E.R.; Bierkens, M.F.P.; Wanders, N.; Sutanudjaja, E.H.; van Beek, L.P.H.; van Vliet, M.T.H. Current Wastewater Treatment Targets Are Insufficient to Protect Surface Water Quality. Commun. Earth Environ. 2022, 3, 221. [Google Scholar] [CrossRef]
- Büttner, O.; Jawitz, J.W.; Birk, S.; Borchardt, D. Why Wastewater Treatment Fails to Protect Stream Ecosystems in Europe. Water Res. 2022, 217, 118382. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J.; Govarthanan, M. Bioremediation of Organic Pollutants: A Mini Review on Current and Critical Strategies for Wastewater Treatment. Arch. Microbiol. 2022, 204, 286. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane Biorefineries Wastewater: Bioremediation Technologies for Environmental Sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef]
- Kasmi, M. Biological Processes as Promoting Way for Both Treatment and Valorization of Dairy Industry Effluents. Waste Biomass Valorization 2018, 9, 195–209. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The Ability of Biologically Based Wastewater Treatment Systems to Remove Emerging Organic Contaminants—A Review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef] [PubMed]
- Manasa, R.L.; Mehta, A. Wastewater: Sources of Pollutants and Its Remediation. In Environmental Biotechnology; Springer: Cham, Switzerland, 2020; Volume 2, pp. 197–219. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Hadibarata, T.; Syafrudin, M.; Yılmaz, M.; Abdullah, S. Microbiological Contaminants in Drinking Water: Current Status and Challenges. Water. Air. Soil Pollut. 2022, 233, 299. [Google Scholar] [CrossRef]
- Gall, A.M.; Mariñas, B.J.; Lu, Y.; Shisler, J.L. Waterborne Viruses: A Barrier to Safe Drinking Water. PLOS Pathog. 2015, 11, e1004867. [Google Scholar] [CrossRef] [PubMed]
- El Moussaouy, A. Environmental Nanotechnology and Education for Sustainability: Recent Progress and Perspective. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 2205–2231. [Google Scholar]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Adisasmito, S.; Pramudita, D.; Sumampouw, G.A.; Mohtar, W.H.M.W.; Indarto, A. Sustainable Applications and Prospects of Nanoadsorbents for Wastewater Treatment. In Adsorption through Advanced Nanoscale Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 533–584. [Google Scholar] [CrossRef]
- Mayo, J.T.; Yavuz, C.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The Effect of Nanocrystalline Magnetite Size on Arsenic Removal. Sci. Technol. Adv. Mater. 2007, 8, 71. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Nutt, M.O.; Hughes, J.B.; Wong, M.S. Designing Pd-on-Au Bimetallic Nanoparticle Catalysts for Trichloroethene Hydrodechlorination. Environ. Sci. Technol. 2005, 39, 1346–1353. [Google Scholar] [CrossRef]
- Lee, J.; Mackeyev, Y.; Cho, M.; Li, D.; Kim, J.H.; Wilson, L.J.; Alvarez, P.J.J. Photochemical and Antimicrobial Properties of Novel C 60 Derivatives in Aqueous Systems. Environ. Sci. Technol. 2009, 43, 6604–6610. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, J.C.T.; Huang, C. Application of Nanosilver Surface Modification to RO Membrane and Spacer for Mitigating Biofouling in Seawater Desalination. Water Res. 2009, 43, 3777–3786. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone Ultrafiltration Membranes Impregnated with Silver Nanoparticles Show Improved Biofouling Resistance and Virus Removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive Deionization (CDI) for Desalination and Water Treatment—Past, Present and Future (a Review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Bogue, R. Nanosensors: A Review of Recent Research. Sens. Rev. 2009, 29, 310–315. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Chen, F.; Yan, T.H.; Bashir, S.; Liu, J.L. Synthesis of Nanomaterials Using Top-down Methods. In Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–60. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Lin, J.M.; Chen, Y.C.; Yang, C.F.; Chen, W. Effect of Substrate Temperature on the Thermoelectric Properties of the Sb2Te3 Thin Films Deposition by Using Thermal Evaporation Method. J. Nanomater. 2015, 2015, 135130. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Zhang, X.; Lyu, H.; Qin, H. Laser Ablation of Polymers: A Review. Polym. Int. 2019, 68, 1391–1401. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc Discharge Synthesis of Carbon Nanotubes: Comprehensive Review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Nat. Rev. Methods Prim. 2021, 1, 5. [Google Scholar] [CrossRef]
- Carlsson, J.O.; Martin, P.M. Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 314–363. ISBN 9780815520313. [Google Scholar]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-Based Nanomaterials for Removal of Chemical and Biological Contaminants from Water: A Review of Mechanisms and Applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Ma, X.; Cambré, S.; Wenseleers, W.; Doorn, S.K.; Htoon, H. Quasiphase Transition in a Single File of Water Molecules Encapsulated in (6,5) Carbon Nanotubes Observed by Temperature-Dependent Photoluminescence Spectroscopy. Phys. Rev. Lett. 2017, 118, 027402. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application Potential of Carbon Nanotubes in Water Treatment: A Review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef]
