Abstract
In this study, triphenylaniline-based porous organic polymers (TPA-POPs) were successfully prepared by the Friedel–Crafts reaction and applied to adsorb malachite green (MG) dye from water. The TPA-POP was characterized using TEM, SEM, FTIR, 13C (CP/MAS) NMR, BET surface area, and XRD analysis. The results exhibited that the TPA-POP has a high surface area (1625.14 m2/g) with pore volume (0.353 cm3/g) and pore radius (1.57 nm) that reflect the high quantity of MG adsorbed on the TPA-POP. The polymer was evaluated as an excellent adsorbent for MG adsorption from water using the batch method. MG dye removal was optimized as 99.60% (at pH: 6.0, adsorbent dosage (m): 0.01 g, temperature (T): 45 °C, and contact time (t): 300 min). The kinetic data follow the Elovich model, while the isotherm data fit the Langmuir model well with uptake capacity (755.72 mg/g) at T: 45 °C. According to thermodynamic parameters, the adsorption process was endothermic and spontaneous. The adsorption of MG on the TPA-POP occurred via different mechanisms (π–π interaction, electrostatic attraction, and hydrogen bonding). Reusability experiments exhibited that the TPA-POP still maintained high removal efficiency (82.12%) after five cycles. In conclusion, the TPA-POP is a promising adsorbent owing to its cost-effectiveness, high adsorption capacity, high surface area, excellent reusability, and efficient MG removal from aqueous media.
1. Introduction
As a result of the rapid development and expansion of industries, the amount of harmful synthetic dyes discharged into aquatic environments is increasing, posing a serious environmental problem. Dyes are extensively applied in various industries like textiles, cosmetics, leather, pharmaceuticals, and paper-making, [1,2,3,4]. The discharge of synthetic organic dyes into the environment poses serious threats to human health and aquatic ecosystems owing to their potential carcinogenicity [5,6,7,8]. Malachite green (MG) is a relatively dangerous pollutant because it exhibits high toxicity, carcinogenicity, and mutagenicity [9]. MG can cause the poisoning of vital organs such as the heart, breast, liver, and kidneys. MG is used to color leather, silk, wool, and acrylic fibers, as well as being used as an antifungal and antibacterial agent [10,11,12]. Therefore, removing malachite green before discharging it into the environment is critical to protecting ecosystems and humans.
Until now, different techniques such as photocatalysis, separation, membrane, oxidation processes, ion exchange, flocculation, sedimentation, coagulation, and adsorption have been developed to eliminate dyes from water [13,14,15,16,17,18,19,20]. However, some of them have drawbacks such as expensive technologies and low removal efficiency. Among these technologies, adsorption is widely used in wastewater treatments because of its simplicity, low cost, environmentally friendly features, and high removal efficiency [13,14,15]. Different adsorbent materials such as clay, polymer, silica, activated carbon, zeolites, biochar, and biomass have been used to remove dyes from polluted water [21,22].
Nowadays, porous organic polymers (POPs) have attracted great attention as efficient adsorbents for CO2 capture and pollutant removal, also due to their abundant surface functionalization, high thermal stability, high porosity, low density, tunable structures, and diversity of synthetic approaches [23,24]. POPs are a type of porous adsorbent material formed from light elements (hydrogen, oxygen, nitrogen, and carbon), which are linked by strong covalent bonds [25]. POPs have been studied for applications in separation [26], gas storage [27], environmental treatment [28,29,30], sensors [31], heterogeneous catalysis [32], and so on. Different porous organic polymers were developed for the elimination of radioactive iodine [30], organic dyes such as methylene blue (MB) [33,34], rhodamine B [35], crystal violet [36], various metal species (Pb(II), Cr2O72-, Hg(II), Cu(II), and UO22+) [37], Hg(II) [38], and the capture of gases like H2 and CO2 [39]. Javad Ghanbari and Akbar Mobinikhaledi developed a new porous organic polymer using Schiff base condensation. The TC-POP was applied to eliminate methyl red dye. They found that the BET surface area and total pore volume of the TC-POP were 108.27 m2/g and 0.1965 cm3/g, respectively. The maximum uptake capacity was 178.57 mg/g at a pH of 4.0, an adsorbent dosage of 8.0 mg, a contact time of 80 min, and a temperature of 45 °C [25]. Zhang et al. prepared POP-SO3H for diquat, paraquat, methylene blue (MB), and rhodamine B (RhB) adsorption from an aqueous solution. They found that the amounts of diquat, paraquat, MB, and RhB adsorbed on POP-SO3H were 60.96, 25.14, 17.29, and 16.12 mg/g, respectively [34].
2,4,6-Triphenylaniline (TPA) is an organic compound found in Alternaria longipes. It contains an amino group attached to one ring of four benzenes. The chelating amino (NH2) groups on the structure of TPA act as adsorption sites for heavy metals and dyes. Therefore, the aim and novelty of this study are the preparation of a new triphenylan-line-based porous organic polymer (TPA-POP) using the Friedel–Craft reaction and assess the ability of TPA-POP for the adsorption of MG dye from an aqueous solution. It is worth mentioning that the surface area of TPA-POP increased markedly by increasing the reaction time of TPA-POP. The TPA-POP was characterized by 13C (CP/MAS) NMR, SEM, TEM, BET, FTIR, and XRD analysis. The influence of different adsorption parameters such as contact time, temperature, adsorbent dose, pH, and initial MG concentration was studied. Additionally, we performed an assessment of the adsorption process using kinetic and isotherm models. The MG adsorption mechanism on the TPA-POP surface was discussed. The regeneration and reusability of TPA-POP were also studied. The adsorption process of MG dye on TPA-POP in terms of experimental conditions, adsorption capacity, BET surface area, and number of cycles were compared with different adsorbents (table in Section 3.6). Finally, the high surface area, MG dye removal efficiency, high adsorption capacity, and excellent reusability make the TPA-POP a promising adsorbent for water treatment.
2. Materials and Methods
2.1. Materials and Instrumentation
Tetrahydrofuran (THF), Malachite green (MG), Methyl blue (MB), 2,4,6-triphenylaniline (TPA, 97%), aluminum chloride (AlCl3), dichloromethane (DCM), and methanol were obtained from Sigma Aldrich (Saint Louis, MO, USA). A Nicolet iS50-FTIR spectrometer (Thermo Scientific, Waltham, MA, USA) was used to record the spectrum of the TPA, TPA-POP, and TPA-POP/MG. Solid-state 13C (CP/MAS) NMR was conducted on a Bruker Avance III 400 NMR spectrometer. The point of zero charge (pHPZC) of the TPA-POP in an aqueous solution was obtained by plotting (pHi–pHf) versus pH initial. The surface area of the TPA-POP was analyzed with the BET technique from the Micromeritics Tristar II 3020 surface area. The morphology of the TPA-POP was analyzed using an SEM (Hitachi Ltd., Tokyo, Japan) and Transmittance electron microscopy (TEM) (TEM: PHILIPS CM120, Philips, Amsterdam, The Netherlands).
2.2. Synthesis of TPA-POP
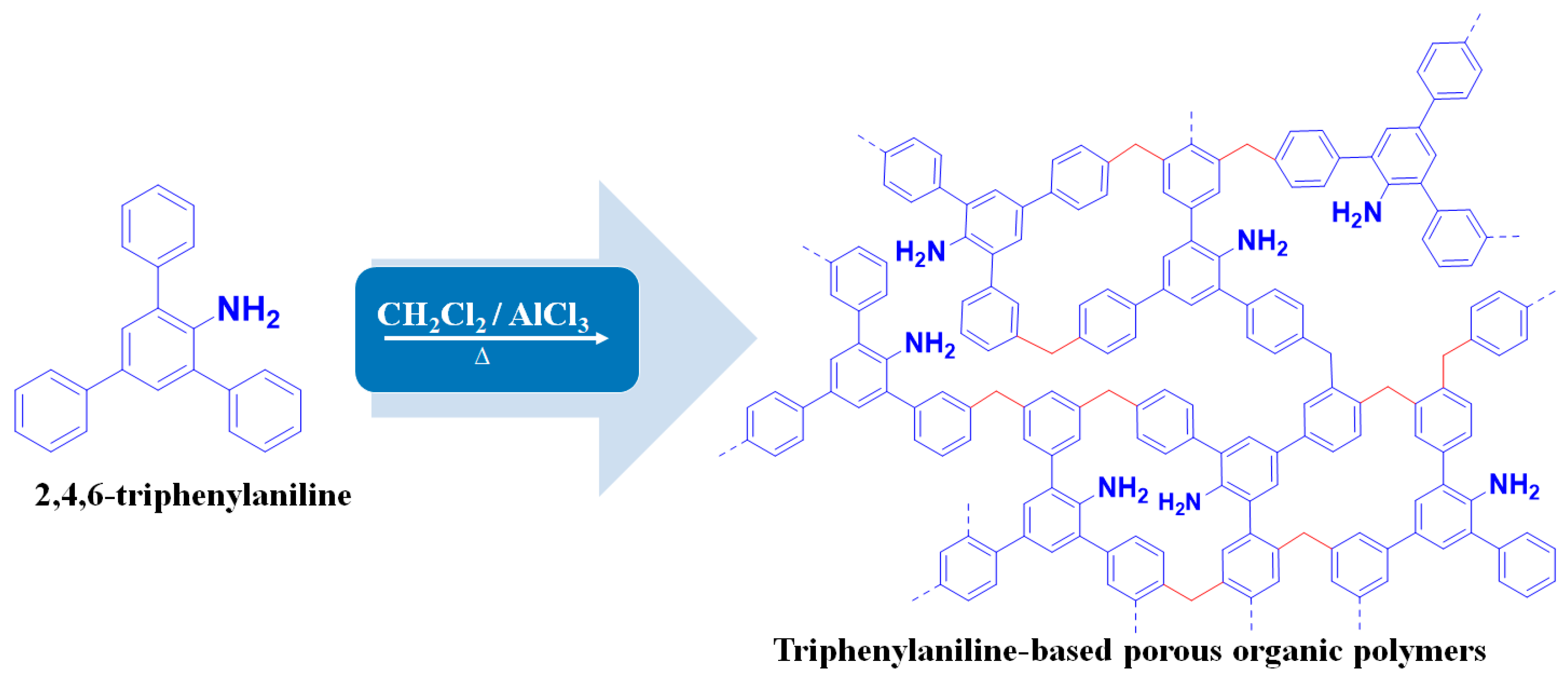
As an additional step from our previous work, this study aimed to obtain the highest porosity of the TPA-POP adsorbent by modifying the reaction time, following the method reported in the literature with some modifications [30]. Typically, 0.50 g of TPA is dissolved in 10 mL of dichloromethane under magnetic stirring for 10 min and then 5 g of AlCl3 is added to it under stirring for 60 min at room temperature (RT). After that, the mixture was stirred vigorously according to the following sequence: for 4 h at RT, for 8 h at 30 °C, for 12 h at 40 °C, for 12 h at 60 °C, and for 24 h at 80 °C. The reaction was conducted under a nitrogen atmosphere. After cooling, the obtained TPA-POP was quenched with 15 mL of HCl: H2O (0.5: 1, ν/ν). The obtained polymer was washed several times with deionized water and methanol, respectively, and purified by the Soxhlet extractor with EtOH for 24 h. Finally, the resulting polymer (TPA-POP) was dried in an oven at 100 °C for 24 h (Figure 1).
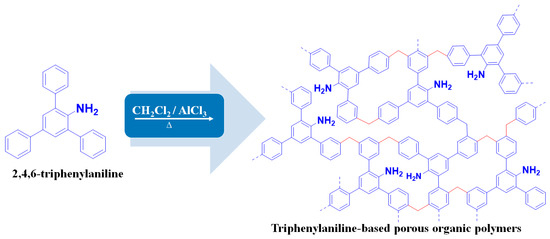
Figure 1.
