Abstract
Algal blooms caused by eutrophication are a major global problem, and the monitoring and prediction of algal densities in lakes are important indicators of eutrophication management. However, the reliability of the commonly used chlorophyll-a (Chl-a) to characterize phytoplankton density in lake environments needs to be further investigated. In this paper, we sampled and analyzed 365 samples from nine plateau lakes in Yunnan Province during the dry and rainy seasons. The Chl-a data measured by the laboratory spectrophotometric method and the portable YSI multi-parameter water quality meter (YSI) directly used in the field were compared, and regression analysis and correlation analysis with phytoplankton density were performed. Most of the Chl-a values measured by the laboratory instrument were greater than those measured by the YSI, and the correlation between the two methods was weak (0.492, p < 0.001). The correlation between Chl-a and phytoplankton density measured by the YSI reached 0.67 (p < 0.001) in the dry season, while the laboratory methods used to measure Chl-a to characterize phytoplankton density may have led to an overestimation of phytoplankton density due to nonspecific sources of Chl-a. However, both methods are relatively inaccurate for characterizing phytoplankton density. For different trophic states of lakes, nutrient concentration changes affect the Chl-a concentration of phytoplankton. During different seasons, changes in the fluorescence intensity of phytoplankton in response to environmental conditions prevent the YSI results from reflecting the authentic phytoplankton density. Furthermore, high species diversity can lead to inconsistent changes in Chl-a and phytoplankton because the content of Chl-a in individual cells of different phytoplankton is different. The relationship between Chl-a and phytoplankton density was species specific. Therefore, when applying Chl-a to characterize phytoplankton density in lakes, it is necessary to consider environmental conditions, phytoplankton community structure and other practical conditions. In addition, laboratory analytical methods and instrumental techniques and instruments need to be improved.
1. Introduction
With the rapid development of industrial and agricultural production and the rapid increase in human influence, the eutrophication of lakes is becoming increasingly serious due to the large amount of nutrients in the water body. Coupled with the impact of climate change, “algal blooms” occur more frequently [1] and affect material circulation and energy flow in aquatic ecosystems, destroying ecological balance [2]. Phytoplankton, as the primary producers of lakes, play an important role in maintaining the balance of aquatic ecosystems, and they have an obvious effect on water eutrophication [3,4]. Therefore, phytoplankton monitoring is beneficial for identifying effective measures to mitigate the harmful effects of “algal blooms” [5,6,7]. Chlorophyll-a (Chl-a) is an essential pigment for photosynthesis and an important component of phytoplankton cells [8]. The concentration of Chl-a is commonly used to characterize phytoplankton density and reflects the intensity of bloom occurrence and eutrophication level of water bodies, as well as the resulting environmental risks [9].
For quantitative phytoplankton analysis, phytoplankton cell counting can be used to quantify the number, classification and composition of phytoplankton. Moreover, it is a direct indicator of phytoplankton determination. However, the testing process is time-consuming and complicated and requires a great deal of effort in terms of algal identification and cell counting for complex environmental samples [10]. In addition to the direct method of cell counting, Chl-a is an indirect indicator for phytoplankton analysis and is currently widely used in environmental studies [8]. Compared with traditional cell counting methods, Chl-a has the advantages of being fast and easy to measure [10]. More importantly, based on hyperspectral remote sensing data, many studies have established inversion models of water quality parameters such as the Chl-a concentration [11,12,13,14]. These models are mainly constructed from the perspective of environmental conditions affecting phytoplankton growth. The Chl-a concentration is used to reflect the total phytoplankton density, to a certain extent, and characterize the intensity of cyanobacterial blooms [15,16]. The lack of in-depth analysis of phytoplankton community structure affects the accuracy of the models. Although the current technology can correlate remote sensing data with Chl-a, it is not possible to accurately estimate the number of algae and the characteristics of algal blooms from remote sensing data without a reliable relationship between Chl-a and phytoplankton, especially algae. Therefore, it is of decisive significance and value to explore how to obtain reliable Chl-a data related to phytoplankton, especially algae.
Some studies have established regression relationships between Chl-a and phytoplankton density to study seasonal changes in phytoplankton [17,18]. However, when using Chl-a to characterize phytoplankton density, it was found that the linear relationship between Chl-a content and phytoplankton density was not always valid, and sometimes the correlation between the two was not even observed. For example, Du et al. [19] found an extremely significant linear relationship between Chl-a and phytoplankton density in Xiamen Port (r = 0.92), but there was no correlation between the two parameters in the Panjiakou and Dayawan reservoirs. The Chl-a concentration in phytoplankton cells varies with phytoplankton species or taxa and is also affected by phytoplankton age, growth rate, light and nutritional conditions [20]. Moreover, studies have shown that the Chl-a concentrations significantly differ among different methods of Chl-a concentration testing [21]. Therefore, whether the uncertainty of this correlation is due to the influence of multiple factors on the Chl-a concentration or the inaccuracy of the measurement methods is still unclear. Given that Chl-a is currently one of the most widely used indicators [11,12,14], it is necessary to discuss the accuracy of its test method and the factors that affect its measurement accuracy to provide a scientific basis for its accurate application in environmental monitoring.
