The Aging Behavior of Polyvinyl Chloride Microplastics by UV/Sodium Percarbonate Oxidation: Efficiency and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Analytical Methods
2.3.1. Characterization Methods
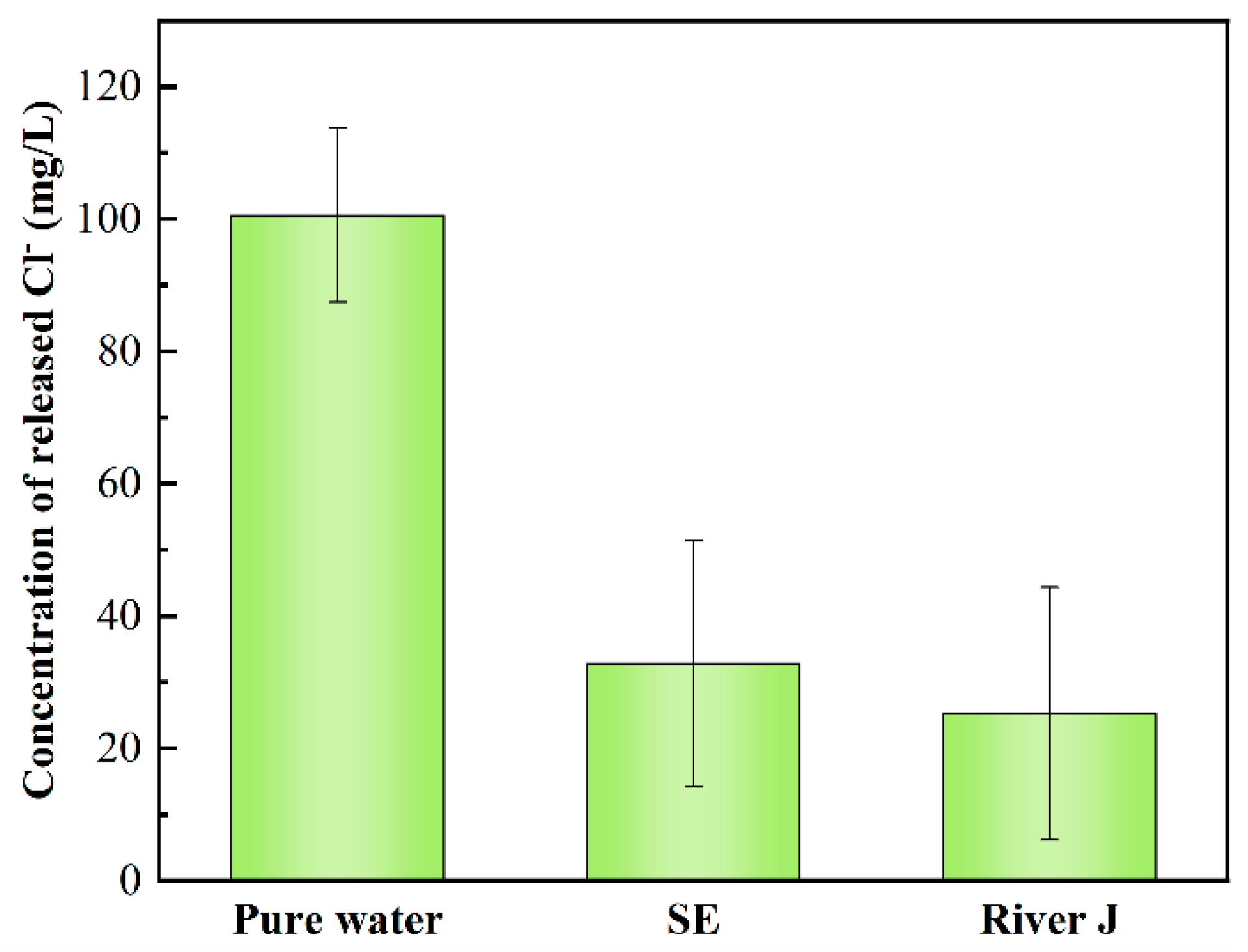
2.3.2. Release of Chloride Ions
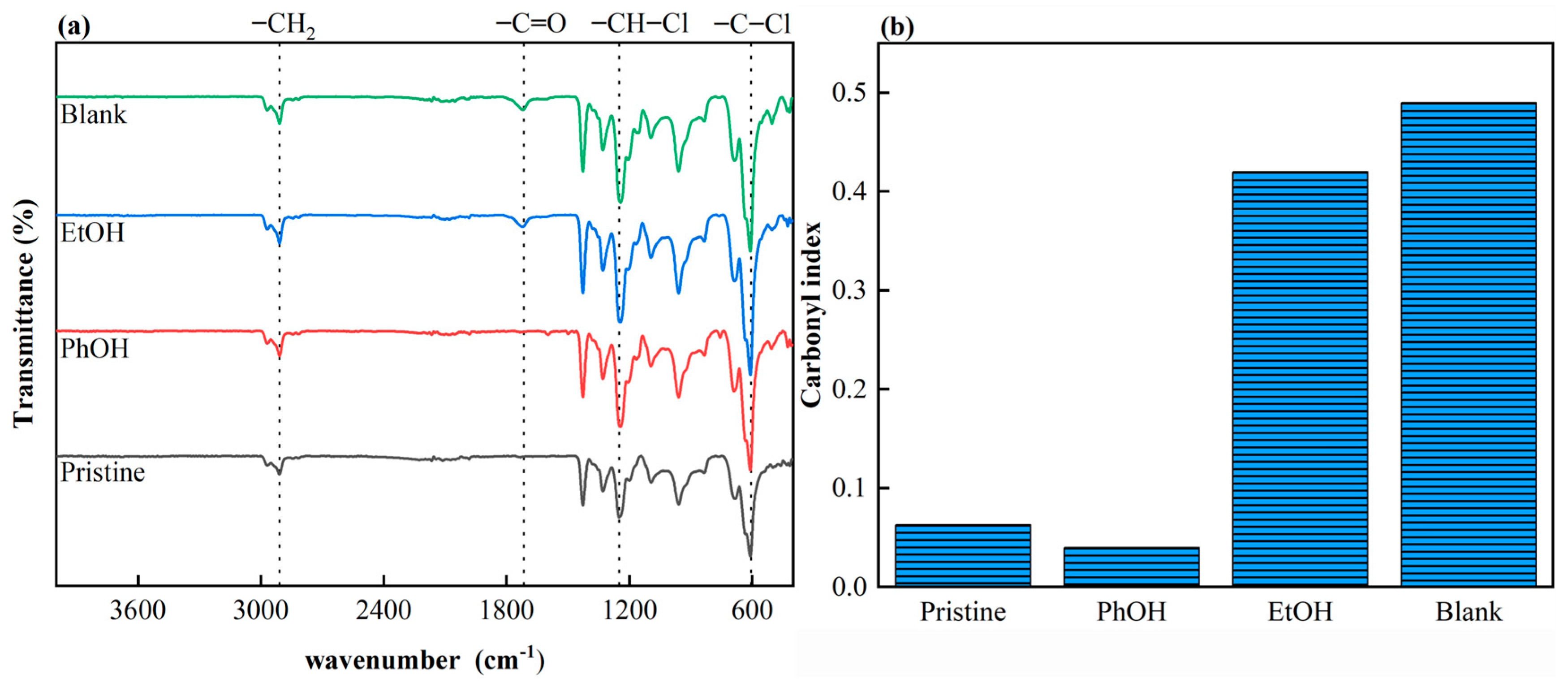
2.3.3. Carbonyl Index
3. Results and Discussion
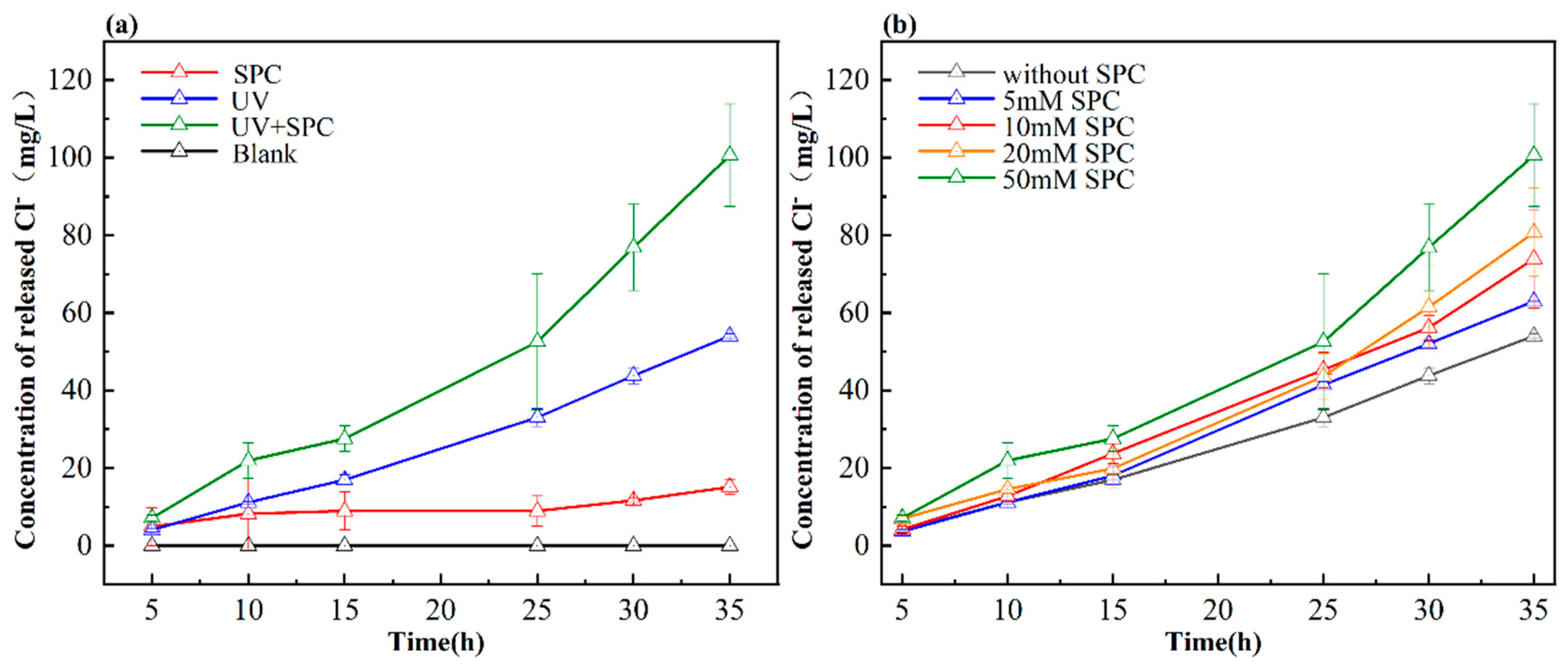
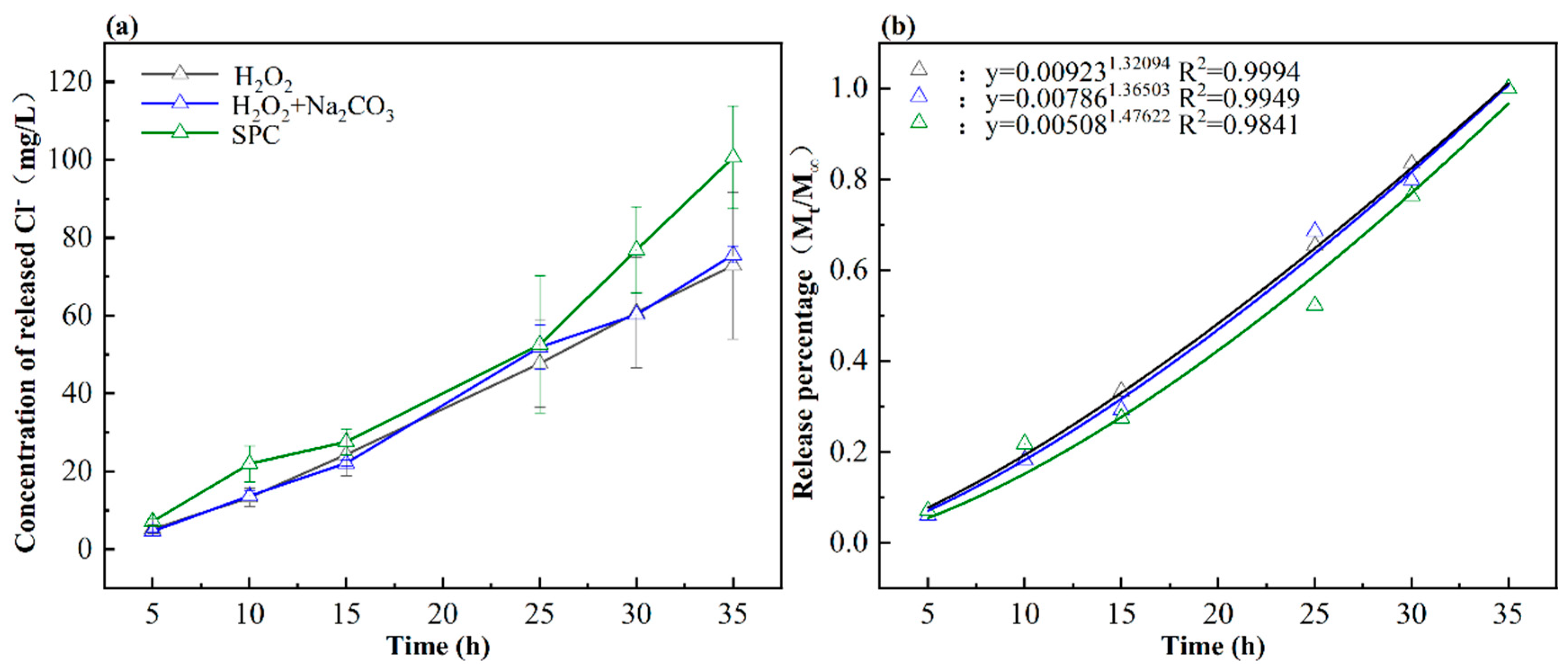
3.1. The Dechlorination of PVC
3.2. Morphology Characterization of PVC
3.2.1. Morphologies
3.2.2. Functional Groups
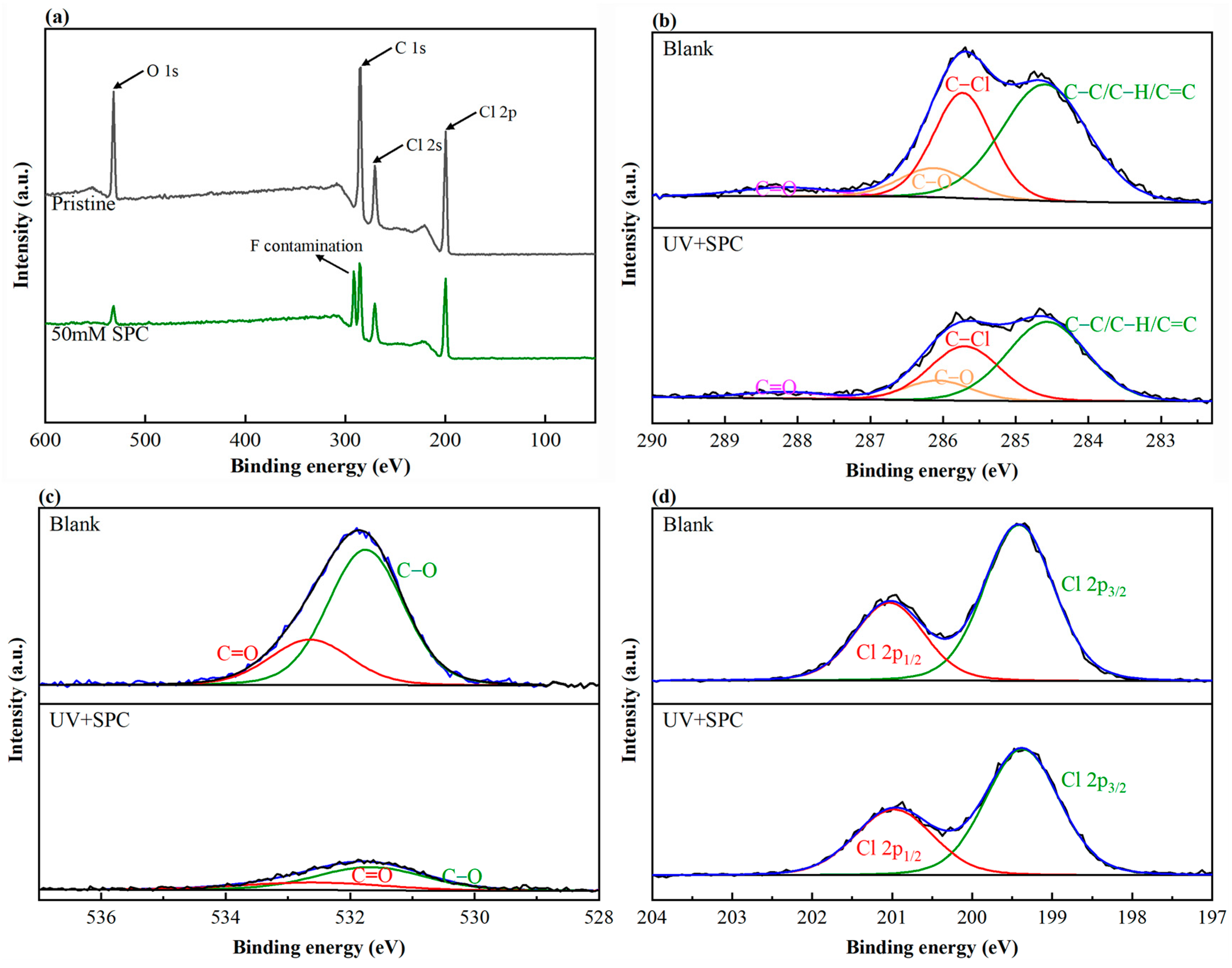
3.2.3. Chemical Composition and Intermediates of PVC
3.3. Oxidation and Dechlorination Mechanism of UV/SPC System for PVC MPs
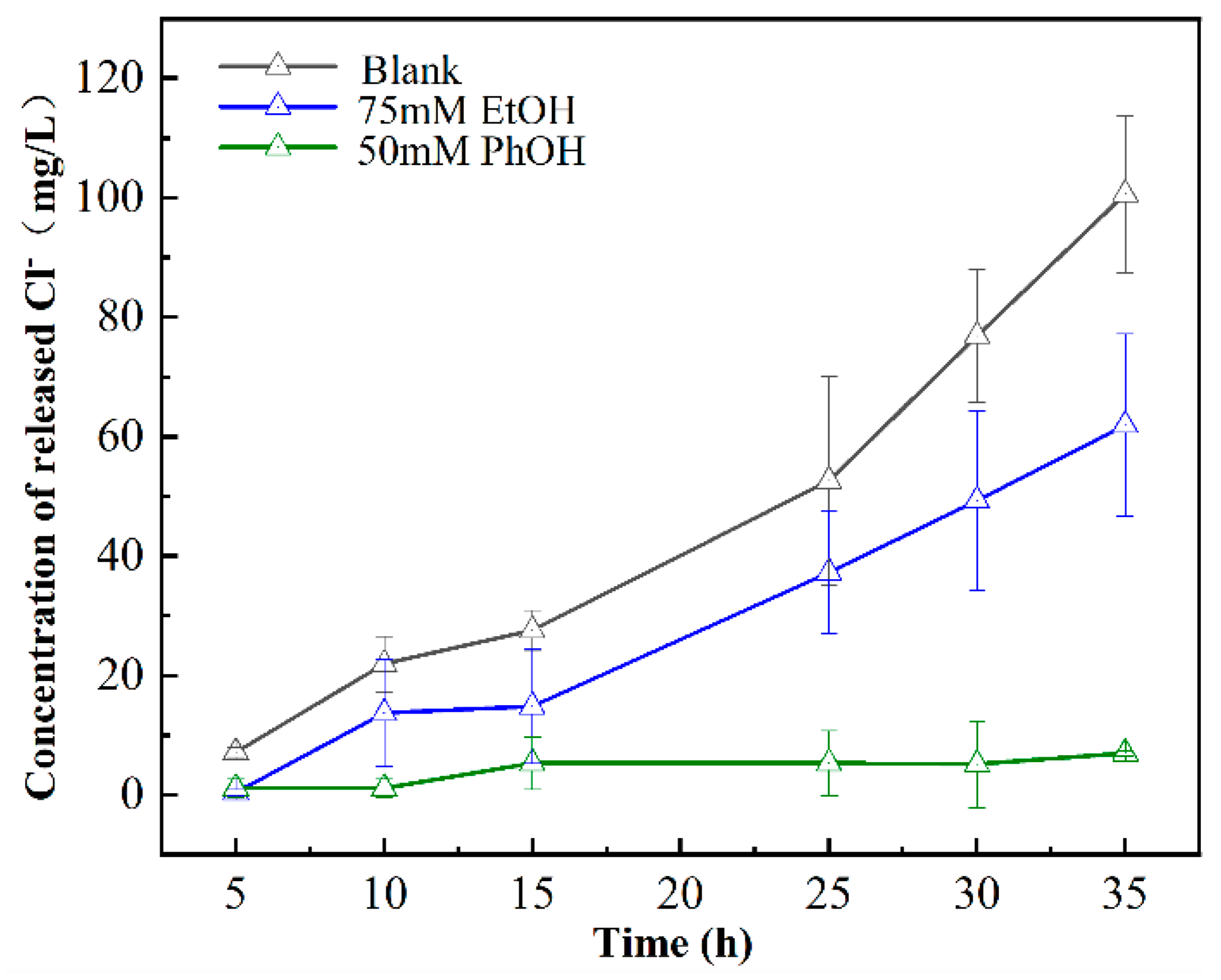
3.3.1. Reactive Species Contribution to Dechlorination
3.3.2. Reactive Species Contribution to Oxidation
3.3.3. Oxidation and Dechlorination Mechanism of PVC MPs in UV/SPC
3.4. Application of UV/SPC in Actual Water Bodies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, N.; Khan, M.H.; Ali, M.; Sidra; Ahmad, S.; Khan, A.; Nabi, G.; Ali, F.; Bououdina, M.; Kyzas, G.Z. Insight into microplastics in the aquatic ecosystem: Properties, sources, threats and mitigation strategies. Sci. Total Environ. 2024, 913, 169489. [Google Scholar] [CrossRef] [PubMed]
- Flores-Munguía, E.J.; Rosas-Acevedo, J.L.; Ramírez-Hernández, A.; Aparicio-Saguilan, A.; Brito-Carmona, R.M.; Violante-González, J. Release of Microplastics from Urban Wastewater Treatment Plants to Aquatic Ecosystems in Acapulco, Mexico. Water 2023, 15, 3643. [Google Scholar] [CrossRef]