- Plata, D.L.; Meshot, E.R.; Reddy, C.M.; Hart, A.J.; Gschwend, P.M. Multiple Alkynes React with Ethylene to Enhance Carbon Nanotube Synthesis, Suggesting a Polymerization-like Formation Mechanism. ACS Nano 2010, 4, 7185–7192. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, M.; Hashim, M.A. Allyl Triphenyl Phosphonium Bromide Based DES-Functionalized Carbon Nanotubes for the Removal of Mercury from Water. Chemosphere 2017, 167, 44–52. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, H.; He, Y.; Long, Z.; Wan, X.; Zeng, G. Novel Amino-Functionalized Fe3O4/Carboxylic Multi-Walled Carbon Nanotubes: One-Pot Synthesis, Characterization and Removal for Cu(II). Russ. J. Appl. Chem. 2016, 89, 1894–1902. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Gharibi, F.; McKay, G.; Gupta, V.K.; Harikaranahalli Puttaiah, S.; Marzban, N. Heavy Metal Adsorption Using PAMAM/CNT Nanocomposite from Aqueous Solution in Batch and Continuous Fixed Bed Systems. Chem. Eng. J. 2018, 346, 258–270. [Google Scholar] [CrossRef]
- Kandah, M.I.; Meunier, J.-L. Removal of Nickel Ions from Water by Multi-Walled Carbon Nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of Divalent Heavy Metal Ions from Water Using Carbon Nanotube Sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption Thermodynamic, Kinetic and Desorption Studies of Pb2+ on Carbon Nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef]
- Atieh, M.A. Removal of Chromium (VI) from Polluted Water Using Carbon Nanotubes Supported with Activated Carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Y.; Ma, H.; Bai, Z.; Qiao, Z.; Ji, D.; Yan, C.; Yan, Y.; Wu, H. Study on Uranium Adsorption Property of Carbon Nanotubes Prepared by Molten Salt Electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 11990–11999. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, N.; Saxena, R. Highly Efficient and Rapid Removal of a Toxic Dye: Adsorption Kinetics, Isotherm, and Mechanism Studies on Functionalized Multiwalled Carbon Nanotubes. Surf. Interfaces 2020, 21, 100639. [Google Scholar] [CrossRef]
- Balarak, D.; Zafariyan, M.; Igwegbe, C.A.; Onyechi, K.K.; Ighalo, J.O. Adsorption of Acid Blue 92 Dye from Aqueous Solutions by Single-Walled Carbon Nanotubes: Isothermal, Kinetic, and Thermodynamic Studies. Environ. Process. 2021, 8, 869–888. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Du, Q.; Peng, X.; Liu, T.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; Zhu, H.; et al. Adsorption of Fluoride from Aqueous Solution by Graphene. J. Colloid Interface Sci. 2011, 363, 348–354. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and Removal of Tetracycline Antibiotics from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Zhang, Q.; Li, C.; Bao, C.; Liu, X.; Xiao, P. Adsorption of Au(III), Pd(II), and Pt(IV) from Aqueous Solution onto Graphene Oxide. J. Chem. Eng. Data 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption Characteristics and Behaviors of Graphene Oxide for Zn(II) Removal from Aqueous Solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Pang, L.; Li, M.; Song, X.; Wen, J.; Zhao, H. Preparation of Reduced Graphene Oxide/Poly(Acrylamide) Nanocomposite and Its Adsorption of Pb(II) and Methylene Blue. Langmuir 2013, 29, 10727–10736. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Weng, W.; Hu, S.R.; Li, S.X.; Huang, Y.H.; Sun, X.; Su, Z.B. Investigation on the Adsorption Properties of Cr(vi) Ions on a Novel Graphene Oxide (GO) Based Composite Adsorbent. J. Mater. Chem. A 2014, 2, 12561–12570. [Google Scholar] [CrossRef]
- Zhang, Y.; Chi, H.; Zhang, W.; Sun, Y.; Liang, Q.; Gu, Y.; Jing, R. Highly Efficient Adsorption of Copper Ions by a PVP-Reduced Graphene Oxide Based On a New Adsorptions Mechanism. Nano-Micro Lett. 2014, 6, 80–87. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide-MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a Graphene-Based Magnetic Nanocomposite for the Removal of an Organic Dye from Aqueous Solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Self-Assembled Cylindrical Graphene-Carbon Nanotube Hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced Graphene Oxide-Based Hydrogels for the Efficient Capture of Dye Pollutants from Aqueous Solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Xie, G.; Xi, P.; Liu, H.; Chen, F.; Huang, L.; Shi, Y.; Hou, F.; Zeng, Z.; Shao, C.; Wang, J. A Facile Chemical Method to Produce Superparamagnetic Graphene Oxide-Fe 3O 4 Hybrid Composite and Its Application in the Removal of Dyes from Aqueous Solution. J. Mater. Chem. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Hadi Najafabadi, H.; Irani, M.; Roshanfekr Rad, L.; Heydari Haratameh, A.; Haririan, I. Removal of Cu2+, Pb2+ and Cr6+ from Aqueous Solutions Using a Chitosan/Graphene Oxide Composite Nanofibrous Adsorbent. RSC Adv. 2015, 5, 16532–16539. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, L.; Xu, M.; Feng, S.; Ding, Y.; Wakeel, M.; Alharbi, N.S.; Chen, C. Amino Siloxane Oligomer Modified Graphene Oxide Composite for the Efficient Capture of U(VI) and Eu(III) from Aqueous Solution. ACS Sustain. Chem. Eng. 2017, 5, 10290–10297. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine Modified Graphene Oxide Hydrogel Composite as an Efficient Adsorbent for Heavy Metal Ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of Graphite Oxide with Magnetic Chitosan for the Preparation of a Nanocomposite Dye Adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N.; Lazaridis, N.K. Selective Separation of Basic and Reactive Dyes by Molecularly Imprinted Polymers (MIPs). Chem. Eng. J. 2009, 149, 263–272. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Kotnala, S. Fabrication of Chitosan-Hydroxyapatite Nano-Adsorbent for Removal of Norfloxacin from Water: Isotherm and Kinetic Studies. Mater. Today Proc. 2022, 61, 143–149. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Kehili, S.; Ali, M.; Musa, H.; Ali, U.; Kutty, S.R.M.; Jagaba, A.H.; Abdullahi, S.S.; Tag-Eldin, E.M.; Mahmud, H.N.M.E. Polymer-Based Nano-Adsorbent for the Removal of Lead Ions: Kinetics Studies and Optimization by Response Surface Methodology. Separations 2022, 9, 356. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for Water Purification: Applications of Nanotechnology Methods in Wastewater Treatment. In Water Purification; Elsevier: Amsterdam, The Netherlands, 2017; pp. 33–74. ISBN 9780128043004. [Google Scholar]
- Farhadi, K. Effect of Nano Silica on Water Quality, Health and Safety in Swimming Pools. Int. J. Appl. Exerc. Physiol. 2012, 1, 24. [Google Scholar]
- Asouhidou, D.; Triantafyllidis, K.; Lazaridis, N.; Matis, K.A. Adsorption of Remazol Red 3BS from Aqueous Solutions Using APTES- and Cyclodextrin-Modified HMS-Type Mesoporous Silicas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 83–90. [Google Scholar] [CrossRef]
- Kotsyuda, S.S.; Tomina, V.V.; Zub, Y.L.; Furtat, I.M.; Lebed, A.P.; Vaclavikova, M.; Melnyk, I.V. Bifunctional Silica Nanospheres with 3-Aminopropyl and Phenyl Groups. Synthesis Approach and Prospects of Their Applications. Appl. Surf. Sci. 2017, 420, 782–791. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, Characterization and Adsorption Studies of Several Heavy Metal Ions on Amino-Functionalized Silica Nano Hollow Sphere and Silica Gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; El-Latif, M.M.A. Nanocomposites of Nanosilica-Immobilized-Nanopolyaniline and Crosslinked Nanopolyaniline for Removal of Heavy Metals. Chem. Eng. J. 2016, 304, 679–691. [Google Scholar] [CrossRef]
- Da’na, E. Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions. Molecules 2022, 27, 3366. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Q.; Gao, J.; Jia, X.; Cai, M.; Liang, Q. Adsorption Properties and Mechanisms of Methylene Blue and Tetracycline by Nano-Silica Biochar Composites Activated by KOH. Chemosphere 2023, 337, 139395. [Google Scholar] [CrossRef] [PubMed]
- Sumesh, E.; Bootharaju, M.S.; Anshup; Pradeep, T. A Practical Silver Nanoparticle-Based Adsorbent for the Removal of Hg2+ from Water. J. Hazard. Mater. 2011, 189, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Lisha, K.P.; Anshup; Pradeep, T. Towards a Practical Solution for Removing Inorganic Mercury from Drinking Water Using Gold Nanoparticles. Gold Bull. 2009, 42, 144–152. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; López, X.; Arbiol, J.; Puntes, V. Citrate-Coated Gold Nanoparticles as Smart Scavengers for Mercury(II) Removal from Polluted Waters. ACS Nano 2012, 6, 2253–2260. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Zhang, G.S. Preparation and Evaluation of Fe-La Composite Oxide Nanoadsorbent for As(III) Removal from Aqueous Solutions. Huanjing Kexue/Environ. Sci. 2014, 35, 4198–4204. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Han, Q.; Yao, X.; Wang, X. Formation of WO3 Nanotube-Based Bundles Directed by NaHSO4 and Its Application in Water Treatment. J. Mater. Chem. A 2013, 1, 1246–1253. [Google Scholar] [CrossRef]
- Pan, S.; Shen, H.; Xu, Q.; Luo, J.; Hu, M. Surface Mercapto Engineered Magnetic Fe3O4 Nanoadsorbent for the Removal of Mercury from Aqueous Solutions. J. Colloid Interface Sci. 2012, 365, 204–212. [Google Scholar] [CrossRef]
- Kozma, G.; Rónavári, A.; Kónya, Z.; Kukovecz, Á. Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanoparticles. ACS Sustain. Chem. Eng. 2016, 4, 291–297. [Google Scholar] [CrossRef]