There are various methods for the determination of Chl-a. The traditional laboratory spectrophotometric method (hereinafter referred to as Lab) is more widely used and has the advantages of mature experimental methods, rigorous processes, standardized operation and relevant industry standards. However, the test procedure is time consuming and requires experienced analysts to ensure good data. Moreover, it cannot be used for continuous monitoring to obtain important real-time data in the early stage of a bloom. A portable YSI multi-parameter water quality meter (YSI6600V2, hereinafter referred to as YSI) can be directly used in the field and has been widely used because it is fast, lightweight and suitable for real-time analysis of a large number of samples [20]. Some researchers compared the Chl-a values measured by the YSI with those determined by spectrophotometry. Most of the values measured by the YSI were low and significantly different from those determined by spectrophotometry [21,22]. However, which of these two methods is reliable for characterizing phytoplankton density has not been assessed. In this study, based on a comparison of these two methods, we further analyzed the factors affecting phytoplankton Chl-a and investigated the accuracy of characterizing phytoplankton density by Chl-a under the influence of different trophic states of lakes, seasonal variations, nutrient concentrations and phytoplankton community structures. This study provides a theoretical basis for the improvement of remote sensing data models, laboratory analysis methods and instruments.
2. Materials and Methods
2.1. Study Area
The Yunnan–Guizhou Plateau is located in the southeastern part of the Tibetan Plateau. Lake Dianchi (LDC), Lake Qilu (LQL), Lake Yilong (LYL), Lake Chenghai (LCH), Lake Yangzong (LYZ), Lake Lugu (LLG), Lake Erhai (LEH), Lake Fuxian (LFX) and Lake Xingyun (LXY) are the nine major lakes on the Yunnan Plateau with areas greater than 30 km2. The Yunnan–Guizhou Plateau, where the nine lakes are located, has obvious dry (from October to April) and rainy seasons (from May to September). The nine plateau lakes play a vital role in water resource regulation and provide habitats for aquatic plants and animals. The population living in the watersheds of the nine plateau lakes is the most densely populated and economically developed region in the province. The rapid economic development of the nine plateau lakes has led to the deterioration of water quality, posing a great threat to the ecological environment. At present, LDC and LXY are dominated by Microcystis genera with frequent cyanobacterial blooms. LQL and LYL are heavily polluted by nutrients and have low transparency [23]. Nutrient salt concentrations reached mesotrophic levels in LEH, LCH and LYZ, of which LYZ was heavily contaminated by arsenic [24]. Although LFX and LLG are in an oligotrophic state, these two lakes have shown a trend toward increasing nutrient salt concentrations in recent years [25,26]. Therefore, the nine plateau lakes in Yunnan are ideal research subjects for studying phytoplankton.
2.2. Sample Collection
A total of 365 water samples were collected from nine lakes in April (in the dry season) and October (in the rainy season) 2020 (Figure 1). Water samples were collected 0.5 m below the surface of each lake, and stratified samples were taken at three sampling points in each lake. Stratified lake water was collected at vertical intervals of 1–2 m from lakes with water depths less than 10 m and at intervals of 5 m from lakes with water depths greater than 10 m. Phytoplankton samples and environmental samples were collected simultaneously. Water samples were stored in Lugo solution for phytoplankton cell counting and species identification. The YSI Multi-parameter Water Quality Monitor (hereafter referred to as YSI) was used to measure Chl-a, water temperature (WT), pH, dissolved oxygen (DO), depth and turbidity in situ, with six measurements at each monitoring point to ensure the accuracy of the data.
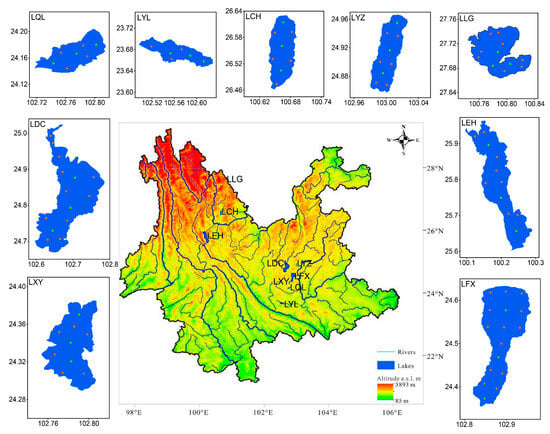
Figure 1.
Location and distribution of sampling points on the nine plateau lakes; yellow dots in the lake indicate sampling points where water samples were collected 0.5 m below the surface, and green dots indicate layered sampling points. (LDC: Lake Dianchi, LQL: Lake Qilu, LYL: Lake Yilong, LCH: Lake Chenghai, LYZ: Lake Yangzong, LLG: Lake Lugu, LEH: Lake Erhai, LFX: Lake Fuxian, LXY: Lake Xingyun).
2.3. Laboratory Spectrophotometric Assay
Water samples (1 L) were collected at each set of sampling points and returned to the laboratory as soon as possible. According to the “National Environmental Protection Standard of the People’s Republic of China” (HJ 897-2017), the experimental detection and analysis of Chl-a were performed via 90% acetone extraction spectrophotometry [10]. These water samples were filtered with a glass fiber filter membrane (0.7 μm aperture, GF/C, Whatman, USA), immersed in 10 mL of 90% acetone for grinding, and then chlorophyll was extracted at 4 °C for 24 h in the dark. Finally, a UV-visible spectrophotometer (UV-2600, Japan) was used to measure the absorbance at 750, 664, 647 and 630 nm. The Chl-a concentrations were calculated by the following formula, according to Jeffrey-Humphrey:
where ρChl-a is the Chl-a content of the sample (μg/L); E750, E664, E647 and E630 are the absorbance values of the acetone extract at wavelengths of 750 nm, 664 nm, 647 nm and 630 nm, respectively; Vacetone is the volume of the acetone extract (mL); VWater Sample is the volume of the water sample filtered (L); and L is the optical range of the cuvette (cm).
