Abstract
Anaerobic digestion of animal manure generates biogas and removes biodegradable organic matter, while most of the nitrogen and phosphorous remains at very high levels after the process. A subsequent microalgae culture in the digestate provides nutrient uptake at very low operational and installation costs. However, the dark color of manure digestate prevents light penetration, reducing the rates of algae growth. Ozonation was researched as a strategy for color removal followed by microalgae culture. Although similar biomass production was achieved in treated and untreated digestates (1.09 vs. 0.99 g L−1), the positive effect of ozonation was evidenced by the significantly higher rates of photosynthetically produced oxygen: 0.804 and 0.18 mg O2 mg−1 TSS min−1, respectively, in ozonated and untreated digestates, revealing a four times higher rate of algae activity. However, this considerable higher activity was not correlated with better performance in nutrient removal since the microalgae treatment was assayed at a considerably reduced scale with a high ratio of illumination per volume. An operational costs analysis revealed that ozonation could be competitive against other strategies of color reduction such as dilution or coagulation/flocculation processes.
1. Introduction
The discharge of untreated agro-livestock wastewaters could potentially endanger ecosystems and human health. The continuous increase in meat demand has resulted in industrialized farming practices that require efficient solutions for waste management [1]. Animal manure wastes present high concentrations of contaminants such as ammoniacal nitrogen (NH4+/NH3) and phosphates (PO43−), which cause eutrophication and pollution of groundwater. Anaerobic digestion is widely used for organic waste treatment with simultaneous bioenergy production and mitigation of greenhouse gases emissions. However, this process only removes organic matter, while the concentrations of nitrogen and phosphorous remain at high levels after the digestion. The final effluent, digestate, is more mineralized than pig slurry and treatments aiming to reduce the nutrient load must be applied prior to discharge [2].
Microalgae culture has been suggested as a secondary treatment after anaerobic processes of wastewater of different origins (domestic, livestock, and industrial) as it can reduce the concentration of pollutants through nutrient assimilation and oxygenation [3] Phycoremediation is a sustainable process that could replace other more expensive wastewater treatments or complement them thanks to the potential of microalgae to absorb carbon, nitrogen, and phosphorous, and concurrently produce a valuable biomass composed of lipids, proteins, and carbohydrates [4]. The growth in microalgae depends on various abiotic factors, such as light intensity, photoperiod, nutrients, temperature, pH, or the presence of toxic compounds in the culture medium [5,6,7].
The light limitation of microalgae cells is often documented due to the large amount of colored compounds present in digested animal manure, namely fulvic and humic compounds and melanoidins. To reduce this light attenuation, chemical and physical pretreatments have been tested, including ultrafiltration, UV light photocatalysis with TiO2 and H2O2, ozone, Fenton systems, coagulation, and flocculation [8,9,10]. Strong dilution has also been referred to as a conventional technique to increase light penetration and to reduce the toxic effects of organic compounds [11,12]. However, the large consumption of blue water can jeopardize the sustainability and viability of the process. In this sense, the analysis of the water footprint has revealed the unsustainability of microalgae culture in several parts of the world [13]. In this scenario, chemical pretreatment aiming to reduce the dark color of digestate has been proposed as one of the most attainable solutions [4,14]. Through the ozonation process, macromolecular organic compounds are broken down thanks to the strong oxidative capacity of ozone. In this sense, the darkness of the liquid is reduced since the structure of the chromophore group is destroyed by ozone. Consequently, after ozone treatment, digestate can be used as a culture medium for microalgae [10]. Although ozone has been applied to digestate and other organic effluents, the efficiency of these systems as a pretreatment prior to algae cultivation must be further improved in order to ensure the integration process [15]. Previously reported experiences have shown increases in microalgae biomass generation when O3 is applied in leachate, with productivities of 12.7 g m−2 in untreated leachate and 18.9 g m−2 in ozonated leachate [8,9,10]. In the case of dark tannery effluents, the microalgae biomass concentration increased from 0.65 to 0.86 g L−1 after 2 h of ozone treatment [4]. However, little attention has been given to the effect of the pretreatment on the net photosynthetic activity. This parameter provides a direct measurement of the light use by the culture and eliminates the possible bias due to the high impact of the experimental setup where microalgae cultures are assayed. In this sense, although color is removed, increasing the availability of light for microalgae growth, if the conditions of the culture system are not representative of the outdoor conditions, the yields and rates can be over- or underestimated. Determination of photosynthetic rates allows for a reliable determination of the light used by microalgae.
In this work, the efficiency of the color removal of swine manure digestate and the positive effect on microalgae photosynthetic activity was tested and evaluated. Microalgae treatment in the treated effluent was tested under simulated outdoor conditions and biomass growth was modeled for a better understanding of the impact on kinetics. In addition, an analysis of the treatment costs was carried out considering operational costs of industrial O3 generator systems.
