Investigation of Kinetic, Equilibrium, and Thermodynamic Modeling of Perfluorooctanoic Acid (PFOA) Adsorption in the Presence of Natural Organic Matter (NOM) by Dielectric Barrier Discharge Plasma-Modified Granular Activated Carbon (GAC)
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Activated GAC with PA
2.3. DBD Plasma Treatment of PA-Activated GAC
2.4. Adsorption Experiment
2.5. PFOA Determination
2.6. Characterization of Plasma-Treated GAC
2.7. Adsorption Kinetic Models
2.7.1. Pseudo-First-Order Model (PFOM)
2.7.2. Pseudo-Second-Order Model (PSOM)
2.7.3. Elovich Model (EM)
2.8. Adsorption Isotherm
2.8.1. Freundlich Isotherm Model (FIM)
2.8.2. Langmuir Isotherm Model (LIM)
2.8.3. Redlich–Peterson (R–P) Isotherm
2.8.4. Sips Isotherm Model (SIM)
2.9. Adsorption Thermodynamics
3. Results and Discussions
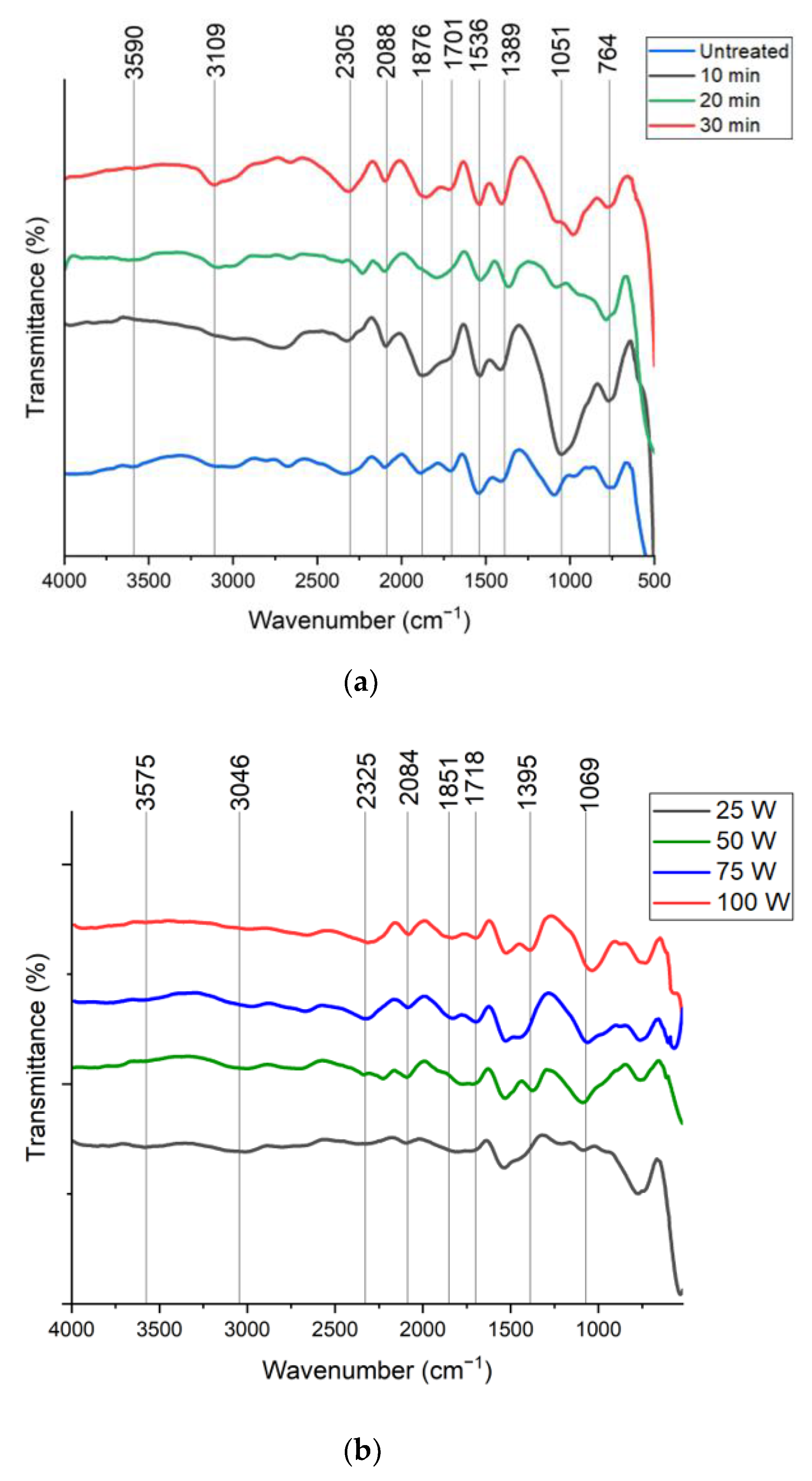
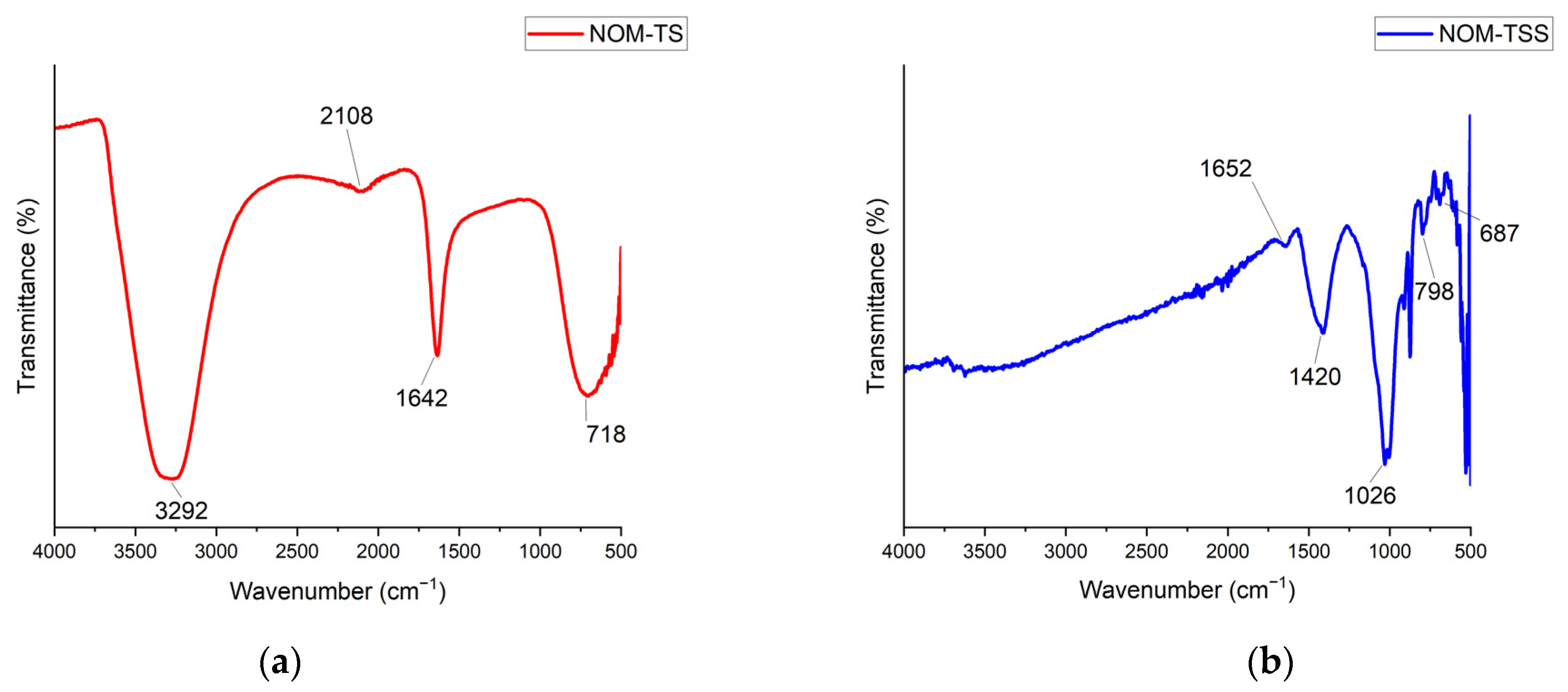
3.1. FTIR Analysis of GAC
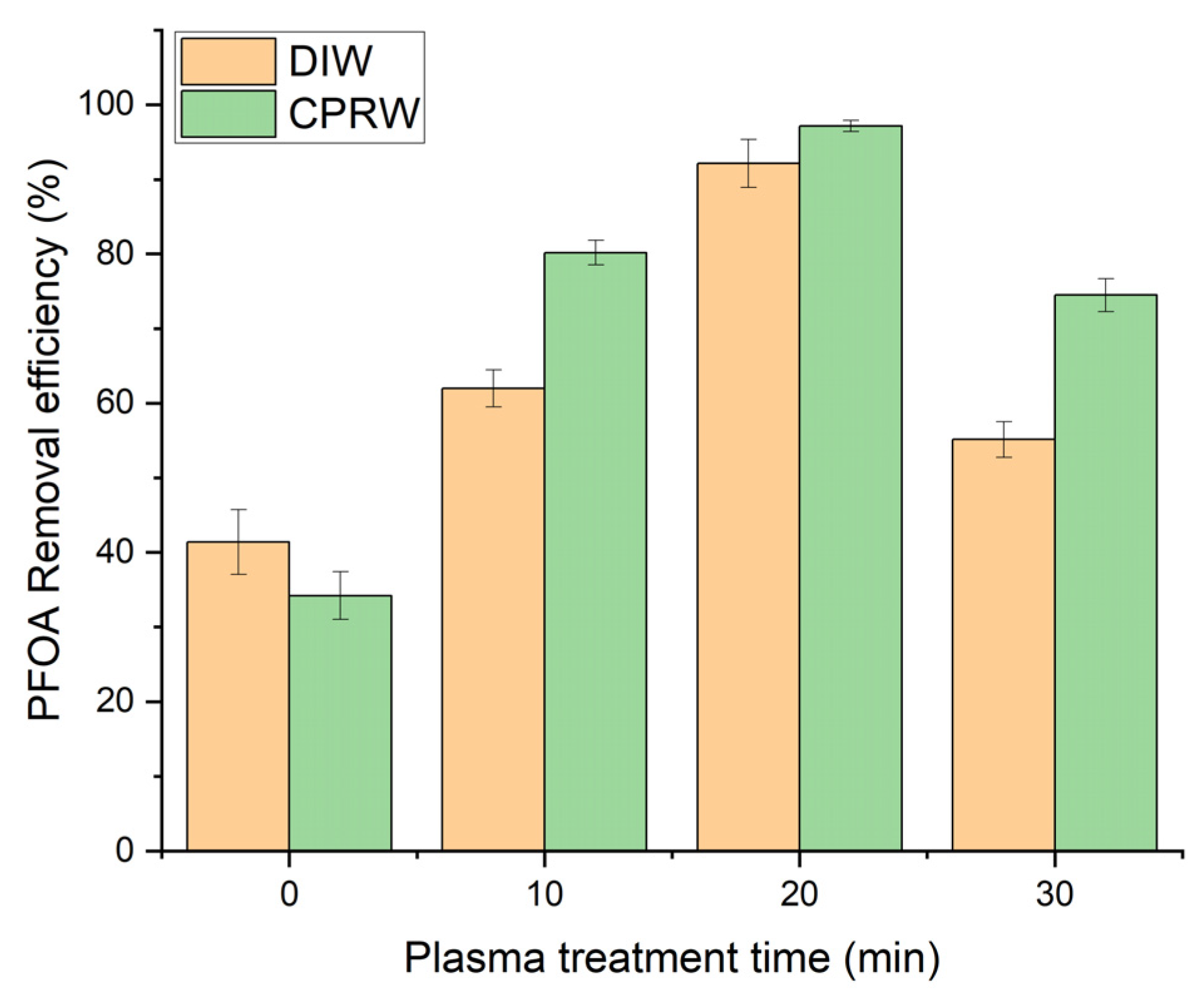
3.2. Effect of Plasma Treatment Time (PTT)
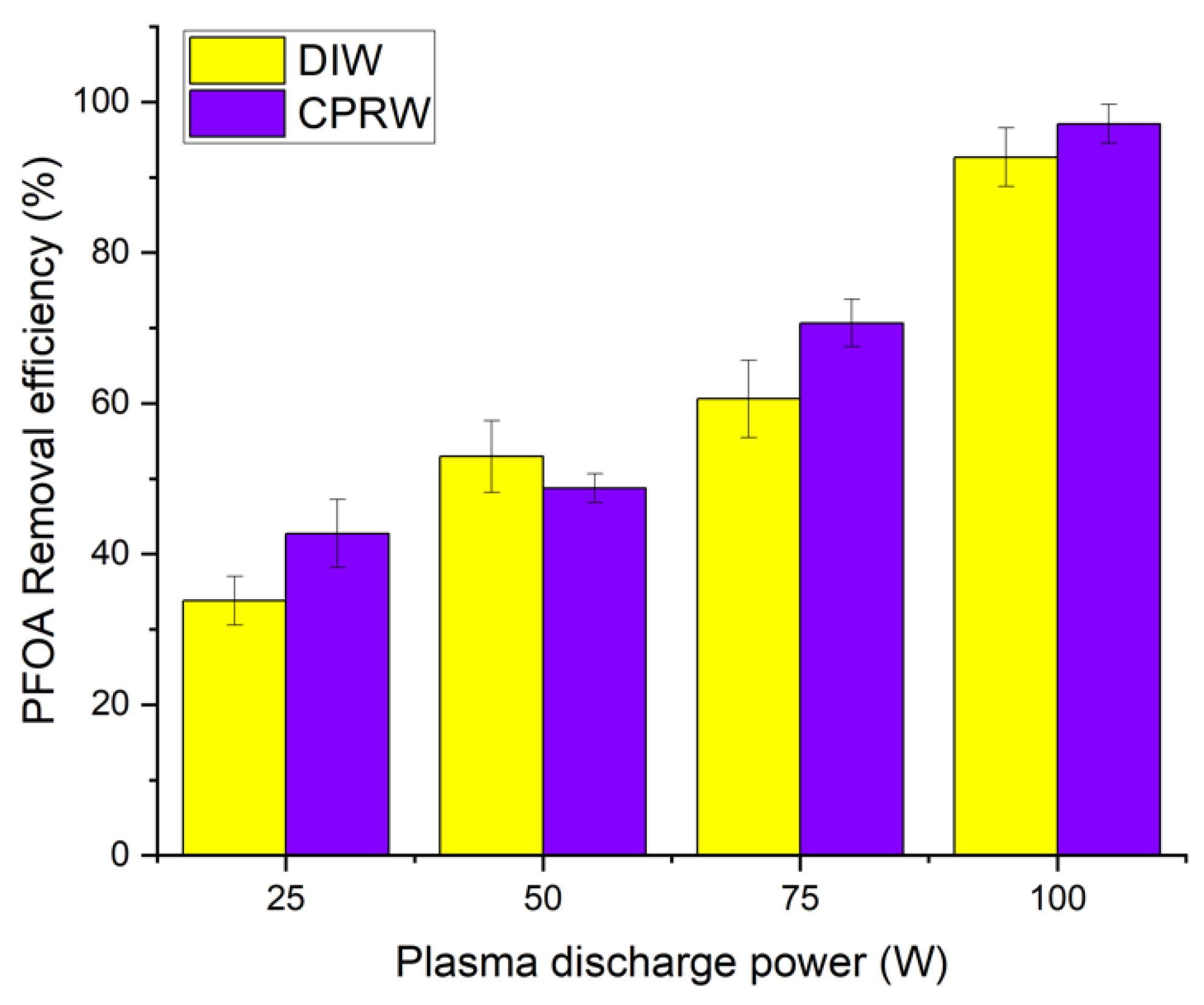
3.3. Effect of Plasma Discharge Power (PDP)
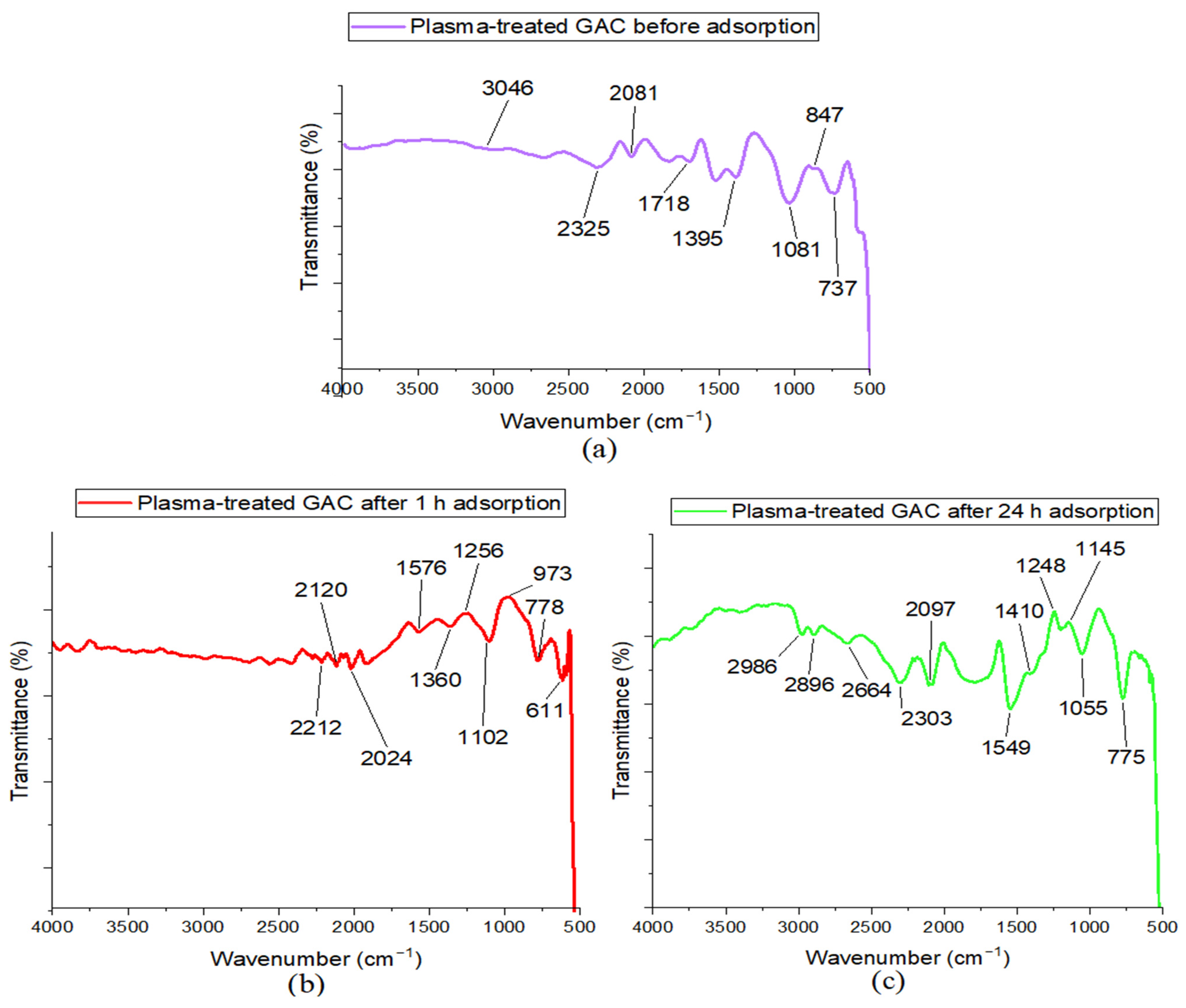
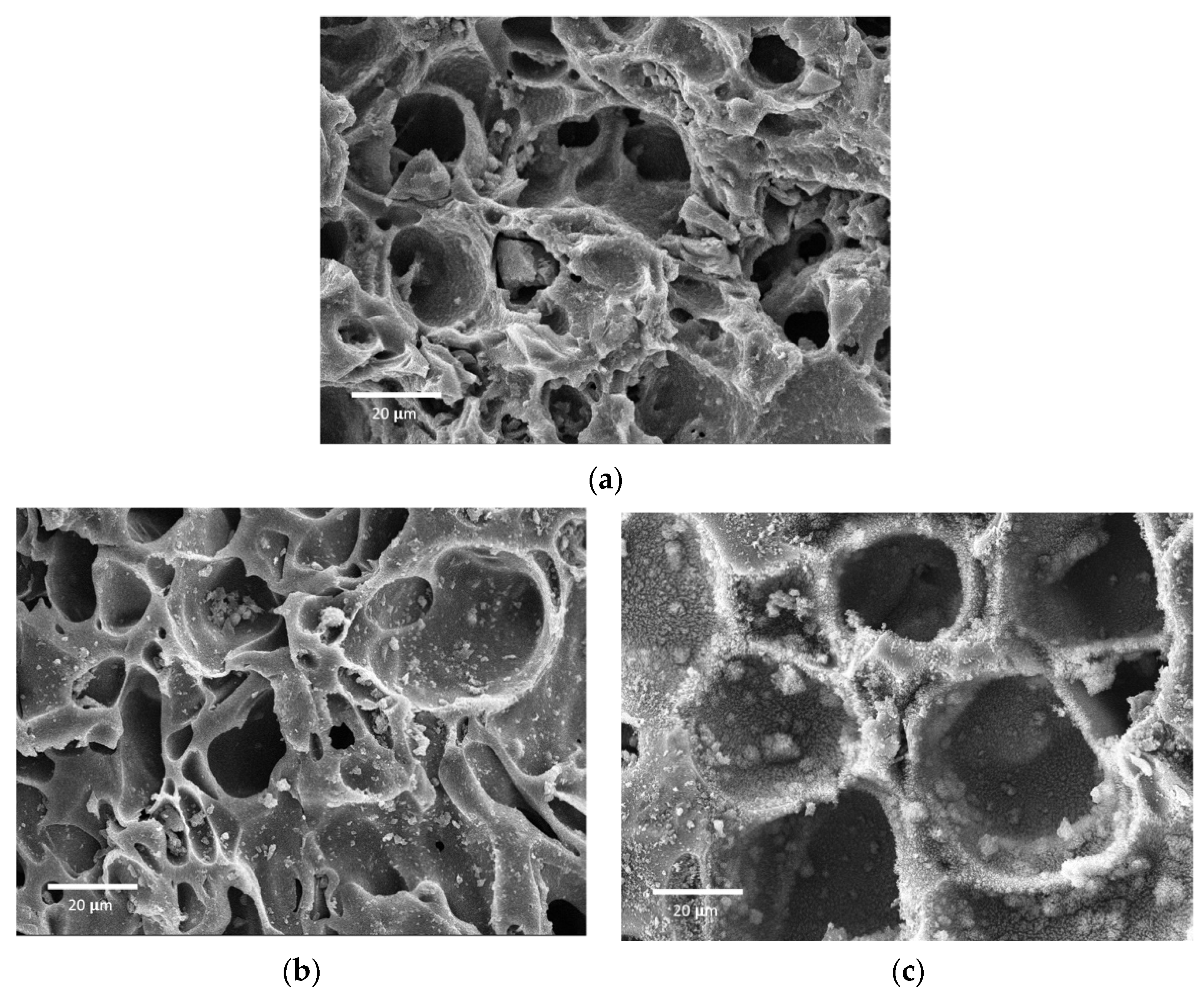
3.4. Characterization Results for the NOM and PFOA Adsorption on the Plasma-Treated GAC
3.5. Adsorption Kinetics
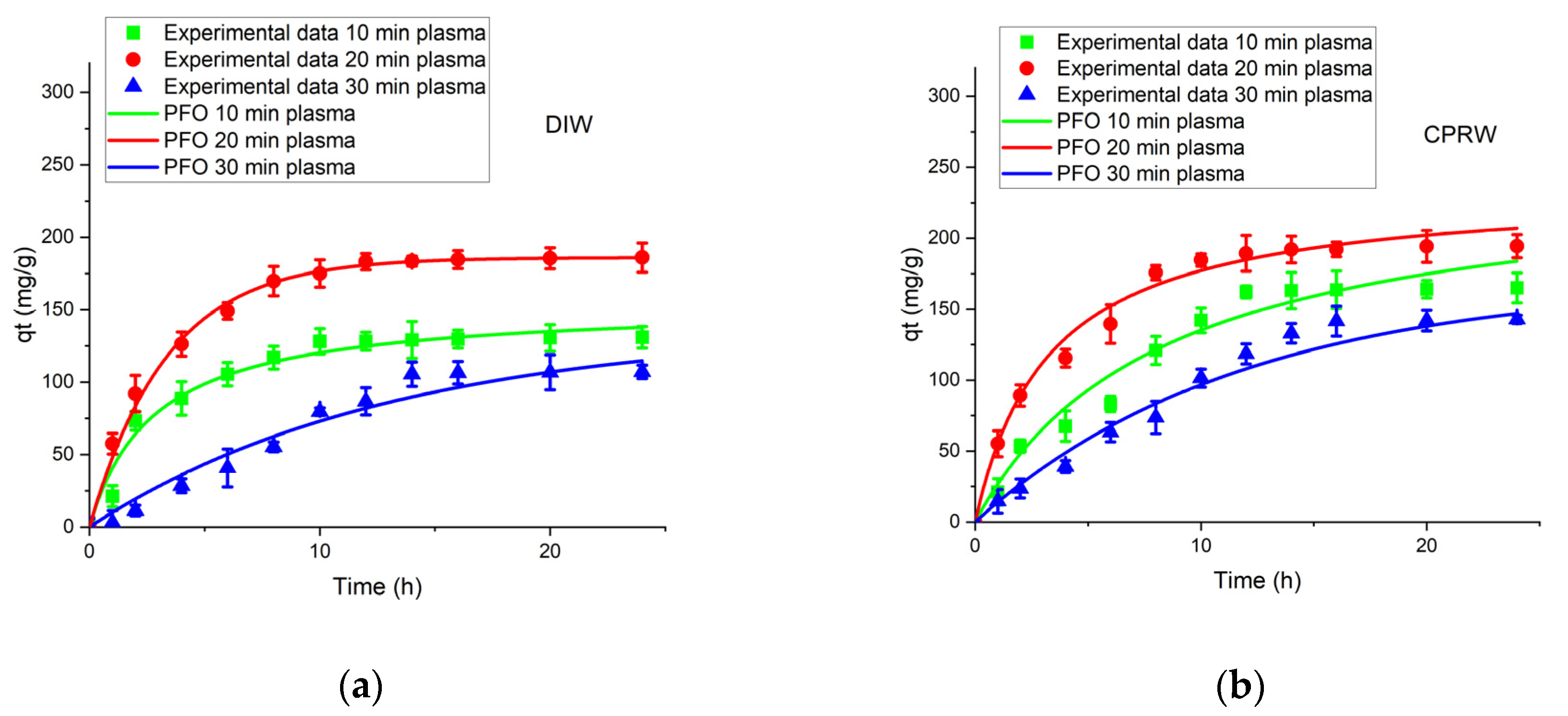
3.5.1. PFOM Adsorption Kinetic
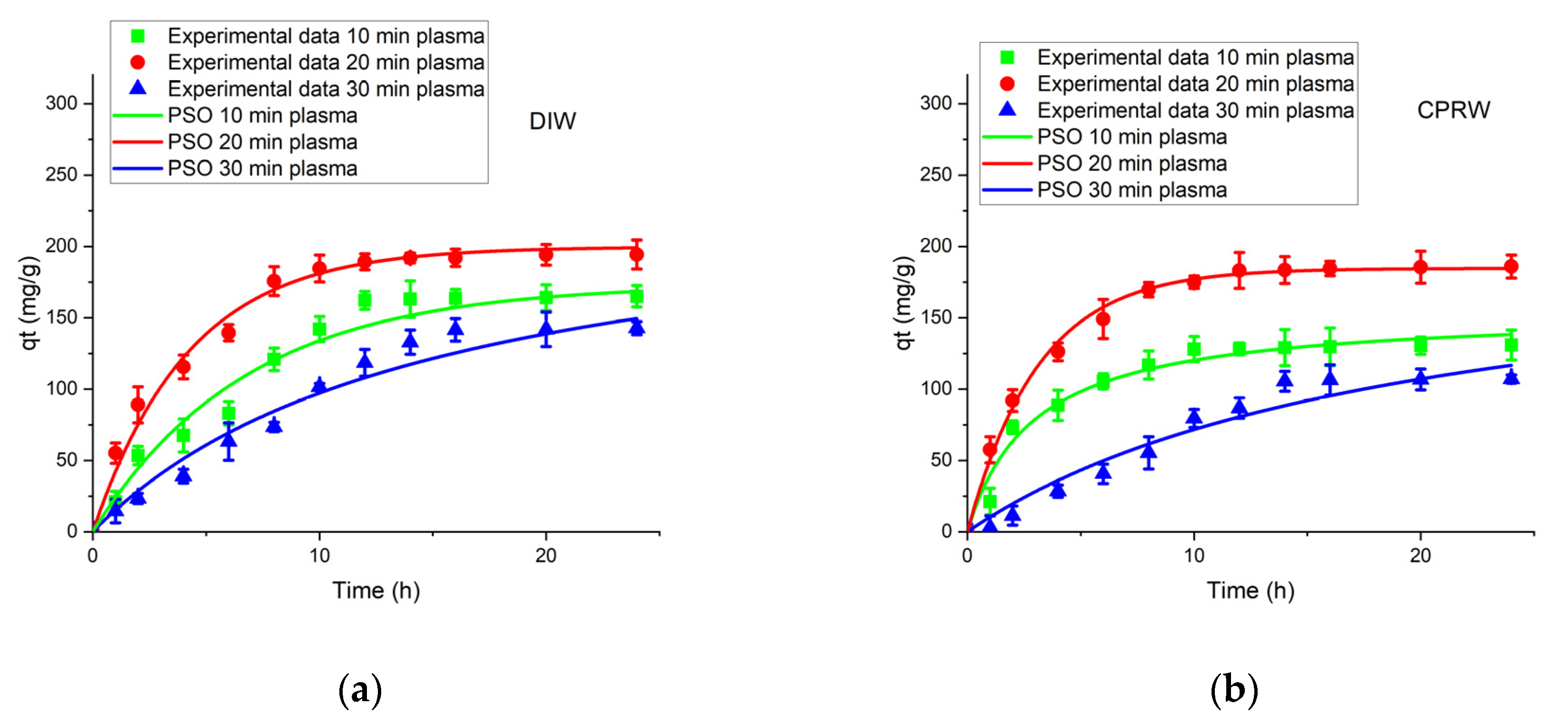
3.5.2. PSOM Adsorption Kinetic
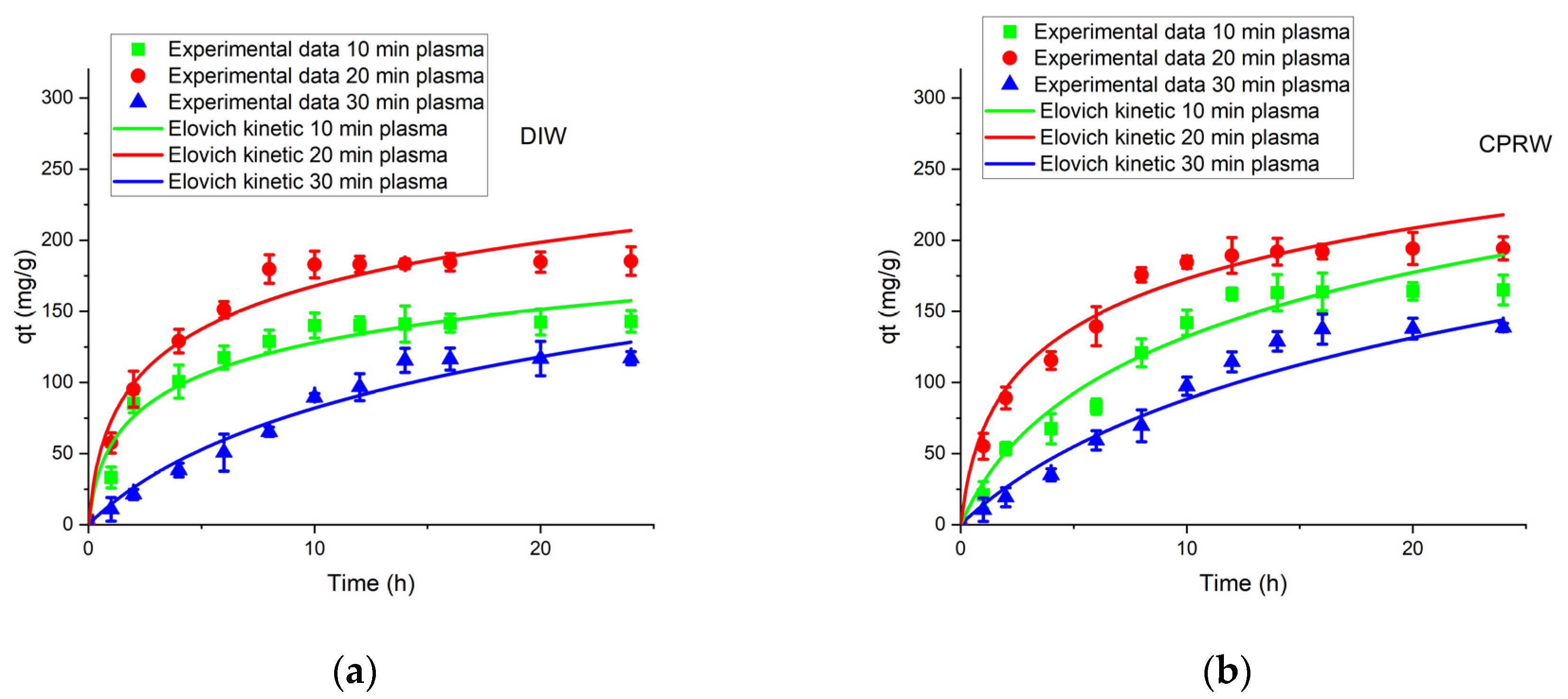
3.5.3. Elovich Kinetic Model (EM)
3.6. Adsorption Isotherm Models
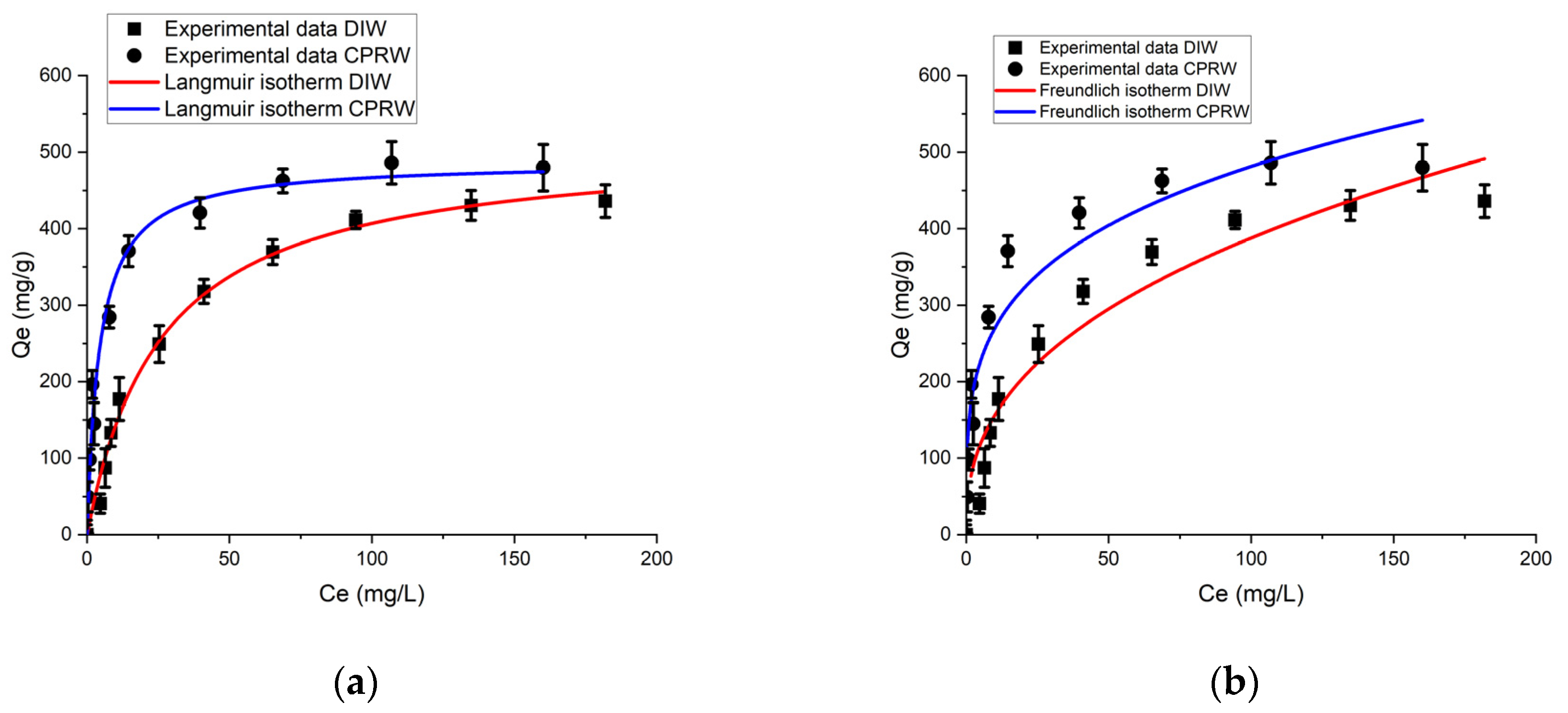
3.6.1. Langmuir Isotherm (LIM) and Freundlich Isotherm (FIM) Analysis
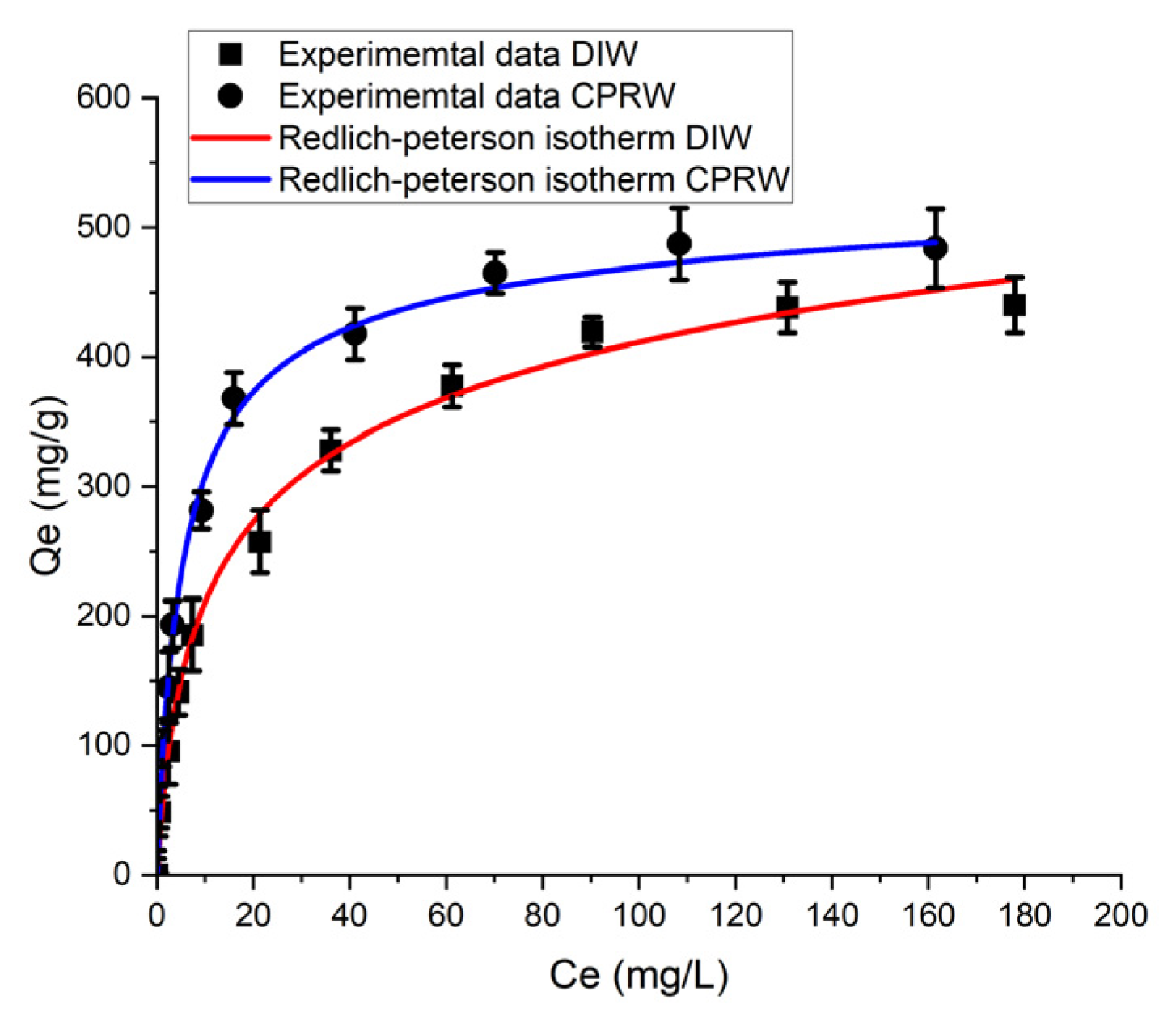
3.6.2. Redlich–Peterson (R–P) Isotherm Model Analysis
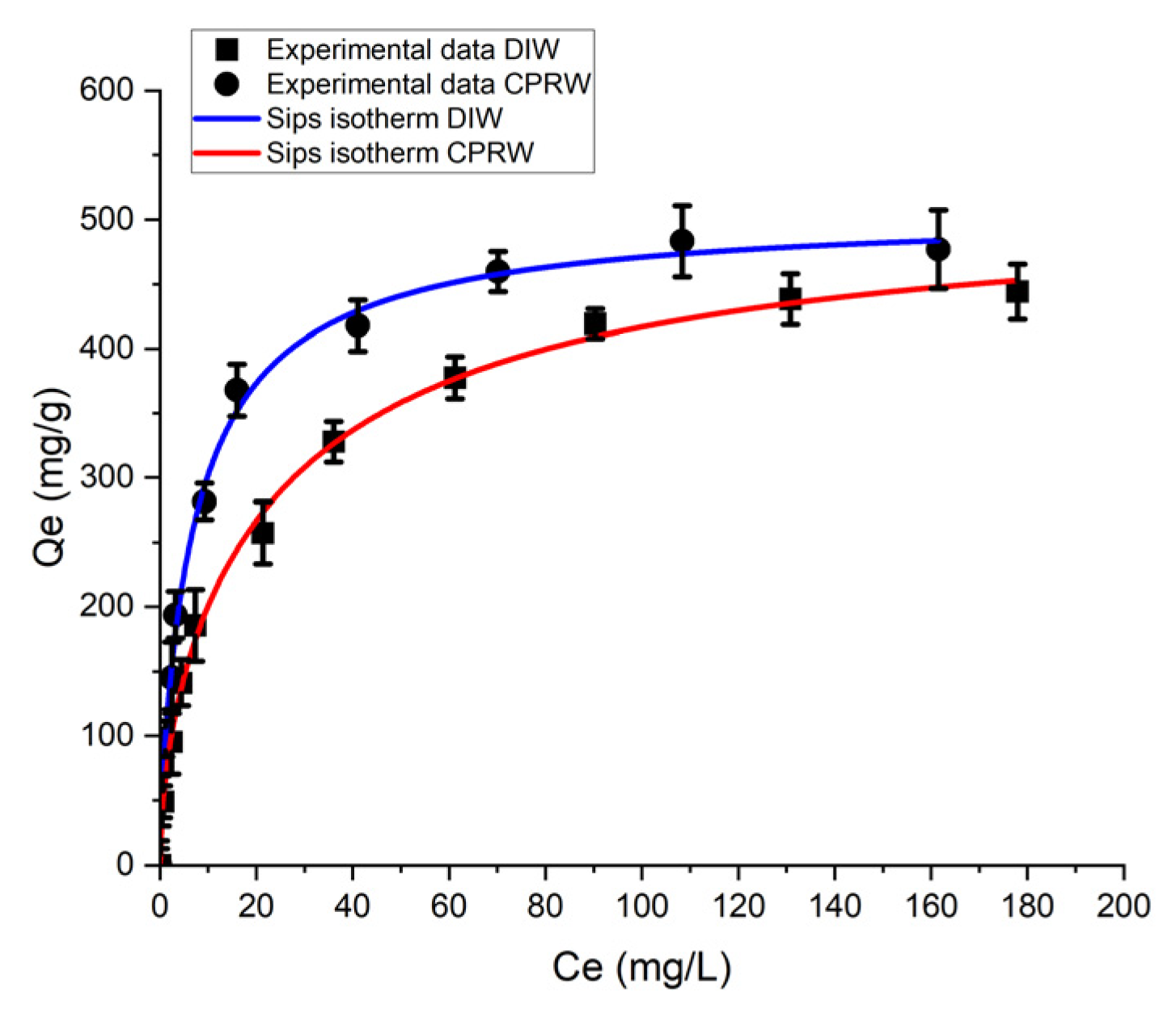
3.6.3. Sips Isotherm Model (SIM) Analysis
3.7. Thermodynamic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, P.; Liu, X.; Hu, L.; Wang, Q.; Xu, P.; Zhang, G. Enhanced degradation of PFOA in water by dielectric barrier discharge plasma in a coaxial cylindrical structure with the assistance of peroxymonosulfate. Chem. Eng. J. 2020, 389, 124381. [Google Scholar] [CrossRef]
