Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
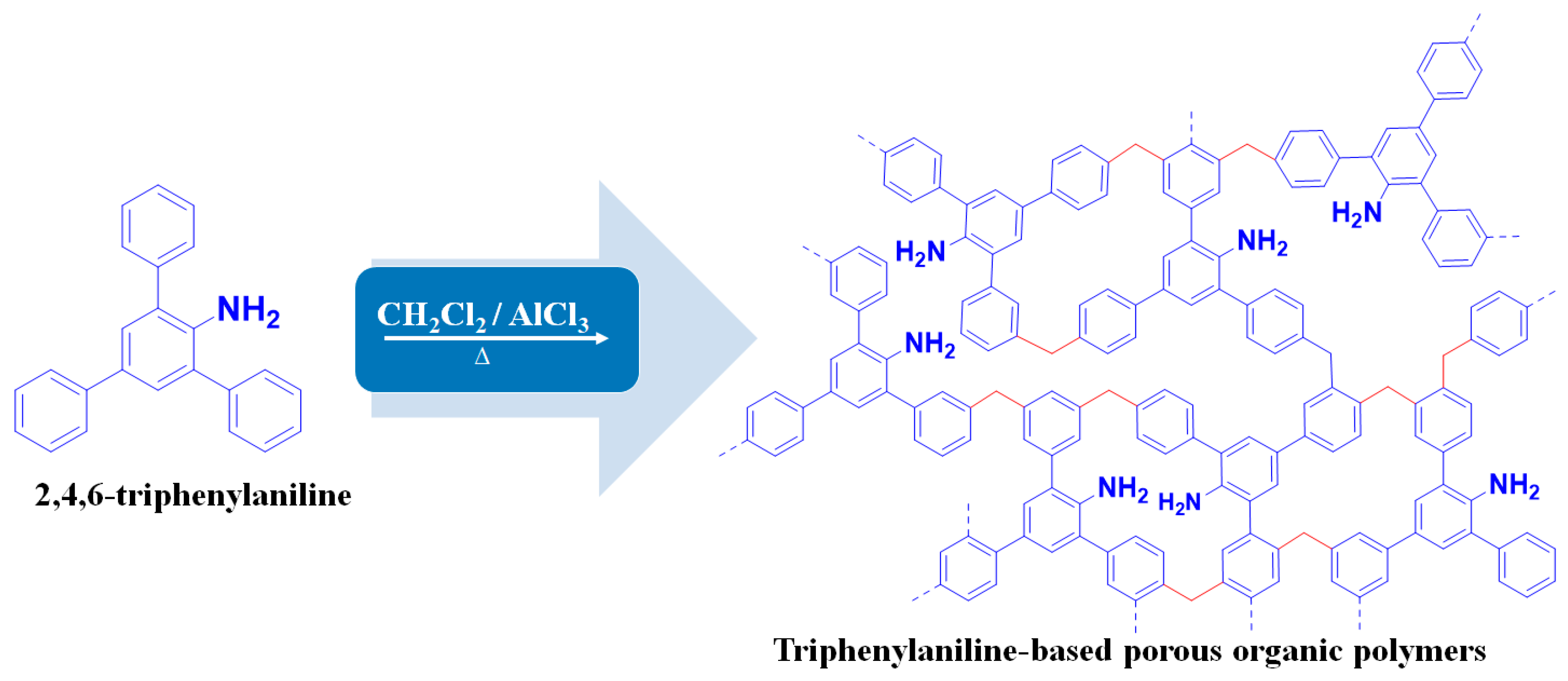
2.2. Synthesis of TPA-POP
2.3. Adsorption Studies
3. Results and Discussion
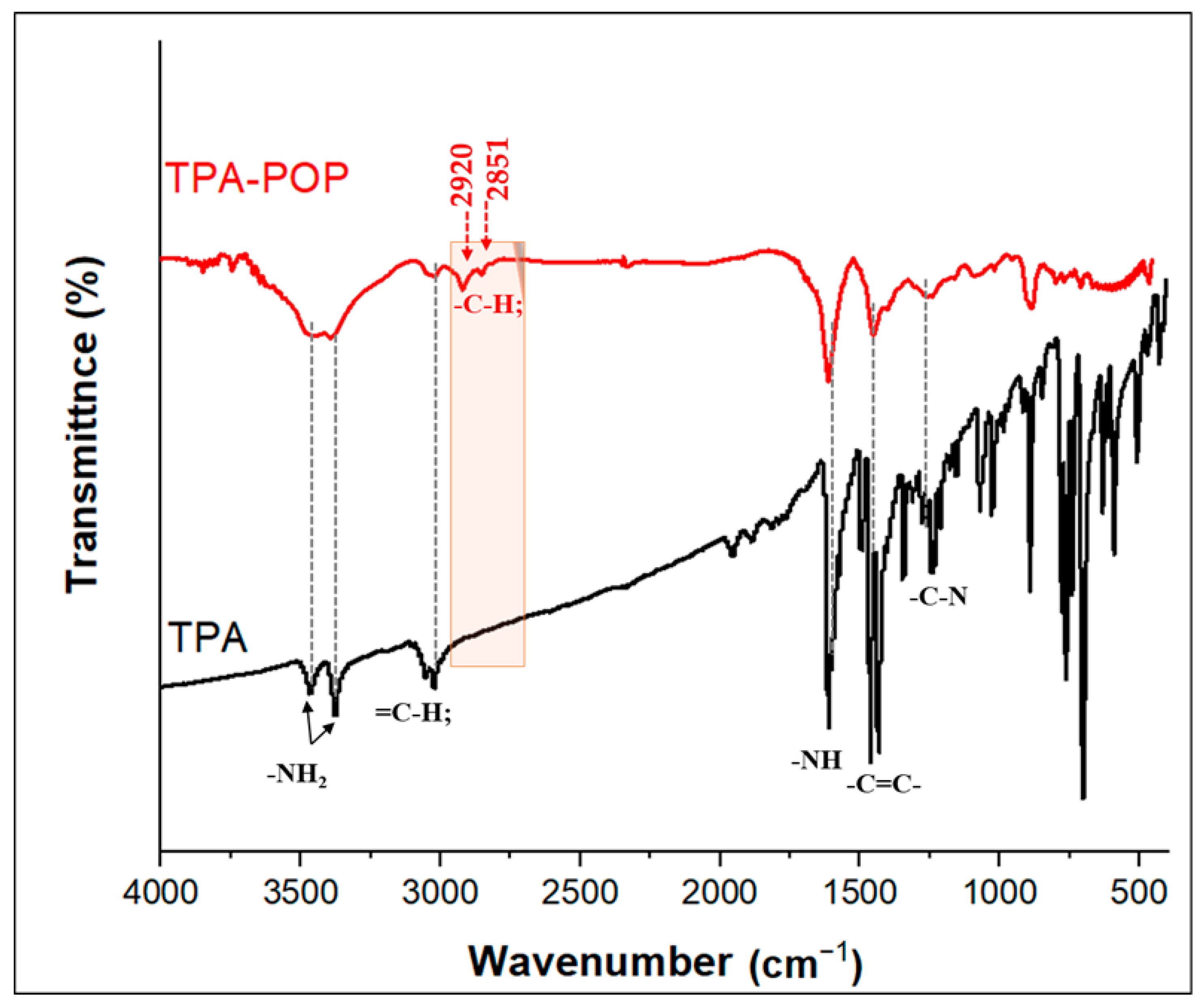
3.1. Characterization of TPA-POP
3.2. Adsorption Studies
3.2.1. Selectivity Study
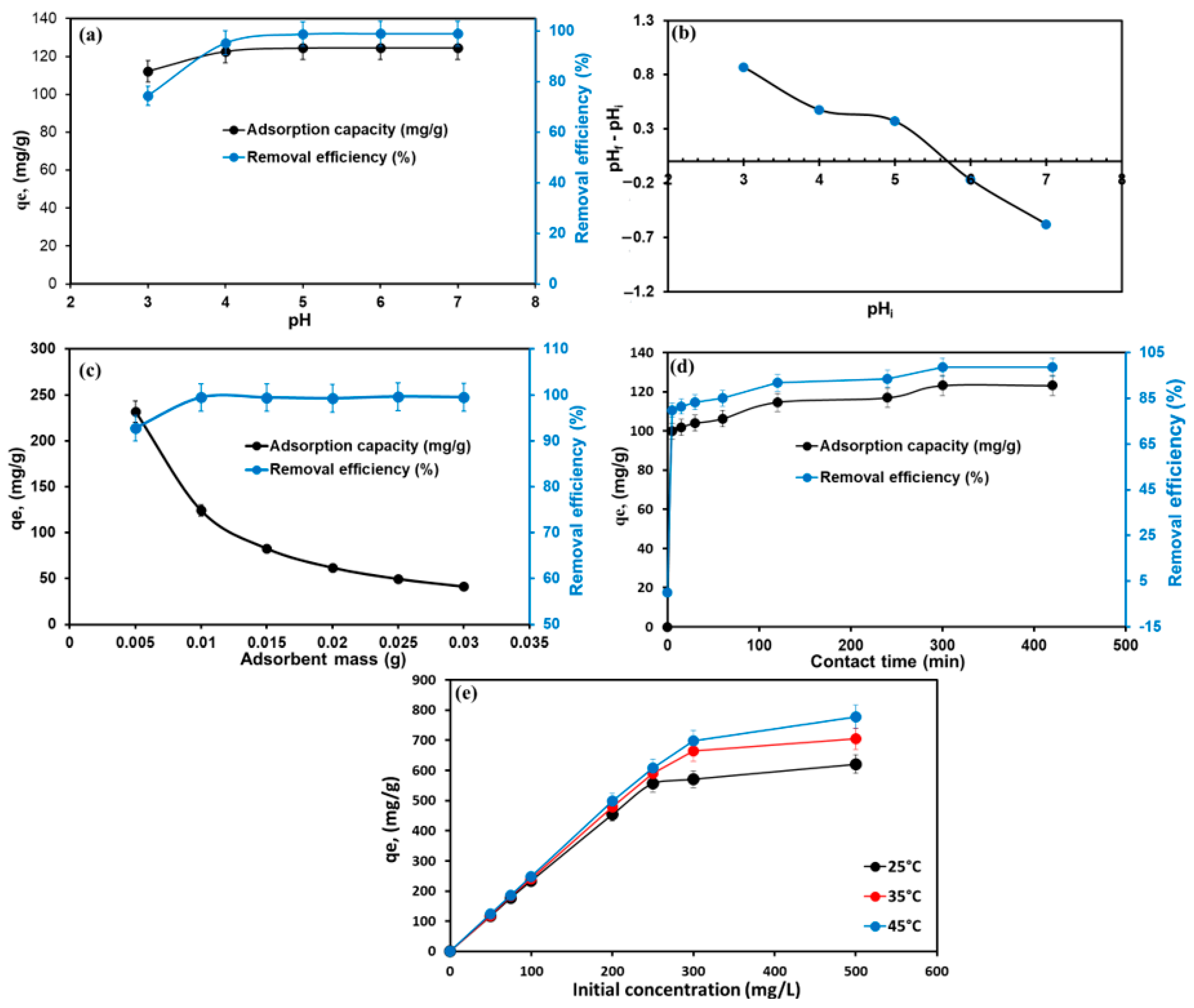
3.2.2. Effect of Factors on MG Adsorption by TPA-POP
3.3. Adsorption Model
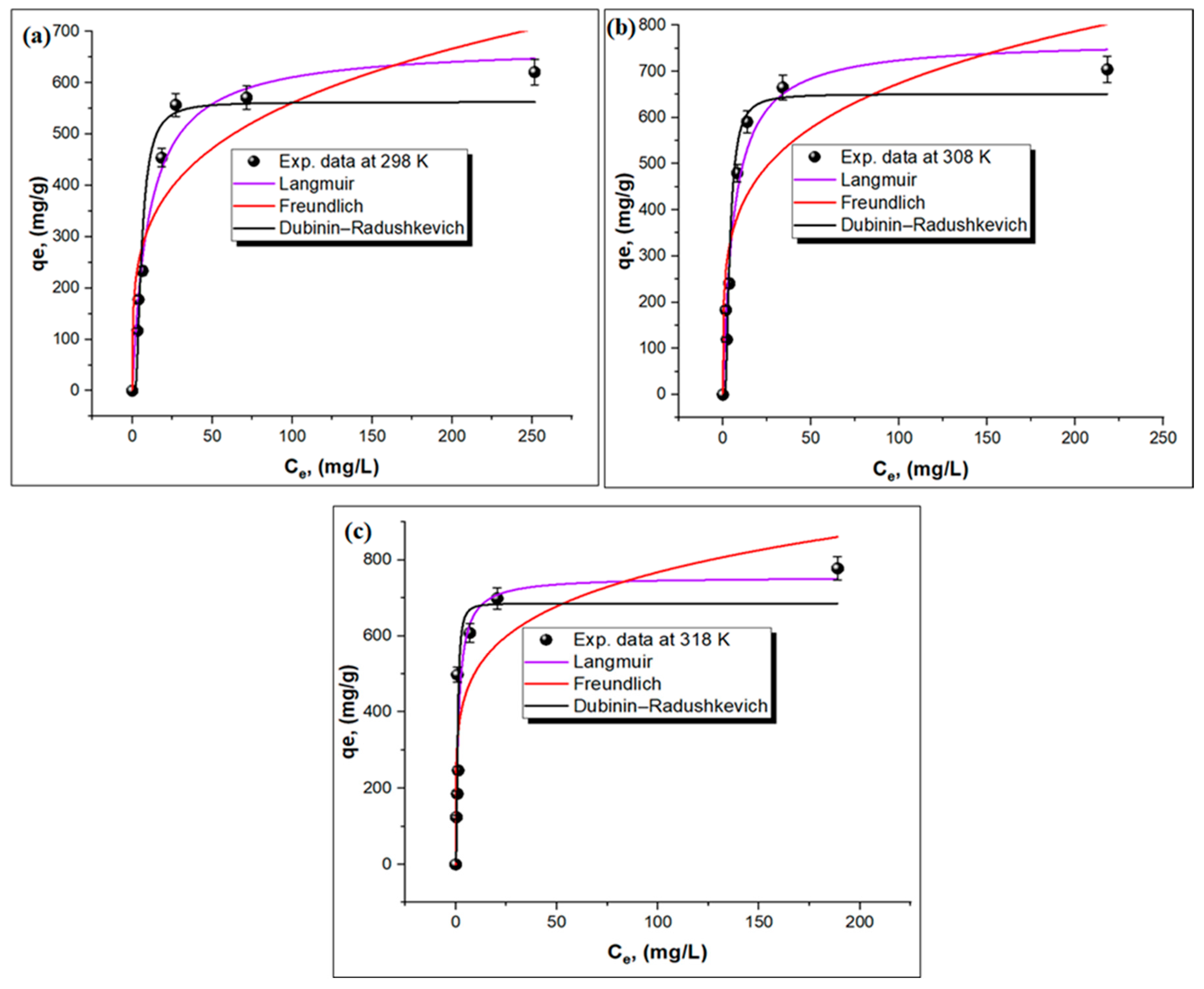
3.3.1. Adsorption Isotherms
3.3.2. Adsorption Kinetics
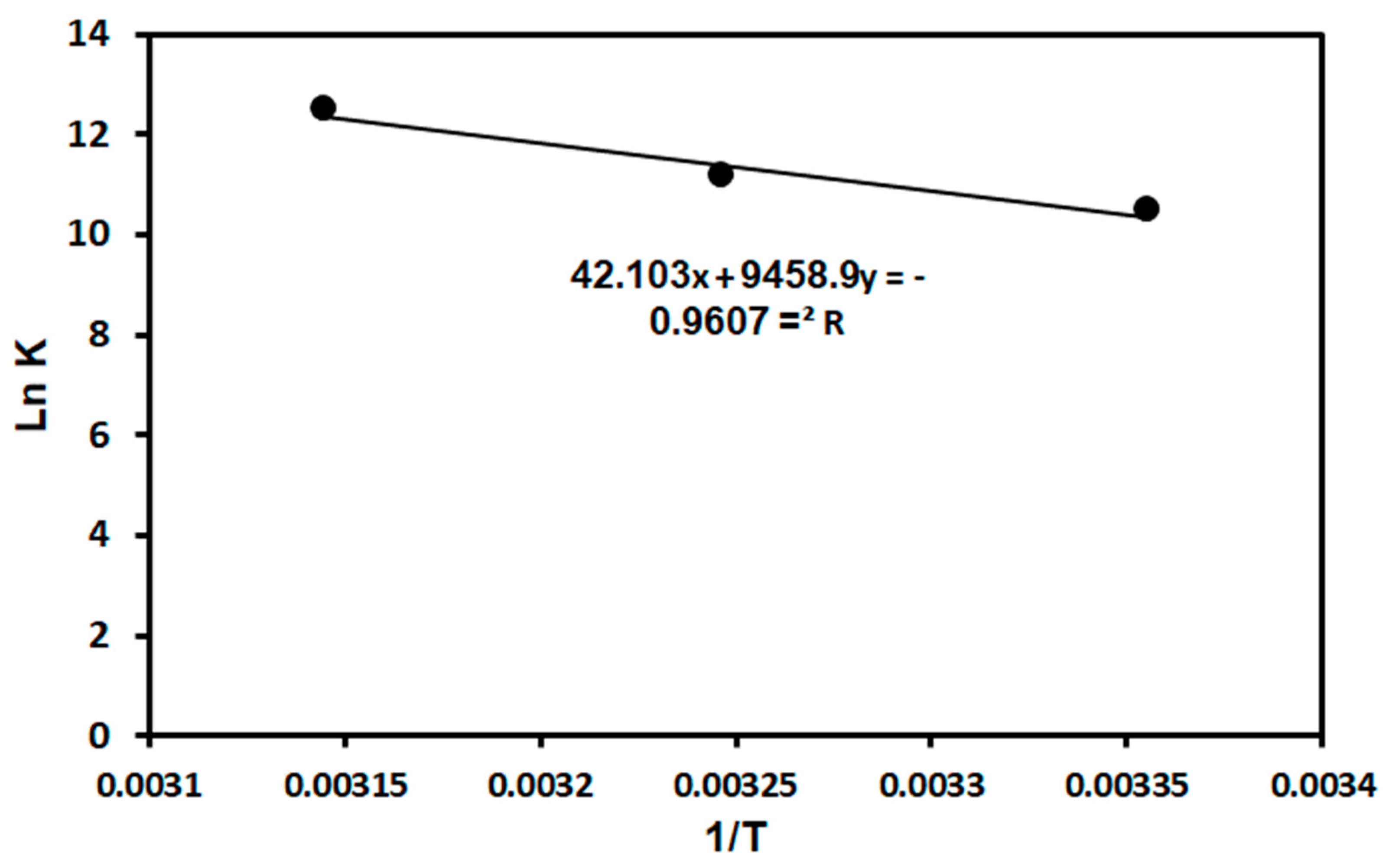
3.3.3. Thermodynamic Studies
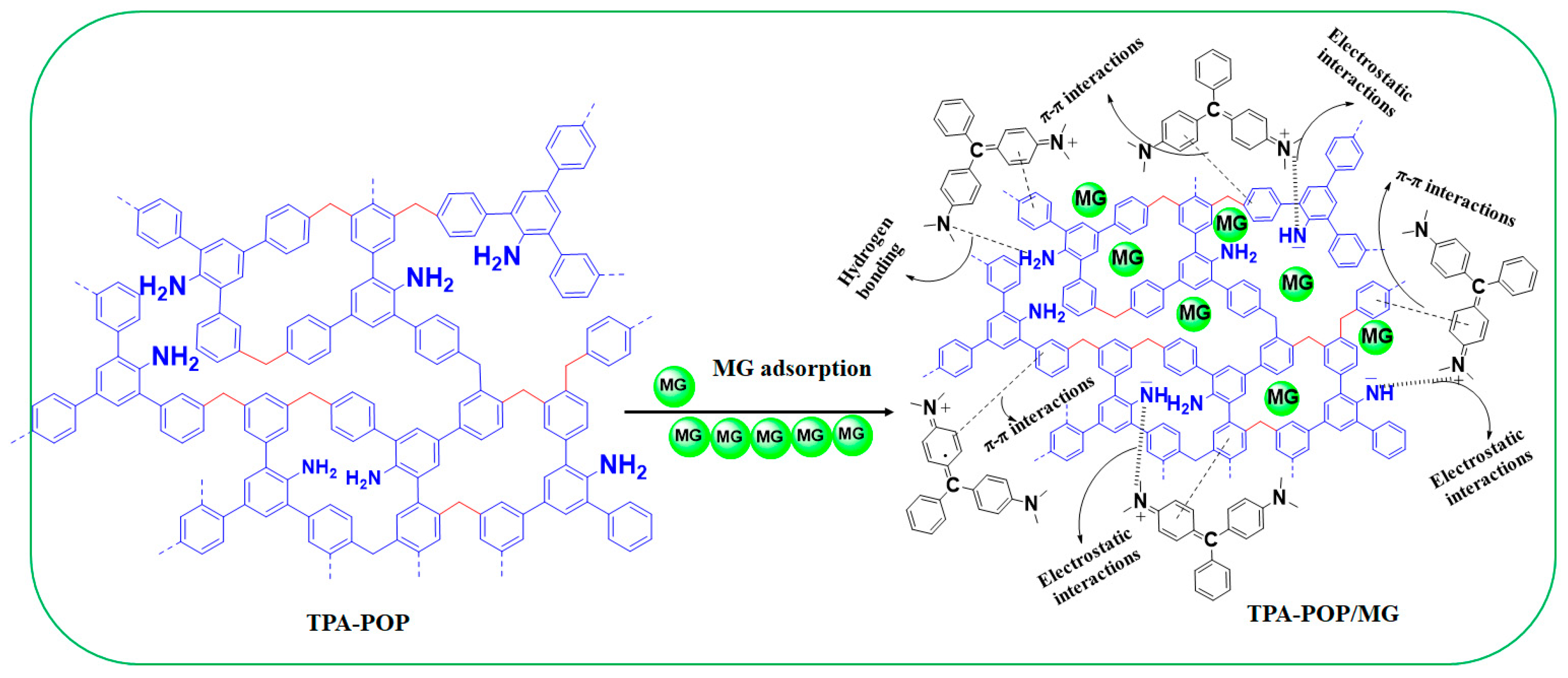
3.4. Proposed Adsorption Mechanism
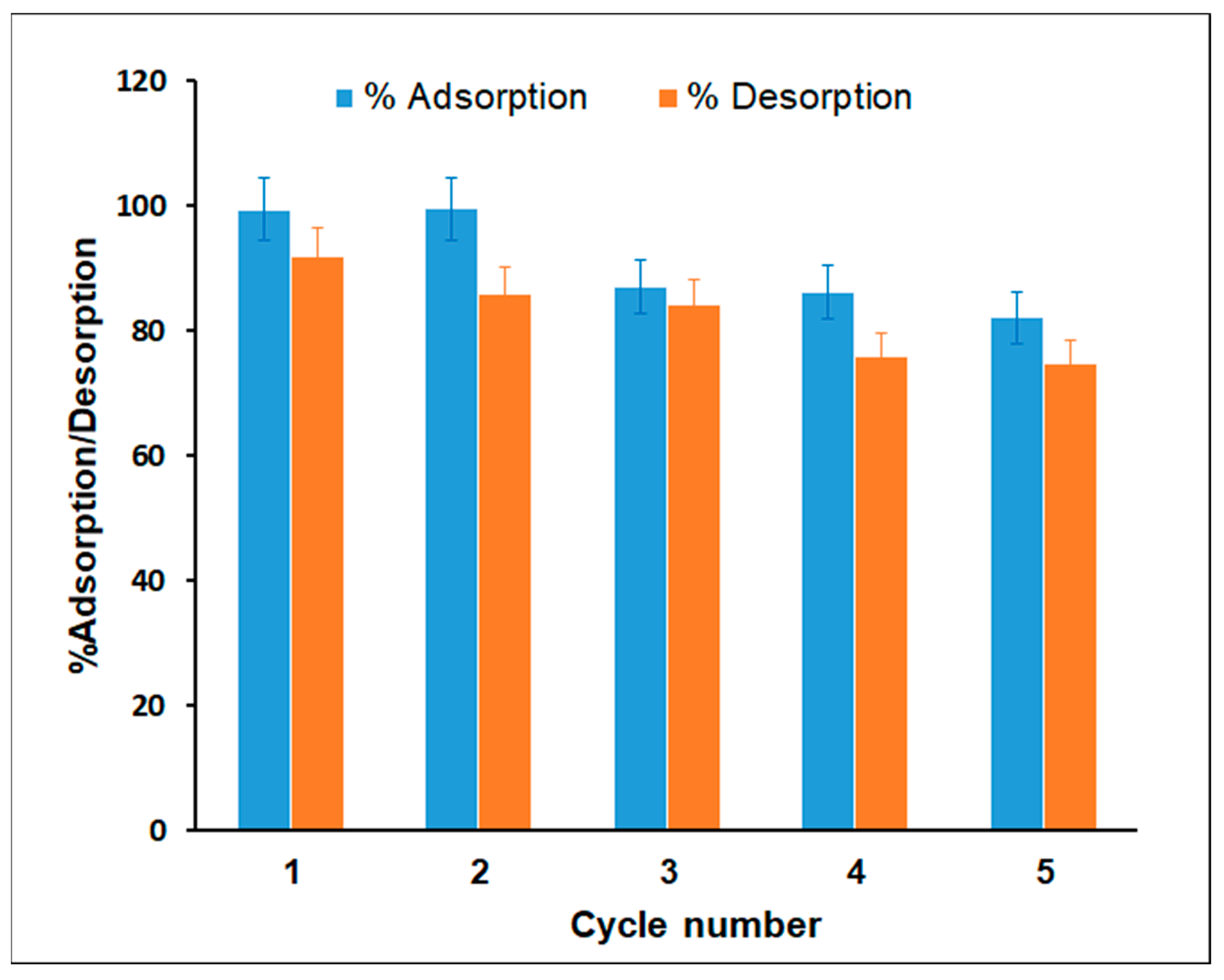
3.5. Regeneration and Reusability of Adsorbent
3.6. Comparison with other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Naushad, M. Advances in the role of natural gums-based hydrogels in water purification, desalination and atmospheric-water harvesting. Int. J. Biol. Macromol. 2022, 222, 2888–2921. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly(acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.H.; Ramadhan, Z.K.; Al-Ansari, N.; Sharma, G.; Naushad, M.; Bathula, C. Precipitation of (Mg/Fe-CTAB)—Layered double hydroxide nanoparticles onto sewage sludge for producing novel sorbent to remove Congo red and methylene blue dyes from aqueous environment. Chemosphere 2022, 291, 132693. [Google Scholar] [CrossRef] [PubMed]
- Chennah, A.; Khan, M.A.; Zbair, M.; Ait Ahsaine, H. NiO/AC Active Electrode for the Electrosorption of Rhodamine B: Structural Characterizations and Kinetic Study. Catalysts 2023, 13, 1009. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Ahmed Alshareef, S.; Abdullah Alqadami, A.; Ali Khan, M.; Alanazi, H.S.; Raza Siddiqui, M.; Jeon, B.-H. Simultaneous co-hydrothermal carbonization and chemical activation of food wastes to develop hydrochar for aquatic environmental remediation. Bioresour. Technol. 2022, 347, 126363. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.A.; Otero, M.; Abdullah, E.C.; Hosomi, M.; Terada, A.; Riya, S. Synthesis of CTAB intercalated graphene and its application for the adsorption of AR265 and AO7 dyes from water. J. Colloid Interface Sci. 2017, 493, 51–61. [Google Scholar] [CrossRef]
- Khan, M.A.; Otero, M.; Kazi, M.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Alothman, Z.A.; Sumbul, S. Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. J. Hazard. Mater. 2019, 365, 759–770. [Google Scholar] [CrossRef]
