Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review
Abstract
1. Introduction
2. Nanoparticles in Environmental Applications
3. Nanoparticle Synthesis Strategies
3.1. Sol–Gel
3.2. Hydrothemal
3.3. Chemical Vapor Condensation of a Metal
3.4. Chemical Reduction Methods
3.5. Co-Precipitation
3.6. Electrochemical Methods
3.7. Precision Milling
| Nanoparticles | Support | Synthesis Techniques | Environmental Applications | Reference |
|---|---|---|---|---|
| Removal of Dyes | ||||
| ZnO | - | Sol–gel co-precipitation in KOH | Methylene blue | [55] |
| Zr-doped Fe2O3 | CoOx | Hydrothermal | Orange II | [59] |
| Ca peroxide | Starch | Precipitation | Methylene blue | [63] |
| TiO2 | g-C3N4(g-CN) nanolayers | Co-precipitation; thermal polymerization | Methylene blue | [64] |
| Au | Au/VO2/CeO2 | Photodeposition | Methylene blue | [69] |
| Magnetite (MN) | Fe3O4@C | Microwave-induced | Methylene blue | [70] |
| Ag | Na0.5Bi0.5TiO3 nanospheres | Chemical solution, hydrothermal | Rhodamine B | [71] |
| HRC-Ag/Agar | Agarose hydrogel | Reduction with KBH4 | Methylene blue, rhodamine B | [72] |
| Cationic polyethyleneimine (PBVPR218) | - | - | Dyes | [73] |
| EC-Ag | Elettaria cardamomum | Reduction with NaBH4 | Methylene blue, rhodamine B | [74] |
| nZVI | Janus particles | Reduction with NaBH4 | Methyl orange | [75] |
| CdAl2O4 | Fe | Co-precipitation | Brilliant blue, brilliant green | [76] |
| CuNiFe2O4 | g-C3N4 | Gel auto combustion | Methylene blue | [77] |
| Black phosphorus (BPQDs)-IO TiO2 | - | Sonication assisted; liquid exfoliation | Rhodamine B, methylene orange | [78] |
| g-C3N4-Fe3O4@KF | Kapok fiber | One step | Rhodamine B | [79] |
| CeO2 | - | Co-precipitation, calcination | Methylene blue | [80] |
| PLA/CMC/GO _f-COOH@Ag | Polymeric matrix | Reduction with ascorbic acid | Methylene blue | [81] |
| (B-GNP) | Graphene | Polyvinyl alcohol film | Atrazine | [82] |
| Magnetite nanoparticles (MNs) | β-cyclodextrin (β-CD) and quaternary ammonium salts | Co-precipitation | Methylene blue, Orange G | [83] |
| C3N5-LDH-Ag | C3N5-LDH | Precipitation in NaOH | Tartrazine | [84] |
| Removal of Organic Compounds | ||||
| Ferrihydrite (Fh) | Non-ionic surfactant Brij L4 | Co-precipitation | Cooking oils | [44] |
| TiO2 | - | Sol–gel | Phenol | [56] |
| Zr-doped Fe2O3 | CoOx | Hydrothermal | Bisphenol A | [59] |
| AuNPs | Au/VO2/CeO2 | Photodeposition | Aromatic alcohols, p-nitrophenol | [69] |
| nZVI | Janus particles | Reduction with NaBH4 | Trichloroethylene | [75] |
| Fe3O4/FeS | Biochar | Pollen pyrolysis | phenol | [85] |
| AA@Fe° | Amino acids | Reduction with KBH4 | Tributyl phosphate, n-dodecane | [86] |
| Biochar | - | Pyrolysis | Bisphenol A | [87] |
| Fe3O4 | rGO | Co-precipitation | 4-Aminophenol | [88] |
| Aptamer-MrGO@Au and ssDNA-AuNP@MBs | BPA aptamer | Reduction with trisodium citrate | Bisphenol A | [89] |
| ZnO@SCF SppCobalt Ferrite | Spinel cobalt ferrite | Precipitation | Phenantrene | [90] |
| Fe⁰@C/surfactant | Carbon | Hydrothermal; carbothermic; reduction with NaBH4 | Nitrobenzene | [91] |
| Zn/FeO | Surfactant foams | Reduction with NaBH4 | Diesel | [92] |
| Removal of Pharmaceutical Compounds | ||||
| CuO and nZVI | - | Sol–gel; precipitation in NaOH; reduction with NaBH4 | Levofloxacin | [57] |
| CuO | - | Co-precipitation; calcination | Metronidazol | [93] |
| Ag-Cu-Li | Multimetal nanorods | Precipitation in NaOH | Antibacterial activity | [94] |
| WS2 | - | Hydrothermal thiourea | Tetracycline | [95] |
| ZnO | Alginate nanofibers | Hydrothermal | Tetracycline | [96] |
| BiFeO3 | - | Combustion | Ofloxacin | [97] |
| TGC/NiCr2O4 | Tubular g-C3N4 (TGC) | Calcination | Tetracycline | [98] |
| ZnO | Carbon cloth (CC) | Hydrothermal | Hydroxychloroquine | [99] |
| Ag/Ag3PO4-VAg | Ag nanocluster with vacancies of Ag (Ag/Ag3PO4-Vag) | Reduction with NaBH4 | Sulfamethoxazole | [100] |
| Au | - | Reduction with NaBH4/320 °C | Penicillin G, sulfamethazine, tetracycline, enrofloxacin, skewered fish | [101] |
| Bi-Ag nanoalloys | - | Solvothermal | Bacterial infections | [102] |
| TiO2NW-CuO- cellulose | Cellulose | Solvothermal | Escherichia coli | [103] |
| Removal of Pesticides | ||||
| Ag (SNP) | - | Reduction with NaBH4 | Miticidal activity | [104] |
| Au | Ovalbumin | Reduction with trisodium citrate | Carbaryl | [105] |
| Au@ZIF-67 | ZIF-67 | Hydrothermal | Thiram, Carbendazim | [106] |
| Chitosan (CS) | - | Ionic gelation | Plant diseases | [107] |
| Cu (ChNC@Cu) | Chitin nanocrystals (ChNC) | TEMPO oxidation | Pesticides | [108] |
| SiO2 | Biopolymers | Sol–gel | Plastic films | [109] |
| ZnO and TiO2 | - | One-pot | Polycyclic aromatic hydrocarbons | [110] |
| Pd-nZVI and S-nZVI | - | Reduction with NaBH4 | Trichloroethylene | [111] |
| Toxicity abatement | ||||
| PbO2 | Humic acid | Chlorination | Toxicity in medaka fish | [112] |
| Metal, metal oxide, carbon, plastic | - | Phytotoxicity | [113] | |
| nFe@Fe3O4, nFe3O4 nFe2O3 | - | Toxic effects on zebra fish | [114] | |
| ZnO and Ag | - | Sol–gel+ reduction with NaBH4 | Acute toxicity in zebra fish | [115] |
| Chemical processes | ||||
| Pt@S-1 | β zeolite | - | Synthesis of naphtha | [116] |
| ZnO | Molasses and urea | Precipitation | Synthesis of fertilizer | [117] |
| OMGR Oximagnesite/green rust | - | Hydrothermal | Phosphate recovery | [118] |
| CrMnFeCoNi-HEO; MgCoNiCuZn-HEO | Spinel-structure sHEO | Sol–gel | Desalination techniques of seawater | [119] |
| RuNi-rGO@ MNCs | N-doped C nanosheets | Reduction with H2 | Hydrogen evolution reaction (HER) | [120] |
| Others | ||||
| TiO2 | Recycled rubber tiles | Sol–gel | Airborne pollution | [121] |
3.8. Commercial Nanoparticles
| Nanoparticles | Support | Environmental Applications | Reference |
|---|---|---|---|
| SiO2@CA MSs | Microspheres | Removal of dyes | [122] |
| UHMWPE/TiO2 | Polyethylene | Removal of methyl orange, methylene blue, Congo red, and tetracycline | [123] |
| nZVI, Fe2O3, Fe3O4 | - | Florfenicol containing cow manure | [124] |
| ZnO | - | Escherichia coli | [125] |
| nZVI | - | Tetracyclines | [126] |
| ZnO and TiO2 | - | Tetranychus urticae and Neoseiulus californicus | [127] |
| NiO | - | Neurotoxicity in zebra fish | [128] |
| ZnO | Film of chitosan | Loaf bread shelf life | [129] |
| nZVI | - | Polycyclic aromatic hydrocarbons | [130] |
| ZnO | - | Gut microbiome alterations in rats | [131] |
| Ag | - | Improved quality of Capsicum annum crops | [132] |
| SiO2, Al2O3, and O-CNT | - | Clofibric acid, acetaminophen, sulfamerazine | [133] |
| nZVI | - | Hydrophobic organic compounds, sediments | [134] |
| ZnO | - | Biofortification of Dracocephalum moldavica | [135] |
| ZnO | Pannonibacter- phragmitetus | Inhibition of microbial Cr(VI) reduction | [136] |
| Se | - | As (III), soybean roots | [137] |
3.9. Emerging Green Alternatives
| Nanoparticles | Support | Reactive Agent | Environmental Applications | Reference |
|---|---|---|---|---|
| Removal of Dyes | ||||
| Ag/Ti | - | Aloe vera L.e. | Rhodamine B | [46] |
| MoO3 and WO3 nanorods | - | Leidenfrost (H2O) with NaOH | Methylene blue | [138] |
| Ag | - | Antidesma acidum L.e. | Congo red, methylene blue | [139] |
| B-doped g-C3N4/TiO2 | g-C3N4 | Spinacia oleracea | Methylene blue | [140] |
| Au | - | Wedelia urticifolia | Rhodamine B | [141] |
| ZnS/Fe3O4 | Carboxymethyl cellulose | Co-precipitation of Fe(II) and Fe(III) | Methylene blue, methyl orange Congo red, and rhodamine B | [142] |
| ZnO | - | Citrus x lemon | Reactive green 19 | [143] |
| IRCFA-PDA@Ag | Iron-rich coal fly ash (IRFA)-Polydopamine (PDA) | Floral | Methylene blue | [144] |
| ZnO, CuO, MnO2 and MgO | - | Leucaena leucocephala | Golden yellow-145, Direct red-31 | [145] |
| nZVI | - | Cow and goat milk | Methyl orange | [146] |
| Au | - | Cell-free filtrate of Penicillium rubens | Methylene blue, phenol red, bromothymol blue, methyl orange | [147] |
| ZnO | Mesoporous carbon | Firecracker waste | Methyl orange | [148] |
| ZnO | - | Aloe vera | Malachite green, Basic violet 3 | [149] |
| ZnO and SiO2 | - | Cyperus alternifolius | Methylene blue | [150] |
| Organic Compounds | ||||
| Pt-SnO2 | rGO-CH | Amaranthus spinosus | Methanol | [151] |
| nZVI | - | Tung, aspen, and holly L.e. | Tetrabromobisphenol A | [152] |
| CeO2 | - | Dillenia indica | 2,2-difphenyl-1- -picrylhydrazyl | [153] |
| GM-Ag | - | Gnetum montanum | 3-nitrophenols and 4-nitrophenols | [154] |
| MgO | - | Jatropha oil | Compounds in air, HC, CO, CO2 | [155] |
| Fe-C, Co-C, and Ni-C | - | Tea residue | Cystine | [156] |
| Removal of Microorganisms | ||||
| ZnO | Brassica oleracea | Gram-negative Bc Escherichia coli | [157] | |
| Ag | Pseudomonas canadensis bacterial isolate | Pseudomonas tolaasii Pt18 | [158] | |
| CuO | Alpinia officinarum | Colletotrichum gloeosporioides | [159] | |
| nZVI | Common mantle | Pathogenic fungi, wheat plants | [160] | |
| Removal of Pharmaceutical Compounds | ||||
| ZnO | - | Aloe vera | Amoxicillin | [149] |
| Ag | - | Passiflora foetida | Antibacterial | [161] |
| Ag | - | Calotropis procera | Fungal and bacterial pathogens | [162] |
| Ag, Cu, and Fe | Catharanthus roseus L.e. | Several anti-inflammatory drugs | [163] | |
| Lignin | Nanocellulose cryogels | Kraft lignin | Diclofenac, metropolol, tramadol, carbamazepine | [164] |
| Removal of Inorganic Compounds | ||||
| MgO | - | Jatropha oil | CO, CO2 in air | [155] |
| Fe-C, Co-C, and Ni-C | - | Tea residue | CrO42¯ | [156] |
| Chitosan | - | Salix subserrata bark extract | As in rats | [165] |
| Magnetite | - | Amla (tree bark) | U(VI) | [166] |
| ZnO | - | Acacia catechu L.e. | As | [167] |
4. Zero-Valent Iron Nanoparticles: Conventional and Eco-Friendly Synthesis
4.1. Zero-Valent Iron Nanoparticles
4.2. Influence of Synthetic Methods for the Preparation of nZVIs
4.3. Stabilization of nZVIs
- Incorporating a second metal into nZVIs.
- Coating the surface of nZVIs with organic substances.
- Synthesizing emulsified nZVIs.
- Fixed support.
- Encapsulation.
- Electrostatic stabilization.
- Steric stabilization.

4.3.1. Incorporating a Second Metal into nZVIs
4.3.2. Coating of nZVI Surfaces
4.3.3. Emulsified nZVIs
4.3.4. Fixed Support
4.3.5. Encapsulation of nZVIs
4.3.6. Electrostatic Stabilization
4.3.7. Steric Stabilization
| FeNPs | Precursor | Support | Size/nm | Morphology | Characterization | Reference |
|---|---|---|---|---|---|---|
| MCMZVI | FeCl3·6H2O+ glucose+CO(NH2)2 | Mesoporous carbon | 20–100 | Uneven size | XRD, SEM, XPS, FTIR, BET | [194] |
| CS-nZVI (core–shell) | FeCl3·6H2O+NaBH4 | - | 15.42–97.57 | Spherical | UV–vis, FTIR, TEM, SEM EDX, XRF | [198] |
| nZVI | FeCl3·6H2O+NaBH4 | - | 34–110 | Spherical | UV, XRD, SEM, EDX, TEM, DSL | [208] |
| nZVI@FSG | FeSO4·7H2O+NaBH4 | Flaxseed gum | 73–87 | Spherical | DLS, FESEM, EDX, FTIR, DR5000 | [216] |
| CMC-S-nZVI | FeSO4·7H2O+NaBH4 | Carboxymethyl cellulose (CMC) | 90 | Spherical | TEM-EDS, UV–vis, PSD, ζ-potential | [218] |
| nZVI/SS/BC | FeSO4·7H2O+NaBH4 | Biochar with stable starch | 45.7–37 | - | SEM, EDS, BET, FTIR, XRD | [220] |
| nZVI | FeSO4·7H2O+KBH4 | Guar gum | 87.4 | Cubic | TEM, XRD, SEM, XPS | [221] |
| nZVI | FeSO4·7H2O+KBH4 | Attapulgite | - | - | FTIR, SEM-EDS, XPS | [225] |
| Fe@SiO2 | FeSO4·7H2O+KBH4 | Silica | 40–50 | Nanospheres | XRS, FTIR, TEM, EDS | [227] |
| nZVI/AC | FeSO4·7H2O+NaBH4 | Activated carbon | 40–100 | Rough | SEM, XRD, BET XPS | [228] |
| CS@nZVI | FeSO4·7H2O+NaBH4 | Chitosan | 13.12 | - | XRD, FESEM, EDS, FTIR | [232] |
| CaCO3-nZVI | FeSO4·7H2O+NaBH4 | CaCO3 | 75–89 | Spherical | SEM-EDX, XRD, TEM, FTIR, XPS, BET | [233] |
| nZVI-A and nZVI-S | FeCl3·6H2O+NaBH4 | Rhamnolipids | 60 and 42 | - | SEM, XRD, FTIR, TG, DLS, ζ-potential | [236] |
| FG-nZVI | FeSO4·7H2O+NaBH4 | Flaxseed gum extract | <100 | Spherical | DLS, FESEM, RDX, FTIR | [237] |
| nZVI | FeCl3·6H2O+NaBH4 | - | 20–60 | Uniform morphology | XRD, SEM, TEM | [238] |
| nZVI | FeSO4·7H2O+NaBH4 | - | 14.3 | - | XRD, SEM, TEM, FTIR | [239] |
| nZVI/GO | Fe (NO3)3·9H2O+NaBH4 | Graphene oxide | 10–20 | Dispersed | SEM, TEM-EDS, FTIR | [240] |
| nZVI-kaol/PES | FeCl3·6H2O+NaBH4 | Kaolin and poly-ethersulfone (PES) | 42 | Ridge and valley | XRD, FESEM, FTIR | [241] |
| NC-nZVI | FeCl3·6H2O+NaBH4 | Nanocelluloses (NC) | 116–200 | Spherical | SEM, TEM-EDX, FTIR, XRD, XPS | [242] |
| Fe@BC | Black liquor lignin and Fenton sludge in one step | Biochar | 20–50 | Notable aggregation | XRD, FTIR, BET, BJH, FESEM, TEM | [243] |
| MFO@nZVI | FeCl3·6H2O+NaBH4 | MnFe2O4 (MFO) hydrogel | - | Mass of spheroidal particles | XRD, SEM, FTIR, UV–vis DRS | [244] |
| CDLA@nZVI and CDCA@nZVI | FeCl3·6H2O+NaBH4 | β-cyclodextrin (CD): CDLA and CDCA | 25 and 30 | Amorphous | NMR, FTIR, HRTEM, DLS, ζ-potential, FESEM, EDAX, VSM, XRD, XPS, TGA | [245] |
| Fe3O4@nZVI-PEI | Fe3O4+FeSO4·7H2O+NaBH4 | Poly ethylenimine | - | - | SEM, TEM, FTIR, XRD, XPS | [246] |
| S-nZVI | FeSO4·7H2O+KBH4 | - | [247] | |||
| nZVI-LBC | FeCl3·6H2O+NaBH4 | Biochar | - | - | FTIR, XRD, TEM, XPS, VSM, BET, ζ-potential | [248] |
| nZVI/GAC | FeSO4·7H2O+NaBH4 | Granular activated carbon (GAC) | 40 | Spherical | SEM, BET, XRD | [249] |
| nZVI/GO | FeCl3·6H2O+NaBH4 | Graphene oxide (GO) | 4.97 | - | SEM-EDS, XRD, FTIR | [250] |
| nZVI/Sch-AP and nZVI/ Sch-CO | FeSO4·7H2O+NaBH4 | Schwermannite (Sch) | 50 | Spherical | SEM, XRD, BET, XPS | [251] |
| A400-nZVI | FeSO4·7H2O+NaBH4 | Polystyrenic gel (Purolite A400) | 75–150 | - | FTIR, SEM, EDAX, XRD, TGA | [252] |
| nZVI@ Zr(OH)4 | FeCl3·6H2O+NaBH4 | Zirconium hydroxide | - | - | TEM-EDS, XRD, BET, FTIR, XPS | [253] |
| pyGA-nZVI | FeCl3·6H2O+NaBH4 | Pyrogallic acid (pyGA) | 40–90 | Spherical | SEM, TEM-EDS, BET, ζ-potential, XRD, FTIR, XPS | [254] |
| Ox-nZVI | FeCl3·6H2O+NaBH4 | Oxalate | 30–40 | Spherical | BET, SEM-EDS, FTIR, XRD, XPS | [255] |
| nZVI 1 and nZVI 2 | FeSO4·7H2O+NaBH4 FeCl3·6H2O+NaBH4 | - | 72 and 38 | Spherical | SEM, TEM-EDS, XRD, BET, XPS, Raman spectroscopy | [256] |
| nZVI/ copper slag | FeCl3·6H2O+NaBH4 | Copper slag | 30 | - | FE-SEM, EDX, XRD, FTIR, BET, VSM, ζ-potential | [257] |
| WPANF/ nZVI | FeCl3·6H2O+NaBH4 | Polyacrylonitrile fiber (WPANF) | 15–50 | - | XRD, FTIR, BET, XPS, SEM-EDS, TEM | [258] |
| nZVI, ds-coated nZVI, and ds-FeS | FeSO4·7H2O+NaBH4 | - | 60.12 and 110 | Spherical | SEM, XRD, FTIR, ζ-potential, DLS | [259] |
| nZVI | Self-combustion of Fe2O3 and NaBH4 | - | 2–8 | Amorphous | XRD, HRTEM, EDS | [260] |
| nZVI | Laser fragmentation in liquids (LFL) ethylene glycol and polyethylene glycol 400 | - | 10.5 and below 3 | - | DLS, LDE, TEM, XPS | [261] |
| CS@BC/ S-nZVI | FeSO4·7H2O+NaBH4 | Chitosan and Biochar | - | - | SEM, BET, FTIR, XRD, XPS | [262] |
| 3D-RGO@nZVI/ Al2O3 | FeSO4·7H2O+NaBH4 | Reduced graphene oxide | <100 | Spherical | SEM, BET, Raman spectroscopy, XRD, XPS | [263] |
| nZVI/n-lignin | FeCl3·6H2O+NaBH4 | Lignin | - | - | TEM, XPS, XRD | [264] |
| BP-S-nZVI | FeCl3+NaBH4+sulfate- reducing bacteria (SRB) | - | - | - | FESEM, TEM, XRD, BET | [265] |
| nZVI-DE | FeCl2·4H2O+NaBH4 | Diatomaceous earth (DE) | 20–40 | Spherical | XRD, SEM, EDX, TEM, BET | [266] |
| CnZVI | FeSO4·7H2O+NaBH4 | - | 80–99 | Spherical | UV, FTIR, XRD, TEM | [267] |
| G-nZVI-BC and C-nZVI-BC | FeSO4·7H2O+NaBH4 | Biochar | - | - | SEM, XRD, FTIR, XRF | [268] |
| Iron oxide | FeCl3·6H2O+FeCl2·4H2O+ NaOH | - | 10 ± 4 | Regular crystalline | TEM | [269] |
| CS/nZVI | FeCl3+NaBH4 | Chitosan | 25 | Spherical | SEM, FTIR, XRD, EDX, VSM, BET, TGA, DSC | [270] |
| BC@nFe-CA | FeSO4·7H2O+NaBH4 | Biochar | - | - | SEM, EDS, FTIR, Raman spectroscopy, XRD, XPS | [271] |
| PDA@Fe/rGO | FeSO4·7H2O+NaBH4 | Reduced graphene oxide (rGO) and polydopamine (PDA) | 51 | - | TEM, XRD, FTIR, XPS, VSM | [272] |
| PDCA@nZVI | FeCl3+NaBH4 | 2,6-pyridinedicarboxylic acid, (PDCA) | 115 | Nanospheres | SEM, EDS, EDX, XRD, FTIR | [273] |
| nZVI | FeSO4·7H2O+NaBH4 | - | - | Spherical | SEM, TEM, BET | [274] |
| nZVI/BC | Pyrolysis (Fe2O3+biochar) | - | 200 | - | XRD, SEM | [275] |
| CMC-nZVI, bare-nZVI, PAA-nZVI, PSM-nZVI, PVP-nZVI | FeCl3+NaBH4 | CMC, PAA, PSM, PVP | 9.53, 65.4, 106.4, 106.6, and 109 | Spherical | SEM-EDX, XRD, FTIR, TEM | [276] |
| nZVI-HPB | FeCl3·6H2O+NaBH4 | Hydrophilic biochar | 52–243 | - | SEM, TEM, XRD, FTIR, XPS | [277] |
| nZVI | FeSO4·7H2O+NaBH4 | - | 20–60 | Spherical | BET, SEM EDX, BET, XRPD, XPS | [278] |
| nZVI/SBA-15 | FeCl3·6H2O+NaBH4 | Mesoporous silica (Santa Bárbara-15) | 50–80 | Spherical | SEM EDS, TEM, BET | [279] |
| nZVI | FeCl3·6H2O+NaBH4 | - | 36 | Regular and irregular | XRD, SEM, EDX, UV–vis | [280] |
| nZVI-chitosan | FeCl3·6H2O+NaBH4 | Chitosan | 15–20 | - | SEM, XRD, TEM, FTIR | [281] |
| nZVI | Bulk iron disks+ solvents+ laser | - | 9.4 and 3.5 | Spherical | TEM EDS, XPS | [282] |
| nZVI/RS | FeSO4·7H2O+NaBH4 | Biochar | - | - | FTIR, XRD, Raman spectra, BET | [283] |
| AHG@nZVI | FeSO4·7H2O+KBH4 | Aluminum hydroxide gel | - | Irregular and rough | SEM, FTIR, XPS, XRD | [284] |
| S-nZVI/GA | FeSO4·7H2O+NaBH4 | Graphene aerogel (GA) | - | Flake-like shell | TEM, SEM, BET, XRD, XPS, FTIR, Raman spectrum | [285] |
| nZVI and SnZVI | FeCl2·4H2O+ FeSO4·7H2O+NaBH4 | - | - | Cross-linked spherical | TEM EDX, XPS, XRD, XAS | [286] |
| Fe0@p-SiO2 | FeCl3·6H2O+KBH4 | SiO2 | 30–40 | - | TEM, XRD, XPS, ζ-potential | [287] |
| Fe/TRGO | Carbothermal GO+ Fe(NO3)3·9H2O | Graphene oxide | - | - | HRTEM, XRD, XPS, Fe Mössbauer spectroscopy | [288] |
| nZVI | FeCl2·4H2O+NaBH4 | - | - | Chain-like | FTIR, SEM, TEM, XRD, XPS | [289] |
| Fe@CQDs MNCs | FeCl3+NaBH4 | - | - | Smooth | FTIR, XRD, SEM, TEM | [290] |
| nZVI | FeCl3·6H2O+NaBH4 | - | - | Irregular and noncircular | XRD, TEM EDS, FESEM | [291] |
| Nanostructured nZVI | FeSO4·7H2O+NaBH4 | - | - | Rough | XRD, SEM, TEM, DLS | [292] |
5. Environmentally Friendly Methods for the Synthesis of nZVI
- Enhanced removal capacity and longevity: The presence of polyphenols in these extracts enhances their removal capabilities because bioactive compounds such as polyphenols have several benzene groups substituted by hydroxyl functional groups in their structure, so they can reduce metal ions to their elemental state, obtaining nanoparticles. They are also low cost or economically viable, reducing the consumption of organic compounds and the generation of toxic products.