- Tosco, T.; Petrangeli Papini, M.; Cruz Viggi, C.; Sethi, R. Nanoscale Zerovalent Iron Particles for Groundwater Remediation: A Review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced Degradation of Halogenated Aliphatics by Zero-Valent Iron. Groundwater 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Bokare, V.; Jung, J.L.; Chang, Y.Y.; Chang, Y.S. Reductive Dechlorination of Octachlorodibenzo-p-Dioxin by Nanosized Zero-Valent Zinc: Modeling of Rate Kinetics and Congener Profile. J. Hazard. Mater. 2013, 250–251, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, Z.-W.; Liu, W.-L.; Xu, X.-H. Synchronous Treatment of Heavy Metal Ions and Nitrate by Zero-Valent Iron. Huanjing Kexue/Environ. Sci. 2009, 30, 775–779. [Google Scholar]
- Seyedi, S.M.; Rabiee, H.; Shahabadi, S.M.S.; Borghei, S.M. Synthesis of Zero-Valent Iron Nanoparticles Via Electrical Wire Explosion for Efficient Removal of Heavy Metals. Clean Soil Air Water 2017, 45, 1600139. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.L.; Sun, Y. Manipulating the Morphology of Nanoscale Zero-Valent Iron on Pumice for Removal of Heavy Metals from Wastewater. Chem. Eng. J. 2015, 263, 55–61. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale Zero Valent Iron and Bimetallic Particles for Contaminated Site Remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V.K. A Novel Reusable Nanocomposite for Complete Removal of Dyes, Heavy Metals and Microbial Load from Water Based on Nanocellulose and Silver Nano-Embedded Pebbles. Environ. Technol. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Batool, M.; Daoush, W.M.; Hussain, M.K. Dye Sequestration Using Biosynthesized Silver Nanoparticles Adsorbent in Aqueous Solutions. Crystals 2022, 12, 662. [Google Scholar] [CrossRef]
- Ahmad, R.; Ejaz, M.O. Synthesis of New Alginate-Silver Nanoparticles/Mica (Alg-AgNPs/MC) Bionanocomposite for Enhanced Adsorption of Dyes from Aqueous Solution. Chem. Eng. Res. Des. 2023, 197, 355–371. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Daniel, G.; Nedelec, J.M.; Gun’Ko, Y.K.; Kessler, V.G. High Surface Area Ordered Mesoporous Nano-Titania by a Rapid Surfactant-Free Approach. J. Mater. Chem. 2012, 22, 20374–20380. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Heavy Metals Removal from Aqueous Solutions Using TiO2, MgO, and Al2O3 Nanoparticles. Chem. Eng. Commun. 2013, 200, 448–470. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Daniel, G.; Kessler, V.G.; Nedelec, J.M. General Facile Approach to Transition-Metal Oxides with Highly Uniform Mesoporosity and Their Application as Adsorbents for Heavy-Metal-Ion Sequestration. Chem. A Eur. J. 2014, 20, 10732–10736. [Google Scholar] [CrossRef] [PubMed]
- Rind, I.K.; Tuzen, M.; Sarı, A.; Lanjwani, M.F.; Memon, N.; Saleh, T.A. Synthesis of TiO2 Nanoparticles Loaded on Magnetite Nanoparticles Modified Kaolinite Clay (KC) and Their Efficiency for As(III) Adsorption. Chem. Eng. Res. Des. 2023, 191, 523–536. [Google Scholar] [CrossRef]
- Kocabaş, Ö.; Yürüm, Y. Synthesis and Characterization of Anatase Nanoadsorbent and Application in Removal of Lead, Copper and Arsenic from Water. Chem. Eng. J. 2013, 225, 625–635. [Google Scholar] [CrossRef]
- Yalçinkaya, Ö.; Kalfa, O.M.; Türker, A.R. Chelating Agent Free-Solid Phase Extraction (CAF-SPE) of Co(II), Cu(II) and Cd(II) by New Nano Hybrid Material (ZrO2/B2O3). J. Hazard. Mater. 2011, 195, 332–339. [Google Scholar] [CrossRef]
- Hua, M.; Jiang, Y.; Wu, B.; Pan, B.; Zhao, X.; Zhang, Q. Fabrication of a New Hydrous Zr(IV) Oxide-Based Nanocomposite for Enhanced Pb(II) and Cd(II) Removal from Waters. ACS Appl. Mater. Interfaces 2013, 5, 12135–12142. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Chen, Y.; Liu, J.; Chen, S.; Cao, J.; Mei, S.; Zhou, Y.; Jing, T. Preparation of Dumbbell Manganese Dioxide/Gelatin Composites and Their Application in the Removal of Lead and Cadmium Ions. J. Hazard. Mater. 2018, 350, 46–54. [Google Scholar] [CrossRef]
- Huangfu, X.; Jiang, J.; Lu, X.; Wang, Y.; Liu, Y.; Pang, S.Y.; Cheng, H.; Zhang, X.; Ma, J. Adsorption and Oxidation of Thallium(I) by a Nanosized Manganese Dioxide. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Saad, A.H.A.; Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; Abd El-latif, M.B. Removal of Toxic Metal Ions from Wastewater Using ZnO@Chitosan Core-Shell Nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 67–75. [Google Scholar] [CrossRef]
- Sheela, T.; Nayaka, Y.A.; Viswanatha, R.; Basavanna, S.; Venkatesha, T.G. Kinetics and Thermodynamics Studies on the Adsorption of Zn(II), Cd(II) and Hg(II) from Aqueous Solution Using Zinc Oxide Nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. Casein Based Biogenic-Synthesized Zinc Oxide Nanoparticles Simultaneously Decontaminate Heavy Metals, Dyes, and Pathogenic Microbes: A Rational Strategy for Wastewater Treatment. J. Chem. Technol. Biotechnol. 2018, 93, 2962–2976. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Seitzhapar, N.; Barsbay, M.; Aimanova, N.A.; Alimkhanova, A.N.; Zheltov, D.A.; Zhumabayev, A.M.; Temirgaziev, B.S.; Almanov, A.A.; Sadyrbekov, D.T. Adsorption Isotherms and Kinetics for Pb(Ii) Ion Removal from Aqueous Solutions with Biogenic Metal Oxide Nanoparticles. RSC Adv. 2023, 13, 26839–26850. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Adsorption of Safranin-O Dye by Copper Oxide Nanoparticles Synthesized from Punica Granatum Leaf Extract. Environ. Technol. 2022, 43, 3047–3063. [Google Scholar] [CrossRef]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J. Superparamagnetic Magnesium Ferrite Nanoadsorbent for Effective Arsenic (III, V) Removal and Easy Magnetic Separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the Efficiency and Mechanism of Cd(II) and Pb(II) Removal from Aqueous Solutions Using MgO Nanoparticles. J. Hazard. Mater. 2015, 299, 664–674. [Google Scholar] [CrossRef]