- Hussain Hakami, A.A.; Ahmed, M.A.; Khan, M.A.; AlOthman, Z.A.; Rafatullah, M.; Islam, M.A.; Siddiqui, M.R. Quantitative Analysis of Malachite Green in Environmental Samples Using Liquid Chromatography-Mass Spectrometry. Water 2021, 13, 2864. [Google Scholar] [CrossRef]
- Yadav, V.K.; Singh, B.; Gacem, A.; Yadav, K.K.; Gnanamoorthy, G.; Alsufyani, T.; Hussein, H.S.; Awwad, N.S.; Verma, R.; Inwati, G.K.; et al. Development of Novel Microcomposite Materials from Coal Fly Ash and Incense Sticks Ash Waste and Their Application for Remediation of Malachite Green Dye from Aqueous Solutions. Water 2022, 14, 3871. [Google Scholar] [CrossRef]
- Ullah, S.; Ur Rahman, A.; Ullah, F.; Rashid, A.; Arshad, T.; Viglašová, E.; Galamboš, M.; Mahmoodi, N.M.; Ullah, H. Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies. Water 2021, 13, 965. [Google Scholar] [CrossRef]
- Alomar, T.S.; AlMasoud, N.; Sharma, G.; ALOthman, Z.A.; Naushad, M. Incorporation of trimetallic nanoparticles to the SiO2 matrix for the removal of methylene blue dye from aqueous medium. J. Mol. Liq. 2021, 336, 116274. [Google Scholar] [CrossRef]
- Nirmaladevi, S.; Palanisamy, N. A comparative study of the removal of cationic and anionic dyes from aqueous solutions using biochar as an adsorbent. Desalin Water Treat 2020, 175, 282–292. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Kumar, A.; Ahamad, T.; Stadler, F.J. Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity. J. King Saud Univ.-Sci. 2020, 32, 1359–1365. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Balakumar, S.; Aruna, P.; Ganesan, S.; Rajendran, S.; Naushad, M. Low cost and quick time absorption of organic dye pollutants under ambient condition using partially exfoliated graphite. J. Water Process Eng. 2020, 34, 101078. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Al-Muhtaseb, A.H.; Khan, A.A.; Khan, A. Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Verma, S.; Kim, K.-H.; Kumar, N.; Bhattacharya, S.S.; Naushad, M.; Dutta, R.K. Amine-amide functionalized graphene oxide sheets as bifunctional adsorbent for the removal of polar organic pollutants. J. Hazard. Mater. 2022, 429, 128308. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Thakur, B.; Vo, D.-V.N.; Gao, B.; Al-Kahtani, A.A.; Stadler, F.J. Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 2021, 416, 125714. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Shihab, A.H.; Naushad, M.; Ahamad, T.; Sharma, G.; Al-Sheetan, K.M. Green synthesis for novel sorbent of sand coated with (Ca/Al)-layered double hydroxide for the removal of toxic dye from aqueous environment. J. Environ. Chem. Eng. 2021, 9, 105342. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Fajal, S.; Dutta, S.; Ghosh, S.K. Porous organic polymers (POPs) for environmental remediation. Mater. Horiz. 2023, 10, 4083–4138. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, X.; Luo, P.; Dai, S.; Zhang, W.; Zhang, Y. Novel Fluorinated Nitrogen-Rich Porous Organic Polymer for Efficient Removal of Perfluorooctanoic Acid from Water. Water 2022, 14, 1010. [Google Scholar] [CrossRef]
- Ghanbari, J.; Mobinikhaledi, A. Synthesis of a novel porous organic polymer containing triazine and cyclohexanone rings as an efficient methyl red adsorbent from aqueous solutions. Sci. Rep. 2023, 13, 12962. [Google Scholar] [CrossRef] [PubMed]
- Sumayli, A.; Alshahrani, S.M.; Alqahtani, A.S. Separation of organic molecules using porous polymeric membranes: Model development using advanced hybrid CFD and artificial intelligence. Ain Shams Eng. J. 2024, 102834. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Zhao, X.; Qin, M.; Li, X.; Jin, W.; Zhang, D. One-pot synthesis of conjugated microporous polymers based on extended molecular graphenes for hydrogen storage. Polymer 2019, 174, 96–100. [Google Scholar] [CrossRef]
- Tadayoni, N.S.; Dinari, M.; Roy, A.; Karimi Abdolmaleki, M. Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants. Polymers 2024, 16, 1543. [Google Scholar] [CrossRef]
- Shi, K.; Song, N.; Zou, Y.; Zhu, S.; Tan, H.; Tian, Y.; Zhang, B.; Yao, H.; Guan, S. Porphyrin-based porous polyimides: Synthesis, porous structure, carbon dioxide adsorption. Polymer 2019, 169, 160–166. [Google Scholar] [CrossRef]
- Liu, C.; Xia, M.; Zhang, M.; Yuan, K.; Hu, F.; Yu, G.; Jian, X. One-pot synthesis of nitrogen-rich aminal-and triazine-based hierarchical porous organic polymers with highly efficient iodine adsorption. Polymer 2020, 194, 122401. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Liu, M.; Zhang, Y.; Wu, C.; Zhang, Y. Porphyrin-functionalized porous polysulfone membrane towards an optical sensor membrane for sorption and detection of cadmium(II). J. Hazard. Mater. 2016, 301, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ni, W.