Schematic illustration of the synthesis of TPA-POP.
2.3. Adsorption Studies
To study the effect of various parameters like initial solution MG concentration (Co: 50–500 mg/L), contact time (5–420 min), adsorbent dose (0.005–0.03 g), pH (3–7), and temperature (25–45 °C) on the elimination of MG dye using the TPA-POP, batch adsorption experiments were applied. In the typical adsorption, 0.01 g of the TPA-POP is added to a flask with 50 mL of a 50 mg/L MG solution. The solution was adjusted to pH: 6.0, and then the samples were shaken at equilibrium time (300 min). After the adsorption, samples were centrifuged, and we measured the remaining concentration of malachite green in the solution using a UV–vis spectrophotometer at λmax = 617 nm. MG removal efficiency (Re%) (Equation (1)) [40] and the amount of MG adsorbed on the TPA-POP (mg/g) (Equation (2)) [41] were calculated by the following equations:
where Co and Ceq,S (mg/L) are the initial MG dye concentration and the equilibrium concentration of the MG solution, respectively, and V (L) and m (g) are the volume of the solution and the weight of the TPA-POP, respectively.
For the regeneration and reusability study, 0.01 g of the TPA-POP adsorbent was brought into contact with the MG dye solution (Co: 50 mg/L, contact time: 300 min, pH: 6.0, shaking speed: 100 rpm). After MG dye adsorption, the MG-loaded TPA-POP was separated by centrifugation at 5000 rpm for 3 min and washed with deionized water. Then, 50 mL of each desorption agent solution (50% acetone, 50% ethanol, and 0.01 M HCl) was added to the MG-loaded TPA-POP. After that, the mixture was agitated for 300 min at 100 rpm, and then the samples were centrifuged and MG in the supernatant was measured. The MG dye’s desorption percentage (desorption %) was calculated using Equation (3) [42]. Five successive adsorption–desorption tests were carried out using the same adsorbent and the best desorbing solution (50% acetone). After each adsorption–desorption cycle, the adsorbents were washed with deionized water before starting the next adsorption cycle.
3. Results and Discussion
3.1. Characterization of TPA-POP
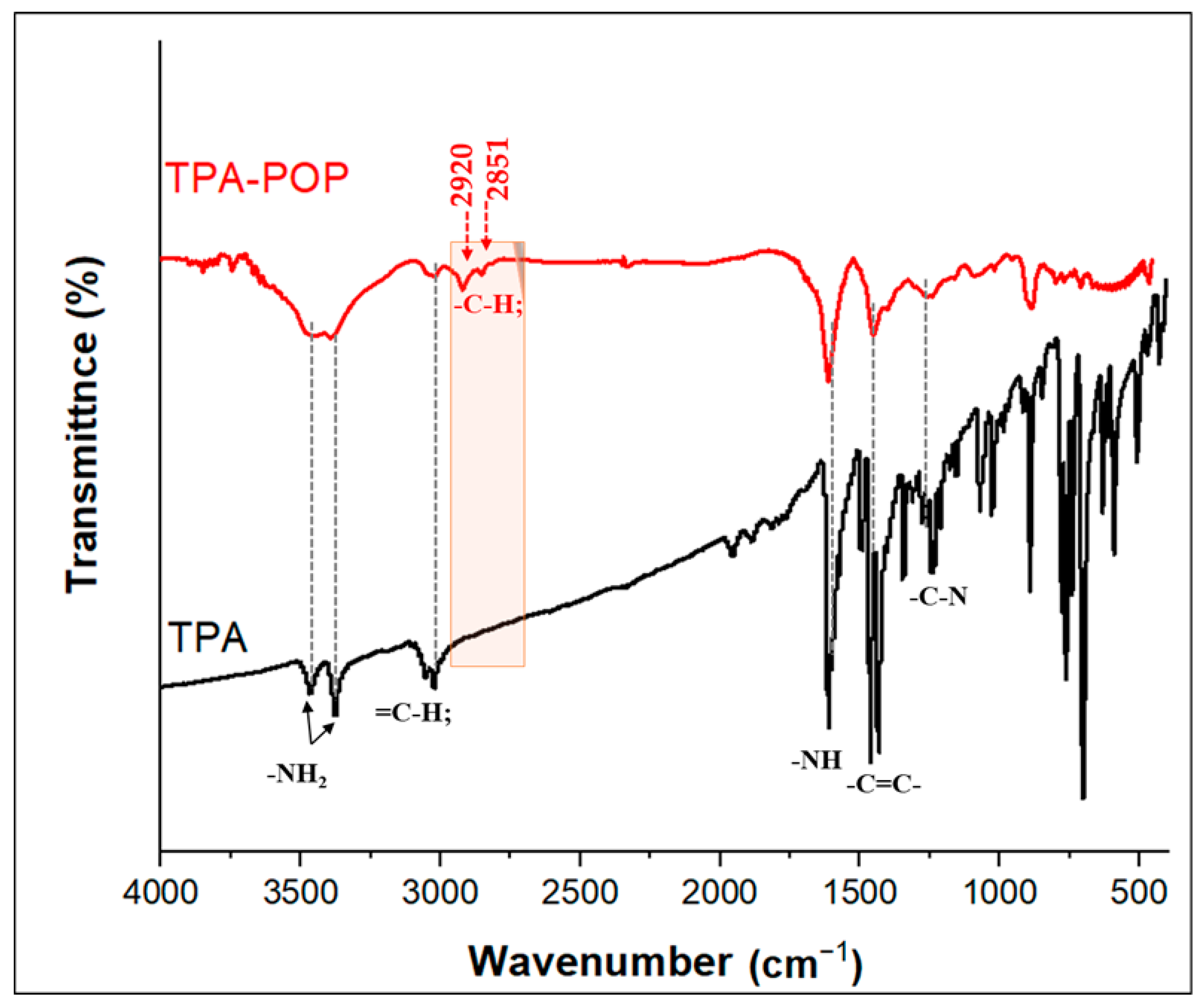
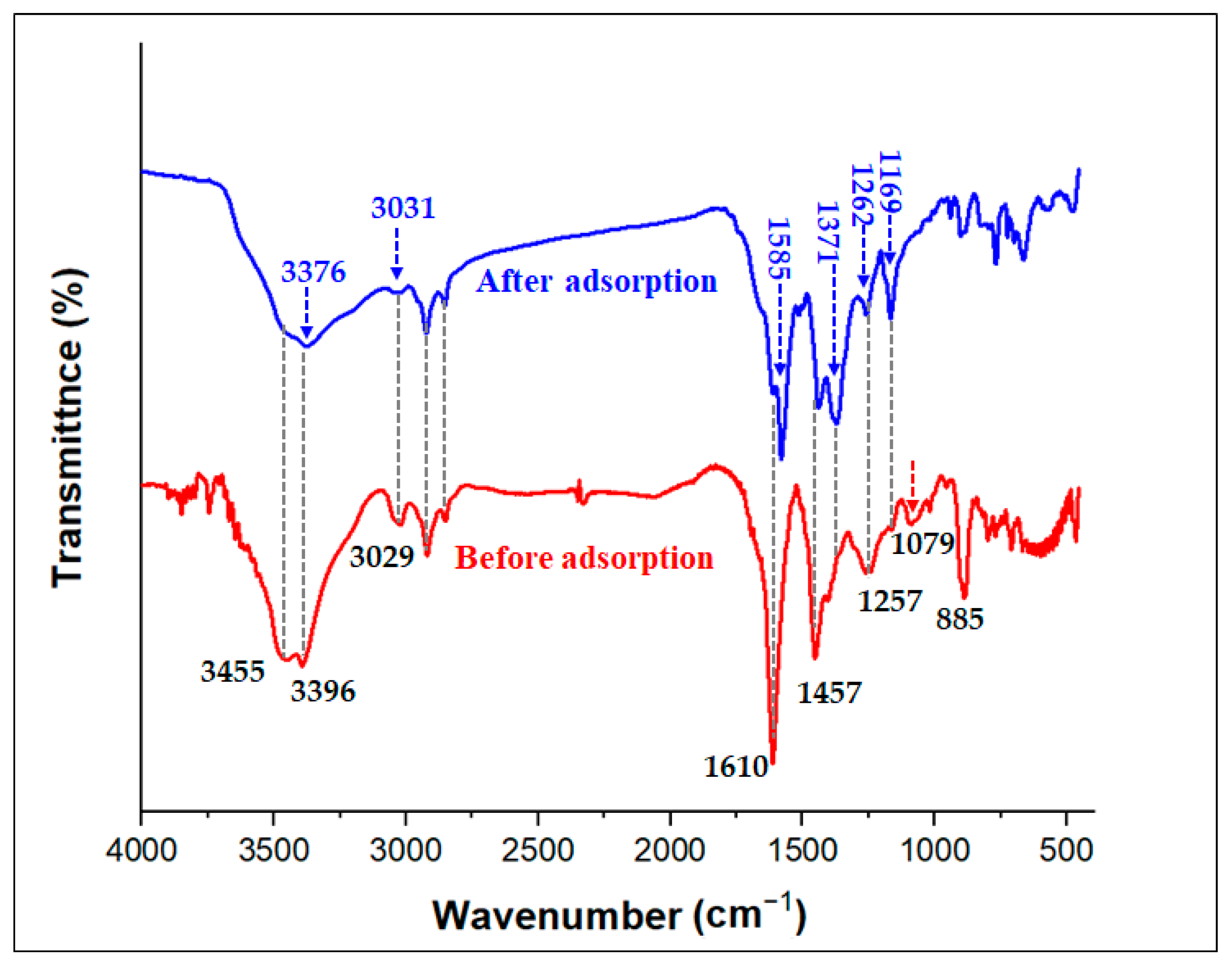
The FTIR spectra of TPA and the TPA-POP are shown in Figure 2. The FTIR spectrum of TPA exhibits two characteristic bands at 3471 and 3377 cm−1 for the NH2 stretching vibration [43]. The bands at 3061 and 3023 cm−1 are assigned to ν(=C-H). The bands at 1614 cm−1 and (1498–1459 cm−1) are assigned to the bending NH and ν(C=C) in the aromatic ring. From the FTIR spectra of the TPA-POP (Figure 2), the characteristic absorption bands at 3455, 3396, 3029, 2920, 2851, 1610, 1457, 1257, 1080, 885, and 706 cm−1 indicate the formation of the polymer. In detail, the two bands at 3455 and 3396 cm−1 are due to the NH2 bond. The bending of NH2 groups appeared in the TPA-POP at 1610 cm−1. The two new characteristic bands at 2920 and 2851 cm−1 are attributed to νasy(CH2) and νsym(CH2), respectively, indicating the successful formation of the polymer [44]. In addition, the bands at 3029 and 1457 cm−1 are attributed to ν (=C-H) and ν(C=C), respectively [45]. The bands ranging from 1084 to 1257 cm−1 are due to –C-N [46].
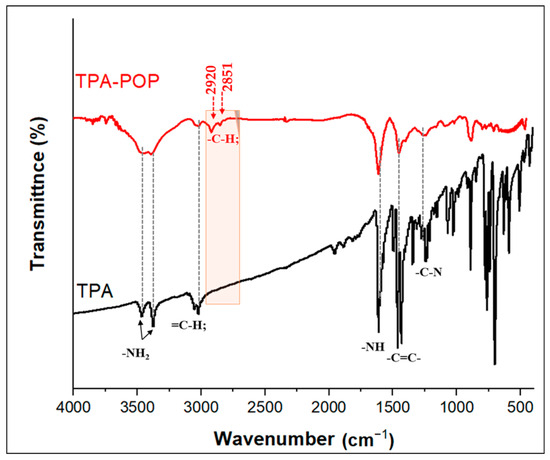
Figure 2.