- Environmentally friendly: This method is hailed as a potential environmentally friendly process, characterized by lower toxicity levels, the absence of aggressive reagents, and the generation of non-harmful by-products.
- Cost-effective: Many authors claim it might provide a low-cost alternative for nanoparticle synthesis.
- Inherent stabilization: The extract matrix often acts as a stabilizer, reducing nanoparticle agglomeration without requiring the addition of dispersants.
- Biomass valorization: The method offers potential for biomass valorization, further contributing to its sustainability.
| FeNPs | Precursor | Support | Size/nm | Morphology | Characterization | Reference |
|---|---|---|---|---|---|---|
| BC-nZVI-BC | Oak+FeCl3 | Biochar | 68–521 | Twister and serpentine | SEM, EDS, XRD, DLS | [230] |
| nZVI, ds-coated-nZVI+ds-FeS | Phoenix dactylifera +FeSO4·7H2O | - | Spherical | SEM, XRD, FTIR, ζ-potential, DLS | [259] | |
| GnZVI | Amaranthus dubius leaf extract+ FeSO4·7H2O | - | 1–3 | Spherical | UV, FTIR, XRD, TEM | [267] |
| G-nZVI-BC and C-nZVI-BC | Green tea residues+FeSO4·7H2O | - | - | - | SEM, XRD, FTIR, XRF | [268] |
| Iron oxide | Cymbopogon citratus extract+ FeCl3·6H2O+Na2CO3 | - | 9 ± 4 | Regular crystalline | TEM | [269] |
| nZVI | Azadirachta indica (neem) Mentha longifolia (mint) L.e.+FeCl3·6H2O | - | Spherical | SEM, TEM, BET | [274] | |
| GT-nZVI | Black tea+FeCl3·6H2O | - | 80 | Regular and irregular | XRD, SEM, EDX, UV–vis | [280] |
| Fe-NP-GV | Mansoa alliacea +FeSO4·7H2O | - | 18.22 | Spherical | XRD, UV–vis, AAS FTIR, TGA | [295] |
| nZVI | Shirazi thyme L.e.+FeSO4·7H2O/ Pistachio green hulls pomegranate/banana/ mango+FeCl3 black tea+ FeCl3·6H2O | - | 40–70 114, 76, 95 | - | - | [298] |
| nZVI | Green tea+ Fe (NO3)3·9H2O | - | 5–45 | Amorphous spherical | TEM, SEM/EDS, XRD, BET | [299] |
| AC/nZVI | Pomegranate peel extract+FeCl2 | Activated carbon | Crystalline | FTIR, XRD, BET, FESEM | [300] | |
| nZVI | Cleistocalyx operculatus L.s+FeCl3 | - | 100 | Spherical | SEM, XRD, FTIR | [301] |
| nZVI @gBC | Carbothermal (sawdust+FeCl3·6H2O) | Graphene | - | - | - | [302] |
| nZVI | Cleistocalyx operculatus L.s+FeCl3 | - | 100 | Spherical | SEM, XRD, FTIR | [303] |
| TP-nZVI/PE | Tea polyphenols | Polyethylene | - | Rough | SEM, TEM, ζ-potential, XRD, FTIR, XPS | [304] |
| Fe@C | Rice powder+ Fe (NO3)3·9H2O | Biomass- derived carbon | 30–150 | Irregular | XRD, FTIR, SEM, TEM, EDS | [305] |
| nZVI@GNPs | Cleistocalyx operculatus Leaf extract+ graphene NPs | Graphene nanoplatelets (GNPs) | 30–100 | Spherical | SEM, XRD, EDS, FTIR | [306] |
| G-nZVI | Ripe mango peels+FeCl3·6H2O | - | - | - | XRD, FTIR, TEM, BET, SEM-EDX | [307] |
| EGnZVI | Eucalyptus grandis+FeSO4·7H2O | - | 50–500 | Spherical | XRD, FTIR, Raman, SEM, TEM/EDS | [308] |
| G-nZVI/B | Green tea+ FeSO4·7H2O | Calcined bentonite | 8 30 | Irregular | BET, SEM, TEM, FTIR, XRD, XPS | [309] |
| nZVI | Corn+ FeCl3·6H2O | - | 150–300 | Spherical | SEM, TEM-EDS, XRD, XPS | [310] |
| EL-nZVI | Eucalyptus L.e.+FeSO4·7H2O | - | 87 | Spherical | SEM, FTIR | [311] |
| SnZVI@HPAC | Green tea+FeSO4·7H2O | - | - | - | SEM-EDS, TGA, FTIR, XRD | [312] |
| SC-nZVI | Green tea waste +FeCl3·6H2O | Silty clay | - | - | FTIR, SEM, XRD, BET, ζ-potential | [313] |
| nZVI/CF | Carbothermal (cotton fiber +Fe (NO3)3·9H2O) | Cotton carbon fiber | - | - | XRD, SEM, BET | [314] |
| nZVI @TP-Mont | Green tea+FeSO4·7H2O | Montmorillonite | 15–30 | Spherical | TEM, XRD, FTIR, SBET, XPS, ζ-potential | [315] |
| RCL-nZVI | Ricinus communis L.e.+FeCl3·6H2O | - | 4.84 25.6 | Irregular | SEM, TEM, FTIR, EDS, XRD, XPS, ζ-potential | [316] |
| B-BT-nZVI | Black tea+ FeCl3 | Bentonite | <50 | - | AFM, SEM, ζ-potential, BET | [317] |
| GT-nZVI@VC | Green tea+ Vit C+ FeSO4·7H2O | - | 100 | - | XRD, TEM, SEM, FTIR | [318] |
| nZVI | Pomegranate peel extract+ FeCl3·6H2O | - | 40–60 | Spherical | UV–visible, FTIR, SEM | [319] |
| k-nZVI | Ruellia tuberosa+FeCl3·6H2O | Kaolin | 20–40 | Spherical | XRD, SEM, TEM, EDS | [320] |
| R-FeNPs | Green tea+FeCl3·6H2O | Resin | 20–40 | Spherical | SEM, TEM, EDS | [321] |
| nZVI @Fe3O4 @HMIMPF6 | Camellia sinensis+ FeCl3·6H2O+ FeCl2·4H2O | Magnetite+ 1-hexyl-3-methylimidazolium hexafluorophosphate | 30 | Spherical | FTIR, XRD, VSM, BET, SEM, TEM | [322] |
| FeNPsJF | Jackfruit peel (JFP) extract+FeCl2 | - | 33 | Irregular spherical | FTIR, TEM, XRD, SEM, EDX | [323] |
| G-nZVI | Pomegranate fruit peel+FeCl3·6H2O | - | 60–75 | Spherical to cubical | UV–vis, XRD, TEM, SEM, DLS, ζ-potential | [324] |
| nZVI coupling with MR-1 | Green tea+ FeSO4·7H2O | Shewanella oneidensis MR-1 | - | - | SEM-EDS, XPS, FTIR, Raman, EEM | [325] |
| RC-nZVI | Ricinus communis seed extract+Fe3+ | - | 20 | Spherical | SEM, TEM, FTIR, XRS, EDS, XRD, XPS, ζ-potential | [326] |
| FeNPs | Denitrifying bacteria+ +FeCl3 | - | - | - | UV–vis, XPS, FTIR, TEM | [327] |
| AC/nZVI | Pomegranate peel extract+FeSO4·7H2O | Activated carbon | - | - | XRD, FTIR, FESEM | [328] |
| Zeolite/ nZVI | Pomegranate peel extract+FeCl2·4H2O | Zeolite | 30 | - | FTIR, FESEM, BET, XRF | [329] |
| BGT-nZVI | Green tea+FeCl3·6H2O | Bentonite | - | - | [330] | |
| PPAC-nZVI | Pomegranate peel extracts+FeCl2·4H2O | Activated carbon | 19–24 | - | FESEM, BET, FTIR | [331] |
| nZVI-RBC | Rice husk+FeSO4·7H2O Rice husk-derived biochar (RBC) | Rice husk-derived biochar (RBC) | 100 | - | SEM EDS, XRD, FTIR, BET, XPS | [332] |
| S-nZVI/AC | Ulva. prolifera+ FeCl2·4H2O | Algal carbon | - | Flower-like | SEM EDS, TEM, XRD, BET, XPS | [333] |
| Fe/N-OB | Carbothermal (hematite oak wood biochar) | - | - | XRD, XPS, SEM EDS, FTIR, BET | [334] | |
| GT-nZVI | Black tea+FeCl3·6H2O | - | 83 | Irregular | XRD, SEM, EDAX | [335] |
| n-ZVI-NPs | Mentha piperita+FeCl3 | - | 5–10 | Spherical | UV–vis, SEM-EDX, DLS | [336] |
| Fe0+ Fe1.91C0.09+ Fe3O4 | Neurospora crassa +urea+FeSO4 | - | 50 | - | SEM EDX, XRD, XPS, BET | [337] |
| TP-ZVI-OB | Green tea+FeCl3·6H2O | Oak wood biochar | - | - | BET, FTIR, SEM, EDS, XPS, XRD | [338] |
| F–Fe0 ads | Ficus sycomorus dry L.e.+FeCl3·6H2O | Wheat bran (B), rice bran (RB), activated charcoal (Ach), and bentonite (Bent) | 2.46–11.49 | Circular | UV–vis, HRTEM | [339] |
| NA-FeNPs | Nephrolepis auriculata+ FeCl3 | - | 40–70 | Spheroidal | TEM, XRD, EDS, XPS, FTIR | [340] |
| GMP-nZVI | Mango peel extract+FeCl3·6H2O | - | 1–10 | - | UV–vis, EDX, XRD, XPS, FTIR | [341] |
| DOX@GTCs-FeNPs | Green tea catechin powder+FeCl3·6H2O | 159 | TEM, UV–vis, AAS, | [342] | ||
| Micro/FeNPs | Mango, rose, Neem L.e. carom seeds, and clove buds+FeCl2·4H2O | Polyvinyl alcohol (PVP) | 75–6500 | Spherical or irregular | SEM, XRD, EDX, FTIR, UV–vis | [343] |
| Iron oxide | Eucalyptus L.e. +FeCl3·6H2O | Cetyltrimethylammonium bromide (CTA) | 80–90 | Spherical | XRD, EDS, FTIR, TGA | [344] |
| FeNPs | Eichhornia crassipes L.s +FeSO4·7H2O | - | - | Rod | UV–vis, SEM, TEMXRD, FTIR | [345] |
| Iron oxide | Cocos nucifera+FeCl3 | - | 10–100 | Clustered | UV–vis, TEM, XRD, XPS | [346] |
| FeNPs | Eucalyptus globulus, Mangifera indica, Syzygium cumini, Psidium guajava+FeCl3·6H2O | - | 38–47 | Irregular | UV–vis, FTIR, FESEM EDS, XRD | [347] |
| VI | Oak L.e.+FeCl3·6H2O | - | 20–100 | Irregular | TEM, EDS, XRD | [348] |
| Fe3O4NPs | Coriandrum sativum L.e. +FeCl3 | - | 20–90 | Spherical | UV–vis, FTIR, XRD SEM EDX | [349] |
| Iron oxide | Lantana camara fruit+FeSO4·7H2O+ FeCl3·6H2O | - | 28 | Spherical | FTIR, TGA, PSA, SEM EDAX, ζ-potential | [350] |
| Iron oxide | Lantana camara L.e.+FeSO4·7H2O | - | 10–20 | Nanorods | XRD, FTIR, SEMEDX, UV–vis | [351] |
| LGFeNPs | Eucalyptus L.e.+ laterite | - | 20–70 | Spherical | FESEM EDX, XRD, FTIR, BET | [352] |
| FeNPs | Eucalyptus L.e.+FeSO4·7H2O | - | 70 ± 20 | Spherical | SEM EDS, FTIRE, XRD, TEM, XPS, XRD, BET | [353] |
| Fe2O3@SiO2 | Zanthoxylum rhetsa +FeCl3·6H2O | SiO2 | 12.2 ± 0.8 | Cluster-like | FTIR, XRD, SEM EDX, HRTEM | [354] |
| SJA-FeNPs | Syzgium jambos +FeCl3 | - | 13.7 ± 5 | Spherical | UV–vis, TEM, XRD, XPS | [355] |
| nZVI | Vaccinium corymbosum +FeCl3·6H2O | - | 52.4 | Irregular | TEM, SEM, BET, XRD | [356] |
| Fe3O4@ZnO | Azadirachta indica(neem)+ FeSO4·7H2O+ Fe (NO3)3·9H2O | ZnO | 38 | Brick-like | XRD, FTIR, SEM EDX, TEM, TGA | [357] |
| Ec-Fe-NPs | Eichhornia crassipes+FeCl3 | - | 20–80 | Amorphous | SEM, EDS, TEM, XPS, FTIR, DLS, ζ-potential | [358] |
| FeNPS | Moringa oleifera+FeCl3 | - | 2.6–6.2 and 3.4–7.4 | Spherical | UV–vis, XRD, FTIR, TEM | [359] |
| Iron oxide | Black tea+FeSO4·7H2O | - | 5–50 | Amorphous | XRD, FTIR, SEM, TEM, EDS | [360] |
| nZVI | Green tea L.e.+FeCl3 | - | 116 | - | FTIR, SEM | [361] |
| FeNPs | Rosa damascene (RD), Thymus vulgaris (TV), and Urtica dioica (UD) +FeCl3·4H2O | - | 100 | Nonuniform | FTIR, SEM, TEM, XRD | [362] |
| FeNPs | Euphorbia cochinchensis +FeCl3 | - | 100 | Spherical | GMS, TEM, XPS, XRD, BET | [363] |
| FeNPs | Eucalyptus L.e. +FeSO4·7H2O | - | 20–80 | Polydisperse | SEM, XRD, XPS, FTIR | [364] |
| Iron oxide | Sapindus mukorossi +Fe(NO3)3·9H2O)+FeCl3 | - | <50 | Nanorods | XRD, FESEM, TEM | [365] |
| Iron oxide nanorods (IONRs) | Mangifera indica L.e. +FeSO4·7H2O | 3.0 ± 0.2 | Nanorods | FESEM, EDX, XRD, TEM | [366] | |
| FeNPS | Mangifera indica, Murraya koenigii, Azadiracta indica, Magnolia champaca +FeSO4·7H2O | AI (96–110), MC (99–129), MIAND MK (100–150) | Spherical | UV–vis, SEM-EDS-FTIR | [367] | |
| GT-Fe NPs and EL-Fe NPs | Eucalyptus L.e.+green tea L.e.+FeSO4·7H2O | 20–80 | Quasi-spherical | SEM, XRD, FTIR | [368] | |
| nZVIs | Waste from citrus juice (orange, lime, lemon and mandarin) +FeSO4·7H2O | 3–300 | Spherical, cylindrical, irregular | TEM, XRD, Mössbauer spectroscopy | [369] | |
| nZVI | Black tea, grape mark vine L.e. +FeCl3·6H2O | 15–45 | - | TEM | [370] | |
| (ZVI) NPs | Terminalia chebula+FeSO4·7H2O | <80 | Amorphous | TEM, XRD, UV–vis, FTIR | [371] | |
| nZVI | 26 tree leaf extracts+FeCl3 | 10–20 | Spherical | TEM | [372] |
6. Dye Pollution in Wastewater and Current Removal Strategies
| NPs | Leaf Extract of Plant | Fe Source | Dye | Concentration (mgL−1) | Removal (%) | React. Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| nZVI | Green tea | Fe (NO3)3∙9H2O | Malachite green | 50 | 93 | 60 | [294] |
| nZVI | Eucalyptus | FeCl3·6H2O | Acid black 194 | 100 | 80.5 | 200 | [296] |
| nZVI | Eucalyptus L.e. | FeSO4·7H2O | Crystal violet | 30 | 97.6 | 1800 | [311] |
| nZVI | Black tea | FeCl3·6H2O | Reactive blue 238 | 49.6 | 90.5 | 60 | [317] |
| nZVI | Ruellia tuberosa | FeCl3·6H2O | Reactive black 5 | 25–400 | 95.3–99.8 | 30 | [320] |
| nZVI | Artocarpus heterophyllus | FeCl3·6H2O | Fuchsin basic | 4 | 87.5 | 20 | [323] |
| nZVI | Ricinus communis | FeCl3·6H2O | Methylene blue | 25–200 | 96.8 | 160 | [326] |
| nZVI | Green tea | FeCl3·6H2O | Reactive blue 238 | 49.5 | 96.2 | 40 | [330] |
| nZVI | Mango peel | FeCl3·6H2O | Methyl orange | 100 | 94.23 | 60 | [341] |
| nZVI | Pomegranate L.e. | FeCl3·6H2O | Malachite green | 156 | 95 | 36 | [396] |
| nZVI | Vine L.e. | FeCl2·4H2O | Orange II | 100 | 80 | 120 | [398] |
| nZVI | Vine L.e. | FeCl2·4H2O | Orange II | 100 | 92 | 200 | [401] |
| nZVI | Tea | FeSO4·7H2O | Malachite green, methylene blue | 200 | 90.7, 90.75 | 30 | [402] |
| nZVI | Camellia sinensis tea | FeCl3·6H2O | Bromothymol blue | 500 | - | 60 | [403] |
| nZVI | Green tea | FeCl2·4H2O | Methylene blue, methyl orange | 50 | 360 | 80 | [404] |
| nZVI | Oolong tea | FeSO4·7H2O | Malachite green | 50 | 75.5 | - | [405] |
| nZVI | Ferula persica | FeSO4·7H2O | Crystal violet | 200 | 99.8 | 210 | [406] |
| nZVI | Catharanthus roseus | FeSO4·7H2O | Methyl orange | 50 | 50 | 360 | [407] |
| α-Fe2O3 | Phyllanthus niruri, Moringa stenopetala | FeCl3·6H2O | Methylene blue | 156 | 92–96 | 36 | [408] |
| nZVI | Chlorophytum comosum | FeCl3·6H2O | Methyl orange | 4 | 77 | 25 | [409] |
| nZVI | Parthenium | FeSO4·7H2O | Crystal violet | 120 | 95 | 30 | [410] |
| Fe3O4 | Thunbergia grandiflora | FeSO4·7H2O | Acid blue 113 | 25 | 94.38 | 210 | [411] |
| Fe2O3 | Raphanus sativus L.e. | FeCl3·6H2O | Methylene blue, methyl red | 100 | 100 | 60 | [412] |
| Fe2O3 | Fan palm, Dombeya wallichii, Pyrus communis | FeSO4·7H2O | Reactive blue | 200 | 94, 81, 88 | 210 | [413] |
| Fe3O4 | Jatropha curcas, Cinnamomum tamala | FeCl2·H2O+ FeCl3 | Methylene blue | 200 | 46.66 | 120 | [414] |
| Iron oxide | Artemisia vulgaris L.e. | FeCl3·6H2O | Methyl orange | 25 | 98.6 | 360 | [415] |
| Iron oxide | Daphne mezereum | FeCl3·6H2O | Methyl orange | 25 | 81 | 360 | [416] |
| Fe3O4 | Fraxinus sinensis Roxb | FeCl3·6H2O+ FeSO4·7H2O | Crystal violet | 4 | 98.57 | 25 | [417] |
| nCluster | Cupressus sempervirens | FeCl3·6H2O | Methyl orange | 25 | 95.8 | 360 | [418] |
| nZVI | Psidium guajava L.e. | FeCl3·6H2O | Methylene blue | 50 | 94 | 5 | [419] |
| nZVI | Hibiscus sabdariffa, roselle flower | FeCl3·6H2O | Rhodamine B | 17 | 100 | 5 | [420] |
| nZVI | Trigonella foenum-graecum | FeCl3·6H2O | Methyl orange | 25 | 95 | 90 | [421] |
| ZVI | Camellia sinensis and pomegranate L.e. | FeSO4·7H2O | Textile wastewater | 2.330 - | >95 pH 8.5 (Pt-Co) | 120 | [422] |
| Fe3O4 | Azolla filiculoides and fig. L.e. | FeCl3·6H2O+ FeSO4·4H2O | Crystal violet Methylene blue | 500 | 100 | 210 | [423] |
| Fe3O4 | Pissum sativum peel | FeCl3·6H2O | Methyl orange | 100 | 96.2 | 360 | [424] |
| nZVI | Eucalyptus | FeSO4·7H2O | Methyl orange | 10 | 99.6 | 180 | [425] |
| Iron oxide | Pomegranate L.e. | FeCl3·6H2O | Congo red | 100 | 93 | 60 | [426] |
| Ni-iron oxide | Moringa oleifera | FeCl3·6H2O + NiCl2·6H2O | Malachite green | 20 | ~91.6 | 25 | [427] |
| Fe3O4 | Green tea L.e. | FeCl3·6H2O+ FeSO4∙ 4H2O | Methylene blue | 3.5 | 95 | 16 | [428] |
| Pd-iron oxide | Pepper | Fe (NO3)3∙9H2O+ FeCl2·4H2O | Acid black, acid brown | 20.2 | 97.85 | 120 | [429] |
| Iron oxide | Cynometra ramiflora fruit | FeCl2·H2O+FeCl3 | Methylene blue | 20 | 100 | 110 | [430] |
| Iron oxide | Cynometra ramiflora | FeSO4·7H2O | Rhodamine B | 134 | 100 | 15 | [431] |
| Iron oxide | S. cumini | Ferrous oxalate | Reactive blue 235 | 10 | 98.75 | 240 | [432] |
| nZVI | Tea | FeSO4·7H2O | Methylene blue | 50 | 85.7, 34.4 | 5 | [433] |
| Fe3O4 | Ridge gourd peels | FeCl3·6H2O | Methylene blue | 120 | 96 | 30 | [434] |
| nZVI | Tieguanyin tea | FeCl3·6H2O | Bromothymol blue | 100 | >90 | 30 | [435] |
| nZVI | Green tea | FeCl3·6H2O | RBB-R, DR80 mixture | 250 | 90 | 20 | [436] |
| Fe3O4 | Maize cob | FeCl2·4H2O+ 2FeCl3·6H2O | Methylene blue | 156 | 99.63 | 36 | [437] |
| nZVI | Eucalyptus tereticornis, Melaleuca nesophila, Rosemarinus officinalis | FeSO4·7H2O | Acid black 194 | 100 | 100 | 200 | [438] |
| nZVI | Green tea, oolong tea, black tea | FeSO4·7H2O | Malachite green, methylene blue | 50 | 81.2, 75.6, 67.1 | 60 | [439] |
| nZVI | Aspalathus linearis | Acid mine drainage | Orange II | 50 | 94 | 30 | [440] |
| nZVI | Calotropis gigantea | Fe (NO3)3·9H2O | Methylene blue | 50–400 | 83.9 | 30 | [441] |
nZVI | Amaranthus dubius L.e. | FeCl3·6H2O | Methyl orange | 20 | 81 | 360 | [442] |
7. Future Perspectives and Challenges
- ▪
- Critical to note is the considerable variability in extracted biomolecules from plant extracts, constituting an unpredictable factor in green chemistry nanoparticle synthesis. The diverse nature of plants, encompassing distinct plant parts and geographical locales, complicates the prognostication of extracted biomolecules and their quantities. Moreover, not all biomolecules involved in nanoparticle synthesis are comprehensively understood. Controllable factors such as solvent, temperature, pH, precursor iron salts, and solution agitation profoundly influence nanoparticle growth, morphology, size, aggregation, coating, and stability within green chemistry procedures.
