Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Brine Water Preparation
2.3. Procedure of Ceramic Support Membrane Fabrication
2.4. Intermediate Layer
2.5. Preparation of the Hydrophobic Membrane
2.6. Characterization of Fabricated Membranes
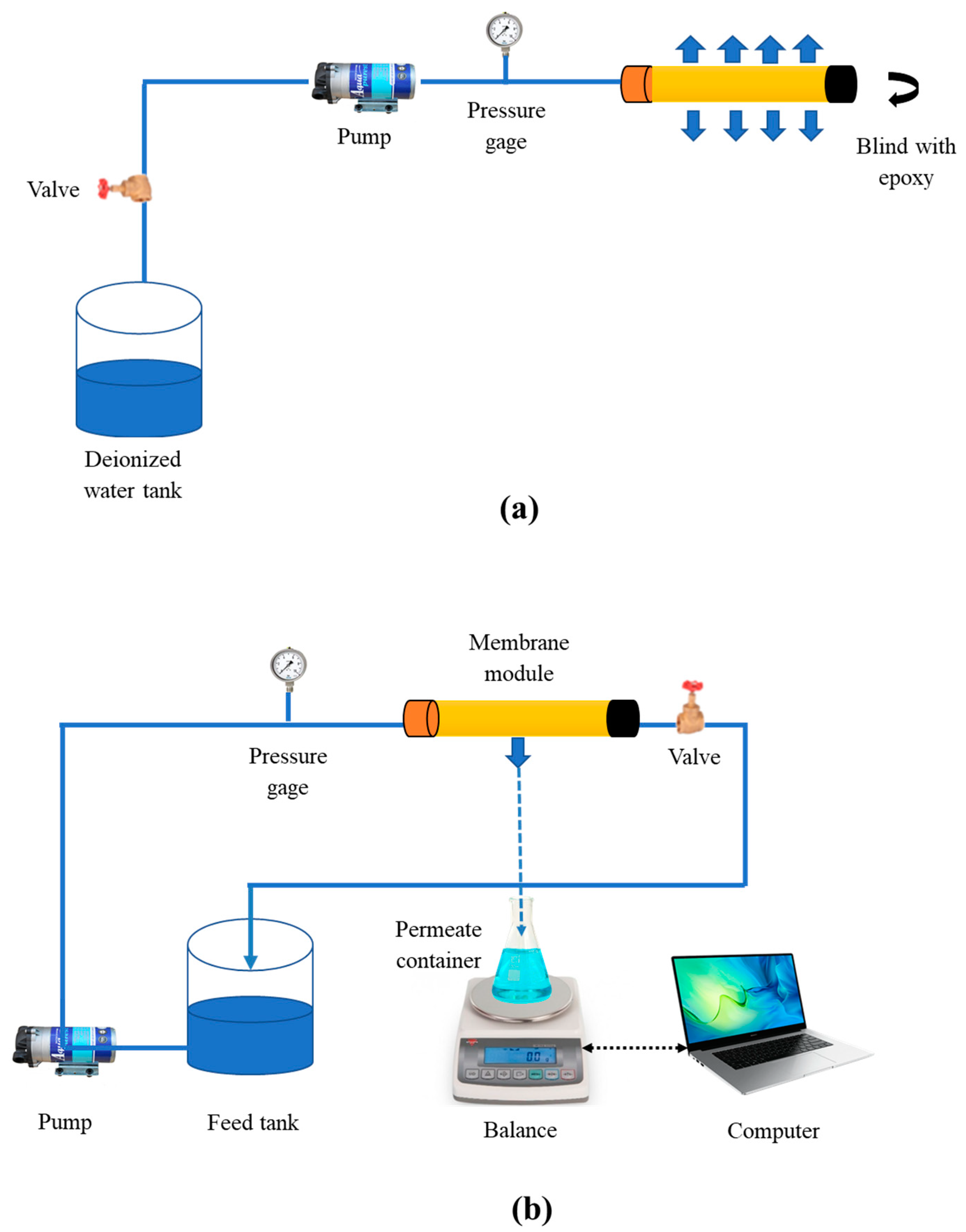
2.7. Performance Test and Apparatus
3. Results and Discussion
3.1. Characterization of the Hydrophobic Ceramic Membranes
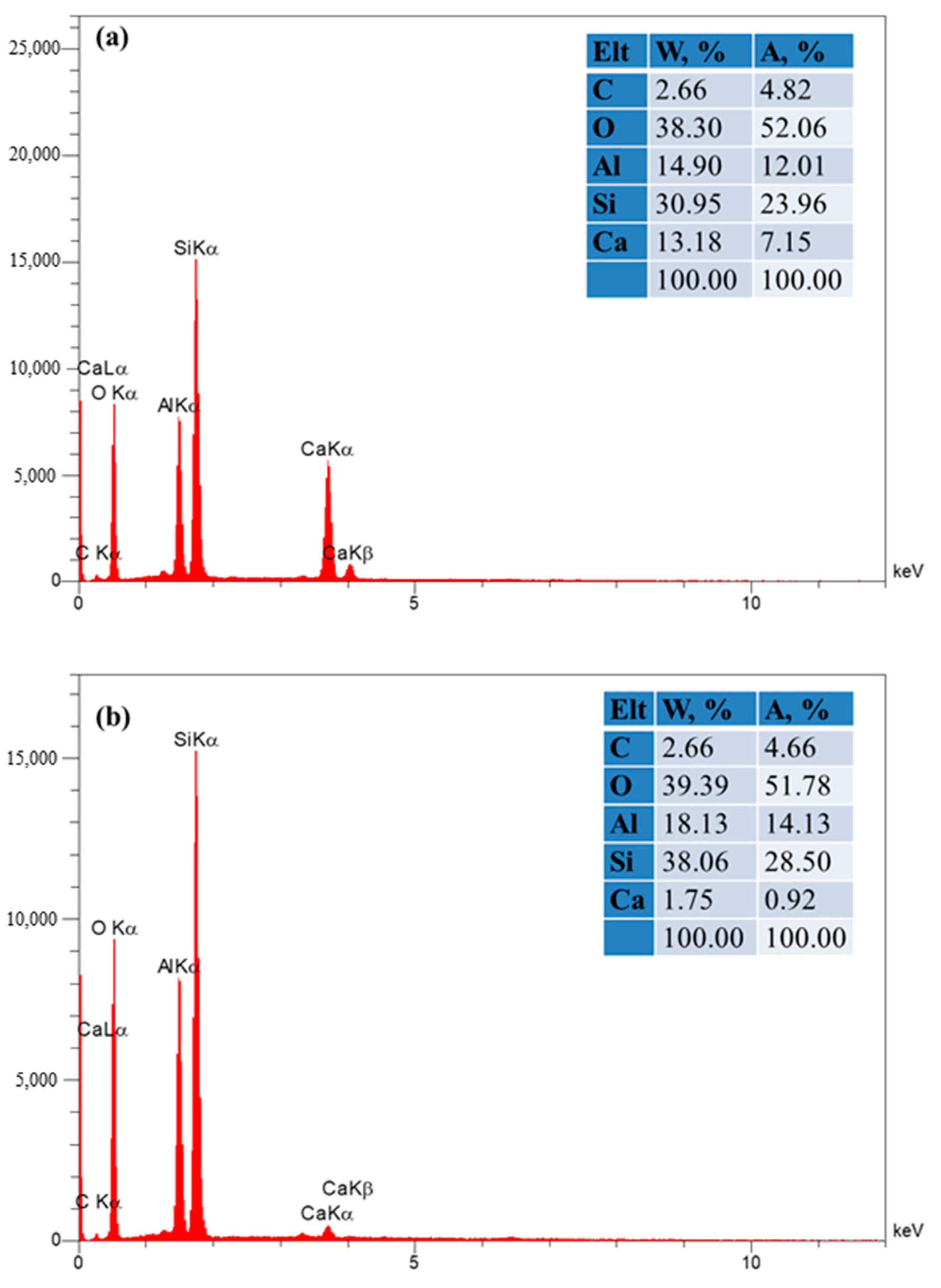
3.1.1. EDS Results
3.1.2. FESEM Results
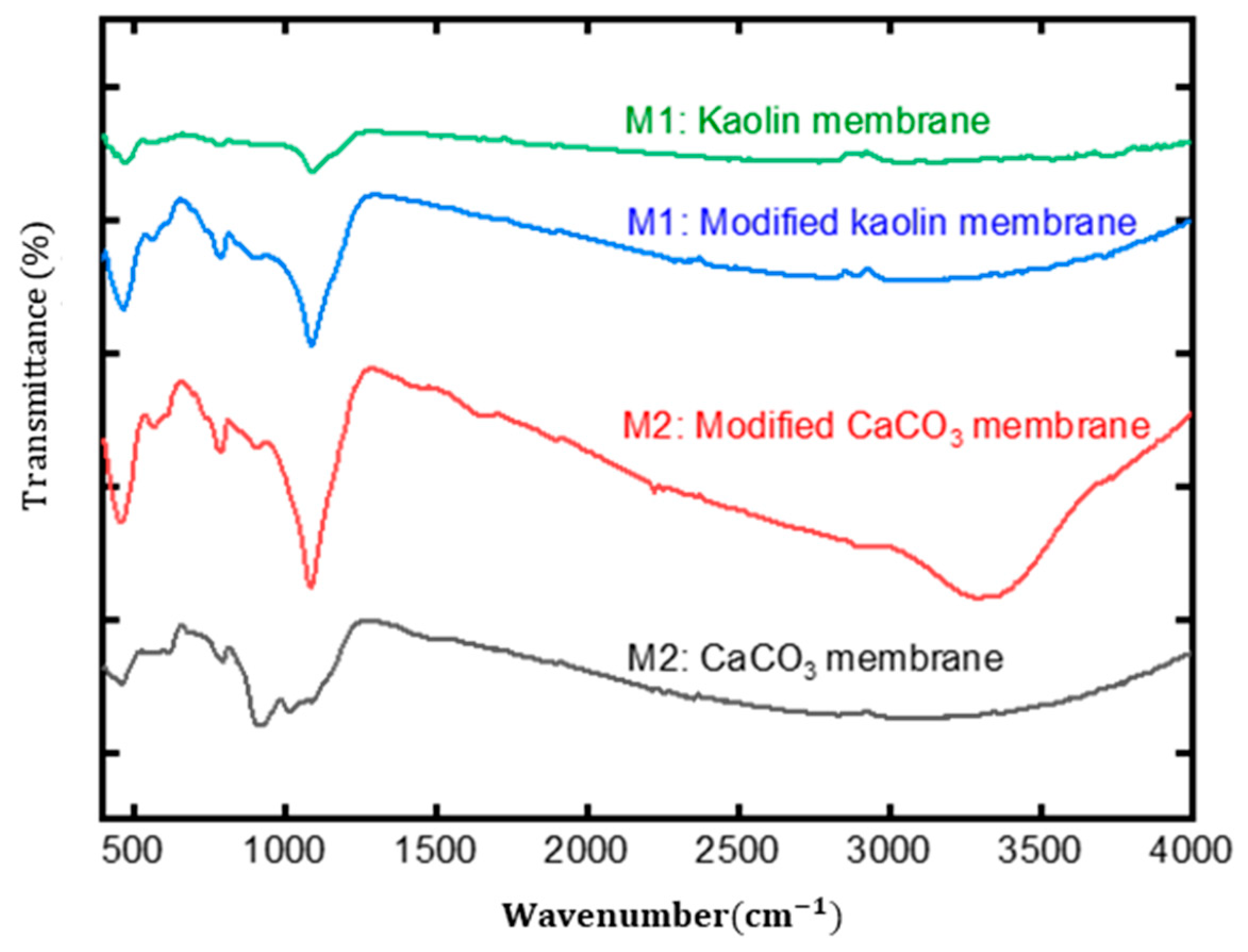
3.1.3. FT-IR Results
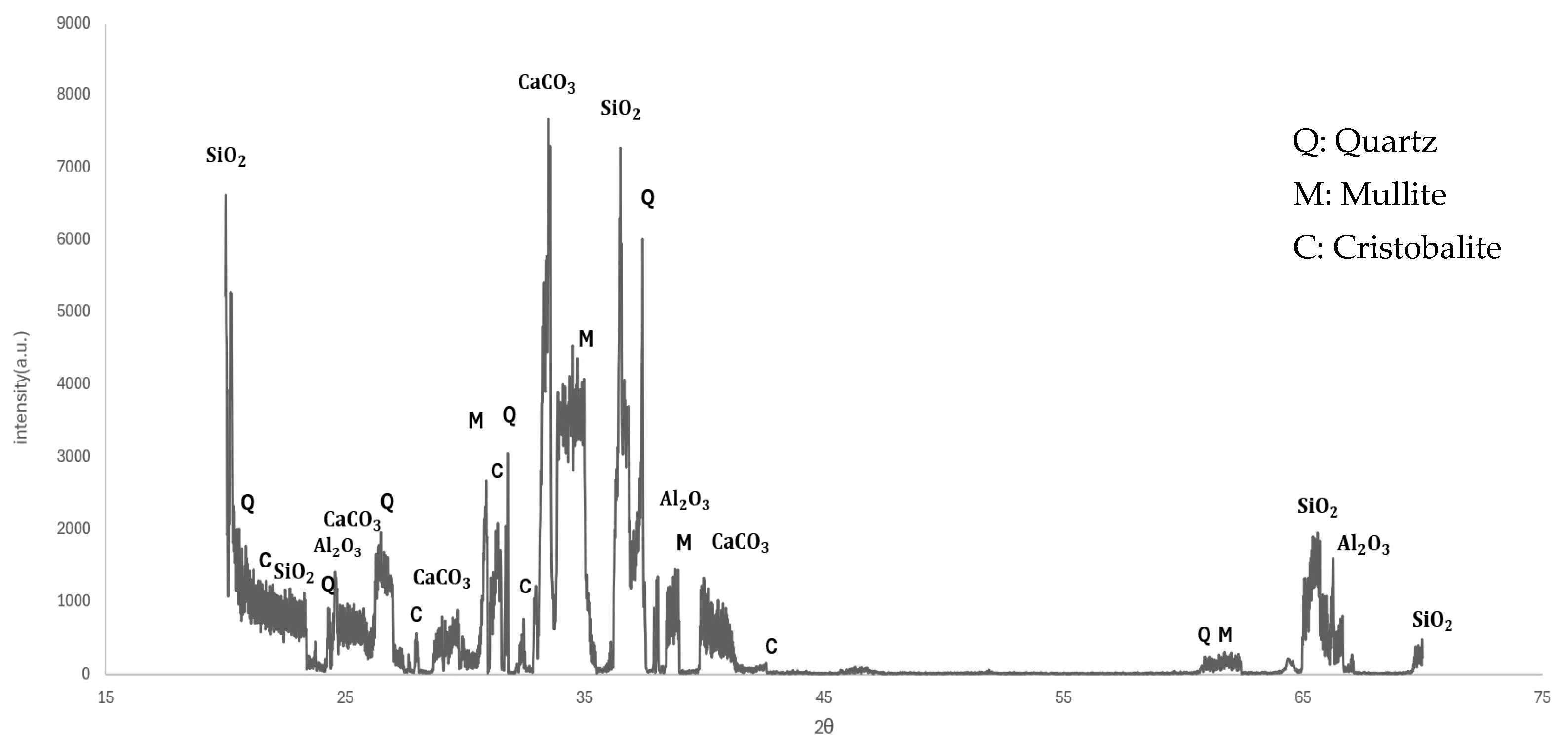
3.1.4. XRD Results
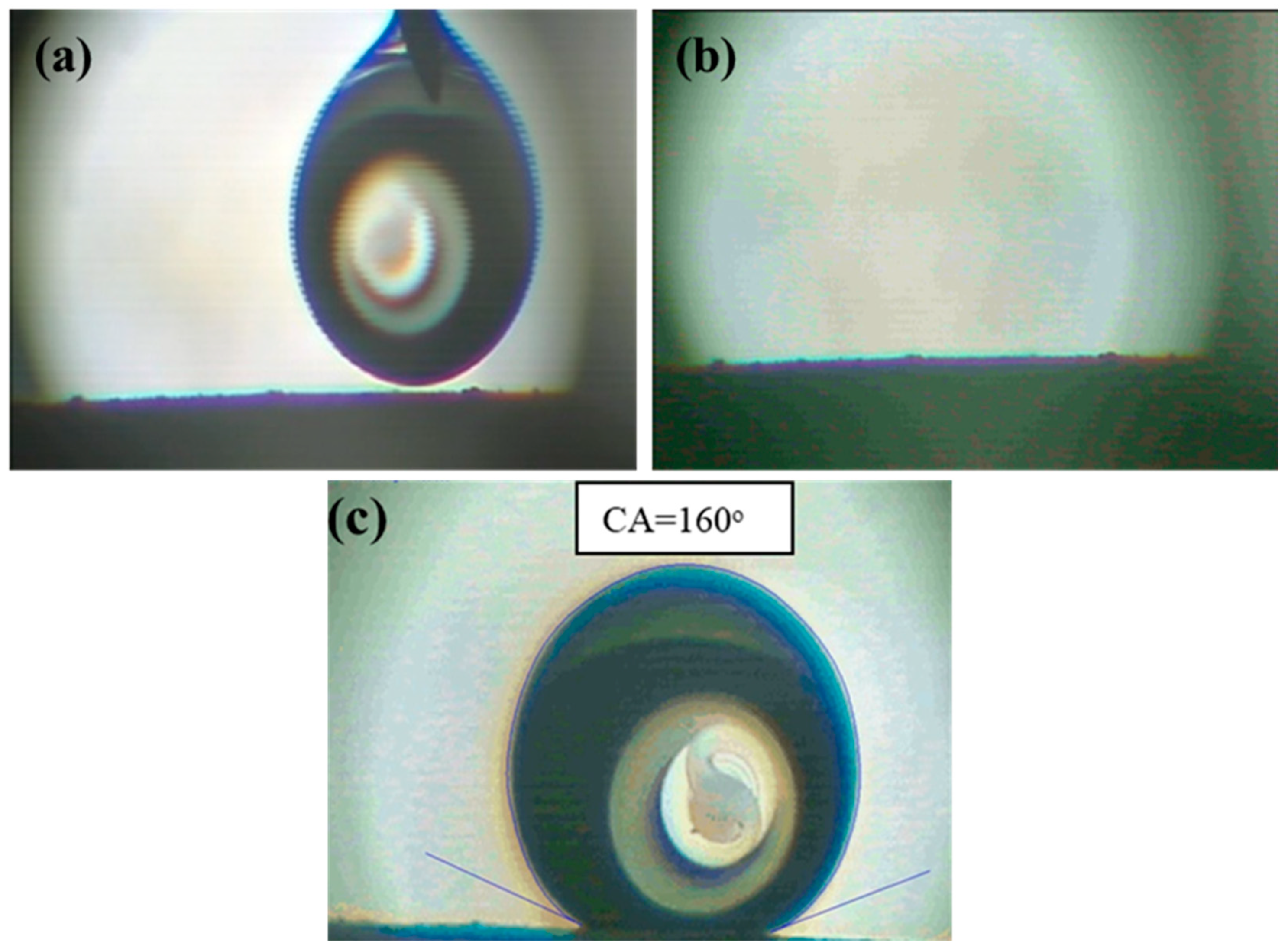
3.1.5. Contact Angle Results
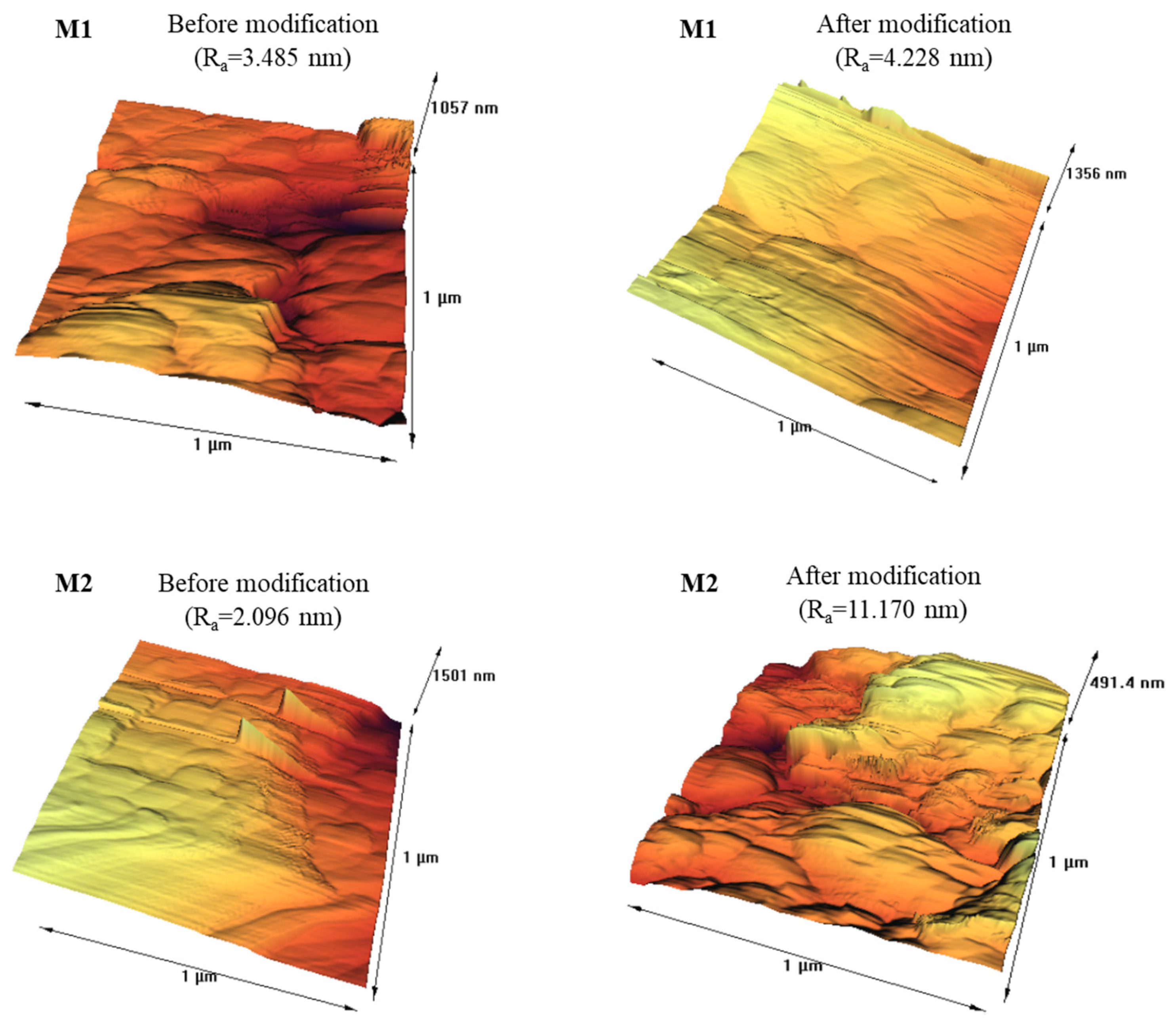
3.1.6. AFM Results
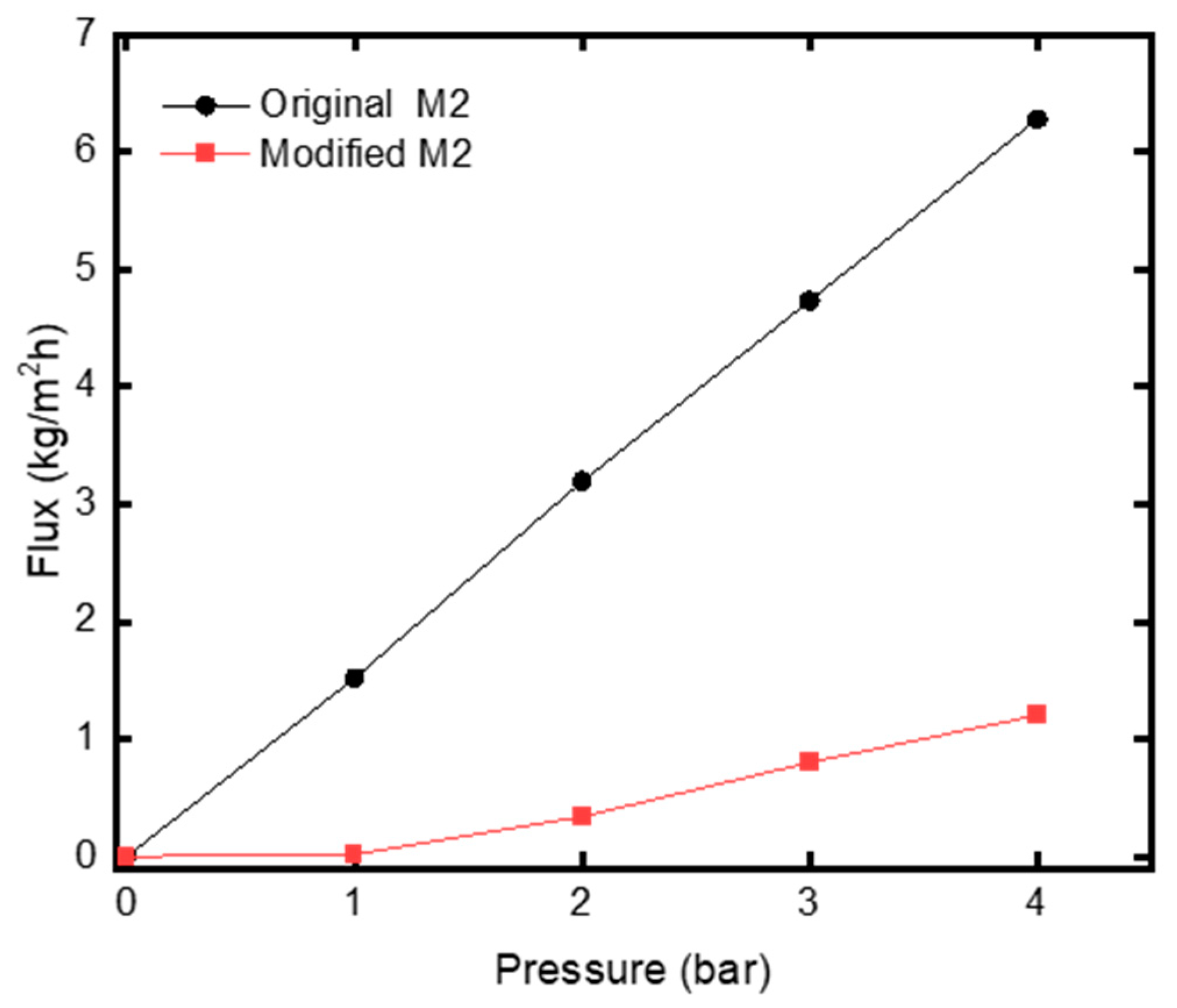
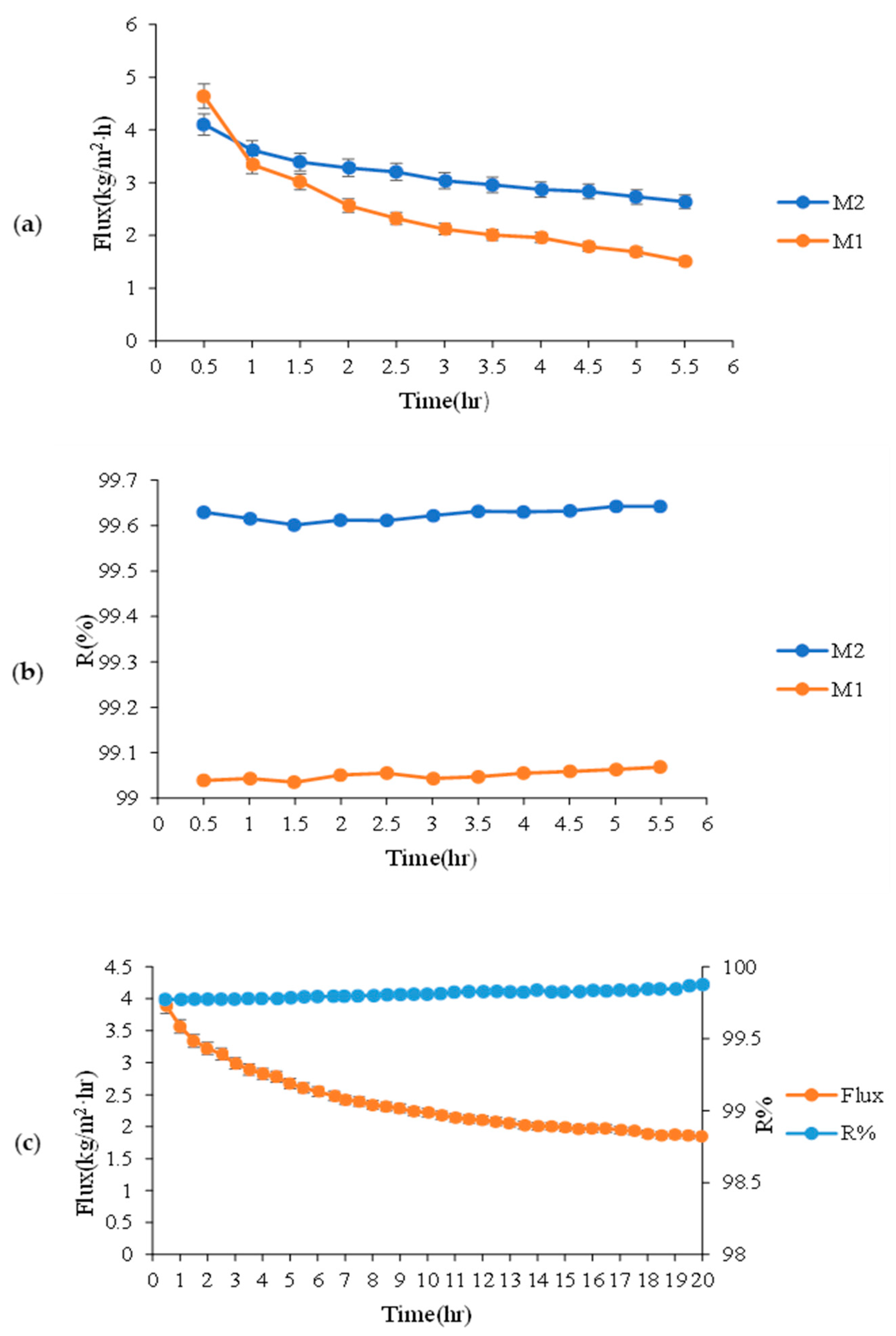
3.2. Effect of Modification on Water Permeation Flux
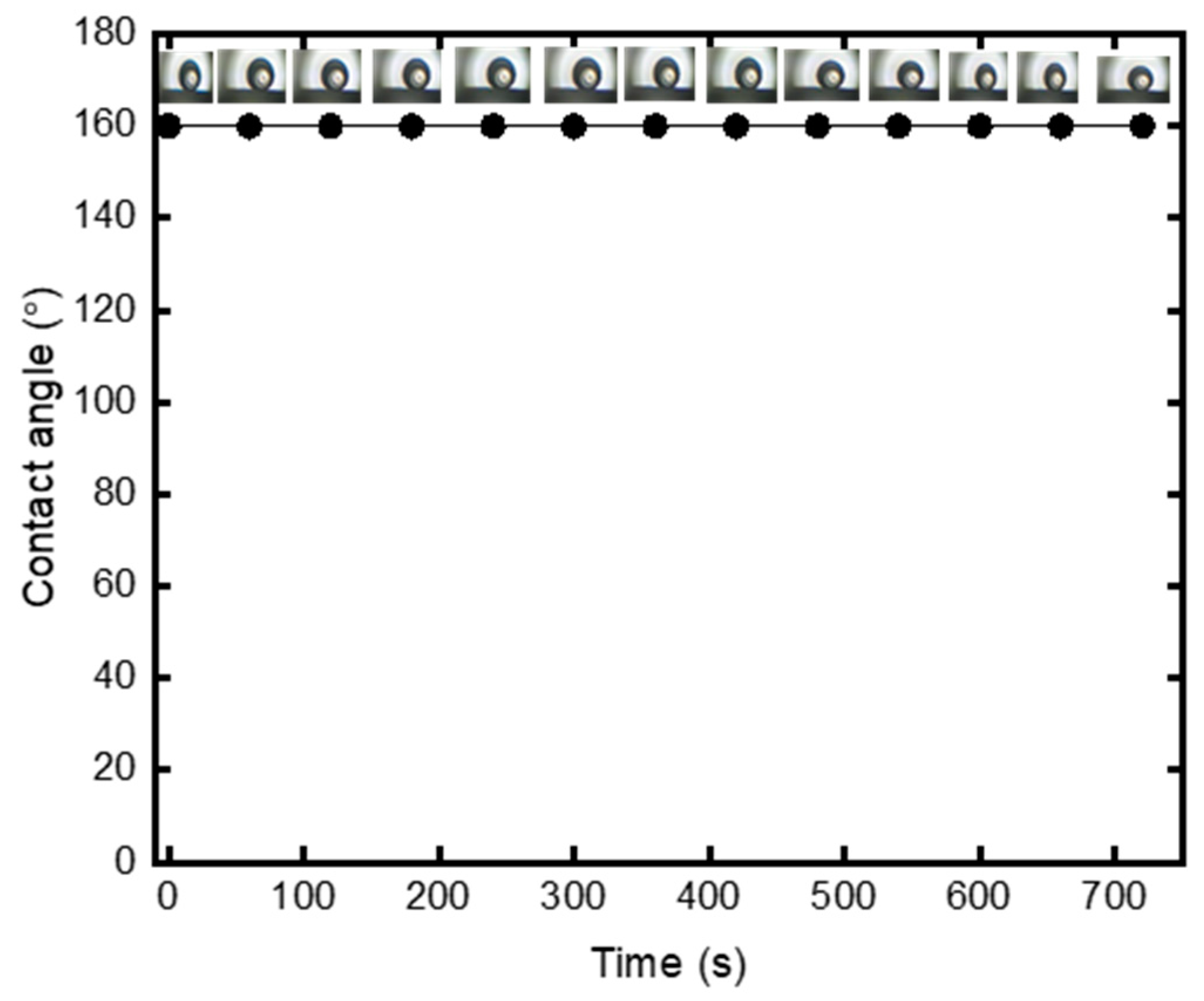
3.3. Modified Membrane Hydrophobicity Durability with Time
3.4. Liquid Entry Pressure
3.5. Evaluation of Fabricated Membrane Performance in MD Application
3.6. Cost Analysis Based on Raw Material and Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hubadillah, S.K.; Tai, Z.S.; Othman, M.H.D.; Harun, Z.; Jamalludin, M.R.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Hydrophobic ceramic membrane for membrane distillation: A mini review on preparation, characterization, and applications. Sep. Purif. Technol. 2019, 217, 71–84. [Google Scholar] [CrossRef]
- Bandar, K.B.; Alsubei, M.D.; Aljlil, S.A.; Darwish, N.B.; Hilal, N. Membrane distillation process application using a novel ceramic membrane for Brackish water desalination. Desalination 2021, 500, 114906. [Google Scholar] [CrossRef]
- Tai, Z.S.; Abd Aziz, M.H.; Othman, M.H.D.; Mohamed Dzahir, M.I.H.; Hashim, N.A.; Koo, K.N.; Hubadillah, S.K.; Ismail, A.F.; A Rahman, M.; Jaafar, J. Ceramic membrane distillation for desalination. Sep. Purif. Rev. 2020, 49, 317–356. [Google Scholar] [CrossRef]
- Alihemati, Z.; Hashemifard, S.; Matsuura, T.; Ismail, A.; Hilal, N. Current status and challenges of fabricating thin film composite forward osmosis membrane: A comprehensive roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Twibi, M.F.; Othman, M.H.D.; Hubadillah, S.K.; Alftessi, S.A.; Adam, M.R.B.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Abd Aziz, M.H. Hydrophobic mullite ceramic hollow fibre membrane (Hy-MHFM) for seawater desalination via direct contact membrane distillation (DCMD). J. Eur. Ceram. Soc. 2021, 41, 6578–6585. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Ferreira, R.K.M.; Ramlow, H.; Marangoni, C.; Machado, R.A.F. A review on the manufacturing techniques of porous hydrophobic ceramic membranes applied to direct contact membrane distillation. Adv. Appl. Ceram. 2021, 120, 336–357. [Google Scholar] [CrossRef]