- Feng, J.; Zou, L.; Wang, Y.; Li, B.; He, X.; Fan, Z.; Ren, Y.; Lv, Y.; Zhang, M.; Chen, D. Synthesis of High Surface Area, Mesoporous MgO Nanosheets with Excellent Adsorption Capability for Ni(II) via a Distillation Treating. J. Colloid Interface Sci. 2015, 438, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Asouhidou, D.D.; Triantafyllidis, K.S.; Lazaridis, N.K.; Matis, K.A. Adsorption of Reactive Dyes from Aqueous Solutions by Layered Double Hydroxides. J. Chem. Technol. Biotechnol. 2012, 87, 575–582. [Google Scholar] [CrossRef]
- Recillas, S.; Colón, J.; Casals, E.; González, E.; Puntes, V.; Sánchez, A.; Font, X. Chromium VI Adsorption on Cerium Oxide Nanoparticles and Morphology Changes during the Process. J. Hazard. Mater. 2010, 184, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Saxena, A.; Rawat, A.S.; Dixit, P.K.; Kumar, R.; Rai, P.K. Surfactant-Free One-Pot Synthesis of Low-Density Cerium Oxide Nanoparticles for Adsorptive Removal of Arsenic Species. Environ. Prog. Sustain. Energy 2018, 37, 221–231. [Google Scholar] [CrossRef]
- Meepho, M.; Sirimongkol, W.; Ayawanna, J. Samaria-Doped Ceria Nanopowders for Heavy Metal Removal from Aqueous Solution. Mater. Chem. Phys. 2018, 214, 56–65. [Google Scholar] [CrossRef]
- Tabesh, S.; Davar, F.; Loghman-Estarki, M.R. Preparation of γ-Al2O3 Nanoparticles Using Modified Sol-Gel Method and Its Use for the Adsorption of Lead and Cadmium Ions. J. Alloys Compd. 2018, 730, 441–449. [Google Scholar] [CrossRef]
- Huang, X.; Yang, J.; Wang, J.; Bi, J.; Xie, C.; Hao, H. Design and Synthesis of Core–Shell Fe3O4@PTMT Composite Magnetic Microspheres for Adsorption of Heavy Metals from High Salinity Wastewater. Chemosphere 2018, 206, 513–521. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent Manganese Feroxyhyte: A Novel Nanoadsorbent Equally Selective for As(III) and As(V) Removal from Drinking Water. Environ. Sci. Technol. 2013, 47, 9699–9705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, L.; Wang, T.-J.; Su, C.-L.; Jin, Y. Synthesis and Properties of a Magnetic Core–Shell Composite Nano-Adsorbent for Fluoride Removal from Drinking Water. Appl. Surf. Sci. 2014, 317, 552–559. [Google Scholar] [CrossRef]
- Norouzian Baghani, A.; Mahvi, A.H.; Gholami, M.; Rastkari, N.; Delikhoon, M. One-Pot Synthesis, Characterization and Adsorption Studies of Amine-Functionalized Magnetite Nanoparticles for Removal of Cr (VI) and Ni (II) Ions from Aqueous Solution: Kinetic, Isotherm and Thermodynamic Studies. J. Environ. Health Sci. Eng. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza, A.Z.M.; Tay, A.S.H.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl-β-Cyclodextrin Conjugated Magnetic Nanoparticles as Nano-Adsorbents for Removal of Copper Ions: Synthesis and Adsorption Studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs Nanocomposites for Rapid Removal of Heavy Metal Ions and Dyes from Water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Peng, Z.; Tan, F.; Wang, X.; Chen, J.; Qiao, X. Facile Fabrication of Fe@MgO Magnetic Nanocomposites for Efficient Removal of Heavy Metal Ion and Dye from Water. Powder Technol. 2018, 326, 393–401. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, F.A. Kinetic Study on Removal of Copper(II) Using Goethite and Hematite Nano-Photocatalysts. J. Colloid Interface Sci. 2010, 347, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Khezami, L.; Ould M’hamed, M.; Lemine, O.M.; Bououdina, M.; Bessadok-Jemai, A. Milled Goethite Nanocrystalline for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution. Desalin. Water Treat. 2016, 57, 6531–6539. [Google Scholar] [CrossRef]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite Nanoparticles for Removal of Heavy Metals from Aqueous Solutions: Synthesis and Characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Adegoke, H.I.; AmooAdekola, F.; Fatoki, O.S.; Ximba, B.J. Adsorption of Cr (VI) on Synthetic Hematite (α-Fe2O3) Nanoparticles of Different Morphologies. Korean J. Chem. Eng. 2014, 31, 142–154. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, L.P.; Pittman, C.U.; Mohan, D. Lead (Pb2+) and Copper (Cu2+) Remediation from Water Using Superparamagnetic Maghemite (γ-Fe2O3) Nanoparticles Synthesized by Flame Spray Pyrolysis (FSP). J. Colloid Interface Sci. 2017, 492, 176–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z. Heavy Metals Removal Using Hydrogel-Supported Nanosized Hydrous Ferric Oxide: Synthesis, Characterization, and Mechanism. Sci. Total Environ. 2017, 580, 776–786. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Steinwinder, T.R.; Zhao, D. Selective Removal of Arsenate from Drinking Water Using a Polymeric Ligand Exchanger. Water Res. 2005, 39, 4993–5004. [Google Scholar] [CrossRef]