FTIR spectra of TPA and TPA-POP.
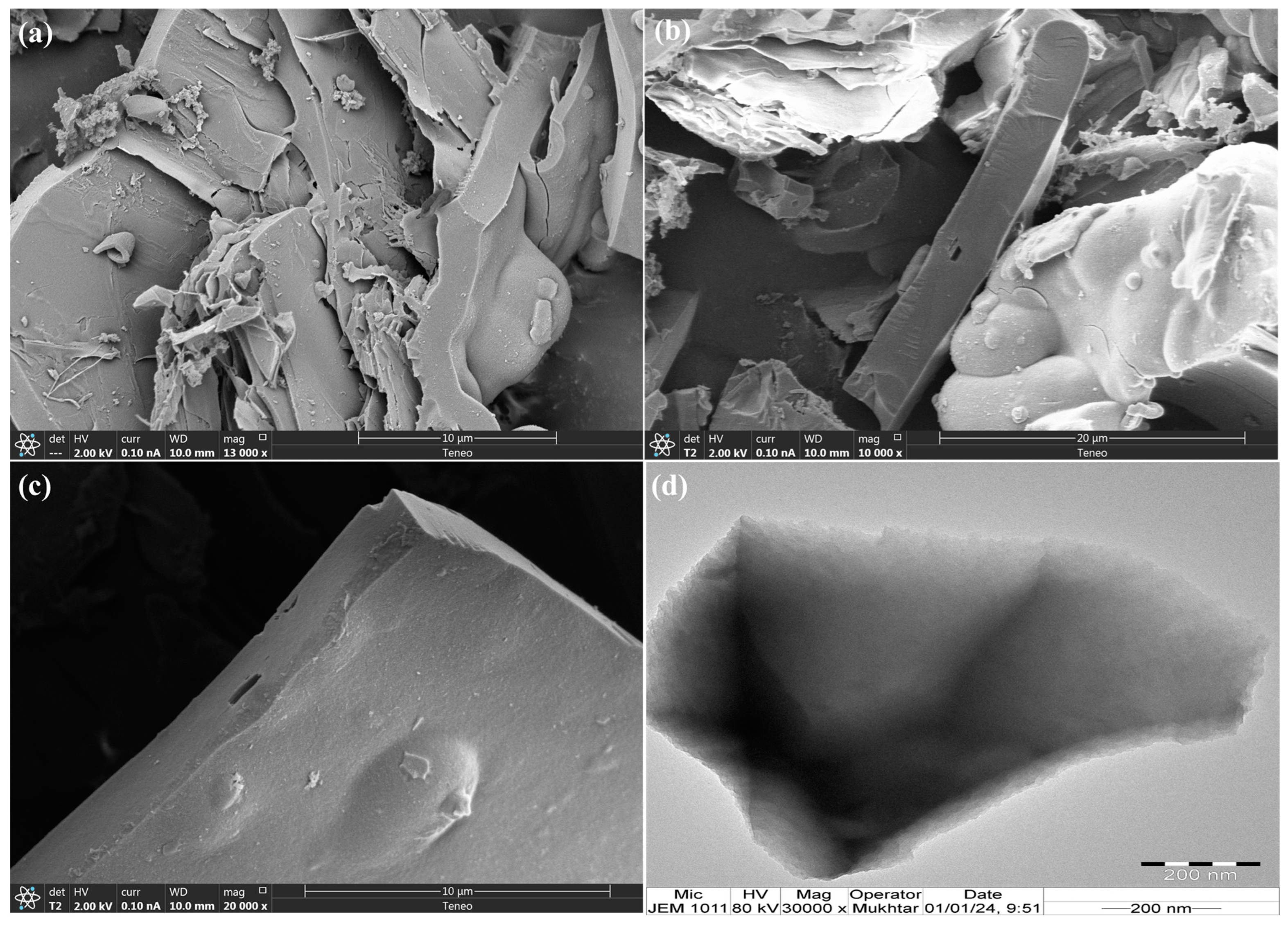
The morphology of the prepared TPA-POP was recorded by SEM and TEM analysis, as displayed in Figure 3. In the SEM image at different magnifications (Figure 3a–c) and the TEM image (Figure 3d), the TPA-POP surface shows block particles with layered structures.
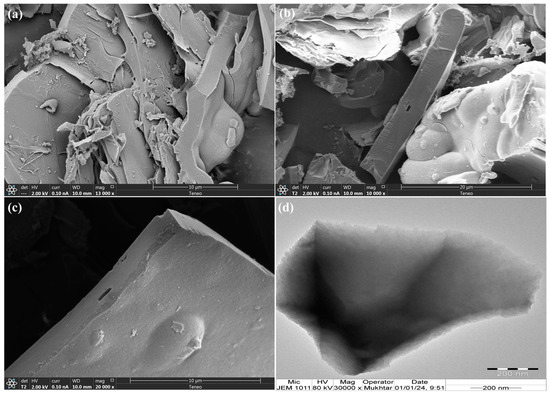
Figure 3.
The SEM images at 10 µm (a), 20 µm (b), and 10 µm (c) and TEM image of TPA-POP (d).
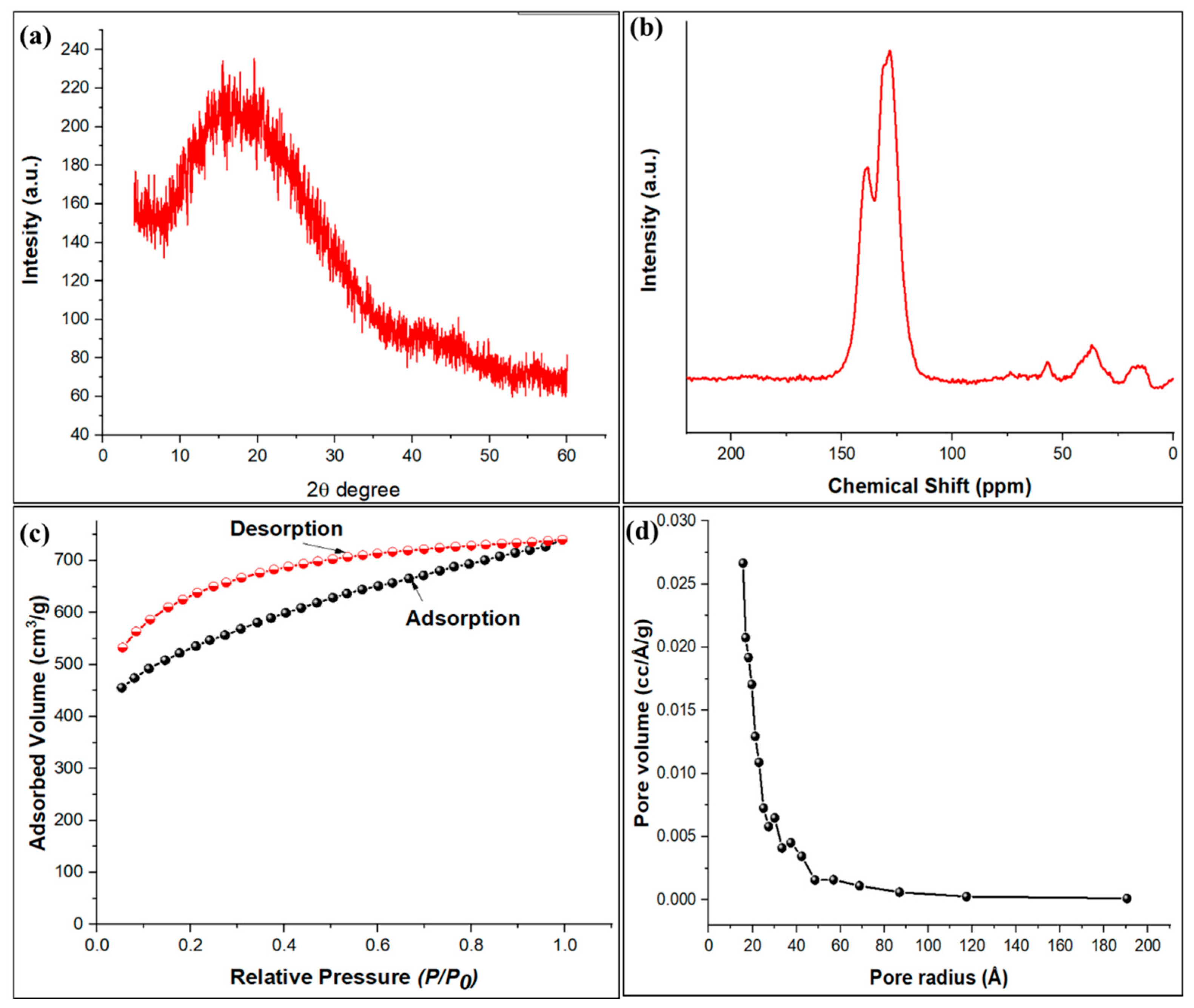
The X-ray diffraction pattern of the TPA-POP shows a broad peak in the 2 θ range of 15.4 to 20.1°, which is assigned to the characteristic of the amorphous organic polymer (Figure 4a). Similar results have been observed for porous polymers that exhibit broad XRD patterns [47,48,49,50]. The 13C (CP/MAS) NMR spectroscopy (Figure 4b) shows that the TPA-POP has three peaks at 128 ppm (substituted aromatic carbon), 138 ppm (unsubstituted aromatic carbon), and 36 ppm (carbon of methylene linkers). Figure 4c displays the nitrogen adsorption–desorption isotherm curve for the TPA-POP, while Figure 4d presents its corresponding pore size distributions. The BET surface area of the TPA-POP was 1625.14 m2/g. The BJH pore size analysis confirmed that the average pores radius is in the 1.57 nm range, which established the microporous nature of the TPA-POP as presented in Figure 4d. In addition, the pore volume value of the TPA-POP was measured as 0.353 cm3/g. The high surface area reflects the high adsorption capacity of TPA-POP toward the removal of MG dye. The obtained surface area was better than other porous organic polymers such as the sulfonic acid-functionalized porous organic polymer (76 m2/g) [51], porous organic polymer-immobilized copper (32.6 m2/g) [47], and the triazine-based porous organic polymer TPOP-2 (105 m2/g) [52].
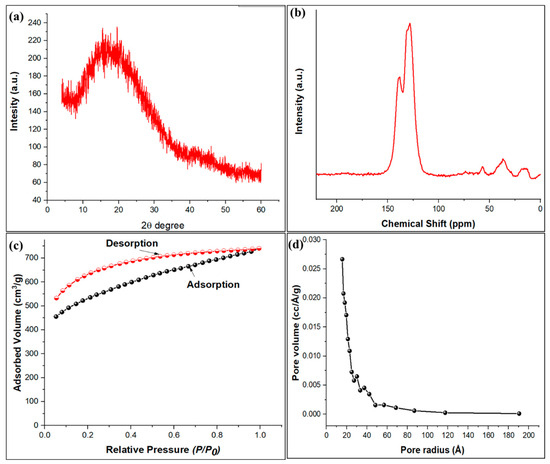
Figure 4.
XRD of TPA-POP (a), solid-state 13C CP/MAS NMR spectra of TPA-POP (b), nitrogen adsorption–desorption isotherm (c), and pore size distribution of TPA-POP (d).
3.2. Adsorption Studies
3.2.1. Selectivity Study
The prepared adsorbent (TPA-POP) was tested for malachite green (MG) and methyl blue (MB) dye adsorption. The selectivity study test was carried out with the following parameters: [Dye]: 20 mg/L, t: 1440 min, m: 0.01 g, T: 298 K, pH: 6.5, and shaking speed: 100 rpm. The results revealed that the removal efficiencies of MB and MG dyes on the TPA-POP were 91.5% and 45.71%, respectively. The high ability of the TPA-POP of MG removal compared to MB dye was due to the difference in the chemical structure of dyes in terms of molecular size and functional groups. Therefore, malachite green dye was selected for detailed adsorption experiments.