-X.; Li, B. Porous Organic Polymer Synthesized by Green Diazo-Coupling Reaction for Adsorptive Removal of Methylene Blue. ACS Omega 2021, 6, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Luo, X.; Gao, Q.; Liu, Y.; Wang, X.; Zhou, S.; Wang, D.; Gu, P.; Li, Z. Superfast removal of dyes and herbicides with triphenylamine-based porous organic polymers by one-step sulfonation and carboxylation. Sep. Purif. Technol. 2023, 327, 124799. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; He, M.; Zhou, N.; Meng, X.; Dai, Z.; Xiong, Y. Photo-tunable ultrafast removal of organic dyes by azobenzene and phosphonium functionalized porous organic polymers. Sep. Purif. Technol. 2024, 335, 126119. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, L.; Gu, P.; Tian, L.; Li, N.; Chen, D.; Marcomini, A.; Xu, Q.; Lu, J. Novel calixarene-based porous organic polymers with superfast removal rate and ultrahigh adsorption capacity for selective separation of cationic dyes. Chem. Eng. J. 2022, 433, 134442. [Google Scholar] [CrossRef]
- Huang, L.; Liu, R.; Yang, J.; Shuai, Q.; Yuliarto, B.; Kaneti, Y.V.; Yamauchi, Y. Nanoarchitectured porous organic polymers and their environmental applications for removal of toxic metal ions. Chem. Eng. J. 2021, 408, 127991. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, P.; Zhu, L.; Hua, M.; Huang, Y.; Chao, Y.; Wu, P.; Qiu, Z.; Zhu, W. Construction of hydrophilic hydroxyl-rich porous organic polymers for efficient removal of heavy metal ions. Inorg. Chem. Commun. 2023, 153, 110821. [Google Scholar] [CrossRef]
- Ansari, M.; Alam, A.; Bera, R.; Hassan, A.; Goswami, S.; Das, N. Synthesis, characterization and adsorption studies of a novel triptycene based hydroxyl azo-nanoporous polymer for environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103558. [Google Scholar] [CrossRef]
- Vallès, V.; López, J.; Fernández de Labastida, M.; Gibert, O.; Leskinen, A.; Koivula, R.T.; Cortina, J.L. Polymeric and inorganic sorbents as a green option to recover critical raw materials at trace levels from sea saltwork bitterns. Green Chem. 2023, 25, 700–719. [Google Scholar] [CrossRef]
- Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Tran, H.N.; Ezzat, A.O.; et al. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water 2024, 16, 243. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.-H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean Prod 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alqadami, A.A.; Melhi, S.; Alhamami, M.A.M.; Fallatah, A.M.; Rizk, M.A. Sulfhydryl Functionalized Magnetic Chitosan as an Efficient Adsorbent for High-Performance Removal of Cd(II) from Water: Adsorption Isotherms, Kinetic, and Reusability Studies. Adsorpt. Sci. Technol. 2022, 2022, 2248249. [Google Scholar] [CrossRef]