2. Materials and Methods
2.1. Digestate and Ozonation System
The digestate used as culture media for the microalgae was obtained from the anaerobic digestion of pig slurry of a farm located in Sauquillo de Boñices, province of Soria, (Spain). For the pretreatment of the digestate, an ozonator was used (6020, Rilize, Gijón, Spain). A Bronkhorst® regulator (Model F-201AV, Ruurlo, The Netherlands) was used to control de flow of ozone. A similar system was described by Merayo et al. [16], followed by a column glass absorption. To calculate the actual ozone consumption in the column, measurements of ozone concentrations at both the inlet and outlet were taken using two real-time ozone analyzers (Model BMT 96-4C Messtechnik (Berlin, Germany). This allowed the determination of the amount of ozone consumed in the process (see Figure S1). The ozone enters through the base of the column through a porous stone diffuser, thus generating microbubbles of this gas, increasing the contact between the ozone and the digestate particles, and obtaining a greater effect in the ozonation process.
2.2. Microalgae Culture System
The microalgae inoculum was withdrawn from an experimental system treating urban wastewater [17] and the microbial population was mainly composed of Desmodesmus opolensis and Scenedesmus vacuolatus [17]. As a light source, two LED boards controlled by two drivers and an Arduino microcontroller were used to simulate the light cycle according to the model described by Gonzalo-Ibrahim et al. [17]. The solar radiation was estimated as a function of its location and position following the models described by [18]. The daily variations in light intensity were converted into polynomial equations corresponding to the conditions simulated. In order to obtain replicable results in outdoor conditions, irradiation and temperature corresponded to inner North Spain conditions (Soria, Castilla y León), with an altitude of 1063 m and a latitude angle of 41°. The calibration and confirmation of light intensity were carried out using a LI-250-LICOR pyranometer [17]. The lamp simulated summer conditions for the area of Soria, Spain, with a maximum light intensity of up to 1700 μmol m−2 s−1 at noon, corresponding to the spring season according to the equations. The temperature ranged between 32 and 26 °C during the day and at night, respectively; see Figure 1.
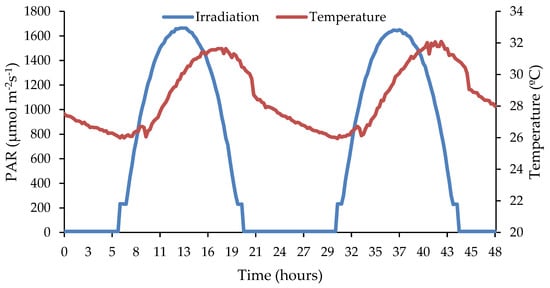
Figure 1.
Irradiance simulation for 2 days under summer conditions.
Six class A Erlenmeyer flasks with a total volume of 250 mL were used as photobioreactors for the cultivation of microalgae. Three untreated digestate controls were compared to three having the treated digestate with O3, thus providing data in triplicate. For the homogenization of the culture broth, a magnetic stirring plate with 9 stirring zones was used. A spectrophotometer (Thermo Spectronic Genesys 10uv, UK) was used to measure the concentration of algal biomass and the absorbance of the treated and untreated sample, with a scanning sweep performed in the wavelength range between 200 and 750 nm.
2.3. Digestate Ozonation
The digestate was centrifuged at 5000 rpm for 15 min to separate the solid phase from the liquid phase. The digestate was introduced into the column without dilution. A total of 1.3 L of digestate was ozonated in triplicate for the tests. The digestate remained in contact with the ozone for 5 h, thus ensuring that color removal was carried out correctly. The ozonator was set at an average concentration of 13.51 mg O3 L−1 and a flow rate of 3 L min−1. The temperature and operating pressure of the reactor were set at 25 °C and 0.95 Bar. The following parameters were measured before and after treatment: percentage of color removal (% CR, pH, chemical oxygen demand (COD), ammonium/ammonia (N-NH4+/N-NH3), nitrates (NO3−), nitrites (NO2−), and phosphates (P-PO43−). In addition, TOC was measured in the raw digestate. For the analysis of the color elimination, a spectrum sweep of the digestate sample was performed with the spectrophotometer. Absorbance was measured between the UV wavelength range of 200 to 400 nm and in the visible range at 450, 475, 500, 550, 600, 650, and 700 nm. To measure the absorbance of the digestate in the spectrophotometer, a dilution of 1:100 was made. Previously, the spectrophotometer calculated the baseline using distilled water, from which it began to measure the absorbances of the samples. The percentage of color removal was calculated following Equation (1) [19]:
where % CR is color removal, Ai is the absorbance of untreated digestate, and Af is the absorbance of the treated digestate (see Figure S2).