The determination of TN and TP in the water samples was carried out in accordance with the national standard method “Monitoring and Analysis Method of Water and Wastewater” (fourth edition) (GB/T11893-1989) [20]. The principle of TN determination is that potassium persulfate will produce potassium bisulfate and atomic oxygen in an aqueous solution above 60 °C, and atomic oxygen can convert nitrogen in the water sample to nitrate at 120 °C. Therefore, the absorbance can be determined by a UV-visible spectrophotometer (UV-2600, Japan, wavelengths of 220 nm and 275 nm), and then the TN content in the sample can be calculated. The principle of TP determination is to use potassium persulfate to dissolve the sample under neutral conditions and oxidize the phosphorus in the water sample to orthophosphate. When orthophosphate reacts with ammonium molybdate, it is immediately reduced with ascorbic acid to form a phosphorous molybdenum blue complex. Finally, the absorbance was measured by a UV-visible spectrophotometer (UV-2600, Japan, wavelength 700 nm), and the TP content in the sample was calculated.
2.4. Phytoplankton Collection and Identification
Phytoplankton samples were stored in Lugol’s solution for phytoplankton cell counting and species identification. All phytoplankton groups were counted in a counting chamber (0.1 mL) under a microscope (Olympus, Japan, 400× magnification). More than 500 cells were counted for each sample. Phytoplankton identification and counting were performed according to relevant classical literature for genus identification [27,28,29].
2.5. Statistical Analysis
The recorded data were evaluated using Microsoft Excel 2016. t-tests were performed on the data using SPSS 23. Scatter plots were drawn using Origin 2023 (Origin Lab, Ltd., Northampton, MA, USA) software, and correlation analysis graphs were generated in R.
3. Results
3.1. Comparative Analysis of Laboratory Instrumental Measurements and YSI Determination of Chl-a
The statistical results of the Chl-a concentration obtained by laboratory instrumental measurements and the YSI show that 90% of the data samples had higher laboratory values than the YSI values, indicating that the overall value of the Lab is higher than that of the YSI (Table 1). A paired t-test (p < 0.001) was performed on the two groups of data, and the results indicated a highly significant difference between the two groups. Correlation analysis between the Lab and YSI Chl-a values revealed a correlation coefficient of 0.492 (p < 0.001). The above results are generally consistent with previous results [21,22]; that is, most of the values measured by the YSI were low and significantly different from those determined by spectrophotometry. Overall, there is a certain correlation between the two methods, indicating that the Chl-a concentration determined by these two methods has a certain accuracy. However, the difference between the two groups of data is very significant, indicating that it may be affected by a variety of factors; one or both methods are not accurate enough, and which method is more accurate needs to be further discussed.

Table 1.
Statistics of the results of the two methods for the determination of Chl-a.
In terms of different seasons, the data were divided into dry and rainy seasons, and regression analysis of the data obtained by the two methods in the two seasons was performed (Figure 2). The results showed that there was a positive correlation between the Chl-a values measured by the two methods in both seasons. The correlation coefficient of the dry season was 0.58 (p < 0.001) greater than that of the rainy season, which was 0.21 (p < 0.001), which indicated that the accuracy of either or both of these methods was greater in the dry season.
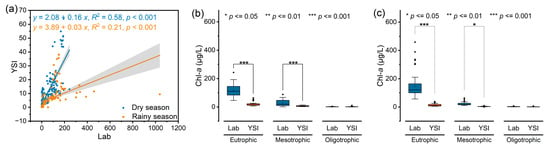
Figure 2.
(a) Linear regression analysis of the two methods; (b) comparison of two methods for the determination of Chl-a in three trophic lakes in the dry season; (c) comparison of two methods for the determination of Chl-a in three trophic lakes in the rainy season.
To explore the effects of different trophic states on the results measured by the two methods, the samples from nine lakes were divided into three groups according to the concentrations of TN and TP. The first group included LQL, LYL, LXY and LDC, which were characterized by high concentrations of TN and TP. The second group consisted of LCH, LEH and LYZ, and the contents of TN and TP were in the middle. The third group included LFX and LLG, which had low TN and TP contents. These grouping results are consistent with most studies showing that LQL, LYL, LXY and LDC are eutrophic lakes; LCH, LEH and LYZ are mesotrophic lakes; and LFX and LLG are oligotrophic lakes [30]. The results revealed that the two methods were influenced by the trophic states of the lakes, with highly significant differences between the Chl-a concentrations measured by the two methods in eutrophic and mesotrophic lakes and no significant differences between the two methods in oligotrophic lakes (Figure 2). Therefore, for eutrophic and mesotrophic lakes, the measurement error increases due to the increased nutrient concentration.
3.2. Accuracy of Chl-a Concentration in Characterizing Phytoplankton Density
The two methods for measuring Chl-a concentrations differ significantly both overall and in lakes during different seasons or with different trophic states. Therefore, to determine which method is most accurate, we compared the Chl-a values with phytoplankton density from different perspectives, such as seasonal variations, trophic states, and phytoplankton community structure.