3.2.2. Effect of Factors on MG Adsorption by TPA-POP
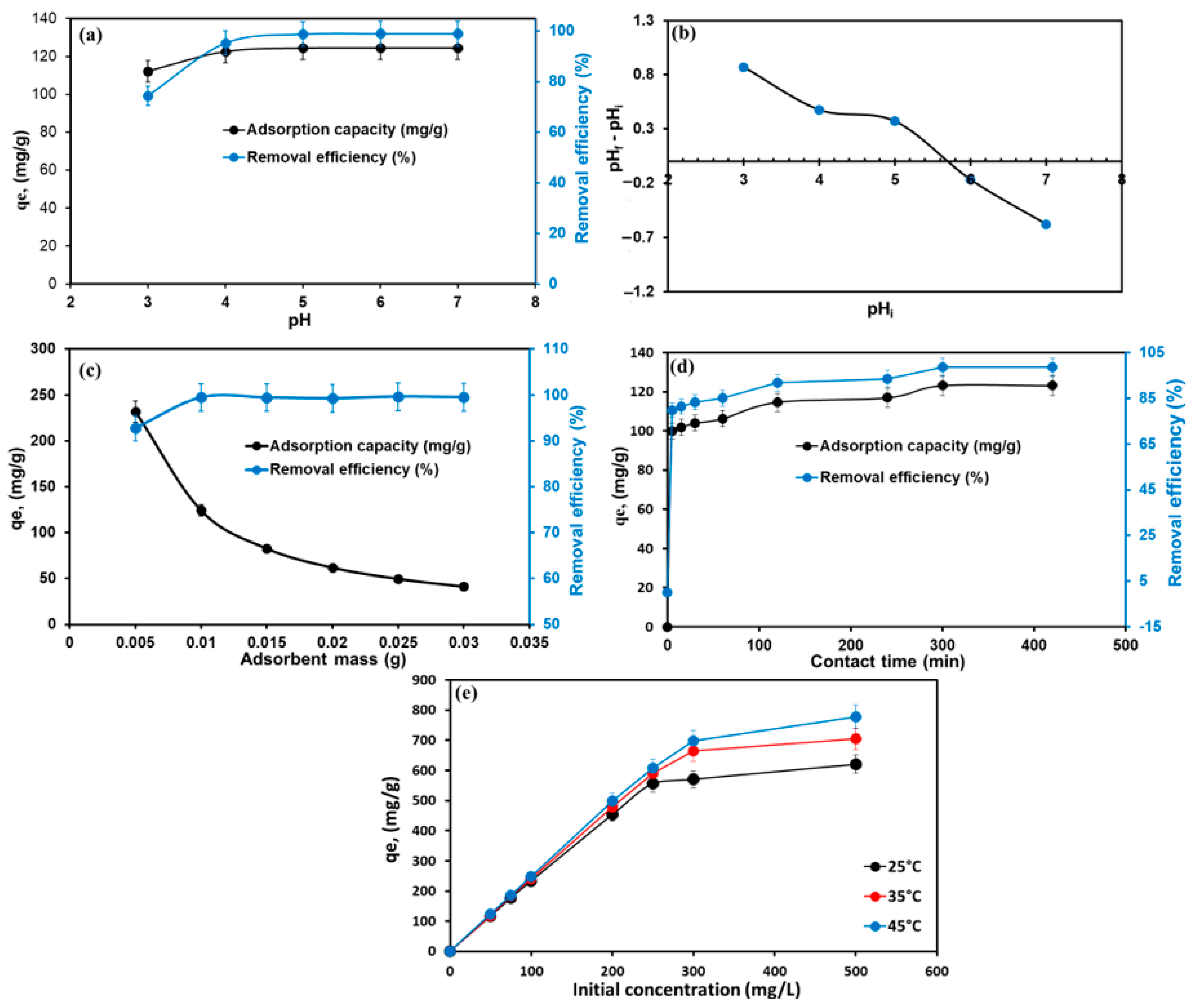
The pH value is an important factor that influences the surface charges of an adsorbent and the structure of dye molecules. MG is unstable under strong alkalis owing to the structure of MG dye changes to a carbinol base at a pH > 8 [53]. Thus, the effect of the initial pH on MG adsorption on the TPA-POP was studied in the range of 3–7 at Co of 50 mg/L, t of 1440 min, T of 298 K, m of 0.01 g, and a shaking speed of 100 rpm, as shown in Figure 5a. The outcomes exhibited that the removal efficiency improved from 74.40% to 95.34% with a rise in initial pH from 3.0 to 4.0, and then gradually increased to reach a maximum (99.06%) at a pH of 6.0. Figure 5b shows the Zeta potential of the TPA-POP adsorbent. The pHpzc of the TPA-POP adsorbent was 5.63; thus, at a pH < pHpzc, the surface charge of the TPA-POP is positive, which implies electrostatic repulsion between the TPA-POP surface and the MG+ dye, resulting in a reduction in MG removal. The increase in uptake capacity with rising initial pH is owing to the reduction in the number of hydrogen ions occupying the active adsorption sites of the TPA-POP adsorbent, which increased the collision probability and electrostatic attraction between the negative charge of the TPA-POP surface and MG+. The high efficiency for MG at pH 3.0 indicates the hydrogen bonding and π–π interaction mechanism involved in the adsorption of MG onto the TPA-POP. The maximum removal efficiency and adsorption capacity were 99.06% and 124.52, respectively, at pH > pHpzc. Therefore, pH 6.0–7.0 was selected for further investigations in this work.
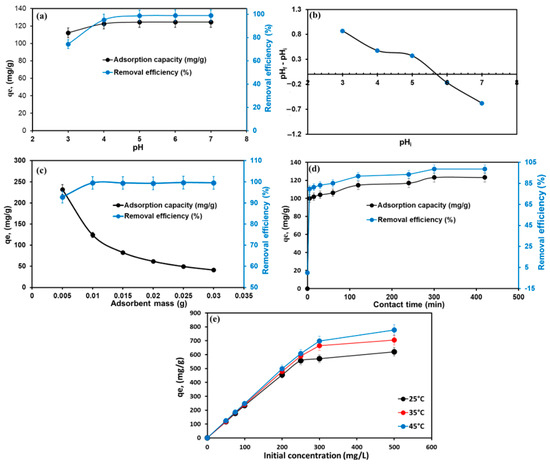
Figure 5.
Effect of pH on MG dye adsorption (a), Zeta potential at different pH (b), effect of adsorbent dosage (c), contact time (d), and inertial MG concentrations on MG dye adsorption on TPA-POP (e).
The impact of the change in TPA-POP dosage for MG removal was studied by changing the mass of the TPA-POP, ranging from 0.005 to 0.03 g at MG of 50 mg/L, t of 1440 min, T of 298 K, pH of 6.0, and shaking speed of 100 rpm, as presented in Figure 5c. The outcome exhibited that the elimination of MG was enhanced from 92.79% to 99.43% with an increase in TPA-POP dosage from 0.005 to 0.01 g and then remained constant as the TPA-POP dosage increased to 0.03 g. This is due to the availability of absorption sites on the TPA-POP. Therefore, 0.01 g of TPA-POP was chosen for further investigations in this work. Similar results were observed for the removal of MG using magnetic hydrochar-grafted chitosan [54].
The effect of contact time on the adsorption process was analyzed at different interval times (5–420 min) at MG of 50 mg/L, T of 298 K, pH of 6.0, and m of 0.01 g, as represented in Figure 5d. The initial rate of MG uptake increased sharply within 5 min, wherein 78.83% of MG was removed, and then gradually increased to reach the maximum (98.51%) within 300 min. The maximum quantity of MG adsorbed and the removal efficiency of MG were 123.11 mg/g and 98.51%, respectively. The fast MG removal at 5 min could be due to the presence of more vacant sites distributed over the TPA-POP surface [55]. Thus, 300 min was designated as the optimum time for further investigations in this work.
The impact of the initial MG concentration on the adsorption process was studied at various concentrations of MG dye (50–500 mg/L) and different temperatures (298–318 K) using 0.01 g of TPA-POP at t of 300 min and pH of 6.0, as represented in Figure 5e. As the concentration of MG increased from 50 to 500 mg/L, the qe was enhanced from 116.94 to 620.72 mg/g. This is attributed to the increase in mass transfer driving force from the solution to the TPA-POP surface. The adsorption efficiency of MG was reduced with higher MG concentrations due to the saturation of the surface-active sites of the TPA-POP with MG molecules as the initial MG dye concentration increased. The maximum MG removal was 99.58% and 97.21% using 200 and 250 mg/L. Figure 5e also shows the influence of temperature on the adsorption process. It was observed that the elimination efficiency of the MG dye improved from 93.55% to 99.42% at 50 mg/L and from 90.80% to 99.58% at 200 mg/L with an increase in temperature from 25 to 45 °C, suggesting that the removal of MG dye was endothermic. The improvement in MG removal efficiency with rising temperatures is because the temperature provides energy for the interaction between the adsorbed MG molecules and the active adsorption sites available for the TPA-POP [56]. Similar results were reported on the elimination of MG dye using MgO-loaded carbon foam [57]. Therefore, the temperature was kept at 45 °C for other investigations in this work.