- Yang, K.-H.; Lin, Y.-C.; Fang, M.-D.; Wu, C.-H.; Panchangam, S.C.; Hong, P.-K.A.; Lin, C.-F. Sorption of Perfluorooctanoic Acid (PFOA) onto Sediment in the Presence of Dissolved Natural Organics. Sep. Sci. Technol. 2013, 48, 1473–1478. [Google Scholar] [CrossRef]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic acid (PFOA)—Main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24, 16. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Akenga, T.A.; Olatunji, O.S. Removal of PFOA and PFOS from aqueous solutions using activated carbon produced from Vitis vinifera leaf litter. Environ. Sci. Pollut. Res. Int. 2017, 24, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Van Wouwe, N.; Yang, J.H.; et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Park, K.Y.; Song, W.J.; Park, Y.M.; Kweon, J.H. Adsorption mechanisms on perfluorooctanoic acid by FeCl(3) modified granular activated carbon in aqueous solutions. Chemosphere 2022, 303 Pt 2, 134965. [Google Scholar] [CrossRef]
- Levchuk, I.; Rueda Marquez, J.J.; Sillanpaa, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-X.; He, W.; Wu, J.-Y.; Wu, W.-J.; Xu, F.-L. Effects of fluorescent dissolved organic matters (FDOMs) on perfluoroalkyl acids (PFAAs) in lake and river water. Sci. Total Environ. 2019, 666, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ateia, M.; Yoshimura, C. Natural organic matter undergoes different molecular sieving by adsorption on activated carbon and carbon nanotubes. Chemosphere 2018, 203, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bjelopavlic, M.; Newcombe, G.; Hayes, R. Adsorption of NOM onto Activated Carbon: Effect of Surface Charge, Ionic Strength, and Pore Volume Distribution. J. Colloid Interface Sci. 1999, 210, 271–280. [Google Scholar] [CrossRef]
- Cheng, W.; Dastgheib, S.A.; Karanfil, T. Adsorption of dissolved natural organic matter by modified activated carbons. Water Res. 2005, 39, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Vidic, R.D.; Suidan, M.T. Role of dissolved oxygen on the adsorptive capacity of activated carbon for synthetic and natural organic matter. Environ. Sci. Technol. 1991, 25, 1612–1618. [Google Scholar] [CrossRef]
- Yu, J.; Lv, L.; Lan, P.; Zhang, S.; Pan, B.; Zhang, W. Effect of effluent organic matter on the adsorption of perfluorinated compounds onto activated carbon. J. Hazard. Mater. 2012, 225–226, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Villota, S.M.; Lei, H.; Villota, E.; Qian, M.; Lavarias, J.; Taylan, V.; Agulto, I.; Mateo, W.; Valentin, M.; Denson, M. Microwave-Assisted Activation of Waste Cocoa Pod Husk by H3PO4 and KOH—Comparative Insight into Textural Properties and Pore Development. ACS Omega 2019, 4, 7088–7095. [Google Scholar] [CrossRef]
