Abstract
Low-concentration antibiotic wastewater is difficult to treat rapidly using conventional photocatalysts. For this reason, this paper simplified the traditional sol–gel method to prepare Fe3+-TiO2/AC composites and characterized the properties of the composites using FT-IR, XRD, SEM, BET, and TEM. The results demonstrated that iron was uniformly dispersed on the surface of the composites, and the activated carbon (AC) was successfully loaded with iron-doped titanium dioxide. Afterward, ciprofloxacin (CIP) was used as the target degradant, and the effects of different activated carbon loadings, iron-doping, pH, initial concentrations, and UV light intensities on the removal of ciprofloxacin were investigated. The repetitive photocatalytic stability of the composites was studied, and the reaction mechanism was explored by using free radical quenching experiments. The results demonstrated that while iron doping reduced the rate at which photogenerated electrons and holes could combine, loading AC increased the usage efficiency of the composites’ adsorption and catalytic active sites. According to the parameter tests, the circumstances that led to the highest CIP degradation efficiency (94.59%) were as follows: 10 mg/L CIP, 0.5 g/L 0.2%Fe3+-TiO2/20%AC, and solution pH of 7 under 40 min of UV light irradiation. In addition, the Fe3+-TiO2/AC composite material has excellent cyclic stability, the degradation rate of CIP can still reach 87.73% at 60 min after four repeated degradation tests under the same conditions. The applicability of this method could be expanded to the treatment of various industrial organic pollutants in water.
1. Introduction
The overuse of antibiotics is a global problem [1] Antibiotics have been found in both sewage treatment facilities and natural water sources. According to Rumeng Wang [2], antibiotics have been detected in at least 5000 µg/L of influent water from sewage treatment plants treating general domestic wastewater, and according to Sicong Su [3], large amounts of antibiotics have also been detected in natural lakes, rivers, and oceans. The accumulation of antibiotics in aquatic environments can cause the rupture of cell membranes in some organisms and have adverse effects on their growth and development [4], endangering the health of organisms and destroying the diversity and stability of ecosystems [5]. This process directly or indirectly endangers human health, so how to effectively degrade antibiotics is an urgent problem to be solved. Ciprofloxacin has risen to prominence as one of the most widely utilized antibiotics globally [6] due to its high bioavailability, broad antimicrobial spectrum, and strong antimicrobial properties, and it plays an important role in aquaculture and medical and other industries. Ciprofloxacin is also highly toxic to some bacteria and microorganisms [7], so the degradation of ciprofloxacin in wastewater treatment is especially critical.
At present, ciprofloxacin is usually treated by biochemical methods during secondary treatment in wastewater treatment plants, but biochemical treatment often cannot completely degrade ciprofloxacin, and the remaining antibiotics still need to be degraded during deep treatment. Xinyan Guo reported that [8] after biochemical treatment with a high concentration of fluoroquinolone antibiotics in a pharmaceutical wastewater treatment plant, 1–3 mg/L of ciprofloxacin still needs to be treated in the deep treatment process, and the characteristics of wastewater in the deep treatment process are that the concentration of pollutants is low, and it requires fast treatment speed and good treatment effects. At present, the deep treatment process of wastewater treatment plants usually involves processes such as sand filtration, adsorption, membrane filtration, high-level oxidation, bioelectrochemical systems, and UV irradiation. These processes, although effective in removing antibiotics from deep treatment, still have limitations. For example, Weifu Yan et al. [9] researched the elimination of antibiotics by a bioelectrochemical system, which can degrade heavy metals and antibiotics, but its biocompatibility and cost limit its application. Marten Klatt [10] used a membrane bioreactor to achieve efficient removal of hospital pathogens, but the biodegradation of some recalcitrant substances was not sufficient. Rizzo [11] reported that UV radiation has a broad spectrum of applications in deep treatment; the radiation generated by UV light can damage cellular DNA and cause the water to produce strong oxidizing groups such as •OH and H2O2 under high-energy radiation to achieve virus inactivation and organic matter degradation in the wastewater of the deep treatment process, and no byproducts are produced, which is a green and widely applicable method [12]. It is a green and widely applicable process. Zewde et al. [13] pointed out that the use of ultraviolet light in the deep treatment of sewage treatment plants over 20 years has greatly increased. Therefore, to better utilize the existing UV resources of sewage plants to achieve further purification of sewage in a more economical, green, and effective way so that the water quality of sewage plant drainage water is further improved is the focus of the research of water treatment scholars.
Since Fujishima et al. [14] discovered in 1972 that titanium dioxide (TiO2) can decompose water to produce hydrogen and oxygen under ultraviolet light, Photocatalysis has found extensive applications across diverse fields, including solar cells, water splitting, and the degradation of pollutants. Therefore, many scholars have conducted extensive research on the photochemical mechanism and fundamental principles of photocatalysis, especially titanium dioxide, through various surface science methods [15]. In the photocatalytic reaction of TiO2, The photocatalytic efficacy is restricted by its wide band gap and the high recombination rate of photogenerated electron–hole pairs. Therefore, many studies have focused on improving its performance by depositing noble metals, doping metals, or nonmetal ions [16]. Doping Fe can not only reduce the band gap of TiO2 but also enhance its light response and utilization efficiency; for example, Swati Sood [17] experimentally corroborated that the energy band gap of Fe3+-doped Fe3+-TiO2 was drastically decreased from 3.2 eV to 2.1–2.5 eV, which improves the light energy utilization of Fe3+-TiO2 and enhances its photocatalytic performance. Furthermore, The similarity in radius between Fe3+ and Ti4+ allowed Fe3+ to infiltrate the lattice of TiO2 and serve as a trapping center. This infiltration diminished the recombination rate of electron–hole pairs, consequently augmenting the photocatalytic performance. Yajun Yang [18] investigated the effect of Fe3+ doping on the grain growth of TiO2 and the morphology of Fe3+ as well as the transformation and phase transition of the existing morphology in the process, and the results showed that Fe3+ can replace Ti4+ and has good electrocatalytic activity for the reduction of CO2 and H2O to CH4. Moreover, iron is abundant in nature, inexpensive, and less biotoxic. Therefore, iron is more suitable as a modified material for photocatalysts than other metal elements.
Currently, many low-concentration pollutants are difficult to degrade quickly when using photocatalytic materials; for example, Shixin Liu [19] treated RhB with Eu-doped high-entropy oxides, and only 10% of the RhB was degraded in 20 min. Cu2−x Se/CdS was used to treat 10 mg/L RhB, only approximately 20% could be treated in 30 min, and the rate of low-concentration pollutants was not effective [20], the catalytic active sites on the surface of pure photocatalytic materials are not fully utilized and cannot reach the optimal degradation state, the photocatalytic materials and adsorbent materials are combined, and the abundant adsorption active sites of the adsorbent materials provide a large mass transfer rate for the catalytic active sites. After the catalytic active sites degrade the pollutants, the adsorption active sites are vacated to continue to adsorb and degrade the pollutant molecules, and the synergistic impact of the two promotes the photocatalytic reaction rate, which has been proven by many scholars to promote photocatalytic performance [21,22,23]. Activated carbon (AC) is a material with a high surface area and porosity (usually 900–1200 m2/g), and its use as a carrier for different photocatalytic materials can provide more active adsorption sites for photocatalysts [24] and higher carrier mobility [25] as well as a wider light absorption range [26]. For example, Chaoneng Ning [27] loaded FeVO4 with biochar, which enlarged the FVC light absorption range by around 100 nm, reduced the band gap to 2.00 eV, and accelerated the electron transport rate of FVC; Peng Feng [28] loaded activated carbon with g-C3N4, which increased the photogenerated carrier separation rate, this decelerated the separation rate of photogenerated electron–hole pairs, while enhancing the photocatalytic efficiency. TiO2 powder easily agglomerates in water, which can be avoided by compositing TiO2 with adsorbent materials. The combination of photocatalysts and activated carbon has been proven by many scholars to improve the photocatalytic performance and solve the agglomeration phenomenon of TiO2 powder [29,30,31].