- Dow, N.; García, J.V.; Niadoo, L.; Milne, N.; Zhang, J.; Gray, S.; Duke, M. Demonstration of membrane distillation on textile waste water: Assessment of long term performance, membrane cleaning and waste heat integration. Environ. Sci. Water Res. Technol. 2017, 3, 433–449. [Google Scholar] [CrossRef]
- Khayet, M.; Souhaimi, M.K.; Matsuura, T. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- He, F.; Gilron, J.; Lee, H.; Song, L.; Sirkar, K.K. Potential for scaling by sparingly soluble salts in crossflow DCMD. J. Membr. Sci. 2008, 311, 68–80. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Fu, K.; Qiu, M.; Xiong, F.; Ding, D.; Cui, Z.; Wang, Z.; Fan, Y.; Drioli, E. Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 2018, 443, 212–220. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, L.; Zhang, J.-W.; Xu, X.; Chen, C.-S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Ramlow, H.; Machado, R.A.F.; Marangoni, C. Direct contact membrane distillation for textile wastewater treatment: A state of the art review. Water Sci. Technol. 2017, 76, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Abdoli, A.; Khosravi, A.; Matsuura, T.; Abbasi, M. Predicting and evaluating the performance of DCMD: The effect of non-ideal morphology and thermal conductivity of porous nanocomposite membranes. Chem. Eng. Res. Des. 2023, 192, 638–652. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, X.-Z.; Fan, M.; Gu, J.-Q.; Hao, L.-Y.; Xu, X.; Chen, C.-S.; Wang, C.-M.; Hao, Y.-Z.; Agathopoulos, S. Porous β-Sialon planar membrane with a robust polymer-derived hydrophobic ceramic surface. J. Membr. Sci. 2017, 535, 63–69. [Google Scholar] [CrossRef]
- Kumar, R.V.; Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Dairy wastewater treatment using a novel low cost tubular ceramic membrane and membrane fouling mechanism using pore blocking models. J. Water Process Eng. 2016, 13, 168–175. [Google Scholar] [CrossRef]