2.4. Microalgae Growth Tests
Quantities of 180 mL of treated and untreated digestate with a dilution of 1:10 were incubated with 20 mL of algae inoculum, resulting in a microalgae inoculum concentration of 10% of the final sample. During the test, absorbance at 550 and 680 nm was measured daily using a spectrophotometer (Thermo Spectronic Genesys 10uv, UK), to monitor algae growth [20]. To calculate the biomass growth of the microalgae in the culture medium, a correlation was made between the absorbance with the microalgae concentration of the culture medium. The concentration was determined by suspended solid analysis while the absorbance was measured using a spectrophotometer at wavelengths of 550 and 680 nm, at different dilutions of algae culture of 100%, 80%, 60%, 40%, 20% and 0%. Control and ozonated tests were carried out in triplicate (see Figure S3). The data obtained on algal concentration were correlated with the Verhulst and Gompertz growth models [21,22]. The Microsoft Excel Solver tool was used to perform the adjustment by means of the least square method. The Verhulst Equation (2) is as follows:
where X is the total concentration (mg biomass L−1), X0 is the initial concentration (mg biomass L−1), Xm is the final concentration (mg biomass L−1), t is the time (hours), and μ is the specific growth rate (h−1). The Gompertz Equation (3) is as follows:
where B is the total concentration (mg biomass L−1), P is the maximum concentration of microalgae (mg biomass L−1), Rm is the specific growth rate (d−1), and λ is the delay phase. The adjustment of the key variables was conducted by means of the least square method and the Solver tool of Microsoft Excel, minimizing the sum of the deviations between the experimental values and the modeled values.
2.5. Light Response Tests
Oxygen production slopes were obtained at different points of distance from the light source, corresponding to different light intensities. The experiment was performed according to the methods previously described by Costache et al. (2013) and Barreiro et al. (2020) [20,23]. A transparent flask of 10 mL (class A) was used to maximize the penetration of incident light into the culture. Dissolved oxygen was monitored by means of a probe (Vernier, USA); see Figure S4. To maintain a constant temperature, the flask was ventilated to avoid cell death due to excess temperature from the light source. Constant agitation was provided to the culture by means of a magnet and a stirring plate; in this way, the microalgae were homogenized and received the same irradiation. Knowing the concentration of the algal biomass in the culture medium, the results are expressed in mg of dissolved oxygen per mg of microalgae per second (mg O2 mg−1 biomass min−1), versus PAR. As a result, a point spread with the values of slope and light intensity was obtained. This was fitted to a function according to the Verhulst and Gompertz models.
2.6. Analytical Procedures
Color removal was determined by reduction in absorbance at different wavelengths by means of a spectrophotometer (Thermo Spectronic Genesys 10uv, UK). TOC was measured in the digestate by the CNS928 25,565 method. % CR, pH, chemical oxygen demand (COD), ammonium/ammonia (NH4+/NH3), nitrates (NO3−), nitrites (NO2−), phosphates (PO43−), and total suspended solids (TSS) were measured according to the APHA standardized methodology [24].
3. Results and Discussion
3.1. Digestate Analysis
An analysis of the digestate before and after pretreatment with ozone was performed, resulting in the parameters presented in Table 1.

Table 1.
Results of analysis for initial digestate and after 5 h ozone treatment.
The digestate was ozonated to eliminate the turbidity provided by the dark compounds, by oxidizing them with ozone, thus reducing the coloration of the effluent. The mass flow calculation revealed an O3 consumption of 4.88 g O3 in 5 h, corresponding to an inlet of 12.2 and outlet of 7.32 g of O3, accounting for a 40% depletion. The ozone decolorization process resulted in a 50.83% of color reduction of the digestate at 475 nm (average white light wavelength). The reduction in color occurred along the entire spectrum, with higher values in the longer wavelength, with an average CR of >52% in the range of 550–700 nm, whereas the minimum CR values were detected in the shorter range of the spectrum, of 28–45%, between 200 and 300 nm. Figure 2 shows the percentage of color elimination in the ozonized digestate for the full spectrum.
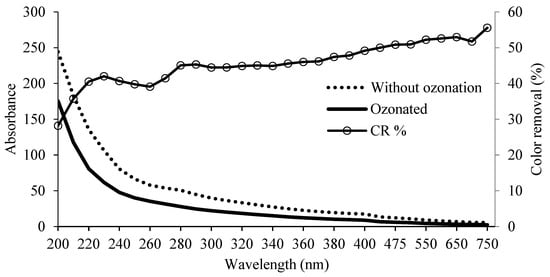
Figure 2.
Percentage of color removal in the spectrum sweep and absorbance of 5 h ozonated and untreated digestate.
