Valorization of Cherry By-Products as Coagulant/Flocculants Combined with Bentonite Clay for Olive Mill Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Olive Mill Wastewater Sampling
2.2. Analytical Determinations of Olive Mill Wastewater
2.3. Preparation and Characterization of Plant-Based Coagulants
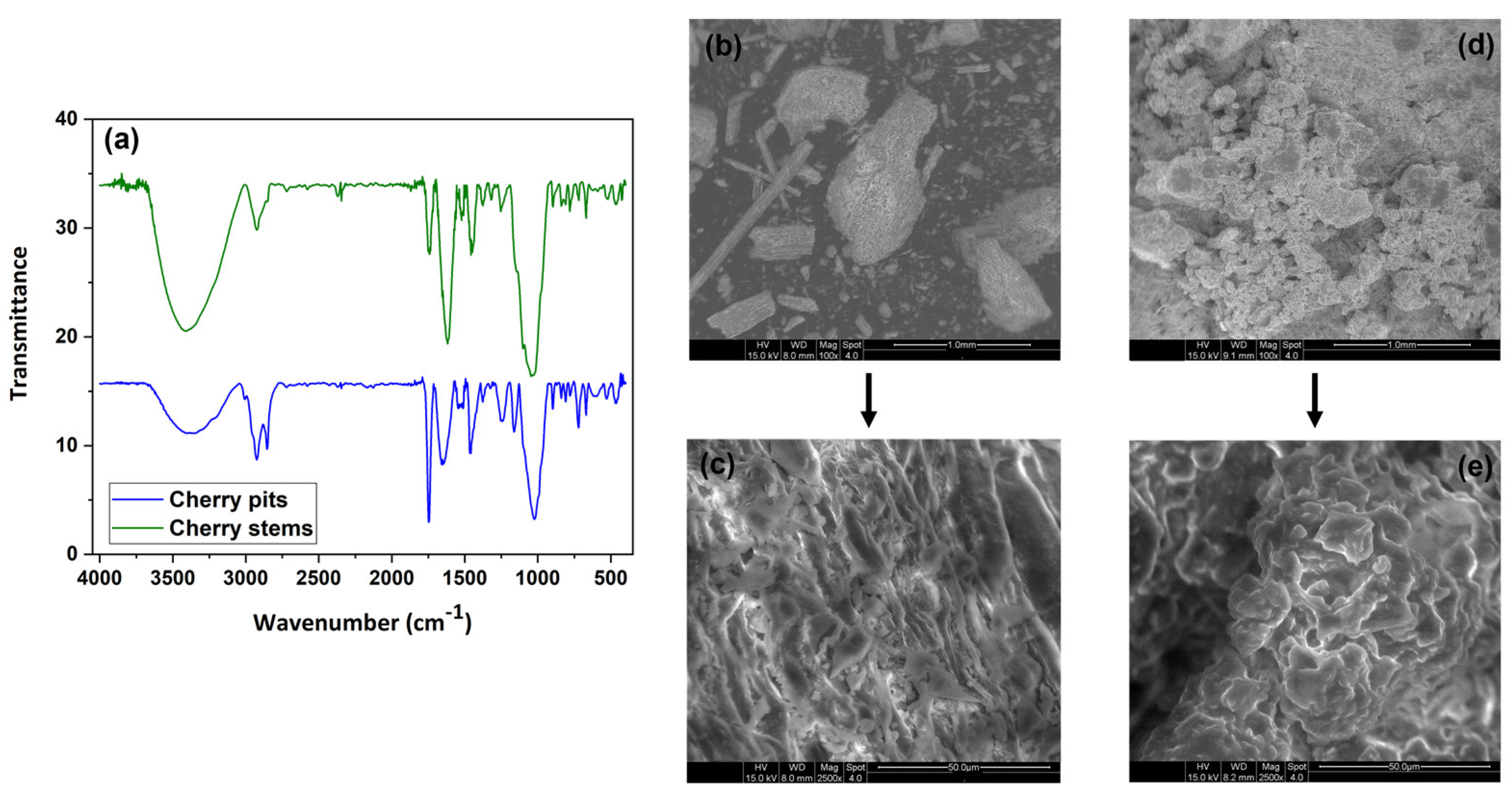
2.3.1. Structural Composition of Cherry By-Products
2.3.2. Microstructural Characterization of the Cherry By-Products
2.3.3. Preparation of Cherry By-Product Extracts
Total Phenolics and Total Flavonoids Content
Quantification of Ortho-Diphenols
Antioxidant Activity (AA)
2.4. Coagulation–Flocculation–Decantation (CFD) Experimental Setup
- A total of 0.1, 0.5, 1.0, and 2.0 g L−1 of PBCs and ferrous sulfate were added to 500 mL of OMW, and the pH varied between 3.0, 5.0, 7.0, 9.0, and 11.0;
- After finding optimal values for the experimental conditions for pH and dosage, we followed the stirring parameter. The stirring process was studied through the variation of fast and slow mix conditions (rpm/min), respectively: 120/1, 150/2, 150/3, 180/3, and 200/2 and 20/30, 50/30, 20/20, 40/17, and 60/30.