- Song, I.-H.; Bae, B.-S.; Ha, J.-H.; Lee, J. Effect of hydraulic pressure on alumina coating on pore characteristics of flat-sheet ceramic membrane. Ceram. Int. 2017, 43, 10502–10507. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Mittal, P.; Jana, S.; Mohanty, K. Synthesis of low-cost hydrophilic ceramic–polymeric composite membrane for treatment of oily wastewater. Desalination 2011, 282, 54–62. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.; Srasra, E. Development of a low cost micro-porous ceramic membrane from kaolin and Alumina, using the lignite as porogen agent. Ceram. Int. 2016, 42, 5089–5093. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Yavna, V.; Daniel, P. DFT theoretical and FT-IR spectroscopic investigations of the plasticity of clay minerals dispersions. J. Mol. Struct. 2016, 1109, 97–105. [Google Scholar] [CrossRef]
- Cabassud, C.; Wirth, D. Membrane distillation for water desalination: How to chose an appropriate membrane? Desalination 2003, 157, 307–314. [Google Scholar] [CrossRef]
- Sarbatly, R.; Chiam, C.-K. Evaluation of geothermal energy in desalination by vacuum membrane distillation. Appl. Energy 2013, 112, 737–746. [Google Scholar] [CrossRef]
- Suárez, F.; Tyler, S.W. Comments on “Evaluation of systems coupling vacuum membrane distillation and solar energy for seawater desalination”. Chem. Eng. J. 2011, 178, 475–476. [Google Scholar] [CrossRef]
- Zrelli, A.; Chaouachi, B. Modeling and simulation of a vacuum membrane distillation plant coupled with solar energy and using helical hollow fibers. Braz. J. Chem. Eng. 2019, 36, 1119–1129. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-enhanced hydrophobic graphene oxide membrane for water desalination via membrane distillation. J. Membr. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Kurniawan, T.A.; El-badawy, T.; Goh, P.S.; Othman, N.H.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Bottlenecks and recent improvement strategies of ceramic membranes in membrane distillation applications: A review. J. Eur. Ceram. Soc. 2022, 42, 5179–5194. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Z.; Chen, J.-H. Preparation and characterisation of the novel hydrophobic FAS/α-Al2O3 composite membrane for membrane distillation. Mater. Res. Innov. 2022, 26, 168–175. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Gani, P.; Sunar, N.M.; Tai, Z.S.; Koo, K.N.; Pauzan, M.A.B.; Ismail, N.J.; Zahari, S.S.N.S. Integrated green membrane distillation-microalgae bioremediation for arsenic removal from Pengorak River Kuantan, Malaysia. Chem. Eng. Process. Process Intensif. 2020, 153, 107996. [Google Scholar] [CrossRef]