The removal of COD was limited to 23%, while color removal reached a considerably higher value of 50.8%, evidencing that some of the organic compounds present in the digestate do not contribute to the dark color. This is the case of volatile fatty acids, which account for more than half of the COD in swine manure and digestates according to García et al. [25]. Significantly higher COD removals have been documented by other researchers. For instance, Liu et al. detected a COD reduction higher than 70%, with O3 dosage greater than 2 g O3 g COD−1 [4]. In the work presented herein, only 1.2 g O3 per g of COD was consumed. In this sense, digested swine manure presents different chromophore groups than industrial wastewater, resulting in lower O3 consumption. In another study, a dose of 1.1 mg O3 mg C−1 was applied to piggery wastewater, resulting in increased light transmittance of 50% and consequently improving microalgae biomass production [26]. Considering the TOC/COD ratio of 1.15/1, the dosage needed in our study was 1.04 g O3 g C−1, which is in the same range as the values reported by Kim et al. [26]. These yields of removal efficiency (in terms of organic carbon, COD, or color) should be considered in order to scale up the treatment process. An ozone generator needs an energy demand between 3.3 and 16 Wh g O3−1 [15,27,28]. The average cost of industrial-scale processing of wastewater through ozonation is around 29 USD m−3 [29].
3.2. Microalgae Growth Monitoring Tests
The effect of color removal was evidenced by a slightly higher biomass concentration reached at the end of the test, after 600 h of incubation, of 1.09 and 0.99 g L−1, in treated and untreated digestate, respectively (Figure 3). However, the impact of ozonation was far more evident in terms of the rate of biomass generation and the reduction in the lag phase, associated with the inhibitory conditions found at the beginning of the experiment. In this sense, ozonated digestate presented algae growth after 71 h of incubation, while untreated assays needed more than 240 h. These results were in agreement with the previously reported experiences. For example, Saranya and Shanthakumar mentioned that a maximum biomass growth of 0.86 g L−1 and a daily specific growth rate of 0.255 d−1 were recorded when C. vulgaris was used in ozonated effluents for 90 min and with a total color removal of 58%. In the same study, the absence of biomass growth was detected in samples that presented limited color removals of 14, 22, and 26% at 10, 20, and 30 min of O3 treatment, respectively [4].
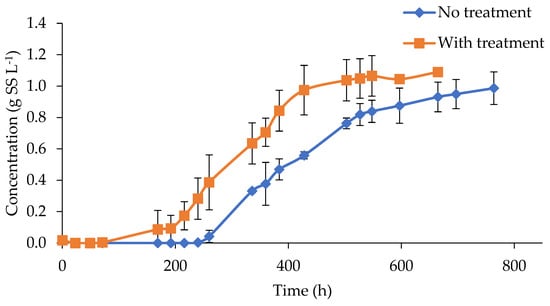
Figure 3.
Microalgae growth in ozonized and non-ozonized digestate.
The kinetics of algal growth allow a better understanding of the positive effects of ozonation. While the Verhulst model is applied to describe population growth under realistic conditions where resources are limited (light or nutrients), the Gompertz model is used to model the growth or decline of algal biomass over time, considering the latency phase at the beginning of the culture (Figure 4).
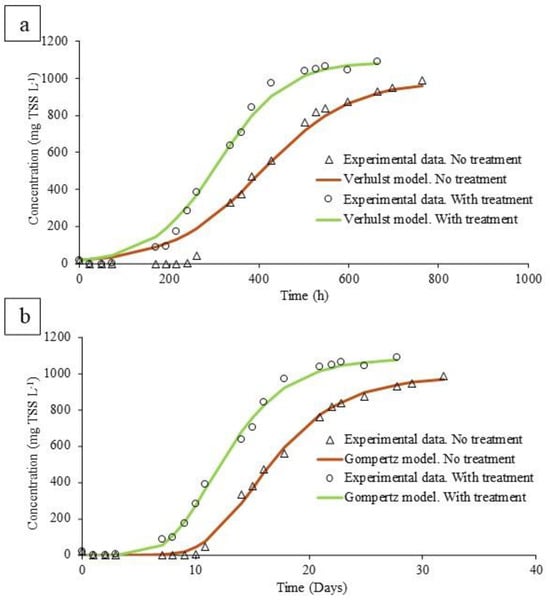
Figure 4.
(a) Algae growth in non-ozonized and ozonized digestate according to the Verhulst model. (b) Algae growth in non-ozonized and ozonized digestate according to the Gompertz model.
Ozonation considerably reduced the inhibition caused by a light limitation; this fact was evidenced by a higher algal generation rate, determined as higher values of the growth rate (GR) according to the two models used (Table 2). According to the Gompertz model, the growth rate increased by 27%, from 84.52 to 107.2 mg L−1 d−1. This trend was also observed in the parameters of the Verhulst model, where the growth rate increased by 44%, from 54.39 to 78.56 mg L−1 d−1. However, the effectiveness of the pretreatment was characterized by a decrease in the lag phase rather than an increase in the growth rate. When using ozonized digestate, the delay time was 4 days, while the raw digestate test needed more than 9 days to show microalgae growth, as a consequence of the strong light limitation. This delay was evidenced in the parameter λ in the Gompertz model, with values of 10.6 and 7.4 in untreated and treated digestate, respectively. In this sense, this reduction in the latency phase could help the viability of the process in continuous mode [4,30]. At this point, it must be stressed that a long lag phase could lead to microalgae washout in a continuously operated process. In this sense, very harsh conditions for microalgae culture would be found in the untreated digested manure.