The sol–gel method is a widely utilized wet chemistry technique within the realm of materials science and engineering. Among various physicochemical techniques, sol–gel methods are highly promising due to their ability to synthesize materials at low temperatures, typically below 100 °C [32]. Sol–gel technology offers several advantages, including its ability to easily monitor and control the shape and size of nanomaterials. This capability enables the production of spherical, fine particles, and nanopowders with uniform size distributions [33].
Therefore, in this paper, the traditional sol–gel method was simplified, and a novel Fe3+-TiO2/AC photocatalyst was synthesized. Through characterization and experiments, the novel photocatalytic material showed strong photocatalytic performance in degrading CIP and could achieve rapid degradation of low-concentration pollutants; this study is expected to provide an experimental basis and theoretical basis for the engineering of photocatalytic materials for subsequent photocatalytic treatment of pharmaceutical wastewater.
2. Materials and Methods
2.1. Experimental Drugs and Reagents
The main reagents used included ciprofloxacin (98%, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) and tetrabutyl titanate (97%, AR, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China); and hydrochloric acid (97%, AR), sodium hydroxide (96%, AR), anhydrous ethanol (99.7%, AR), and iron (III) nitrate nanohydrate (98.5%, Fe(NO3)3·9H2O), which were obtained from Tianjin Yongda Chemical Reagent Co., Tianjin, China. The water used in the experiments was deionized water, and the activated carbon (AC) was coconut shell activated carbon (iodine value 1200).
The main instruments used were a constant-temperature stirring water bath (Jintan Chengdong Xinrui Instrument Factory, Model: LKTC-B1-T, Jintan, China); a benchtop low-speed centrifuge (Hunan Xiangli Scientific Instrument Co., Ltd., Model: TD6M, Changsha, China); a UV-visible spectrophotometer (Unocal (Shanghai) Instrument Co., Ltd., Model: UV-4800, Shanghai, China); a 40 W, 254 nm, 29 W, 254 nm UV lamp; an electric blast drying oven (Yuyao Xingchen Instrument Factory, Model: XGQ-2000, Yuyao, China); and a tube furnace (Hefei Kejing Material Technology Co., Ltd., Model: OTF-1200X, Hefei, China).
2.2. Preparation of Fe3+-TiO2/AC Composites
- Pretreatment of AC: The predetermined quantity of activated carbon (AC) was immersed in 1 mol/L hydrochloric acid for a duration of 24 h, following which the acid was drained off, distilled water was poured into a beaker containing this AC, the mixture was heated to boiling, the water was filtered out, and the process was repeated 2 times. Finally, the AC was then dried at a temperature of 100 °C for 12 h to complete the pretreatment for backup [34].
- For the preparation of the composite materials, 40 mL of anhydrous ethanol and 15 mL of tetrabutyl titanate were poured into a 200 mL beaker to mix well and stirred for 30 min in air with a cling film. A certain amount of Fe(NO3)3-9H2O was then dissolved in 20 mL of anhydrous ethanol and stirred for 20 min in air with cling film. The latter solution was quickly poured into the former solution and stirred for 2 h in air with cling film. After air treatment for 2 h, a certain amount of pretreated activated carbon was added, and the mixture was stirred for another 2 h.
The mixed solution was then put in a 30 °C water bath with an open mouth and stirred for approximately 15 h, and the stirring was stopped. The gel was placed at room temperature for aging for 5 h and then put into an electric blast drying oven and dried at 120 °C for 14 h to produce the Fe3+-TiO2/AC dry gel. The resulting solid material was then ground into powder and placed into a tube furnace for calcination with nitrogen as the protective gas against oxidation. The rate of temperature increase was 5 °C per minute with calcination at 500 °C for 5 h to obtain the Fe3+-TiO2/AC composite material [35].The detailed composition of synthesized samples is presented in Table 1.

Table 1.
Detailed composition of synthesized samples.
2.3. Characterization of Photocatalytic Materials
X-ray diffraction (XRD) analysis was performed with an diffractometer (PANalytical B.V., Model: Aeris, Almelo, Netherlands) to determine the crystalline phase of the composites. Scanning electron microscopy (SEM) was also carried out using a field emission multifunctional scanning electron microscope (Nippon Electric (Electronic) Company, Model: JSM-IT700HR, Tokyo, Japan)with a current of 20 kV. X-ray energy dispersive spectroscopy (EDS) was used to analyze the elemental composition of the materials. Fourier transform infrared spectroscopy (FT-IR) was performed with a FT-IR spectrometer (Thermo Fisher Scientific, Model: Nicolet IS10, Shanghai, China). Specific surface and pore size analysis (BET) was performed with a -Specific surface and pore size analyzer (Beijing Guoyi Precision Measurement Technology Co, Model:V-Sorb 4804 TP, Beijing, China). The degassing temperature was 150 °C. Transmission electron microscopy analysis was performed using High Resolution Transmission Electron Microscope(Nippon Electric (Electronic) Company, Model: JEM-2010, Tokyo, Japan).
2.4. Experimental Approach to Fe3+-TiO2/AC Photocatalysis
The photocatalytic reaction was conducted in a custom photocatalytic reactor, and samples were collected at regular intervals. The absorbance of ciprofloxacin was measured at 277 nm with a total reaction duration of 120 min. The photocatalytic reaction was carried out in a homemade photocatalytic reactor by varying the AC loading of Fe3+-TiO2/AC (5%, 10%, 20%, and 30%, mass percentage) and the doping of Fe3+ (0.1%, 0.2%, 0.3%, 0.4%, 1%, molar percentage) to investigate the optimal ratio of the materials; changing the solution pH (3, 5, 7, 9, 11), the initial concentration of pollutants (1–10 mg/L), and the intensity of light (40 W, 29 W) to investigate the variation in the pollutant removal rate under the influence of different factors; and finally, the photocatalytic stability of the composites. The degradation rate of pollutants can be calculated using Equation (1):
where is the ciprofloxacin degradation rate; C0 is the initial concentration of the solution, in mg/L; and Ct is the concentration of the solution at time t, in mg/L.
The effect of reactive oxygen species (ROS) on the photocatalytic degradation of ciprofloxacin (CIP) was confirmed by radical quenching experiments. In a photocatalytic reaction, the corresponding ROS are inhibited by adding different ROS quenchers to the reaction. In this study, 100 μM tert-butanol (TBA), tert-benzoquinone (PQB), and ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) were employed to quench hydroxyl (•OH), superoxide radical (O2−), and h+ groups [36,37].
3. Results and Discussion
3.1. XRD Analysis
To investigate the effect of compounding TiO2 with different materials on the crystal growth of TiO2, TiO2, Fe3+-TiO2, TiO2/AC, and Fe3+-TiO2/AC were prepared and compared via XRD analyses, and the results are shown in Figure 1. Comparing the XRD patterns of the four materials with the standard card, it was found that the characteristic peaks of the anatase phase appeared in the four materials, and TiO2 was in the anatase phase, which indicated that the doping of Fe3+ with AC loading did not have a large effect on the crystalline growth of TiO2. There were no significant characteristic peaks of the Fe material in the plots of Fe3+-TiO2 or Fe3+-TiO2/AC, indicating that the doped Fe3+ was very well dispersed in TiO2, which confirmed the successful doping of Fe3+ [38] and was also illustrated in the subsequent EDS plots. The peak shift of the TiO2/AC profile was mainly to demonstrate the presence of activated carbon. The loading of activated carbon can lead to the accumulation of electrons on the surface of titanium dioxide, thereby increasing the order of the titanium dioxide lattice, reducing the voids between atoms, and reducing the crystal size of titanium dioxide so that activated carbon can be wrapped on the surface of titanium dioxide [39].