3.3. Adsorption Model
3.3.1. Adsorption Isotherms
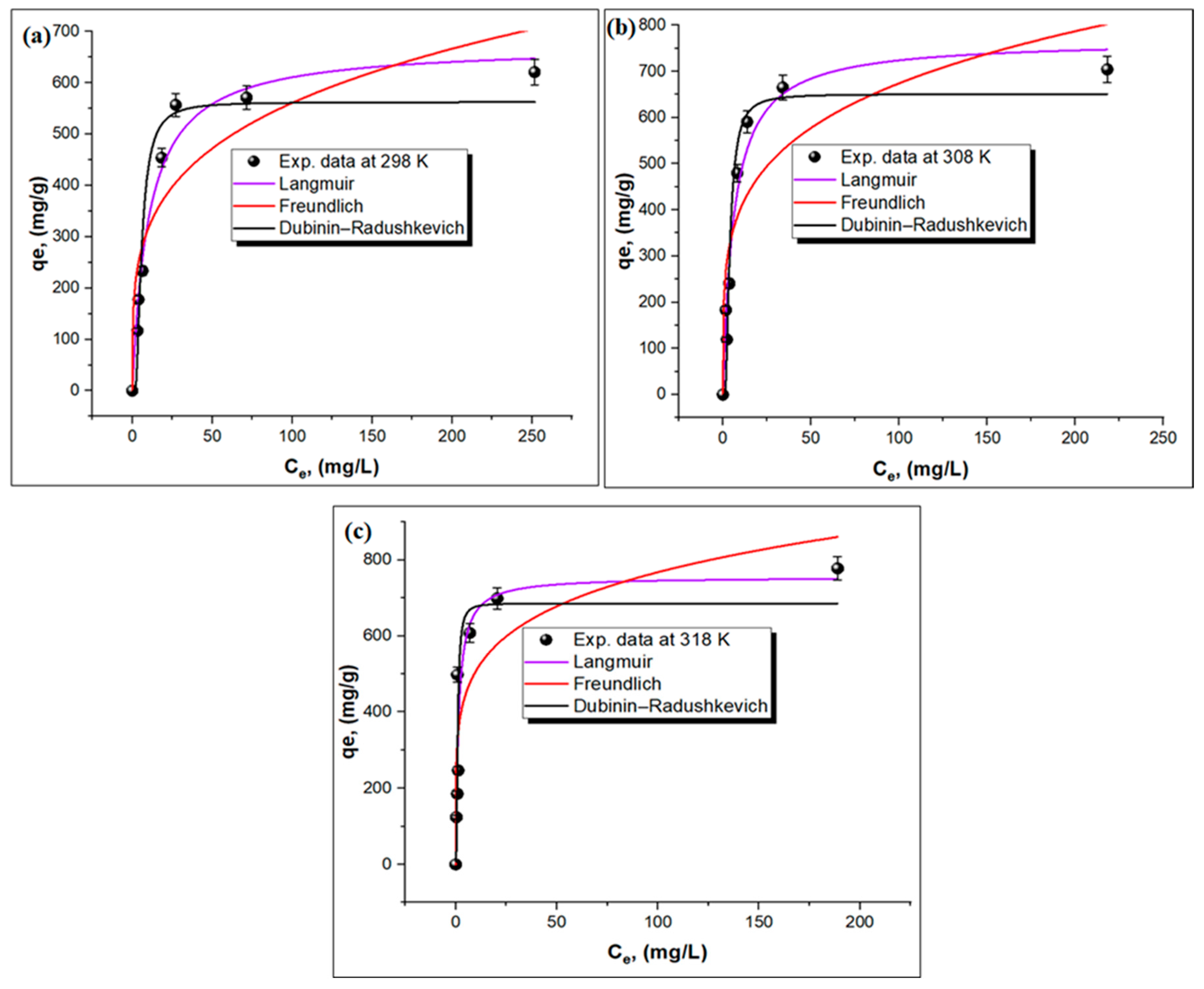
Three isotherm models, namely, Dubinin–Radushkevich (D-R), Freundlich, and Langmuir models, were applied to determine the maximum uptake capacity of the TPA-POP and the adsorption mechanism of MG. The equations of these models are shown in Supplementary Materials (Text S1). Figure 6a–c shows the curve-fitting plots of the isotherm models, while the values of isotherm parameters are displayed in Table 1. The higher R2 value obtained from the Langmuir model (0.97412) is better than those of the Dubinin-R2 (0.94564) and Freundlich (0.8137) models, indicating a monolayer adsorption process. The qm was found to be 755.72 mg/g at 45 °C. The n values ranged between 4.02 and 4.57, which indicates that the adsorption process is favorable [58]. The mean free energy (Ea) values were in the range of 0.206–0.988 kJ/mol (Table 1), which is Ea < 8 kJ/mol [59], indicating that the adsorption of malachite green onto the TPA-POP is in the physical mode [54].
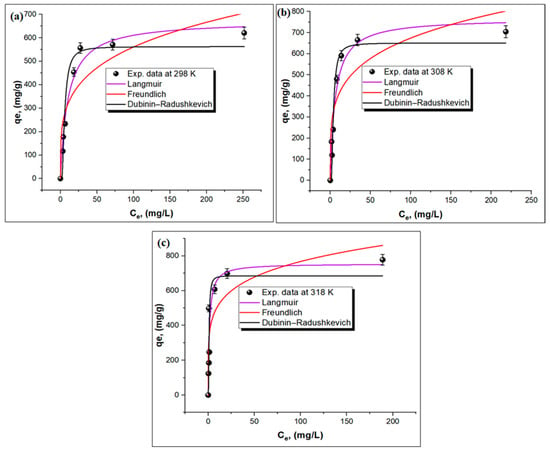
Figure 6.
Isotherm models at different temperature: 298 K (a), 308 K (b), and 318 K (c) for MG adsorption on TPA-POP.

Table 1.
Parameters of isotherm equations for MG adsorption on TPA-POP.
3.3.2. Adsorption Kinetics
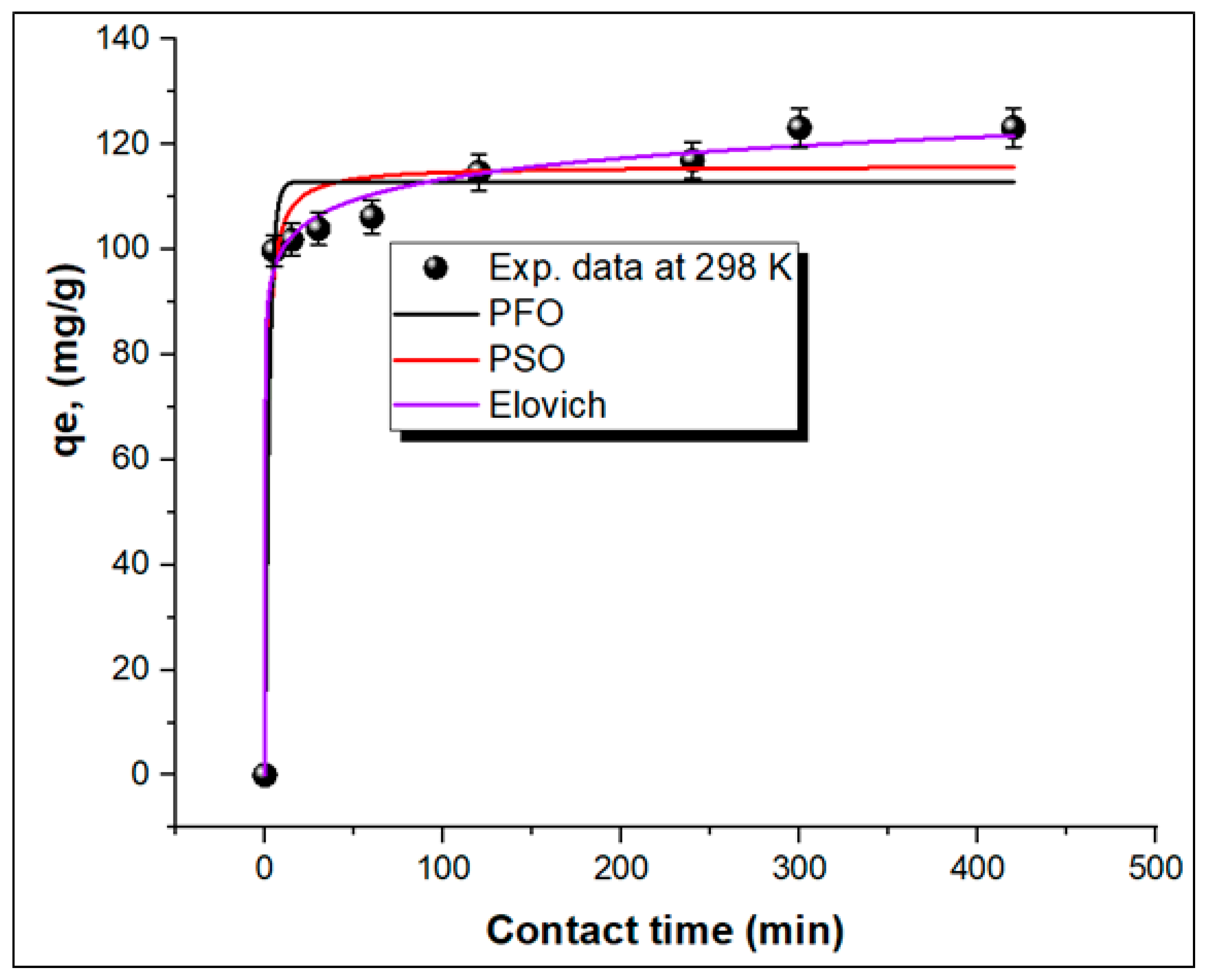
Three nonlinear kinetic models, namely, pseudo-first-order (PFO), Elovich, and pseudo-second-order (PSO) models, were used to determine the mechanism of adsorption of MG onto the TPA-POP. The equations for these kinetic models are shown in Supplementary Materials (Text S2). Figure 7 shows the curve plots of the kinetic models, and their parameters are listed in Table 2. The higher regression factor (R2) value obtained from the Elovich kinetic model (0.99468) is better than PFO (0.97067) and PSO (0.95353) models, suggesting that the adsorption of MG on the TPA-POP may be of the chemisorption type [20,60,61].
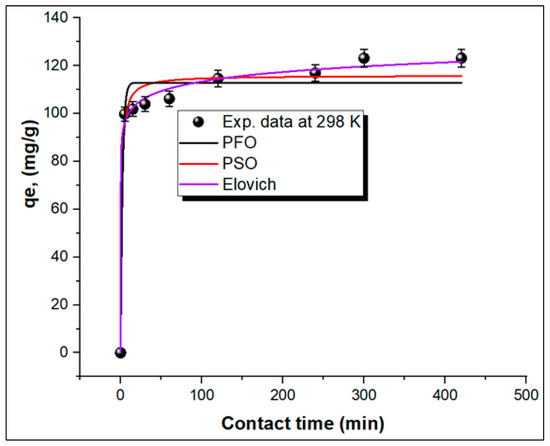
Figure 7.
Nonlinear kinetic models for MG adsorption on TPA-POP.

Table 2.
Parameters of kinetic equations for MG adsorption on TPA-POP.
3.3.3. Thermodynamic Studies
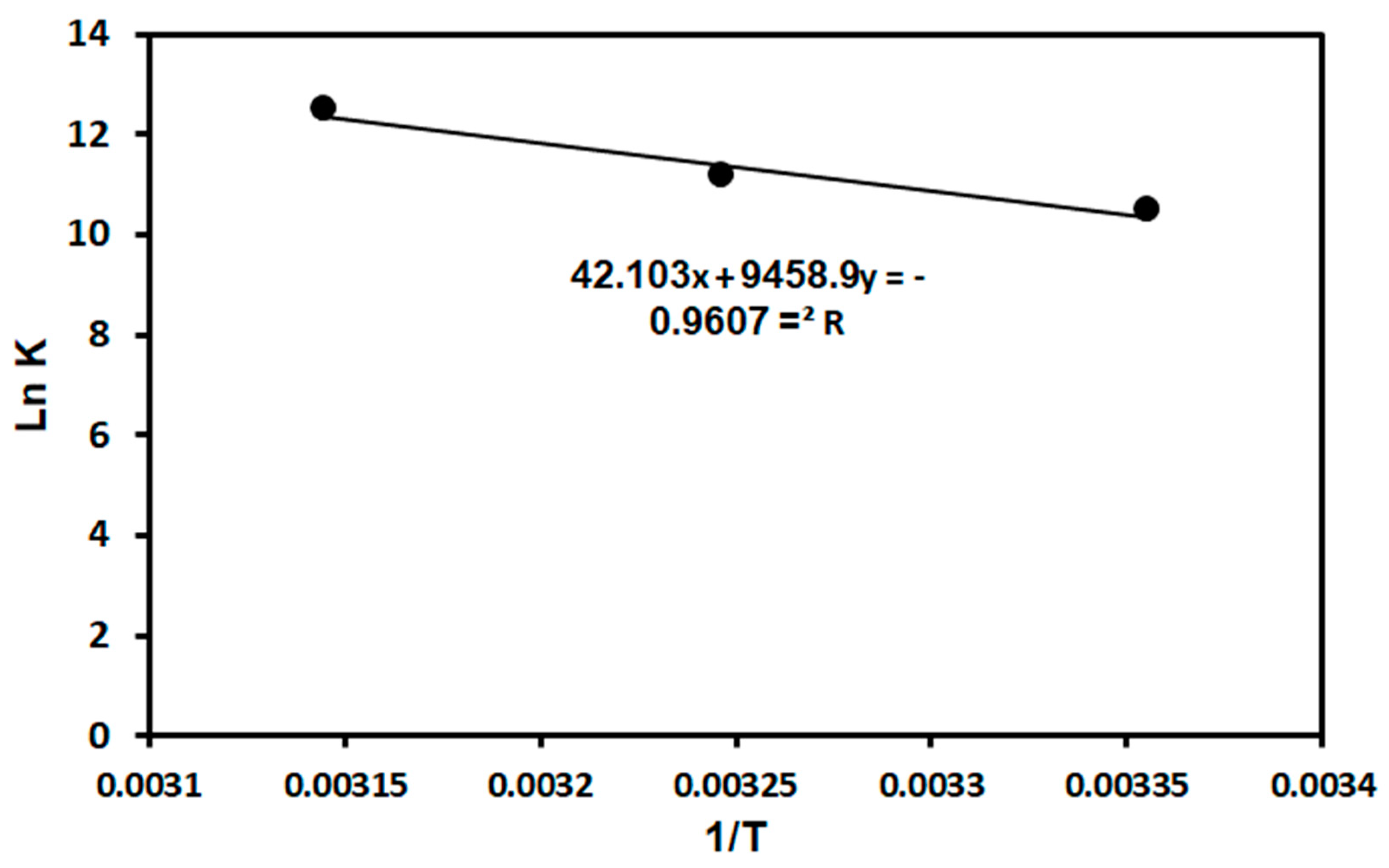
Thermodynamics parameters such as the standard entropy change (ΔS°), enthalpy change (ΔH°), and Gibb’s free energy (ΔG°) were used to determine the spontaneity and feasibility of MG adsorption on the TPA-POP. The values of ΔG° were calculated using Equation (4), while the ΔH° and ΔS° values were calculated using the slope and intercept of the Van’t Hoff Equation (5) [62,63,64], respectively. The Van’t Hoff linear plot of ln versus 1/T is represented in Figure 8.
