Long-Term Stability of Low-Cost IoT System for Monitoring Water Quality in Urban Rivers
Abstract
1. Introduction
2. Materials and Methods
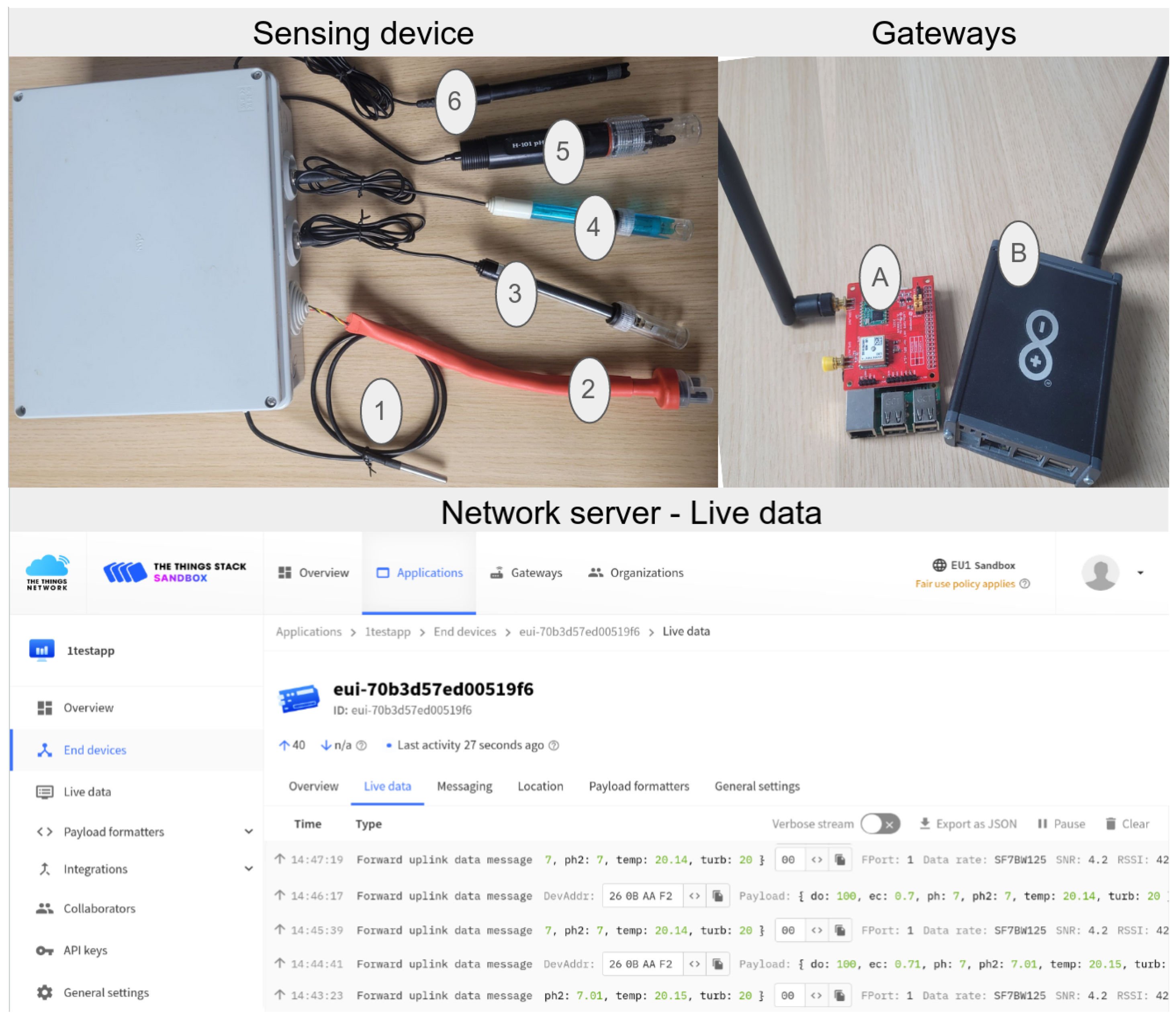
2.1. Prototype Design
2.1.1. Low-Cost Sensors
2.1.2. Reference Sensors
2.2. Specifications and Price
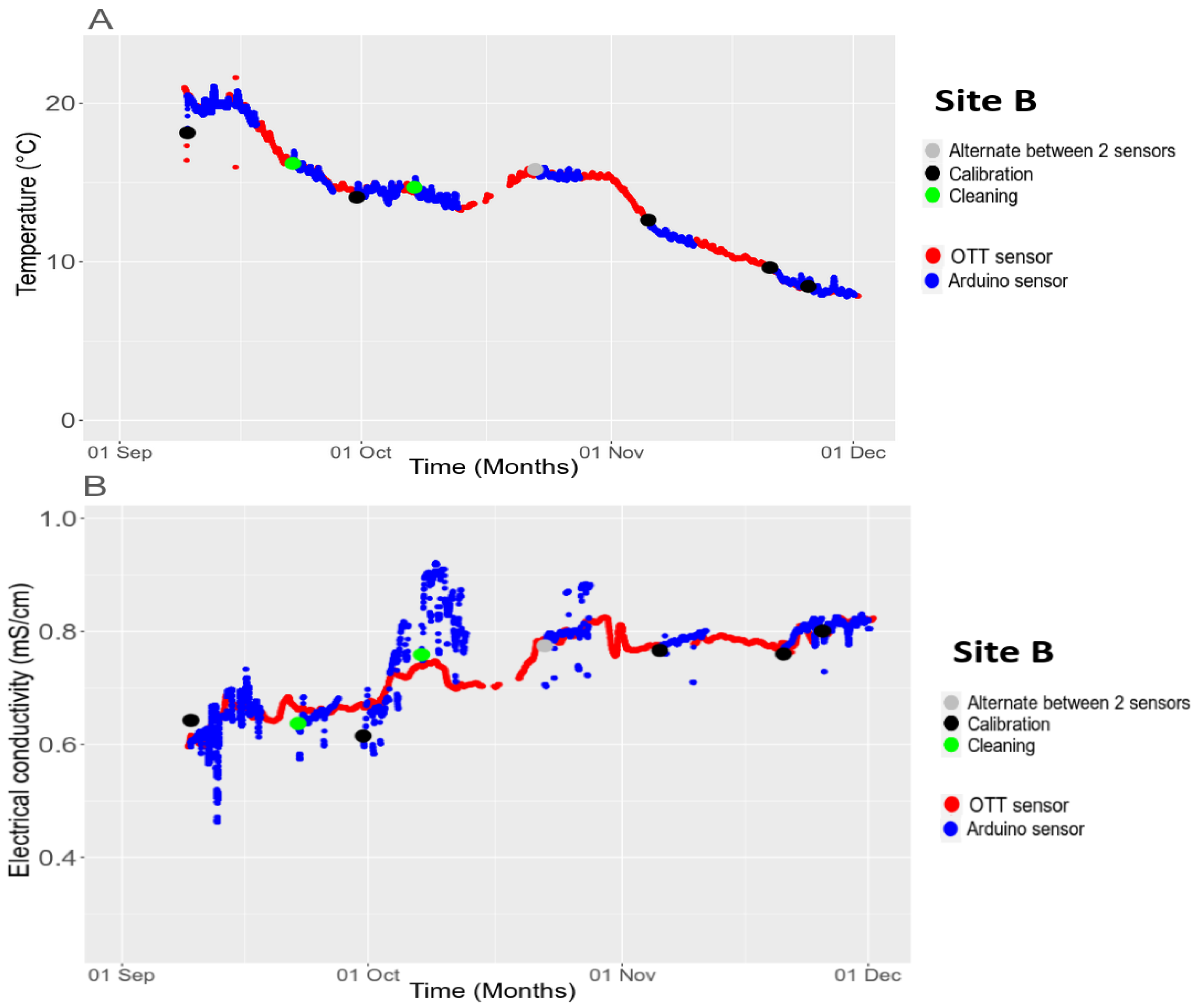
2.3. Cleaning and Calibration
2.4. LoRa Gateway
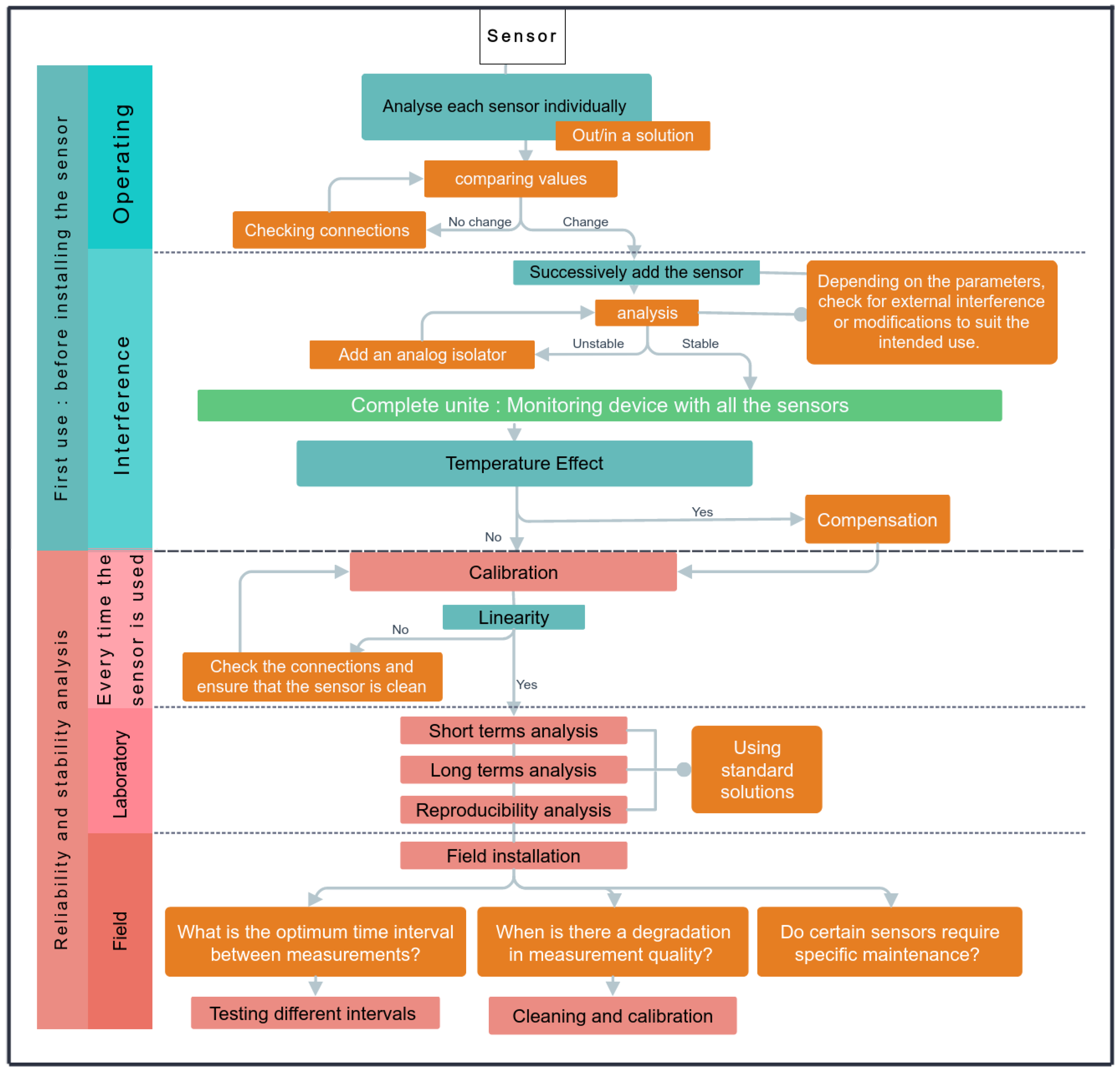
2.5. Sensor Validation
2.5.1. Accuracy
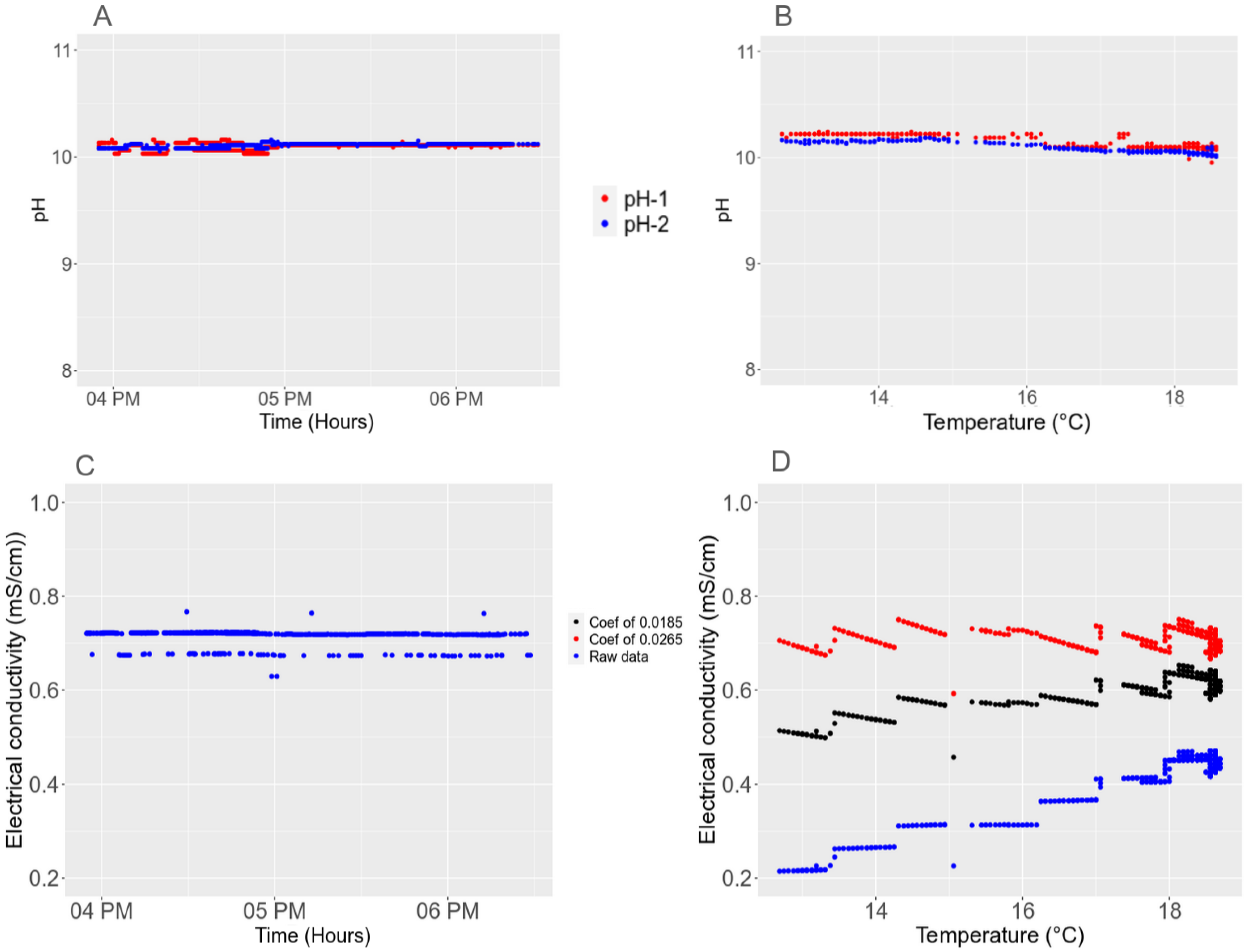
2.5.2. Temperature Effect
2.5.3. Temporal Stability in the Laboratory
2.5.4. Temporal Stability in the Field
3. Results and Discussion
3.1. Accuracy of the Sensors