- Vanraes, P.; Nikiforov, A.Y.; Leys, C. Electrical Discharge in Water Treatment Technology for Micropollutant Decomposition. In Plasma Science and Technology—Progress in Physical States and Chemical Reactions; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Takaki, K.; Shimizu, M.; Mukaigawa, S.; Fujiwara, T. Effect of Electrode Shape in Dielectric Barrier Discharge Plasma Reactor for NOx Removal. IEEE Trans. Plasma Sci. 2004, 32, 32–38. [Google Scholar] [CrossRef]
- Duan, S.; Liu, X.; Wang, Y.; Meng, Y.; Alsaedi, A.; Hayat, T.; Li, J. Plasma surface modification of materials and their entrapment of water contaminant: A review. Plasma Process. Polym. 2017, 14, 1600218. [Google Scholar] [CrossRef]
- Yang, S.; Okada, N.; Nagatsu, M. The highly effective removal of Cs⁺ by low turbidity chitosan-grafted magnetic bentonite. J. Hazard Mater. 2016, 301, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Ö.; Saka, C. Surface modification with cold plasma application on kaolin and its effects on the adsorption of methylene blue. Appl. Clay Sci. 2013, 85, 96–102. [Google Scholar] [CrossRef]
- Naseh, M.V.; Khodadadi, A.A.; Mortazavi, Y.; Pourfayaz, F.; Alizadeh, O.; Maghrebi, M. Fast and clean functionalization of carbon nanotubes by dielectric barrier discharge plasma in air compared to acid treatment. Carbon 2010, 48, 1369–1379. [Google Scholar] [CrossRef]
- Monte Blanco, S.P.D.; Scheufele, F.B.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Marin, P.; Kroumov, A.D.; Borba, C.E. Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem. Eng. J. 2017, 307, 466–475. [Google Scholar] [CrossRef]
- Ta, A.T.; Babel, S. Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals. Chemosphere 2020, 257, 127234. [Google Scholar] [CrossRef] [PubMed]
- Molle, F. Scales and power in river basin management: The Chao Phraya River in Thailand 1. Geogr. J. 2007, 173, 358–373. [Google Scholar] [CrossRef]
- Shih, K.; Wang, F. Adsorption Behavior of Perfluorochemicals (PFCs) on Boehmite: Influence of Solution Chemistry. Procedia Environ. Sci. 2013, 18, 106–113. [Google Scholar] [CrossRef]