- Yang, H.; Yan, Y.; Yu, Y.; He, Y.; Fu, B.; Wang, J. Distribution, sources, migration, influence and analytical methods of microplastics in soil ecosystems. Ecotoxicol. Environ. Saf. 2022, 243, 114009. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric microplastics in the Northwestern Pacific Ocean: Distribution, source, and deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Li, Q.; Xia, X.; Zhang, H. The effects of land use types on microplastics in river water: A case study on the mainstream of the Wei River, China. Environ. Monit. Assess. 2024, 196, 349. [Google Scholar] [CrossRef] [PubMed]
- Samandra, S.; Mescall, O.J.; Plaisted, K.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci. Total Environ. 2022, 837, 155329. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cortes, M.; Armstrong-Altrin, J.S. Textural characteristics and abundance of microplastics in Tecolutla beach sediments, Gulf of Mexico. Environ. Monit. Assess. 2022, 194, 752. [Google Scholar] [CrossRef]
- Rathore, C.; Saha, M.; de Boer, J.; Desai, A.; Gupta, P.; Naik, A.; Subha, H.Y. Unraveling the land-based discharge of microplastics from sewers to oceans—A comprehensive study and risk assessment in wastewaters of Goa, India. Sci. Total Environ. 2024, 913, 169621. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, M.; Yan, B.; Wei, J.; Liu, F.; Li, Q.; Bo, Y. Characteristics of microplastics in the atmosphere of Anyang City. Environ. Monit. Assess. 2024, 196, 350. [Google Scholar] [CrossRef]
- Razaviarani, V.; Saudagar, A.; Gallage, S.; Shrinath, S.; Arab, G. Comprehensive investigation on microplastics from source to sink. Clean Technol. Environ. Policy 2024. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Wang, Y.; Yu, F.; Ma, J. Adsorption behavior of Cu(II) and Cr(VI) on aged microplastics in antibiotics-heavy metals coexisting system. Chemosphere 2022, 291, 132794. [Google Scholar] [CrossRef] [PubMed]