3.2. Reproducibility of the Sensors
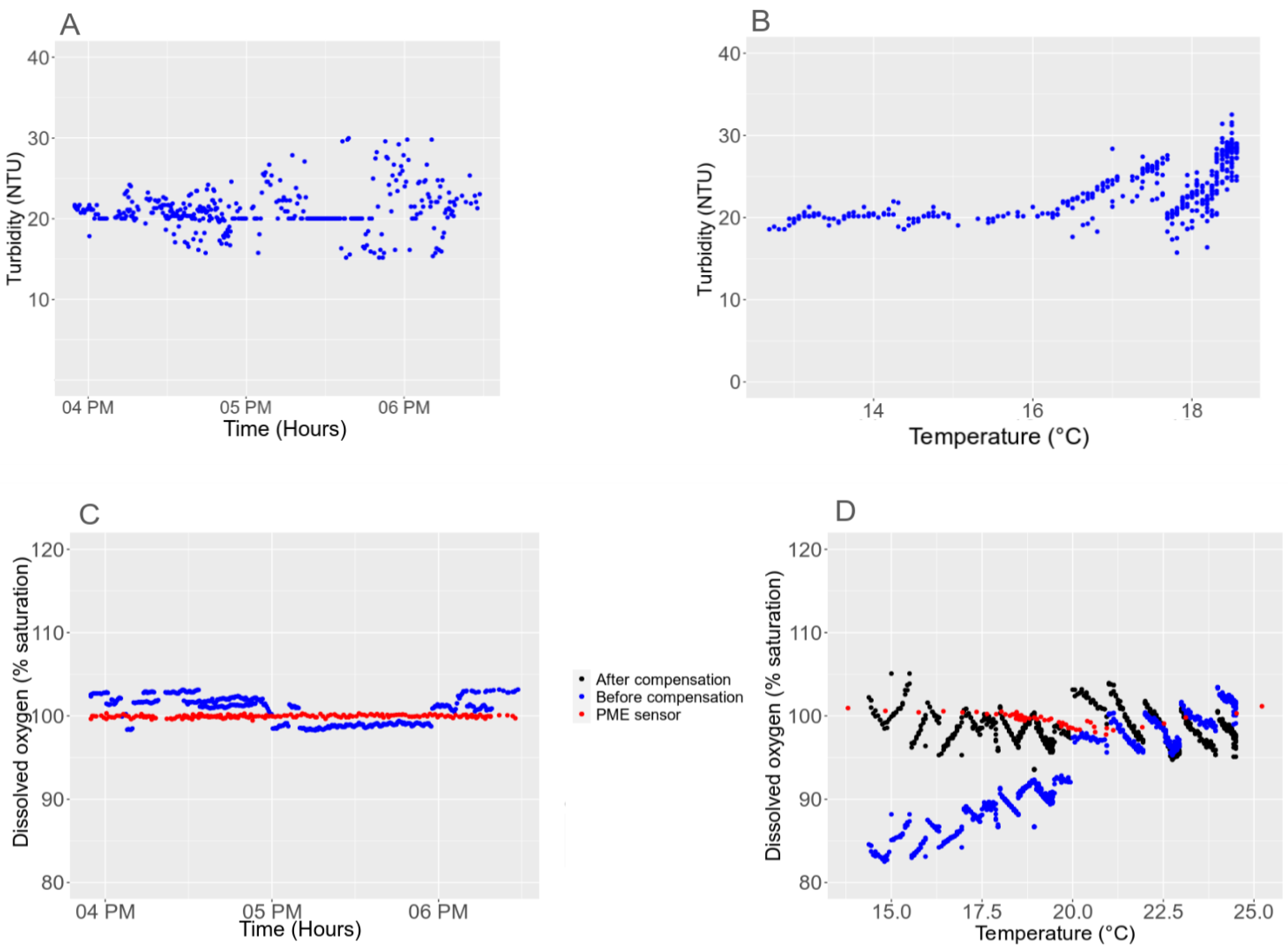
3.3. Sensitivity to the Environment
3.4. Temporal Stability in the Laboratory
3.4.1. Short-Term Stability
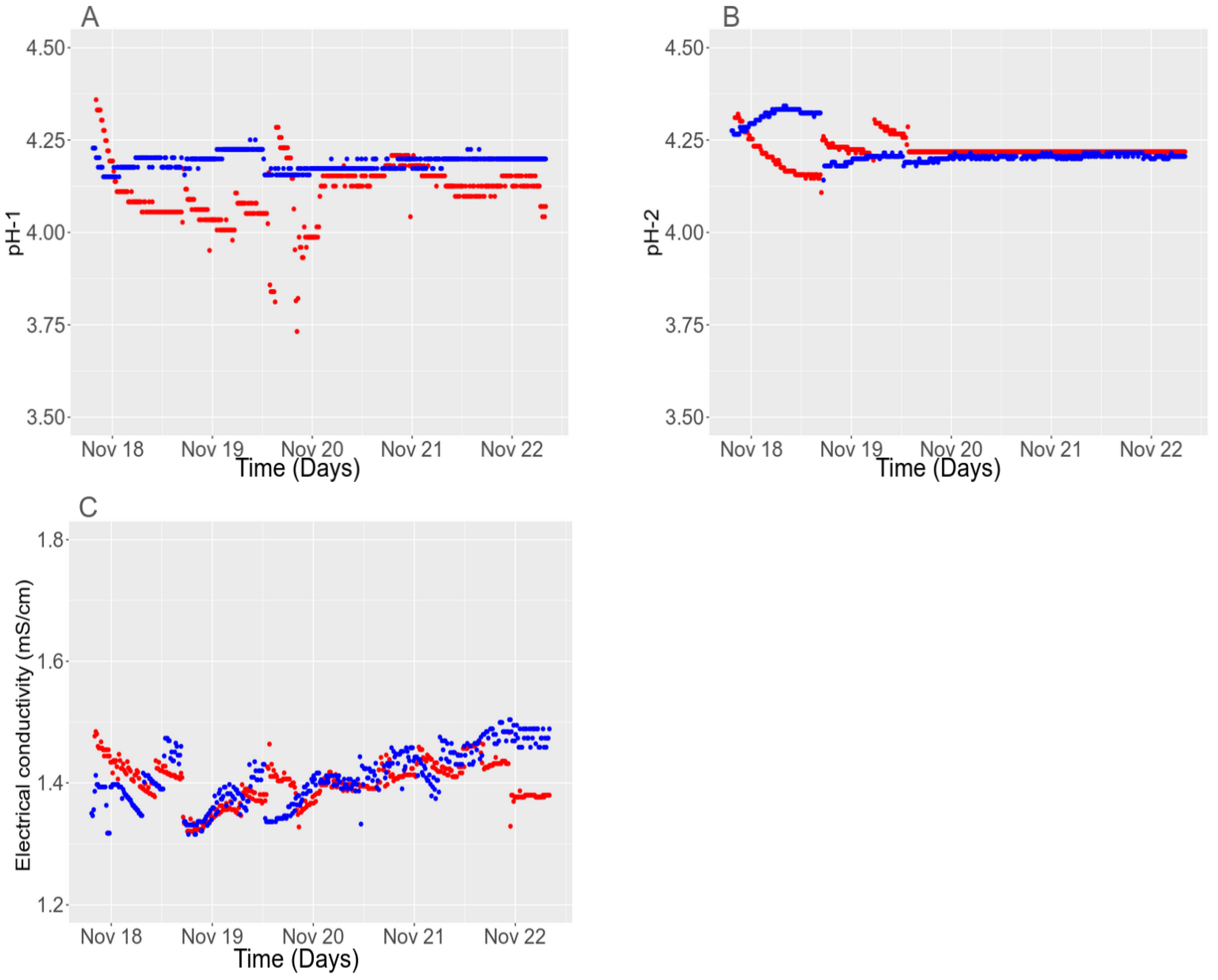
3.4.2. Detection and Removal of Outlier for Long-Term Series
3.4.3. Long-Term Stability
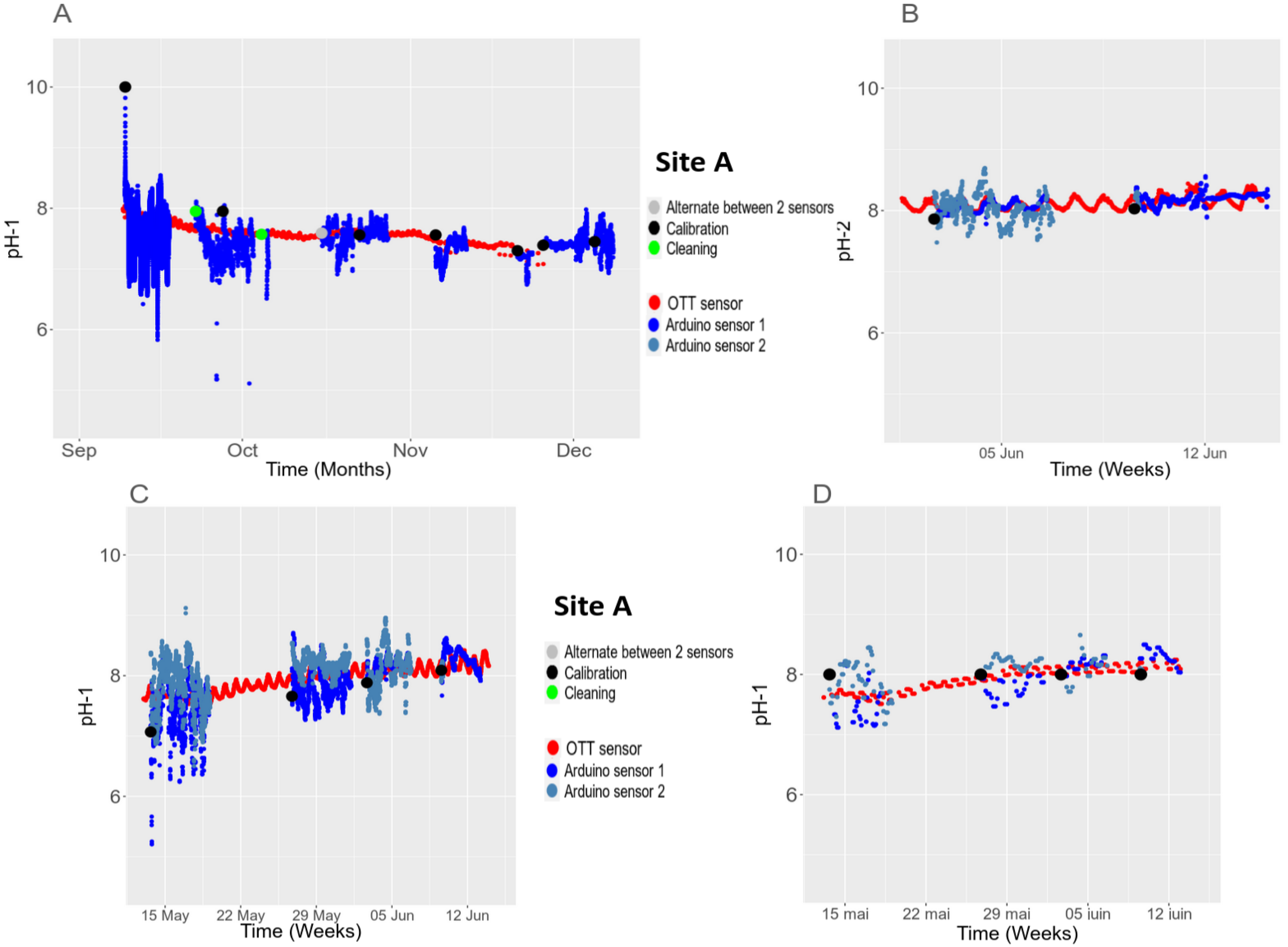
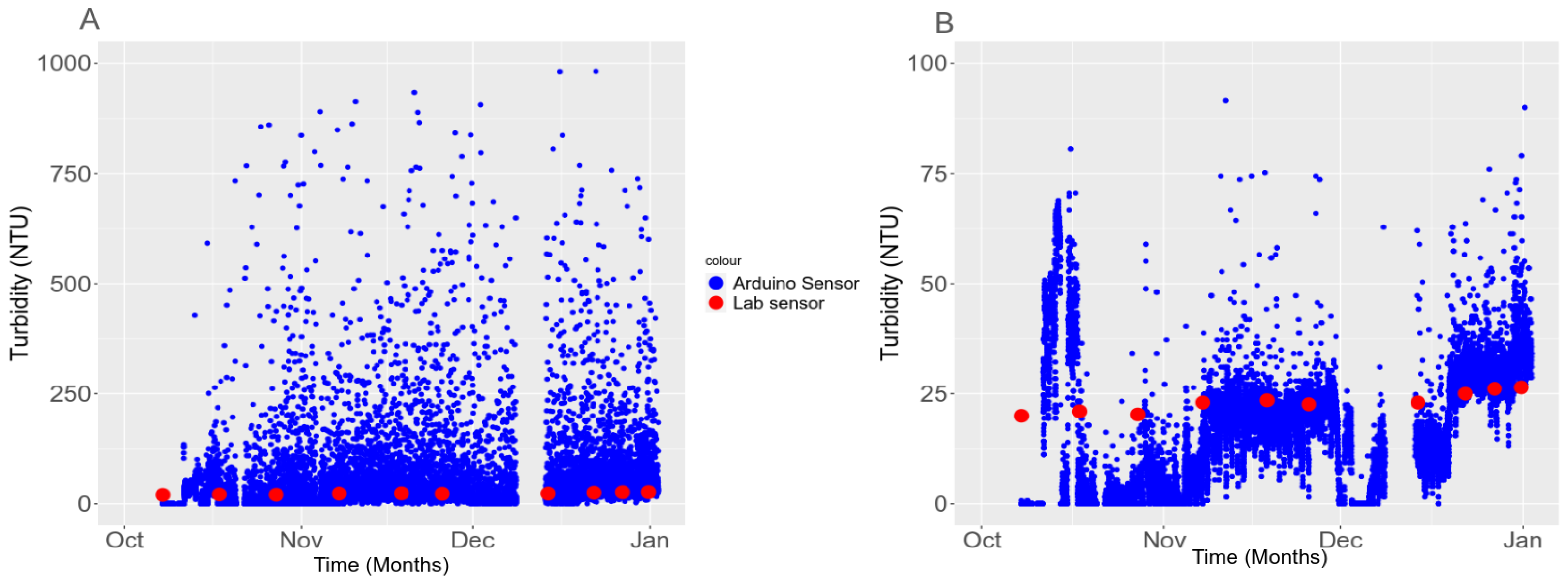
3.5. In Situ Validation
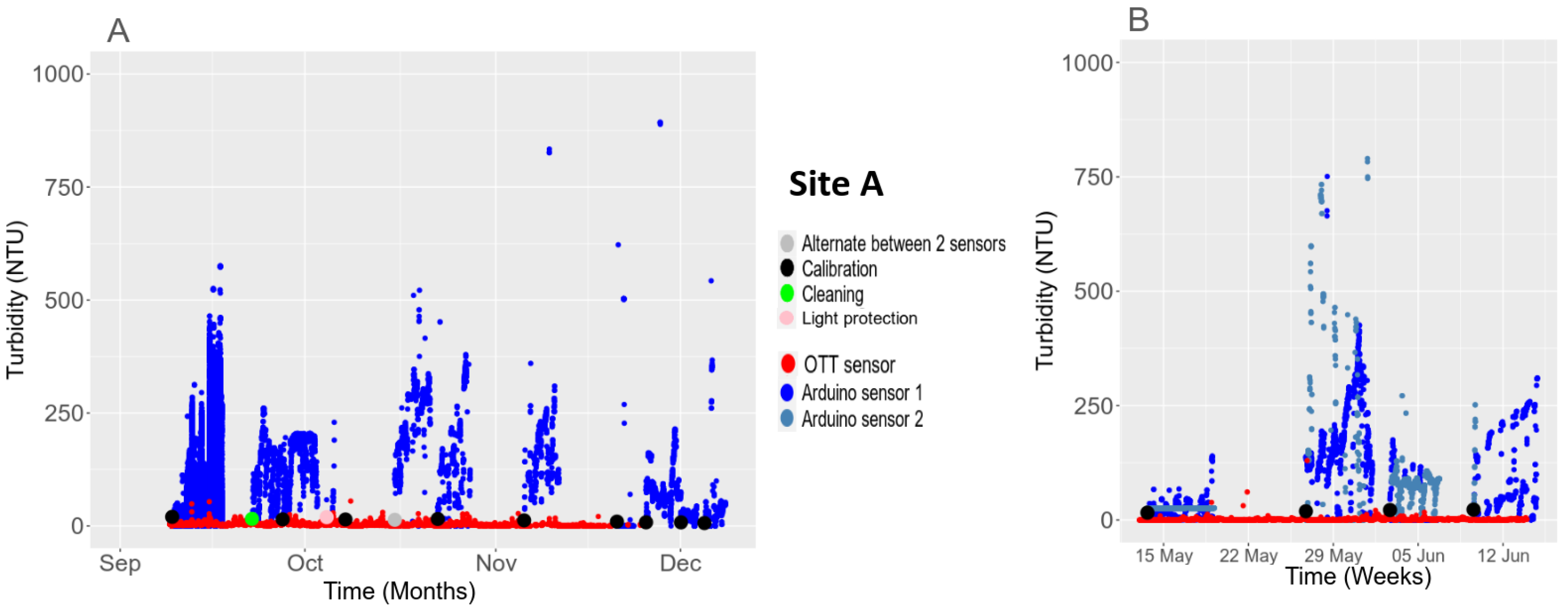
3.5.1. Light Interference with the Turbidity Sensor
3.5.2. Temporal Stability in the Field
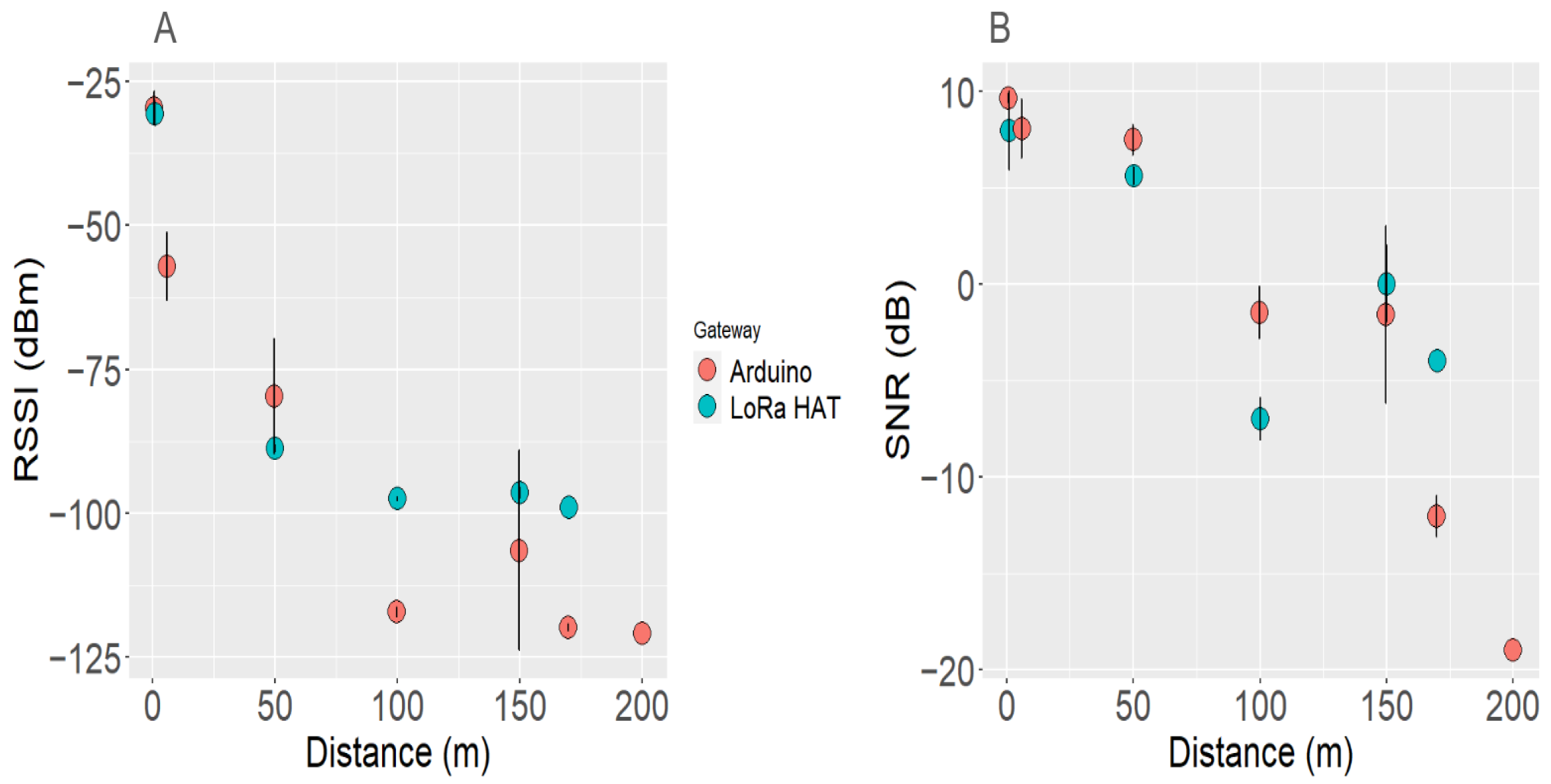
3.6. LoRa Gateway Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A