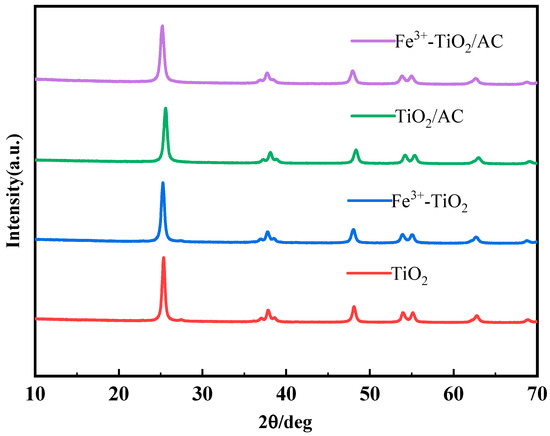
Figure 1.
XRD patterns of TiO2, Fe3+-TiO2, TiO2/AC, and Fe3+-TiO2/AC.
3.2. FT-IR Analysis
The composites’ FT-IR spectra are illustrated in Figure 2. The peak at 3425 cm−1 is attributed to the stretching vibration of the O-H bond, while the peak at 1630 cm−1 is associated with the bending vibration of H-O-H [28]. The absorption peaks at 2931 cm−1 and 1388 cm−1 are attributed to the C-H stretching vibration peaks, and the peaks near 1105 cm−1 are attributed to the C-OH stretching vibration peaks [35]. The peaks of Fe3+-TiO2/AC are significantly weaker than those of pure TiO2, which indicates that there is an enrichment of functional groups, such as hydroxyl and carbon groups, on the surface of the carbon material. This indicates that during the sol–gel synthesis process of Fe3+-TiO2/AC, AC is coloaded with Fe3+-TiO2, enriching the surface with these functional groups [40]. The absorption peak near 500 cm−1 can be attributed to the vibrational absorption peak of the Ti-O-Ti skeleton [41], indicating that the vibrational peaks of the Ti-O and Fe-O bonds of the Fe3+-TiO2/AC materials synthesized via the simplified sol–gel method appear at low wavenumbers. Since the radii of Fe3+ and Ti4+ are similar, their vibrational peaks overlap with those of Ti-O, suggesting that Fe3+ is introduced into the TiO2 lattice through substitution during material synthesis [18,42].
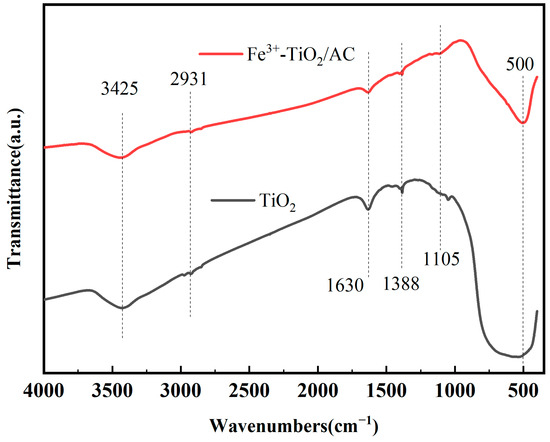
Figure 2.
FT-IR spectra of Fe3+-TiO2/AC and TiO2.
3.3. SEM/EDS and TEM Analysis
The surface structure of composites has a very important role in their adsorption and photocatalytic properties. The effects of different AC loading ratios on the surface microstructure of the composites can be observed in Figure 3. Electron microscopy revealed that milled Fe3+-TiO2/AC has a laminar structure at 5000× magnification, the edges and surfaces of the three materials are rough, and the surfaces of the composites are rougher as the AC loading ratio increases, which promotes the degradation of pollutants by Fe3+-TiO2/AC; however, it is not clear that the higher the AC loading ratio is, the faster the degradation efficiency of Fe3+-TiO2/AC on pollutants. However, it is not the case that the higher the AC loading ratio is, the faster the degradation efficiency of Fe3+-TiO2/AC on pollutants, which may be because excessive AC loading affects the absorption of light by Fe3+-TiO2, which was also proven in subsequent experiments. From the EDS patterns, it can be seen that the surface of the Ti composites was almost completely covered and that Fe was uniformly distributed on the surface of the composites, indicating that the composite of Fe and titanium dioxide was very successful. Although the distribution of C on the surface of the composites is uneven, the Fe3+-TiO2/AC synthesized by the sol–gel method is not conducive to the formation of a uniform morphology, which also proves the successful recombination of Fe3+-TiO2 and AC. The TEM image (Figure 4a) confirmed the successful loading of AC on Fe3+-TiO2, which has an approximately spherical structure, in agreement with the results of Esteves et al. [43]. From the HRTEM image (Figure 4b), a lattice spacing of 0.355 nm matching to TiO2 was observed, which is consistent with the XRD observations, and furthermore, a unique interface was formed at the interface between AC and Fe3+-TiO2, not only facilitated the successful synthesis of Fe3+-TiO2/AC but also established a pathway for swift electron transfer [27].
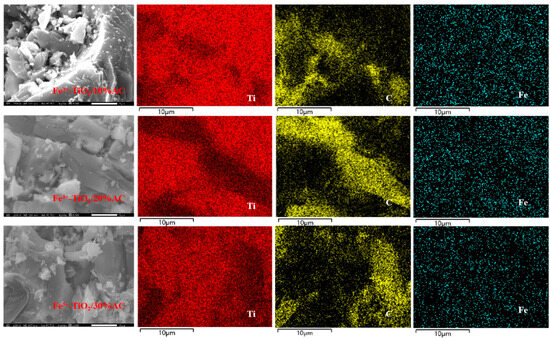
Figure 3.
SEM/EDS patterns of milled Fe3+-TiO2/AC with different AC loading ratios.
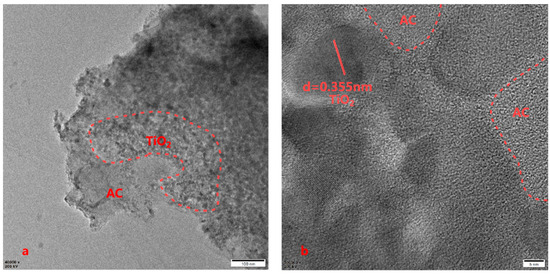
Figure 4.
TEM (a) and HRTEM (b) images of Fe3+-TiO2/AC.
3.4. BET Analysis
The pores and other characteristics of AC, TiO2, and Fe3+-TiO2/AC, were explored by N2 adsorption-desorption isotherms, and the findings are illustrated in Figure 5. Following the IUPAC classification, the adsorption-desorption isotherms of all the samples were class IV adsorption isotherms, which indicated that the obtained samples were all rich in mesoporous structural features. For Fe3+-TiO2/AC, the mutual composite of Fe3+-TiO2 and AC decreased its specific surface area relative to that of the pure AC phase, and its adsorption-desorption isotherm and pore size were between those of TiO2 and AC, which also indicated the successful composite of Fe3+-TiO2 and AC. The rich specific surface area and pore size of Fe3+-TiO2/AC provided the photocatalyst with more abundant adsorption active sites, which is favorable for its mass transfer rate and thus improved photocatalytic efficiency. The rich specific surface area and pore size of Fe3+-TiO2/AC provided the photocatalyst with more abundant adsorption active sites, which was conducive to its mass transfer rate and thus improved the photocatalytic efficiency. This result also confirms the successful complexation of Fe3+-TiO2 with AC according to the EDS patterns.
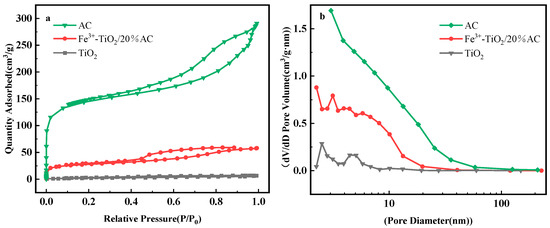
Figure 5.
Adsorption-desorption isotherm plot of N2 of AC, Fe3+-TiO2/AC, and TiO2 (a) with pore size distribution (b).
