Enhanced Adsorption of Aqueous Pb(II) by Acidic Group-Modified Biochar Derived from Peanut Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AMBC Preparation
2.3. Acid–Base Titration
2.4. Characterization
2.5. Adsorption Test
3. Results and Discussion
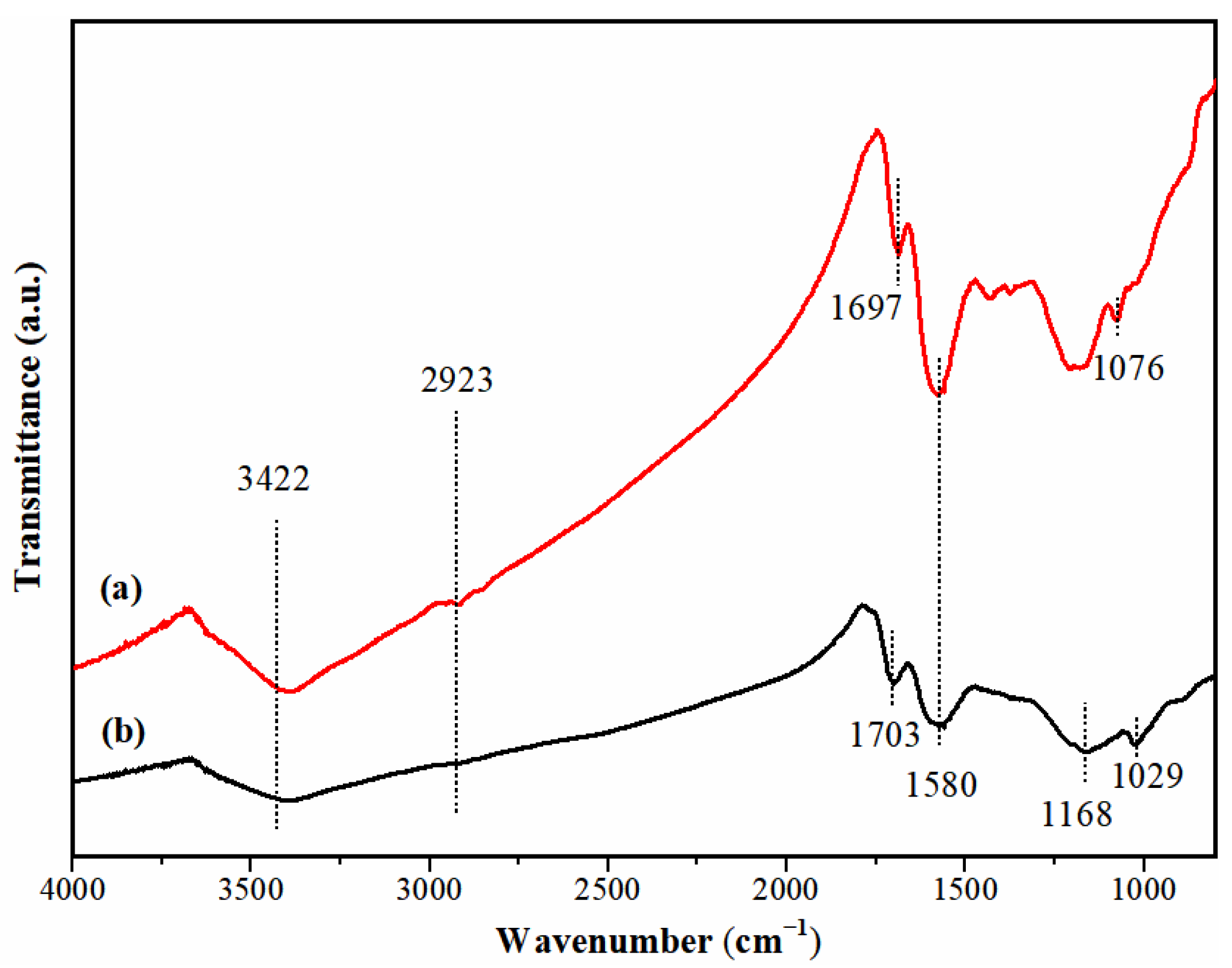
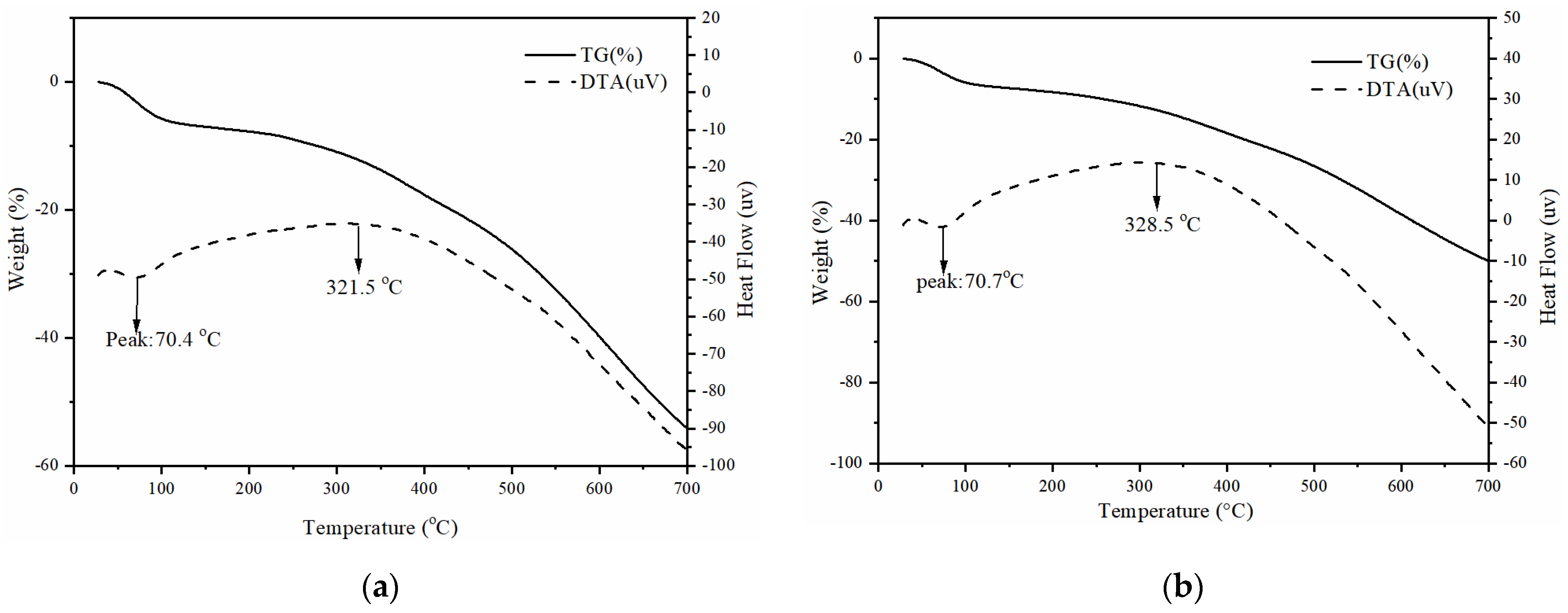
3.1. Characterization of Adsorbents
3.2. Pb(II) Adsorption by AMBC
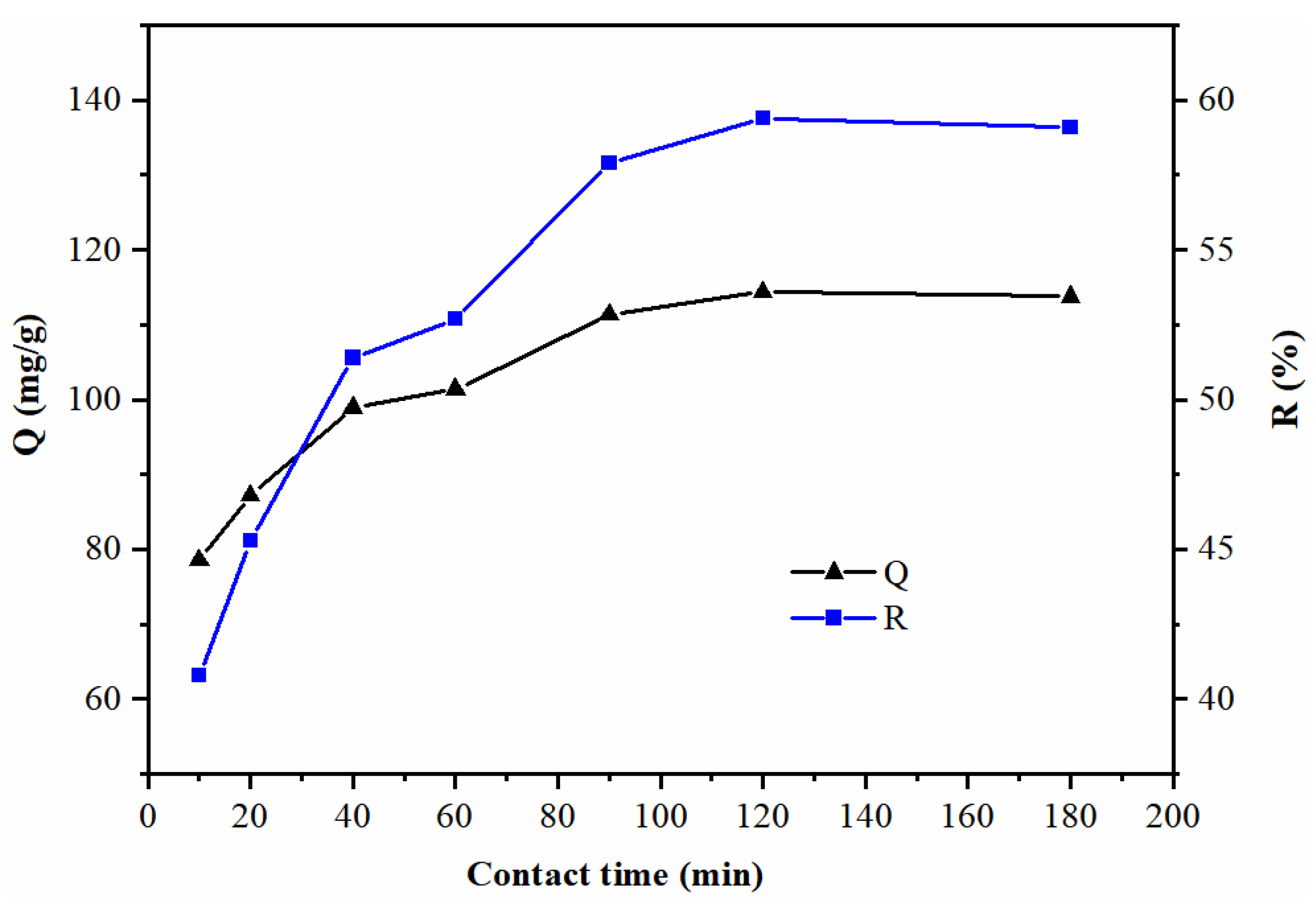
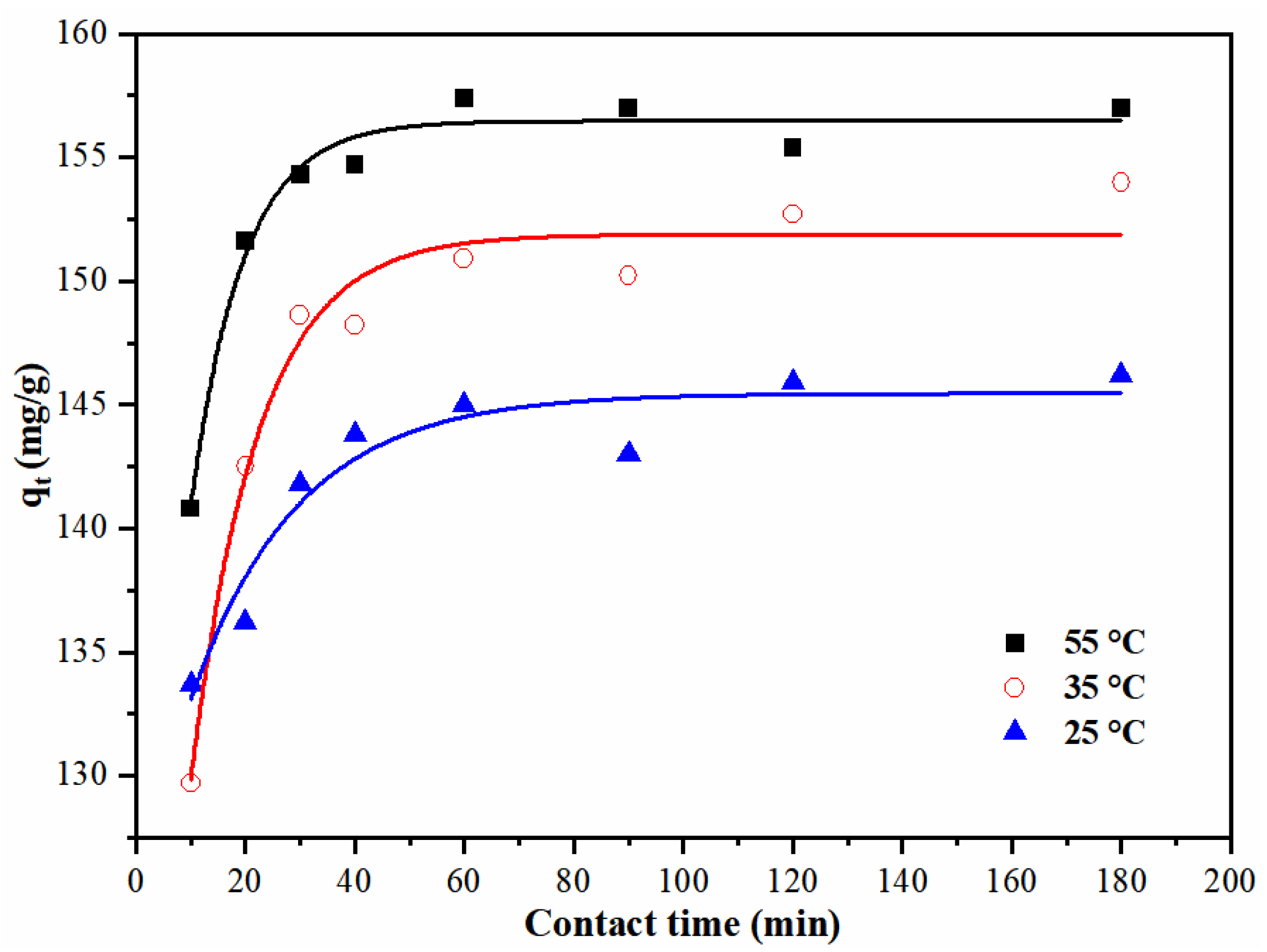
3.2.1. Effects of Contact Time
3.2.2. Effects of pH Value
3.2.3. Effects of the Dosage of AMBC
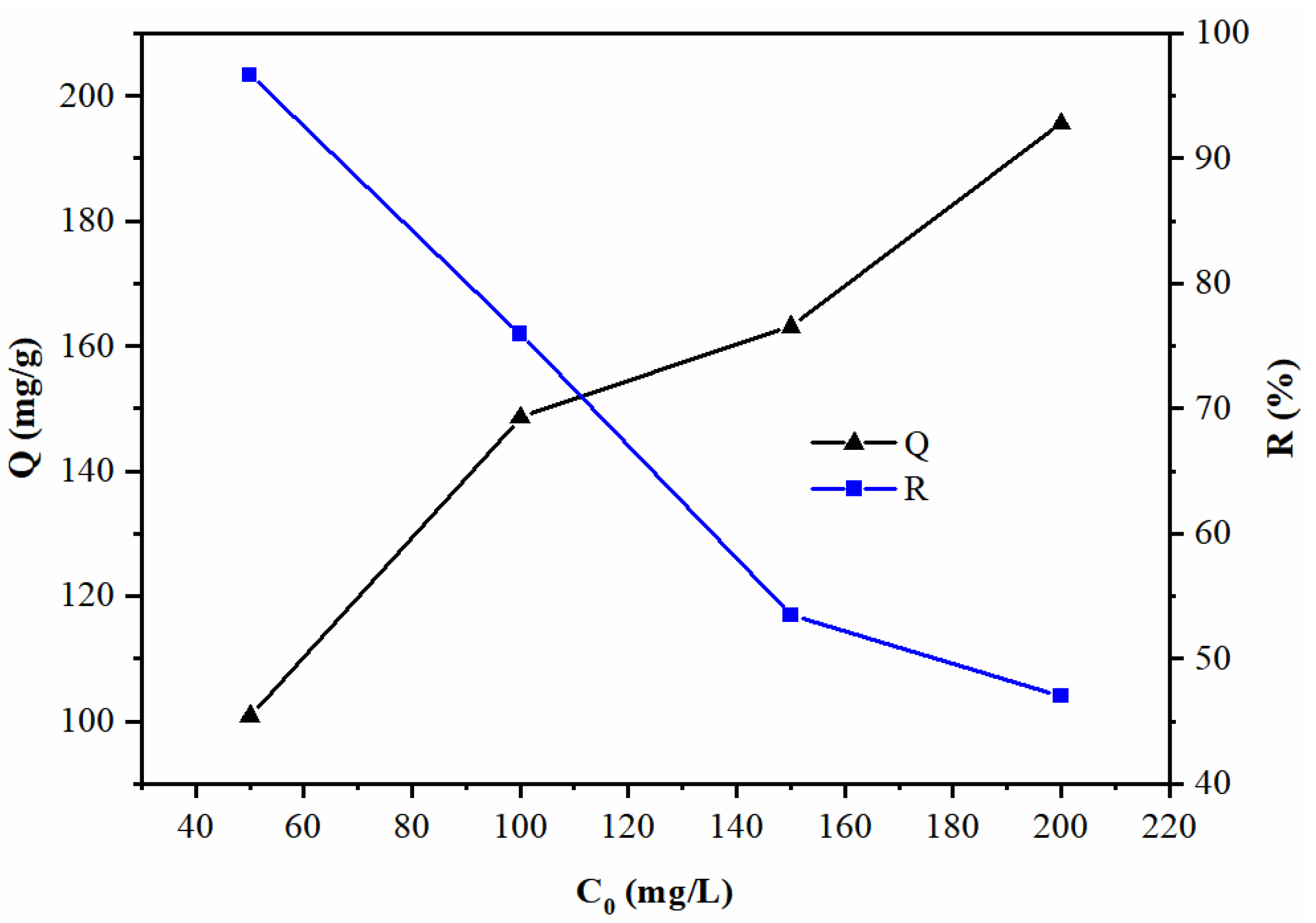
3.2.4. Effects of Initial Pb(II) Concentration
3.3. Comparison of Pre-AMBC and AMBC with Other Adsorbents
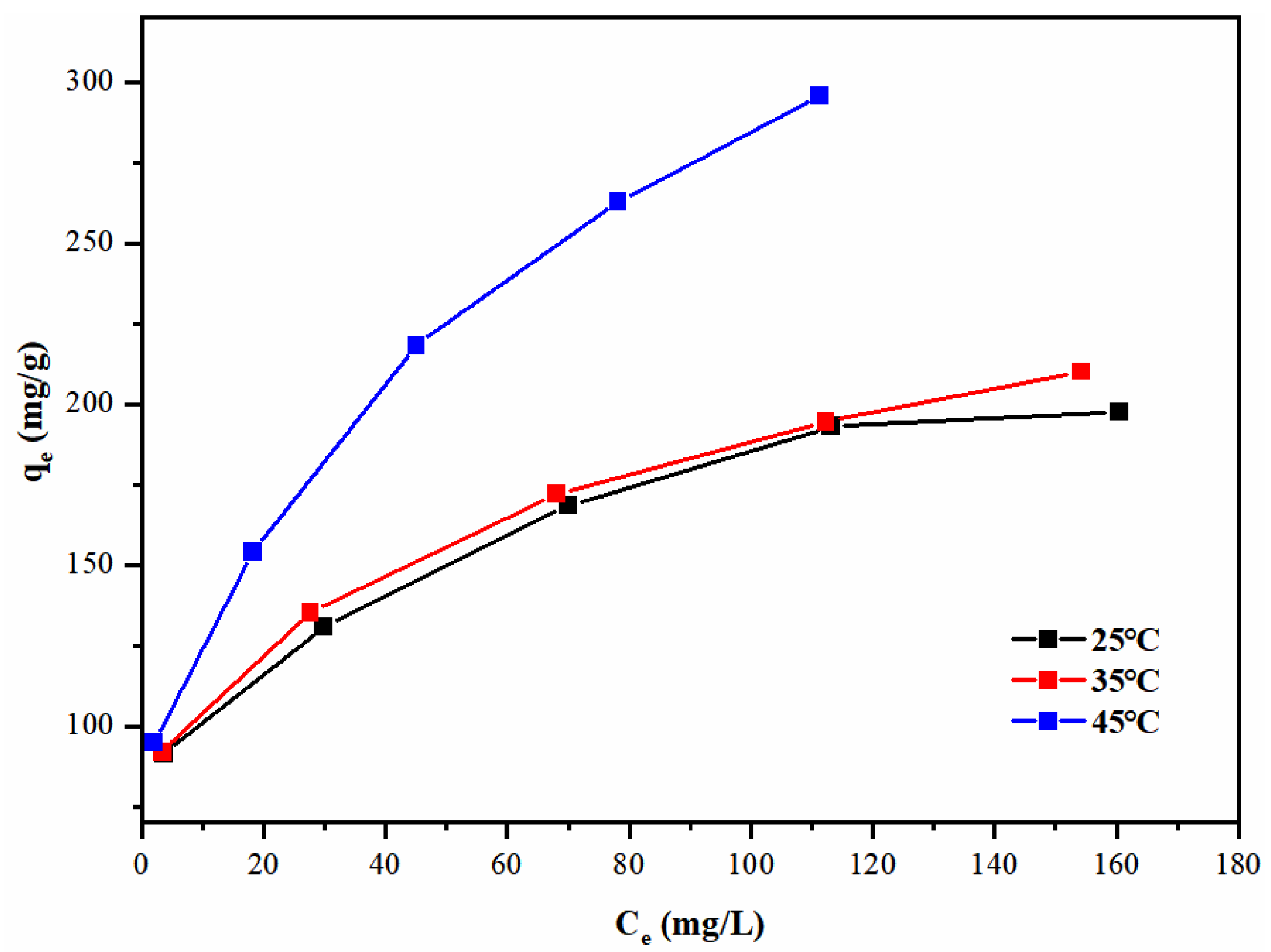
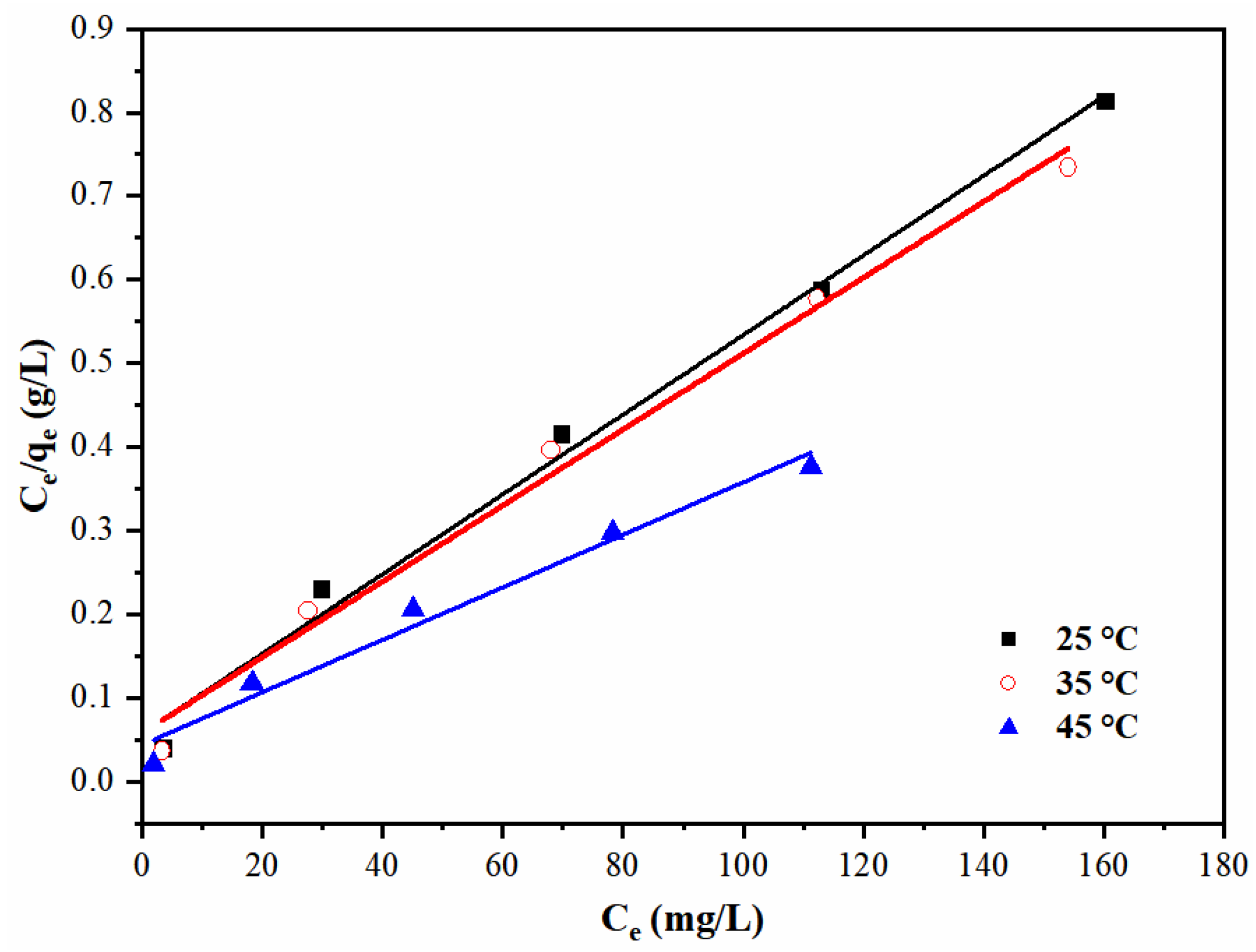
3.4. Pb(II) Adsorption Isotherms
3.5. Thermodynamic Study of the Adsorption
3.6. Kinetics Study of the Adsorption
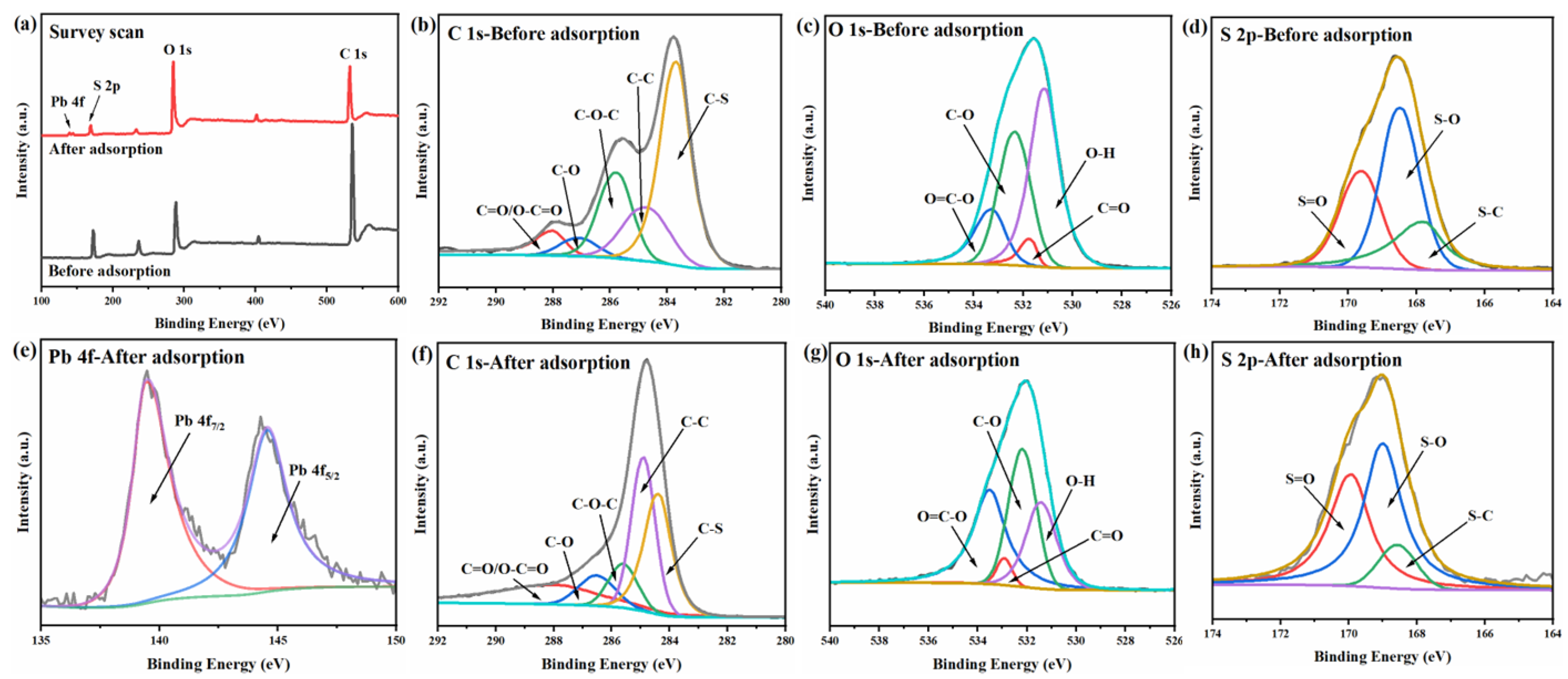
3.7. Possible Adsorption Mechanisms
4. Conclusions
- (1)
- The optimal adsorption performance of AMBC for heavy metal ions in water occurred when the initial concentration of Pb(II) was 100 mg/L, the pH was 5, the dosage of the adsorbent was 0.5 g/L, and the contact time was 120 min. Under these optimal conditions, the Pb(II) removal ratio was 76.0% and the adsorption capacity was 148.6 mg/g, which was much better than the pre-AMBC.
- (2)
- It is believed that the higher acid content of the AMBC, along with the introduction of the -SO3H groups, imparts greater electronegativity, stronger complexation, and more active adsorption sites to the AMBC, thereby enhancing its ability to adsorb Pb(II) in aqueous solution.
- (3)