- ▪
- Furthermore, it is imperative to recognize the industrial implications of nanoparticle synthesis, necessitating large-scale production. Consequently, material supply assumes critical importance, with the agrifood industry wielding significant influence due to the pronounced variability influenced by geographical location and climate.
- ▪
- A standardized protocol is imperative for achieving greater reproducibility in nanoparticle synthesis concerning size and shape, thereby optimizing dye decontamination processes in the environment. Furthermore, the development of methodologies aimed at minimizing waste generation or enabling waste reuse is critical for fostering more sustainable processes. The extraction processes from agrifood industries entail significant solvent consumption, resulting in solid residues, necessitating a reduction in energy consumption. Analogous to numerous other fields, the reutilization and recycling of these by-product streams are pivotal for practical industrial implementation.
- ▪
- Regarding applications, substantial challenges persist. Ongoing efforts are directed towards exploring novel applications in diverse sectors such as food, textiles, healthcare, and construction, extending beyond environmental objectives.
- ▪
- In the realm of water purification, wastewater treatment plants are transitioning towards pollutant mineralization and biorefinery models, aiming to derive commercially viable products. Accordingly, pollutant removal processes must align with this philosophy and be seamlessly integrated into wastewater treatment plants. This mandates that new nanoparticles, acting as catalysts, should adsorb and convert pollutants into products that can be easily desorbed at low energy costs.
- ▪
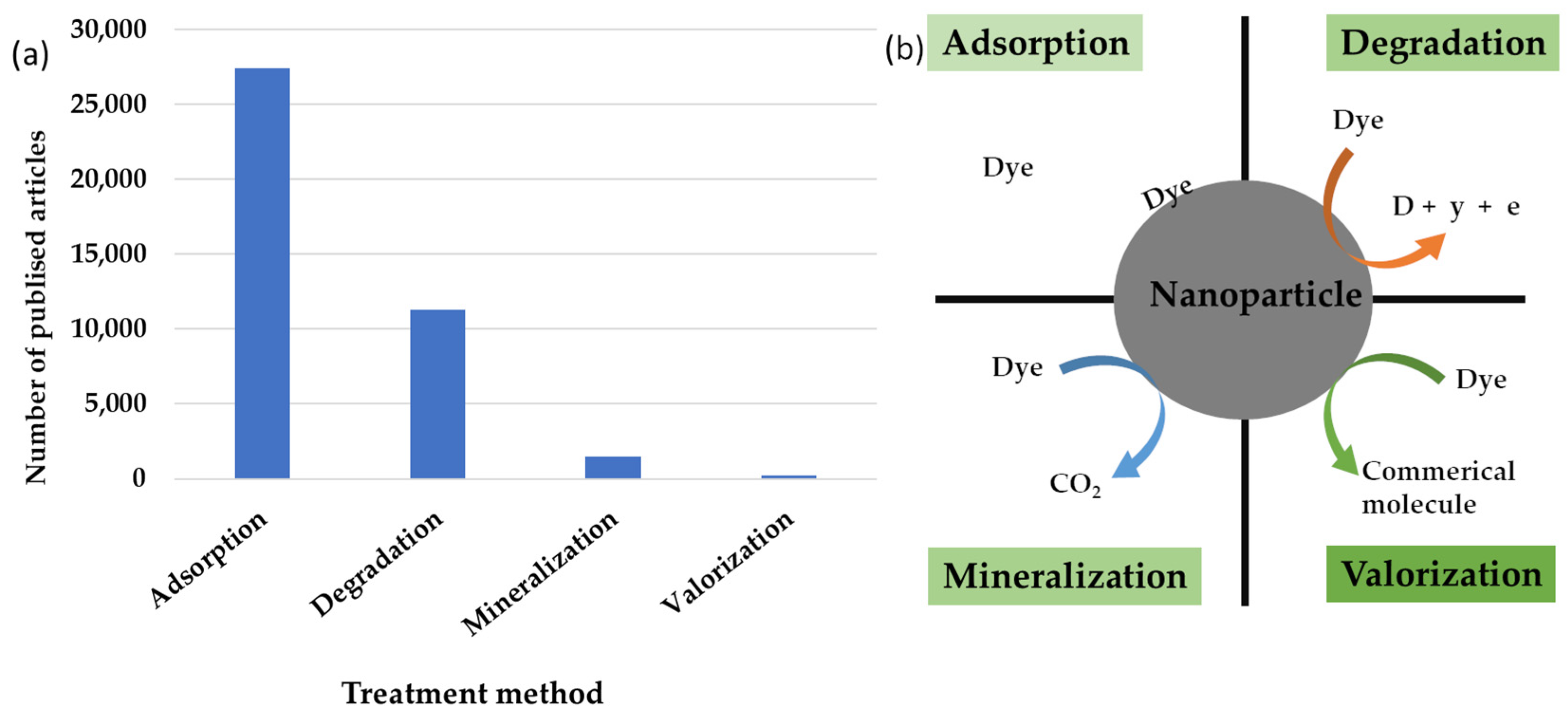
- The authors underscore the potential for the wastewater industry to operate as a biorefinery, extracting commercial products from wastewater. However, these treatment methodologies are still nascent, as depicted in Figure 6.
- ▪
- It is noteworthy that a mere 242 articles, less than 0.001%, focus on dye recovery, with the majority concentrating on repurposing waste for dye treatment using alternative methods. Only a fraction of these articles address the recovery of solid waste from the textile industry [443,444]. Marazzi et al. [445] propose the use of algae for the treatment of dyes and their subsequent transformation into energy in biogas plants through anaerobic digestion.
- ▪
- Since clean, high-quality water is a valuable and essential commodity, and given that one of the main and most obvious parameters indicating water quality is its color, the application of iron nanoparticles as an available technology for dye removal is easy to use, cost-effective, and very efficient. The two main removal processes are adsorption and decolorization. This implies that studies should be directed to investigate the optimal conditions of these processes, such as the influence of the effects of initial dye concentration, pH, temperature, as well as nanoparticle size, morphology, and dosage, and to generate new general trends based on these studies.
- ▪
- A plethora of literature exists on environmentally friendly methods, often associated with biomass or bio-resources as feedstock sources. While these methodologies hold significant promise, presuming their environmental friendliness without thorough environmental impact assessments would be erroneous [446]. Thus, it is imperative to evaluate the environmental footprint of the chosen method to ensure its sustainability and assess its economic viability for industrialization. Furthermore, methods employing industrial chemical reagents can be rendered environmentally friendly provided energy consumption is minimized, and by-products or residues are recycled or integrated into a circular economy framework.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaki, M.S.; Abou Zaid, A.A.; Abdelzaher, M.F.; Shalaby, S.I. Clinicopathological Changes in Fish Exposed to Pollutants. Life Sci. J. 2014, 11, 271–278. [Google Scholar]
- Dwivedi, S.; Shikha, D. Water Pollution: Causes, Effects and Control. Biochem. Cell Arch. 2016, 16, 96–102. [Google Scholar]
- Agoha, E.E.C. Crude Oil in Drinking Water: Chitosan Intervention. IFMBE Proc. 2019, 68, 741–743. [Google Scholar] [CrossRef]
- Larios-Meoño, J.F.; Morales, Y.; Gonzalez-Taranco, C.; Mougenot, B. Impact of Water Quality on the Economy and Health of Latin America: The Case of Peru and Colombia. In Proceedings of the 33rd International Business Information Management Association Conference, IBIMA 2019: Education Excellence and Innovation Management through Vision 2020, Granada, Spain, 10–11 April 2019; pp. 8960–8969. [Google Scholar]
- Gupta, J.K.; Shah, K.; Mishra, P. Inadmissible Planktons in Potable Water: A Potential Risk for Human Health. Curr. Sci. 2020, 119, 1627–1632. [Google Scholar] [CrossRef]
- Russ, J.; Zaveri, E.; Desbureaux, S.; Damania, R.; Rodella, A.-S. The Impact of Water Quality of GDP Growth: Evidence from around the World. Water Secur. 2022, 17, 100130. [Google Scholar] [CrossRef]
- du Plessis, A. Global Water Quality Challenges; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Das, J.; Sarkar, S.; Saha, K.; Roy, S.; Nag, S. Emerging Environmental Contaminants: Sources, Consequences, and Future Challenges. In Bioremediation Technologies: For Wastewater and Sustainable Circular Bioeconomy; Walter de Gruyter: Berlin, Germany, 2023; ISBN 978-3-11101-682-5. [Google Scholar] [CrossRef]
- Snyder, S.A.; Anumol, T. Emerging Chemical Contaminants: How Chemical Development Outpaces Impact Assessment. In Still Only One Earth: Progress in the 40 Years Since the First UN Conference on the Environment; Royal Society of Chemistry: London, UK, 2015; Volume 2015, ISBN 978-1-78262-076-1. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Becker, J.A. A Review of Emerging Contaminants in Water: Classification, Sources, and Potential Risks. In Impact of Water Pollution on Human Health and Environmental Sustainability; IGI Global: Pennsylvania, PA, USA, 2019; ISBN 978-1-79981-211-1. [Google Scholar] [CrossRef]
- Sivasubramaniyan, S.G.; Kandasamy, S.; Manickam, N.K. Biotechnological Approaches for Removal of Emerging Contaminants. In Biotechnology for Zero Waste: Emerging Waste Management Techniques; Wiley: Hoboken, NJ, USA, 2021; ISBN 978-3-52783-206-4. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Ngeno, E.; Shikuku, V.O. Emerging Contaminants: Pollution Control and Abatement. In Research Anthology on Emerging Techniques in Environmental Remediation; IGI Global: Pennsylvania, PA, USA, 2022; Volume 2, ISBN 978-1-66843-886-2. [Google Scholar] [CrossRef]
- Lofrano, G.; Sacco, O.; Venditto, V.; Carotenuto, M.; Libralato, G.; Guida, M.; Meric, S.; Vaiano, V. Occurrence and Potential Risks of Emerging Contaminants in Water. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12818-334-2. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide Cases of Water Pollution by Emerging Contaminants: A Review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A Review of What Is an Emerging Contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Noguera-Oviedo, K.; Aga, D.S. Lessons Learned from More than Two Decades of Research on Emerging Contaminants in the Environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Jankowska, A.; Ejsmont, A.; Galarda, A.; Goscianska, J. The Outcome of Human Exposure to Environmental Contaminants. Importance of Water and Air Purification Processes. In Sustainable Materials for Sensing and Remediation of Noxious Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-32399-425-5. [Google Scholar] [CrossRef]
- Rahman, M.; Tabassum, Z. Biotechnological Approach to Treat Textile Dyeing Effluents: A Critical Review Analysing the Practical Applications. Text. Leather Rev. 2024, 7, 124–152. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zhang, T.; Liu, S.; Ho, S.S.H.; Tian, J.; Su, H.; Zhang, Y.; Wang, L.; Wu, T.; et al. Elaborations of the Influencing Factors on the Formation of Secondary Inorganic Aerosols in a Heavily Polluted Urban Area of China. J. Environ. Sci. 2024, 138, 406–417. [Google Scholar] [CrossRef]
- Bai, Y.; Lin, H.; Wang, C.; Wang, Q.; Qu, J. Digitalizing River Aquatic Ecosystems. J. Environ. Sci. 2024, 137, 677–680. [Google Scholar] [CrossRef]
- Brown, A.M.; Bass, A.M.; Skiba, U.; MacDonald, J.M.; Pickard, A.E. Urban Landscapes and Legacy Industry Provide Hotspots for Riverine Greenhouse Gases: A Source-to-Sea Study of the River Clyde. Water Res. 2023, 236, 119969. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.-H.; Wang, J.J. Biochar-Based Materials as Remediation Strategy in Petroleum Hydrocarbon-Contaminated Soil and Water: Performances, Mechanisms, and Environmental Impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of Nanoplastics in the Aquatic Environment and Possible Impacts on Aquatic Organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef]
- Castaño-Ortiz, J.; Gil-Solsona, R.; Ospina-Álvarez, N.; Alcaraz-Hernández, J.; Farré, M.; León, V.; Barceló, D.; Santos, L.; Rodríguez-Mozaz, S. Fate of Pharmaceuticals in the Ebro River Delta Region: The Combined Evaluation of Water, Sediment, Plastic Litter, and Biomonitoring. Sci. Total Environ. 2024, 906, 167467. [Google Scholar] [CrossRef]
- Khezami, F.; Gómez-Navarro, O.; Barbieri, M.V.; Khiari, N.; Chkirbene, A.; Chiron, S.; Khadhar, S.; Pérez, S. Occurrence of Contaminants of Emerging Concern and Pesticides and Relative Risk Assessment in Tunisian Groundwater. Sci. Total Environ. 2024, 906, 167319. [Google Scholar] [CrossRef]
- Hung, V.Q.; Egodawatta, P.; Gallage, C.; Dawes, L.; Nguyen-Xuan, T.; Nguyen-Viet, T.; Bui-Tien, T.; Nguyen-Quang, T.; De Roeck, G. Leaching Mechanism of Metals from Recycled Concrete Aggregates (RCA) and Potentially Environmental Issues. In Proceedings of the 4th International Conference on Sustainability in Civil Engineering, Hanoi, Vietnam, 25–27 November 2024; Volume 344, ISBN 978-981-99-2344-1. [Google Scholar] [CrossRef]
- Tudor, V.C.; Stoicea, P.; Chiurciu, I.-A.; Soare, E.; Iorga, A.M.; Dinu, T.A.; David, L.; Micu, M.M.; Smedescu, D.I.; Dumitru, E.A. The Use of Fertilizers and Pesticides in Wheat Production in the Main European Countries. Sustainability 2023, 15, 3038. [Google Scholar] [CrossRef]
- Bukomeko, H.; Taulya, G.; Schut, A.G.T.; van de Ven, G.W.J.; Kubiriba, J.; Giller, K. Evaluating Combined Effects of Pesticide and Crop Nutrition (with N, P, K and Si) on Weevil Damage in East African Highland Bananas. PLoS ONE 2023, 18, e0282493. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.A.; Ahad, U.; Inayatullah, M.; Ali, U.; Ahmed, P. Evaluation of Water Quality Status of Pohru Watershed, Kashmir Valley, Jammu and Kashmir, India. Water Air Soil Pollut. 2023, 234, 154. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manage. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Anandita; Raees, K.; Shahadat, M.; Ali, S.W. Mechanistic Interaction of Microbe in Dye Degradation and the Role of Inherently Modified Organisms: A Review. Water Conserv. Sci. Eng. 2023, 8, 43. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.; Shi, X.; Fan, M.; Luo, J.; Wei, X. Nanoscale Zero-Valent Metals: A Review of Synthesis, Characterization, and Applications to Environmental Remediation. Environ. Sci. Pollut. Res. 2016, 23, 17880–17900. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale Zero-Valent Iron: Future Prospects for an Emerging Water Treatment Technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Hasirci, V.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.; Basmanav, F.B.; Aydin, E. Nanobiomaterials: A Review of the Existing Science and Technology, and New Approaches. J. Biomater. Sci. 2006, 17, 1241–1268. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A Review on Nanomaterials for Environmental Remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Mi, J.L.; Nørby, P.; Bremholm, M.; Becker, J.; Iversen, B.B. The Formation Mechanism of Bimetallic PtRu Alloy Nanoparticles in Solvothermal Synthesis. Nanoscale 2015, 7, 16170–16174. [Google Scholar] [CrossRef] [PubMed]
- Veronico, L.; Gentile, L. Removal of Pollutants by Ferrihydrite Nanoparticles Combined with Brij L4 Self-Assembled Nanostructures. ACS Appl. Nano Mater. 2023, 6, 720–728. [Google Scholar] [CrossRef]
- Ganesan, S.; Lakshmisankar, S.; Deepak, S.; Selvakumar, M.; Venkatesan, S.P.; Hemanandh, J. Environmental Impact of One-Step Hydrothermal Green Synthesized ZnO Nanoparticles in DI Diesel Engine Performance Analysis Using Duel Fuel. Nanotechnol. Environ. Eng. 2023, 8, 361–375. [Google Scholar] [CrossRef]
- Muraro, P.C.L.; Wouters, R.D.; Pavoski, G.; Espinosa, D.C.R.; Ruiz, Y.P.M.; Galembeck, A.; Rech, V.C.; da Silva, W.L. Ag/TiNPS Nanocatalyst: Biosynthesis, Characterization and Photocatalytic Activity. J. Photochem. Photobiol. A Chem. 2023, 439, 114598. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and Removal of Titanium at Full Scale Wastewater Treatment Plants: Implications for TiO2 Nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments. Acc. Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef]
- Gallocchio, F.; Biancotto, G.; Moressa, A.; Pascoli, F.; Pretto, T.; Toffan, A.; Arcangeli, G.; Montesi, F.; Peters, R.; Ricci, A. Bioaccumulation and in Vivo Formation of Titanium Dioxide Nanoparticles in Edible Mussels. Food Chem. 2020, 323, 126841. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.S.N.; Kumar, T.R.P.; Rajasimman, M.; Hosseini-Bandegharaei, A.; et al. A Focus to Green Synthesis of Metal/Metal Based Oxide Nanoparticles: Various Mechanisms and Applications towards Ecological Approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Alzahrani, H.A.H.; Almulaiky, Y.Q.; Alsaiari, A.O. The Photocatalytic Dye Degradation of Methylene Blue (MB) by Nanostructured ZnO under UV Irradiation. Phys. Scr. 2023, 98, 045703. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Al-Sabahi, J.; Nejad, S.A.T.; Heydari, M.; Al-Abri, M.; Namaeighasemi, A. Visible-Light-Driven Super-Active Sn and GO Single- and Sn/Cu Co-Doped Nanophotocatalysts for Phenol Degradation: Thin-Film Printability, Thermal Stability, and Cytotoxicity Assay. J. Ind. Eng. Chem. 2023, 120, 514–528. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H.; El-Sesy, M.E. Adsorptive Removal of Levofloxacin and Antibiotic Resistance Genes from Hospital Wastewater by Nano-Zero-Valent Iron and Nano-Copper Using Kinetic Studies and Response Surface Methodology. Bioresour. Bioprocess. 2023, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, R.; Vignesh, S.; Balachandar, R.; Suganthi, S.; Raj, V.; Ramasundaram, S.; Kalyana Sundar, J.; Shkir, M.; Oh, T.H. Construction of Novel G-C3N4 Coupled Efficient Bi2O3 Nanoparticles for Improved Z-Scheme Photocatalytic Removal of Environmental Wastewater Contaminant: Insight Mechanism. J. Environ. Manag. 2023, 330, 117134. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Anushkkaran, P.; Hwang, I.S.; Chae, W.S.; Lee, H.H.; Choi, S.H.; Mahadik, M.A.; Jang, J.S. Synergistic Role of In-Situ Zr-Doping and Cobalt Oxide Cocatalysts on Photocatalytic Bacterial Inactivation and Organic Pollutants Removal over Template-Free Fe2O3 Nanorods. Chemosphere 2023, 310, 136825. [Google Scholar] [CrossRef]
- Choi, C.J.; Dong, X.L.; Kim, B.K. Characterization of Fe and Co Nanoparticles Synthesized by Chemical Vapor Condensation. Scr. Mater. 2001, 44, 2225–2229. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Nano-Fe0 Encapsulated in Microcarbon Spheres: Synthesis, Characterization, and Environmental Applications. ACS Appl. Mater. Interfaces 2012, 4, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Zhu, K.; Wang, X. Nanoscale Zero-Valent Iron Particles Modified on Reduced Graphene Oxides Using a Plasma Technique for Cd(II) Removal. J. Taiwan. Inst. Chem. Eng. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- Amerhaider Nuar, N.N.; Siti Nurul, S.N.A.; Choong, T.S.Y.; Mat Azmi, I.D.; Abdul Romli, N.A.; Abdullah, L.C.; Chiang, P.C.; Li, F. Synthesis of Calcium Peroxide Nanoparticles with Starch as a Stabilizer for the Degradation of Organic Dye in an Aqueous Solution. Polymers 2023, 15, 1327. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Manikandan, V.; Rajkumar, C.; Hatamleh, A.A.; Alnafisi, B.K.; Easwaran, G.; Liu, X.; Sivakumar, K.; Kim, H. Constructing Z-Scheme g-C3N4/TiO2 Heterostructure for Promoting Degradation of the Hazardous Dye Pollutants. Chemosphere 2023, 311, 136928. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Jiang, Z.; Wang, J.; Yao, Z. A Facile Preparation of Hierarchical Dendritic Zero-Valent Iron for Fenton-like Degradation of Phenol. Catal. Commun. 2017, 100, 57–61. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Elshobary, M.; Abdullah, E.; Abdel-Basset, R.; Metwally, M. Application of a Novel Biological-Nanoparticle Pretreatment to Oscillatoria Acuminata Biomass and Coculture Dark Fermentation for Improving Hydrogen Production. Microb. Cell Fact. 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Ribas, D.; Pešková, K.; Jubany, I.; Parma, P.; Černik, M.; Benito, J.A.; Martí, V. High Reactive Nano Zero-Valent Iron Produced via Wet Milling through Abrasion by Alumina. Chem. Eng. J. 2019, 366, 235–245. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on Nano Zerovalent Iron (NZVI): From Synthesis to Environmental Applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Gui, S.; Chen, G.; Wang, Y.; Wang, Z.; Zheng, X.; Meng, S.; Ruan, C.; Chen, S. Photothermal Synergistic Engineering of CeO2 and Au Co-Modified VO2 for Efficient and Selective Oxidation of Aromatic Alcohols. Appl. Surf. Sci. 2023, 611, 155616. [Google Scholar] [CrossRef]
- Li, W.W.; Cheng, L.; Liu, J.; Yang, S.Y.; Zan, S.T.; Zhao, G.C. Recyclable Magnetic Fe3O4@C for Methylene Blue Removal under Microwave-Induced Reaction System. Chemosphere 2023, 310, 136821. [Google Scholar] [CrossRef]
- Shi, X.; Li, L.; Cao, W.; Xuan, X.; Zheng, J.; Wang, C. Piezoelectric Assisted Photocatalytic Degradation of Dyes by Plasmon Ag Nanoparticles Modified Na0.5Bi0.5TiO3 Nanospheres. Mater. Lett. 2023, 335, 133827. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Song, Y.; Xu, X.; Cai, M.; Li, P.; Yuan, W.; Xiahou, Y. Functionalized Agarose Hydrogel with in Situ Ag Nanoparticles as Highly Recyclable Heterogeneous Catalyst for Aromatic Organic Pollutants. Environ. Sci. Pollut. Res. 2023, 30, 43950–43961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, K.; Liu, X.; Qiao, X.; Wang, J. Simplified and Efficient Inkjet Printing of Cotton Fabrics Using Cationic Colored Nanoparticles. Ind. Crops Prod. 2023, 193, 116217. [Google Scholar] [CrossRef]