3.5. Effect of AC Loading on the Degradation of CIP by Fe3+-TiO2/AC
Under the specified conditions of a ciprofloxacin concentration of 10 mg/L, 0.5 g/L photocatalyst, a light intensity of 40 W, 254 nm ultraviolet light, and a solution pH of 7, CIP was degraded by Fe3+-TiO2/AC (1% Fe3+) with prepared loaded AC mass ratios of 5%, 10%, 20%, and 30%, as well as by pure TiO2. The effects of different AC loadings on the degradation of CIP solution by Fe3+-TiO/AC were investigated.
As shown in Figure 6, pure TiO2 degraded CIP during the process from the inception of the reaction to 120 min, signifying that the photocatalytic reaction has significant degradation efficacy for CIP. However, the degradation efficiencies of all Fe3+-TiO2/AC composites markedly surpassed that of pure TiO2, while the highest photocatalytic activity was observed for the Fe3+-TiO2/AC composites loaded with 20% AC and 30% AC, which may be attributed to the presence of a moderate amount of carbonaceous material, crystalline structure, high specific surface area, and significant porosity in the composites. An appropriate amount of AC effectively increases the adsorption of ciprofloxacin molecules on the TiO2 surface, so that the ciprofloxacin molecules form a local high-concentration state on the surface of the photocatalysts. These molecules occupy more catalytic active sites on the catalyst surface and undergo a redox effect with more active radicals generated by TiO2, thus increasing the photocatalytic efficiency, which also suggests that the loading amount of AC has important significance.
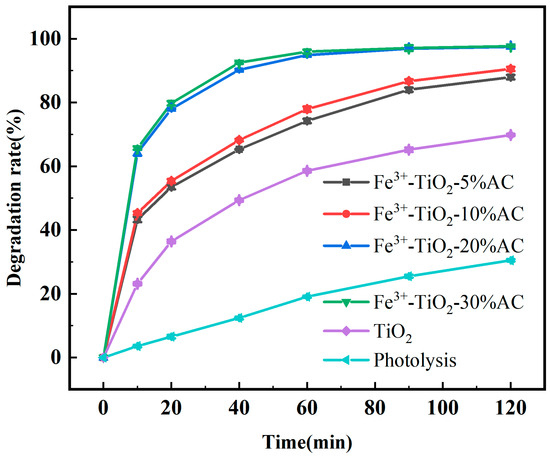
Figure 6.
Degradation rate of CIP by Fe3+-TiO2/AC with different AC loadings.
Furthermore, Figure 6 demonstrates that the CIP degradation rate increased with increasing AC loading, in which the CIP degradation rate notably surged from 10% AC loading to 20% AC loading, indicating that the increase in AC increased both the adsorption of CIP and the concentration of CIP on the surface of TiO2. However, when the AC loading increased from 20% to 30%, the CIP degradation rate did not significantly increase, possibly because too much AC affected the contact area between TiO2 and light, which resulted in a shading effect and inhibited the catalyst’s UV light absorption, thereby diminishing the efficiency of the photocatalytic reaction.
3.6. Effect of Fe3+ Doping on the Fe3+-TiO2/AC Degradation of CIP
Under the conditions of ciprofloxacin at a mass concentration of 10 mg/L, 0.5 g/L photocatalyst, a light intensity of 40 W, a 254 nm UV, and a solution pH of 7, CIP was degraded by Fe3+ doped with molar ratios of 0.1%, 0.2%, 0.3%, 0.4%, and 1% (20% AC) to investigate the effects of different amounts of the Fe3+ dopant on Fe3+-TiO2/AC on the degradation of the CIP solution.
As Figure 7 shows, the photocatalytic degradation rates of CIP using Fe3+-TiO2/AC doped with 0.1%, 0.2%, 0.3%, 0.4%, and 1% Fe3+ were 95.10%, 97.14%, 95.43%, 92.59%, and 89.86%, respectively, of that of the CIP solution after 60 min of the photocatalytic reaction, whereas the photocatalytic efficiency of Fe3+-TiO2/AC doped with 1% Fe3+ was lower than TiO2/AC. This fully signifies that the appropriate amount of Fe3+ can act as an effective electron and hole trap to capture photogenerated electrons from the conduction band and effectively reduce the complexation of electron–hole pairs, and at the same time, the doping of the appropriate amount of Fe3+ can introduce more oxygen vacancies on the surface of the crystal lattice and TiO2, which facilitates the adsorption of water and the formation of hydroxyl groups on the surface [44]. Furthermore, this Fe3+ doping improves the absorption and utilization of light by TiO2, enhancing its photocatalytic activity [45].
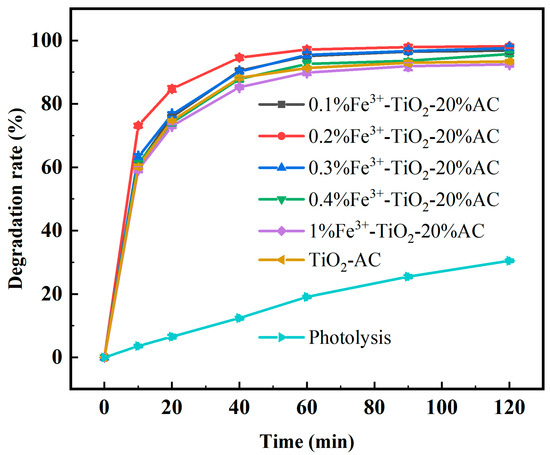
Figure 7.
Degradation rate of CIP by Fe3+-TiO2/AC with different Fe3+ doping levels.
As shown in Figure 7, the highest degradation rate of CIP was observed when 0.2% Fe3+ was doped, whereas the photocatalytic reaction efficiency decreased after more than 0.2% Fe3+ was doped because, when too much Fe3+ is doped, Fe3+ acts as a recombination center for photogenerated electrons and holes, which reduces its photocatalytic activity instead [46]. Additionally, higher Fe3+ content may lead to the occupation of AC pores by Fe3+, which reduces the effective specific surface area of Fe3+-TiO2/AC. This reduction in surface area promotes catalyst agglomeration and causes shading effects, further reducing the photocatalytic activity of the material.
3.7. Effect of pH on CIP Degradation by Fe3+-TiO2/AC
Under the conditions of ciprofloxacin at a mass concentration of 10 mg/L, 0.5 g/L of photocatalyst (0.2% Fe3+-TiO2/20% AC), and a light intensity of 40 W, 254 nm UV, the desired pH was adjusted with both hydrochloric acid and sodium hydroxide to obtain mixed solutions with pH values of 3, 5, 7, 9, and 11, respectively, to explore the effect of pH in the photocatalytic system on the Fe3+-TiO2/AC catalytic degradation effect of the CIP solution.
As shown in Figure 8, the degradation rate of CIP increased from 79.85% to 88.39% at pH 3–5 for 60 min of reaction; when the pH was 7, the degradation rate of CIP reached the highest value of 97.14%; and as the pH further increased from 9 to 11, the degradation rate of CIP decreased from 95.52% to 67.17%. An alkaline or acidic solution pH is not favorable for the photocatalytic degradation of CIP. This is because the solution pH affects the catalyst surface charge [47], the adsorption rate of CIP [35], and the separation of functional groups in the active site of the catalyst [44]. At pH = 7, CIP in a solution mainly exists in molecular form [48], and electrostatic adsorption is enhanced, which increases its concentration on the surface of TiO2, thus leading to the highest degradation rate. In an alkaline solution, CIP is prone to crystallize on the catalyst surface [49]. In an alkaline solution, CIP easily crystallizes on the catalyst surface, which affects the degradation rate. In acidic environments, the concentration of H+ increases, which leads to a decrease in the concentration of strong oxidizing hydroxyl radicals (•OH) generated by the reaction with H+, thus decreasing the degradation efficiency of the photocatalyst.