- Abihssira-Garcia, I.S.; Kogel, T.; Gomiero, A.; Kristensen, T.; Krogstad, M.; Olsvik, P.A. Distinct polymer-dependent sorption of persistent pollutants associated with Atlantic salmon farming to microplastics. Mar. Pollut. Bull. 2022, 180, 113794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Weerasinghe, K.D.I.; Mayor, D.J.; Lampitt, R. Microplastics in commercial marine fish species in the UK—A case study in the River Thames and the River Stour (East Anglia) estuaries. Sci. Total Environ. 2024, 915, 170170. [Google Scholar] [CrossRef] [PubMed]
- Benjaminsen, S.C.; Dehnhard, N.; Herzke, D.; Johnsen, A.; Anker-Nilssen, T.; Bourgeon, S.; Collard, F.; Langset, M.; Christensen-Dalsgaard, S.; Gabrielsen, G.W. The challenges of opportunistic sampling when comparing prevalence of plastics in diving seabirds: A multi-species example from Norway. Mar. Pollut. Bull. 2024, 199, 116037. [Google Scholar] [CrossRef] [PubMed]
- Aranda, D.A.; Sindou, P.; Rodriguez, J.V.C.; Saldana, G.M.; Coronado, R.F.V.; Gonzalez, W.D.N.; Diaz, M.E.; Escalante, V.C. A non-invasive method of microplastics pollution quantification in green sea turtle Chelonia mydas of the Mexican Caribbean. Mar. Pollut. Bull. 2024, 200, 116092. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Jia, Z.; Wei, W.; Wang, Y.; Chang, Y.; Lei, R.; Che, Y. Occurrence characteristics and risk assessment of microplastics in agricultural soils in the loess hilly gully area of Yan’an, China. Sci. Total Environ. 2024, 912, 169627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Shao, T.; Wang, R.; Dong, Z.; Xing, B. Antibiotics and microplastics in manure and surrounding soil of farms in the Loess Plateau: Occurrence and correlation. J. Hazard. Mater. 2024, 465, 133434. [Google Scholar] [CrossRef]