- The adsorption system followed a pseudo-second-order kinetic model and reached an equilibrium after 90 min. Chemisorption was the rate-limiting step. The adsorption isothermal data showed that the equilibrium adsorption of Pb(II) by AMBC increased with increasing adsorption temperatures, indicating a heat-absorbing process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ifthikar, J.; Shahib, I.I.; Sellaoui, L.; Jawad, A.; Zhao, M.; Chen, Z.; Chen, Z. pH tunable anionic and cationic heavy metal reduction coupled adsorption by thiol cross-linked composite: Physicochemical interpretations and fixed-bed column mathematical model study. Chem. Eng. J. 2020, 401, 126041. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Baruwa, S.O.; Ogunfowokan, A.O.; Ebenso, E.E. Speciation study of the heavy metals in commercially available recharge cards coatings in Nigeria and the health implication. Toxicol. Rep. 2014, 1, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Shoukry, A.F.; Shuaib, N.M.; Ajadi, A.A. Homogeneous precipitation at solid/solution interface as a novel chemical route for synthesis of nanoparticles: Application to Cd(II) and Pb(II) sulfides. J. Exp. Nanosci. 2017, 13, 39–49. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Mahmood, M.; Chen, D.-Y. Enhanced adsorption of aqueous Pb(II) by modified biochar produced through pyrolysis of watermelon seeds. Sci. Total Environ. 2021, 784, 147136. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.; Martins, P.M.; Fernandes, M.M.; Costa, P.; Ferdov, S.; Botelho, G.; Lanceros-Mendez, S. Reusable nanocomposite-filters for arsenite and arsenate dual real effluents remediation in an up-scaled membrane reactor. J. Hazard. Mater. 2022, 440, 129756. [Google Scholar] [CrossRef]
- Botello-González, J.; Cerino-Córdova, F.J.; Dávila-Guzmán, N.E.; Salazar-Rábago, J.J.; Soto-Regalado, E.; Gómez-González, R.; Loredo-Cancino, M. Ion exchange modeling of the competitive adsorption of Cu(II) and Pb(II) using chemically modified solid waste coffee. Water Air Soil Pollut. 2019, 230, 73. [Google Scholar] [CrossRef]