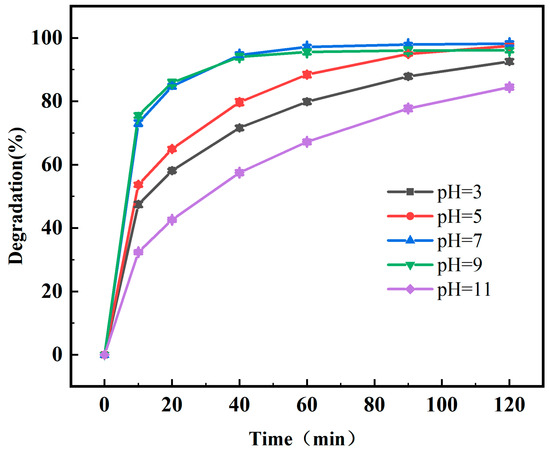
Figure 8.
Degradation rate of CIP by 0.2% Fe3+-TiO2/20% AC at different pH values.
3.8. Effect of Initial Concentration on the Degradation of CIP by Fe3+-TiO2/AC
The initial concentration of CIP was set at 1, 3, 5, 7, and 10 mg/L with the addition of a 0.5 g/L photocatalyst (0.2% Fe3+-TiO2/20% AC), a light intensity of 40 W, a 254 nm UV, and a pH of 7. The effect of the initial concentration on the degradation of Fe3+-TiO2/AC was explored.
From Figure 9, it can be observed that at the ciprofloxacin concentration of 1 mg/L, the degradation rate of CIP basically did not increase after reaching 87.02% in 5 min, indicating that the catalyst could not degrade CIP when the ciprofloxacin concentration in the solution was too low, because the low ciprofloxacin concentration is not helpful to the accumulation of CIP on the surface of the photocatalyst. It reduces the concentration of the reactants accumulated on the surface of the photocatalyst, leading to the ineffective utilization of the catalytic active sites on the surface of the photocatalyst, thus failing to achieve the ideal degradation effect [50]. The degradation rate of the ciprofloxacin concentration at 3 mg/L was the highest, after which the degradation rate of ciprofloxacin by photocatalysis decreased. If the amount of photocatalyst in a solution remains constant and the concentration of CIP in a solution increases, the quantity of adsorption and catalytic active sites provided by the photocatalyst surface is fixed. The probability of collision of CIP molecules in solution with the adsorption active site and the catalytic active site would also increase. However, the photocatalyst could not provide a higher quantity of active sites for the photocatalytic reaction with CIP molecules, resulting in a downward trend in the photocatalytic degradation rate [51].
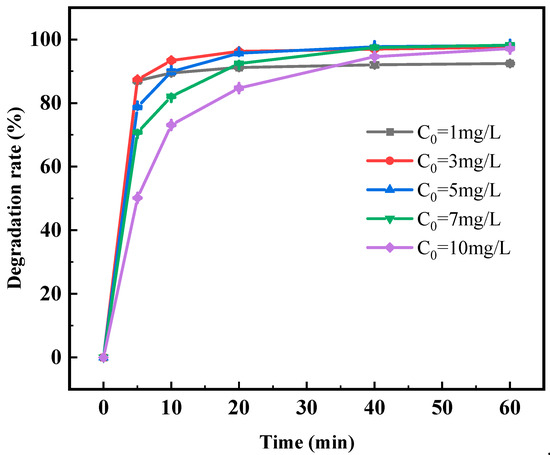
Figure 9.
Degradation rate of CIP by 0.2% Fe3+-TiO2/20% AC at different initial CIP concentrations.
3.9. Effect of Light Intensity on CIP Degradation by Fe3+-TiO2/AC
Under the conditions of a CIP solution with a mass concentration of 10 mg/L, 0.5 g/L photocatalyst (0.2% Fe3+-TiO2/20% AC), and pH = 7, the light intensity was set to 40 W, 254 nm, and 29 W, 254 nm to investigate the effect of light intensity on the degradation of CIP.
As shown in Figure 10. The CIP degradation efficiency increased with increasing light intensity, and its corresponding degradation rate was the highest at a light intensity of 40 W, reaching 97.14% after 60 min. This is because the higher the light intensity is, the more the photocatalytic material can absorb photons and excite more photogenerated electron–hole pairs. However, it can also be seen from Figure 10 that the difference in the degradation rate of CIP between 40 W and 29 W illumination was obvious before 40 min of reaction, but the difference between the two decreased after 40 min. This shows that for this experimental application, to achieve the expected degradation rate of CIP, two modes, high-power UV light-short reaction time or low-power UV light-long reaction time, can be selected.
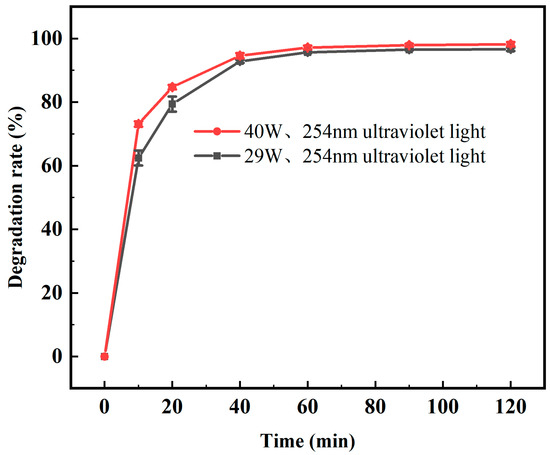
Figure 10.
Degradation rate of CIP by 0.2% Fe3+-TiO2/20% AC at different light intensities.
3.10. Kinetic Analysis of the Photocatalytic Degradation Reaction
In the kinetic fitting of the photocatalytic reaction, the pH, initial concentration of ciprofloxacin, and intensity of UV light were fitted with the time t of the photocatalytic stage as the abscissa and ln(Ct/C0) as the ordinate, and Figure 11a,c,e were obtained. The kobs rate of change of reaction kinetics was then fitted with the number of changes as the abscissa and the kobs rate of change as the ordinate, and Figure 11b,d,f were obtained. Table 2, Table 3 and Table 4 show the kinetic parameters of the photocatalytic reaction for different factors.
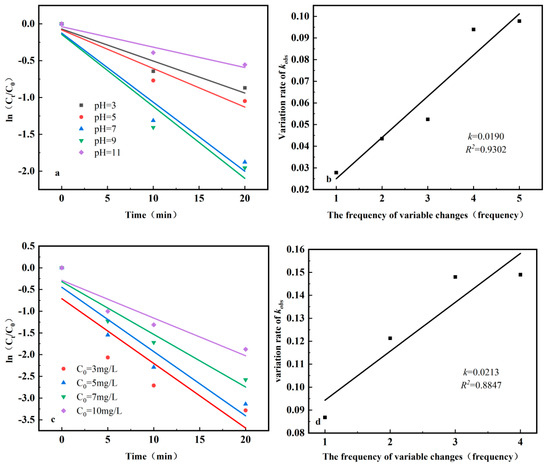
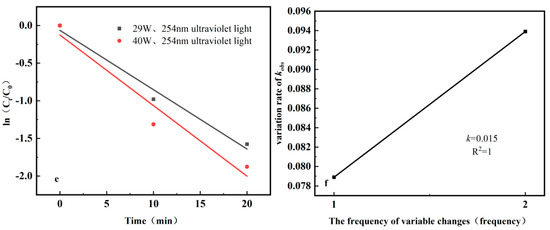
Figure 11.
Fitting curve of the photocatalytic reaction kinetics. (a) Fitting curves under different pH conditions; (b) Fitting curves of kobs change rates at different pH values; (c) Fitting curves of different initial concentrations; (d) Fitting curves of kobs change rate at different initial concentrations; (e) Fitting curves at different UV intensities; (f) Fitting curves of kobs change rate under different UV intensities.

Table 2.
Kinetic parameters of the photocatalytic reaction at different pH values.

Table 3.
Kinetic parameters of the photocatalytic reaction at different initial concentrations.

Table 4.
Kinetic parameters of the photocatalytic reaction under different UV light intensities.