- Al Lafi, A.G.; Hay, J.N. 2D-COS-FTIR analysis of high molecular weight poly (N-vinyl carbazole) undergoing phase separation on purification and thermal annealing. J. Mol. Struct. 2019, 1175, 152–162. [Google Scholar] [CrossRef]
- Ranganathan, N.; Mahalingam, G. 2,4,6-Triphenylaniline nanoemulsion formulation, optimization, and its application in type 2 diabetes mellitus. J. Cell Physiol. 2019, 234, 22505–22516. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, B.; Çağlı, M.; Tülek, R.; Teke, A. Synthesis and optical characterization of bipod carbazole derivatives. Heterocycl. Commun. 2020, 26, 148–156. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, H.; Yang, L.; Fan, L.; Xiao, Y.; Chen, J.; Wang, W. Porous organic polymer immobilized copper nanoparticles as heterogeneous catalyst for efficient benzylic C–H bond oxidation. J. Saudi Chem. Soc. 2022, 26, 101397. [Google Scholar] [CrossRef]
- Zhu, X.; Xue, D.; Gu, L.; Li, W.; Xie, A.; Wang, Z. Pyrene-based sulfonated organic porous materials for rapid adsorption of cationic dyes in water. Environ. Technol. 2023, 44, 2795–2806. [Google Scholar] [CrossRef]
- Trandafir, M.M.; Pop, L.; Hӑdade, N.D.; Hristea, I.; Teodorescu, C.M.; Krumeich, F.; van Bokhoven, J.A.; Grosu, I.; Parvulescu, V.I. Spirobifluorene-based Porous Organic Polymers as Efficient Porous Supports for Pd and Pt for Selective Hydrogenation. ChemCatChem 2019, 11, 538–549. [Google Scholar] [CrossRef]
- Sebati, W.; Ray, S.S. Advances in Nanostructured Metal-Encapsulated Porous Organic-Polymer Composites for Catalyzed Organic Chemical Synthesis. Catalysts 2018, 8, 492. [Google Scholar] [CrossRef]
- Halder, M.; Bhanja, P.; Islam, M.M.; Chatterjee, S.; Khan, A.; Bhaumik, A.; Islam, S.M. Porous organic polymer as an efficient organocatalyst for the synthesis of biofuel ethyl levulinate. Mol. Catal. 2020, 494, 111119. [Google Scholar] [CrossRef]
- Kundu, S.K.; Bhaumik, A. A triazine-based porous organic polymer: A novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes. RSC Adv. 2015, 5, 32730–32739. [Google Scholar] [CrossRef]