- García-Fernández, L.; Wang, B.; García-Payo, M.; Li, K.; Khayet, M. Morphological design of alumina hollow fiber membranes for desalination by air gap membrane distillation. Desalination 2017, 420, 226–240. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Fang, H.; Wang, J.-W.; Hao, L.-Y.; Xu, X.; Chen, C.-S. Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J. Membr. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Investigation of the stability of metal oxide powders and ceramic membranes grafted by perfluoroalkylsilanes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 109–117. [Google Scholar] [CrossRef]
- Dhore, R.; Murthy, G.S. Per/polyfluoroalkyl substances production, applications and environmental impacts. Bioresour. Technol. 2021, 341, 125808. [Google Scholar] [CrossRef]
- Calvert, L.; Green, M.P.; De Iuliis, G.N.; Dun, M.D.; Turner, B.D.; Clarke, B.O.; Eamens, A.L.; Roman, S.D.; Nixon, B. Assessment of the emerging threat posed by perfluoroalkyl and polyfluoroalkyl substances to Male reproduction in humans. Front. Endocrinol. 2022, 12, 799043. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, G.; Das, I.I.; Mohanty, A.K.; Kumar, R.; Sahoo, L.; Sundaray, J.K. Poly-and Perfluoroalkyl Substances (PFAS): Do They Matter to Aquatic Ecosystems? Toxics 2023, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Liu, X.; Guo, F. Membrane distillation using surface modified multi-layer porous ceramics. Int. J. Heat Mass Transf. 2019, 129, 764–772. [Google Scholar] [CrossRef]
- Falamaki, C.; Naimi, M.; Aghaie, A. Dual behavior of CaCO3 as a porosifier and sintering aid in the manufacture of alumina membrane/catalyst supports. J. Eur. Ceram. Soc. 2004, 24, 3195–3201. [Google Scholar] [CrossRef]
- Jafari, B.; Rezaei, E.; Abbasi, M.; Hashemifard, S.A.; Sillanpää, M. Application of mullite-zeolite-alumina microfiltration membranes coated by SiO2 nanoparticles for separation of oil-in-water emulsions. J. Eur. Ceram. Soc. 2022, 42, 6005–6014. [Google Scholar] [CrossRef]
- Ren, T.; Tang, G.; Yuan, B.; Yang, Y.; Yan, Z.; Ma, L.; Huang, X. Hexadecyltrimethoxysilane-Modified SiO2 Nanoparticle-Coated Halloysite Nanotubes Embedded in Silicone–Acrylic Polymer Films as Durable Fluorine-Free Superhydrophobic Coatings. ACS Appl. Nano Mater. 2020, 3, 5807–5815. [Google Scholar] [CrossRef]
- Alftessi, S.A.; Othman, M.H.D.; Adam, M.R.B.; Farag, T.M.; Tai, Z.S.; Raji, Y.O.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Bakar, S.A. Hydrophobic silica sand ceramic hollow fiber membrane for desalination via direct contact membrane distillation. Alex. Eng. J. 2022, 61, 9609–9621. [Google Scholar] [CrossRef]