As shown in Figure 11a–d, the rates of change in pH and initial concentration of ciprofloxacin in kobs were 0.0190 and 0.0213, respectively, over 20 min, with little difference. This suggests that the pH and initial ciprofloxacin concentration have similar effects on the reaction during this time. However, as shown in Figure 11, the degradation rate stabilized at 20 min for different initial concentrations of ciprofloxacin, the degradation rate exceeded 80%, and the maximum difference in degradation rate between different initial concentrations of ciprofloxacin was approximately 10%. However, the results in Figure 11 show that under the influence of different pH values, the degradation rates at different pH values are still quite different after 20 min, and the maximum difference in the degradation rates at different pH values can reach approximately 40%.
As shown in Figure 11e,f, the rate of change of different UV light intensities on kobs over a 20 min period is 0.015, which is lower than the effect of pH and the initial concentration of ciprofloxacin. Figure 11 shows that the degradation rate under different UV light intensities was approximately 5% at 20 min. Therefore, the effect of different UV light intensities on the degradation rate was less pronounced than that of pH and initial ciprofloxacin concentration. Finally, it can be concluded that the degrees of influence on the photocatalytic degradation rate are the pH, initial ciprofloxacin concentration, and UV light intensity.
Generally, the factors considered according to the degree of degradation of the contaminant are pH, initial concentration of ciprofloxacin, and ultraviolet light intensity when the Fe3+-TiO2/AC material is used for the degradation of ciprofloxacin.
3.11. Photocatalytic Repeat Stability of Fe3+-TiO2/AC
In practical applications, the stability of the degradation efficacy of photocatalysts for repeated recycling is an important index for evaluating the advantages and disadvantages of photocatalytic materials. To test the reusability of the homemade composite photocatalyst, a photocatalytic reaction was carried out with a CIP solution with a mass concentration of 5 mg/L, 0.5 g/L photocatalyst (0.2% Fe3+-TiO2/20% AC), a pH of 7, and UV light with illumination intensities of 40 W and a wavelength of 254 nm. Then, the photocatalysts used were centrifuged, washed, and dried, and photocatalytic degradation was carried out under the same experimental conditions. Photocatalytic degradation was carried out to investigate the photocatalytic stability of Fe3+-TiO2/AC, and the experimental results are shown in Figure 12.
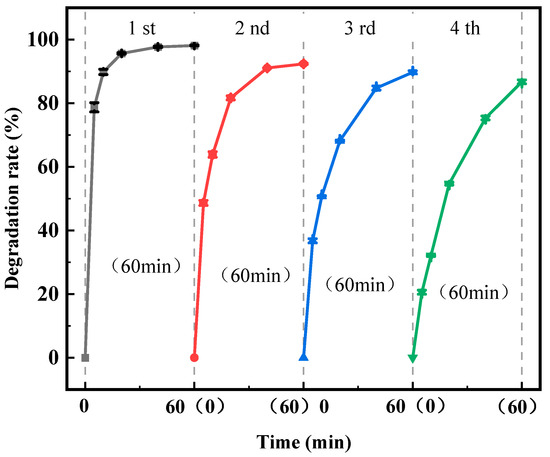
Figure 12.
Degradation rate of CIP by 0.2% Fe3+-TiO2/20% AC for four consecutive cycles.
As shown in Figure 12, the Fe3+-TiO2/AC photocatalyst showed a slight decrease in the degradation rate, with degradation efficiencies of 96.12%, 92.37%, 89.73%, and 86.73%, respectively, for CIP at 60 min after four cycles of use, but the results still indicated that the photocatalyst had excellent photocatalytic stability, suggesting that it had good degradation stability for CIP and was economical. The photocatalytic stability of the photocatalyst slightly decreased.
4. Fe3+-TiO2/AC Degradation Mechanism of CIP
According to the photocatalytic principle of Fe3+-TiO2/AC (as shown in Figure 13), the TiO2 semiconductor has a valence band (VB) full of electrons and a conduction band (CB) full of energy but lacking electrons, with an energy gap (Eg) between the two. When the semiconductor is irradiated by a light source (hv ≥ Eg), the electrons in the valence band are excited to jump to the conduction band to generate photogenerated electrons (e−), while holes are formed in the VB (hvb+), and hvb+ migrates to the surface of the semiconductor to form hydroxyl radicals (•OH); e− in the CB is captured by the O2 on the surface of the semiconductor to form superoxide radicals (O2− •), and the •OH and O2− • can oxidize any organic matter adsorbed on the surface of the semiconductor to produce H2O and CO2 [52]. Due to the similarity of the radii of Fe3+ and Ti4+, Fe3+ can easily replace Ti4+ in TiO2, which leads to distortion of the TiO2 lattice, preventing the grain from growing properly and leading to an increase in the specific surface area of TiO2 [53]. Moreover, after doping with Fe3+, part of the hvb+ and e− in the VB and CB will migrate to Fe3+ to form Fe4+ and Fe2+, which will act as traps for electrons and holes and increase the lifespan of photogenerated charge carriers, and due to the instability of Fe4+ and Fe2+, the trapped charges will migrate to the surface of Ti4+ to generate strong oxidants, thus elevating the photocatalytic activity [54], which enhances the photocatalytic reaction activity. This principle can also be explained by the following equation. The loaded AC provides enhanced adsorption performance after loading the composite material.
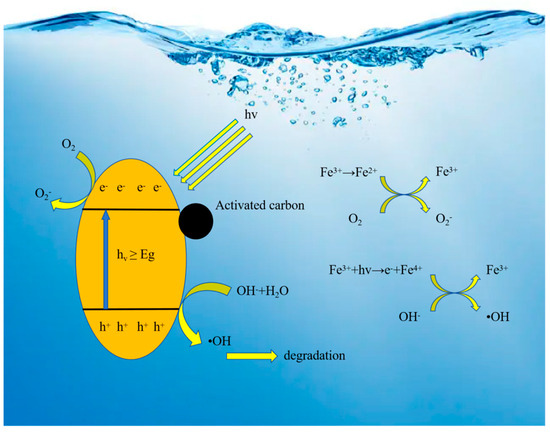
Figure 13.
Process schematic diagram of the Fe3+-TiO2/AC adsorption-photocatalysis process.
When the photocatalyst degrades CIP, the adsorption and degradation processes occur simultaneously. In the adsorption stage, the concentration gradient of CIP on the surface of the adsorption active sites in the internal void structure of the composite material increases, and then the mass transfer gradient increases so that more CIP molecules can be adsorbed on the surface of Fe3+-TiO2/AC, which promotes the rate of the catalytic reaction. The adsorption stage is carried out under the action of the UV light source, and photocatalytic degradation is carried out at the same time. At the same time, after the degradation of pollutants by the photocatalytic material, the AC active sites can vacate, and thus, the AC can continue to adsorb and degrade the pollutant molecules. The Fe3+-TiO2/AC surface can maintain a high concentration of pollutants, thus realizing the efficient degradation of CIP by the composite photocatalytic material.
In addition, to determine the main agents contributing to the degradation of CIP, a series of radical quenching experiments were carried out using TBA, PQB, and EDTA-Na2 as scavengers of •OH, O2−, and H+. Clearly, the introduction of scavengers inhibited the degradation of CIP. As shown in Figure 14, the degradation rates of CIP were reduced to 77.49% and 82.28% after the addition of tert-butanol and EDTA-Na2, respectively, and the inhibition rate of p-benzoquinone against CIP was only 4.33%. The results showed that the free radicals that played a major role in the degradation of CIP were •OH and h+, and the roles of the above agents were •OH > h+ > O2−.
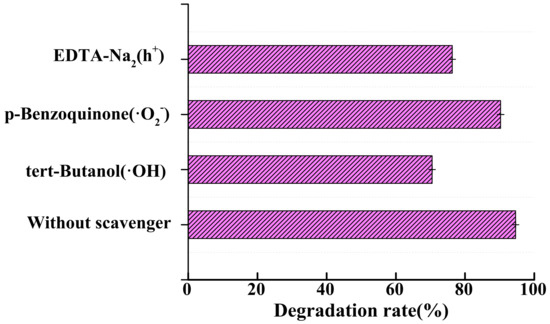
Figure 14.