Table 2.
Microalgae culture parameters for the Gompertz and Verhulst models using treated and untreated digestate.
These results evidenced the viability of the microalgae treatment of digestate without the requirement of a strong dilution. In previous studies, digestate from settled and centrifuged cattle manure was used for the cultivation of Scenedesmus and Chlorella using a very strong dilution between 1:100 and 1:588 [31]. Uggetti et al. used a 1:20 dilution of digested manure prior to microalgae growth [32]. In the case of cow manure digestate, a 1:50 dilution was applied in order to reduce inhibition [33]. This experiment showed an improvement in the growth of microalgae when using pretreatment with ozonation for the decolorization of 1.3 L of digestate diluted at 1:10. Considering the average energy consumption of an industrial ozonator (7 Wh g O3) [34,35,36], this pretreatment requires 65.7 Wh per liter of raw digestate (before dilution). The full-scale implementation of this process could result in a significant reduction in the amount of water required to dilute livestock waste, thus promoting the optimal development of microalgae cultivation. Ozonation does not require operation costs additional to electricity; on the contrary, other digestate pretreatments involve reagent consumption, such as H2O2 in photocatalysis systems, sorbents in filtration, or coagulants and flocculants [14,37]. According to the Verhulst model, it is estimated that a residence time between 3.2 and 6.2 days is required for a maximum microalgae biomass formation and optimum digestate treatment, in terms of nutrient removal. These values are in accordance with the conventional microalgae growth systems applied at an industrial scale in raceway ponds or high-rate algae ponds [38].
3.3. Light Response Test
In order to evaluate the impact of the proposed pretreatment on the light availability for the microalgae, the photosynthetic activity of the cultures was measured as the production of dissolved oxygen (D.O.) at different light conditions. This procedure has been previously used to evaluate the efficiency of microalgae cultures in light utilization [20,39]. Figure 5 plots the D.O. production at different light intensities in both ozonated and untreated digestate. In both cases, the response showed the typical light response occurring in three zones: photolimitation between 50 and 300 μmol m−2 s−1, light saturation around 326 μmol m−2 s−1, and slight photoinhibition from 300 to 2200 μmol m−2 s−1. The points evaluated in the transition zone from photolimitation to photosaturation were characterized by a larger deviation as a consequence of the large impact of a small variation in the irradiance level. However, the treated digestate presented a considerably higher value of oxygen production in the light saturation conditions, namely 0.804 vs. 0.18 mg O2 min−1 per gram of biomass (determined as TSS), respectively, in treated and untreated samples. This is due to the greater light penetration into the clarified digestate and the microalgae being able to take advantage of more irradiance from the light source [40]. In previous research, similar values were obtained in terms of photosynthetic activity when using synthetic culture media without color [20]. Costache et al. found values of 1.14 mg O2 g TSS −1 min−1 under light intensity of 400 µmol m−2 s−1. These authors also reported constant rates of photosynthesis until 1000 µmol m−2 s−1 and a progressive reduction as a consequence of photoinhibition at higher irradiance levels. On the other hand, using urban wastewater as the culture [23], the maximum oxygen production was 0.54 ± 0.02 mg O2 g TSS−1 min−1. In the work presented here, without applying the treatment to the digestate, the photosynthetic rate was significantly below the potential reported (approximately 5 times lower), evidencing the strong light limitation conditions imposed by the dark digestate. However, when applying the ozone pretreatment, the rate approached the maximum reported photosynthesis potential (70% values reported in a mineral medium).
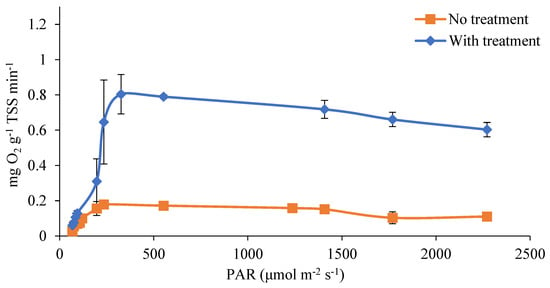
Figure 5.
Dissolved oxygen production curves in treated and untreated digestate.
3.4. Nutrient Removal
The nutrient removal graphs for treated and untreated digestate are shown below. (see Figure 6).