3.2.1. Effects of Seasonal Variations
The Chl-a values measured by Lab and YSI in the dry season and the rainy season were correlated with phytoplankton density according to regression analysis (Figure 3). The results showed that the Chl-a values measured by the two methods were positively correlated with phytoplankton density in both seasons. In the dry season, the correlation coefficient between phytoplankton density and Chl-a measured by Lab was 0.57 (p < 0.001), and that between phytoplankton density and Chl-a measured by the YSI was 0.67 (p < 0.001). In the rainy season, the correlation coefficient between phytoplankton density and Chl-a measured by Lab was 0.62 (p < 0.001), and that between phytoplankton density and Chl-a measured by the YSI was 0.66 (p < 0.001). The correlation coefficient between the Chl-a value and phytoplankton density measured by the YSI was greater than that between the Chl-a value and phytoplankton density measured by the laboratory. These results indicated that the two methods can characterize phytoplankton density under different seasonal conditions, and the YSI is more accurate.
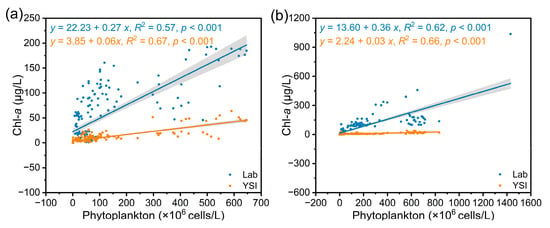
Figure 3.
Linear regression analysis of Chl-a and phytoplankton density measured by two methods. (a) Dry season; (b) rainy season.
3.2.2. Effects of Trophic States of the Lakes
Phytoplankton Chl-a was affected by nutrients, and all correlation coefficients between Chl-a and phytoplankton density were lower after the lakes were grouped according to nutrient concentrations than before grouping (Figure 4). The correlation coefficients between laboratory instrumental data and phytoplankton density ranged from 0.19 to 0.37; the correlation coefficients between data obtained by the YSI and phytoplankton density ranged from 0.10 to 0.54. The correlation coefficients between Chl-a and phytoplankton density were as follows: eutrophic lake > oligotrophic lake > mesotrophic lake. These results indicated that Chl-a is more accurate at characterizing phytoplankton density in eutrophic lakes. However, we also acknowledge that after grouping, the small sample sizes of mesotrophic and oligotrophic lakes may have contributed to the inaccuracy of these results.
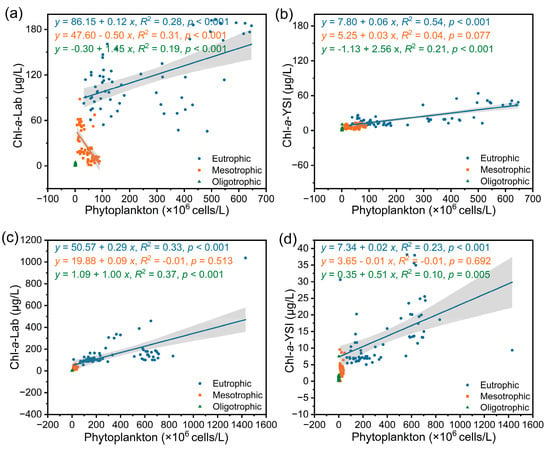
Figure 4.
Linear regression analysis between the Chl-a values measured by the two methods and phytoplankton density. (a) Dry season, Lab; (b) dry season, YSI; (c) rainy season, Lab; (d) rainy season, YSI.
3.2.3. Effects of Phytoplankton Community Structures in Lakes
There were great differences in the phytoplankton community structure in the nine lakes, and there were also great differences in the phytoplankton community structure in each lake between the two seasons [31]. In both seasons, four eutrophic lakes, LQL, LYL, LXY and LDC, and three mesotrophic lakes, LCH, LEH and LYZ, were dominated by cyanobacteria with a single dominant phytoplankton species. In the dry season, the oligotrophic lake LFX was dominated by cyanobacteria, chlorophytes, diatoms and chrysophytes, while in the rainy season, LFX was dominated by cyanobacteria, chlorophytes and diatoms with a variety of phytoplankton species. In the dry season, the LLG was dominated by diatoms, while in the rainy season, the LLG was dominated by chlorophyte and diatom species (Figure 5).
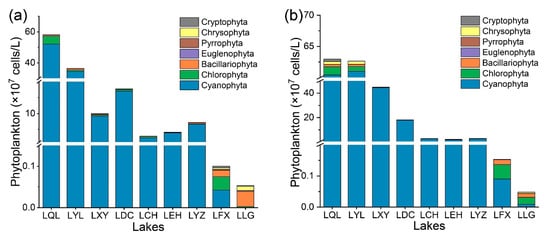
Figure 5.
Phytoplankton species composition in nine lakes. (a) Dry season; (b) rainy season.
According to the dominant species of phytoplankton in each lake, the samples from lakes with the same dominant species were divided into groups. In both seasons, the first group included LQL, LYL, LXY, LDC, LCH, LEH and LYZ, with cyanobacteria as the single dominant species. In the dry season, the second group was LFX, which had diverse phytoplankton species. The third group was LLG, with diatoms as the single dominant species. In the rainy season, the second group included LFX and LLG, with diverse phytoplankton species and chlorophytes among the dominant species. The third group was the LLG, which had diverse phytoplankton species. In lakes with cyanobacteria as the single dominant species, there was a positive correlation between the Chl-a values measured by the two methods and phytoplankton density. In the dry season, the correlation coefficient between phytoplankton density and Chl-a measured by Lab was 0.45 (p < 0.001), and that between phytoplankton density and Chl-a measured by the YSI was 0.67 (p < 0.001). In the rainy season, the correlation coefficient between phytoplankton density and Chl-a measured by Lab was 0.55 (p < 0.001), and that between phytoplankton density and Chl-a measured by the YSI was 0.58 (p < 0.001). In lakes with diatoms as the single dominant species, there was a positive correlation between Chl-a measured by the YSI and phytoplankton density, and the correlation coefficient was 0.60 (p < 0.001); however, there was no correlation with Lab. In lakes with diverse phytoplankton species, Chl-a measured by both methods in lakes with diverse phytoplankton species had low correlation coefficients with phytoplankton density, and the correlations were weak or uncorrelated (Figure 6). The above results indicated that in the samples with cyanobacteria as the dominant species, the Chl-a concentration was more accurate for characterizing phytoplankton density, and the YSI method was better. Second, in the samples with diatoms as the dominant species, the characterization of Chl-a determined by the YSI method was also more accurate.