- Rasouli, Y.; Abbasi, M.; Hashemifard, S.A. Investigation of in-line coagulation-MF hybrid process for oily wastewater treatment by using novel ceramic membranes. J. Clean. Prod. 2017, 161, 545–559. [Google Scholar] [CrossRef]
- Yousefi, M.; Abbasi, M.; Akrami, M.; Sillanpää, M. Pre-Treatment and Turbidity Reduction of Sea Waters Using New Composite Ceramic Microfiltration Membranes with Iron Oxide Additive. Water 2022, 14, 3475. [Google Scholar] [CrossRef]
- Jafari, B.; Abbasi, M.; Hashemifard, S.A.; Sillanpää, M. Elaboration and characterization of novel two-layer tubular ceramic membranes by coating natural zeolite and activated carbon on mullite-alumina-zeolite support: Application for oily wastewater treatment. J. Asian Ceram. Soc. 2020, 8, 848–861. [Google Scholar] [CrossRef]
- Abid, M.B.; Wahab, R.A.; Salam, M.A.; Moujdin, I.A.; Gzara, L. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.M.; Salehi, M.B. Enhancing hydrocarbon productivity via wettability alteration: A review on the application of nanoparticles. Rev. Chem. Eng. 2019, 35, 531–563. [Google Scholar] [CrossRef]
- Morrow, N.R. Wettability and its effect on oil recovery. J. Pet. Technol. 1990, 42, 1476–1484. [Google Scholar] [CrossRef]
- Chen, H.; Muros-Cobos, J.L.; Amirfazli, A. Contact angle measurement with a smartphone. Rev. Sci. Instrum. 2018, 89, 035117. [Google Scholar] [CrossRef] [PubMed]
- Huhtamaki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Magili, S.T.; Kaigamma, I. Adsorption of phenol over bio-based silica/calcium carbonate (CS-SiO2/CaCO3) nanocomposite synthesized from waste eggshells and rice husks. PeerJ Phys. Chem. 2021, 3, e17. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Nagam, V.; Bobbu, P.; Ghosh, S.B.; Tartte, V. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl. Nanosci. 2015, 5, 801–807. [Google Scholar] [CrossRef]
- Suegama, P.H.; Aoki, I.V. Electrochemical behavior of carbon steel pre-treated with an organo functional bis-silane filled with copper phthalocyanine. J. Braz. Chem. Soc. 2008, 19, 744–754. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Zhong, X.-W.; Liu, Z.-Q.; Chen, S.; Li, N. Preparation and Enhancement of Thermal Conductivity of Heat Transfer Oil-Based MoS2Nanofluids. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Zimnoch, J.ú.H. Fourier transform infrared and Raman characterization of silica-based materials. Appl. Mol. Spectrosc. Curr. Res. Chem. Biol. Sci. 2016, 32, 137–144. [Google Scholar]