- Zahid, M.; Segar, A.S.M.; Al-Majmaie, S.; Shather, A.H.; Khan, M.F.; Alguno, A.C.; Capangpangan, R.Y.; Ismail, A. Elettaria Cardamomum Seed Extract Synthesized Silver Nanoparticles for Efficient Catalytic Reduction of Toxic Dyes. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100809. [Google Scholar] [CrossRef]
- Pandey, K.; Saha, S. Encapsulation of Zero Valent Iron Nanoparticles in Biodegradable Amphiphilic Janus Particles for Groundwater Remediation. J. Hazard. Mater. 2023, 445, 130501. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, G.; Kumar, P.S.; Akilandeswari, S.; Rangasamy, G.; Mandal, A.; Shankar, V.U.; Ramya, M.; Nirmala, K.; Thirumalai, K. A Synergistic Consequence of Catalyst Dosage, PH Solution and Reactive Species of Fe-Doped CdAl2O4 Nanoparticles on the Degradation of Toxic Environmental Pollutants. Chemosphere 2023, 318, 137919. [Google Scholar] [CrossRef] [PubMed]
- Waheed, I.F.; Hamad, M.A.; Jasim, K.A.; Gesquiere, A.J. Degradation of Methylene Blue Using a Novel Magnetic CuNiFe2O4/g-C3N4 Nanocomposite as Heterojunction Photocatalyst. Diam. Relat. Mater. 2023, 133, 109716. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; He, Y.; Sun, L.; Hou, P.; Gan, Z.; Yu, L.; Dong, L. Design and Synthesis of Black Phosphorus Quantum Dot Sensitized Inverse Opal TiO2 Photonic Crystal with Outstanding Photocatalytic Activities. Appl. Surf. Sci. 2023, 609, 155442. [Google Scholar] [CrossRef]
- Zheng, C.; Song, X.; Gan, Q.; Lin, J. High-Efficiency Removal of Organic Pollutants by Visible-Light-Driven Tubular Heterogeneous Micromotors through a Photocatalytic Fenton Process. J. Colloid. Interface Sci. 2023, 630, 121–133. [Google Scholar] [CrossRef]
- Gadge, S.; Tamboli, A.; Shinde, M.; Fouad, H.; Terashima, C.; Chauhan, R.; Gosavi, S. Sonocatalytic Degradation of Methylene Blue Using Spindle Shaped Cerium Oxide Nanoparticles. J. Solid. State Electrochem. 2023, 27, 2005–2015. [Google Scholar] [CrossRef]
- Al-Arjan, W.S. Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment. Polymers 2023, 15, 340. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, S.; Hu, H.; He, Z.; Dong, F.; Tang, J.; Lu, X.; Wang, L.; Song, S.; Ma, J. Catalytic Ozonation of Atrazine with Stable Boron-Doped Graphene Nanoparticles Derived from Waste Polyvinyl Alcohol Film: Performance and Mechanism. Chem. Eng. J. 2023, 455, 140316. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, H.; Wang, Y.; Xiao, Y.; Cheng, Y. Quaternary Ammonium Groups Modified Magnetic Cyclodextrin Polymers for Highly Efficient Dye Removal and Sterilization in Water Purification. Molecules 2023, 28, 167. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Khazani, Y.; Khaloo, S.S.; Ghalkhani, M. Highly Effectual Photocatalytic Degradation of Tartrazine by Using Ag Nanoparticles Decorated on Zn-Cu-Cr Layered Double Hydroxide@ 2D Graphitic Carbon Nitride (C3N5). Environ. Sci. Pollut. Res. 2023, 30, 12903–12915. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; He, Y.; Liu, X.; He, C. Honeycomb-like Biochar Framework Coupled with Fe3O4/FeS Nanoparticles as Efficient Heterogeneous Fenton Catalyst for Phenol Degradation. J. Environ. Sci. 2024, 136, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ming, F.; Wang, J.; Xu, L. Amino Acids Modified Nanoscale Zero-Valent Iron: Density Functional Theory Calculations, Experimental Synthesis and Application in the Fenton-like Degradation of Organic Solvents. J. Environ. Sci. 2024, 135, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Qi, Z.; Dong, W.; Chen, Q.; Wu, M.; Yi, P.; Pan, B.; Xing, B. Aggregation of Biochar Nanoparticles and the Impact on Bisphenol A Sorption: Experiments and Molecular Dynamics Simulations. Sci. Total Environ. 2023, 875, 162724. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, L. A Carbon Based-Screen-Printed Electrode Amplified with Two-Dimensional Reduced Graphene/Fe3O4 Nanocomposite as Electroanalytical Sensor for Monitoring 4-Aminophenol in Environmental Fluids. Chemosphere 2023, 323, 138238. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cui, J.; Wang, Y.; Jia, J. An Ultrasensitive Electrochemical Biosensor for Bisphenol A Based on Aptamer-Modified MrGO@AuNPs and SsDNA-Functionalized AuNP@MBs Synergistic Amplification. Chemosphere 2023, 311, 137154. [Google Scholar] [CrossRef]
- Naderi, A.; Hasham Firooz, M.; Gharibzadeh, F.; Giannakis, S.; Ahmadi, M.; Rezaei Kalantary, R.; Kakavandi, B. Anchoring ZnO on Spinel Cobalt Ferrite for Highly Synergic Sono-Photo-Catalytic, Surfactant-Assisted PAH Degradation from Soil Washing Solutions. J. Environ. Manag. 2023, 326, 116584. [Google Scholar] [CrossRef]
- Li, S.; Feng, D.; Liu, J.; Liu, Q.; Tang, J. Surfactant-Enhanced Reduction of Soil-Adsorbed Nitrobenzene by Carbon-Coated NZVI: Enhanced Desorption and Mechanism. Sci. Total Environ. 2023, 856, 159186. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, I.; Chowdhury, A.; Rao, A.; Roy, B.; Chattopadhyay, P. Assessment of Bimetallic Zn/Fe0 Nanoparticles Stabilized Tween-80 and Rhamnolipid Foams for the Remediation of Diesel Contaminated Clay Soil. J. Environ. Manag. 2023, 325, 116596. [Google Scholar] [CrossRef] [PubMed]
- Banu, T.; Rashid, M.H.; Tofail, S.A.M.; Haq, E.U.; Gulshan, F. Photocatalytic Degradation of Metronidazole (MNZ) Antibiotic by CuO Nanoparticles for Environmental Protection from Pharmaceutical Pollution. Surf. Interface Anal. 2023, 55, 430–436. [Google Scholar] [CrossRef]
- Rawashdeh, R.Y.; Qabaja, G.; Albiss, B.A. Antibacterial Activity of Multi-Metallic (Ag–Cu–Li) Nanorods with Different Metallic Combination Ratios against Staphylococcus Aureus. BMC Res. Notes 2023, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Arshad, M.; Chuang, Y.; Hong, Y.L.; Nguyen, T.B.; Wu, C.H.; Chen, C.W.; Dong, C. Di Facile Fabrication of Efficient Tungsten Disulfide Nanoparticles for Enhanced Photocatalytic Removal of Tetracycline (TC) and Pb (II) Photoreduction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131004. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Li, L.; Jia, L. Fabrication of Alginate-Based Nanofibers Loaded with ZnO Nanoparticles for Adsorption of Tetracyclines from Environmental Waters. Mater. Today Commun. 2023, 34, 105214. [Google Scholar] [CrossRef]
- Gupta, G.; Kansal, S.K.; Umar, A.; Akbar, S. Visible-Light Driven Excellent Photocatalytic Degradation of Ofloxacin Antibiotic Using BiFeO3 Nanoparticles. Chemosphere 2023, 314, 137611. [Google Scholar] [CrossRef] [PubMed]
- Hemmati-Eslamlu, P.; Habibi-Yangjeh, A.; Khataee, A. Anchoring Spinel NiCr2O4 Nanoparticles on Tubular G-C3N4: Efficacious p-n Heterojunction Photocatalysts for Removal of Tetracycline Hydrochloride under Visible Light. J. Alloys Compd. 2023, 932, 167571. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.J.; Murugesan, T.; Anthuvan, A.J.; Kumar, R.R.; Ahmed, F.; Arshi, N. Structural Growth of Zinc Oxide Nanograins on Carbon Cloth as Flexible Electrochemical Platform for Hydroxychloroquine Detection. Chemosphere 2023, 312, 137186. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Zhang, T.; Zhang, J.; Xia, D.; Du, Y.; Jiang, Y.; Lin, K. Construction of Ag Nanocluster-Modified Ag3PO4 Containing Silver Vacancies via in-Situ Reduction: With Enhancing the Photocatalytic Degradation Activity of Sulfamethoxazole. J. Colloid. Interface Sci. 2023, 629, 989–1002. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. Gold-Nanoparticle-Based Multiplex Immuno-Strip Biosensor for Simultaneous Determination of 83 Antibiotics. Nano Res. 2023, 16, 1259–1268. [Google Scholar] [CrossRef]
- Ma, S.; Luo, X.; Kong, J.; Li, X.; Cao, Z.; Wang, X.; Cai, W.; Wang, L.; Ran, G. Plasmonic Silver Loaded Hybrid Bi-Ag Nanoalloys for Highly Efficient Disinfection by Enhancing Photothermal Performance and Interface Capability. Chem. Eng. J. 2022, 450, 138016. [Google Scholar] [CrossRef]
- Ahmed Shehab, M.; Szőri-Dorogházi, E.; Szabó, S.; Valsesia, A.; Chauhan, T.; Koós, T.; Muránszky, G.; Szabó, T.; Hernadi, K.; Németh, Z. Virus and Bacterial Removal Ability of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Arab. J. Chem. 2023, 16, 104388. [Google Scholar] [CrossRef]
- Ghani, S.B.A.; Al-Azzazy, M.M.; Alhewairini, S.S.; Al-Deghairi, M.A. The Miticidal Activity of Silver Nanoparticles towards Date Palm Mite (Oligonychus Afrasiaticus (McGregor)): Efficacy, Selectivity, and Risk Assessment. Braz. J. Biol. 2022, 84, e261262. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zhu, J.; Yang, B.; Peng, L.; Lou, S. Ovalbumin-Coated Gold Nanoparticles with Interesting Colloidal Stability for Colorimetric Detection of Carbaryl in Complex Media. Food Chem. 2023, 403, 134485. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, N.B.; Ly, N.H.; Joo, S.W.; Vasseghian, Y. Core-Shell Au@ZIF-67-Based Pollutant Monitoring of Thiram and Carbendazim Pesticides. Environ. Pollut. 2023, 317, 120775. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Thepbandit, W.; Saengchan, C.; Papathoti, N.K.; Treekoon, J.; Kamkaew, A.; Phansak, P.; Buensanteai, K. The Effect of Chitosan Nanoparticle Formulations for Control of Leaf Spot Disease on Cassava. Phytoparasitica 2023, 51, 621–636. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X.; Zou, A.; Shi, Z.; Xiang, S.; Xu, J.; Cai, L.; Huang, J.; Sun, X. Chitin Nanocrystals Supported Copper: A New Nanomaterial with High Activity with P. Syringae Pv. Tabaci. Pest. Manag. Sci. 2023, 79, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahari, H.; Zhang, Z.; Jafari, S.M. Role of Silica (SiO2) Nano/Micro-Particles in the Functionality of Degradable Packaging Films/Coatings and Their Application in Food Preservation. Trends Food Sci. Technol. 2023, 133, 75–86. [Google Scholar] [CrossRef]
- Bouzidi, I.; Sellami, B.; Boulanger, A.; Joyeux, C.; Harrath, A.H.; Albeshr, M.F.; Pacioglu, O.; Boufahja, F.; Beyrem, H.; Mougin, K. Metallic Nanoparticles Affect Uptake of Polycyclic Aromatic Hydrocarbons and Impacts in the Mediterranean Mussels Mytilus Galloprovincialis. Mar. Pollut. Bull. 2023, 188, 114641. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Z.; Xia, C.; Zheng, J.; Gu, Y.; He, F. A Quantitative Study of the Effects of Particle’ Properties and Environmental Conditions on the Electron Efficiency of Pd and Sulfidated Nanoscale Zero-Valent Irons. Sci. Total Environ. 2022, 853, 158469. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.A.; Chen, P.J. Exploring Specific Biomarkers Regarding Neurobehavioral Toxicity of Lead Dioxide Nanoparticles in Medaka Fish in Different Water Matrices. Sci. Total Environ. 2023, 856, 159268. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in Transport and Toxicity of Nanoparticles in Plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Sun, X.; Shi, Y.; Chen, L.; Wang, L.; Cai, H.; Han, C.; Liao, T.; Yang, C.; Zuo, Z.; et al. The Valence State of Iron-Based Nanomaterials Determines the Ferroptosis Potential in a Zebrafish Model. Sci. Total Environ. 2023, 855, 158715. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubian, M.; Naeemi, A.S.; Moradi-Shoeili, Z.; Tyler, C.R.; Mansouri, B. Toxicity of Silver Nanoparticles in the Presence of Zinc Oxide Nanoparticles Differs for Acute and Chronic Exposures in Zebrafish. Arch. Environ. Contam. Toxicol. 2023, 84, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, H.; Shao, Z.; Zhou, H.; Lu, J.; Chen, J.; Huang, C.; Zhang, S.; Liu, X.; Xia, L.; et al. Converting Plastic Wastes to Naphtha for Closing the Plastic Loop. J. Am. Chem. Soc. 2023, 145, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Haider, G.; Zia, M.; Shah, G.A.; Iqbal, Z.; Hayat, A. Development and Testing of Zinc Sulfate and Zinc Oxide Nanoparticle-Coated Urea Fertilizer to Improve N and Zn Use Efficiency. Front. Plant Sci. 2023, 13, 1058219. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Xu, J.; Yang, K.; Lin, D. Novel Oxymagnesite/Green Rust Nanohybrids for Selective Removal and Slow Release of Phosphate in Water. Sci. Total Environ. 2023, 856, 159207. [Google Scholar] [CrossRef] [PubMed]
- Triolo, C.; Santangelo, S.; Petrovičovà, B.; Musolino, M.G.; Rincón, I.; Atxirika, A.; Gil, S.; Belaustegui, Y. Evaluation of the Specific Capacitance of High-Entropy Oxide-Based Electrode Materials in View of Their Use for Water Desalination via Capacitive Method. Appl. Sci. 2023, 13, 721. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, L.; Ouyang, Y.; Xu, F.; Zou, Y.; Chu, H.; Zhang, K.; Li, B.; Pan, H. Improved Electrochemical Water Splitting by RuNi Nanoparticles Supported on RGO@mesoporous N-Doped Carbon Nanosheets. J. Alloys Compd. 2023, 937, 168334. [Google Scholar] [CrossRef]
- Benjak, P.; Radetić, L.; Tomaš, M.; Brnardić, I.; Radetić, B.; Špada, V.; Grčić, I. Rubber Tiles Made from Secondary Raw Materials with Immobilized Titanium Dioxide as Passive Air Protection. Processes 2023, 11, 125. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shi, W.D.; Yan, W.C. Electric Field Assisted Assembly of Nanoparticle Loaded Microspheres toward Industrial Applications for Organic Dye Removal. Sep. Purif. Technol. 2023, 306, 122565. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Tu, Y.; Liu, Y.; Huang, J.; Yin, X.; Feng, Y. Preparation of Reusable UHMWPE/TiO2 Photocatalytic Microporous Membrane Reactors for Efficient Degradation of Organic Pollutants in Water. Sep. Purif. Technol. 2023, 305, 122515. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.; Shu, L.; Ke, S.; He, Q.; Zhao, Q.; Ke, Q. The Disinhibition Effect of Iron-Based Particles on Anaerobic Digestion of Florfenicol-Containing Cow Manure: Performance and Mechanism. Environ. Res. 2023, 223, 115471. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, A.; Borymski, S.; Adamek, A.; Sułowicz, S. The Influence of ZnO Nanoparticles on Horizontal Transfer of Resistance Genes in Lab and Soil Conditions. Environ. Res. 2023, 223, 115420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, H.; Yang, K.; Zhu, L. Attenuation of Tetracyclines and Related Resistance Genes in Soil When Exposed to Nanoscale Zero-Valent Iron. J. Hazard. Mater. 2023, 448, 130867. [Google Scholar] [CrossRef] [PubMed]
- Senbill, H.; Hassan, S.M.; Eldesouky, S.E. Acaricidal and Biological Activities of Titanium Dioxide and Zinc Oxide Nanoparticles on the Two-Spotted Spider Mite, Tetranychus Urticae Koch (Acari: Tetranychidae) and Their Side Effects on the Predatory Mite, Neoseiulus Californicus (Acari: Phytoseiidae). J. Asia Pac. Entomol. 2023, 26, 102027. [Google Scholar] [CrossRef]
- Wang, Z.; Bi, Y.; Li, K.; Song, Z.; Pan, C.; Zhang, S.; Lan, X.; Foulkes, N.S.; Zhao, H. Nickel Oxide Nanoparticles Induce Developmental Neurotoxicity in Zebrafish by Triggering Both Apoptosis and Ferroptosis. Environ. Sci. Nano 2023, 10, 640–655. [Google Scholar] [CrossRef]
- Bakouei, M.; Sadeghizadeh Yazdi, J.; Ehrampoush, M.H.; Salari, M.; Madadizadeh, F. The Effect of Active Chitosan Films Containing Bacterial Cellulose Nanofiber and ZnO Nanoparticles on the Shelf Life of Loaf Bread. J. Food Qual. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Alvan, Z.B.A.; Asgari, H.M.; Amanipoor, H.; Buazar, F.; Motaghed, S. Evaluation of the Effects of Zero-Valent Iron Nanoparticles in the Treatment of Soils Polluted with Refinery Effluent Hydrocarbons. Water Air Soil. Pollut. 2023, 234, 40. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Evaluation of the Gut Microbiome Alterations in Healthy Rats after Dietary Exposure to Different Synthetic ZnO Nanoparticles. Life Sci. 2023, 312, 121250. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, M.; Khalid, N.; Nazir, A.; Irshad, M.K.; Hakami, O.; Basahi, M.A.; Alamri, S.; Hashem, M.; Noman, A. Foliar Application of Silver Nanoparticles Mitigated Nutritional and Biochemical Perturbations in Chilli Pepper Fertigated with Domestic Wastewater. Plant Physiol. Biochem. 2023, 194, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Du, C.; Wang, Y.; He, K.; Zheng, H.; Xue, Z.; Chen, Q.; Li, X. Various Hydrogen Bonds Make Different Fates of Pharmaceutical Contaminants on Oxygen-Rich Nanomaterials. Environ. Pollut. 2023, 316, 120572. [Google Scholar] [CrossRef] [PubMed]
- Albarano, L.; Toscanesi, M.; Trifuoggi, M.; Guida, M.; Lofrano, G.; Libralato, G. In Situ Microcosm Remediation of Polyaromatic Hydrocarbons: Influence and Effectiveness of Nano-Zero Valent Iron and Activated Carbon. Environ. Sci. Pollut. Res. 2023, 30, 3235–3251. [Google Scholar] [CrossRef] [PubMed]
- Nekoukhou, M.; Fallah, S.; Abbasi-Surki, A.; Pokhrel, L.R.; Rostamnejadi, A. Improved Efficacy of Foliar Application of Zinc Oxide Nanoparticles on Zinc Biofortification, Primary Productivity and Secondary Metabolite Production in Dragonhead. J. Clean. Prod. 2022, 379, 134803. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, C.; Liu, Y.; Tan, X.; Li, X.; Shi, Y.; Ding, C. The Interaction of ZnO Nanoparticles, Cr(VI), and Microorganisms Triggers a Novel ROS Scavenging Strategy to Inhibit Microbial Cr(VI) Reduction. J. Hazard. Mater. 2023, 443, 130375. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S.; et al. Physiological and Transcriptomic Study Reveal SeNPs-Mediated AsIII Stress Detoxification Mechanisms Involved Modulation of Antioxidants, Metal Transporters, and Transcription Factors in Glycine Max L. (Merr.) Roots. Environ. Pollut. 2023, 317, 120637. [Google Scholar] [CrossRef] [PubMed]
- Moghazy, M.A. Leidenfrost Green Synthesis Method for MoO3 and WO3 Nanorods Preparation: Characterization and Methylene Blue Adsorption Ability. BMC Chem. 2023, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, S.; Kumar, K.J.; Daimari, J.; Mondal, A.; Kalita, S.; Dey, K.S.; Deka, A.K. Biosynthesis of Silver Nanoparticles Using Antidesma Acidum Leaf Extract: Its Application in Textile Organic Dye Degradation. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100769. [Google Scholar] [CrossRef]
- Faryad, S.; Azhar, U.; Tahir, M.B.; Ali, W.; Arif, M.; Sagir, M. Spinach-Derived Boron-Doped g-C3N4/TiO2 Composites for Efficient Photo-Degradation of Methylene Blue Dye. Chemosphere 2023, 320, 138002. [Google Scholar] [CrossRef]
- Rather, M.Y.; Shincy, M.; Sundarapandian, S. Photocatalytic Degradation of Rhodamine-B by Phytosynthesized Gold Nanoparticles. Int. J. Environ. Sci. Technol. 2023, 20, 4073–4084. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Custódio, D.A.C.; Dorneles, E.M.S.; Coura, F.M.; Carvalho, I.C.; Lage, A.P.; Mansur, H.S. Nanoplexes of ZnS Quantum Dot-Poly-L-Lysine/Iron Oxide Nanoparticle-Carboxymethylcellulose for Photocatalytic Degradation of Dyes and Antibacterial Activity in Wastewater Treatment. Int. J. Biol. Macromol. 2023, 231, 123363. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nancy; Singh, G.; Singh, J. Sustainable Synthesis of Biogenic ZnO NPs for Mitigation of Emerging Pollutants and Pathogens. Environ. Res. 2023, 219, 114952. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Singh, N.B.; Agarwal, A. Iron-Rich Coal Fly Ash-Polydopamine-Silver Nanocomposite (IRCFA-PDA-Ag NPs): Tailored Material for Remediation of Methylene Blue Dye from Aqueous Solution. Environ. Monit. Assess. 2023, 195, 322. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.; Ali, K.; Naqvi, S.A.Z.; Maqsood, M.A.; Bashir, M.Z.; Noreen, S. Biosynthesis of Leucaena Leucocephala Leaf Mediated ZnO, CuO, MnO2, and MgO Based Nano-Adsorbents for Reactive Golden Yellow-145 (RY-145) and Direct Red-31 (DR-31) Dye Removal from Textile Wastewater to Reuse in Agricultural Purpose. Sep. Purif. Technol. 2023, 306, 122527. [Google Scholar] [CrossRef]