where R is the universal gas constant (J/mol. K), T (K) is the absolute temperature, and K°e is the equilibrium constant (L/mol) calculated by multiplying the Langmuir constant (KL) by the molecular weight of MG dye (g/mol) and then 1000 [62,63,64]. The thermodynamic data are displayed in Table 3. The outcomes showed that MG adsorption on the TPA-POP was endothermic and spontaneous due to the positive and negative values of ΔH° and ΔG°, respectively. Similar results were observed for MG adsorption using AC [65]. The values of ΔH° for physical and chemical adsorption fall in the range of 2.1–20.9 kJ/mol and 80–200 kJ/mol, respectively [66]. From Table 3, the value of ΔH° for MG dye is 78.64 KJ/mol, which is higher than 20.9 but less than 80 kJ/mol, indicating the physicochemical sorption of MG dye on the TPA-POP.
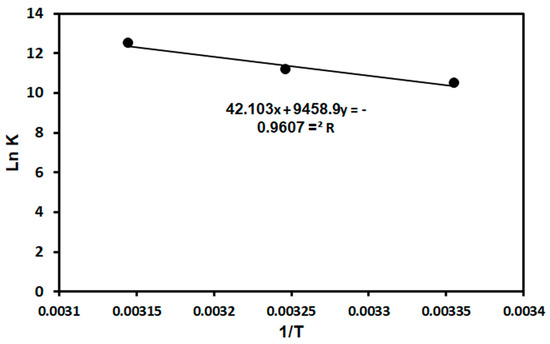
Figure 8.
Van’t Hoff linear plot for MG adsorption on TPA-POP.

Table 3.
Parameters of thermodynamic equations for MG adsorption on TPA-POP.
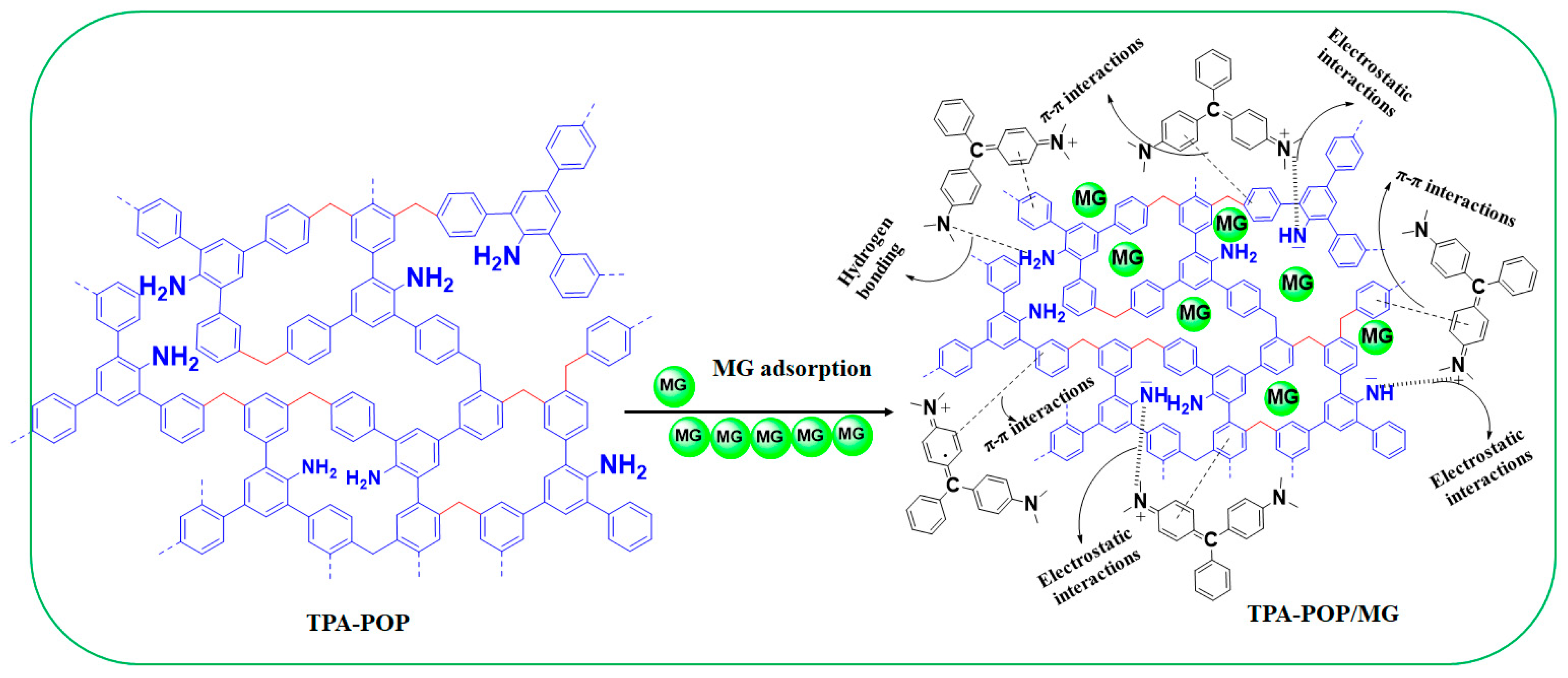
3.4. Proposed Adsorption Mechanism
The results from the kinetics, isotherm, thermodynamic, FTIR analysis, and pH values explain the probable adsorption mechanism of MG dye by the TPA-POP. Based on the effect of the initial pH, the high removal of MG (74.40%) at pH 3.0 indicates that H-bonding and π–π interaction mechanisms participate in the adsorption of MG dye. Hydrogen bonding occurs between hydrogen-rich nitrogen of the amino group over the surface of the TPA-POP and nitrogen atoms of N(CH3)2 over the MG dye. π–π interactions occur between benzene rings over the TPA-POP surface and aromatic rings of the MG molecule. In addition, the increase in uptake capacity with increasing pH also indicated the electrostatic interaction participation in MG adsorption. According to the FTIR spectrum, the spectra of TPA-POP/MG showed a decrease and an increase in the band positions with slight shifting due to the binding of MG onto the TPA-POP surface. After MG adsorption (Figure 9), the spectra of TPA-POP/MG showed a decrease and an increase in the band positions with slight shifting indicating the binding of MG dye onto the TPA-POP surface. The adsorption bands for NH2 stretching and NH bending were reduced in intensity and shifted to 3376 and 1585 cm−1, respectively, due to the interaction between the hydrogen-rich nitrogen of the amino group over the surface of the TPA-POP and the nitrogen atoms of N(CH3)2 over the surface of MG dye by H-bonding or electrostatic interactions. The adsorption band for =C-H stretching was reduced in intensity and shifted to 3031 cm−1 after MG adsorption due to the interaction between benzene rings over the TPA-POP surface and aromatic rings of the MG molecule by π–π interactions. The increase in intensity of the band at 1169 cm−1 is owing to the interaction of the –C-N bond with cationic MG [67]. Finally, the adsorption mechanism of MG onto the TPA-POP can be explained by three mechanisms, namely, hydrogen bonding, electrostatic interaction, and π–π interaction, as shown in Figure 10.
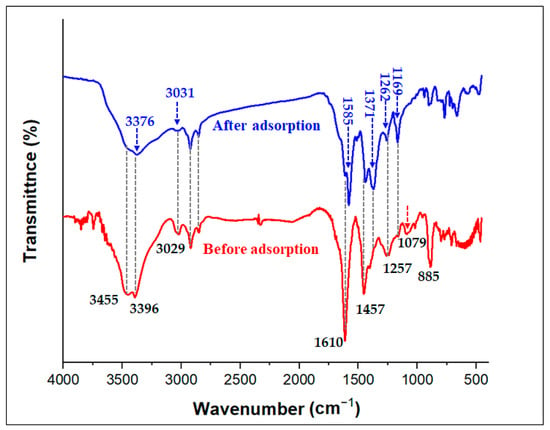
Figure 9.
FTIR spectrum of TPA-POP before and after MG adsorption.
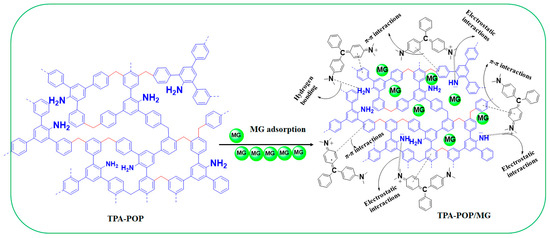
Figure 10.
Proposed adsorption mechanism of MG on TPA-POP.