- Jozanikohan, G.; Abarghooei, M.N. The Fourier transform infrared spectroscopy (FTIR) analysis for the clay mineralogy studies in a clastic reservoir. J. Pet. Explor. Prod. Technol. 2022, 12, 2093–2106. [Google Scholar] [CrossRef]
- Fang, H.; Gao, J.; Wang, H.; Chen, C. Hydrophobic porous alumina hollow fiber for water desalination via membrane distillation process. J. Membr. Sci. 2012, 403, 41–46. [Google Scholar] [CrossRef]
- Yusof, N.; Rana, D.; Ismail, A.F.; Matsuura, T. Microstructure of polyacrylonitrile-based activated carbon fibers prepared from solvent-free coagulation process. J. Appl. Res. Technol. 2016, 14, 54–61. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the fouling/scaling resistance of superhydrophobic/omniphobic membranes in membrane distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Rezaei, M.; Alsaati, A.; Warsinger, D.M.; Hell, F.; Samhaber, W.M. Long-running comparison of feed-water scaling in membrane distillation. Membranes 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.G.; Scherer, C.M.; Fisch, A.G.; Rodrigues, M.A.S. Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics. Membranes 2021, 11, 958. [Google Scholar] [CrossRef]
- Song, L.; Ma, Z.; Liao, X.; Kosaraju, P.B.; Irish, J.R.; Sirkar, K.K. Pilot plant studies of novel membranes and devices for direct contact membrane distillation-based desalination. J. Membr. Sci. 2008, 323, 257–270. [Google Scholar] [CrossRef]
- Meng, Y.; Zhong, Q.; Liu, Y.; Yan, Z.; Liang, Y.; Chang, H.; Liang, H.; Vidic, R.D. Evaluating membrane cleaning for organic fouling in direct contact membrane distillation. J. Clean. Prod. 2023, 410, 137319. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Skuse, C.; Zaragoza, G.; Gryta, M.; Gorgojo, P. Membrane cleaning and pretreatments in membrane distillation–a review. Chem. Eng. J. 2021, 422, 129696. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, Y.; Huang, L.; Yan, Z.; Qu, F.; Liang, H. Mineral scaling induced membrane wetting in membrane distillation for water treatment: Fundamental mechanism and mitigation strategies. Water Res. 2023, 247, 120807. [Google Scholar] [CrossRef] [PubMed]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Khemakhem, S.; Amar, R.B. Modification of Tunisian clay membrane surface by silane grafting: Application for desalination with Air Gap Membrane Distillation process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 79–85. [Google Scholar] [CrossRef]