- Long, J.; Yuvaraja, G.; Zhou, S.; Mo, J.; Li, H.; Luo, D.; Chen, D.Y.; Kong, L.; Subbaiah, M.V.; Reddy, G.M. Inactive Fusarium Fungal strains (ZSY and MJY) isolation and application for the removal of Pb(II) ions from aqueous environment. J. Ind. Eng. Chem. 2019, 72, 442–452. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Hsu, P.-C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/Alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef]
- Zarandona, A.; Salazar, H.; Insausti, M.; Lanceros-Méndez, S.; Zhang, Q. Sonophotocatalytic removal of organic dyes in real water environments using reusable BiSI@PVDF-HFP nanocomposite membranes. Chemosphere 2024, 357, 142069. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Queirós, J.M.; Salazar, H.; Valverde, A.; Botelho, G.; Fernández de Luis, R.; Teixeira, J.; Martins, P.M.; Lanceros-Mendez, S. Reusable composite membranes for highly efficient chromium removal from real water matrixes. Chemosphere 2022, 307, 135922. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, F.; Li, J.; Chen, J.; Tang, M. The effects of different factors on the removal mechanism of Pb(II) by biochar-supported carbon nanotube composites. RSC Adv. 2020, 10, 5988–5995. [Google Scholar] [CrossRef] [PubMed]
- Moja, T.N.; Bunekar, N.; Mojaki, S.; Mishra, S.B.; Tsai, T.Y.; Hwang, S.S.; Mishra, A.K. Polypropylene-polypropylene-grafted-maleic anhydride-montmorillonite clay nanocomposites for Pb(II) removal. J. Inorg. Organomet. Polym Mater. 2018, 28, 2799–2811. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Valenca, R.; Berger, A.W.; Yu, I.K.M.; Xiong, X.; Saunders, T.M.; Tsang, D.C.W. Plenty of room for carbon on the ground: Potential applications of biochar for stormwater treatment. Sci. Total Environ. 