References
- Bunsen, J.; Berger, M.; Finkbeiner, M. Planetary boundaries for water—A review. Ecol. Indic. 2021, 121, 107022. [Google Scholar] [CrossRef]
- Whelan, M.J.; Linstead, C.; Worrall, F.; Ormerod, S.J.; Durance, I.; Johnson, A.C.; Johnson, D.; Owen, M.; Wiik, E.; Howden, N.J.; et al. Is water quality in British rivers “better than at any time since the end of the Industrial Revolution”? Sci. Total Environ. 2022, 843, 157014. [Google Scholar] [CrossRef]
- Sutadian, A.D.; Muttil, N.; Yilmaz, A.G.; Perera, B. Development of river water quality indices—A review. Environ. Monit. Assess. 2016, 188, 58. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Pereira, E.M.; Cardoso, J.S. Machine learning interpretability: A survey on methods and metrics. Electronics 2019, 8, 832. [Google Scholar] [CrossRef]
- Whelan, M.; Ramos, A.; Villa, R.; Guymer, I.; Jefferson, B.; Rayner, M. A new conceptual model of pesticide transfers from agricultural land to surface waters with a specific focus on metaldehyde. Environ. Sci. Process. Impacts 2020, 22, 956–972. [Google Scholar] [CrossRef]
- WHO. World Health Organization. 2018. Available online: https://www.who.int/docs/default-source/wash-documents/who-recommendations-on-ec-bwd-august-2018.pdf (accessed on 16 July 2021).