- Luo, D.; Wang, Z.; Liao, Z.; Chen, G.; Ji, X.; Sang, Y.; Qu, L.; Chen, Z.; Wang, Z.; Dahlgren, R.A.; et al. Airborne microplastics in urban, rural and wildland environments on the Tibetan Plateau. J. Hazard. Mater. 2024, 465, 133177. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y.; Sui, Q.; Zhou, Y. Mechanism and characterization of microplastic aging process: A review. Front. Environ. Sci. Eng. 2023, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Turk, T.; Skalar, T.; Zupancic, M.; Korosin, N.C.; Marinsek, M.; Olivero-Verbel, J.; Kalcikova, G. An extensive characterization of various environmentally relevant microplastics—Material properties, leaching and ecotoxicity testing. Sci. Total Environ. 2021, 773, 145576. [Google Scholar] [CrossRef] [PubMed]
- Hanun, J.N.; Hassan, F.; Theresia, L.; Chao, H.-R.; Bu, H.M.; Rajendran, S.; Kataria, N.; Yeh, C.-F.; Show, P.L.; Khoo, K.S.; et al. Weathering effect triggers the sorption enhancement of microplastics against oxybenzone. Environ. Technol. Innov. 2023, 30, 103112. [Google Scholar] [CrossRef]
- Majewski, K.; Mantell, S.C.; Bhattacharya, M. Relationship between morphological changes and mechanical properties in HDPE films exposed to a chlorinated environment. Polym. Degrad. Stab. 2020, 171, 109027. [Google Scholar] [CrossRef]
- Chang, J.; Liang, J.; Fang, W.; Zhang, H.; Zhang, Y.; Zhao, H.; Zhang, R.; Zhang, P.; Zhang, G. Adsorption behaviors and bioavailability of tetrabromobisphenol A in the presence of polystyrene microplastic in soil: Effect of microplastics aging. Environ. Pollut. 2023, 334, 122156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, Y.; Su, F.; Qian, J.; Liu, S. Adsorption of levofloxacin by ultraviolet aging microplastics. Chemosphere 2023, 343, 140196. [Google Scholar] [CrossRef]