2018, 625, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Kongsuwan, A.; Patnukao, P.; Pavasant, P. Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J. Ind. Eng. Chem. 2009, 15, 465–470. [Google Scholar] [CrossRef]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Huang, D.; Li, P.; Tang, J.; Meng, F. Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts. Green Process. Synth. 2021, 10, 687–699. [Google Scholar] [CrossRef]
- Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J.N.; Hayashi, S.; Domen, K. A carbon material as a strong protonic acid. Angew. Chem. Int. Ed. 2004, 43, 2955–2958. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Mou, L.; Wu, M.; Wang, Y.; Cai, W.; Tan, F. Preparation of the porous carbon-based solid acid from starch for efficient degradation of chitosan to D-glucosamine. Int. J. Biol. Macromol. 2022, 209, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.F.; Abo El Naga, A.O.; El Saied, M.; AbuBaker, M.M.; Shaban, S.A.; El Kady, F.Y. Potato peel waste-derived carbon-based solid acid for the esterification of oleic acid to biodiesel. Environ. Technol. Innov. 2021, 21, 101355. [Google Scholar] [CrossRef]
- Valle-Vigón, P.; Sevilla, M.; Fuertes, A.B. Sulfonated mesoporous silica-carbon composites and their use as solid acid catalysts. Appl. Surf. Sci. 2012, 261, 574–583. [Google Scholar] [CrossRef]
- Xue, W.; Zhao, H.; Yao, J.; Li, F.; Wang, Y. Esterification of cyclohexene with formic acid over a peanut shell-derived carbon solid acid catalyst. Chin. J. Catal. 2016, 37, 769–777. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Ninomiya, S.; Sasaki, M.; Quitain, A.; Kida, T.; Saldaña, M.D.A. Carbon-based solid acid catalyst derived from undaria pinnatifida and its application in esterification. Algal Res. 2021, 55, 102272. [Google Scholar] [CrossRef]