- Koc, M.; Donten, M.A.; Musijowski, J.; Guo, X.; Fauland, A.; Lankmayr, E.; Trojanowicz, M. Application of gas chromatography to determination of Total Organic Fluorine after defluorination of perfluorooctanoic acid as a model compound. Croat. Chem. Acta 2011, 84, 399–406. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handlingar. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; Wase, D.A.J.; CF Forster, C. Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA 1996, 22, 219–224. [Google Scholar]
- El-Khaiary, M.I.; Malash, G.F.; Ho, Y.-S. On the use of linearized pseudo-second-order kinetic equations for modeling adsorption systems. Desalination 2010, 257, 93–101. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk 1962, 2, 209–216. [Google Scholar]
- Ho, Y.S. Isotherms for the sorption of lead onto peat: Comparison of linear and non-linear methods. Pol. J. Environ. Stud. 2006, 15, 81–86. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part i. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Latour, R.A. The Langmuir isotherm: A commonly applied but misleading approach for the analysis of protein adsorption behavior. J. Biomed. Mater. Res. Part A 2015, 103, 949–958. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 2004, 16, 490–495. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Huang, H.; Li, R.; Lyu, Z.; Cheng, Y.; Zhao, B.; Jiang, Z.; Zhang, Y.; Xiong, F. Comparative study of methane adsorption of Middle-Upper Ordovician marine shales in the western Ordos Basin, NW China: Insights into impacts of moisture on thermodynamics and kinetics of adsorption. Chem. Eng. J. 2022, 446, 137411. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into adsorption thermodynamics. Thermodynamics 2011, 16, 349–364. [Google Scholar]
- Adane, B.; Siraj, K.; Meka, N. Kinetic, equilibrium and thermodynamic study of 2-chlorophenol adsorption onto Ricinus communis pericarp activated carbon from aqueous solutions. Green Chem. Lett. Rev. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Tian, H.; Li, T.; Zhang, T.; Xiao, X. Characterization of methane adsorption on overmature Lower Silurian–Upper Ordovician shales in Sichuan Basin, southwest China: Experimental results and geological implications. Int. J. Coal Geol. 2016, 156, 36–49. [Google Scholar] [CrossRef]