2.5. Adsorption Experimental Setup
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Plant-Based Coagulants Powder
3.2. Coagulation–Flocculation–Decantation Experiments
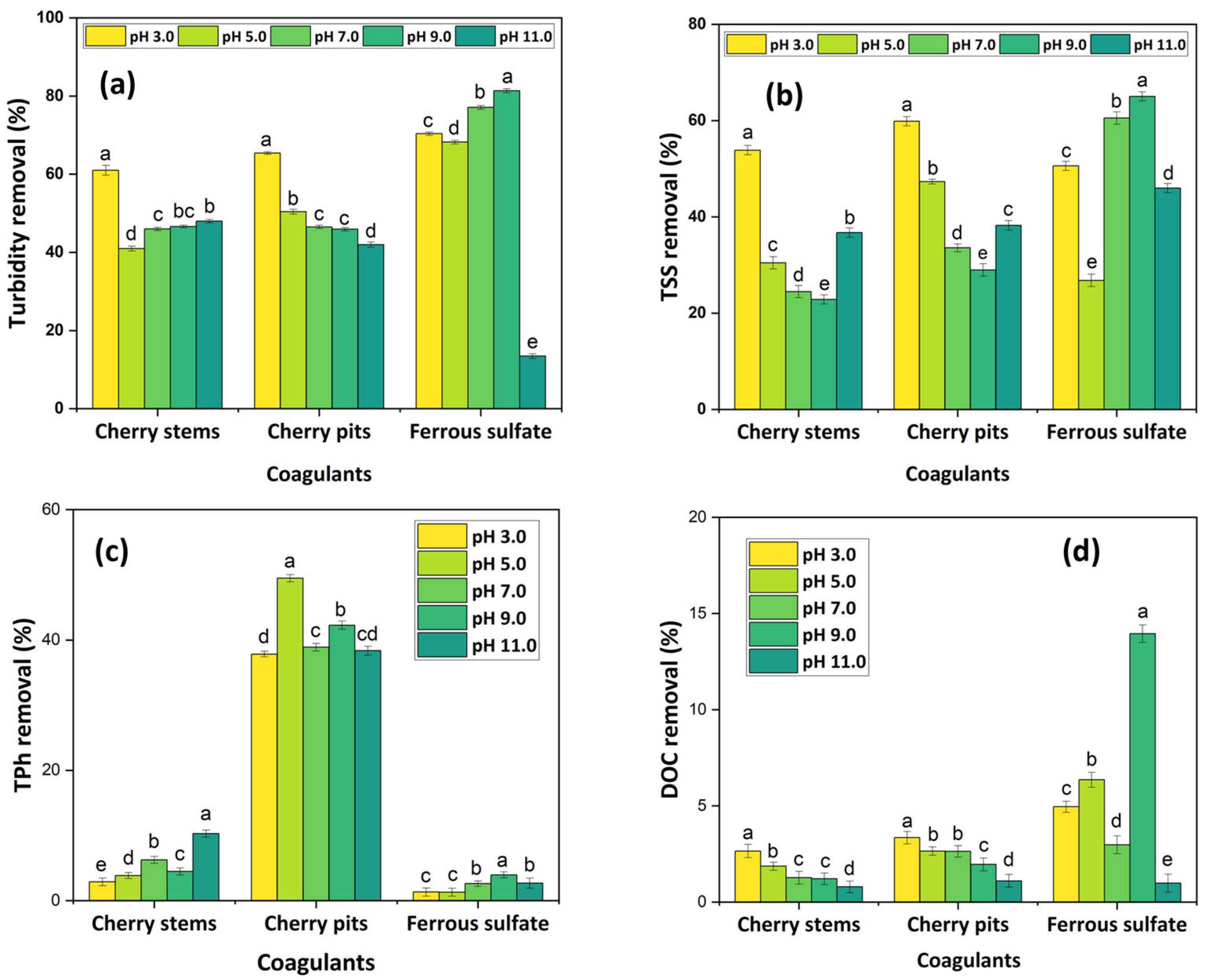
3.2.1. Influence of pH
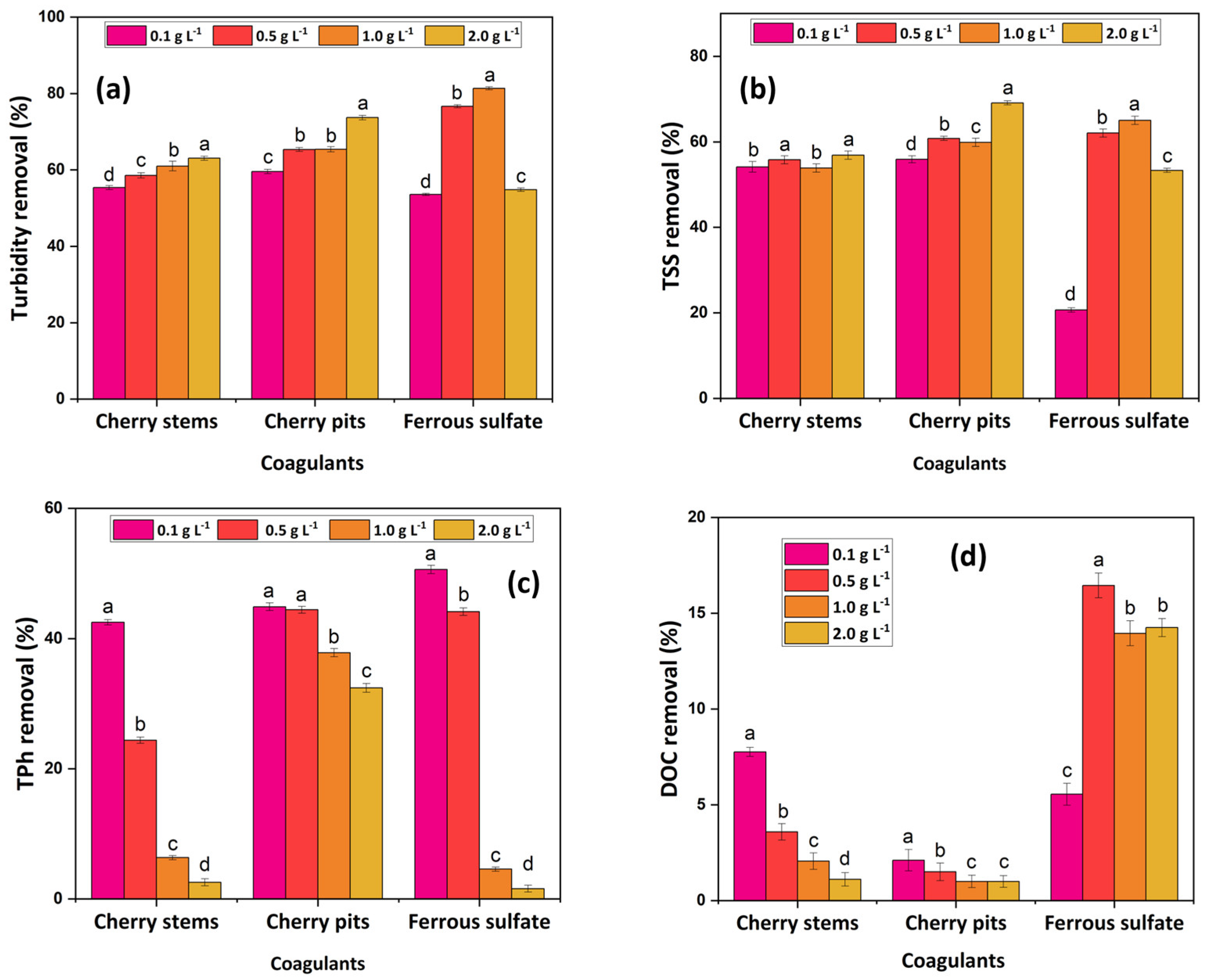
3.2.2. Coagulant Dosage Effect
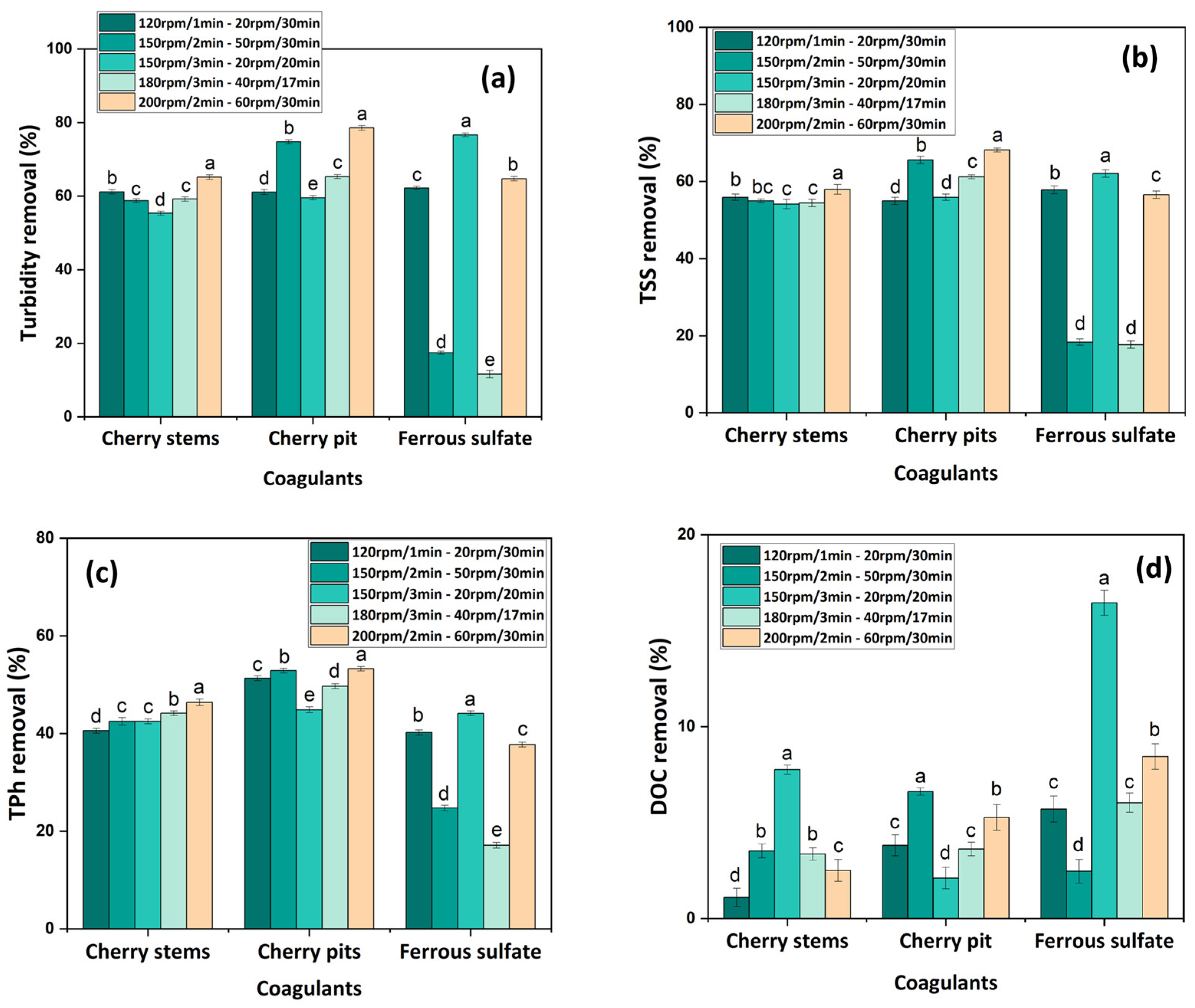
3.2.3. Agitation Effect
3.3. Adsorption Experiments with Bentonite
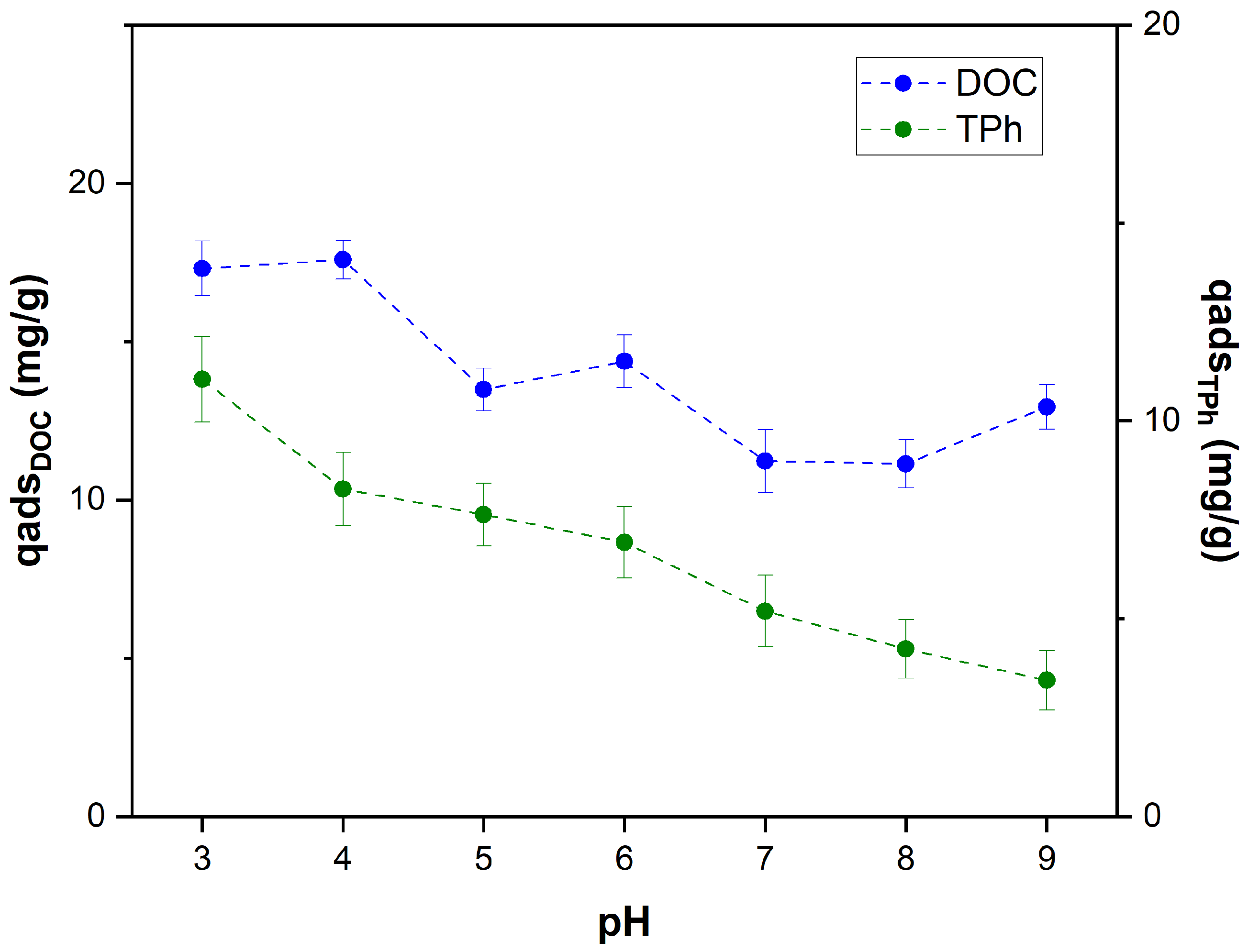
3.3.1. Effect of pH
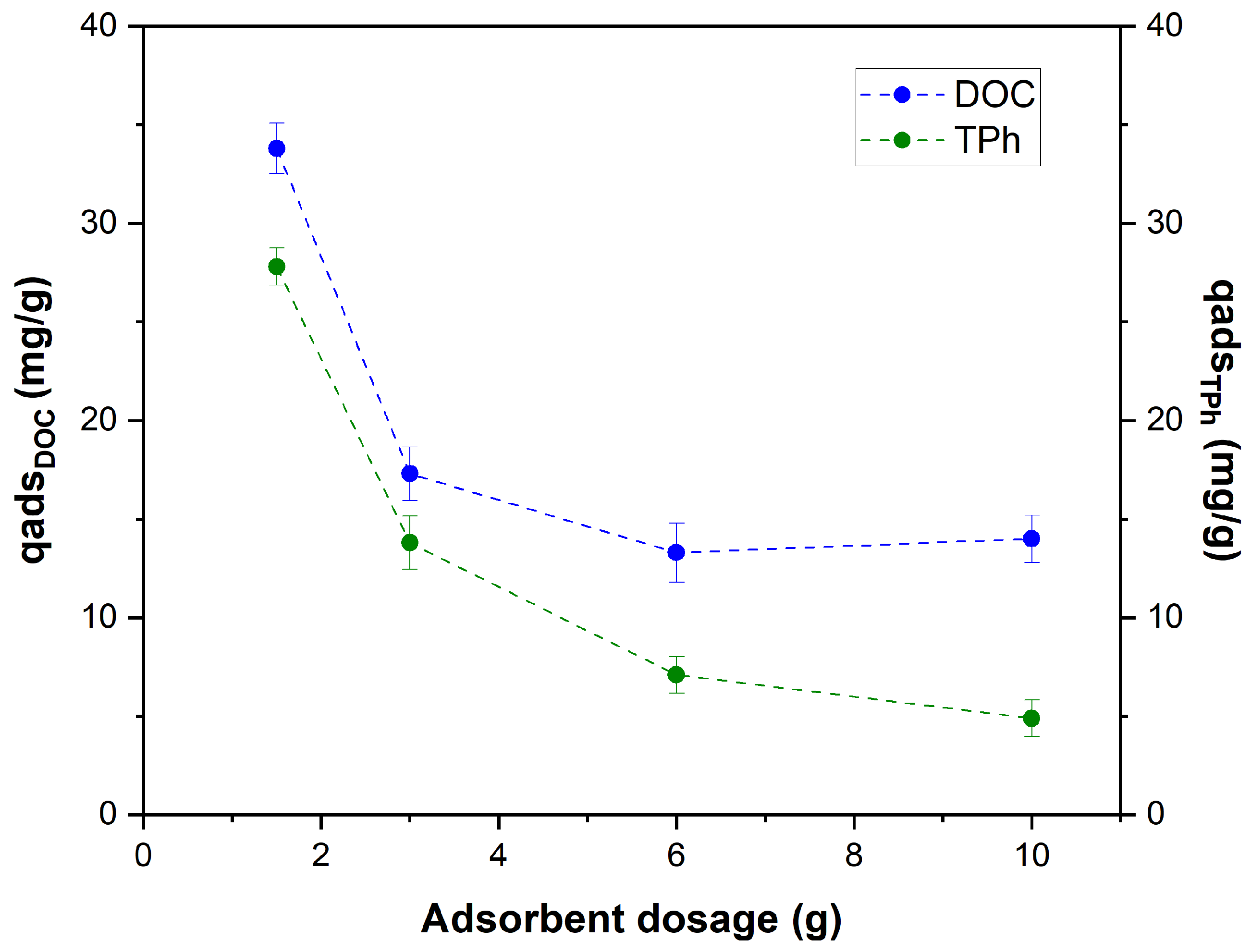
3.3.2. Dosage of Bentonite Effect
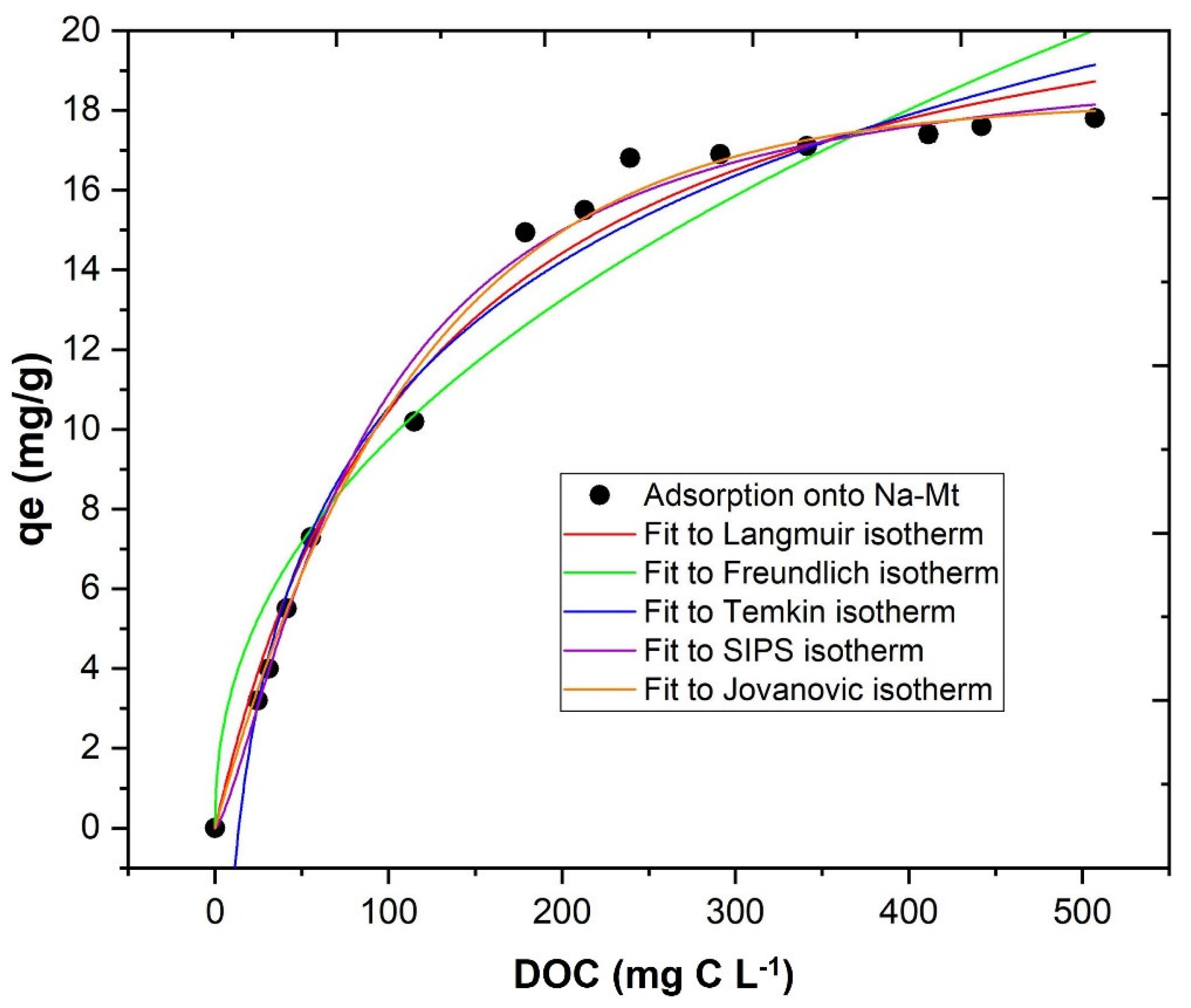
3.3.3. Influence of DOC Initial
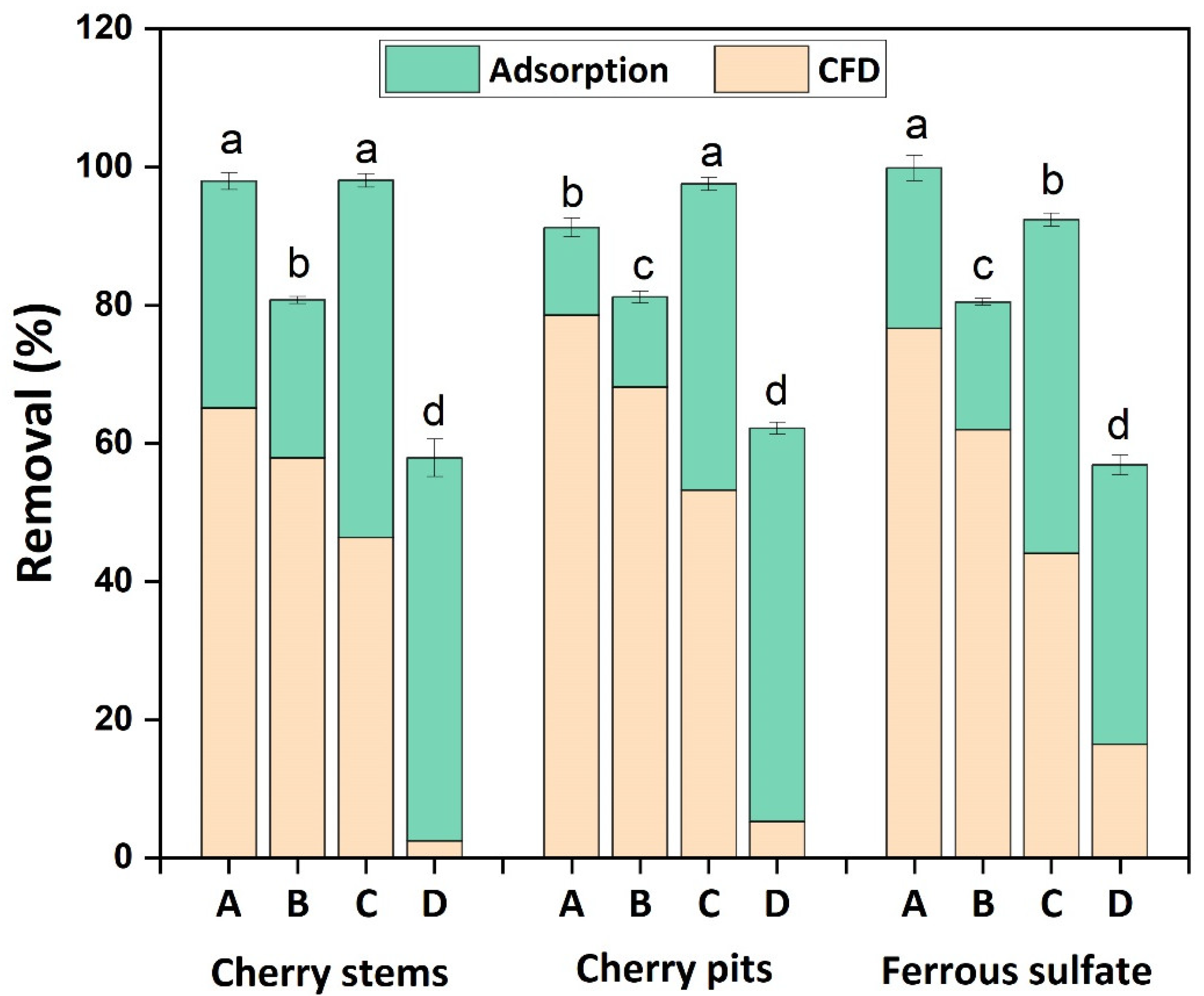
3.4. Combination of Coagulation–Flocculation–Decantation and Adsorption Processes
4. Conclusions
- The PBC is a complex matrix. Sweet cherry by-products are carbon-based materials with amino acids, aromatic compounds, lignin, and minerals, such as potassium, calcium, and iron, in their constitution. They are rich in bioactive compounds and have antioxidant activity;