- Higai, D.; Lee, C.; Lang, J.; Qian, E.W. Saccharification of cellulose using biomass-derived activated carbon-based solid acid catalysts. Fuel Process. Technol. 2021, 215, 106738. [Google Scholar] [CrossRef]
- Yu, W.; Hu, J.; Yu, Y.; Ma, D.; Gong, W.; Qiu, H.; Hu, Z.; Gao, H.-w. Facile preparation of sulfonated biochar for highly efficient removal of toxic Pb(II) and Cd(II) from wastewater. Sci. Total Environ. 2021, 750, 141545. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, X. Peanut shell activated carbon: Characterization, surface modification and adsorption of Pb2+ from aqueous solution. Chin. J. Chem. Eng. 2008, 16, 401–406. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Zhu, X. Preparation of solid acid catalyst from rice husk char and its catalytic performance in esterification. Chin. J. Catal. 2013, 34, 1674–1682. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, L.; Li, B.; Yang, X. Synthesis and characteristics of lignin-derived solid acid catalysts for microcrystalline cellulose hydrolysis. Korean J. Chem. Eng. 2016, 34, 110–117. [Google Scholar] [CrossRef]
- Farabi, M.S.A.; Ibrahim, M.L.; Rashid, U.; Taufiq-Yap, Y.H. Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energy Convers. Manag. 2019, 181, 562–570. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F.M. Sulfonated carbons from olive stones as catalysts in the microwave-assisted etherification of glycerol with tert-butyl alcohol. Mol. Catal. 2020, 488, 110921. [Google Scholar] [CrossRef]
- Lim, S.; Yap, C.Y.; Pang, Y.L.; Wong, K.H. Biodiesel synthesis from oil palm empty fruit bunch biochar derived heterogeneous solid catalyst using 4-benzenediazonium sulfonate. J. Hazard. Mater. 2020, 390, 121532. [Google Scholar] [CrossRef]