- Huo, L.; Zeng, X.; Su, S.; Bai, L.; Wang, Y. Enhanced Removal of As (V) from Aqueous Solution Using Modified Hydrous Ferric Oxide Nanoparticles. Sci. Rep. 2017, 7, 40765. [Google Scholar] [CrossRef]
- Moussa, S.I.; Sheha, R.R.; Saad, E.A.; Tadros, N.A. Synthesis and Characterization of Magnetic Nano-Material for Removal of Eu3+ Ions from Aqueous Solutions. J. Radioanal. Nucl. Chem. 2013, 295, 929–935. [Google Scholar] [CrossRef]
- Afshar, A.; Sadjadi, S.A.S.; Mollahosseini, A.; Eskandarian, M.R. Polypyrrole-Polyaniline/Fe3O4 Magnetic Nanocomposite for the Removal of Pb(II) from Aqueous Solution. Korean J. Chem. Eng. 2016, 33, 669–677. [Google Scholar] [CrossRef]
- Su, Y.; Adeleye, A.S.; Huang, Y.; Sun, X.; Dai, C.; Zhou, X.; Zhang, Y.; Keller, A.A. Simultaneous Removal of Cadmium and Nitrate in Aqueous Media by Nanoscale Zerovalent Iron (NZVI) and Au Doped NZVI Particles. Water Res. 2014, 63, 102–111. [Google Scholar] [CrossRef]
- Zarime, N.A.; Yaacob, W.Z.W.; Jamil, H. Removal of Heavy Metals Using Bentonite Supported Nano-Zero Valent Iron Particles. In Proceedings of the AIP Conference Proceedings, Selangor, Malaysia, 4 April 2018; American Institute of Physics Inc.: College Park, MD, USA; Volume 1940. [Google Scholar]
- Huang, D.L.; Chen, G.M.; Zeng, G.M.; Xu, P.; Yan, M.; Lai, C.; Zhang, C.; Li, N.J.; Cheng, M.; He, X.X.; et al. Synthesis and Application of Modified Zero-Valent Iron Nanoparticles for Removal of Hexavalent Chromium from Wastewater. Water Air Soil Pollut. 2015, 226, 1–14. [Google Scholar] [CrossRef]
- Sepehri, S.; Kanani, E.; Abdoli, S.; Rajput, V.D.; Minkina, T.; Asgari Lajayer, B. Pb(II) Removal from Aqueous Solutions by Adsorption on Stabilized Zero-Valent Iron Nanoparticles—A Green Approach. Water 2023, 15, 222. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Omer, A.M.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Eltaweil, A.S. Zero-Valent Iron Supported-Lemon Derived Biochar for Ultra-Fast Adsorption of Methylene Blue. Biomass Convert. Biorefinery 2024, 14, 1697–1709. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lazaridis, N.K. Low-Swelling Chitosan Derivatives as Biosorbents for Basic Dyes. Langmuir 2008, 24, 4791–4799. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Sudha, P.N.; Anil, S. Removal of the Heavy Metal Ion Chromiuim(VI) Using Chitosan and Alginate Nanocomposites. Int. J. Biol. Macromol. 2017, 104, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Asouhidou, D.; Triantafyllidis, K.; Lazaridis, N.; Matis, K.A.; Kim, S.-S.; Pinnavaia, T. Sorption of Reactive Dyes from Aqueous Solutions by Ordered Hexagonal and Disordered Mesoporous Carbons. Microporous Mesoporous Mater. 2009, 117, 257–267. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Zadpour, B.; Ahmadi, M. New Synthetic Mercaptoethylamino Homopolymer-Modified Maghemite Nanoparticles for Effective Removal of Some Heavy Metal Ions from Aqueous Solution. J. Ind. Eng. Chem. 2015, 21, 1160–1166. [Google Scholar] [CrossRef]
- Zendehdel, M.; Shoshtari-Yeganeh, B.; Cruciani, G. Removal of Heavy Metals and Bacteria from Aqueous Solution by Novel Hydroxyapatite/Zeolite Nanocomposite, Preparation, and Characterization. J. Iran. Chem. Soc. 2016, 13, 1915–1930. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Gupta, A. Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects. Membranes 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Maximous, N.; Nakhla, G.; Wong, K.; Wan, W. Optimization of Al2O3/PES Membranes for Wastewater Filtration. Sep. Purif. Technol. 2010, 73, 294–301. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’Asti, V.; Piaggio, P. Preparation and Properties of Novel Organic-Inorganic Porous Membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Effect of TiO2 Nanoparticles on Fouling Mitigation of Ultrafiltration Membranes for Activated Sludge Filtration. J. Memb. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M.V. Influence of Zeolite Crystal Size on Zeolite-Polyamide Thin Film Nanocomposite Membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Deng, B. Fabrication of Polyamide Thin-Film Nano-Composite (PA-TFN) Membrane with Hydrophilized Ordered Mesoporous Carbon (H-OMC) for Water Purifications. J. Memb. Sci. 2011, 375, 46–54. [Google Scholar] [CrossRef]
- Chen, X.; Hong, L.; Xu, Y.; Ong, Z.W. Ceramic Pore Channels with Inducted Carbon Nanotubes for Removing Oil from Water. ACS Appl. Mater. Interfaces 2012, 4, 1909–1918. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S. Fabrication and Characterization of Polyamide Thin Film Nanocomposite (TFN) Nanofiltration Membrane Impregnated with TiO2 Nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Gehrke, I. Environmental Friendly Recycling of Strategic Metals; Annual Report; Fraunhofer UMSICHT: Oberhausen, Germany, 2014. [Google Scholar]