- Pan, X.; Zuo, G.; Su, T.; Cheng, S.; Gu, Y.; Qi, X.; Dong, W. Polycarboxylic magnetic polydopamine sub-microspheres for effective adsorption of malachite green. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 106–113. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Magnetic hydrochar grafted-chitosan for enhanced efficient adsorption of malachite green dye from aqueous solutions: Modeling, adsorption behavior, and mechanism analysis. Int. J. Biol. Macromol. 2024, 254, 127767. [Google Scholar] [CrossRef]
- Sahraei, R.; Hemmati, K.; Ghaemy, M. Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water. RSC Adv. 2016, 6, 72487–72499. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide decorated with cellulose and copper nanoparticle as an efficient adsorbent for the removal of malachite green. Int. J. Biol. Macromol. 2021, 167, 23–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, T.; Lin, Q.; Fang, C. Facile preparation of robust dual MgO-loaded carbon foam as an efficient adsorbent for malachite green removal. Environ. Res 2021, 195, 110698. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Adsorptive performance of a new magnetic hydrochar nanocomposite for highly efficient removal of cadmium ions from water: Mechanism, modeling, and reusability studies. Environ. Technol. Innov 2023, 32, 103404. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Kumar, A.; Chakraborty, S.; Naushad, M.; Islam, R.U.; Bhattacharya, T.; Datta, S. Removal of toxic dye from dye-laden wastewater using a new nanocomposite material: Isotherm, kinetics and adsorption mechanism. Chemosphere 2022, 308, 136413. [Google Scholar] [CrossRef]
- Sivashankar, R.; Sathya, A.B.; Vasantharaj, K.; Sivasubramanian, V. Magnetic composite an environmental super adsorbent for dye sequestration—A review. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 36–49. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Anastopoulos, I. Response to “Some remarks on a critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the van’t Hoff equation for calculation of thermodynamic parameters of adsorption—Journal of Molecular Liquids 273 (2019) 425–434. J. Mol. Liq. 2019, 280, 298–300. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol Eng. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic Graphene Oxide: Effect of Preparation Route on Reactive Black 5 Adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Melhi, S.; Algamdi, M.; Alqadami, A.A.; Khan, M.A.; Alosaimi, E.H. Fabrication of magnetically recyclable nanocomposite as an effective adsorbent for the removal of malachite green from water. Chem. Eng. Res. Des. 2022, 177, 843–854. [Google Scholar] [CrossRef]