- Mouchel, J.M.; Lucas, F.S.; Moulin, L.; Wurtzer, S.; Euzen, A.; Haghe, J.P.; Rocher, V.; Azimi, S.; Servais, P. Bathing activities and microbiological river water quality in the Paris area: A long-term perspective. Seine River Basin Handb. Environ. Chem. 2020, 90, 323–354. [Google Scholar]
- Yaroshenko, I.; Kirsanov, D.; Marjanovic, M.; Lieberzeit, P.A.; Korostynska, O.; Mason, A.; Frau, I.; Legin, A. Real-time water quality monitoring with chemical sensors. Sensors 2020, 20, 3432. [Google Scholar] [CrossRef]
- Farouk, M.I.H.Z.; Latip, M.F.A.; Jamil, Z. Integrated water quality monitoring system and iot technology for surface water monitoring. AIP Conf. Proc. 2022, 2532, 050002. [Google Scholar]
- McGrane, S.J. Impacts of urbanisation on hydrological and water quality dynamics, and urban water management: A review. Hydrol. Sci. J. 2016, 61, 2295–2311. [Google Scholar] [CrossRef]
- Zhu, Q.; Cherqui, F.; Bertrand-Krajewski, J.L. End-user perspective of low-cost sensors for urban stormwater monitoring: A review. Water Sci. Technol. 2023, 87, 2648–2684. [Google Scholar] [CrossRef]
- Kannel, P.R.; Lee, S.; Lee, Y.S.; Kanel, S.R.; Khan, S.P. Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ. Monit. Assess. 2007, 132, 93–110. [Google Scholar] [CrossRef]
- Wang, Z.A.; Moustahfid, H.; Mueller, A.V.; Michel, A.P.M.; Mowlem, M.; Glazer, B.T.; Mooney, T.A.; Michaels, W.; McQuillan, J.S.; Robidart, J.C.; et al. Advancing Observation of Ocean Biogeochemistry, Biology, and Ecosystems with Cost-Effective in situ Sensing Technologies. Front. Mar. Sci. 2019, 6, 519. [Google Scholar] [CrossRef]
- Bogdan, R.; Paliuc, C.; Crisan-Vida, M.; Nimara, S.; Barmayoun, D. Low-Cost Internet-of-Things Water-Quality Monitoring System for Rural Areas. Sensors 2023, 23, 3919. [Google Scholar] [CrossRef]
- Wuijts, S.; Friederichs, L.; Hin, J.A.; Schets, F.M.; Van Rijswick, H.F.; Driessen, P.P. Governance conditions to overcome the challenges of realizing safe urban bathing water sites. Int. J. Water Resour. Dev. 2022, 38, 554–578. [Google Scholar] [CrossRef]
- Wuijts, S.; De Vries, M.; Zijlema, W.; Hin, J.; Elliott, L.R.; Dirven-van Breemen, L.; Scoccimarro, E.; de Roda Husman, A.M.; Külvik, M.; Frydas, I.S.; et al. The health potential of urban water: Future scenarios on local risks and opportunities. Cities 2022, 125, 103639. [Google Scholar] [CrossRef]
- de Camargo, E.T.; Spanhol, F.A.; Slongo, J.S.; da Silva, M.V.R.; Pazinato, J.; de Lima Lobo, A.V.; Coutinho, F.R.; Pfrimer, F.W.D.; Lindino, C.A.; Oyamada, M.S.; et al. Low-Cost Water Quality Sensors for IoT: A Systematic Review. Sensors 2023, 23, 4424. [Google Scholar] [CrossRef]
- Huan, J.; Li, H.; Wu, F.; Cao, W. Design of water quality monitoring system for aquaculture ponds based on NB-IoT. Aquac. Eng. 2020, 90, 102088. [Google Scholar] [CrossRef]
- Hong, W.J.; Shamsuddin, N.; Abas, E.; Apong, R.A.; Masri, Z.; Suhaimi, H.; Gödeke, S.H.; Noh, M.N.A. Water quality monitoring with arduino based sensors. Environments 2021, 8, 6. [Google Scholar] [CrossRef]
- Gowri, D.; Prasad, S.S.; Kiran, O.; Mysaiah, M.M. Water quality level monitoring system using arduino. J. Eng. Sci. 2023, 14, 226–229. [Google Scholar]
- Sekhar, K.C.; Venkatesh, B.; Reddy, K.S.; Giridhar, G.; Nithin, K.; Eshwar, K. IoT-based Realtime Water Quality Management System using Arduino Microcontroller. Turk. J. Comput. Math. Educ. (TURCOMAT) 2023, 14, 783–792. [Google Scholar]
- Cheniti, M.; Abdesslam, B.; Kaouthar, R. An Arduino-Based Water Quality Monitoring System Using pH, Temperature, Turbidity, and TDS Sensors. Available online: https://www.researchgate.net/profile/Cheniti-Mohamed/publication/371608557_An_Arduino-based_Water_Quality_Monitoring_System_using_pH_Temperature_Turbidity_and_TDS_Sensors/links/648c186bc41fb852dd0a0b70/An-Arduino-based-Water-Quality-Monitoring-System-using-pH-Temperature-Turbidity-and-TDS-Sensors.pdf (accessed on 13 December 2023).
- Hacker, J. Assessing the Ability of Arduino-Based Sensor Systems to Monitor Changes in Water Quality. Ph.D. Thesis, Georgia Southern University, Statesboro, GA, USA, 2023. [Google Scholar]
- Trevathan, J.; Schmidtke, S.; Read, W.; Sharp, T.; Sattar, A. An IoT general-purpose sensor board for enabling remote aquatic environmental monitoring. Internet Things 2021, 16, 100429. [Google Scholar] [CrossRef]
- Wong, Y.J.; Nakayama, R.; Shimizu, Y.; Kamiya, A.; Shen, S.; Muhammad Rashid, I.Z.; Nik Sulaiman, N.M. Toward industrial revolution 4.0: Development, validation, and application of 3D-printed IoT-based water quality monitoring system. J. Clean. Prod. 2021, 324, 129230. [Google Scholar] [CrossRef]
- Abotaleb, M. Authenticated WiFi-Based Wireless Data Transmission from Multiple Sensors through a Laboratory Stand Based on Collaboration between ATMEGA2560 and ESP32 Microcontrollers. Sci. J. Gdyn. Marit. Univ. 2023, 27–41. [Google Scholar] [CrossRef]
- Randika, M.; Amarasooriya, A.; Weragoda, S. Development of an arduino-based low-cost turbidity and electric conductivity meter for wastewater characterization. Larhyss J. 2022, 51, 115–127. [Google Scholar]
- Dfrobot. Available online: https://www.dfrobot.com/ (accessed on 1 September 2023).