- Zhou, T.; Song, S.; Min, R.; Liu, X.; Zhang, G. Advances in chemical removal and degradation technologies for microplastics in the aquatic environment: A review. Mar. Pollut. Bull. 2024, 201, 116202. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Yan, Z.; Liu, J.; Wu, D.; Wang, Y. Microplastic degradation by hydroxy-rich bismuth oxychloride. J. Hazard. Mater. 2021, 405, 124247. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Min, Y.; Shi, P. Aging of polystyrene microplastics by UV/Sodium percarbonate oxidation: Organic release, mechanism, and disinfection by-product formation. J. Hazard. Mater. 2024, 464, 132934. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2023, 442, 130014. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Zhou, L.; Sun, H.; Suvorova, A.; Odedairo, T.; Zhu, Z.; Shao, Z.; Wang, S. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl. Catal. B Environ. 2016, 194, 7–15. [Google Scholar] [CrossRef]
- Li, L.; Guo, R.; Zhang, S.; Yuan, Y. Sustainable and effective degradation of aniline by sodium percarbonate activated with UV in aqueous solution: Kinetics, mechanism and identification of reactive species. Environ. Res. 2022, 207, 112176. [Google Scholar] [CrossRef]
- Sindelar, H.R.; Brown, M.T.; Boyer, T.H. Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources. Chemosphere 2014, 105, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, Y.G.; Jin, C.; Yang, D.; Zhang, Y.; Progress, M. Removal of tetracycline by ultraviolet/sodium percarbonate (UV/SPC) advanced oxidation process in water. Environ. Res. 2024, 247, 118260. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Duan, X.; O’Shea, K.; Dionysiou, D.D. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qian, Y.; Chen, J.; Zhang, Y.; Zhou, X. A critical review of solid peroxides in environmental remediation and water purification: From properties to field applications. Chem. Eng. J. 2023, 465, 142424. [Google Scholar] [CrossRef]