- The cherry-based coagulants achieved better performance at acidic conditions, with a concentration of 0.1 g L−1 and stirring conditions of 200 rpm/2 min–60 rpm/30 min. The CFD process was shown to be more efficient in the removal of turbidity and TSS, achieving 65.2 and 78.6% turbidity removal and 58.0 and 68.2% TSS removal, respectively, for CSs and CPs;
- The adsorption process with bentonite achieved high removal of DOC (12.6%) and TPh (54.4%) in pH 3.0 and with 3.0 g L−1. Concerning adsorption isothermal models, the best fitting was provided by the Jovanovic model (r2 = 0.994);
- The adsorption process, after the CFD process, overall, improves removal rates, despite this increase being more significant in DOC removal rates, specifically from 2.5 and 5.3% for 58.0 and 62.2% for CSs and CPs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomakou, N.; Goula, A.M. Treatment of Olive Mill Wastewater by Adsorption of Phenolic Compounds. Rev. Environ. Sci. Biotechnol. 2021, 20, 839–863. [Google Scholar] [CrossRef]
- FAOSTAT Data. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 September 2023).
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment Technologies of Palm Oil Mill Effluent (POME) and Olive Mill Wastewater (OMW): A Brief Review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Donner, M.; Erraach, Y.; López-i-Gelats, F.; Manuel-i-Martin, J.; Yatribi, T.; Radić, I.; El Hadad-Gauthier, F. Circular Bioeconomy for Olive Oil Waste and By-Product Valorisation: Actors’ Strategies and Conditions in the Mediterranean Area. J. Environ. Manag. 2022, 321, 115836. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.M.; Rodrigues, C.S.D.; Maldonado-Hódar, F.J.; Madeira, L.M. Treatment of High-Strength Olive Mill Wastewater by Combined Fenton-like Oxidation and Coagulation/Flocculation. J. Environ. Chem. Eng. 2019, 7, 103252. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Castro-Silva, S.; Martins, R.C. Olive Oil Extraction Industry Wastewater Treatment by Coagulation and Fenton’s Process. J. Water Process Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- García, C.A.; Hodaifa, G. Real Olive Oil Mill Wastewater Treatment by Photo-Fenton System Using Artificial Ultraviolet Light Lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation–Flocculation–Decantation and Ozonation Processes for Winery Wastewater Treatment. Int. J. Environ. Res. Public Health 2021, 18, 8882. [Google Scholar] [CrossRef] [PubMed]