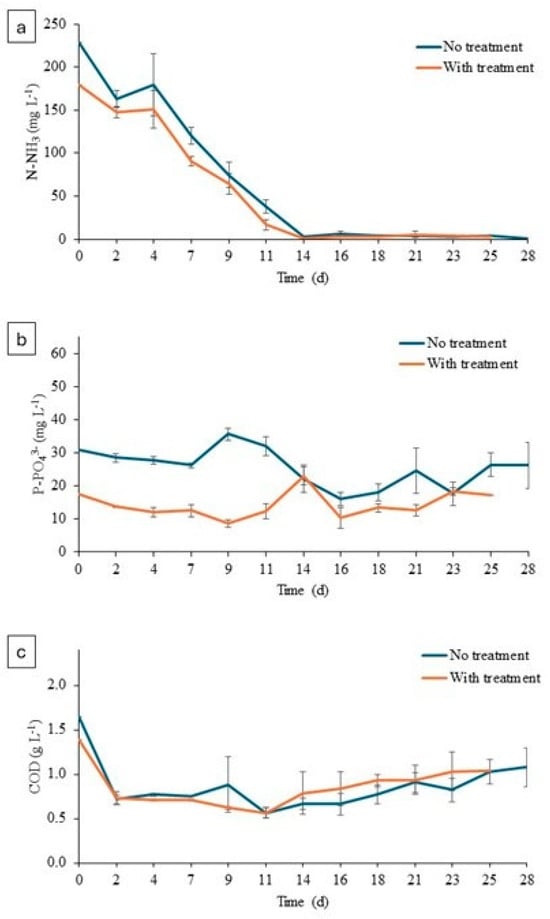
Figure 6.
(a) Concentration of N-NH3 in microalgae cultures. (b) Concentration of P-PO43− in microalgae cultures. (c) Concentration of COD in microalgae cultures.
Ammoniacal nitrogen concentration decreased throughout the experiment in both experiments (treated and untreated). A higher ammonia concentration was observed at the beginning of the experiment in the untreated digestate than in the treated digestate, 229 ± 10.12 mg NH3 L−1 versus 179 ± 0.01 mg NH3 L−1. This fact was related to the ammoniacal oxidation to nitrite and nitrate during the O3 addition, and it could be considered an additional positive effect since high levels of NH3 result in microalgae inhibition. Therefore, the inherent NH3 oxidation could improve the algae pretreatment. At the end of the experiment, values of 0.78 ± 0.85 mg NH3 L−1 and 3 ± 0.98 mg NH3 L−1 were reached in the untreated and treated digestate, respectively. As shown in Figure 6b, the phosphate concentration at the beginning of the culture was higher in the untreated digestate (30.90 ± 1.29 mg PO43− L−1) than in the treated digestate (17.36 ± 0.30 mg P-PO43− L−1). This reduction in phosphate was clearly related to the variation in pH values as a consequence of the ozonation. Raw digestate presented a pH value of 7.65 and that of treated effluent increased to 8.27 (Table 1). This alkalinization could induce phosphate precipitation during the ozone addition [41,42]. Microalgae pretreatment resulted in phosphorous removal of 7.48 ± 17.42% and 24.17 ± 16.07% in O3-treated and control experiments, respectively. As for the COD concentration, at the beginning of the experiment the value in the untreated digestate was 1.65 ± 0.04 g COD L−1, and at the end the value it was 1.08 ± 0.22 g COD L−1; in the treated digestate, it increased from 1.40 ± 0.08 g COD L−1 to 1.03± 0.22 g COD L−1. These values correspond to 50% COD removal, which is in accordance to previously reported experiences with ozonated organic effluents [5].
4. Conclusions
Ozonation is an efficient and achievable pretreatment that reduces inhibition of microalgae in digested swine manure. Color was efficiently removed (total removal of 50.83%), increasing the rates of biomass generation by only 11%. However, the chemical treatment presented a higher impact on the rates of photosynthetic activity determined as oxygen production, resulting in a 440% increase. According to the Gompertz model, the algal biomass took 19 days less time to grow in the treated digestate than in the untreated digestate. A preliminary cost analysis suggests that only 65 kWh of electricity would be needed for the treatment of a liter of digestate. This reduction in energy cost will result in considerable blue water savings through the reduction in the dilution applied before microalgae cultivation. These results suggest the viability of the ozone treatments of dark colored effluents. Thus, a future experimental step could include a continuously operated ozonator followed by a microalgae culture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16121740/s1, Figure S1. Ozonator (left) and absorption column (right); Figure S2. On the left ozonated digestate on the right without ozonation; Figure S3. Growth of microalgae with digestate at the entrance and exit of the ozonator; Figure S4. On the left, light source. On the right, stirring plate, photobioreactor and oxygen sensor.