- Gautam, I.; Grady, T.; Fernando, H. Degradation of the Dye Methyl Orange Using Cow and Goat Milk Iron Nanoparticles. Green Chem. Lett. Rev. 2023, 16, 2174818. [Google Scholar] [CrossRef]
- Bhandari, Y.; Varma, S.; Sawant, A.; Beemagani, S.; Jaiswal, N.; Chaudhari, B.P.; Vamkudoth, K.R. Biosynthesis of Gold Nanoparticles by Penicillium Rubens and Catalytic Detoxification of Ochratoxin A and Organic Dye Pollutants. Int. Microbiol. 2023, 26, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.B.; Dhengale, S.D.; Nille, O.S.; Jadhav, S.S.; Gore, A.H.; Bhosale, T.R.; Birajdar, N.B.; Kolekar, S.S.; Kolekar, G.B.; Anbhule, P.V. Template Free Synthesis of Mesoporous Carbon from Fire Cracker Waste and Designing of ZnO-Mesoporous Carbon Photocatalyst for Dye (MO) Degradation. Inorg. Chem. Commun. 2023, 147, 110242. [Google Scholar] [CrossRef]
- Manikanika; Chopra, L. Photo-Degradation of Dyes and Drugs Using Aloe Vera Synthesized Zinc Oxide Nanoparticles—A Review. Mater. Today Proc. 2023, 72, 1613–1617. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Le, P.T.; Nguyen, T.A.; Doan, N.N.; No, K. Biochar from Cyperus Alternifolius Linn.: From a Waste of Phytoremediation Processing to Efficient Depolluting Agent. Environ. Sci. Pollut. Res. 2023, 30, 1898–1907. [Google Scholar] [CrossRef]
- Aryafar, A.; Ekrami-Kakhki, M.S.; Naeimi, A. Enhanced Electrocatalytic Activity of Pt-SnO2 Nanoparticles Supported on Natural Bentonite-Functionalized Reduced Graphene Oxide-Extracted Chitosan from Shrimp Wastes for Methanol Electro-Oxidation. Sci. Rep. 2023, 13, 3597. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yu, S.; Xue, N.; Li, T.; Sun, M.; Zhang, L. Activation of Iron Based Persulfate Heterogeneous Nano Catalyst Using Plant Extract for Removal of Tetrabromobisphenol A from Soil. J. Environ. Chem. Eng. 2023, 11, 109493. [Google Scholar] [CrossRef]
- Das, S.; Panicker, N.J.; Rather, M.A.; Mandal, M.; Sahu, P.P. Green Synthesis of Cerium Oxide Nanoparticles Using Dillenia Indica Aqueous Extract and Its Anti-Oxidant Activity. Bull. Mater. Sci. 2023, 46, 3. [Google Scholar] [CrossRef]
- Doan, V.D.; Le, V.T.; Tran, D.L.; Nguyen, T.L.H.; Nguyen, D.C.; Nguyen, A.T.; Le, V.T. Catalytic Reduction of Nitrophenols Using Gnetum Montanum Extract Capped Silver Nanoparticles. Mol. Catal. 2023, 534, 112804. [Google Scholar] [CrossRef]
- Ganesan, S.; Nijohn, J.M.; lakshmisankar, S.; Venkatesan, S.P.; Hemanandh, J.; Muniappan, P. Environmental Analysis of One-Step Hydrothermal Green Synthesized MgO Nanoparticles on VCR Diesel Engine Performance Using Jatropha Oil and Lemongrass Oil. Nanotechnol. Environ. Eng. 2023, 8, 599–613. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Tan, Y.; Fu, L.; Qing, W. Preparation of Transition Metal Ions (Fe2+, Co2+ and Ni2+) Doped Carbon Nanoparticles from Biowaste for Cystine and Cr(VI) Detection and Fluorescence Ink. Inorg. Chem. Commun. 2023, 147, 110220. [Google Scholar] [CrossRef]
- Wali, S.; Zahra, M.; Okla, M.K.; Wahidah, H.A.; Tauseef, I.; Haleem, K.S.; Farid, A.; Maryam, A.; Abdelgawad, H.; Adetunji, C.O.; et al. Brassica Oleracea L. (Acephala Group) Based Zinc Oxide Nanoparticles and Their Efficacy as Antibacterial Agent. Braz. J. Biol. 2024, 84, e259351. [Google Scholar] [CrossRef]
- Ghasemi, S.; Harighi, B.; Ashengroph, M. Biosynthesis of Silver Nanoparticles Using Pseudomonas Canadensis, and Its Antivirulence Effects against Pseudomonas Tolaasii, Mushroom Brown Blotch Agent. Sci. Rep. 2023, 13, 3668. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhu, W.; Lu, Y.; Ren, Y.; Gu, J.; Song, Y.; He, J. Alpinia Officinarum Mediated Copper Oxide Nanoparticles: Synthesis and Its Antifungal Activity against Colletotrichum Gloeosporioides. Environ. Sci. Pollut. Res. 2023, 30, 28818–28829. [Google Scholar] [CrossRef]
- Nikishina, M.B.; Ivanova, E.V.; Tretyakova, A.V.; Mukhtorov, L.G.; Atroshchenko, Y.M. Study of Biological Activity Colloidal Solutions of Iron Synthesized on the Basis of Aqueous Cuff Extract. Sib. J. Life Sci. Agric. 2022, 14, 388–403. [Google Scholar] [CrossRef]
- Palanisamy, D.S.; Gounder, B.S.; Selvaraj, K.; Kandhasamy, S.; Alqahtani, T.; Alqahtani, A.; Chidambaram, K.; Arunachalam, K.; Alkahtani, A.M.; Chandramoorthy, H.C.; et al. Synergistic Antibacterial and Mosquitocidal Effect of Passiflora Foetida Synthesized Silver Nanoparticles. Braz. J. Biol. 2024, 84, 263391. [Google Scholar] [CrossRef] [PubMed]
- Nawab, R.; Ali, M.; Haroon, U.; Kamal, A.; Akbar, M.; Anwar, F.; Ahmed, J.; Chaudhary, H.J.; Iqbal, A.; Hashem, M.; et al. Calotropis procera (L.) Mediated Synthesis of AgNPs and Their Application to Control Leaf Spot of Hibiscus rosa-sinensis (L.). Braz. J. Biol. 2024, 84, 261123. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.J.; Nibir, A.I.S.; Ahmed, F.R.S. Biosensing and Anti-Inflammatory Effects of Silver, Copper and Iron Nanoparticles from the Leaf Extract of Catharanthus Roseus. Beni Suef Univ. J. Basic Appl. Sci. 2023, 12, 26. [Google Scholar] [CrossRef]
- Agustin, M.B.; Lehtonen, M.; Kemell, M.; Lahtinen, P.; Oliaei, E.; Mikkonen, K.S. Lignin Nanoparticle-Decorated Nanocellulose Cryogels as Adsorbents for Pharmaceutical Pollutants. J. Environ. Manag. 2023, 330, 117210. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.I.; Tawfeek, N.; El-Shiekh, R.A.; Khalil, H.M.A.; Mahmoud, M.Y.; Bakr, A.F.; Zaafar, D.; Farrag, N.; Wink, M.; El-Shazly, A.M. Salix Subserrata Bark Extract-Loaded Chitosan Nanoparticles Attenuate Neurotoxicity Induced by Sodium Arsenate in Rats in Relation with HPLC–PDA-ESI–MS/MS Profile. AAPS PharmSciTech 2023, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Sar, S.K.; Jindal, M.K. Plant Mediated Magnetic Nano Composite as Promising Scavenger’s Radionuclides for the Efficient Remediation in Aqueous Medium. Chemosphere 2023, 312, 137246. [Google Scholar] [CrossRef] [PubMed]
- Karthik Chandan, A.; Neha Mallika, G.; Bala Narsaiah, T. A Green Approach to Arsenic Removal Using ZnO Nanoparticles Synthesized from Acacia Catechu Leaf Extract. Mater. Today Proc. 2023, 72, 110–119. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Lin, N.; Wang, R.; Wang, M.; Zhang, X. Iron-Based Materials for Simultaneous Removal of Heavy Metal(Loid)s and Emerging Organic Contaminants from the Aquatic Environment: Recent Advances and Perspectives. Environ. Pollut. 2022, 299, 118871. [Google Scholar] [CrossRef] [PubMed]
- Papolu, P.; Bhogi, A. Green Synthesis of Various Metal Oxide Nanoparticles for the Environmental Remediation—An Overview. Mater. Today Proc. 2023, 92, 924–927. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, K.; Shoji, R. A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (NZVI) from Fe2O3 for Water Remediation Application. Minerals 2023, 13, 1385. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Carvalho, F.; Fernandes, E. Iron Oxide Nanoparticles: An Insight into Their Biomedical Applications. Curr. Med. Chem. 2015, 22, 1808–1828. [Google Scholar] [CrossRef] [PubMed]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Gautam, S.; Bansal, D.; Bhatnagar, D.; Sharma, C.; Goyal, N. Synthesis of Iron-Based Nanoparticles by Chemical Methods and Their Biomedical Applications. In Oxides for Medical Applications; Woodhead Publishing: Sawston, UK, 2023; ISBN 978-0-32390-538-1. [Google Scholar] [CrossRef]
- Manohar, A.K.; Yang, C.; Malkhandi, S.; Prakash, G.K.S.; Narayanan, S.R. Enhancing the Performance of the Rechargeable Iron Electrode in Alkaline Batteries with Bismuth Oxide and Iron Sulfide Additives. J. Electrochem. Soc. 2013, 160, A2078–A2084. [Google Scholar] [CrossRef]
- Hayashi, K.; Maeda, Y.; Suzuki, T.; Sakamoto, H.; Kugimiya, T.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A. Development of Iron-Based Rechargeable Batteries with Sintered Porous Iron Electrodes. ECS Trans. 2016, 75, 111–116. [Google Scholar] [CrossRef]
- Yang, C.; Manohar, A.K.; Narayanan, S.R. A High-Performance Sintered Iron Electrode for Rechargeable Alkaline Batteries to Enable Large-Scale Energy Storage. J. Electrochem. Soc. 2017, 164, A418–A429. [Google Scholar] [CrossRef]
- Wei, D.; Darcel, C. Iron Catalysis in Reduction and Hydrometalation Reactions. Chem. Rev. 2019, 119, 2550–2610. [Google Scholar] [CrossRef] [PubMed]
- Rydel-Ciszek, K.; Pacześniak, T.; Zaborniak, I.; Błoniarz, P.; Surmacz, K.; Sobkowiak, A.; Chmielarz, P. Iron-Based Catalytically Active Complexes in Preparation of Functional Materials. Processes 2020, 8, 1683. [Google Scholar] [CrossRef]
- Palomo, J.M. Design of Iron Nanostructured Catalysts. In Iron Catalysis; World Scientific: Singapore, 2021; Volume 19. [Google Scholar] [CrossRef]
- Franken, T.; Heel, A. Are Fe Based Catalysts an Upcoming Alternative to Ni in CO2 Methanation at Elevated Pressure? J. CO2 Util. 2020, 39, 101175. [Google Scholar] [CrossRef]
- Phuong, D.H.L.; Alsaiari, M.; Pham, C.Q.; Hieu, N.H.; T․ Pham, T.-P.; Rajamohan, N.; Pham, D.D.; Vo, D.-V.N.; Trinh, T.H.; Setiabudi, H.D.; et al. Carbon Dioxide Reforming of Methane over Modified Iron-Cobalt Alumina Catalyst: Role of Promoter. J. Taiwan. Inst. Chem. Eng. 2024, 155, 105253. [Google Scholar] [CrossRef]
- Guo, N.; Zhu, S. Iron-Catalyzed Hydrogenation Reactions. Chin. J. Org. Chem. 2015, 35, 1383–1398. [Google Scholar] [CrossRef]
- Cheng, Y.; Pham, H.; Huo, J.; Johnson, R.; Datye, A.K.; Shanks, B. High Activity Pd-Fe Bimetallic Catalysts for Aqueous Phase Hydrogenations. Mol. Catal. 2019, 477, 110546. [Google Scholar] [CrossRef]
- Amiri, R.; Bourezgui, A.; Djeridi, W.; Dappozze, F.; Houas, A.; Guillard, C.; Elsellami, L. Surface Modification of TiO2 with a Less Expensive Metal (Iron) to Exploit Solar Energy in Photocatalysis: An Ecological and Economical Solution. Int. J. Hydrog. Energy 2024, 51, 638–647. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; Zhou, R.; Wang, J. Hydroxylamine Enhanced Activation of Peroxymonosulfate by Fe(III)/Cu(II) Bimetallic for High-Efficiency Degradation of AO7. Water Sci. Technol. 2022, 85, 2038–2050. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, S.; Li, J.; Gao, M. Preparation of CuFeO2 as Heterogeneous Fenton-like Catalyst and Its Degradation Performance on Ofloxacin in the Water. Chin. J. Environ. Eng. 2022, 16, 2144–2155. [Google Scholar] [CrossRef]
- Hou, Q.; Qin, L.; Peng, X.; Zhou, C.; Li, X.; Zhang, J.; Gao, L. Insight to an Efficient and Magnetic α-Fe2O3/γ-Fe2O3/Cu2O Hybrid Catalysis for Peroxymonosulfate: Preparation, Performance, and Mechanism. Res. Chem. Intermed. 2022, 48, 3753–3772. [Google Scholar] [CrossRef]
- Mendoza-Carrasco, R.; Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C.; Gómez-Serrano, V. Preparation of High-Quality Activated Carbon from Polyethyleneterephthalate (PET) Bottle Waste. Its Use in the Removal of Pollutants in Aqueous Solution. J. Environ. Manag. 2016, 181, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, X.; Zhang, Y.; Liu, N.; Hu, X. Corrosion Behaviors and Kinetics of Nanoscale Zero-Valent Iron in Water: A Review. J. Environ. Sci. 2024, 135, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Dias-Ferreira, C.; Ribeiro, A.B.; Pamukcu, S. Influence of Electrolyte and Voltage on the Direct Current Enhanced Transport of Iron Nanoparticles in Clay. Chemosphere 2014, 99, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Grieger, K.D.; Fjordbøge, A.; Hartmann, N.B.; Eriksson, E.; Bjerg, P.L.; Baun, A. Environmental Benefits and Risks of Zero-Valent Iron Nanoparticles (NZVI) for in Situ Remediation: Risk Mitigation or Trade-Off? J. Contam. Hydrol. 2010, 118, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, B.F. Recent Advances in Soil Remediation Using Nano Zero-Valent Iron (NZVI) Activated Peroxydisulfate (PDS) and Peroxymonosulfate (PMS). In Advances in Environmental Research; Nova Publishers: Hauppauge, NY, USA, 2023; Volume 94, ISBN 979-8-88697-665-6. [Google Scholar]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of Organic Compounds by Nanoscale Zero-Valent Iron and Its Composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, H.; Lv, F.; Yang, Y.; Chen, C.; Yang, T.; Zheng, H.; Wu, D. Preparation of NZVI Embedded Modified Mesoporous Carbon for Catalytic Persulfate to Degradation of Reactive Black 5. Front. Environ. Sci. Eng. 2021, 15, 98. [Google Scholar] [CrossRef]
- Soubh, A.M.; Abdoli, M.A.; Ahmad, L.A. Optimizing the Removal of Methylene Blue from Aqueous Solutions Using Persulfate Activated with Nanoscale Zero Valent Iron (nZVI) Supported by Reduced Expanded Graphene Oxide (REGO). Environ. Health Eng. Manag. 2021, 8, 15–24. [Google Scholar] [CrossRef]
- Li, Y.-T.; Liu, X.-Y.; Li, X.; Liu, H.; Du, W.-Y.; Chen, J.-L. Oxidative Degradation of Rhodamine B Solution with NZVI Persulfate Activation. Environ. Eng. Res. 2024, 29, 230405. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Molecular Oxygen Activation with Nano Zero-Valent Iron for Aerobic Degradation of Organic Contaminants and the Performance Enhancement. Acta Chim. Sin. 2017, 75, 538–543. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O.; Dada, F.E.; Bello, O.S.; Ogunlaja, A.S. Bottom-up Approach Synthesis of Core-Shell Nanoscale Zerovalent Iron (CS-NZVI): Physicochemical and Spectroscopic Characterization with Cu(II) Ions Adsorption Application. MethodsX 2020, 7, 100976. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, W.-X. Fine Structural Features of Nanoscale Zero-Valent Iron Characterized by Spherical Aberration Corrected Scanning Transmission Electron Microscopy (CS-STEM). Analyst 2014, 139, 4512–4518. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.K.; Rohith, V.K.; Theertharaman, G.; Navaneethan, M.; Balakumar, S. Probing the Influence of Liquid Nitrogen Assisted Chemical Reduction on the Nature of Passivation Layer, Magnetic Properties, and Cr (VI) Remediation Performance of Nanoscale Zero Valent Iron. J. Environ. Chem. Eng. 2023, 11, 109096. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, W.; Chen, X.; Fan, J.; Jiang, W.; Wang, L.; Jiang, W.; Zhang, W.-X.; Yang, J. Porous-Carbon-Confined Formation of Monodisperse Iron Nanoparticle Yolks toward Versatile Nanoreactors for Metal Extraction. Chem. Eur. J. 2018, 24, 15663–15668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Elliott, D.W.; Zhang, W.X. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid. State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Yan, W.; Herzing, A.A.; Kiely, C.J.; Zhang, W.-X. Nanoscale Zero-Valent Iron (NZVI): Aspects of the Core-Shell Structure and Reactions with Inorganic Species in Water. J. Contam. Hydrol. 2010, 118, 96–104. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Nemati, Z.; Khurshid, H.; Alonso, J.; Phan, M.H.; Mukherjee, P.; Srikanth, H. From Core/Shell to Hollow Fe/γ-Fe2O3 Nanoparticles: Evolution of the Magnetic Behavior. Nanotechnology 2015, 26, 405705. [Google Scholar] [CrossRef] [PubMed]
- De Montferrand, C.; Hu, L.; Milosevic, I.; Russier, V.; Bonnin, D.; Motte, L.; Brioude, A.; Lalatonne, Y. Iron Oxide Nanoparticles with Sizes, Shapes and Compositions Resulting in Different Magnetization Signatures as Potential Labels for Multiparametric Detection. Acta Biomater. 2013, 9, 6150–6157. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Siddiqui, V.U.; Akram, M.K.; Siddiqi, W.A.; Khan, A.; Al-Romaizan, A.N.; Hussein, M.A.; Puttegowda, M. Synthesis of Atmospherically Stable Zero-Valent Iron Nanoparticles (nZVI) for the Efficient Catalytic Treatment of High-Strength Domestic Wastewater. Catalysts 2022, 12, 26. [Google Scholar] [CrossRef]
- Carpenter, E.E.; Calvin, S.; Stroud, R.M.; Harris, V.G. Passivated Iron as Core-Shell Nanoparticles. Chem. Mater. 2003, 15, 3245–3246. [Google Scholar] [CrossRef]
- Barreto-Rodrigues, M.; Silveira, J.; Zazo, J.A.; Rodriguez, J.J. Synthesis, Characterization and Application of Nanoscale Zero-Valent Iron in the Degradation of the Azo Dye Disperse Red 1. J. Environ. Chem. Eng. 2017, 5, 628–634. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Recent Developments in Surface Modification of Nano Zero-Valent Iron (NZVI): Remediation, Toxicity and Environmental Impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Idris, D.S.; Roy, A. Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review. Crystals 2023, 13, 637. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Li, B.; Wang, L.; Chu, S.; Luo, M.; Liu, J. Zeolite Supported Fe/Ni Bimetallic Nanoparticles for Simultaneous Removal of Nitrate and Phosphate: Synergistic Effect and Mechanism. Chem. Eng. J. 2018, 347, 669–681. [Google Scholar] [CrossRef]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of Heavy Metal Lead(II) Using Nanoscale Zero-Valent Iron with Different Preservation Methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of Active Hydrogen Species on Palladium Nanoparticles for an Enhanced Electrocatalytic Hydrodechlorination of 2,4-Dichlorophenol in Water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Sangani, M.M.M.; Yavari, M.A.; Baghdadi, M. Optimization of a Simple Continuous System for the Preparation of Zero-Valent Iron Nanoparticles Coated with Flaxseed Gum: Effect of Groundwater Quality on the Aggregation. Environ. Technol. Innov. 2023, 30, 103119. [Google Scholar] [CrossRef]
- Parimala, L.; Santhanalakshmi, J. Studies on the Iron Nanoparticles Catalyzed Reduction of Substituted Aromatic Ketones to Alcohols. J. Nanopart. 2014, 2014, 156868. [Google Scholar] [CrossRef]
- Nunez Garcia, A.; Boparai, H.K.; de Boer, C.V.; Chowdhury, A.I.A.; Kocur, C.M.D.; Austrins, L.M.; Herrera, J.; O’Carroll, D.M. Fate and Transport of Sulfidated Nano Zerovalent Iron (S-nZVI): A Field Study. Water Res. 2020, 170, 115319. [Google Scholar] [CrossRef] [PubMed]
- Kania, G.; Sternak, M.; Jasztal, A.; Chlopicki, S.; Błażejczyk, A.; Nasulewicz-Goldeman, A.; Wietrzyk, J.; Jasiński, K.; Skórka, T.; Zapotoczny, S.; et al. Uptake and Bioreactivity of Charged Chitosan-Coated Superparamagnetic Nanoparticles as Promising Contrast Agents for Magnetic Resonance Imaging. Nanomedicine 2018, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ge, C.; Li, X.; Li, L.; Wang, B.; Lin, A.; Yang, W. Does Soluble Starch Improve the Removal of Cr(VI) by nZVI Loaded on Biochar? Ecotoxicol. Environ. Saf. 2021, 208, 111552. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tielong, L.; Fangchun, L.; Jinjuan, Z. Synthesis of Guar Gum Stabilized Nanoscale Zero-Valent Iron for Cr (VI) Removal in Water. IOP Conf. Ser. Earth Environ. Sci. 2021, 647, 012101. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Reductive Immobilization of Chromate in Water and Soil Using Stabilized Iron Nanoparticles. Water. Res. 2007, 41, 2101–2108. [Google Scholar] [CrossRef]