- Balbinoti, J.R.; Junior, R.E.S.; Sousa, L.B.F.; Bassetti, F.d.J.; Balbinoti, T.C.V.; Jorge, L.M.M.; Jorge, R.M.M. Plant-Based Coagulants for Food Industry Wastewater Treatment. J. Water Process Eng. 2023, 52, 103525. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the Art and Sustainability of Natural Coagulants in Water and Wastewater Treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Badawi, A.K.; Zaher, K. Hybrid Treatment System for Real Textile Wastewater Remediation Based on Coagulation/Flocculation, Adsorption and Filtration Processes: Performance and Economic Evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- Vuppala, S.; Shaik, R.U.; Stoller, M. Multi-Response Optimization of Coagulation and Flocculation of Olive Mill Wastewater: Statistical Approach. Appl. Sci. 2021, 11, 2344. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Maurya, S.; Daverey, A. Evaluation of Plant-Based Natural Coagulants for Municipal Wastewater Treatment. 3 Biotech 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Saritha, V.; Karnena, M.K.; Dwarapureddi, B.K. “Exploring Natural Coagulants as Impending Alternatives towards Sustainable Water Clarification”—A Comparative Studies of Natural Coagulants with Alum. J. Water Process Eng. 2019, 32, 100982. [Google Scholar] [CrossRef]
- Martins, R.B.; Jorge, N.; Lucas, M.S.; Raymundo, A.; Barros, A.I.R.N.A.; Peres, J.A. Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 4134. [Google Scholar] [CrossRef]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) by-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Pinto, E.; Amaro, F.; Flores-Félix, J.D.; Almeida, A.; de Pinho, P.G.; Falcão, A.; Alves, G.; Silva, L.R. Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods 2022, 11, 751. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Silva, A.S. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- INE Estatística Sobre Portugal e Europa. Available online: https://www.pordata.pt/portugal/superficie+das+principais+arvores+de+fruto+e+oliveiras-3365 (accessed on 20 August 2023).