- Lawtae, P.; Tangsathitkulchai, C. The Use of High Surface Area Mesoporous-Activated Carbon from Longan Seed Biomass for Increasing Capacity and Kinetics of Methylene Blue Adsorption from Aqueous Solution. Molecules 2021, 26, 6521. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption Using Modified Orange Peel as Adsorbent. Adv. Mater. Res. 2011, 236–238, 237–240. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martínez-Hernández, A.L.; Castaño, V.M.; Rivera-Armenta, J.L.; Medellín-Rodríguez, F.; Velasco-Santos, C. Effects on the Thermo-Mechanical and Crystallinity Properties of Nylon 6,6 Electrospun Fibres Reinforced with One Dimensional (1D) and Two Dimensional (2D) Carbon. Materials 2013, 6, 3494–3513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, Y.; Ning, K.; Niu, X.; Tan, S.; Su, P. Remediation of perfluoroalkyl substances in landfill leachates by electrocoagulation. CLEAN Soil Air Water 2014, 42, 1740–1743. [Google Scholar] [CrossRef]
- Cui, J.; Deng, Y. Hydrated Electron Degradation of PFOA Laden on Ion-Exchange Resins in the Presence of Natural Organic Matter. ACS ES&T Eng. 2022, 3, 86–93. [Google Scholar]
- Schütze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Tao, X.; Liu, D.; Song, J.; Ye, Q.; Xu, D. Plasma modification of ZnMgAl-LDHs for adsorption property improvement. J. Taiwan Inst. Chem. Eng. 2017, 74, 281–288. [Google Scholar] [CrossRef]
- Sazali, N.; Harun, Z.; Sazali, N. A review on batch and column adsorption of various adsorbent towards the removal of heavy metal. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 67, 66–88. [Google Scholar]
- Kancharla, S.; Alexandridis, P.; Tsianou, M. Sequestration of per-and polyfluoroalkyl substances (PFAS) by adsorption: Surfactant and surface aspects. Curr. Opin. Colloid Interface Sci. 2022, 58, 101571. [Google Scholar] [CrossRef]
- Abdulla, H.A.N.; Minor, E.C.; Dias, R.F.; Hatcher, P.G. Changes in the compound classes of dissolved organic matter along an estuarine transect: A study using FTIR and 13C NMR. Geochim. Cosmochim. Acta 2010, 74, 3815–3838. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wang, X.; Wang, W.; Liu, Y.; Ren, M.; Xiao, H.; Liu, X. Characterization of Conformation and Locations of C–F Bonds in Graphene Derivative by Polarized ATR-FTIR. Anal. Chem. 2016, 88, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Sahara, T.; Wongsawaeng, D.; Ngaosuwan, K.