- I2C ADS1115 16-Bit ADC Module. Available online: https://www.dfrobot.com/product-1730.html (accessed on 25 November 2023).
- Analog Electrical Conductivity Meter V2. Available online: https://wiki.dfrobot.com/Gravity__Analog_Electrical_Conductivity_Sensor___Meter_V2__K=1__SKU_DFR0300 (accessed on 11 November 2023).
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Reston, VA, USA, 1985; Volume 2254.
- DS18B20 Arduino Temperature Sensor. Available online: https://wiki.dfrobot.com/Waterproof_DS18B20_Digital_Temperature_Sensor__SKU_DFR0198_ (accessed on 25 November 2023).
- pH Meter V2. Available online: https://wiki.dfrobot.com/Gravity__Analog_pH_Sensor_Meter_Kit_V2_SKU_SEN0161-V2 (accessed on 24 November 2023).
- pH Meter 2 V2. Available online: https://wiki.dfrobot.com/Analog_pH_Meter_Pro_SKU_SEN0169 (accessed on 24 November 2023).
- Hakim, W.; Hasanah, L.; Mulyanti, B.; Aminudin, A. Characterization of turbidity water sensor SEN0189 on the changes of total suspended solids in the water. J. Phys. Conf. Ser. 2019, 1280, 022064. [Google Scholar] [CrossRef]
- For Arduino. A.T.S. Available online: https://www.dfrobot.com/product-1394.html (accessed on 15 November 2023).
- Analog Disslved Oxygen Sensor. Available online: https://wiki.dfrobot.com/Gravity__Analog_Dissolved_Oxygen_Sensor_SKU_SEN0237 (accessed on 20 November 2023).
- Villeneuve, V.; Légaré, S.; Painchaud, J.; Vincent, W. Dynamique et modélisation de l’oxygène dissous en rivière. Rev. Des. Sci. De L’eau 2006, 19, 259–274. [Google Scholar] [CrossRef][Green Version]
- Trevathan, J.; Read, W.; Schmidtke, S. Towards the Development of an Affordable and Practical Light Attenuation Turbidity Sensor for Remote Near Real-Time Aquatic Monitoring. Sensors 2020, 20, 1993. [Google Scholar] [CrossRef]
- Arduino Pro Gateway. Available online: https://www.cascoantiguopro.com/fr/oceanographie/capteurs-oceanographie/minidot-logger.html (accessed on 3 May 2023).
- Hydrolab DS5X, D. M.W.Q.M. Available online: https://www.ott.com/download/user-manual-hydrolab-ds5x-ds5-and-ms5-water-quality-multiprobes-1/ (accessed on 4 January 2024).
- D.L.S. Available online: https://wiki1.dragino.com/index.php/Lora_Shield (accessed on 4 November 2023).
- R.P.L.H. Available online: https://fr.farnell.com/seeed-studio/113990254/gps-lora-hat-for-raspberry-pi/dp/3498581?gad_source=5&cjevent=6c2f6bd47f0811ee816c1cda0a18ba74&cjdata=MXxZfDB8WXww&CMP=AFC-CJ-FR-1765328&gross_price=true&source=CJ (accessed on 1 November 2023).
- A.P.G.D. Available online: https://docs.arduino.cc/retired/kits/pro-gateway (accessed on 1 November 2023).
- Tsanousa, A.; Xefteris, V.R.; Meditskos, G.; Vrochidis, S.; Kompatsiaris, I. Combining RSSI and Accelerometer Features for Room-Level Localization. Sensors 2021, 21, 2723. [Google Scholar] [CrossRef]
- Audéoud, H.J.; Heusse, M.; Duda, A. Single reception estimation of wireless link quality. In Proceedings of the 2020 IEEE 31st Annual International Symposium on Personal, Indoor and Mobile Radio Communications, London, UK, 31 August–3 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–7. [Google Scholar]
- Guidara, A.; Fersi, G.; Jemaa, M.B.; Derbel, F. A new deep learning-based distance and position estimation model for range-based indoor localization systems. Ad Hoc Netw. 2021, 114, 102445. [Google Scholar] [CrossRef]
- Guillot-Le Goff, A.; Angelotti, N.; Carmigniani, R.; Souza, G.C.; Saad, M.; Dubois, P.; Vinçon-Leite, B. Prévision de la qualité Microbiologique des Milieux Aquatiques: Modélisation Hydrodynamique Pour Anticiper des Épisodes de Contamination Microbiologique sur des Sites de Baignade Urbaine. In Proceedings of the Novatech 2023: 11e Conférence Internationale sur l’Eau dans la Ville; 2023. Available online: https://hal.science/hal-04176992/ (accessed on 5 December 2023).
- Venelinov, T. Comparison of ISO 21748 and ISO 11352 standards for measurement uncertainty estimation in water analysis. Ovidius Univ. Ann. Ser. Civ. Eng. 2016, 18, 187–192. [Google Scholar]
- Moyón Rivera, C.W.; Ordóñez Berrones, D.K. Construcción de un Prototipo de Red de Nodos Inteligentes Para Supervisar la Calidad y Niveles del Agua Potable en los Tanques de Reserva de EP-EMAPAR. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2019. [Google Scholar]
- Saputra, R.E.; Irawan, B.; Nugraha, Y.E. System design and implementation automation system of expert system on hydroponics nutrients control using forward chaining method. In Proceedings of the 2017 IEEE Asia Pacific Conference on Wireless and Mobile (APWiMob), Bandung, Indonesia, 28–29 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 41–46. [Google Scholar]
- Rozaq, I.A.; Setyaningsih, N.Y.D.; Gunawan, B. Pengkondisian Sinyal Sensor Salinitas Dfr0300 Menggunakan Arduino Due. 2020. Available online: https://www.unisbank.ac.id/ojs/index.php/sendi_u/article/view/8022 (accessed on 10 April 2023).
- Hayashi, M. Temperature-electrical conductivity relation of water for environmental monitoring and geophysical data inversion. Environ. Monit. Assess. 2004, 96, 119–128. [Google Scholar] [CrossRef]
- Jeroschewski, P.; Zur Linden, D. A flow system for calibration of dissolved oxygen sensors. Fresenius’ J. Anal. Chem. 1997, 358, 677–682. [Google Scholar] [CrossRef]
- Clesceri, L.S. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; Volume 9. [Google Scholar]
- Keller, G.V.; Frank, C. Electrical Methods in Geophysical Prospecting; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Hitchman, M.L. Measurement of Dissolved Oxygen; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Boards. Available online: https://support.arduino.cc/hc/en-us/articles/360016076980-What-is-the-operating-temperature-range-for-Arduino-boards (accessed on 10 April 2023).
- Alimorong, F.M.L.S.; Apacionado, H.A.D.; Villaverde, J.F. Arduino-based multiple aquatic parameter sensor device for evaluating pH, turbidity, conductivity and temperature. In Proceedings of the 2020 IEEE 12th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 3–7 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–5. [Google Scholar]
- Saha, S.; Rajib, R.H.; Kabir, S. IoT based automated fish farm aquaculture monitoring system. In Proceedings of the 2018 International Conference on Innovations in Science, Engineering and Technology (ICISET), Chittagong, Bangladesh, 27–28 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 201–206. [Google Scholar]
- Méndez-Barroso, L.; Rivas-Márquez, J.; Sosa-Tinoco, I.; Robles-Morúa, A. Design and implementation of a low-cost multiparameter probe to evaluate the temporal variations of water quality conditions on an estuarine lagoon system. Environ. Monit. Assess. 2020, 192, 710. [Google Scholar] [CrossRef]
- Xu, S.; Lu, B.; Baldea, M.; Edgar, T.F.; Wojsznis, W.; Blevins, T.; Nixon, M. Data cleaning in the process industries. Rev. Chem. Eng. 2015, 31, 453–490. [Google Scholar] [CrossRef]
- Le Deunf, J.; Debese, N.; Schmitt, T.; Billot, R. A review of data cleaning approaches in a hydrographic framework with a focus on bathymetric multibeam echosounder datasets. Geosciences 2020, 10, 254. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C. Time series data cleaning: A survey. IEEE Access 2019, 8, 1866–1881. [Google Scholar] [CrossRef]
- Sedaghat, L.; Hersey, J.; McGuire, M.P. Detecting spatio-temporal outliers in crowdsourced bathymetry data. In Proceedings of the Second ACM SIGSPATIAL International Workshop on Crowdsourced and Volunteered Geographic Information, Orlando, FL, USA, 5 November 2013; pp. 55–62. [Google Scholar]
- Yang, J.; Rahardja, S.; Fränti, P. Mean-shift outlier detection and filtering. Pattern Recognit. 2021, 115, 107874. [Google Scholar] [CrossRef]
- Bianco, A.M.; Garcia Ben, M.; Martinez, E.; Yohai, V.J. Outlier detection in regression models with arima errors using robust estimates. J. Forecast. 2001, 20, 565–579. [Google Scholar] [CrossRef]
- Liu, H.; Shah, S.; Jiang, W. On-line outlier detection and data cleaning. Comput. Chem. Eng. 2004, 28, 1635–1647. [Google Scholar] [CrossRef]
- Shi, M.; Ma, J.; Zhang, K. The Impact of Water Temperature on In-Line Turbidity Detection. Water 2022, 14, 3720. [Google Scholar] [CrossRef]
- Sensor. Available online: https://www.hemmis.com/docs/hydrolab/MS5_DS5_DS5X_low_F.pdf (accessed on 5 January 2024).