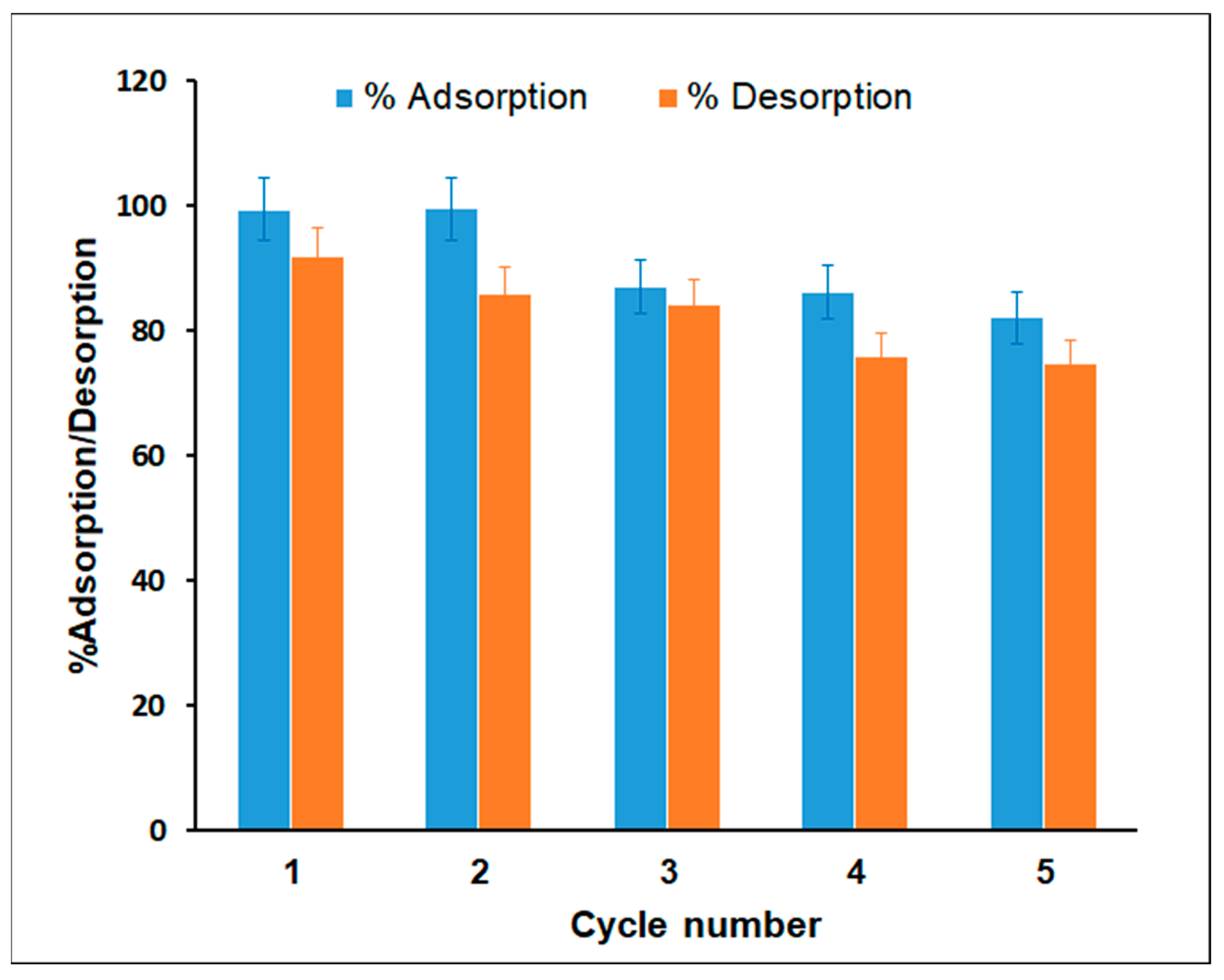
3.5. Regeneration and Reusability of Adsorbent
The regeneration and recycling of prepared adsorbents determines their practicality and cost-effectiveness. To assess the possible desorption of MG from the TPA-POP adsorbent and the reusability of the TPA-POP as an adsorbent, three different chemical reagents, including 0.1 M HCl, 50% ethanol, and 50% acetone, were examined as eluents for the desorption of MG dye from the TPA-POP. The results show that the acetone exhibited a 90% desorption percentage compared to 83% and 76% for 50% ethanol/water and 50% acetone/water, respectively. The high desorption percentage may be due to the weak and reversible physical bonds between the MG dye and the TPA-POP adsorbent. Five successive adsorption–desorption tests were carried out using 50% acetone as an eluent, as depicted in Figure 11. The MG removal efficiency was still greater than 82.12% after the TPA-POP adsorbent was recycled five times, indicating that the MG adsorption of the TPA-POP occurred due to the physisorption mechanism. The decrease in the removal efficiency of MG dye during the desorption process may be due to the decrease in active sites of the adsorbent. These results indicate that the prepared TPA-POP adsorbent is cost-effective for MG adsorption from aqueous solutions. Similar results have been demonstrated by Amin et al. for MG dye desorption from sulfonated-triptycene (TRIP-SO3H) using acetone. They found that the MG adsorption on TRIP-SO3H was reduced to 88.52% after five cycles [68].
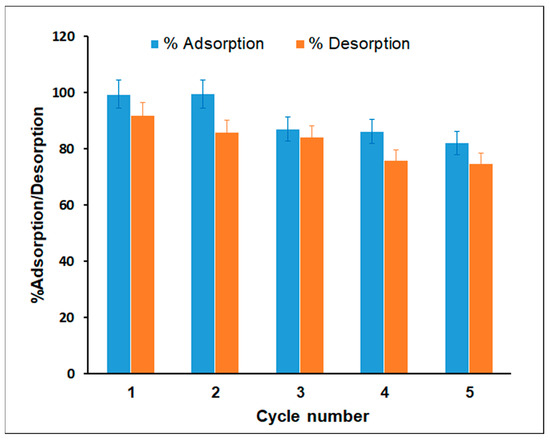
Figure 11.
TPA-POP reusability of MG.
3.6. Comparison with other Adsorbents
The comparison of maximum uptake capacity obtained using the Langmuir isotherm for MG adsorption on the TPA-POP, as well as the BET surface area, and the experimental conditions for different adsorbents are listed in Table 4. The TPA-POP adsorbent shows a high adsorption affinity towards MG dye compared to other previous studies. These results may be due to the high surface area and rich nitrogen present in the amino group over the surface of the TPA-POP, which act as adsorption sites for MG dye. In addition, the TPA-POP adsorbent exhibits good reusability for up to five adsorption–desorption cycles, indicating that the TPA-POP is an interesting alternative adsorbent to eliminate malachite green dye from water environments.

Table 4.
Comparison of the experimental conditions and maximum uptake capacity of the TPA-POP with other different adsorbents for removal of MG dye.
4. Conclusions
A new adsorbent of triphenylaniline-based porous organic polymers (TPA-POPs) was successfully prepared. The prepared TPA-POP was used to remove MG dye from water. FTIR, 13C CP/MAS NMR, and BET surface area results confirmed the successful formation of the TPA-POP adsorbent. The TPA-POP adsorbent had a superior BET surface area of 1625.14 m2/g with an average microspore volume of 0.353 cm3/g and a pore radius of 1.57 nm, reflecting the high amount of MG adsorbed on the TPA-POP. The results showed that the maximum MG elimination efficiency and uptake capacity were 99.60% and 755.72 mg/g, respectively, at optimal parameter values (pH: 6.0, m: 0.01 g, T: 45 °C, and time: 300 min). The kinetic data fit the Elovich kinetic model well, while the isothermal data were best fitted to the Langmuir model, revealing monolayer adsorption. The thermodynamic study showed that MG adsorption onto the TPA-POP was endothermic and spontaneous. The TPA-POP can be reused five times with a removal efficiency of up to 82.12%. H-bonding, electrostatic interactions, and π–π were the adsorption mechanisms of MG. Finally, the results exhibited that the TPA-POP has a high ability to remove MG attributed to its superior high surface, high porosity, and tunable features in its structure and function.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16131869/s1, Adsorption isotherms (Text S1) and kinetics models (Text S2). References [74,75,76,77,78,79] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.M.; methodology, S.M. and A.A.A.; software, A.A.A.; validation, E.H.A. and G.M.I.; formal analysis, B.E.-G. and E.H.A.; investigation, B.E.-G., G.M.I. and M.A.B.; resources, E.M.E.; data curation, E.M.E. and M.A.B.; writing—original draft preparation, A.A.A.; writing—review and editing, A.A.A.; supervision, S.M.; project administration, S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Graduate Studies and Scientific Research at the University of Bisha through the Fast-Track Research Support Program.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Naushad, M. Advances in the role of natural gums-based hydrogels in water purification, desalination and atmospheric-water harvesting. Int. J. Biol. Macromol. 2022, 222, 2888–2921. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly(acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.H.; Ramadhan, Z.K.; Al-Ansari, N.; Sharma, G.; Naushad, M.; Bathula, C. Precipitation of (Mg/Fe-CTAB)—Layered double hydroxide nanoparticles onto sewage sludge for producing novel sorbent to remove Congo red and methylene blue dyes from aqueous environment. Chemosphere 2022, 291, 132693. [Google Scholar] [CrossRef] [PubMed]
- Chennah, A.; Khan, M.A.; Zbair, M.; Ait Ahsaine, H. NiO/AC Active Electrode for the Electrosorption of Rhodamine B: Structural Characterizations and Kinetic Study. Catalysts 2023, 13, 1009. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Ahmed Alshareef, S.; Abdullah Alqadami, A.; Ali Khan, M.; Alanazi, H.S.; Raza Siddiqui, M.; Jeon, B.-H. Simultaneous co-hydrothermal carbonization and chemical activation of food wastes to develop hydrochar for aquatic environmental remediation. Bioresour. Technol. 2022, 347, 126363. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.A.; Otero, M.; Abdullah, E.C.; Hosomi, M.; Terada, A.; Riya, S. Synthesis of CTAB intercalated graphene and its application for the adsorption of AR265 and AO7 dyes from water. J. Colloid Interface Sci. 2017, 493, 51–61. [Google Scholar] [CrossRef]
- Khan, M.A.; Otero, M.; Kazi, M.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Alothman, Z.A.; Sumbul, S. Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. J. Hazard. Mater. 2019, 365, 759–770. [Google Scholar] [CrossRef]
- Hussain Hakami, A.A.; Ahmed, M.A.; Khan, M.A.; AlOthman, Z.A.; Rafatullah, M.; Islam, M.A.; Siddiqui, M.R. Quantitative Analysis of Malachite Green in Environmental Samples Using Liquid Chromatography-Mass Spectrometry. Water 2021, 13, 2864. [Google Scholar] [CrossRef]
- Yadav, V.K.; Singh, B.; Gacem, A.; Yadav, K.K.; Gnanamoorthy, G.; Alsufyani, T.; Hussein, H.S.; Awwad, N.S.; Verma, R.; Inwati, G.K.; et al. Development of Novel Microcomposite Materials from Coal Fly Ash and Incense Sticks Ash Waste and Their Application for Remediation of Malachite Green Dye from Aqueous Solutions. Water 2022, 14, 3871. [Google Scholar] [CrossRef]
- Ullah, S.; Ur Rahman, A.; Ullah, F.; Rashid, A.; Arshad, T.; Viglašová, E.; Galamboš, M.; Mahmoodi, N.M.; Ullah, H. Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies. Water 2021, 13, 965. [Google Scholar] [CrossRef]
- Alomar, T.S.; AlMasoud, N.; Sharma, G.; ALOthman, Z.A.; Naushad, M. Incorporation of trimetallic nanoparticles to the SiO2 matrix for the removal of methylene blue dye from aqueous medium. J. Mol. Liq. 2021, 336, 116274. [Google Scholar] [CrossRef]
- Nirmaladevi, S.; Palanisamy, N. A comparative study of the removal of cationic and anionic dyes from aqueous solutions using biochar as an adsorbent. Desalin Water Treat 2020, 175, 282–292. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Kumar, A.; Ahamad, T.; Stadler, F.J. Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity. J. King Saud Univ.-Sci. 2020, 32, 1359–1365. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Balakumar, S.; Aruna, P.; Ganesan, S.; Rajendran, S.; Naushad, M. Low cost and quick time absorption of organic dye pollutants under ambient condition using partially exfoliated graphite. J. Water Process Eng. 2020, 34, 101078. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Al-Muhtaseb, A.H.; Khan, A.A.; Khan, A. Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Verma, S.; Kim, K.-H.; Kumar, N.; Bhattacharya, S.S.; Naushad, M.; Dutta, R.K. Amine-amide functionalized graphene oxide sheets as bifunctional adsorbent for the removal of polar organic pollutants. J. Hazard. Mater. 2022, 429, 128308. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Thakur, B.; Vo, D.-V.N.; Gao, B.; Al-Kahtani, A.A.; Stadler, F.J. Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 2021, 416, 125714. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Shihab, A.H.; Naushad, M.; Ahamad, T.; Sharma, G.; Al-Sheetan, K.M. Green synthesis for novel sorbent of sand coated with (Ca/Al)-layered double hydroxide for the removal of toxic dye from aqueous environment. J. Environ. Chem. Eng. 2021, 9, 105342. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Fajal, S.; Dutta, S.; Ghosh, S.K. Porous organic polymers (POPs) for environmental remediation. Mater. Horiz. 2023, 10, 4083–4138. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, X.; Luo, P.; Dai, S.; Zhang, W.; Zhang, Y. Novel Fluorinated Nitrogen-Rich Porous Organic Polymer for Efficient Removal of Perfluorooctanoic Acid from Water. Water 2022, 14, 1010. [Google Scholar] [CrossRef]
- Ghanbari, J.; Mobinikhaledi, A. Synthesis of a novel porous organic polymer containing triazine and cyclohexanone rings as an efficient methyl red adsorbent from aqueous solutions. Sci. Rep. 2023, 13, 12962. [Google Scholar] [CrossRef] [PubMed]
- Sumayli, A.; Alshahrani, S.M.; Alqahtani, A.S. Separation of organic molecules using porous polymeric membranes: Model development using advanced hybrid CFD and artificial intelligence. Ain Shams Eng. J. 2024, 102834. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Zhao, X.; Qin, M.; Li, X.; Jin, W.; Zhang, D. One-pot synthesis of conjugated microporous polymers based on extended molecular graphenes for hydrogen storage. Polymer 2019, 174, 96–100. [Google Scholar] [CrossRef]