- Wang, C.; Xian, Z.; Jin, X.; Liang, S.; Chen, Z.; Pan, B.; Wu, B.; Ok, Y.S.; Gu, C. Photo-aging of polyvinyl chloride microplastic in the presence of natural organic acids. Water Res. 2020, 183, 116082. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.N.H.; Murata, S.; Artok, L. Statistical Distribution Characteristics of Pyridine Transport in Coal Particles and a Series of New Phenomenological Models for Overshoot and Nonovershoot Solvent Swelling of Coal Particles. Energy Fuels 1999, 13, 518–528. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, B.; Xie, F.; Zhang, X.; Zhou, A.; Wang, S.; Yue, X. Study on the preparation and feasibility of a novel adding-type biological slow-release carbon source. J. Environ. Manag. 2022, 316, 115236. [Google Scholar] [CrossRef]
- Miranda, M.N.; Sampaio, M.J.; Tavares, P.B.; Silva, A.M.T.; Pereira, M.F.R. Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors. Sci. Total Environ. 2021, 796, 148914. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Li, S.; Zhao, M.; Wangmu, Q.; Ding, R.; Xiao, C.; Guo, X. The aging behavior of polyvinyl chloride microplastics promoted by UV-activated persulfate process. J. Hazard. Mater. 2022, 424, 127461. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xian, Z.N.; Yue, W.; Yin, C.F.; Zhou, N.Y. Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 2023, 318, 137944. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal carbonization of medical wastes and lignocellulosic biomass for solid fuel production from lab-scale to pilot-scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef]