- Berge, N.D.; Ramsburg, C.A. Iron-Mediated Trichloroethene Reduction within Nonaqueous Phase Liquid. J. Contam. Hydrol. 2010, 118, 105–116. [Google Scholar] [CrossRef]
- Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.S.; Gavaskar, A.; et al. Field Demonstration of DNAPL Dehalogenation Using Emulsified Zero-Valent Iron. Environ. Sci. Technol. 2005, 39, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, L.; Chen, Y.; Ouyang, D.; Han, L.; Shang, X.; Li, J.; Gu, M.; Chen, M. Nanoscale Zero-Valent Iron Supported by Attapulgite Produced at Different Acid Modification: Synthesis Mechanism and the Role of Silicon on Cr(VI) Removal. Chemosphere 2021, 267, 129183. [Google Scholar] [CrossRef] [PubMed]
- Zarime, N.‘A.; Solemon, B.; Wan Yaacob, W.Z.; Jamil, H.; Che Omar, R.; Oyekanmi, A.A. Effectiveness of Artificially Synthesized Granitic Residual Soil-Supported Nano Zero-Valent Iron (Gr-ZVI) as Effective Heavy Metal Contaminant Adsorbent. Inorganics 2023, 11, 131. [Google Scholar] [CrossRef]
- Tajabadi, M.; Rahmani, I.; Mirkazemi, S.M.; Goran Orimi, H. Insights into the Synthesis Optimization of Fe@SiO2 Core-Shell Nanostructure as a Highly Efficient Nano-Heater for Magnetic Hyperthermia Treatment. Adv. Powder Technol. 2022, 33, 103366. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Ma, K.; Qin, Q. Facile Synthesis of High Iron Content Activated Carbon-Supported Nanoscale Zero-Valent Iron for Enhanced Cr(VI) Removal in Aqueous Solution. Chemosphere 2022, 291, 132709. [Google Scholar] [CrossRef] [PubMed]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic Zeolite Synthesis for Efficient Removal of Cesium in a Lab-Scale Continuous Treatment System. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Leovac Maćerak, A.; Kulić Mandić, A.; Pešić, V.; Tomašević Pilipović, D.; Bečelić-Tomin, M.; Kerkez, D. “Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment. Molecules 2023, 28, 1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate Nanostructures of Organic Molecular Materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef]
- Kumari, N.; Behera, M.; Singh, R. Facile Synthesis of Biopolymer Decorated Magnetic Coreshells for Enhanced Removal of Xenobiotic Azo Dyes through Experimental Modelling. Food Chem. Toxicol. 2023, 171, 113518. [Google Scholar] [CrossRef]
- Antony, J.; Meera, V.; Raphael, V.P.; Vinod, P. Facile Encapsulation of Nano Zero-Valent Iron with Calcium Carbonate: Synthesis, Characterization and Application for Iron Remediation. J. Environ. Health Sci. Eng. 2022, 20, 915–930. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic Stabilization of Charged Colloidal Particles with Adsorbed Polyelectrolytes of Opposite Charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.C.; Salazar-Bryam, A.M.; Piazza, R.D.; Carvalho dos Santos, C.; Jafelicci, M.; Marques, R.F.C.; Contiero, J. Rhamnolipids as Green Stabilizers of nZVI and Application in the Removal of Nitrate From Simulated Groundwater. Front. Bioeng. Biotechnol. 2022, 10, 794460. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Ali, B.H.; Shahin, M.S.; Baghdadi, M. The Removal of Cr(VI) from Aqueous and Saturated Porous Media by Nanoscale Zero-Valent Iron Stabilized with Flaxseed Gum Extract: Synthesis by Continuous Flow Injection Method. Korean J. Chem. Eng. 2022, 39, 2217–2228. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Bera, S.; Paul, S.; Mondal, S.; Saha, A.; Roy, S. Synthesis and Characterization of Inorganic Nanoparticles Luminophores for Environmental Remediation. 4open 2022, 5, 19. [Google Scholar] [CrossRef]
- Bhawna; Acharya, A.D.; Kaur, S. Rapid Reductive Degradation of Dye Contaminated Water by Using a Core-Shell Nano Zerovalent Iron (NZVI). J. Indian Chem. Soc. 2022, 99, 100598. [Google Scholar] [CrossRef]
- Moreno-Bárcenas, A.; Arizpe-Zapata, J.A.; Rivera Haro, J.A.; Sepúlveda, P.; Garcia-Garcia, A. Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems. Nanomaterials 2022, 12, 3974. [Google Scholar] [CrossRef]
- Selvan, B.K.; Thiyagarajan, K.; Das, S.; Jaya, N.; Jabasingh, S.A.; Saravanan, P.; Rajasimman, M.; Vasseghian, Y. Synthesis and Characterization of Nano Zerovalent Iron-Kaolin Clay (NZVI-Kaol) Composite Polyethersulfone (PES) Membrane for the Efficacious As2O3 Removal from Potable Water Samples. Chemosphere 2022, 288, 132405. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hu, X.; Gu, T.; Zhang, W.X.; Deng, Z. Nanocelluloses Affixed Nanoscale Zero-Valent Iron (NZVI) for Nickel Removal: Synthesis, Characterization and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 107466. [Google Scholar] [CrossRef]
- Xia, J.; Shen, Y.; Zhang, H.; Hu, X.; Mian, M.M.; Zhang, W.H. Synthesis of Magnetic NZVI@biochar Catalyst from Acid Precipitated Black Liquor and Fenton Sludge and Its Application for Fenton-like Removal of Rhodamine B Dye. Ind. Crops Prod. 2022, 187, 115449. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhou, Y.; Ma, H.; Cheng, X.; Wei, L.; Hou, Z. MFO@NZVI/Hydrogel for Sulfasalazine Degradation: Performance, Mechanism and Degradation Pathway. Sep. Purif. Technol. 2022, 282, 120054. [Google Scholar] [CrossRef]
- Shukla, F.; Kikani, T.; Khan, A.; Thakore, S. α-Hydroxy Acids Modified β-Cyclodextrin Capped Iron Nanocatalyst for Rapid Reduction of Nitroaromatics: A Sonochemical Approach. Int. J. Biol. Macromol. 2022, 209, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Akoto, J.D.; Chai, F.; Repo, E.; Yang, Z.; Wang, D.; Zhao, F.; Liao, Q.; Chai, L. Polyethyleneimine Stabilized Nanoscale Zero-Valent Iron-Magnetite (Fe3O4@nZVI-PEI) for the Enhanced Removal of Arsenic from Acidic Aqueous Solution: Performance and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108589. [Google Scholar] [CrossRef]
- Cheng, F.; Hou, J.; Wu, J.; Xu, Y.; Miao, L.; Xia, J.; Huo, Z. Kinetics, Products and Pathways for the Removal of Pentachlorophenol (PCP) by Sulfidated Nanoscale Zero-Valent Iron (S-NZVI). Environ. Sci. 2022, 8, 2567–2579. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Omer, A.M.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Eltaweil, A.S. Zero-Valent Iron Supported-Lemon Derived Biochar for Ultra-Fast Adsorption of Methylene Blue. Biomass Convers. Biorefin. 2022, 14, 1697–1709. [Google Scholar] [CrossRef]
- Okonji, S.O.; Achari, G.; Pernitsky, D. Removal of Organoselenium from Aqueous Solution by Nanoscale Zerovalent Iron Supported on Granular Activated Carbon. Water 2022, 14, 987. [Google Scholar] [CrossRef]
- Idham, M.F.; Falyouna, O.; Eljamal, R.; Maamoun, I.; Eljamal, O. Chloramphenicol Removal from Water by Various Precursors to Enhance Graphene Oxide–Iron Nanocomposites. J. Water Process Eng. 2022, 50, 103289. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, G.; Tao, X.; Wen, Z.; Dang, Z. A Collaborative Strategy for Elevated Reduction and Immobilization of Cr(VI) Using Nano Zero Valent Iron Assisted by Schwertmannite: Removal Performance and Mechanism. J. Hazard. Mater. 2022, 422, 126952. [Google Scholar] [CrossRef] [PubMed]
- Orbuleţ, O.D.; Dăncilă, A.M.; Căprărescu, S.; Modrogan, C.; Purcar, V. Nitrates Removal from Simulated Groundwater Using Nano Zerovalent Iron Supported by Polystyrenic Gel. Polymers 2023, 15, 61. [Google Scholar] [CrossRef]
- Fan, X.; Ma, L.; Liu, S.; Xie, Y.; Lu, S.; Tan, Z.; Ji, J.; Fu, M.L.; Yuan, B.; Hu, Y.-B. Facile Synthesis of Lattice-Defective and Recyclable Zirconium Hydroxide Coated Nanoscale Zero-Valent Iron for Robust Arsenite Removal. Sep. Purif. Technol. 2022, 302, 122085. [Google Scholar] [CrossRef]
- Wen, J.; Fu, W.; Ding, S.; Zhang, Y.; Wang, W. Pyrogallic Acid Modified Nanoscale Zero-Valent Iron Efficiently Removed Cr(VI) by Improving Adsorption and Electron Selectivity. Chem. Eng. J. 2022, 443, 136510. [Google Scholar] [CrossRef]
- Dai, G.; Li, X.; Fu, H.; Wang, F.; Cui, Z.; Zhao, R.; Wang, L. A Novel Oxalated Zero-Valent Iron Nanoparticle for Pb(II) Removal from Aqueous Solution: Performance and Synergistic Mechanisms. Sep. Purif. Technol. 2022, 302, 122017. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Huo, Y.; Liu, H.; Li, J.; Huang, T.; Ye, R.; Li, W. Impacts of Shell Structure on Nitrate-Reduction Activity and Air Stability of Nanoscale Zero-Valent Iron. Environ. Sci. Pollut. Res. 2022, 29, 80683–80692. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseininia, J.; Sabbaghi, S.; Mirbagheri, N.S.; Zerafat, M.M. Treatment of As-Contaminated Drinking Water Using a Nano Zero-Valent Iron/Copper Slag Nanocomposite. J. Water Process Eng. 2022, 49, 103011. [Google Scholar] [CrossRef]
- Liu, J.; Shi, S.; Shu, J.; Li, C.; He, H.; Xiao, C.; Dong, X.; He, Y.; Liao, J.; Liu, N.; et al. Synthesis and Characterization of Waste Commercially Available Polyacrylonitrile Fiber-Based New Composites for Efficient Removal of Uranyl from U(VI)–CO3 Solutions. Sci. Total Environ. 2022, 822, 153507. [Google Scholar] [CrossRef] [PubMed]
- Shanableh, A.; Bhattacharjee, S.; Sadik, S. Evaluating Iron-Based Nanoparticles for Ciprofloxacin Removal: Date Seed Extract as a Biostabilizing and a Bioreducing Agent. J. Water Process Eng. 2021, 44, 102419. [Google Scholar] [CrossRef]
- Pillai, R.; Balan, R.; Lekshmi, I.C.; Milina, K. Facile Auto-Combustion Synthesis and Characterization of Stable Amorphous Nanoscale Zero-Valent Iron (NZVI). Int. J. Self-Propagating High-Temp. Synth. 2021, 30, 251–256. [Google Scholar] [CrossRef]
- Havelka, O.; Cvek, M.; Urbánek, M.; Łukowiec, D.; Jašíková, D.; Kotek, M.; Černík, M.; Amendola, V.; Torres-Mendieta, R. On the Use of Laser Fragmentation for the Synthesis of Ligand-Free Ultra-Small Iron Nanoparticles in Various Liquid Environments. Nanomaterials 2021, 11, 1538. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, M.; Hu, X.; Chen, Y.; Li, Y.; Xu, X.; Zhang, R.; Yang, X.; Tang, C.; Hu, X. A Novel Preparation of S-NZVI and Its High Efficient Removal of Cr(VI) in Aqueous Solution. J. Hazard. Mater. 2021, 416, 125924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, L.; Shen, H.; Wang, J. Construction of Three-Dimensional Reduced Graphene Oxide Wrapped NZVI Doped with Al2O3 as the Ternary Fenton-like Catalyst: Optimization, Characterization and Catalytic Mechanism. Sci. Total Environ. 2021, 780, 146576. [Google Scholar] [CrossRef]
- Peng, F.Y.; Wang, P.W.; Liao, W.; Yu, I.S. Lignin Biopolymer for the Synthesis of Iron Nanoparticles and the Composite Applied for the Removal of Methylene Blue. Polymers 2021, 13, 3847. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Yang, X.; Zhang, X.; Wang, Y.; Cai, X.; Chen, H. Bioprecipitation Facilitates the Green Synthesis of Sulfidated Nanoscale Zero-Valent Iron Particles for Highly Selective Dechlorination of Trichloroethene. J. Environ. Chem. Eng. 2021, 9, 106050. [Google Scholar] [CrossRef]
- Flores-Rojas, E.; Schnabel, D.; Justo-Cabrera, E.; Solorza-Feria, O.; Poggi-Varaldo, H.M.; Breton-Deval, L. Using Nano Zero-Valent Iron Supported on Diatomite to Remove Acid Blue Dye: Synthesis, Characterization, and Toxicology Test. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Jismy, A.; Meera, V.; RaphaelVinod, P. Comparative Study on Iron Removal Using Chemically and Greenly Synthesised Zero-Valent Iron Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1114, 012082. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, C.; Ai, D.; Fan, Z. Activation of Persulfate by Green Nano-Zero-Valent Iron-Loaded Biochar for the Removal of p-Nitrophenol: Performance, Mechanism and Variables Effects. J. Hazard. Mater. 2021, 417, 126106. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Ruiz, D.A.; Meramo-Hurtado, S.I.; González-Delgado, Á.D.; Herrera, A. Environmental Sustainability Evaluation of Iron Oxide Nanoparticles Synthesized via Green Synthesis and the Coprecipitation Method: A Comparative Life Cycle Assessment Study. ACS Omega 2021, 6, 12410–12423. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Sohrabi, M.R.; Motiee, F.; Davallo, M. Organophosphorus Diazinon Pesticide Removing from Aqueous Solution by Zero-Valent Iron Supported on Biopolymer Chitosan: RSM Optimization Methodology. J. Polym. Environ. 2021, 29, 103–120. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, R.; Wang, P.; Wang, Q.; Wan, S.; Huang, S.; Su, R.; Song, Y.; Yang, X.; Fu, X. Synthesis of a Novel Biochar-Supported Polycarboxylic Acid-Functionalized Nanoiron Oxide-Encapsulated Composite for Wastewater Treatment: Removal of Cd(II), EDTA and Cd-EDTA. J. Mater. Sci. 2021, 56, 18031–18049. [Google Scholar] [CrossRef]
- Farooq, U.; Zhuang, J.; Wang, X.; Lyu, S. A Recyclable Polydopamine-Functionalized Reduced Graphene Oxide/Fe Nanocomposite (PDA@Fe/RGO) for the Enhanced Degradation of 1,1,1-Trichloroethane. Chem. Eng. J. 2021, 403, 126405. [Google Scholar] [CrossRef]
- Rončević, S.; Nemet, I.; Zagorec, V.; Selmani, A. A Facile Size Tunable One-Pot Synthesis of Dipicolinate@nZVI Core-Shell Nanoparticles: Material Properties for Trace Cadmium Ion Removal. New J. Chem. 2020, 44, 17840–17848. [Google Scholar] [CrossRef]
- Francy, N.; Shanthakumar, S.; Chiampo, F.; Sekhar, Y.R. Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results. Processes 2020, 8, 1453. [Google Scholar] [CrossRef]
- Fan, J.; Chen, X.; Xu, Z.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. One-Pot Synthesis of NZVI-Embedded Biochar for Remediation of Two Mining Arsenic-Contaminated Soils: Arsenic Immobilization Associated with Iron Transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef] [PubMed]
- Eljamal, R.; Eljamal, O.; Maamoun, I.; Yilmaz, G.; Sugihara, Y. Enhancing the Characteristics and Reactivity of NZVI: Polymers Effect and Mechanisms. J. Mol. Liq. 2020, 315, 113714. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous Biochar-Nanoscale Zero-Valent Iron Composites: Synthesis, Characterization and Application for Lead Ion Removal. Scie. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef] [PubMed]
- Mdlovu, N.V.; Lin, K.S.; Hsien, M.J.; Chang, C.J.; Kunene, S.C. Synthesis, Characterization, and Application of Zero-Valent Iron Nanoparticles for TNT, RDX, and HMX Explosives Decontamination in Wastewater. J. Taiwan. Inst. Chem. Eng. 2020, 114, 186–198. [Google Scholar] [CrossRef]
- Mortazavian, S.; Bandala, E.R.; Bae, J.H.; Chun, D.; Moon, J. Assessment of P-Nitroso Dimethylaniline (PNDA) Suitability as a Hydroxyl Radical Probe: Investigating Bleaching Mechanism Using Immobilized Zero-Valent Iron Nanoparticles. Chem. Eng. J. 2020, 385, 123748. [Google Scholar] [CrossRef]
- Karam, A.; Zaher, K.; Mahmoud, A.S. Comparative Studies of Using Nano Zerovalent Iron, Activated Carbon, and Green Synthesized Nano Zerovalent Iron for Textile Wastewater Color Removal Using Artificial Intelligence, Regression Analysis, Adsorption Isotherm, and Kinetic Studies. Air Soil Water Res. 2020, 13, 1178622120908273. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karri, R.R.; Alimohammadi, M.; Nazmara, S.; Zarei, A.; Saeedi, Z. Insights into Endocrine-Disrupting Bisphenol-A Adsorption from Pharmaceutical Effluent by Chitosan Immobilized Nanoscale Zero-Valent Iron Nanoparticles. J. Mol. Liq. 2020, 311, 113317. [Google Scholar] [CrossRef]
- Lahoz, R.; Natividad, E.; Mayoral, Á.; Rentenberger, C.; Díaz-Fernández, D.; Félix, E.J.; Soriano, L.; Kautek, W.; Bomati-Miguel, O. Pursuit of Optimal Synthetic Conditions for Obtaining Colloidal Zero-Valent Iron Nanoparticles by Scanning Pulsed Laser Ablation in Liquids. J. Ind. Eng. Chem. 2020, 81, 340–351. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Ouyang, D.; Yan, J.; Qian, L.; Han, L.; Chen, M.; Li, J.; Gu, M. Mechanistic Insights into Adsorptive and Oxidative Removal of Monochlorobenzene in Biochar-Supported Nanoscale Zero-Valent Iron/Persulfate System. Chem. Eng. J. 2020, 400, 125811. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Ma, J.; Ning, P. Novel Synthesis of Aluminum Hydroxide Gel-Coated Nano Zero-Valent Iron and Studies of Its Activity in Flocculation-Enhanced Removal of Tetracycline. J. Environ. Sci. 2020, 89, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Bin, Q.; Lin, B.; Zhu, K.; Shen, Y.; Man, Y.; Wang, B.; Lai, C.; Chen, W. Superior Trichloroethylene Removal from Water by Sulfide-Modified Nanoscale Zero-Valent Iron/Graphene Aerogel Composite. J. Environ. Sci. 2020, 88, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Avellan, A.; Li, H.; Clark, E.A.; Henkelman, G.; Kaegi, R.; Lowry, G.V. Iron and Sulfur Precursors Affect Crystalline Structure, Speciation, and Reactivity of Sulfidized Nanoscale Zerovalent Iron. Environ. Sci. Technol. 2020, 54, 13294–13303. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, J.; Tang, J.; Wang, Y.; Niu, Y.; Lu, H. Research on the Characterization, Reactivity, and Transportability of Porous Silicon-Coated Nanoscale Zero-Valent Iron. Environ. Sci. Pollut. Res. 2020, 27, 31567–31577. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Kadam, R.G.; Tuček, J.; Sofer, Z.; Bouša, D.; Varma, R.S.; Gawande, M.B.; Zbořil, R. Fe(0)-Embedded Thermally Reduced Graphene Oxide as Efficient Nanocatalyst for Reduction of Nitro Compounds to Amines. Chem. Eng. J. 2020, 382, 122469. [Google Scholar] [CrossRef]
- Sawafta, R.; Shahwan, T. A Comparative Study of the Removal of Methylene Blue by Iron Nanoparticles from Water and Water-Ethanol Solutions. J. Mol. Liq. 2019, 273, 274–281. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of Toxic Dye from Aqueous Environment Using Monometallic and Bimetallic Quantum Dots Based Nanocomposites. J. Clean. Prod. 2016, 172, 2919–2930. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-Valent Iron Nanoparticles for Methylene Blue Removal from Aqueous Solutions and Textile Wastewater Treatment, with Cost Estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, I.; Levina, V.; Leybo, D.; Masov, V.; Tagirov, M.; Kuznetsov, D. Synthesis, Characterization and Reactivity of Nanostructured Zero-Valent Iron Particles for Degradation of Azo Dyes. Int. J. Nanosci. 2017, 16, 1750017. [Google Scholar] [CrossRef]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green Synthesis and Environmental Application of Iron-Based Nanomaterials and Nanocomposite: A Review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef]
- Plachtová, P.; Medříková, Z.; Zbořil, R.; Tuček, J.; Varma, R.S.; Maršálek, B. Iron and Iron Oxide Nanoparticles Synthesized with Green Tea Extract: Differences in Ecotoxicological Profile and Ability to Degrade Malachite Green. ACS Sustain. Chem. Eng. 2018, 6, 8679–8687. [Google Scholar] [CrossRef]