Author Contributions
Conceptualization, I.d.G., D.H. and R.M.; methodology, C.R.P.; software, C.R.P.; validation, A.G.Á., D.H., A.G. and I.d.G.; formal analysis, C.R.P.; investigation, C.R.P.; resources, D.H.; data curation, C.R.P.; writing—original draft preparation, C.R.P.; writing—review and editing, A.G.Á.; visualization, I.d.G.; supervision, I.d.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Regional Government of Castilla y León, LIFE program through LIFE SMART AgroMobility (LIFE19 CCM/ES/001206). Grant PID 2020-114918RB-I00 funded by PHOTOPREBIO project, reference number MCIN/AEI/10.13039/501100011033.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
To the short stay grant, the Universidad Politécnica de Madrid, and the Universidad Complutense de Madrid.
Conflicts of Interest
The authors declare no conflict of interest.
Acronym and Symbol List
| Acronym | Symbol | Meaning | |
| Af | Absorbance final | μmol | Photon micromoles |
| Ai | Absorbance initial | min | Minutes |
| COD | Chemical oxygen demand | mg | Milligrams |
| CR | Color removal | L | Liters |
| DO | Dissolved oxygen | h | Hours |
| GR | Growth rate | nm | Nanometers |
| LED | Light emitting diode | °C | Degrees Celsius |
| NTK | Total nitrogen Kjeldahl | Xo | Initial biomass concentration |
| PAR | Photosynthetically active radiation | Xm | Final biomass concentration |
| RPM | Revolutions per minute | λ | Delay phase |
| Td | Time delay | Af | Absorbance final |
| TOC | Total organic carbon | Ai | Absorbance initial |
| TSS | Total suspended solids | g | Grams |
| UV | Ultra violet | Wh | Watt hours |
| s | Seconds | ||
| m | meters |
References
- Ardakani, Z.; Aragrande, M.; Canali, M. Global antimicrobial use in livestock farming: An estimate for cattle, chickens, and pigs. Animal 2024, 18, 101060. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, D.; Zeng, W.; Yang, J. Removal of refractory organics from piggery bio-treatment effluent by the catalytic ozonation process with piggery biogas residue biochar as the catalyst. Sci. Total Environ. 2020, 734, 139448. [Google Scholar] [CrossRef]
- Liu, L.; Lin, X.; Luo, L.; Yang, J.; Luo, J.; Liao, X.; Cheng, H. Biosorption of copper ions through microalgae from piggery digestate: Optimization, kinetic, isotherm and mechanism. J. Clean. Prod. 2021, 319, 128724. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. An integrated approach for tannery effluent treatment with ozonation and phycoremediation: A feasibility study. Environ. Res. 2020, 183, 109163. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. Effect of culture conditions on biomass yield of acclimatized microalgae in ozone pre-treated tannery effluent: A simultaneous exploration of bioremediation and lipid accumulation potential. J. Environ. Manag. 2020, 273, 111129. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Rawat, B.S.; Kumar, P.; Kumar, N.; Upadhyay, S.; Chetana, S.; Gururani, P.; Kimothi, S. Sustainable synthetic approach and applications of ZnO/r-GO in the adsorption of toxic Pb2+ and Cr6+ ions. Inorg. Chem. Commun. 2022, 145, 110040. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P. A mini review on heavy metal contamination in vegetable crops. Int. J. Environ. Anal. Chem. 2023, 1–12. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R. Treatment of landfill leachate using different configurations of ultrasonic reactors combined with advanced oxidation processes. Sep. Purif. Technol. 2019, 211, 10–18. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Hu, R.; Chang, H.; Tang, X.; Huang, X.; Cheng, C.; Zhong, N.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean. Prod. 2020, 248, 119182. [Google Scholar] [CrossRef]
- de Godos, I.; Vargas, V.A.; Blanco, S.; González, M.C.; Soto, R.; García-Encina, P.A.; Becares, E.; Muñoz, R. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour. Technol. 2010, 101, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Guieysse, B.; Béchet, Q.; Shilton, A. Variability and uncertainty in water demand and water footprint assessments of fresh algae cultivation based on case studies from five climatic regions. Bioresour. Technol. 2012, 128C, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- John; Brookes, A.; Carra, I.; Jefferson, B.; Jarvis, P. Microbubbles and their application to ozonation in water treatment: A critical review exploring their benefit and future application. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1561–1603. [Google Scholar] [CrossRef]
- Merayo, N.; Hermosilla, D.; Blanco, L.; Cortijo, L.; Blanco, Á. Assessing the application of advanced oxidation processes, and their combination with biological treatment, to effluents from pulp and paper industry. J. Hazard. Mater. 2013, 262, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.G.G.; Gómez, V.A.; Torre, R.M.; de Godos Crespo, I. Scale-down of high-rate algae ponds systems for urban wastewater reuse. J. Water Process Eng. 2023, 56, 104342. [Google Scholar] [CrossRef]