- Amin, M.O.; Al-Hetlani, E.; Antonangelo, A.R.; Zhou, H.; Carta, M. Ultrasonic-assisted removal of cationic and anionic dyes residues from wastewater using functionalized triptycene-based polymers of intrinsic microporosity (PIMs). Appl. Water Sci. 2023, 13, 131. [Google Scholar] [CrossRef]
- Sirach, R.; Dave, P.N. β -Cyclodextrin polymer/zinc ferrite nanocomposite: Synthesis, characterization, and adsorption application for the removal of malachite green and Congo red. J. Hazard. Mater. Adv. 2023, 10, 100300. [Google Scholar] [CrossRef]
- Joshy, D.; Chamundi, P.J.; Kuruvangattu Puthenveettil, N.; Ismail, Y.A.; Periyat, P. Mechanistic investigation of mesoporous Mg2+ doped CeO2 encapsulated Fe3O4 core-shells for the selective adsorptive removal of malachite green. Results Eng. 2023, 20, 101409. [Google Scholar] [CrossRef]
- El Hadj Ali, Y.A.; Hejji, L.; Seddik, N.B.; Azzouz, A.; Pérez-Villarejo, L.; Stitou, M.; Sonne, C. Remediation of malachite-green dye from textile wastewater using biosorbent almond shell-based cellulose. J. Mol. Liq. 2024, 399, 124435. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, B.-B.; You, L.-X.; Ren, B.-Y.; He, Y.-K.; Ding, F.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes. J. Mater. Chem. A 2019, 7, 393–404. [Google Scholar] [CrossRef]
- He, Y.; Fu, X.; Li, B.; Zhao, H.; Yuan, D.; Na, B. Highly Efficient Organic Dyes Capture Using Thiol-Functionalized Porous Organic Polymer. ACS Omega 2022, 7, 17941–17947. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal Proceedings of the Academy of Sciences. Phys. Chem. Sect. USSR 1947, 55, 331. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]


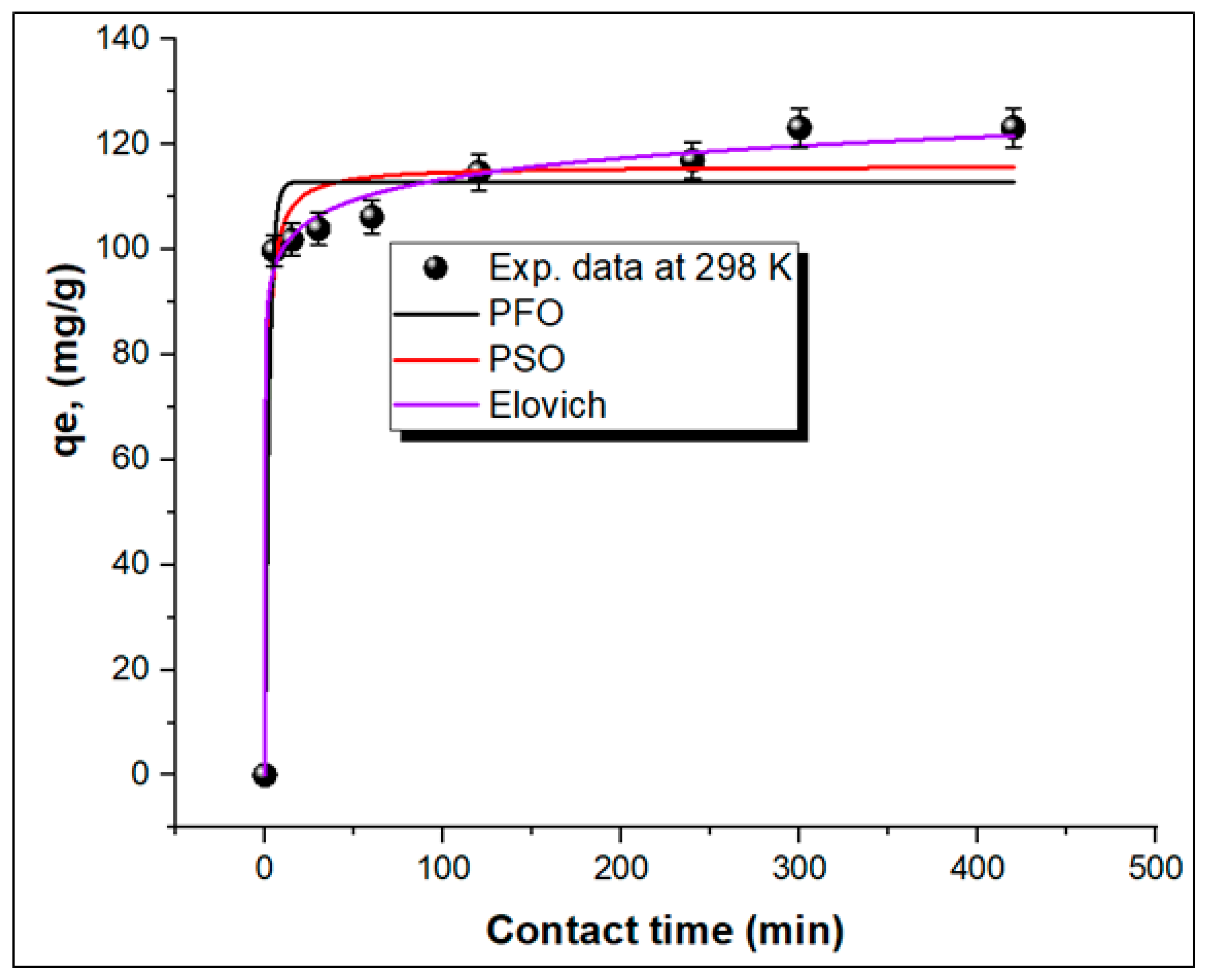

| Adsorbent | T (K) | qe,exp. (mg/g) | Langmuir | Freundlich | Dubinin-R | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm, (mg/g) | KL (L/mg) | R2 | Kf, (mg/g) (L/mg)1/n | n | R2 | qs, mg/g | KD-R (mol2 KJ−2) | E (kJ mol−1) | R2 | |||
| TPA-POP | 298 | 620.72 | 673.81 | 0.096 | 0.97412 | 178.39 | 4.02 | 0.8137 | 562.72 | 11.67 | 0.206 | 0.94564 |
| 308 | 704.34 | 768.24 | 0.163 | 0.96025 | 241.20 | 4.48 | 0.7600 | 650.91 | 5.450 | 0.302 | 0.92086 | |
| 318 | 777.65 | 755.72 | 0.719 | 0.96915 | 335.74 | 5.57 | 0.7650 | 685.55 | 0.512 | 0.988 | 0.80417 | |
| Adsorbent | Co (mg/L) | qe,exp. (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe, cal. (mg/g) | K1 (1/min) | R2 | qe, cal. (mg/g) | K2 (g/mg-min) | R2 | α (mg/g min) | Β (mg/g) | R2 | |||