- Kujawa, J. From nanoscale modification to separation-The role of substrate and modifiers in the transport properties of ceramic membranes in membrane distillation. J. Membr. Sci. 2019, 580, 296–306. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Pauzan, M.A.B.; Hisam, N.A.S.M.; Othman, M.H.D.; Adam, M.R.; Iwamoto, Y.; Puteh, M.H.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Superhydrophobic ball clay based ceramic hollow fibre membrane via universal spray coating method for membrane distillation. Sep. Purif. Technol. 2022, 288, 120574. [Google Scholar] [CrossRef]
- Lanjewar, T.; Satyakam, A.; Varma, M.N. Low-Cost Hydrophobic Cenosphere Ceramic Membrane for the Desalination Application Using Direct Contact Membrane Distillation. Arab. J. Sci. Eng. 2022, 47, 6445–6460. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R. How to make membrane distillation greener: A review of environmentally friendly and sustainable aspects. Green Chem. 2024, 26, 164–185. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation using low-grade energy for desalination: A review. J. Environ. Chem. Eng. 2021, 9, 105818. [Google Scholar] [CrossRef]
- Nandi, B.; Uppaluri, R.; Purkait, M. Preparation and characterization of low cost ceramic membranes for micro-filtration applications. Appl. Clay Sci. 2008, 42, 102–110. [Google Scholar] [CrossRef]
- Cassano, A.; Marchio, M.; Drioli, E. Clarification of blood orange juice by ultrafiltration: Analyses of operating parameters, membrane fouling and juice quality. Desalination 2007, 212, 15–27. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Biomass assisted microfiltration of chromium (VI) using Baker’s yeast by ceramic membrane prepared from low cost raw materials. Desalination 2012, 285, 239–244. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low cost ceramic membranes for mosambi juice clarification. Desalination 2013, 317, 32–40. [Google Scholar] [CrossRef]




| Chemical Composition | Value |
|---|---|
| SiO2 | 61.62 |
| TiO2 | 0.40 |
| Al2O3 | 24–25 |
| Fe2O3 | 0.45–0.65 |
| K2O | 0.40 |
| Na2O | 0.50 |
| L.O.I | 9.5–10 |
| Parameter | Value |
|---|---|
| EC (mS/cm) | 60 ± 0.1 |
| TDS (ppm) | 42,000 ± 95 |
| pH | 8.8 ± 0.1 |
| Salinity (PSU) Turbidity (NTU) | 40.5 ± 0.2 11.65 ± 0.1 |
| Property of M1 | Before Modification | After Modification |
| Porosity (%) | 42 | 36 |
| Surface roughness (Ra) (nm) | 3.485 | 4.228 |
| Contact angle (°) | 0 | 156 |
| LEP (bar) | 0 | ~1.5 |