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Highly effective removal of perfluorooctanoic acid (PFOA) in water with DBD-plasma-enhanced rice husks. Sci. Rep. 2023, 13, 13210. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Grythe, K.F.; Hansen, F.K. Surface modification of EPDM rubber by plasma treatment. Langmuir 2006, 22, 6109–6124. [Google Scholar] [CrossRef] [PubMed]
- Somashekara, D.; Mulky, L. Sequestration of Contaminants from Wastewater: A Review of Adsorption Processes. ChemBioEng Rev. 2023, 10, 491–509. [Google Scholar] [CrossRef]
- Back, D.; Shaffer, B.T.; Loper, J.E.; Philmus, B. Untargeted Identification of Alkyne-Containing Natural Products Using Ruthenium-Catalyzed Azide Alkyne Cycloaddition Reactions Coupled to LC-MS/MS. J. Nat. Prod. 2022, 85, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Inwati, G.K.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Future Sci. OA 2017, 3, FSO239. [Google Scholar] [CrossRef] [PubMed]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption in natural water: Role of activated carbon pore size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of Polar and Nonpolar Organic Chemicals to Carbon Nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef]
- Maibaum, L.; Dinner, A.R.; Chandler, D. Micelle Formation and the Hydrophobic Effect. J. Phys. Chem. B 2004, 108, 6778–6781. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; DeMarco, J.; Owen, D.M.; Randtke, S.J. Selected processes for removing NOM: An overview. J. Am. Water Work. Assoc. 1995, 87, 64–77. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Anah, L.; Astrini, N. Isotherm adsorption studies of Ni (II) ion removal from aqueous solutions by modified carboxymethyl cellulose hydrogel. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012017. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Choy, K.K.; Porter, J.F.; McKay, G. Langmuir isotherm models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. J. Chem. Eng. Data 2000, 45, 575–584. [Google Scholar] [CrossRef]
- Li, X.-J.; Yan, C.-J.; Luo, W.-J.; Gao, Q.; Zhou, Q.; Liu, C.; Zhou, S. Exceptional cerium(III) adsorption performance of poly(acrylic acid) brushes-decorated attapulgite with abundant and highly accessible binding sites. Chem. Eng. J. 2016, 284, 333–342. [Google Scholar] [CrossRef]