- Abo El Naga, A.O.; El Saied, M.; Shaban, S.A.; El Kady, F.Y. Fast removal of diclofenac sodium from aqueous solution using sugar cane bagasse-derived activated carbon. J. Mol. Liq. 2019, 285, 9–19. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, Y.M.; Andoshe, D.M.; Desissa, T.D. Composite of bentonite/CoFe2O4/hydroxyapatite for adsorption of Pb(II). Mater. Res. Express 2020, 7, 115501. [Google Scholar] [CrossRef]
- Lv, M.; Du, Y.; Zhang, T.; Du, X.; Yin, X. Cassava starch-based thermo-responsive Pb(II)-imprinted material: Preparation and adsorption performance on Pb(II). Polymers 2022, 14, 828. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Huang, B.; Dong, H.; Huang, J.; Yang, Z.; Wei, J.; Hu, L.; Zhang, Q. Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chem. Eng. J. 2016, 284, 247–259. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Mandal, S.; Das, S.K. The sorption of lead(II) ions on rice husk ash. J. Hazard. Mater. 2009, 163, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Serrano, V.; Macias-Garcia, A.; Espinosa-Mansilla, A.; Valenzuela-Calahorro, C. Adsoprtion of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 1998, 32, 1–4. [Google Scholar] [CrossRef]
- Cheraghi, E.; Ameri, E.; Moheb, A. Continuous biosorption of Cd(II) ions from aqueous solutions by sesame waste: Thermodynamics and fixed-bed column studies. Desalin. Water Treat. 2015, 57, 6936–6949. [Google Scholar] [CrossRef]
- Semerjian, L. Equilibrium and kinetics of cadmium adsorption from aqueous solutions using untreated Pinus halepensis sawdust. J. Hazard. Mater. 2010, 173, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, J.; Jin, Q.; Li, Z.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, H. Sludge-based biochar activation to enhance Pb(II) adsorption. Fuel 2019, 252, 101–108. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb(II) on activated carbon prepared from polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, S.; Song, J.; Zhao, Y.; Yang, F. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J. Hazard. Mater. 2020, 389, 122115. [Google Scholar] [CrossRef]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Soliman, A.M.; Elwy, H.M.; Thiemann, T.; Majedi, Y.; Labata, F.T.; Al-Rawashdeh, N.A.F. Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated palm tree leaves. J. Taiwan Inst. Chem. Eng. 2016, 58, 264–273. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, P.; Zhu, X.; Li, H.; Deng, Q.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Highly efficient alkylation using hydrophobic sulfonic acid-functionalized biochar as a catalyst for synthesis of high-density biofuels. ACS Sustain. Chem. Eng. 2019, 7, 14973–14981. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem. Eng. J. 2018, 334, 1055–1064. [Google Scholar] [CrossRef]







| Adsorbent | SBET a (m2/g) | VP b (cm3/g) | DP c (nm) | Acid Density (mmol/g) | |||
|---|---|---|---|---|---|---|---|
| Total | Ph-OH | -SO3H | -COOH | ||||
| Pre-AMBC | 832.6 | 3.99 × 10−1 | 2.2 | 1.26 | 0.05 | - | 1.21 |
| AMBC | 329.3 | 2.67 × 10−2 | 2.1 | 2.28 | 0.33 | 0.52 | 1.43 |
| Adsorbents | Q (mg/g) | R (%) |
|---|---|---|
| Pre-AMBC | 83.1 | 39.7 |
| AMBC | 148.6 | 76.0 |
| Adsorbents | Qmax (mg/g) | Activator | Experiment Conditions | |||||
|---|---|---|---|---|---|---|---|---|
| pH | T (°C) | Dosage (g/L) | Equilibration Time (min) | Initial Concentration (mg/g) | Ref. | |||
| AMBC | 148.6 | H2SO4 | 5 | 25 | 0.5 | 120 | 100 | This study |
| C-KOH | 57.5 | KOH | 5 | 25 | 2 | 35 | 100 | [47] |
| SBC | 191.1 | H2SO4 | 4.5 | - | 2 | 5 | 200 | [27] |
| HP-BC | 60.9 | H2O2 | 5 | 25 | - | 500 | 100 | [5] |