- Vieira, Y.; Netto, M.S.; Lima, É.C.; Anastopoulos, I.; Oliveira, M.L.S.; Dotto, G.L. An Overview of Geological Originated Materials as a Trend for Adsorption in Wastewater Treatment. Geosci. Front. 2022, 13, 101150. [Google Scholar] [CrossRef]
- Guimarães, V.; Lucas, M.S.; Peres, J.A. Combination of Adsorption and Heterogeneous Photo-Fenton Processes for the Treatment of Winery Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 31000–31013. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Vezentsev, A.I.; Gorbunova, N.M.; Sokolovskiy, P.V.; Mar’inskikh, S.G.; Chub, A.V.; Chau, N.H.; Greish, A.A. On the Adsorption Mechanism of Copper Ions on Bentonite Clay. Russ. Chem. Bull. 2022, 71, 651–655. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 1999; ISBN 0875532357. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Garcia, B.; Coelho, J.; Costa, M.; Pinto, J.; Paiva-Martins, F. A Simple Method for the Determination of Bioactive Antioxidants in Virgin Olive Oils. J. Sci. Food Agric. 2013, 93, 1727–1732. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Optimization of Ultrasound Assisted Extraction of Polyphenols from Meghalayan Cherry Fruit (Prunus Nepalensis) Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Approach. J. Food Meas. Charact. 2021, 15, 119–133. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Fragata, A.; Guiné, R.; Esteves, B. Influence of Pre-Hydrolysis on the Chemical Composition of Prunus Avium Cherry Seeds. Agronomy 2022, 12, 280. [Google Scholar] [CrossRef]
- Siejak, P.; Smułek, W.; Nowak-Karnowska, J.; Dembska, A.; Neunert, G.; Polewski, K. Bird Cherry (Prunus padus) Fruit Extracts Inhibit Lipid Peroxidation in PC Liposomes: Spectroscopic, HPLC, and GC–MS Studies. Appl. Sci. 2022, 12, 7820. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Agro-Industrial Wastewater Treatment with Acacia Dealbata Coagulation/Flocculation and Photo-Fenton-Based Processes. Recycling 2022, 7, 54. [Google Scholar] [CrossRef]
- ELsayed, E.M.; Nour El-Den, A.A.; Elkady, M.F.; Zaatout, A.A. Comparison of Coagulation Performance Using Natural Coagulants against Traditional Ones. Sep. Sci. Technol. 2021, 56, 1779–1787. [Google Scholar] [CrossRef]
- Pap, S.; Radonić, J.; Trifunović, S.; Adamović, D.; Mihajlović, I.; Vojinović Miloradov, M.; Turk Sekulić, M. Evaluation of the Adsorption Potential of Eco-Friendly Activated Carbon Prepared from Cherry Kernels for the Removal of Pb2+, Cd2+ and Ni2+ from Aqueous Wastes. J. Environ. Manag. 2016, 184, 297–306. [Google Scholar] [CrossRef]
- Saritha, V.; Srinivas, N.; Srikanth Vuppala, N.V. Analysis and Optimization of Coagulation and Flocculation Process. Appl. Water Sci. 2017, 7, 451–460. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, C.-W.; Liang, J.-Y. Effect of Esterification Condensation on the Folin-Ciocalteu Method for the Quantitative Measurement of Total Phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Enhancement of EDDS-Photo-Fenton Process with Plant-Based Coagulants for Winery Wastewater Management. Environ. Res. 2023, 229, 116021. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.Z.; Madehi, N.; Yunus, R. Coagulative Behaviour of Jatropha curcas and Its Performance in Wastewater Treatment. Environ. Prog. Sustain. Energy 2017, 36, 1709–1718. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S. Revolutionary Technique for Sustainable Plant-Based Green Coagulants in Industrial Wastewater Treatment—A Review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Amuda, O.S.; Alade, A. Coagulation/Flocculation Process in the Treatment of Abattoir Wastewater. Desalination 2006, 196, 22–31. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Combined Organic Coagulants and Photocatalytic Processes for Winery Wastewater Treatment. J. Environ. Manag. 2023, 326, 116819. [Google Scholar] [CrossRef] [PubMed]
- Amuda, O.S.; Amoo, I.A. Coagulation/Flocculation Process and Sludge Conditioning in Beverage Industrial Wastewater Treatment. J. Hazard. Mater. 2007, 141, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kenea, D.; Denekew, T.; Bulti, R.; Olani, B.; Temesgen, D.; Sefiw, D.; Beyene, D.; Ebba, M.; Mekonin, W. Investigation on Surface Water Treatment Using Blended Moringa Oleifera Seed and Aloe Vera Plants as Natural Coagulants. S. Afr. J. Chem. Eng. 2023, 45, 294–304. [Google Scholar] [CrossRef]