- Wu, F.-C.; Liu, B.-L.; Wu, K.-T.; Tseng, R.-L. A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Girish, C. Various isotherm models for multicomponent adsorption: A review. Int. J. Civ. Eng. Technol. 2017, 8, 80–86. [Google Scholar]
- Tzabar, N.; ter Brake, H.J.M. Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 2016, 22, 901–914. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yan, C.; Jiang, W.; Chang, C. The Langmuir monolayer adsorption model of organic matter into effective pores in activated carbon. J. Colloid Interface Sci. 2013, 389, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilibria 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef] [PubMed]
- Myers, A. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
| Operational Condition | Adsorption Kinetics | Adsorption Isotherm | Thermodynamics |
|---|---|---|---|
| Temperature (°C) | 25 | 25 | 25–65 |
| Contact time (h) | 0–24 | 24 | 24 |
| Stirring speed (rpm) | 300 | 300 | 300 |
| Adsorbent mass (g) | 0.05 | 0.05 | 0.05 |
| Initial PFOA concentration (mg/L) | 100 | 50–400 | 100 |
| Solution volume (mL) | 100 | 100 | 100 |
| PTT (min) | PFOM Parameter for DIW | PFOM Parameter for CPRW | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/h) | R2 | qe (mg/g) | k1 (1/h) | R2 | |
| 10 | 131.45 | 0.00227 | 0.972 | 163.97 | 5.02 × 10−4 | 0.965 |
| 20 | 185.93 | 0.2983 | 0.982 | 211.56 | 0.0013 | 0.975 |
| 30 | 107.62 | 0.5482 | 0.952 | 153.48 | 0.00197 | 0.959 |
| PTT (min) | PSOM Parameter for DIW | PSOM Parameter for CPRW | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k2 (g/mg·h) | R2 | qe (mg/g) | k2 (g/mg·h) | R2 | |
| 10 | 139.57 | 0.0018 | 0.975 | 154.68 | 0.137 | 0.994 |
| 20 | 184.86 | 0.254 | 0.987 | 191.43 | 0.311 | 0.995 |
| 30 | 111.37 | 0.071 | 0.977 | 140.59 | 0.078 | 0.962 |
| PTT (min) | EM Parameter for DIW | EM Parameter for CPRW | ||||
|---|---|---|---|---|---|---|
| α (mg/g·s) | β (g/mg) | R2 | α (mg/g·s) | β (g/mg) | R2 | |
| 10 | 36.65 | 0.0226 | 0.908 | 58.16 | 0.014 | 0.951 |
| 20 | 126.038 | 0.017 | 0.905 | 95.91 | 0.019 | 0.924 |
| 30 | 14.585 | 0.010 | 0.975 | 12.55 | 0.0093 | 0.968 |
| Solution | LIM Parameters | FIM Parameters | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (L/mg) | 1/n | R2 | |