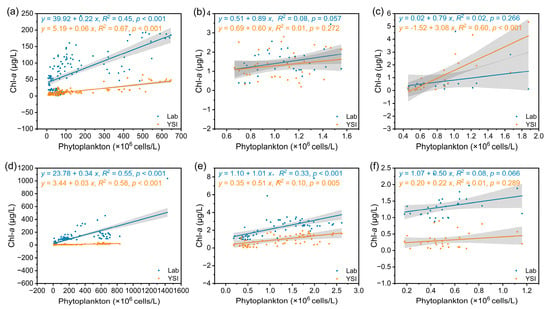
Figure 6.
Linear regression analysis of Chl-a and phytoplankton density. (a) Dry season, lakes with cyanobacteria as a single dominant species; (b) dry season, lakes with diverse species; (c) dry season, lakes with a single dominant species of diatom; (d) rainy season, lakes with a single dominant species of cyanobacteria; (e) rainy season, lakes with diverse species composition with chlorophytes among the dominant species; (f) rainy season, lakes with diverse species.
3.3. Analysis of the Factors Influencing the Chl-a Concentration
In terms of the physicochemical properties of lake water, correlation analysis revealed a significant positive correlation between Chl-a and TP in both seasons in the three trophic states of the lakes. In eutrophic lakes, there was a significant positive correlation between Chl-a and TN, but there was no significant correlation between Chl-a and TN in mesotrophic and oligotrophic lakes. This indicated that phytoplankton growth was limited by TN and TP in eutrophic lakes, and TP was the limiting factor for phytoplankton growth in mesotrophic and oligotrophic lakes [32]. In the three trophic states of the lakes, there was a significant positive correlation between Chl-a and WT, DO and pH in both seasons and a significant negative correlation between Chl-a and depth. These significant correlations were related to the growth environment of phytoplankton, which require light and high water temperatures and grow mainly at the lake surface [6,33]. In the three trophic states of the lakes, there was a positive or significant positive correlation between Chl-a and turbidity in the two seasons, and only a weak negative correlation appeared in the oligotrophic lakes. This is mainly because the turbidity of the water body will be affected by the phytoplankton density in the water. The greater the phytoplankton density, the greater the turbidity of the water body and the greater the measured Chl-a [34].
For biological factors, there were dominant species but no correlations with the Lab of Chl-a in the rainy season in either mesotrophic or oligotrophic lakes, such as cyanobacteria in mesotrophic lakes and diatoms in oligotrophic lakes. In mesotrophic and oligotrophic lakes, there were also dominant species but no correlations with the Chl-a concentration measured by the YSI, such as cyanobacteria in the dry season in mesotrophic lakes, cyanobacteria and chrysophytes in the dry season in oligotrophic lakes, and diatoms in the rainy season (Figure 7). The above phenomenon indicated that the Chl-a concentration is independent of the dominant species of phytoplankton in the lake.
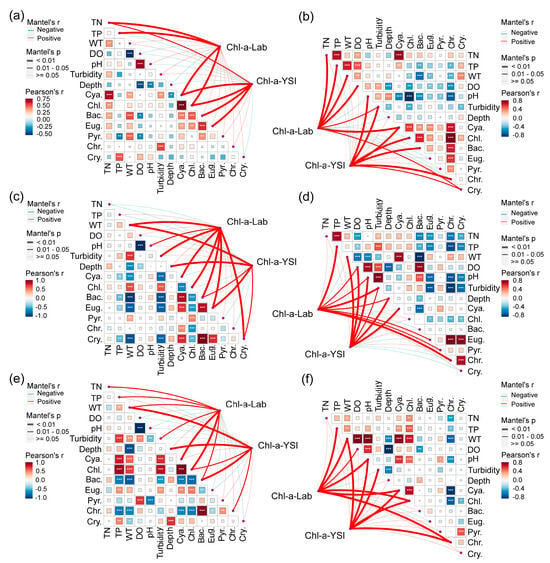
Figure 7.
Correlation analysis between the measured parameters and the Chl-a concentration measured by the two methods. (a) Dry season, eutrophic lakes; (b) rainy season, eutrophic lakes; (c) dry season, mesotrophic lakes; (d) rainy season, mesotrophic lakes; (e) dry season, oligotrophic lakes; (f) rainy season, oligotrophic lakes. The thickness of the line indicates the strength of the correlation; an asterisk indicates a significant correlation: * p < = 0.05, ** p < = 0.01, *** p < = 0.001. Cya.: Cyanophyta; Chl.: Chlorophyta; Bac.: Bacillariophyta; Eug.: Euglenophyta; Pyr.: Pyrrophyta; Chr.: Chrysophyta; Cry.: Cryptophyta.