- Pervez, M.N.; Yeo, W.S.; Mishu, M.M.R.; Talukder, M.E.; Roy, H.; Islam, M.S.; Zhao, Y.; Cai, Y.; Stylios, G.K.; Naddeo, V. Electrospun Nanofiber Membrane Diameter Prediction Using a Combined Response Surface Methodology and Machine Learning Approach. Sci. Rep. 2023, 13, 9679. [Google Scholar] [CrossRef] [PubMed]
- Cloete, T.E. Nanotechnology in Water Treatment Applications; Kwaadsteniet, M.d., Botes, M., Lopez-Romero, J.M., Eds.; Caister Academic Press: Norfolk, UK, 2010; ISBN 9781904455660. [Google Scholar]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon Nanotube Membranes for Water Purification: A Bright Future in Water Desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Tabe, S.; Ismail, A.F. Preparation and Characterization of Electro-Spun Nanofiber Membranes and Their Possible Applications in Water Treatment. Sep. Purif. Technol. 2013, 102, 118–135. [Google Scholar] [CrossRef]
- Rahman, M.M. Selective Swelling and Functionalization of Integral Asymmetric Isoporous Block Copolymer Membranes. Macromol. Rapid Commun. 2021, 42, 2100235. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Memb. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, J.; Zeng, Y.; Zhou, H.; Liu, G.; Liu, Y.; Zeng, L.; Jian, J.; Yuan, Z. Preparation and Performance of Poly(4-Vinylpyridine)-b-Polysulfone-b-Poly(4-Vinylpyridine) Triblock Copolymer/Polysulfoneblend Membrane for Separation of Palladium (II) from Electroplating Wastewaters. J. Hazard. Mater. 2020, 384, 121277. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly Permeable Polymeric Membranes Based on the Incorporation of the Functional Water Channel Protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.H.; Akter, T.; Kalagara, S. Recent Advances in Photocatalytic Treatment of Pollutants in Aqueous Media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic Systems as an Advanced Environmental Remediation: Recent Developments, Limitations and New Avenues for Applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nawaz, T.; Nabi, G.; Sagir, M.; Khan, M.I.; Malik, N. Role of Nanophotocatalysts for the Treatment of Hazardous Organic and Inorganic Pollutants in Wastewater. Int. J. Environ. Anal. Chem. 2022, 102, 491–515. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Wang, C. K4Nb6O17/Fe3N/α-Fe2O3/C3N4 as an Enhanced Visible Light-Driven Quaternary Photocatalyst for Acetamiprid Photodegradation, CO2 Reduction, and Cancer Cells Treatment. Appl. Surf. Sci. 2021, 544, 148939. [Google Scholar] [CrossRef]
- El Mragui, A.; Zegaoui, O.; Esteves da Silva, J.C.G. Elucidation of the Photocatalytic Degradation Mechanism of an Azo Dye under Visible Light in the Presence of Cobalt Doped TiO2 Nanomaterials. Chemosphere 2021, 266, 128931. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.H.; Thaweesak, S.; Yun, J.H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient Photocatalytic Degradation of Malachite Green Dye Using Facilely Synthesized Cobalt Oxide Nanomaterials Using Citric Acid and Oleic Acid. J. Phys. Chem. Solids 2021, 155, 110125. [Google Scholar] [CrossRef]
- Asadzadeh Patehkhor, H.; Fattahi, M.; Khosravi-Nikou, M. Synthesis and Characterization of Ternary Chitosan–TiO2–ZnO over Graphene for Photocatalytic Degradation of Tetracycline from Pharmaceutical Wastewater. Sci. Rep. 2021, 11, 24177. [Google Scholar] [CrossRef]
- Yi, C.; Liao, Q.; Deng, W.; Huang, Y.; Mao, J.; Zhang, B.; Wu, G. The Preparation of Amorphous TiO2 Doped with Cationic S and Its Application to the Degradation of DCFs under Visible Light Irradiation. Sci. Total Environ. 2019, 684, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic Degradation of Selected Pharmaceuticals Using Green Fabricated Zinc Oxide Nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Fattahi, M.; Anvaripour, B.; Jorfi, S. Photocatalytic Decontamination of Phenol and Petrochemical Wastewater through ZnO/TiO2 Decorated on Reduced Graphene Oxide Nanocomposite: Influential Operating Factors, Mechanism, and Electrical Energy Consumption. RSC Adv. 2018, 8, 40035–40053. [Google Scholar] [CrossRef] [PubMed]
- Vikesland, P.J. Nanosensors for Water Quality Monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-Dot-Tagged Microbeads for Multiplexed Optical Coding of Biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Gupta, A.; Mishra, A. Recent Advances on Nano-Adsorbents and Nanomembranes for the Remediation of Water. J. Clean. Prod. 2021, 322, 129051. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Hadibarata, T.; Yuniarto, A.; Sathishkumar, P.; Abdel-Salam, E.M.; Alatar, A.A. Role of Nanocatalyst in the Treatment of Organochlorine Compounds–A Review. Chemosphere 2021, 268, 128873. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K.; Arias, J.L.; Scherf, U.; El-Hammadi, M.M.; Zhou, Y.; et al. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Kloos, J.; Joosten, N.; Schenning, A.; Nijmeijer, K. Self-Assembling Liquid Crystals as Building Blocks to Design Nanoporous Membranes Suitable for Molecular Separations. J. Memb. Sci. 2021, 620, 118849. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Sikdar, S.K.; Costa, C.A.V.; Caetano, N.S. Sustainability Considerations about Microalgae for Biodiesel Production. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 745–757. ISBN 9781461433484. [Google Scholar]