- Prasad, A.S. Iron Oxide Nanoparticles Synthesized by Controlled Bio-Precipitation Using Leaf Extract of Garlic Vine (Mansoa Alliacea). Mater. Sci. Semicond. Process 2016, 53, 79–83. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, C.; Fang, C.; Mallavarapu, M. Dye Removal Using Iron-Polyphenol Complex Nanoparticles Synthesized by Plant Leaves. Environ. Technol. Innov. 2014, 1–2, 29–34. [Google Scholar] [CrossRef]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of Iron-Based Nanoparticles by Green Tea Extract and Their Degradation of Malachite. Ind. Crops Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Cáceres-Jensen, L.; Castro-Rojas, J.; Poblete-Grant, P.; Bolan, N.; de la Luz Mora, M. NZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials 2023, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Panić, S.; Petronijević, M.; Vukmirović, J.; Grba, N.; Savić, S. Green Synthesis of Nanoscale Zero-Valent Iron Aggregates for Catalytic Degradation of Textile Dyes. Catal. Lett. 2023, 153, 3605–3619. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Sher, F.; Afshin, S.; Hamzezadeh, A.; Ahmadi, S.; Azhar, O.; Rastegar, A.; Ghosh, S.; Poureshgh, Y. Green Synthesis of Zero-Valent Iron Nanoparticles and Loading Effect on Activated Carbon for Furfural Adsorption. Chemosphere 2022, 287, 132114. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Dang, T.D.; Hoang Binh, K.; Nguyen, T.M.; Xuan, T.N.; La, D.D.; Kumar Nadda, A.; Chang, S.W.; Nguyen, D.D. Green Synthesis of Highly Stable Zero-Valent Iron Nanoparticles for Organic Dye Treatment Using Cleistocalyx Operculatus Leaf Extract. Sustain. Chem. Pharm. 2022, 25, 100598. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Gao, F.; Yu, C.; Li, S.; Lyu, H.; Sun, H. Tetrahydrofuran Aided Self-Assembly Synthesis of NZVI@gBC Composite as Persulfate Activator for Degradation of 2,4-Dichlorophenol. Chem. Eng. J. 2022, 431, 134063. [Google Scholar] [CrossRef]
- Van Hoang, N.; Nguyen-Thi, L.; Kim, G.M.; Dang, T.D.; Ngoc Toan, V.; La, D.D. Green Synthesis of Zero-Valent Iron Nanoparticles by Cleistocalyx Operculatus Leaf Extract Using Microfluidic Device for Degradation of the Rhodamine B Dye. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 045007. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X. Green Synthesis of Modified Polyethylene Packing Supported Tea Polyphenols-NZVI for Nitrate Removal from Wastewater: Characterization and Mechanisms. Sci. Total Environ. 2022, 806, 150596. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, J.; Zhang, W.; Wu, W.; Wei, Z.; Chen, M.; Yan, J.; Qian, L.; Han, L.; Li, J.; et al. Hydrothermally Assisted Synthesis of Nano Zero-Valent Iron Encapsulated in Biomass-Derived Carbon for Peroxymonosulfate Activation: The Performance and Mechanisms for Efficient Degradation of Monochlorobenzene. Sci. Total Environ. 2022, 829, 154645. [Google Scholar] [CrossRef] [PubMed]
- Van Hoang, N.; Thi Xuan Quynh, N.; Dang, T.D.; Nguyen Xuan, T.; Ngoc Toan, V.; Duc La, D. Green Synthesis of Fe/Graphene Nanocomposite Using Cleistocalyx Operculatus Leaf Extract as a Reducing Agent: Removal of Pollutants (RhB Dye and Cr6+ Ions) in Aqueous Media. ChemistrySelect 2022, 7, e202203499. [Google Scholar] [CrossRef]
- Deewan, R.; Yan, D.Y.S.; Khamdahsag, P.; Tanboonchuy, V. Remediation of Arsenic-Contaminated Water by Green Zero-Valent Iron Nanoparticles. Environ. Sci. Pollut. Res. 2022, 30, 90352–90361. [Google Scholar] [CrossRef] [PubMed]
- Puiatti, G.A.; de Carvalho, J.P.; de Matos, A.T.; Lopes, R.P. Green Synthesis of Fe0 Nanoparticles Using Eucalyptus Grandis Leaf Extract: Characterization and Application for Dye Degradation by a (Photo)Fenton-like Process. J. Environ. Manag. 2022, 311, 114828. [Google Scholar] [CrossRef] [PubMed]
- Hür, C.; Erken, E. Assessment of Green Tea-Enabled Iron/Calcined Bentonite Nanocomposites for Phosphate Removal and Recovery. J. Environ. Chem. Eng. 2022, 10, 108519. [Google Scholar] [CrossRef]
- Sun, H.; Hua, Y.; Zhao, Y. Synchronous Efficient Reduction of Cr (VI) and Removal of Total Chromium by Corn Extract / Fe (III) System. Environ. Sci. Pollut. Res. 2022, 29, 28552–28564. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xue, C.; Guo, S.; Owens, G.; Chen, Z. Effects of Green Synthesized and Commercial NZVI on Crystal Violet Degradation by Burkholderia Vietnamiensis C09V: Dose-Dependent Toxicity and Biocompatibility. Chemosphere 2021, 279, 130612. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liu, Y.; Cheng, L.; Jiang, Z.; Zhang, G.; Deng, F.; Wang, L.; Han, W.; Zhang, Y. Green Synthesis of Hydrophilic Activated Carbon Supported Sulfide NZVI for Enhanced Pb(II) Scavenging from Water: Characterization, Kinetics, Isotherms and Mechanisms. J. Hazard. Mater. 2021, 403, 123607. [Google Scholar] [CrossRef]
- Kadhum, S.T.; Alkindi, G.Y.; Albayati, T.M. Eco Friendly Adsorbents for Removal of Phenol from Aqueous Solution Employing Nanoparticle Zero-Valent Iron Synthesized from Modified Green Tea Bio-Waste and Supported on Silty Clay. Chin. J. Chem. Eng. 2021, 36, 19–28. [Google Scholar] [CrossRef]
- Tan, W.; Ruan, Y.; Diao, Z.; Song, G.; Su, M.; Hou, L.; Chen, D.; Kong, L.; Deng, H. Removal of Levofloxacin through Adsorption and Peroxymonosulfate Activation Using Carbothermal Reduction Synthesized NZVI/Carbon Fiber. Chemosphere 2021, 280, 130626. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Xu, N.; Ye, Z.; Yang, H.; Huangfu, X. Synthesis of Montmorillonite-Supported Nano-Zero-Valent Iron via Green Tea Extract: Enhanced Transport and Application for Hexavalent Chromium Removal from Water and Soil. J. Hazard. Mater. 2021, 419, 126461. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; El-Khouly, M.E.; Eltaweil, A.S. Efficient Adsorptive Removal of Tetracycline from Aqueous Solution Using Phytosynthesized Nano-Zero Valent Iron. J. Saudi Chem. Soc. 2021, 25, 101365. [Google Scholar] [CrossRef]
- Al Kindi, G.Y.; Hassan, A.K.; Yahya, D.G.H.; Alhaidri, H.A. The Nanoparticles Zero-Valent Synthesis by Black Tea Extract to Remove Rb 238 Using Synthetic and Natural Wastewater by Packed Bed Reactor. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012092. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, N.; Li, D.; Qian, J.; Du, C.; Chen, M. Vitamin C Mediates the Activation of Green Tea Extract to Modify Nanozero-Valent Iron Composites: Enhanced Transport in Heterogeneous Porous Media and the Removal of Hexavalent Chromium. J. Hazard. Mater. 2021, 411, 125042. [Google Scholar] [CrossRef] [PubMed]
- Salmani, M.H.; Abedi, M.; Mozaffari, S.A.; Mahvi, A.H.; Sheibani, A.; Jalili, M. Simultaneous Reduction and Adsorption of Arsenite Anions by Green Synthesis of Iron Nanoparticles Using Pomegranate Peel Extract. J. Environ. Health Sci. Eng. 2021, 19, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Khunjan, U.; Kasikamphaiboon, P. Green Synthesis of Kaolin-Supported Nanoscale Zero-Valent Iron Using Ruellia Tuberosa Leaf Extract for Effective Decolorization of Azo Dye Reactive Black 5. Arab. J. Sci. Eng. 2021, 46, 383–394. [Google Scholar] [CrossRef]
- Panagou, I.; Noutsopoulos, C.; Mystrioti, C.; Barka, E.; Koumaki, E.; Kalli, M.; Malamis, S.; Papassiopi, N.; Mamais, D. Assessing the Performance of Environmentally Friendly-Produced Zerovalent Iron Nanoparticles to Remove Pharmaceuticals from Water. Sustainability 2021, 13, 12708. [Google Scholar] [CrossRef]
- Mousazadeh, S.; Shariati, S.; Yousefi, M.; Baniyaghoob, S.; Kefayati, H. Hexavalent Chromium Removal Using Ionic Liquid Coated Magnetic Nano Zero-Valent Iron Biosynthesized by Camellia Sinensis Extract. Int. J. Environ. Res. 2021, 15, 1017–1036. [Google Scholar] [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green Synthesis of Iron Nanoparticles Using Artocarpus Heterophyllus Peel Extract and Their Application as a Heterogeneous Fenton-like Catalyst for the Degradation of Fuchsin Basic Dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with Zero-Valent Iron Synthesized Using Pomegranate Peel Waste: A “Green” Approach for Yield Enhancement in Oryza Sativa L. Cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Du, Y.; Chen, S.; Zhang, F.; Zhan, W.; Du, D.; Zhang, T.C. Nanoscale Zero-Valent Iron Coupling with Shewanella Oneidensis MR-1 for Enhanced Reduction/Removal of Aqueous Cr(VI). Sep. Purif. Technol. 2021, 277, 119488. [Google Scholar] [CrossRef]
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green Synthesis of Nano-Zero-Valent Iron Using Ricinus Communis Seeds Extract: Characterization and Application in the Treatment of Methylene Blue-Polluted Water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Y.; He, X.; Zhang, H.; Wei, J.; Zhu, L. Microbiological Synthesis of Denitrifying Bacteria-Iron Nanopartical Composite Material and Its Eminent Performance in Removal of Nitrate-N. Sep. Purif. Technol. 2021, 267, 118663. [Google Scholar] [CrossRef]
- Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezadeh, A.; Fazlzadeh, M. Green Preparation of Activated Carbon from Pomegranate Peel Coated with Zero-Valent Iron Nanoparticles (NZVI) and Isotherm and Kinetic Studies of Amoxicillin Removal in Water. Environ. Sci. Pollut. Res. 2020, 27, 36732–36743. [Google Scholar] [CrossRef] [PubMed]
- Rashtbari, Y.; Américo-Pinheiro, J.H.P.; Bahrami, S.; Fazlzadeh, M.; Arfaeinia, H.; Poureshgh, Y. Efficiency of Zeolite Coated with Zero-Valent Iron Nanoparticles for Removal of Humic Acid from Aqueous Solutions. Water Air Soil Pollut. 2020, 231, 514. [Google Scholar] [CrossRef]
- Hassan, A.K.; Al-Kindi, G.Y.; Ghanim, D. Green Synthesis of Bentonite-Supported Iron Nanoparticles as a Heterogeneous Fenton-like Catalyst: Kinetics of Decolorization of Reactive Blue 238 Dye. Water Sci. Eng. 2020, 13, 286–298. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A Novel, Eco-Friendly and Green Synthesis of PPAC-ZnO and PPAC-NZVI Nanocomposite Using Pomegranate Peel: Cephalexin Adsorption Experiments, Mechanisms, Isotherms and Kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Zhao, H.; Wang, W. Pyrolytic Production of Zerovalent Iron Nanoparticles Supported on Rice Husk-Derived Biochar: Simple, in Situ Synthesis and Use for Remediation of Cr(VI)-Polluted Soils. Sci. Total Environ. 2020, 708, 134479. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Wu, J. One-Pot Synthesis of Magnetic Algal Carbon/Sulfidated Nanoscale Zerovalent Iron Composites for Removal of Bromated Disinfection by-Product. Chemosphere 2020, 250, 126257. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.; Yang, Y.; Li, J.; Wang, S.; Lv, J.; Tang, R. Novel Carbothermal Synthesis of Fe, N Co-Doped Oak Wood Biochar (Fe/N-OB) for Fast and Effective Cr(VI) Removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124926. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Farag, R.S.; Elshfai, M.M. Reduction of Organic Matter from Municipal Wastewater at Low Cost Using Green Synthesis Nano Iron Extracted from Black Tea: Artificial Intelligence with Regression Analysis. Egypt. J. Pet. 2020, 29, 9–20. [Google Scholar] [CrossRef]
- Shad, S.; Belinga-Desaunay-Nault, M.F.A.; Sohail; Bashir, N.; Lynch, I. Removal of Contaminants from Canal Water Using Microwave Synthesized Zero Valent Iron Nanoparticles. Environ. Sci. 2020, 6, 3057–3065. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Wang, T.; Chen, C.; Gadd, G.M. Iron Coral: Novel Fungal Biomineralization of Nanoscale Zerovalent Iron Composites for Treatment of Chlorinated Pollutants. Chem. Eng. J. 2020, 402, 126263. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, X.; Liu, N.; Lv, J.; Yang, Y. Enhanced Removal of Aqueous Cr(VI) by a Green Synthesized Nanoscale Zero-Valent Iron Supported on Oak Wood Biochar. Chemosphere 2020, 245, 125542. [Google Scholar] [CrossRef] [PubMed]
- Romeh, A.A.; Ibrahim Saber, R.A. Green Nano-Phytoremediation and Solubility Improving Agents for the Remediation of Chlorfenapyr Contaminated Soil and Water. J. Environ. Manag. 2020, 260, 110104. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Tu, G.; Tsang, P.E.; Xiao, S.; Fang, Z. Green Synthesis of Iron-Based Nanoparticles from Extracts of Nephrolepis Auriculata and Applications for Cr(VI) Removal. Mater. Lett. 2019, 234, 388–391. [Google Scholar] [CrossRef]
- Desalegn, B.; Megharaj, M.; Chen, Z.; Naidu, R. Green Synthesis of Zero Valent Iron Nanoparticle Using Mango Peel Extract and Surface Characterization Using XPS and GC-MS. Heliyon 2019, 5, e01750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, Z.; Chen, G.; Ma, X.; Su, W.; Cui, X.; Li, X. DOX-Assisted Functionalization of Green Tea Polyphenol Nanoparticles for Effective Chemo-Photothermal Cancer Therapy. J. Mater. Chem. B 2019, 7, 4066–4078. [Google Scholar] [CrossRef]
- Afsheen, S.; Tahir, M.B.; Iqbal, T.; Liaqat, A.; Abrar, M. Green Synthesis and Characterization of Novel Iron Particles by Using Different Extracts. J. Alloys Compd. 2018, 732, 935–944. [Google Scholar] [CrossRef]
- Gan, L.; Lu, Z.; Cao, D.; Chen, Z. Effects of Cetyltrimethylammonium Bromide on the Morphology of Green Synthesized Fe3O4 Nanoparticles Used to Remove Phosphate. Mater. Sci. Eng. C 2018, 82, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Jagathesan, G.; Rajiv, P. Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Eichhornia Crassipes Leaf Extract and Assessing Their Antibacterial Activity. Biocatal. Agric. Biotechnol. 2018, 13, 90–94. [Google Scholar] [CrossRef]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. A Green Synthetic Route to Phenolics Fabricated Magnetite Nanoparticles from Coconut Husk Extract: Implications to Treat Metal Contaminated Water and Heavy Metal Stress in Oryza Sativa L. J. Clean Prod. 2018, 174, 355–366. [Google Scholar] [CrossRef]
- Rana, A.; Kumari, N.; Tyagi, M.; Jagadevan, S. Leaf-Extract Mediated Zero-Valent Iron for Oxidation of Arsenic (III): Preparation, Characterization and Kinetics. Chem. Eng. J. 2018, 347, 91–100. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green Zero-Valent Iron Nanoparticles for the Degradation of Amoxicillin. Int. J. Environ. Sci. Technol. 2017, 14, 1109–1118. [Google Scholar] [CrossRef]
- Sathya, K.; Saravanathamizhan, R.; Baskar, G. Ultrasound Assisted Phytosynthesis of Iron Oxide Nanoparticle. Ultrason. Sonochem. 2017, 39, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Nithya, K.; Sathish, A.; Senthil Kumar, P.; Ramachandran, T. Fast Kinetics and High Adsorption Capacity of Green Extract Capped Superparamagnetic Iron Oxide Nanoparticles for the Adsorption of Ni(II) Ions. J. Ind. Eng. Chem. 2018, 59, 230–241. [Google Scholar] [CrossRef]
- Rajiv, P.; Bavadharani, B.; Kumar, M.N.; Vanathi, P. Synthesis and Characterization of Biogenic Iron Oxide Nanoparticles Using Green Chemistry Approach and Evaluating Their Biological Activities. Biocatal. Agric. Biotechnol. 2017, 12, 45–49. [Google Scholar] [CrossRef]
- Sangami, S.; Manu, B. Synthesis of Green Iron Nanoparticles Using Laterite and Their Application as a Fenton-like Catalyst for the Degradation of Herbicide Ametryn in Water. Environ. Technol. Innov. 2017, 8, 150–163. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Y.; Tan, J.; Owens, G.; Chen, Z. Removal of Cr(VI) from Aqueous Solutions via Reduction and Absorption by Green Synthesized Iron Nanoparticles. J. Clean. Prod. 2018, 176, 929–936. [Google Scholar] [CrossRef]
- Saikia, I.; Hazarika, M.; Hussian, N.; Das, M.R.; Tamuly, C. Biogenic Synthesis of Fe2O3@SiO2 Nanoparticles for Ipso-Hydroxylation of Boronic Acid in Water. Tetrahedron Lett. 2017, 58, 4255–4259. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, H.; Xu, Y.; Yuan, M.; Jing, X.; Huang, J.; Li, Q.; Sun, D. Ultra-Efficient Removal of Chromium from Aqueous Medium by Biogenic Iron Based Nanoparticles. Sep. Purif. Technol. 2017, 174, 466–473. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Angélica Rubio, M.; Reyes, C.; Arancibia-Miranda, N. Preparation of Nanoscale Iron (Oxide, Oxyhydroxides and Zero-Valent) Particles Derived from Blueberries: Reactivity, Characterization and Removal Mechanism of Arsenate. Ecotoxicol. Environ. Saf. 2017, 145, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Madhubala, V.; Kalaivani, T. Phyto and Hydrothermal Synthesis of Fe3O4@ZnO Core-Shell Nanoparticles Using Azadirachta Indica and Its Cytotoxicity Studies. Appl. Surf. Sci. 2018, 449, 584–590. [Google Scholar] [CrossRef]
- Wei, Y.; Fang, Z.; Zheng, L.; Tsang, E.P. Biosynthesized Iron Nanoparticles in Aqueous Extracts of Eichhornia Crassipes and Its Mechanism in the Hexavalent Chromium Removal. Appl. Surf. Sci. 2017, 399, 322–329. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green Synthesis of Iron Nanoparticles Using Moringa Oleifera Extracts and Their Applications: Removal of Nitrate from Water and Antibacterial Activity against Escherichia Coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Alwarthan, A. Uptake of Propranolol on Ionic Liquid Iron Nanocomposite Adsorbent: Kinetic, Thermodynamics and Mechanism of Adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Gottimukkala, K.S.V.; Harika-Reddy, P.; Deeveka, Z. Green Synthesis of Iron Nanoparticles Using Green Tea Leaves Extract. J. Nanomed. Biother. Discov. 2017, 7, 1–4. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A Novel Green Synthesis of Zero Valent Iron Nanoparticles (NZVI) Using Three Plant Extracts and Their Efficient Application for Removal of Cr(VI) from Aqueous Solutions. Adv. Powder Technol. 2017, 28, 122–130. [Google Scholar] [CrossRef]
- Guo, M.; Weng, X.; Wang, T.; Chen, Z. Biosynthesized Iron-Based Nanoparticles Used as a Heterogeneous Catalyst for the Removal of 2,4-Dichlorophenol. Sep. Purif. Technol. 2017, 175, 222–228. [Google Scholar] [CrossRef]
- Weng, X.; Jin, X.; Lin, J.; Naidu, R.; Chen, Z. Removal of Mixed Contaminants Cr(VI) and Cu(II) by Green Synthesized Iron Based Nanoparticles. Ecol. Eng. 2016, 97, 32–39. [Google Scholar] [CrossRef]
- Jassal, V.; Shanker, U.; Gahlot, S. Green Synthesis of Some Iron Oxide Nanoparticles and Their Interaction with 2-Amino, 3-Amino and 4-Aminopyridines. Mater. Today Proc. 2016, 3, 1874–1882. [Google Scholar] [CrossRef]
- Al-Ruqeishi, M.S.; Mohiuddin, T.; Al-Saadi, L.K. Green Synthesis of Iron Oxide Nanorods from Deciduous Omani Mango Tree Leaves for Heavy Oil Viscosity Treatment. Arab. J. Chem. 2019, 12, 4084–4090. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K.; Katte, S.Y. Green Synthesis of Iron Nanoparticles Using Different Leaf Extracts for Treatment of Domestic Waste Water. J. Clean. Prod. 2016, 139, 1425–1435. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesized Iron Nanoparticles by Green Tea and Eucalyptus Leaves Extracts Used for Removal of Nitrate in Aqueous Solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Machado, S.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Utilization of Food Industry Wastes for the Production of Zero-Valent Iron Nanoparticles. Sci. Total Environ. 2014, 496, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Stawiński, W.; Slonina, P.; Pinto, A.R.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Application of Green Zero-Valent Iron Nanoparticles to the Remediation of Soils Contaminated with Ibuprofen. Sci. Total Environ. 2013, 461–462, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, K.; Mandal, B.K.; Siva Kumar, K.; Sreedhara Reddy, P.; Sreedhar, B. Biobased Green Method to Synthesise Palladium and Iron Nanoparticles Using Terminalia Chebula Aqueous Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green Production of Zero-Valent Iron Nanoparticles Using Tree Leaf Extracts. Sci. Total Environ. 2013, 445–446, 1–8. [Google Scholar] [CrossRef]