4. Discussion
4.1. Potential Reasons for Discrepancies between Laboratory Instruments and the YSI
The results of the data analysis showed that most of the Chl-a values measured by the laboratory instrument were greater than those measured by the YSI, and the correlation between the two methods was weak (Figure 2). To compare the accuracy of the two methods for predicting phytoplankton, linear regression analysis was performed on the Chl-a measured by the two methods and phytoplankton density, and the YSI was more strongly correlated than the Lab (Figure 3). The possible reason for this difference was the nonspecific source of Chl-a. In addition to phytoplankton, Chl-a can also be produced by aquatic plants and terrestrial plants, and the use of Lab to measure Chl-a to characterize phytoplankton density may lead to an overestimation of phytoplankton cell density.
Previous studies revealed that in the case of low Chl-a concentrations, YSI results were more accurate, and the measurement error increased with increasing concentration [21,22]. Therefore, in this study, there was a highly significant difference between the two methods for measuring Chl-a in eutrophic and mesotrophic lakes, while there was no significant difference in Chl-a in oligotrophic lakes (Figure 2).
The principle used by the YSI is the fluorescence method [20,35], and that used by the laboratory is the spectrophotometric method [36,37]. The YSI is based on the fluorescence principle of photosynthetic pigments unique to blue-green algae [35], so the determination of blue-green algae density is relatively accurate; as a result, the Chl-a concentration is more accurate for characterizing phytoplankton density in eutrophic lakes (Figure 4). In fact, in terms of instrument accuracy, both methods are relatively inaccurate due to overlapping absorption bands and the presence of degradation products [18]. Moreover, the Chl-a concentration of phytoplankton cells is affected by external and internal factors, including environmental changes and phytoplankton community structure changes [38]. The above factors all affect the characterization of phytoplankton density by the Chl-a concentration.
4.2. Factors Affecting the Effect of Chl-a on Phytoplankton Density
The concentrations of Chl-a in the different lakes greatly differed, and the results of grouping all the samples according to trophic state revealed that Chl-a was positively correlated with phytoplankton density only in the eutrophic lakes in the dry season (Figure 4). In other periods, the correlation between Chl-a and the phytoplankton density at all nutrient levels weakened or even had no correlation (Figure 4). The possible reason was that the change in nutrient concentration can affect the Chl-a concentration of phytoplankton. That is, under nutrient-limited conditions, the relative Chl-a concentration decreases with increasing total cell volume [39,40], which in turn affects the Chl-a characterization of phytoplankton density. On the other hand, in eutrophic lakes, the phytoplankton density is high, and the Chl-a concentration is high, while in mesotrophic or even oligotrophic lakes, the phytoplankton density is low, and the Chl-a concentration is low, which is susceptible to interference from Chl-a produced by aquatic and terrestrial plants, thus affecting the characterization of phytoplankton density by Chl-a [40].
In this study, the correlation between Chl-a and phytoplankton density differed between the dry season and the rainy season (Figure 3). Laboratory spectrophotometry revealed a significant positive correlation between the Chl-a concentration and phytoplankton density in both seasons (Figure 3). However, due to nutrient restriction, the content of Chl-a in algal cells decreased in the dry season [21], resulting in a weaker correlation between the two than in the rainy season (Figure 3). In the rainy season, precipitation will bring a large amount of nutrients from runoff into the lake, resulting in a rapid increase in nutrient concentration [41]. The correlations between TN, TP and Chl-a also confirmed that nutrient concentration had an effect on Chl-a (Figure 7). For physical factors, Chl-a was significantly positively correlated with WT, DO, pH and turbidity and negatively correlated with water depth (Figure 7), which is consistent with the findings of most studies [42,43]. In different seasons, the WT, DO, pH and turbidity change significantly due to the increase and decrease in temperature and precipitation [44]. For example, the fluorescence intensity of phytoplankton increased with decreasing temperature, and as the depth of the lake increased, the light decreased and the algal cells fluoresced more in the dark environment than in the light transmission zone of the water column [21]. Therefore, the change in the fluorescence intensity of phytoplankton in response to environmental conditions prevents the YSI results from reflecting the authentic phytoplankton density.
In addition, the phytoplankton community structure in the different lakes varied greatly (Figure 5). In mesotrophic lakes, the correlation between Chl-a and phytoplankton density was minimal or even negative (Figure 6). The possible reason was that phytoplankton species diversity has an impact on the Chl-a concentration of phytoplankton because the Chl-a concentrations in individual cells of different phytoplankton species are different, and high species diversity will lead to inconsistent changes in Chl-a and phytoplankton [20,45]. In this study, LEH identified the greatest number of phytoplankton taxa in mesotrophic lakes (Figure 5). Thus, Chl-a affected the characterization of phytoplankton density, showing the lowest correlation with phytoplankton density (Figure 6). This finding is similar to the results of Du et al. [19]. On the other hand, a large number of studies have also shown that when there is a single dominant phytoplankton species in water, the Chl-a content generally increases proportionally with increasing phytoplankton density, which is significantly and positively correlated with each other [39,46]. In this study, there was a significant positive correlation between Chl-a and phytoplankton density in lakes with a single dominant species of cyanobacteria, while there was little or no correlation between Chl-a and phytoplankton density in lakes with diverse dominant species (Figure 6). This phenomenon also indicated that the relationship between Chl-a and phytoplankton density was species specific [39]. This is due to environmental changes (nutrient salts, temperature, light, etc.). However, the absence of a correlation between the Chl-a concentration and a particular dominant species indicated that Chl-a is not affected by a particular dominant species (Figure 7).