| M-RH-AC | 134.9 | HNO3 | 5.5 | - | 1 | 90 | 180 | [43] |
| PLAC | 98.4 | H3PO4 | 5.0 | 25 | 0.6 | 30 | 80 | [48] |
| Temperature (°C) | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | RL | R2 | KF (mg/g) | 1/n | R2 | |
| 25 | 0.0716 | 210.1 | 0.046~0.195 | 0.98947 | 68.117 | 0.211 | 0.97800 |
| 35 | 0.0786 | 220.3 | 0.048~0.203 | 0.98606 | 69.611 | 0.215 | 0.98978 |
| 45 | 0.0826 | 318.5 | 0.053~0.218 | 0.96706 | 75.708 | 0.280 | 0.97686 |
| Temperature (°C) | Kd | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/mol.K) |
|---|---|---|---|---|
| 25 | 5.304 | −4.14 | 3.248 | 0.025 |
| 35 | 5.334 | −4.29 | ||
| 45 | 4.764 | −4.13 |
| Temperature °C | qe mg/g | kads g/(min.mg) | R2 |
|---|---|---|---|
| 25 | 147.0 | 5.53 × 10−3 | 0.99989 |
| 35 | 155.3 | 6.92 × 10−3 | 0.99991 |
| 55 | 157.5 | 8.71 × 10−3 | 0.99992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, C.; Wang, Z.; Li, F.; Li, J.; Xue, W.; Zhao, X. Enhanced Adsorption of Aqueous Pb(II) by Acidic Group-Modified Biochar Derived from Peanut Shells. Water 2024, 16, 1871. https://doi.org/10.3390/w16131871
Wu Y, Li C, Wang Z, Li F, Li J, Xue W, Zhao X. Enhanced Adsorption of Aqueous Pb(II) by Acidic Group-Modified Biochar Derived from Peanut Shells. Water. 2024; 16(13):1871. https://doi.org/10.3390/w16131871
Chicago/Turabian StyleWu, Yumeng, Ci Li, Zhimiao Wang, Fang Li, Jing Li, Wei Xue, and Xinqiang Zhao. 2024. "Enhanced Adsorption of Aqueous Pb(II) by Acidic Group-Modified Biochar Derived from Peanut Shells" Water 16, no. 13: 1871. https://doi.org/10.3390/w16131871
APA StyleWu, Y., Li, C., Wang, Z., Li, F., Li, J., Xue, W., & Zhao, X. (2024). Enhanced Adsorption of Aqueous Pb(II) by Acidic Group-Modified Biochar Derived from Peanut Shells. Water, 16(13), 1871. https://doi.org/10.3390/w16131871