- Mathur, N.; Bhatnagar, P.; Verma, H. Genotoxicity of Vegetables Irrigated by Industrial Wastewater. J. Environ. Sci. 2006, 18, 964–968. [Google Scholar] [CrossRef]
- Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules 2023, 28, 5954. [Google Scholar] [CrossRef] [PubMed]
- Ataei, M.; Maghsoudi, A.S.; Hassani, S. Dyes and Colorants. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 3, ISBN 978-0-12824-315-2. [Google Scholar] [CrossRef]
- Mustroph, H. Streptocyanine Dyes. Phys. Sci. Rev. 2021, 6, 137–147. [Google Scholar] [CrossRef]
- Mustroph, H. Polymethine Dyes. Phys. Sci. Rev. 2020, 5, 20190084. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Croce, R.; Cinà, F.; Lombardo, A.; Crispeyn, G.; Cappelli, C.I.; Vian, M.; Maiorana, S.; Benfenati, E.; Baderna, D. Aquatic Toxicity of Several Textile Dye Formulations: Acute and Chronic Assays with Daphnia Magna and Raphidocelis Subcapitata. Ecotoxicol. Environ. Saf. 2017, 144, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bharadvaja, N. Microorganisms: A Remedial Source for Dye Pollution. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12821-014-7. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes 2023, 11, 855. [Google Scholar] [CrossRef]
- Gita, S.; Shukla, S.P.; Deshmukhe, G.; Choudhury, T.G.; Saharan, N.; Singh, A.K. Toxicity Evaluation of Six Textile Dyes on Growth, Metabolism and Elemental Composition (C, H, N, S) of Microalgae Spirulina Platensis: The Environmental Consequences. Bull. Environ. Contam. Toxicol. 2021, 106, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Dave, S.; Das, J. Photocatalytic Degradation of Dyes in Textile Effluent: A Green Approach to Eradicate Environmental Pollution. In The Future of Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12822-956-9. [Google Scholar] [CrossRef]
- Atalay, S.; Ersöz, G. Hybrid Application of Advanced Oxidation Processes to Dyes’ Removal. In Green Chemistry and Water Remediation: Research and Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12817-742-6. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A Critical Review on Advances in the Practices and Perspectives for the Treatment of Dye Industry Wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Li, Z.-D.; Cheng, P.; Zhang, H.-X.; Bao, C.-H.; Zhu, X.-M.; Shao, B.-Y.; Ma, J. Perspectives on Dye Wastewater Treatment. Oxid. Commun. 2015, 38, 1470–1479. [Google Scholar]
- Bayineni, V.K. Bioremediation of Toxic Dyes for Zero Waste. In Biotechnology for Zero Waste: Emerging Waste Management Techniques; Wiley: Hoboken, NJ, USA, 2021; ISBN 978-3-52783-206-4. [Google Scholar] [CrossRef]
- Roy, M.; Saha, R. Dyes and Their Removal Technologies from Wastewater: A Critical Review. In Intelligent Environmental Data Monitoring for Pollution Management; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12819-671-7. [Google Scholar] [CrossRef]
- Thakare, S.R.; Thakare, J.; Kosankar, P.T.; Pal, M.R. A Chief, Industrial Waste, Activated Red Mud for Subtraction of Methylene Blue Dye from Environment. Mater. Today Proc. 2019, 29, 822–827. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B.; Authilingam, S.; Sivakumar, S.V. A Comprehensive Review of Effective Adsorbents Used for the Removal of Dyes from Wastewater. Curr. Anal. Chem. 2022, 18, 255–268. [Google Scholar] [CrossRef]
- Ernawati, L.; Reza, M.; Synthia, A.C.; Kartikasari, D.A.; Maharsih, I.K.; Halim, A. Role of Chemical Activating Agent on the Characteristics of Activated Carbon Derived from Fruit-Peel Waste for Aqueous Dye Removal. Key Eng. Mater. 2022, 937, 165–180. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Tiwari, A.; Shankar, R.; Khare, P. Recent Trends of Hybrid Systems and Their Importance in Dye Degradation. In Photocatalytic Degradation of Dyes; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12823-876-9. [Google Scholar] [CrossRef]
- Yew, G.Y.; Tan, X.; Chew, K.W.; Chang, J.-S.; Tao, Y.; Jiang, N.; Show, P.L. Thermal-Fenton Mechanism with Sonoprocessing for Rapid Non-Catalytic Transesterification of Microalgal to Biofuel Production. Chem. Eng. J. 2021, 408, 127264. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, S.; Pan, X.; Zhou, F.; Sun, Y.; Liu, M.; Zhang, D.; Zhang, L. Fe Nanoparticles Synthesized by Pomegranate Leaves for Treatment of Malachite Green. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2022, 37, 350–354. [Google Scholar] [CrossRef]
- Kirti; Kamsonlian, S.; Agarwal, V. Review on Synthesis of Plant-Mediated Green Iron Nanoparticles and Their Application for Decolorization of Dyes. Mater. Today Proc. 2022, 78, 99–107. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Biomolecules in Grape Leaf Extract Involved in One-Step Synthesis of Iron-Based Nanoparticles. RSC Adv. 2014, 4, 53467–53474. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. Characterization of Bimetallic Fe/Pd Nanoparticles by Grape Leaf Aqueous Extract and Identification of Active Biomolecules Involved in the Synthesis. Sci. Total Environ. 2016, 562, 526–532. [Google Scholar] [CrossRef]
- Monga, Y.; Kumar, P.; Sharma, R.K.; Filip, J.; Varma, R.S.; Zbořil, R.; Gawande, M.B. Sustainable Synthesis of Nanoscale Zerovalent Iron Particles for Environmental Remediation. ChemSusChem 2020, 13, 3288–3305. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. The Mechanism for Degrading Orange II Based on Adsorption and Reduction by Ion-Based Nanoparticles Synthesized by Grape Leaf Extract. J. Hazard. Mater. 2015, 296, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, A.; Ma, J.; Fu, M. Facile Green Synthesis of Functional Nanoscale Zero-Valent Iron and Studies of Its Activity toward Ultrasound-Enhanced Decolorization of Cationic Dyes. Chemosphere 2017, 166, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of Bromothymol Blue by “greener” Nano-Scale Zero-Valent Iron Synthesized Using Tea Polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyaci, E.; Eroĝlu, A.E.; Scott, T.B.; Hallam, K.R. Green Synthesis of Iron Nanoparticles and Their Application as a Fenton-like Catalyst for the Degradation of Aqueous Cationic and Anionic Dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of Iron-Based Nanoparticles Using Oolong Tea Extract for the Degradation of Malachite Green. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, J.; Motamedi, E.; Naghavi, M.R.; Ghafoori, M. Removal of Crystal Violet from Water Using β-Cyclodextrin Functionalized Biogenic Zero-Valent Iron Nanoadsorbents Synthesized via Aqueous Root Extracts of Ferula Persica. J. Hazard. Mater. 2019, 367, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A.K.; Kumar, G.; Emran, T.B. Antibacterial and Dye Degradation Activity of Green Synthesized Iron Nanoparticles. J. Nanomater. 2022, 2022, 3636481. [Google Scholar] [CrossRef]
- Haseena, S.; Shanavas, S.; Ahamad, T.; Alshehri, S.M.; Baskaran, P.; Duraimurugan, J.; Acevedo, R.; Khan, M.A.M.; Anbarasan, P.M.; Jayamani, N. Investigation on Photocatalytic Activity of Bio-Treated α-Fe2O3 Nanoparticles Using Phyllanthus Niruri and Moringa Stenopetala Leaf Extract against Methylene Blue and Phenol Molecules: Kinetics, Mechanism and Stability. J. Environ. Chem. Eng. 2021, 9, 104996. [Google Scholar] [CrossRef]
- Shaker Ardakani, L.; Alimardani, V.; Tamaddon, A.M.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron-Based Nanoparticles Using Chlorophytum Comosum Leaf Extract: Methyl Orange Dye Degradation and Antimicrobial Properties. Heliyon 2021, 7, e06159. [Google Scholar] [CrossRef]
- Rawat, S.; Samreen, K.; Nayak, A.K.; Singh, J.; Koduru, J.R. Fabrication of Iron Nanoparticles Using Parthenium: A Combinatorial Eco-Innovative Approach to Eradicate Crystal Violet Dye and Phosphate from the Aqueous Environment. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100426. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Narasimhan, M.K.; Pugazhendhi, A.; Selvaraj, R. Structural Characterization and Adsorptive Ability of Green Synthesized Fe3O4 Nanoparticles to Remove Acid Blue 113 Dye. Surf. Interfaces 2021, 23, 100947. [Google Scholar] [CrossRef]
- Balu, P.; Asharani, I.V.; Thirumalai, D. Catalytic Degradation of Hazardous Textile Dyes by Iron Oxide Nanoparticles Prepared from Raphanus Sativus Leaves’ Extract: A Greener Approach. J. Mater. Sci. Mater. Electron. 2020, 31, 10669–10676. [Google Scholar] [CrossRef]
- Noreen, S.; Mustafa, G.; Ibrahim, S.M.; Naz, S.; Iqbal, M.; Yaseen, M.; Javed, T.; Nisar, J. Iron Oxide (Fe2O3) Prepared via Green Route and Adsorption Efficiency Evaluation for an Anionic Dye: Kinetics, Isotherms and Thermodynamics Studies. J. Mater. Res. Technol. 2020, 9, 4206–4217. [Google Scholar] [CrossRef]
- Das, C.; Sen, S.; Singh, T.; Ghosh, T.; Paul, S.S.; Kim, T.W.; Jeon, S.; Maiti, D.K.; Im, J.; Biswas, G. Green Synthesis, Characterization and Application of Natural Product Coated Magnetite Nanoparticles for Wastewater Treatment. Nanomaterials 2020, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Jadidi Kouhbanani, M.A.; Beheshtkhoo, N.; Amani, A.M.; Taghizadeh, S.; Beigi, V.; Zakeri Bazmandeh, A.; Khalaf, N. Green Synthesis of Iron Oxide Nanoparticles Using Artemisia Vulgaris Leaf Extract and Their Application as a Heterogeneous Fenton-like Catalyst for the Degradation of Methyl Orange. Mater. Res. Express 2018, 5, 115013. [Google Scholar] [CrossRef]
- Beheshtkhoo, N.; Kouhbanani, M.A.J.; Savardashtaki, A.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron Oxide Nanoparticles by Aqueous Leaf Extract of Daphne Mezereum as a Novel Dye Removing Material. Appl. Phys. A Mater. Sci. Process 2018, 124, 363. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Sultan, M.; Naz, I. Green Synthesis of Phytogenic Magnetic Nanoparticles and Their Applications in the Adsorptive Removal of Crystal Violet from Aqueous Solution. Arab. J. Sci. Eng. 2018, 43, 6245–6259. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Green Synthesized Nanoclusters of Ultra-Small Zero Valent Iron Nanoparticles as a Novel Dye Removing Material. Sci. Total Environ. 2018, 621, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Somchaidee, P.; Tedsree, K. Green Synthesis of High Dispersion and Narrow Size Distribution of Zero-Valent Iron Nanoparticles Using Guava Leaf (Psidium guajava L.) Extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035006. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A. Green Synthesis of Zero-Valent Fe-Nanoparticles: Catalytic Degradation of Rhodamine B, Interactions with Bovine Serum Albumin and Their Enhanced Antimicrobial Activities. J. Photochem. Photobiol. B 2018, 180, 259–267. [Google Scholar] [CrossRef]
- Radini, I.A.; Hasan, N.; Malik, M.A.; Khan, Z. Biosynthesis of Iron Nanoparticles Using Trigonella Foenum-Graecum Seed Extract for Photocatalytic Methyl Orange Dye Degradation and Antibacterial Applications. J. Photochem. Photobiol. B 2018, 183, 154–163. [Google Scholar] [CrossRef]
- Ozkan, Z.Y.; Cakirgoz, M.; Kaymak, E.S.; Erdim, E. Rapid Decolorization of Textile Wastewater by Green Synthesized Iron Nanoparticles. Water Sci. Technol. 2018, 77, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Shariati, S.; Besharati, N. Adsorption of Crystal Violet and Methylene Blue on Azolla and Fig Leaves Modified with Magnetite Iron Oxide Nanoparticles. Int. J. Environ. Res. 2017, 11, 197–206. [Google Scholar] [CrossRef]
- Prasad, C.; Yuvaraja, G.; Venkateswarlu, P. Biogenic Synthesis of Fe3O4 Magnetic Nanoparticles Using Pisum Sativum Peels Extract and Its Effect on Magnetic and Methyl Orange Dye Degradation Studies. J. Magn. Magn. Mater. 2017, 424, 376–381. [Google Scholar] [CrossRef]
- Weng, X.; Guo, M.; Luo, F.; Chen, Z. One-Step Green Synthesis of Bimetallic Fe/Ni Nanoparticles by Eucalyptus Leaf Extract: Biomolecules Identification, Characterization and Catalytic Activity. Chem. Eng. J. 2017, 308, 904–911. [Google Scholar] [CrossRef]
- Prasad, C.; Karlapudi, S.; Venkateswarlu, P.; Bahadur, I.; Kumar, S. Green Arbitrated Synthesis of Fe3O4 Magnetic Nanoparticles with Nanorod Structure from Pomegranate Leaves and Congo Red Dye Degradation Studies for Water Treatment. J. Mol. Liq. 2017, 240, 322–328. [Google Scholar] [CrossRef]
- Prasad, C.; Sreenivasulu, K.; Gangadhara, S.; Venkateswarlu, P. Bio Inspired Green Synthesis of Ni/Fe3O4magnetic Nanoparticles Using Moringa Oleifera Leaves Extract: A Magnetically Recoverable Catalyst for Organic Dye Degradation in Aqueous Solution. J. Alloys Compd. 2017, 700, 252–258. [Google Scholar] [CrossRef]
- Singh, K.K.; Senapati, K.K.; Sarma, K.C. Synthesis of Superparamagnetic Fe3O4 Nanoparticles Coated with Green Tea Polyphenols and Their Use for Removal of Dye Pollutant from Aqueous Solution. J. Environ. Chem. Eng. 2017, 5, 2214–2221. [Google Scholar] [CrossRef]
- Khaghani, S.; Ghanbari, D.; Khaghani, S. Green Synthesis of Iron Oxide-Palladium Nanocomposites by Pepper Extract and Its Application in Removing of Colored Pollutants from Water. J. Nanostruct. 2017, 7, 175–182. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile Synthesis of Magnetic Iron Oxide Nanoparticles Using Inedible Cynometra Ramiflora Fruit Extract Waste and Their Photocatalytic Degradation of Methylene Blue Dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Groiss, S.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Structural Characterization, Antibacterial and Catalytic Effect of Iron Oxide Nanoparticles Synthesised Using the Leaf Extract of Cynometra Ramiflora. J. Mol. Struct. 2017, 1128, 572–578. [Google Scholar] [CrossRef]
- Natarajan, E.; Ponnaiah, G.P. Optimization of Process Parameters for the Decolorization of Reactive Blue 235 Dye by Barium Alginate Immobilized Iron Nanoparticles Synthesized from Aluminum Industry Waste. Environ. Nanotechnol. Monit. Manag. 2017, 7, 73–88. [Google Scholar] [CrossRef]
- Lin, J.; Weng, X.; Dharmarajan, R.; Chen, Z. Characterization and Reactivity of Iron Based Nanoparticles Synthesized by Tea Extracts under Various Atmospheres. Chemosphere 2017, 169, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Cheera, P.; Karlapudi, S.; Sellola, G.; Ponneri, V. A Facile Green Synthesis of Spherical Fe3O4 Magnetic Nanoparticles and Their Effect on Degradation of Methylene Blue in Aqueous Solution. J. Mol. Liq. 2016, 221, 993–998. [Google Scholar] [CrossRef]
- Xin, H.; Yang, X.; Liu, X.; Tang, X.; Weng, L.; Han, Y. Biosynthesis of Iron Nanoparticles Using Tie Guanyin Tea Extract for Degradation of Bromothymol Blue. J. Nanotechnol. 2016, 2016, 4059591. [Google Scholar] [CrossRef]
- Truskewycz, A.; Shukla, R.; Ball, A.S. Iron Nanoparticles Synthesized Using Green Tea Extracts for the Fenton-like Degradation of Concentrated Dye Mixtures at Elevated Temperatures. J. Environ. Chem. Eng. 2016, 4, 4409–4417. [Google Scholar] [CrossRef]
- Tan, K.A.; Morad, N.; Teng, T.T.; Norli, I. Synthesis of Magnetic Nanocomposites (AMMC-Fe3O4) for Cationic Dye Removal: Optimization, Kinetic, Isotherm, and Thermodynamics Analysis. J. Taiwan. Inst. Chem. Eng. 2015, 54, 96–108. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Megharaj, M. Characterization of Iron-Polyphenol Nanoparticles Synthesized by Three Plant Extracts and Their Fenton Oxidation of Azo Dye. ACS Sustain. Chem. Eng. 2014, 2, 1022–1025. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Iron Nanoparticles by Various Tea Extracts: Comparative Study of the Reactivity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef]
- Kimpiab, E.; Kapiamba, K.F.; Folifac, L.; Oyekola, O.; Petrik, L. Synthesis of Stabilized Iron Nanoparticles from Acid Mine Drainage and Rooibos Tea for Application as a Fenton-like Catalyst. ACS Omega 2022, 7, 24423–24431. [Google Scholar] [CrossRef]
- Sravanthi, K.; Ayodhya, D.; Yadgiri Swamy, P. Green Synthesis, Characterization of Biomaterial-Supported Zero-Valent Iron Nanoparticles for Contaminated Water Treatment. J. Anal. Sci. Technol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Harshiny, M.; Iswarya, C.N.; Matheswaran, M. Biogenic Synthesis of Iron Nanoparticles Using Amaranthus Dubius Leaf Extract as a Reducing Agent. Powder Technol. 2015, 286, 744–749. [Google Scholar] [CrossRef]
- Parmakoğlu, E.Ü.; Çay, A.; Yanık, J. Valorization of Solid Wastes from Textile Industry as an Adsorbent Through Activated Carbon Production. AATCC J. Res. 2023, 10, 133–143. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Song, Y.; Kim, H.Y.; Nguyen, D.T.; Bae, H.J. Converting Textile Waste into Value-Added Chemicals: An Integrated Bio-Refinery Process. Environ. Sci. Ecotechnol. 2023, 15, 100238. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, F.; Fornaroli, R.; Clagnan, E.; Brusetti, L.; Ficara, E.; Bellucci, M.; Mezzanotte, V. Wastewater from Textile Digital Printing as a Substrate for Microalgal Growth and Valorization. Bioresour. Technol. 2023, 375, 128828. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from Biomass: Technological versus Environmental Feasibility. A Review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
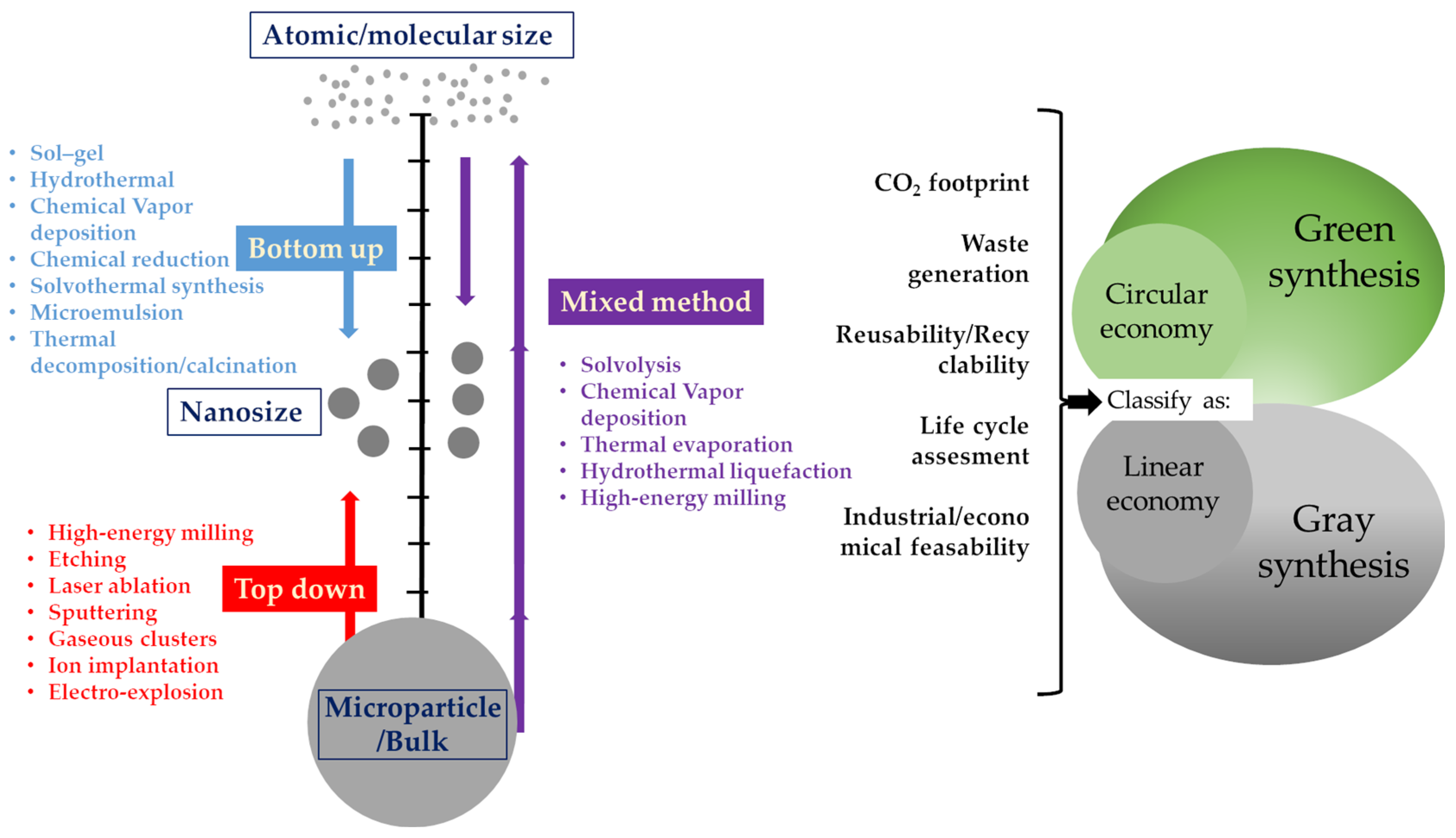

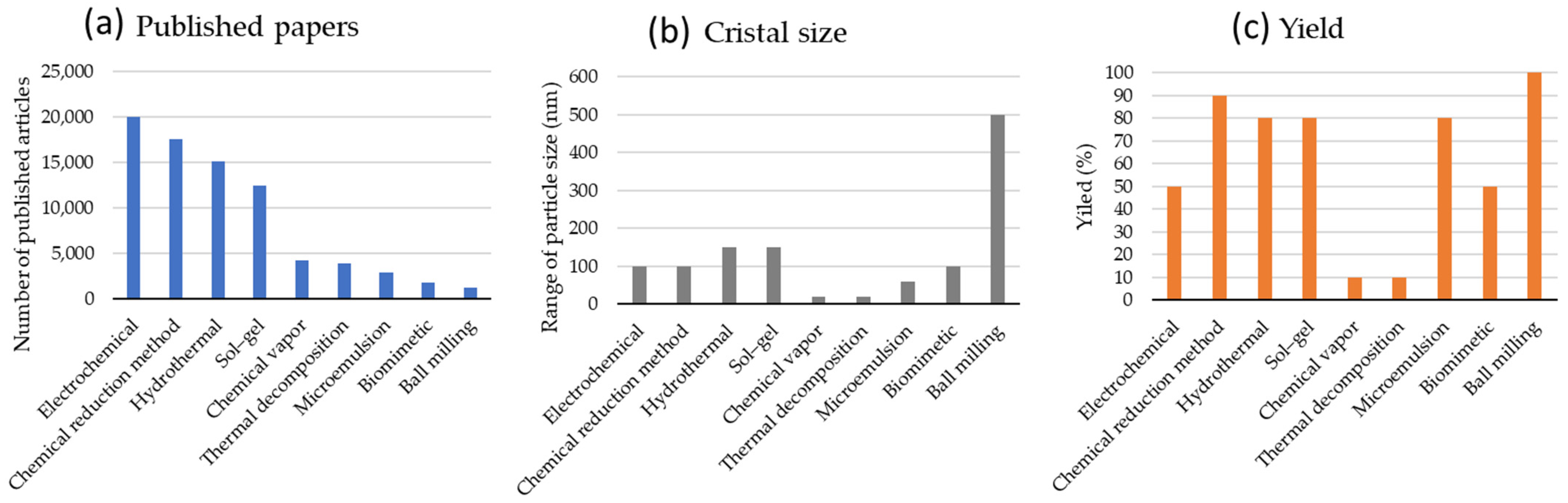

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rasero, C.; Montes-Jimenez, V.; Alexandre-Franco, M.F.; Fernández-González, C.; Píriz-Tercero, J.; Cuerda-Correa, E.M. Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water 2024, 16, 1607. https://doi.org/10.3390/w16111607
Rodríguez-Rasero C, Montes-Jimenez V, Alexandre-Franco MF, Fernández-González C, Píriz-Tercero J, Cuerda-Correa EM. Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water. 2024; 16(11):1607. https://doi.org/10.3390/w16111607
Chicago/Turabian StyleRodríguez-Rasero, Cristina, Vicente Montes-Jimenez, María F. Alexandre-Franco, Carmen Fernández-González, Jesús Píriz-Tercero, and Eduardo Manuel Cuerda-Correa. 2024. "Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review" Water 16, no. 11: 1607. https://doi.org/10.3390/w16111607
APA StyleRodríguez-Rasero, C., Montes-Jimenez, V., Alexandre-Franco, M. F., Fernández-González, C., Píriz-Tercero, J., & Cuerda-Correa, E. M. (2024). Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water, 16(11), 1607. https://doi.org/10.3390/w16111607