Photocatalytic activity of 0.2%Fe3+-TiO2/20%AC towards the mineralization of CIP with different types of scavengers.
Gupta et al. [55] noted that •OH produced by holes is susceptible to CIP attack, leading to the breakdown of CIP molecules into various intermediates that are eventually converted to CO2 and H2O [56]. Wen et al. proposed that the mineralization process of CIP involves the destruction of the piperazine-based moiety, defluorination, the destruction of quinolone groups and benzene rings, loss of piperazine rings, and hydroxylation to produce compounds with lower molecular weights. These reactions go through a series of steps and eventually mineralize to CO2, H2O, NO3−, and F−.
5. Conclusions
This paper simplified the conventional sol–gel method to prepare Fe3+-TiO2/AC composites and characterized the properties of the composites using FT-IR, XRD, SEM, BET, and TEM. The results demonstrated that iron was uniformly dispersed on the surface of the composites, and the activated carbon (AC) was successfully loaded with iron-doped titanium dioxide. All of the titanium dioxide showed an anatase phase, in addition, the activated carbon with a high specific surface area provided the composites with a high specific surface area, which increased the adsorption capacity of the composites and improved the utilization of catalytic active sites of the composites through mass transfer, which in turn improved the photocatalytic activity. According to the parameter tests, the circumstances that led to the highest CIP degradation efficiency (94.59%) were as follows: 10 mg/L CIP, 0.5 g/L 0.2%Fe3+-TiO2/20%AC, and solution pH of 7 under 40 min of UV light irradiation. In addition, the Fe3+-TiO2/AC composite material has excellent cyclic stability, the degradation rate of CIP can still reach 87.73% at 60 min after four repeated degradation tests under the same conditions. The applicability of this method could be expanded to the treatment of various industrial organic pollutants in water.
Author Contributions
Methodology, Y.Y.; Software, Y.Y.; Writing—original draft, Y.Y.; Writing—review & editing, J.C.; Supervision, J.C. and F.Z.; Funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (21676178).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, Fate, and Mass Balance of Different Classes of Pharmaceuticals and Personal Care Products in an Anaerobic-Anoxic-Oxic Wastewater Treatment Plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ji, M.; Zhai, H.; Guo, Y.; Liu, Y. Occurrence of Antibiotics and Antibiotic Resistance Genes in WWTP Effluent-Receiving Water Bodies and Reclaimed Wastewater Treatment Plants. Sci. Total Environ. 2021, 796, 148919. [Google Scholar] [CrossRef]
- Su, S.; Li, C.; Yang, J.; Xu, Q.; Qiu, Z.; Xue, B.; Wang, S.; Zhao, C.; Xiao, Z.; Wang, J.; et al. Distribution of Antibiotic Resistance Genes in Three Different Natural Water Bodies—A Lake, River and Sea. Int. J. Environ. Res. Public Health IJERPH 2020, 17, 552. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, Y.; Zhao, K.; Li, C.; Liu, B.; Li, M.; Lyu, C.; Sun, L.; Zhong, S. Occurrence, Bioaccumulation, Metabolism and Ecotoxicity of Fluoroquinolones in the Aquatic Environment: A Review. Toxics 2023, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Ohore, O.E.; Wei, Y.; Wang, Y.; Nwankwegu, A.S.; Wang, Z. Tracking the Influence of Antibiotics, Antibiotic Resistomes, and Salinity Gradient in Modulating Microbial Community Assemblage of Surface Water and the Ecological Consequences. Chemosphere 2022, 305, 135428. [Google Scholar] [CrossRef] [PubMed]
- Kanth, S.; Malgar Puttaiahgowda, Y.; Gupta, S.; T., S. Recent Advancements and Perspective of Ciprofloxacin-Based Antimicrobial Polymers. J. Biomater. Sci. Polym. Ed. 2023, 34, 918–949. [Google Scholar] [CrossRef] [PubMed]
- Załęska-Radziwiłł, M.; Affek, K.; Rybak, J. Ecotoxicity of Chosen Pharmaceuticals in Relation to Micro-Organisms—Risk Assessment. Desalin. Water Treat. 2014, 52, 3908–3917. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Z.; Zhang, Y.; Kong, X.; Kong, D.; Shan, Z.; Wang, N. Removal Mechanisms for Extremely High-Level Fluoroquinolone Antibiotics in Pharmaceutical Wastewater Treatment Plants. Environ. Sci. Pollut. Res. 2017, 24, 8769–8777. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The Effect of Bioelectrochemical Systems on Antibiotics Removal and Antibiotic Resistance Genes: A Review. Chem. Eng. J. 2019, 358, 1421–1437. [Google Scholar] [CrossRef]
- Klatt, M.; Beyer, F.; Einfeldt, J. Hospital Wastewater Treatment and the Role of Membrane Filtration—Removal of Micropollutants and Pathogens: A Review. Water Sci. Technol. 2022, 86, 2213–2232. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for Antibiotic Resistant Bacteria and Genes Spread into the Environment: A Review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Park, E.; Mancl, K.M.; Lee, J.; Tuovinen, O.H. Batch UV Disinfection for Small Flow Onsite Wastewater Treatment. Appl. Eng. Agric. 2020, 36, 717–725. [Google Scholar] [CrossRef]
- Zewde, A.A.; Li, Z.; Zhang, L.; Odey, E.A.; Xiaoqin, Z. Utilisation of Appropriately Treated Wastewater for Some Further Beneficial Purposes: A Review of the Disinfection Method of Treated Wastewater Using UV Radiation Technology. Rev. Environ. Health 2020, 35, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X.; Zhang, N.; Shao, Y.; Xu, C.C. Enhanced Photocatalytic Performance of Iron oxides@HTCC Fabricated from Zinc Extraction Tailings for Methylene Blue Degradation: Investigation of the Photocatalytic Mechanism. Int. J. Miner. Metall. Mater. 2023, 30, 2364–2374. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly Effective Fe-Doped TiO 2 Nanoparticles Photocatalysts for Visible-Light Driven Photocatalytic Degradation of Toxic Organic Compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Wang, J.; Zheng, W.; Cao, Y. Doping and Transformation Mechanisms of Fe3+ Ions in Fe-Doped TiO2. CrystEngComm 2017, 19, 1100–1105. [Google Scholar] [CrossRef]
- Liu, S.; Du, M.; Ge, Y.; Li, Z.; Srivastava, G.P.; Wang, J.; Wei, T.; Zou, Y.; Li, X.; Li, Y.; et al. Enhancement of High Entropy Oxide (La0.2Nd0.2Sm0.2Gd0.2Y0.2)2Zr2O7 Mechanical and Photocatalytic Properties via Eu Doping. J. Mater. Sci. 2022, 57, 7863–7876. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS Composite Photocatalyst with Enhanced Visible Light Photocatalysis Activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled Adsorption and Photocatalysis of g-C3N4 Based Composites: Material Synthesis, Mechanism, and Environmental Applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, H.; Pei, W.; Liu, W.; Shi, F.; Li, Y.; Huo, Y. Integrated Photocatalysis-Adsorption-Membrane Separation in Rotating Reactor for Synergistic Removal of RhB. Chemosphere 2021, 270, 129424. [Google Scholar] [CrossRef]