| Property of M2 | Before Modification | After Modification |
| Porosity (%) | 65 | 53 |
| Surface roughness (Ra) (nm) | 2.096 | 11.17 |
| Contact angle (°) | 0 | 160 |
| LEP (bar) | 0 | ~1.5 |
| Average pore size (µm) | 0.95 | ~0.40 |
| Maximum pore size (µm) | 0.95 | ~0.40 |
| Parameter | Value |
|---|---|
| EC (µS/cm) | 133 |
| TDS (mg/L) | 86 |
| pH | 6.66 |
| Salinity (psu) | 0 |
| Turbidity (NTU) | 0.80 |
| Membrane | Hydrophobic Agent | Feed Solution | Feed/Permeate Temperature (°C) | Flux (kg/m2·h) | Salt Rejection (R%) | Membrane Configuration | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| alumina and zirconia membranes | FAS | NaCl (0.01–1 molarity) | 95/5 | 0.87–5.4 | ~100 | Tubular | DCMD | [74] |
| Tunisian clay | FAS | NaCl solution | 90/5 | 3.2–6.45 | 99 | planar | AGMD | [75] |
| Titania | FAS | NaCl solution | 90/5 | 1.2 | N/A | Tubular | AGMD | [40] |
| Al2O3/TiO2/ ZrO2 | HDTMS | NaCl (0.25–1 M) | 72/5 | 0.31–8.95 | 99 | Tubular | AGMD | [76] |
| Ball clay | ZnO nanoparticles with T-PFOS | NaCl solution | 80/10 | 6.2 | >99.8 | Hollow fibre | DCMD | [77] |
| Cenosphere | PDMS | NaCl solution | 85/5 | 1–13 | 99 | N/A | DCMD | [78] |
| Alumina layer on the composite mullite CaCO3 synthesis membrane | HDTMS | 3.5 wt% NaCl solution | 68/15 | 2.2–3.6 | 99.60 | Tubular | DCMD | This work |
| Cost of Raw Material ($/kg) | Composite Membrane Preparation Cost ($/m2) | Cost of Consumption Energy in Sintering Process ($/m2) | Total Ceramic Membrane Preparation Cost ($/m2) | DCMD Energy Consumption ($/lit) |
|---|---|---|---|---|
| 0.1 | 0.45 | 0.09 | 0.54 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, J.; Abbasi, M.; Hashemifard, S.A.; Dizge, N.; Dibaj, M.; Akrami, M. Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination. Water 2024, 16, 1593. https://doi.org/10.3390/w16111593
Zare J, Abbasi M, Hashemifard SA, Dizge N, Dibaj M, Akrami M. Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination. Water. 2024; 16(11):1593. https://doi.org/10.3390/w16111593
Chicago/Turabian StyleZare, Javad, Mohsen Abbasi, Seyed Abdollatif Hashemifard, Nadir Dizge, Mahdieh Dibaj, and Mohammad Akrami. 2024. "Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination" Water 16, no. 11: 1593. https://doi.org/10.3390/w16111593
APA StyleZare, J., Abbasi, M., Hashemifard, S. A., Dizge, N., Dibaj, M., & Akrami, M. (2024). Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination. Water, 16(11), 1593. https://doi.org/10.3390/w16111593