According to the above analysis, in the field environment, a significant correlation between phytoplankton density and Chl-a can be observed only when the external environmental conditions are stable and when the dominant phytoplankton species are single. However, when the phytoplankton community is altered, the dominant species become more numerous, and the environmental conditions change, which can lead to a weakening or even disappearance of the relationship between phytoplankton density and Chl-a [19,47].
5. Conclusions
In our work, we compared the determination of Chl-a by the commonly used laboratory spectrophotometric method and the portable YSI multi-parameter water quality meter directly used in the field. Most of the Chl-a values measured by the laboratory instrument were greater than those measured by the YSI, and the correlation between the two methods was weak (0.492, p < 0.001). The correlation between Chl-a and phytoplankton density measured by the YSI reached 0.67 (p < 0.001) in the dry season, while the laboratory methods used to measure Chl-a to characterize phytoplankton density may have led to an overestimation of phytoplankton density due to nonspecific sources of Chl-a. However, both methods are relatively inaccurate for characterizing phytoplankton density. The content of Chl-a in phytoplankton cells is affected by both external and internal factors. For different trophic states of lakes, nutrient concentration changes affect the Chl-a concentration of phytoplankton. We also found that the effect of TN on laboratory-measured Chl-a was largely dependent on the trophic state of the lake, with TN having a positive effect on Chl-a in eutrophic lakes but not in mesotrophic or hypotrophic lakes. During different seasons, changes in the fluorescence intensity of phytoplankton in response to environmental conditions prevent the YSI results from reflecting the authentic phytoplankton density. Physical factors, such as WT, DO, pH and light, all have effects on Chl-a. Furthermore, high species diversity can lead to inconsistent changes in Chl-a and phytoplankton because the content of Chl-a in individual cells of different phytoplankton is different. The number of phytoplankton taxa identified by LEH in mesotrophic lakes was the highest, and the correlation between Chl-a and phytoplankton density was the lowest. The relationship between Chl-a and phytoplankton density was species specific. In this study, there was a significant positive correlation between Chl-a and phytoplankton density in lakes with a single dominant species of cyanobacteria, while there was little or no correlation between Chl-a and phytoplankton density in lakes with diverse dominant species. Due to the complexity and variability of the phytoplankton community structure in Yunnan Plateau lakes, it is very difficult to characterize phytoplankton density by using Chl-a because most lakes can hardly meet the stringent prerequisites of a stable external environment and a single dominant phytoplankton species. To improve the reliability of the sample analysis results, the best index should be selected according to the specific situation in practical applications. Therefore, the two methods for determining Chl-a can only approximate phytoplankton density in some specific situations. In future studies, on the one hand, it will be necessary to update the instruments and equipment, such as improving the fluorescence test accuracy of the YSI for photosynthetic pigments in various algae. On the other hand, it is also necessary to establish an integrated index system for a more accurate characterization of phytoplankton density in lake environments.
Author Contributions
Conceptualization, methodology, supervision, resources, foundation acquisition, writing—review and editing, H.Z.; formal analysis, writing—original draft preparation, J.W. and D.L.; investigation and data curation, J.W., L.D., Z.Y., Y.Z. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NSFC (Grant No. U2202207), the Special Project for Social Development of Yunnan Province (Grant No. 202103AC100001) and the 14th Graduate Research Innovation Project of Yunnan University (Grant No. KC-22222984).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to the teachers and students who participated in the field collection of the data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H. Lake Management and Eutrophication Mitigation: Coming down to Earth—In Situ Monitoring, Scientific Management and Well-Organized Collaboration Are Still Crucial. Water 2022, 14, 2878. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, T.; Chen, W.; Zheng, G.; Shum, C.K.; Yang, K.; Jia, Y. Regional differences of lake evolution across China during 1960s–2015 and its natural and anthropogenic causes. Remote Sens. Environ. 2019, 221, 386–404. [Google Scholar] [CrossRef]
- Qi, J.; Deng, L.; Song, Y.; Qi, W.; Hu, C. Nutrient Thresholds Required to Control Eutrophication: Does It Work for Natural Alkaline Lakes? Water 2022, 14, 2674. [Google Scholar] [CrossRef]
- Li, D.; Chang, F.; Wen, X.; Duan, L.; Zhang, H. Seasonal Variations in Water Quality and Algal Blooming in Hypereutrophic Lake Qilu of Southwestern China. Water 2022, 14, 2611. [Google Scholar] [CrossRef]