- Mata, M.T.; Martins, A.A.; Costa, A.V.C.; Sikdar, K.S. Nanotechnology and Sustainability-Current Status and Future Challenges. In Life Cycle Analysis of Nanoparticles. Risk, Assessment, and Sustainability; DEStech Publications: Lancaster, PA, USA, 2015; pp. 271–306. [Google Scholar]
- Lens, P.N.L. Nanotechnology for Water and Wastewater Treatment; IWA Publishing: London UK, 2013; Volume 12. [Google Scholar] [CrossRef]
- Arancon, R.A.D.; Tao Zhang, Y.; Luque, R. Nanotechnology Management for a Safer Work Environment. Pure Appl. Chem. 2014, 86, 1159–1168. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of Nanomaterial Aging and Transformations through the Life Cycle of Nano-Enhanced Products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Bernd, N. Pollution Prevention and Treatment Using Nanotechnology. In Nanotechnology; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Saleem, H.; Zaidi, S.J. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials 2020, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, Mineral Nanoparticles, and Earth Systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Qiu, Y.; Yi, X.; Von Dem Bussche, A.; Kane, A.; Gao, H.; Koski, K.; Hurt, R. Biological and Environmental Interactions of Emerging Two-Dimensional Nanomaterials. Chem. Soc. Rev. 2016, 45, 1750–1780. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, Characterization and Quantification of Inorganic Engineered Nanomaterials: A Review of Techniques and Methodological Approaches for the Analysis of Complex Samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.P.; Von Der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 Nanoparticles from Sunscreens into Surface Waters: A One-Year Survey at the Old Danube Recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Kim, S. Influence of Nanotoxicity on Human Health and Environment: The Alternative Strategies. Rev. Environ. Contam. Toxicol. 2017, 242, 61–104. [Google Scholar] [CrossRef]
- Aitken, R.J.; Hankin, S.M.; Ross, B.; Tran, C.L.; Stone, V.; Fernandes, T.F.; Donaldson, K.; Duffin, R.; Chaudhry, Q.; Wilkins, T.A.; et al. A Review of Completed and Near Completed Environment, Health and Safety Research on Nanomaterials and Nanotechnology; Institute of Occupational Medicine (IOM): Edinburgh, UK, 2009. [Google Scholar]
- Som, C.; Nowack, B.; Krug, H.F.; Wick, P. Toward the Development of Decision Supporting Tools That Can Be Used for Safe Production and Use of Nanomaterials. Acc. Chem. Res. 2013, 46, 863–872. [Google Scholar] [CrossRef]
- Krug, H.F.; Wick, P. Nanotoxicology: An Interdisciplinary Challenge. Angew. Chem. Int. Ed. 2011, 50, 1260–1278. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union 2006, 396, 1–849. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj (accessed on 18 June 2024).
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Rasmussen, K.; González, M.; Kearns, P.; Sintes, J.R.; Rossi, F.; Sayre, P. Review of Achievements of the OECD Working Party on Manufactured Nanomaterials’ Testing and Assessment Programme. From Exploratory Testing to Test Guidelines. Regul. Toxicol. Pharmacol. 2016, 74, 147–160. [Google Scholar] [CrossRef] [PubMed]
- GadelHak, Y.; El-Azazy, M.; Shibl, M.F.; Mahmoud, R.K. Cost Estimation of Synthesis and Utilization of Nano-Adsorbents on the Laboratory and Industrial Scales: A Detailed Review. Sci. Total Environ. 2023, 875, 162629. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Bahadur, N.; Kansal, A. Techno-Economic Assessment of Integrated Photochemical AOPs for Sustainable Treatment of Textile and Dyeing Wastewater. J. Water Process Eng. 2023, 56, 104302. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. A Prototype of Textile Wastewater Treatment Using Coagulation and Adsorption by Fe/Cu Nanoparticles: Techno-Economic and Scaling-up Studies. Nanomater. Nanotechnol. 2021, 11, 18479804211041181. [Google Scholar] [CrossRef]
- Cinelli, M.; Coles, S.R.; Sadik, O.; Karn, B.; Kirwan, K. A Framework of Criteria for the Sustainability Assessment of Nanoproducts. J. Clean. Prod. 2016, 126, 277–287. [Google Scholar] [CrossRef]
- Roco, M.C. Converging Science and Technology at the Nanoscale: Opportunities for Education and Training. Nat. Biotechnol. 2003, 21, 1247–1249. [Google Scholar] [CrossRef]
- Yawson, R.M. Skill Needs and Human Resources Development in the Emerging Field of Nanotechnology. J. Vocat. Educ. Train. 2010, 62, 285–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).