- Duffie, J.A.; Beckman, W.A.; McGowan, J. Solar Engineering of Thermal Processes. Am. J. Phys. 1985, 53, 382. [Google Scholar] [CrossRef]
- Depraetere, O.; Foubert, I.; Muylaert, K. Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis. Bioresour. Technol. 2013, 148, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Costache, T.A.; Acien Fernandez, F.G.; Morales, M.M.; Fernández-Sevilla, J.M.; Stamatin, I.; Molina, E. Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 7627–7637. [Google Scholar] [CrossRef] [PubMed]
- Frunzo, L.; Garra, R.; Giusti, A.; Luongo, V. Modeling biological systems with an improved fractional Gompertz law. Commun. Nonlinear Sci. Numer. Simul. 2019, 74, 260–267. [Google Scholar] [CrossRef]
- De Lauro, E.; De Martino, S.; De Siena, S.; Giorno, V. Stochastic roots of growth phenomena. Phys. A Stat. Mech. Its Appl. 2014, 401, 207–213. [Google Scholar] [CrossRef]
- Barreiro-Vescovo, S.; González-Fernández, C.; Ballesteros, M.; de Godos, I. Activity determination of an algal-bacterial consortium developed during wastewater treatment based on oxygen evolution. J. Water Process Eng. 2020, 36, 101278. [Google Scholar] [CrossRef]
- Apha. Standard Methods for the Examination of Water and Wastewater; Apha: Cincinnati, OH, USA, 1985. [Google Scholar]
- Álvaro, A.G.; Palomar, C.R.; Valenzuela, E.I.; Redondo, D.H.; Torre, R.M.; de Godos Crespo, I. Microbial analysis of anaerobic digester reveals prevalence of manure microbiota. J. Water Process Eng. 2024, 60, 105162. [Google Scholar] [CrossRef]
- Kim, H.C.; Choi, W.J.; Maeng, S.K.; Kim, H.J.; Kim, H.S.; Song, K.G. Ozonation of piggery wastewater for enhanced removal of contaminants by S. quadricauda and the impact on organic characteristics. Bioresour. Technol. 2014, 159, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Magara, Y.; Itoh, M.; Morioka, T. Application of ozone to water treatment and power consumption of ozone generating systems. Progress Nucl. Energy 1995, 29, 175–182. [Google Scholar] [CrossRef]
- Jodzis, S.; Zięba, M. Energy efficiency of an ozone generation process in oxygen. Analysis of a pulsed DBD system. Vacuum 2018, 155, 29–37. [Google Scholar] [CrossRef]
- Tripathi, P.; Tiwari, S.; Tiwari, H.; Sonwani, R.K.; Singh, R.S. Techno-economic assessment of coupling ozonation and biodegradation process for the dye wastewater treatment. J. Water Process Eng. 2023, 56, 104286. [Google Scholar] [CrossRef]
- Das, C.; Ramaiah, N.; Pereira, E.; Naseera, K. Efficient bioremediation of tannery wastewater by monostrains and consortium of marine Chlorella sp. and Phormidium sp. Int. J. Phytoremed. 2018, 20, 284–292. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: A review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Al-Mallahi, J.; Ishii, K.; Sato, M.; Ochiai, S. Static supply of different simulated flue gases for native microalgae cultivation in diluted cow manure digestate. J. Environ. Manag. 2023, 335, 117557. [Google Scholar] [CrossRef] [PubMed]
- Depurador Húmedo. Available online: https://www.kairos-engineering.it/en/ozone-generator (accessed on 27 March 2024).
- Fabricante de Equipos de Ozono e Integradores de Sistemas de Ozono Póngase en Contacto con Oxidation Technologies Ozone Integration Experts. Available online: https://www.oxidationtech.com/contact-us-101.html (accessed on 27 March 2024).
- Ozone Generators–Lenntech. Available online: https://www.lenntech.com/otozone.htm (accessed on 27 March 2024).
- Riaño, B.; Coca, M.; García-González, M.C. Evaluation of Fenton method and ozone-based processes for colour and organic matter removal from biologically pre-treated swine manure. Chemosphere 2014, 117, 193–199. [Google Scholar] [CrossRef] [PubMed]
- de Godos, I.; Arbib, Z.; Lara, E.; Cano, R.; Muñoz, R.; Rogalla, F. Wastewater treatment in algal systems. In Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment; International Water Association: London, UK, 2017; pp. 76–95. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Cho, C.W.; Yun, Y.S. Oxygen evolution rate of photosynthetic microalga Haematococcus pluvialis depending on light intensity and quality. Stud. Surf. Sci. Catal. 2006, 159, 157–160. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Kendir, S.; Franzreb, M. Synergies of pH-induced calcium phosphate precipitation and magnetic separation for energy-efficient harvesting of freshwater microalgae. Bioresour. Technol. 2024, 391, 129964. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Shelef, G. Algal autoflocculation—Verification and proposed mechanism. Biotechnol. Bioeng. 1984, 26, 142–147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).