- Lin, J.; Yan, D.; Fu, J.; Chen, Y.; Ou, H. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020, 186, 116360. [Google Scholar] [CrossRef]
- Dong, S.; Yan, X.; Yue, Y.; Li, W.; Luo, W.; Wang, Y.; Sun, J.; Li, Y.; Liu, M.; Fan, M. H2O2 concentration influenced the photoaging mechanism and kinetics of polystyrene microplastic under UV irradiation: Direct and indirect photolysis. J. Clean. Prod. 2022, 380, 135046. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q. Oxidation of sulfamethazine by a novel CuS/calcium peroxide/tetraacetylethylenediamine process: High efficiency and contribution of oxygen-centered radicals. Chem. Eng. J. 2022, 446, 136882. [Google Scholar] [CrossRef]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ni, B.-J.; Zheng, X.; Lu, B.; Chen, Z.; Gao, Y.; Zhang, Y.; Zhang, S.; Luo, G. The changes of microplastics’ behavior in adsorption and anaerobic digestion of waste activated sludge induced by hydrothermal pretreatment. Water Res. 2022, 221, 118744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, K.; Zhang, Y.; Wang, Y.; Wang, F.; Zhang, G.; Dionysiou, D.D. Magnetically recoverable MgFe2O4/conjugated polyvinyl chloride derivative nanocomposite with higher visible-light photocatalytic activity for treating Cr(VI)-polluted water. Sep. Purif. Technol. 2020, 236, 116272. [Google Scholar] [CrossRef]
- Jasmin, J.-P.; Miserque, F.; Dumas, E.; Vickridge, I.; Ganem, J.-J.; Cannizzo, C.; Chaussé, A. XPS and NRA investigations during the fabrication of gold nanostructured functionalized screen-printed sensors for the detection of metallic pollutants. Appl. Surf. Sci. 2017, 397, 159–166. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, L.; Wu, Y.; Zhu, S.; Zhou, S.; Shi, Z.; Dionysiou, D.D. Degradation of contaminants of emerging concern in UV/Sodium percarbonate Process: Kinetic understanding of carbonate radical and energy consumption evaluation. Chem. Eng. J. 2022, 442, 135995. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, T.; Zhang, J.; Wei, R.; You, S.; Xu, Y. Facile synthesis of oxygen vacancies enriched alpha-Fe(2)O(3) for peroxymonosulfate activation: A non-radical process for sulfamethoxazole degradation. J. Hazard. Mater. 2021, 419, 126447. [Google Scholar] [CrossRef]



| Original pH | UV-Vis Absorbance @ 254 nm | Cl− (mg/L) | (mg/L) | (mg/L) | (mg/L) | |
|---|---|---|---|---|---|---|
| River J | 8.56 | 0.315 | 107.38 | 11.70 | 64.85 | 0.09 |
| SE | 7.12 | 0.061 | 22.64 | 2.27 | 58.27 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Liu, Z.; He, J.; Wu, Y.; Wang, Q. The Aging Behavior of Polyvinyl Chloride Microplastics by UV/Sodium Percarbonate Oxidation: Efficiency and Mechanism. Water 2024, 16, 1529. https://doi.org/10.3390/w16111529
Su L, Liu Z, He J, Wu Y, Wang Q. The Aging Behavior of Polyvinyl Chloride Microplastics by UV/Sodium Percarbonate Oxidation: Efficiency and Mechanism. Water. 2024; 16(11):1529. https://doi.org/10.3390/w16111529
Chicago/Turabian StyleSu, Luhan, Zhongwen Liu, Jia He, Yan Wu, and Qingguo Wang. 2024. "The Aging Behavior of Polyvinyl Chloride Microplastics by UV/Sodium Percarbonate Oxidation: Efficiency and Mechanism" Water 16, no. 11: 1529. https://doi.org/10.3390/w16111529
APA StyleSu, L., Liu, Z., He, J., Wu, Y., & Wang, Q. (2024). The Aging Behavior of Polyvinyl Chloride Microplastics by UV/Sodium Percarbonate Oxidation: Efficiency and Mechanism. Water, 16(11), 1529. https://doi.org/10.3390/w16111529