- Demetillo, A.T.; Japitana, M.V.; Taboada, E.B. A system for monitoring water quality in a large aquatic area using wireless sensor network technology. Sustain. Environ. Res. 2019, 29, 12. [Google Scholar] [CrossRef]
- Analog Electrical Conductivity Meter Pro. Available online: https://wiki.dfrobot.com/SKU_SEN0451_Gravity_Analog_Electrical_Conductivity_Sensor_PRO_K_1 (accessed on 24 May 2024).
- Gusri, A.J.; Harmadi, H. Rancang Bangun Alat Penguras Air pada Wadah Penampungan Berbasis Turbidity Sensor SEN0189. J. Fis. Unand 2021, 10, 330–336. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, S.; Han, D.; Fu, G.; Solomatine, D.; Zheng, Y. A comprehensive review on the design and optimization of surface water quality monitoring networks. Environ. Model. Softw. Environ. Data News 2020, 132, 104792. [Google Scholar] [CrossRef]
- Sendra, S.; Parra, L.; Jimenez, J.M.; Garcia, L.; Lloret, J. LoRa-based network for water quality monitoring in coastal areas. Mob. Netw. Appl. 2023, 28, 65–81. [Google Scholar] [CrossRef]
- Zourmand, A.; Hing, A.L.K.; Hung, C.W.; AbdulRehman, M. Internet of things (IoT) using LoRa technology. In Proceedings of the 2019 IEEE International Conference on Automatic Control and Intelligent Systems (I2CACIS), Selangor, Malaysia, 29 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 324–330. [Google Scholar]
- Petrariu, A.I.; Lavric, A.; Coca, E. Lorawan gateway: Design, implementation and testing in real environment. In Proceedings of the 2019 IEEE 25th International Symposium for Design and Technology in Electronic Packaging (SIITME), Cluj-Napoca, Romania, 23–26 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 49–53. [Google Scholar]
- Casado-Vara, R.; Prieto-Castrillo, F.; Corchado, J.M. A game theory approach for cooperative control to improve data quality and false data detection in WSN. Int. J. Robust Nonlinear Control 2018, 28, 5087–5102. [Google Scholar] [CrossRef]





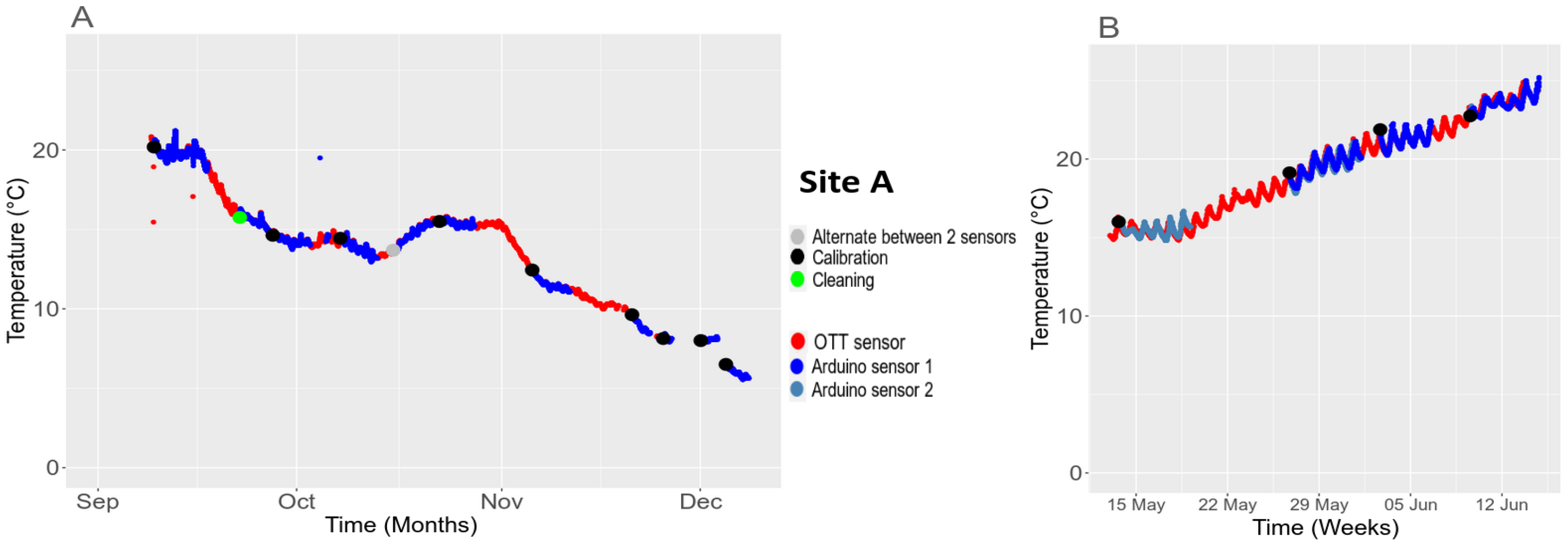
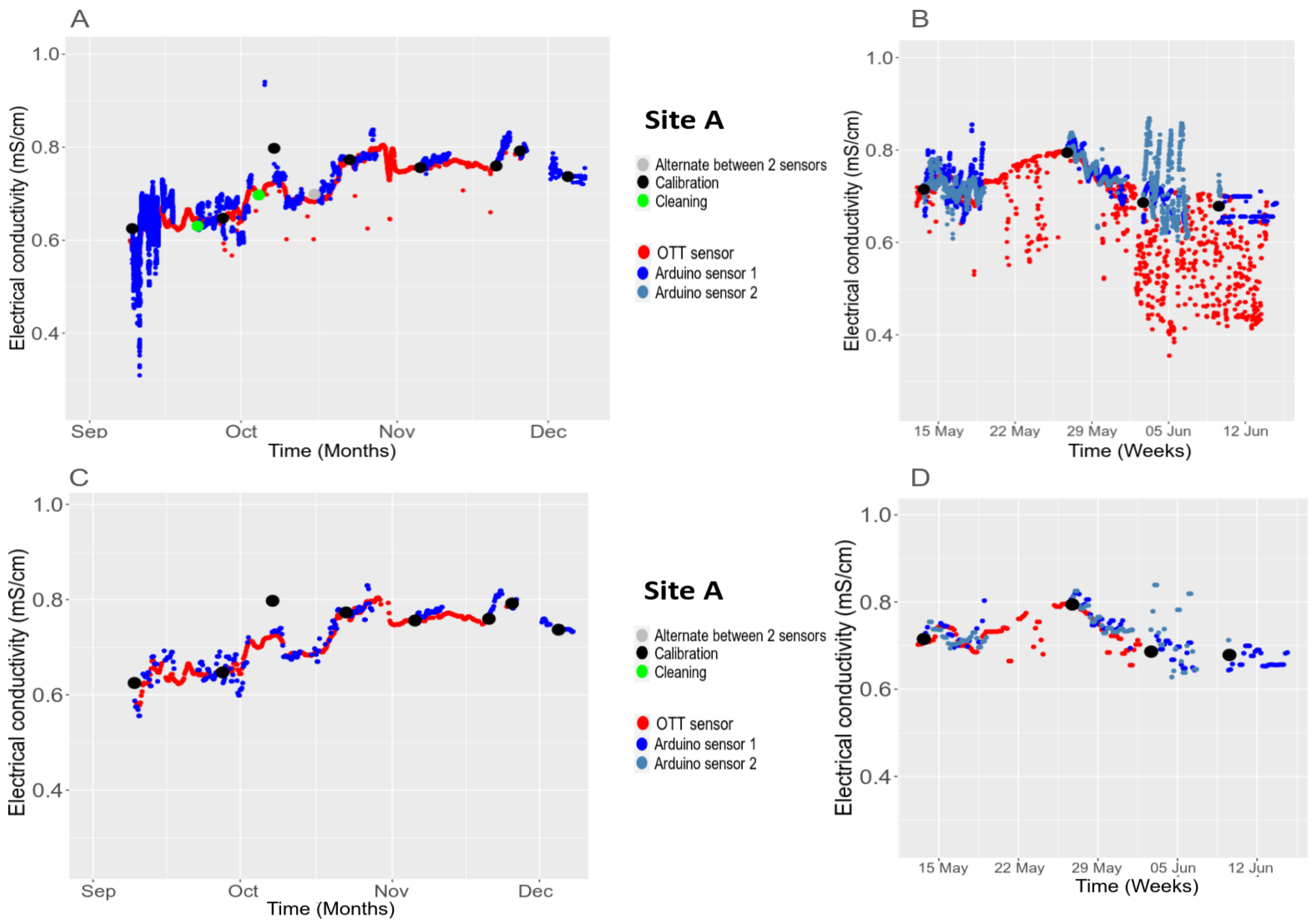
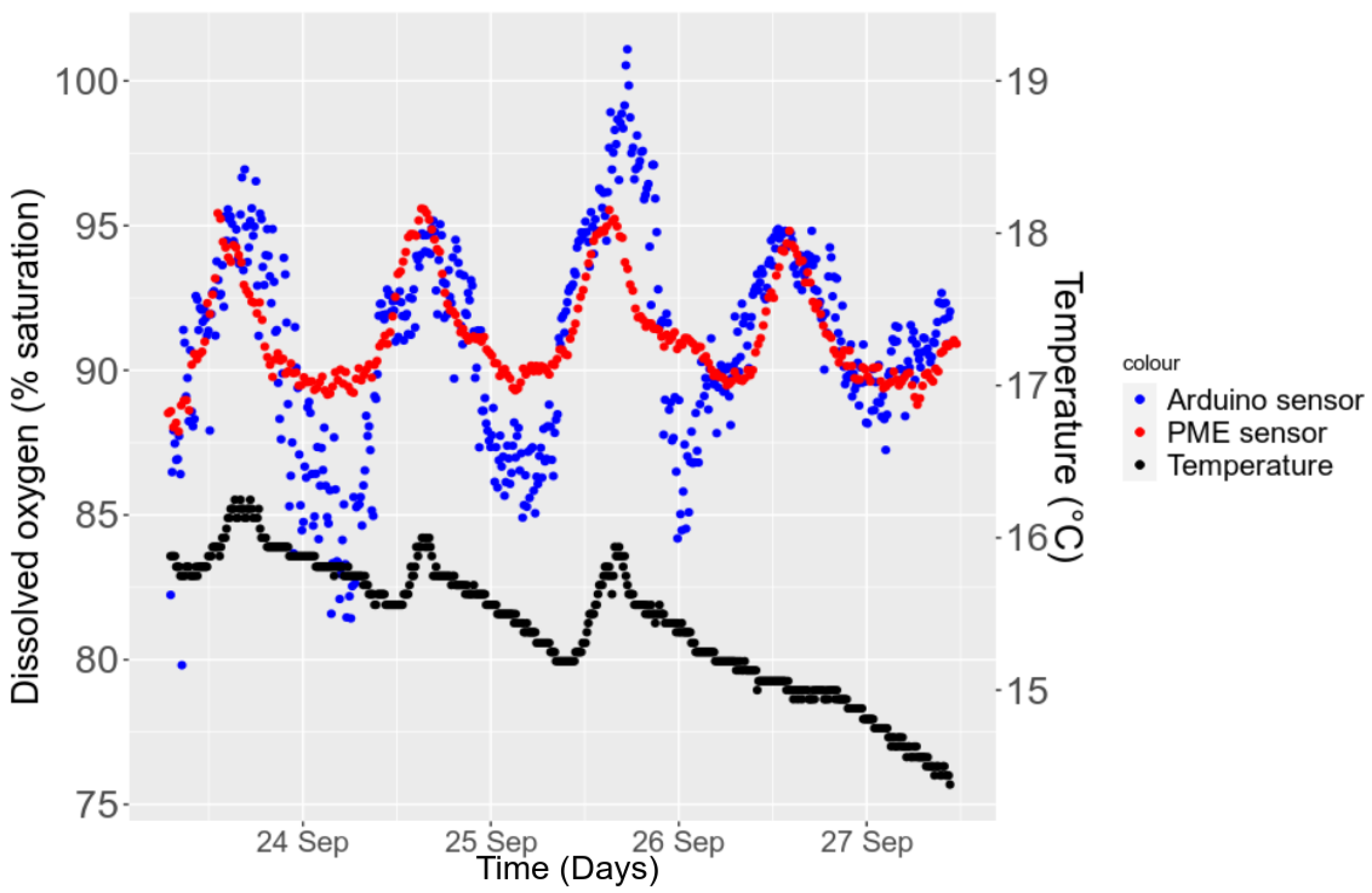
| Parameters | Temperature [9,32] (°C) | pH-1 [33] | pH-2 [34] | Conductivity [30] (mS·cm−1) | Turbidity [35,36] (NTU) | Dissolved Oxygen [37,38] (%) |
|---|---|---|---|---|---|---|
| Sensor, DFRobot, Shanghai, China | DS18B20 | SEN0161-V2 | SEN0169-V2 | DFR0300 | SEN0189 | SEN0237-A |
| Detection range | −10 to 85 | 0 to 14 | 0 to 20 | 0 to 1000 | 0 to 100 | |
| Resolution | 0.010 | 0.010 | 0.001 | 1.000 | 0.050 | |
| Measurement Accuracy | ±0.5 | ±0.5 | ±0.1 | ±1.0 | ±3.6 | ±2.0 |
| Price (EUR) | 8 | 39 | 65 | 70 | 9 | 148 |
| Parameters | Temperature (°C) | pH | Conductivity (mS·cm−1) | Turbidity (NTU) |
|---|---|---|---|---|
| Detection range | −5 to 50 | 0 to 14 | 0 to 100 | 0 to 3000 |
| Resolution | 0.01 | 0.01 | 0.0001 | 0.10 |