- Tadayoni, N.S.; Dinari, M.; Roy, A.; Karimi Abdolmaleki, M. Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants. Polymers 2024, 16, 1543. [Google Scholar] [CrossRef]
- Shi, K.; Song, N.; Zou, Y.; Zhu, S.; Tan, H.; Tian, Y.; Zhang, B.; Yao, H.; Guan, S. Porphyrin-based porous polyimides: Synthesis, porous structure, carbon dioxide adsorption. Polymer 2019, 169, 160–166. [Google Scholar] [CrossRef]
- Liu, C.; Xia, M.; Zhang, M.; Yuan, K.; Hu, F.; Yu, G.; Jian, X. One-pot synthesis of nitrogen-rich aminal-and triazine-based hierarchical porous organic polymers with highly efficient iodine adsorption. Polymer 2020, 194, 122401. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Liu, M.; Zhang, Y.; Wu, C.; Zhang, Y. Porphyrin-functionalized porous polysulfone membrane towards an optical sensor membrane for sorption and detection of cadmium(II). J. Hazard. Mater. 2016, 301, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ni, W.-X.; Li, B. Porous Organic Polymer Synthesized by Green Diazo-Coupling Reaction for Adsorptive Removal of Methylene Blue. ACS Omega 2021, 6, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Luo, X.; Gao, Q.; Liu, Y.; Wang, X.; Zhou, S.; Wang, D.; Gu, P.; Li, Z. Superfast removal of dyes and herbicides with triphenylamine-based porous organic polymers by one-step sulfonation and carboxylation. Sep. Purif. Technol. 2023, 327, 124799. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; He, M.; Zhou, N.; Meng, X.; Dai, Z.; Xiong, Y. Photo-tunable ultrafast removal of organic dyes by azobenzene and phosphonium functionalized porous organic polymers. Sep. Purif. Technol. 2024, 335, 126119. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, L.; Gu, P.; Tian, L.; Li, N.; Chen, D.; Marcomini, A.; Xu, Q.; Lu, J. Novel calixarene-based porous organic polymers with superfast removal rate and ultrahigh adsorption capacity for selective separation of cationic dyes. Chem. Eng. J. 2022, 433, 134442. [Google Scholar] [CrossRef]
- Huang, L.; Liu, R.; Yang, J.; Shuai, Q.; Yuliarto, B.; Kaneti, Y.V.; Yamauchi, Y. Nanoarchitectured porous organic polymers and their environmental applications for removal of toxic metal ions. Chem. Eng. J. 2021, 408, 127991. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, P.; Zhu, L.; Hua, M.; Huang, Y.; Chao, Y.; Wu, P.; Qiu, Z.; Zhu, W. Construction of hydrophilic hydroxyl-rich porous organic polymers for efficient removal of heavy metal ions. Inorg. Chem. Commun. 2023, 153, 110821. [Google Scholar] [CrossRef]
- Ansari, M.; Alam, A.; Bera, R.; Hassan, A.; Goswami, S.; Das, N. Synthesis, characterization and adsorption studies of a novel triptycene based hydroxyl azo-nanoporous polymer for environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103558. [Google Scholar] [CrossRef]
- Vallès, V.; López, J.; Fernández de Labastida, M.; Gibert, O.; Leskinen, A.; Koivula, R.T.; Cortina, J.L. Polymeric and inorganic sorbents as a green option to recover critical raw materials at trace levels from sea saltwork bitterns. Green Chem. 2023, 25, 700–719. [Google Scholar] [CrossRef]
- Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Tran, H.N.; Ezzat, A.O.; et al. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water 2024, 16, 243. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.-H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean Prod 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alqadami, A.A.; Melhi, S.; Alhamami, M.A.M.; Fallatah, A.M.; Rizk, M.A. Sulfhydryl Functionalized Magnetic Chitosan as an Efficient Adsorbent for High-Performance Removal of Cd(II) from Water: Adsorption Isotherms, Kinetic, and Reusability Studies. Adsorpt. Sci. Technol. 2022, 2022, 2248249. [Google Scholar] [CrossRef]
- Al Lafi, A.G.; Hay, J.N. 2D-COS-FTIR analysis of high molecular weight poly (N-vinyl carbazole) undergoing phase separation on purification and thermal annealing. J. Mol. Struct. 2019, 1175, 152–162. [Google Scholar] [CrossRef]
- Ranganathan, N.; Mahalingam, G. 2,4,6-Triphenylaniline nanoemulsion formulation, optimization, and its application in type 2 diabetes mellitus. J. Cell Physiol. 2019, 234, 22505–22516. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, B.; Çağlı, M.; Tülek, R.; Teke, A. Synthesis and optical characterization of bipod carbazole derivatives. Heterocycl. Commun. 2020, 26, 148–156. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, H.; Yang, L.; Fan, L.; Xiao, Y.; Chen, J.; Wang, W. Porous organic polymer immobilized copper nanoparticles as heterogeneous catalyst for efficient benzylic C–H bond oxidation. J. Saudi Chem. Soc. 2022, 26, 101397. [Google Scholar] [CrossRef]
- Zhu, X.; Xue, D.; Gu, L.; Li, W.; Xie, A.; Wang, Z. Pyrene-based sulfonated organic porous materials for rapid adsorption of cationic dyes in water. Environ. Technol. 2023, 44, 2795–2806. [Google Scholar] [CrossRef]
- Trandafir, M.M.; Pop, L.; Hӑdade, N.D.; Hristea, I.; Teodorescu, C.M.; Krumeich, F.; van Bokhoven, J.A.; Grosu, I.; Parvulescu, V.I. Spirobifluorene-based Porous Organic Polymers as Efficient Porous Supports for Pd and Pt for Selective Hydrogenation. ChemCatChem 2019, 11, 538–549. [Google Scholar] [CrossRef]
- Sebati, W.; Ray, S.S. Advances in Nanostructured Metal-Encapsulated Porous Organic-Polymer Composites for Catalyzed Organic Chemical Synthesis. Catalysts 2018, 8, 492. [Google Scholar] [CrossRef]
- Halder, M.; Bhanja, P.; Islam, M.M.; Chatterjee, S.; Khan, A.; Bhaumik, A.; Islam, S.M. Porous organic polymer as an efficient organocatalyst for the synthesis of biofuel ethyl levulinate. Mol. Catal. 2020, 494, 111119. [Google Scholar] [CrossRef]
- Kundu, S.K.; Bhaumik, A. A triazine-based porous organic polymer: A novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes. RSC Adv. 2015, 5, 32730–32739. [Google Scholar] [CrossRef]
- Pan, X.; Zuo, G.; Su, T.; Cheng, S.; Gu, Y.; Qi, X.; Dong, W. Polycarboxylic magnetic polydopamine sub-microspheres for effective adsorption of malachite green. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 106–113. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Magnetic hydrochar grafted-chitosan for enhanced efficient adsorption of malachite green dye from aqueous solutions: Modeling, adsorption behavior, and mechanism analysis. Int. J. Biol. Macromol. 2024, 254, 127767. [Google Scholar] [CrossRef]
- Sahraei, R.; Hemmati, K.; Ghaemy, M. Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water. RSC Adv. 2016, 6, 72487–72499. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide decorated with cellulose and copper nanoparticle as an efficient adsorbent for the removal of malachite green. Int. J. Biol. Macromol. 2021, 167, 23–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, T.; Lin, Q.; Fang, C. Facile preparation of robust dual MgO-loaded carbon foam as an efficient adsorbent for malachite green removal. Environ. Res 2021, 195, 110698. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Adsorptive performance of a new magnetic hydrochar nanocomposite for highly efficient removal of cadmium ions from water: Mechanism, modeling, and reusability studies. Environ. Technol. Innov 2023, 32, 103404. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Kumar, A.; Chakraborty, S.; Naushad, M.; Islam, R.U.; Bhattacharya, T.; Datta, S. Removal of toxic dye from dye-laden wastewater using a new nanocomposite material: Isotherm, kinetics and adsorption mechanism. Chemosphere 2022, 308, 136413. [Google Scholar] [CrossRef]
- Sivashankar, R.; Sathya, A.B.; Vasantharaj, K.; Sivasubramanian, V. Magnetic composite an environmental super adsorbent for dye sequestration—A review. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 36–49. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Anastopoulos, I. Response to “Some remarks on a critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the van’t Hoff equation for calculation of thermodynamic parameters of adsorption—Journal of Molecular Liquids 273 (2019) 425–434. J. Mol. Liq. 2019, 280, 298–300. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol Eng. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic Graphene Oxide: Effect of Preparation Route on Reactive Black 5 Adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Melhi, S.; Algamdi, M.; Alqadami, A.A.; Khan, M.A.; Alosaimi, E.H. Fabrication of magnetically recyclable nanocomposite as an effective adsorbent for the removal of malachite green from water. Chem. Eng. Res. Des. 2022, 177, 843–854. [Google Scholar] [CrossRef]
- Amin, M.O.; Al-Hetlani, E.; Antonangelo, A.R.; Zhou, H.; Carta, M. Ultrasonic-assisted removal of cationic and anionic dyes residues from wastewater using functionalized triptycene-based polymers of intrinsic microporosity (PIMs). Appl. Water Sci. 2023, 13, 131. [Google Scholar] [CrossRef]
- Sirach, R.; Dave, P.N. β -Cyclodextrin polymer/zinc ferrite nanocomposite: Synthesis, characterization, and adsorption application for the removal of malachite green and Congo red. J. Hazard. Mater. Adv. 2023, 10, 100300. [Google Scholar] [CrossRef]
- Joshy, D.; Chamundi, P.J.; Kuruvangattu Puthenveettil, N.; Ismail, Y.A.; Periyat, P. Mechanistic investigation of mesoporous Mg2+ doped CeO2 encapsulated Fe3O4 core-shells for the selective adsorptive removal of malachite green. Results Eng. 2023, 20, 101409. [Google Scholar] [CrossRef]
- El Hadj Ali, Y.A.; Hejji, L.; Seddik, N.B.; Azzouz, A.; Pérez-Villarejo, L.; Stitou, M.; Sonne, C. Remediation of malachite-green dye from textile wastewater using biosorbent almond shell-based cellulose. J. Mol. Liq. 2024, 399, 124435. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, B.-B.; You, L.-X.; Ren, B.-Y.; He, Y.-K.; Ding, F.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes. J. Mater. Chem. A 2019, 7, 393–404. [Google Scholar] [CrossRef]
- He, Y.; Fu, X.; Li, B.; Zhao, H.; Yuan, D.; Na, B. Highly Efficient Organic Dyes Capture Using Thiol-Functionalized Porous Organic Polymer. ACS Omega 2022, 7, 17941–17947. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal Proceedings of the Academy of Sciences. Phys. Chem. Sect. USSR 1947, 55, 331. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).