| DIW | 487.68 | 0.038 | 0.990 | 62.88 | 0.395 | 0.813 |
| CPRW | 512.49 | 0.224 | 0.982 | 151.13 | 0.251 | 0.816 |
| Solution | R–P Model Parameters | ||
|---|---|---|---|
| KRP (L/mg) | R2 | ||
| DIW | 65.91 | 0.256 | 0.994 |
| CPRW | 100.92 | 0.332 | 0.995 |
| Solution | Sips Model Parameter | |||
|---|---|---|---|---|
| qmax (mg/g) | KS | n | R2 | |
| DIW | 506.35 | 0.020 | 0.431 | 0.996 |
| CPRW | 520.44 | 0.091 | 0.796 | 0.997 |
| Temp. (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol·K) |
|---|---|---|---|
| 298 | −5.42 ± 2.07 | 17.46 | 0.08 |
| 318 | −7.29 ± 1.88 | ||
| 338 | −8.48 ± 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahara, T.; Wongsawaeng, D.; Ngaosuwan, K.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Investigation of Kinetic, Equilibrium, and Thermodynamic Modeling of Perfluorooctanoic Acid (PFOA) Adsorption in the Presence of Natural Organic Matter (NOM) by Dielectric Barrier Discharge Plasma-Modified Granular Activated Carbon (GAC). Water 2024, 16, 1499. https://doi.org/10.3390/w16111499
Sahara T, Wongsawaeng D, Ngaosuwan K, Kiatkittipong W, Hosemann P, Assabumrungrat S. Investigation of Kinetic, Equilibrium, and Thermodynamic Modeling of Perfluorooctanoic Acid (PFOA) Adsorption in the Presence of Natural Organic Matter (NOM) by Dielectric Barrier Discharge Plasma-Modified Granular Activated Carbon (GAC). Water. 2024; 16(11):1499. https://doi.org/10.3390/w16111499
Chicago/Turabian StyleSahara, Thera, Doonyapong Wongsawaeng, Kanokwan Ngaosuwan, Worapon Kiatkittipong, Peter Hosemann, and Suttichai Assabumrungrat. 2024. "Investigation of Kinetic, Equilibrium, and Thermodynamic Modeling of Perfluorooctanoic Acid (PFOA) Adsorption in the Presence of Natural Organic Matter (NOM) by Dielectric Barrier Discharge Plasma-Modified Granular Activated Carbon (GAC)" Water 16, no. 11: 1499. https://doi.org/10.3390/w16111499
APA StyleSahara, T., Wongsawaeng, D., Ngaosuwan, K., Kiatkittipong, W., Hosemann, P., & Assabumrungrat, S. (2024). Investigation of Kinetic, Equilibrium, and Thermodynamic Modeling of Perfluorooctanoic Acid (PFOA) Adsorption in the Presence of Natural Organic Matter (NOM) by Dielectric Barrier Discharge Plasma-Modified Granular Activated Carbon (GAC). Water, 16(11), 1499. https://doi.org/10.3390/w16111499