- Javier Fonseca-Bermudez, O.; Giraldo, L.; Sierra-Ramirez, R.; Carlos Moreno-Pirajan, J. Removal of Hydrogen Sulfide from Biogas by Adsorption and Photocatalysis: A Review. Environ. Chem. Lett. 2023, 21, 1059–1073. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 Nanomaterials for Photocatalytic Degradation of Pollutants: A Review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of Pharmaceuticals and Personal Care Products in Water Treatment Using Carbonaceous-TiO2 Composites: A Critical Review of Recent Literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Park, S.-J. Recent Advances in Carbonaceous Photocatalysts with Enhanced Photocatalytic Performances: A Mini Review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef]
- Ning, C.; Cui, J.; Zhang, F.; Xiangli, P.; Wei, L.; Yang, D.; Liang, Y.; Cui, J. Enhanced Degradation of Ofloxacin by Activation of Peroxymonosulfate with Biochar-FeVO4 under Visible Light Assistance: The Role of Biochar in Mediating Charge Transfer and Mechanism Insight. Appl. Surf. Sci. 2024, 652, 159236. [Google Scholar] [CrossRef]
- Feng, P.; Cui, K.; Hai, Z.; Wang, J.; Wang, L. Facile Synthesis of Activated Carbon Loaded g-C3N4 Composite with Enhanced Photocatalytic Performance under Visible Light. Diam. Relat. Mater. 2023, 136, 109921. [Google Scholar] [CrossRef]
- Rangkooy, H.; Tanha, F.; Jaafarzadeh, N.; Mohammadbeigi, A. The Influence of ZnO-SnO 2 Nanoparticles and Activated Carbon on the Photocatalytic Degradation of Toluene Using Continuous Flow Mode. Med. Gas. Res. 2017, 7, 260. [Google Scholar] [CrossRef]
- Yuan, R.; Guan, R.; Zheng, J. Effect of the Pore Size of TiO2-Loaded Activated Carbon Fiber on Its Photocatalytic Activity. Scr. Mater. 2005, 52, 1329–1334. [Google Scholar] [CrossRef]
- Wang, G.J.; Wang, D.M. Titanium Dioxide Supported on Activated Carbon Performance of Wastewater. AMR 2011, 239–242, 3319–3322. [Google Scholar]
- Malekshahi Byranvand, M.; Nemati Kharat, A.; Fatholahi, L.; Malekshahi Beiranvand, Z. A Review on Synthesis of Nano-TiO2 via Different Methods. J. Nanostruct. 2013, 3, 1–9. [Google Scholar]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, H.; Dong, Y.; Zhan, F.; Liao, M.; Pan, B.; Wang, C. Polymerization Synthesis of Semi-Coke Activated Carbon Modified Carbon Nitride Nanoscrolls with Boosted Photocatalytic H2 Production. Mol. Catal. 2024, 556, 113918. [Google Scholar] [CrossRef]
- Loo, W.W.; Pang, Y.L.; Lim, S.; Wong, K.H.; Lai, C.W.; Abdullah, A.Z. Enhancement of Photocatalytic Degradation of Malachite Green Using Iron Doped Titanium Dioxide Loaded on Oil Palm Empty Fruit Bunch-Derived Activated Carbon. Chemosphere 2021, 272, 129588. [Google Scholar] [CrossRef]
- Wu, Z.; He, X.; Gao, Z.; Xue, Y.; Chen, X.; Zhang, L. Synthesis and Characterization of Ni-Doped Anatase TiO2 Loaded on Magnetic Activated Carbon for Rapidly Removing Triphenylmethane Dyes. Environ. Sci. Pollut. Res. 2021, 28, 3475–3483. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Zhao, L.; Guo, L.-H.; Zhang, H.; Chen, F.-J.; Yu, W.-C. Roles of Reactive Oxygen Species (ROS) in the Photocatalytic Degradation of Pentachlorophenol and Its Main Toxic Intermediates by TiO2/UV. J. Hazard. Mater. 2019, 369, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ren, J.; Huo, Y.; Bian, Z.; Li, H. Nanocrystalline Fe/TiO2 Visible Photocatalyst with a Mesoporous Structure Prepared via a Nonhydrolytic Sol−Gel Route. J. Phys. Chem. C 2007, 111, 18965–18969. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.-C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Lourenço, V.S.; Carvalho, W.A. Removal of Phenol in Seawater by Heterogeneous Photocatalysis Using Activated Carbon Materials Modified with TiO2. Catal. Today 2022, 388–389, 247–258. [Google Scholar] [CrossRef]
- Le Thi Thanh, T.; Nguyen Thi, L.; Tran Dinh, T.; Nguyen Van, N. Enhanced Photocatalytic Degradation of Rhodamine B Using C/Fe Co-Doped Titanium Dioxide Coated on Activated Carbon. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Eshaghi, A.; Moradi, H. Optical and Photocatalytic Properties of the Fe-Doped TiO2 Nanoparticles Loaded on the Activated Carbon. Adv. Powder Technol. 2018, 29, 1879–1885. [Google Scholar] [CrossRef]
- Esteves, M.; Fernandez-Werner, L.; Pla Cid, C.C.; Pelegrini, S.; Pasa, A.A.; Faccio, R.; Mombru, A.W. Optical, Electrical and Structural Properties of Fe Doped Sodium Titanate Nanostructures. Appl. Surf. Sci. 2021, 552, 149534. [Google Scholar] [CrossRef]
- Rosa, D.; Abbasova, N.; Di Palma, L. Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review. Nanomaterials 2024, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Ren, Z.; Wang, P.; Liu, J.; Jing, L.; Fu, H. Study on the Mechanisms of Photoinduced Carriers Separation and Recombination for Fe3+–TiO2 Photocatalysts. Appl. Surf. Sci. 2007, 253, 4390–4395. [Google Scholar] [CrossRef]
- Velázquez-Martínez, S.; Silva-Martínez, S.; Pineda-Arellano, C.A.; Jiménez-González, A.; Salgado-Tránsito, I.; Morales-Pérez, A.A.; Peña-Cruz, M.I. Modified Sol-Gel/Hydrothermal Method for the Synthesis of Microsized TiO2 and Iron-Doped TiO2, Its Characterization and Solar Photocatalytic Activity for an Azo Dye Degradation. J. Photochem. Photobiol. A Chem. 2018, 359, 93–101. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Imam, S.S.; Adnan, R.; Mohd Kaus, N.H.; Us Saqib, N. Influence of Various Operational Parameters on the Photocatalytic Degradation of Ciprofloxacin in Aqueous Media: A Short Review. Toxin Rev. 2023, 42, 655–670. [Google Scholar] [CrossRef]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of Transformation Product Formation on pH during Photolytic and Photocatalytic Degradation of Ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; Yang, B.; Jiang, Z.; Yang, J. Synthesis of Phosphotungstic Acid/S-Doped g-C3N4 Photocatalyst and Its Photocatalytic Degradation of Organic Pollutants in Aqueous Solutions. Mater. Sci.-Medzg. 2023, 29, 135–141. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Li, M.; Wang, X.; Qi, X.; Li, W.; Xue, M.; Wang, Y.; Han, F. Synergetic Effect between Adsorption and Photodegradation on rGO/TiO2/ACF Composites for Dynamic Toluene Gaseous Removal. Environ. Sci. Pollut. Res. 2020, 27, 9866–9881. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Fu, S.-H.; Tseng, J.-J.; Chu, H.; Ko, T.-H. Study on Photocatalytic Degradation of Gaseous Dichloromethane Using Pure and Iron Ion-Doped TiO2 Prepared by the Sol–Gel Method. Chemosphere 2007, 66, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Delekar, S.D.; Yadav, H.M.; Hankare, P.P. Iron–Doped Anatase Titania Nanostructures: Synthesis and Characterization. CNANO 2013, 9, 235–240. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic Degradation of Rhodamine B and Real Textile Wastewater Using Fe-Doped TiO2 Anchored on Reduced Graphene Oxide (Fe-TiO2/rGO): Characterization and Feasibility, Mechanism and Pathway Studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K. Photocatalytic Performance of 3D Engineered Chitosan Hydrogels Embedded with Sulfur-Doped C3N4/ZnO Nanoparticles for Ciprofloxacin Removal: Degradation and Mechanistic Pathways. Int. J. Biol. Macromol. 2022, 198, 87–100. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photocatalytic Degradation of Ciprofloxacin by a Novel Z-Scheme CeO2-Ag/AgBr Photocatalyst: Influencing Factors, Possible Degradation Pathways, and Mechanism Insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).