- Muslim, W.A.; Al-nasri, S.K.; Albayati, T.M.; Salih, I.K. Investigation of Bentonite Clay Minerals as a Natural Adsorbents for Cs-137 Real Radioactive Wastewater Treatment. Desalin. Water Treat. 2024, 317, 100121. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, S.; Hao, F. Differential Adsorption of Clay Minerals: Implications for Organic Matter Enrichment. Earth-Sci. Rev. 2023, 246, 104598. [Google Scholar] [CrossRef]
- El Azzouzi, L.; El Aggadi, S.; Ennouhi, M.; Ennouari, A.; Kabbaj, O.K.; Zrineh, A. Removal of the Amoxicillin Antibiotic from Aqueous Matrices by Means of an Adsorption Process Using Kaolinite Clay. Sci. Afr. 2022, 18, e01390. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Jorge, N.; Fernandes, J.R.; Lucas, M.S.; Peres, J.A. Textile Dye Removal by Acacia Dealbata Link. Pollen Adsorption Combined with UV-A/NTA/Fenton Process. Top. Catal. 2022, 65, 1045–1061. [Google Scholar] [CrossRef]
- Jovanović, D.S. Physical Adsorption of Gases-I: Isotherms for Monolayer and Multilayer Adsorption. Kolloid-Zeitschrift Zeitschrift für Polym. 1969, 235, 1203–1213. [Google Scholar] [CrossRef]

| Parameter | OMW | |
|---|---|---|
| Values | Units | |
| pH | 4.6 ± 0.1 | Sorensen scale |
| Conductivity | 3.8 ± 0.2 | mS cm−1 |
| Turbidity | 2400 ± 36 | NTU |
| Total suspended solids—TSS | 3.7 ± 0.3 | g L−1 |
| Dissolved organic carbon—DOC | 8.2 ± 0.1 | g C L−1 |
| Chemical oxygen demand—COD | 21.2 ± 0.8 | g O2 L−1 |
| Biochemical oxygen demand—BOD5 | 4.0 ± 0.5 | g O2 L−1 |
| Biodegradability—BOD5/COD | 0.19 ± 0.03 | - |
| Total polyphenols—TPh | 1.5 ± 0.2 | g gallic acid L−1 |
| Coagulant | K+ | Ca2+ | Mg2+ | Na+ | Fe2+ | Cu2+ |
|---|---|---|---|---|---|---|
| Cherry pits | 342.67 ± 6.63 b | 98.31 ± 10.94 b | 83.60 ± 16.51 a | 13.12 ± 3.84 b | 6.11 ± 0.29 | 0.57 ± 0.04 |
| Cherry stems | 741.43 ± 29.20 a | 329.88 ± 43.57 a | 15.69 ± 1.79 b | 45.98 ± 7.96 a | 6.17 ± 0.26 | 0.53 ± 0.01 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Coagulant | Total Phenolics (mg GAE g−1) | Total Flavonoids (mg CE g−1) | Ortho-Diphenols (mg CAE g−1) | DPPH (μg Trolox g−1) |
|---|---|---|---|---|
| Cherry pits | 3.59 ± 0.24 b | 0.23 ± 0.03 b | 0.61 ± 0.01 b | 3.10 ± 0.15 b |
| Cherry stems | 10.63 ± 0.06 a | 13.98 ± 2.09 a | 2.37 ± 0.11 a | 27.88 ± 0.23 a |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Isothermal Model | Equation | Model Parameters | Bentonite Adsorption |
|---|---|---|---|
| Langmuir | qm (mg g−1) | 23.236 | |
| KL (L g−1) | 0.00818 | ||
| r2 | 0.985 | ||
| Freundlich | Kf (mg g−1 (mg L−1)−n) | 1.264 | |
| n | 0.443 | ||
| r2 | 0.939 | ||
| SIPS | Ks | 0.012 | |
| qm (mol kg−1) | 19.794 | ||
| n | 1.356 | ||
| r2 | 0.992 | ||
| Temkin | Kt (L g−1) | 0.073 | |
| β (J mol−1) | 5.300 | ||
| r2 | 0.981 | ||
| Jovanovic | qm (mg g−1) | 18.219 | |
| b (L g−1) | 0.009 | ||
| r2 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, A.R.; Afonso, S.; Jorge, N.; Oliveira, I.V.; Gonçalves, B.; Peres, J.A.; Lucas, M.S. Valorization of Cherry By-Products as Coagulant/Flocculants Combined with Bentonite Clay for Olive Mill Wastewater Treatment. Water 2024, 16, 1530. https://doi.org/10.3390/w16111530
Teixeira AR, Afonso S, Jorge N, Oliveira IV, Gonçalves B, Peres JA, Lucas MS. Valorization of Cherry By-Products as Coagulant/Flocculants Combined with Bentonite Clay for Olive Mill Wastewater Treatment. Water. 2024; 16(11):1530. https://doi.org/10.3390/w16111530
Chicago/Turabian StyleTeixeira, Ana R., Sílvia Afonso, Nuno Jorge, Ivo V. Oliveira, Berta Gonçalves, José A. Peres, and Marco S. Lucas. 2024. "Valorization of Cherry By-Products as Coagulant/Flocculants Combined with Bentonite Clay for Olive Mill Wastewater Treatment" Water 16, no. 11: 1530. https://doi.org/10.3390/w16111530
APA StyleTeixeira, A. R., Afonso, S., Jorge, N., Oliveira, I. V., Gonçalves, B., Peres, J. A., & Lucas, M. S. (2024). Valorization of Cherry By-Products as Coagulant/Flocculants Combined with Bentonite Clay for Olive Mill Wastewater Treatment. Water, 16(11), 1530. https://doi.org/10.3390/w16111530