| TPA-POP | 50 | 123.14 | 115.90 | 0.00775 | 0.97067 | 112.93 | 0.42295 | 0.95353 | 2.01 E7 | 0.17352 | 0.99468 |
| Adsorbent | Temperature (K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|
| TPA-POP | 298 | −25.95 | 78.64 | 350.04 |
| 313 | −28.57 | |||
| 328 | −32.98 |
| Adsorbent | Experimental Conditions | BET (m2/g) | Micropore Volume (cm3/g) and Pore Radius (nm) | qmax, (mg/g) | Number of Cycles with (% Adsorption) | Reference |
|---|---|---|---|---|---|---|
| TRIP-SO3H | Co: 25–150 mg/L; pH: 4.18; t = 20 min; m = 5 mg; V = 0.01 L; T:25 °C | 1145 | - | 303.03 | 5 cycles (88.52%) | [68] |
| β-Cyclodextrin polymer/zinc ferrite | Co: 500 mg/L; t = 15 min; pH: 6.0; m = 0.01 g; V = 0.02 L; T:30 °C; shaking speed: 100 rpm | 6.97 | 0.0164 and 1.7 | 649.4 | 5 cycles (91%) | [69] |
| Fe3O4@Mg2+ doped CeO2 | Co: 2–6 mg/L; t = 60 min; pH >7.0; m = 25 m; V = 0.05 L; shaking speed: 250 rpm | 105.9 | 0.192 and 7.27 | 60.53 | 4 cycles (82.4%) | [70] |
| Almond shell-based cellulose | Co: 60 mg/L; pH: 6.8; t = 125 min; m = 10 mg; T:20 °C; shaking speed: 150 rpm | 0.3087 | 0.000152 and 4.81 | 118 | - | [71] |
| Si-POP-2 | Co: 550 mg/L; pH = 5.0; T:35 °C; t = 240 min; m = 10 mg; shaking speed: 160 rpm | 234 | 0.33 and 1.3 | 757 | - | [72] |
| TPP-SH | Co: 1−1000 mg/L; pH = 7.0; T:25 °C; m = 2.5 mg; V = 5 mL | 607.11 | - | 689.6 | 5 cycles (>90) | [73] |
| TPA-POP | Co: 50−500 mg/L; pH = 6.0; t = 300 min; T:45 °C; m = 0.01 g, shaking speed: 100 rpm | 1625.14 | 0.353 and 1.57 | 755.72 | 5 cycles (82.12%) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melhi, S.; Alqadami, A.A.; Alosaimi, E.H.; Ibrahim, G.M.; El-Gammal, B.; Bedair, M.A.; Elnaggar, E.M. Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies. Water 2024, 16, 1869. https://doi.org/10.3390/w16131869
Melhi S, Alqadami AA, Alosaimi EH, Ibrahim GM, El-Gammal B, Bedair MA, Elnaggar EM. Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies. Water. 2024; 16(13):1869. https://doi.org/10.3390/w16131869
Chicago/Turabian StyleMelhi, Saad, Ayoub Abdullah Alqadami, Eid H. Alosaimi, Gehan M. Ibrahim, Belal El-Gammal, Mahmoud A. Bedair, and Elsayed M. Elnaggar. 2024. "Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies" Water 16, no. 13: 1869. https://doi.org/10.3390/w16131869
APA StyleMelhi, S., Alqadami, A. A., Alosaimi, E. H., Ibrahim, G. M., El-Gammal, B., Bedair, M. A., & Elnaggar, E. M. (2024). Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies. Water, 16(13), 1869. https://doi.org/10.3390/w16131869