| Measurement Accuracy | ±0.100 | ±0.200 | ±0.001 | ±1.000 |
| Price (EUR) | 480 | 380 | 1540 |
| Parameters | Temperature (°C) | pH-1 | pH-2 | Conductivity (mS · cm−1) | Turbidity (NTU) | Dissolved Oxygen (%) |
|---|---|---|---|---|---|---|
| Number of measures (n) | 368 | 30 | 30 | 44 | 77 | 20 |
| Standard solutions | Temperature from 5 to 30 | 4, 7 and 10 | 4 standards from 0.22 to 1.42 | 7 standards from 0 to 800 | 0 and 100 | |
| Linearity (units 1–2) | 0.999 | 0.999 | 0.999 | 0.998–0.993 | 0.998 | 0.999 |
| Slope of the curve | ||||||
| Unit 1 | 0.999 | 0.938 | 0.959 | 1.060 | 0.947 | 1.038 |
| Unit 2 | 0.999 | 0.950 | 0.984 | 1.083 | 0.916 | |
| Repeatability | ||||||
| Unit 1 | 0.01 | 0.02 | 0.01 | 0.02 | 3.66 | 1.74 |
| Unit 2 | 0.01 | 0.02 | 0.01 | 0.02 | 3.69 | |
| Reproducibility | 0.03 | 0.02 | 0.01 | 0.02 | 3.54 | |
| Parameters | Temperature | pH-1 | pH-2 | Conductivity | Turbidity | Dissolved Oxygen (%) | |
|---|---|---|---|---|---|---|---|
| (°C) | () | (NTU) | Arduino Sensor | PME Sensor | |||
| Average | 0.78 | 0.04 | 0.03 | 0.04 | 4.68 | 2.33 | 0.31 |
| Min–Max | 0.38–1.01 | 0.02–0.08 | 0.02–0.06 | 0.02–0.08 | 3.89–5.46 | 1.55–3.71 | 0.16–0.59 |
| Parameters | Temperature | pH-1 * | pH-2 | Conductivity | Turbidity | Turbidity ** | Dissolved Oxygen (%) | |
|---|---|---|---|---|---|---|---|---|
| (°C) | () | (NTU) | (NTU) | Arduino Sensor | PME Sensor | |||
| Standard deviation | 1.91 | 0.04 | 0.04 | 0.03 | 64.85 | 13.23 | 12.42 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naloufi, M.; Abreu, T.; Souihi, S.; Therial, C.; Rodrigues, N.A.d.P.; Le Goff, A.G.; Saad, M.; Vinçon-Leite, B.; Dubois, P.; Delarbre, M.; et al. Long-Term Stability of Low-Cost IoT System for Monitoring Water Quality in Urban Rivers. Water 2024, 16, 1708. https://doi.org/10.3390/w16121708
Naloufi M, Abreu T, Souihi S, Therial C, Rodrigues NAdP, Le Goff AG, Saad M, Vinçon-Leite B, Dubois P, Delarbre M, et al. Long-Term Stability of Low-Cost IoT System for Monitoring Water Quality in Urban Rivers. Water. 2024; 16(12):1708. https://doi.org/10.3390/w16121708
Chicago/Turabian StyleNaloufi, Manel, Thiago Abreu, Sami Souihi, Claire Therial, Natália Angelotti de Ponte Rodrigues, Arthur Guillot Le Goff, Mohamed Saad, Brigitte Vinçon-Leite, Philippe Dubois, Marion Delarbre, and et al. 2024. "Long-Term Stability of Low-Cost IoT System for Monitoring Water Quality in Urban Rivers" Water 16, no. 12: 1708. https://doi.org/10.3390/w16121708
APA StyleNaloufi, M., Abreu, T., Souihi, S., Therial, C., Rodrigues, N. A. d. P., Le Goff, A. G., Saad, M., Vinçon-Leite, B., Dubois, P., Delarbre, M., Kennouche, P., & Lucas, F. S. (2024). Long-Term Stability of Low-Cost IoT System for Monitoring Water Quality in Urban Rivers. Water, 16(12), 1708. https://doi.org/10.3390/w16121708