- Tebbs, E.J.; Remedios, J.J.; Harper, D.M. Remote sensing of chlorophyll-a as a measure of cyanobacterial biomass in Lake Bogoria, a hypertrophic, saline–alkaline, flamingo lake, using Landsat ETM+. Remote Sens. Environ. 2013, 135, 92–106. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.; Zhu, Y.; Wang, S.; Duan, C.; Yang, C.; Tang, J. Hysteresis effects of meteorological variation-induced algal blooms: A case study based on satellite-observed data from Dianchi Lake, China (1988–2020). Sci. Total Environ. 2022, 812, 152558. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Duan, L.; Liu, Q.; Zhang, Y.; Zhang, X.; Liu, F.; Zhang, H. Spatiotemporal Changes in Water Quality Parameters and the Eutrophication in Lake Erhai of Southwest China. Water 2022, 14, 3398. [Google Scholar] [CrossRef]
- Huang, C.C.; Li, Y.M.; Sun, D.Y.; Le, C.F. Retrieval of Microcystis aentginosa percentage from high turbid and eutrophia inland water: A case study in Taihu Lake. IEEE Trans. Geosci. Remote Sens. 2011, 49, 4090–4100. [Google Scholar] [CrossRef]
- Du, S.; Huang, X.; Zang, C. Study on the Correlation Research between Indicators of Phytoplankton Standing Stock I: Chlorophyll A and Algal Density. Water Resour. Water Eng. 2011, 22, 40–44. [Google Scholar]
- Koponen, S.; Pulliainen, J.; Kallio, K.; Hallikainen, M. Lake water quality classification with airborne hyperspectral spectrometer and simulated MERIS data. Remote Sens. Environ. 2002, 79, 51–59. [Google Scholar] [CrossRef]
- Yang, Y.P.; Wang, Q.; Wen, J.G. Quantitative remote sensing inversion methods of chlorophyll-a concentration in Taihu Lake based on TM data. Geogr. Geo-Inf. Sci. 2006, 22, 5–8. [Google Scholar]
- Wang, Z.; Zou, H.; Yang, G.; Zhang, H.; Zhuang, Y. Spatial-temporal characteristics of chlorophyll-a and its relationship with environmental factors in Lake Taihu. Lake Sci. 2014, 26, 567–575. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Li, N.; Sun, X.; Wang, W.; Qin, B.; Zhu, G. Capturing the rapid intraday change of cyanobacterial bloom by land-based hyperspectral remote sensing in Lake Taihu. Lake Sci. 2021, 33, 1951–1960. [Google Scholar]
- Obenour, D.R.; Gronewold, A.D.; Stow, C.A.; Scavia, D. Using a B ayesian hierarchical model to improve Lake Erie cyanobacteria bloom forecasts. Water Resour. Res. 2014, 50, 7847–7860. [Google Scholar] [CrossRef]
- Zhu, G.; Shi, K.; Li, W.; Li, N.; Zou, W.; Guo, C.; Qin, B. Seasonal forecast method of cyanobacterial bloom intensity in eutrophic Lake Taihu. China. Lake Sci. 2020, 32, 1421–1431. [Google Scholar]
- Vörös, L.; Padisák, J. Phytoplankton biomass and chlorophyll-an in some shallow lakes in Central Europe. Hydrobiologia 1991, 215, 111–119. [Google Scholar] [CrossRef]
- Lehman, P.W. Comparison of chlorophyll A and carotenoid pigments as predictors of phytoplankton biomass. Mar. Biol. 1981, 65, 237–244. [Google Scholar] [CrossRef]
- Du, S.L.; Huang, S.L.; Zang, C.J.; Wu, M.; Gao, F.; Lin, C.; Guo, Y.; Luo, Y. Study on the Correlation Research between Indicators of Phytoplankton Standing Stock II: Chlorophyll A and Algal Density. Water Resour. Water Eng. 2011, 22, 44–49. [Google Scholar]
- Sun, L.; Zhong, Y.; Zhang, D.M.; Zhu, L.; Dai, S.G.; Zhuang, Y.Y. Changes of algal communities in water body with different proportions of nitrogen and phosphorus. Appl. Ecol. 2006, 17, 1218–1223. [Google Scholar]
- Liu, Y.; Chen, Y.; Deng, J. Discussion on accuracy and errors for phytoplankton chlorophyll-a concentration analysis using YSI (multiparameter water analyzer). J. Lake Sci. 2010, 22, 965–968. [Google Scholar]
- Song, T.; Zhu, B.; Yan, F.; Xu, C. Comparison of Onsite YSI6600V2 Multi-Parameter Water Quality Meter and Laboratory Spectrophotometry for the Determination of Lake Algal Chlorophyll. Environ. Monit. Forewarning 2016, 8, 14–18. [Google Scholar]
- Yu, Z.; Yang, K.; Luo, Y.; Yang, Y. Secchi depth inversion and its temporal and spatial variation analysis—A case study of nine plateau lakes in Yunnan Province of China. Appl. Earth Obs. Geoinf. 2021, 100, 102344. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, F.; Duan, L.; Wu, H.; Zhang, H. The industrial pollution history inferred by stable Pb isotope in the chronological sedimentary record of a plateau lake, Yunnan Province, southwestern of China. Coast. Res. 2020, 115, 641–647. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, E.; Zhang, E.; Nath, B.; Bindler, R.; Liu, J.; Shen, J. Organic carbon burial in a large, deep alpine lake (Southwest China) in response to changes in climate, land use and nutrient supply over the past 100 years. Catena 2021, 202, 105240. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Liu, Z. Seasonal variations of thermal stratification in Lake Lugu. Highlights Sci. Online 2014, 7, 2441–2448. [Google Scholar]
- Zhou, Q.; Zhang, Y.; Li, K.; Huang, L.; Yang, F.; Zhou, Y.; Chang, J. Seasonal and spatial distributions of euphotic zone and long-term variations in water transparency in a clear oligotrophic Lake Fuxian, China. Environ. Sci. 2018, 72, 185–197. [Google Scholar] [CrossRef]
- Seuront, L.; Schmitt, F.; Lagadeuc, Y.; Schertzer, D.; Lovejoy, S.; Frontier, S. Multifractal analysis of phytoplankton biomass and temperature in the ocean. Geophys. Res. Lett. 1996, 23, 3591–3594. [Google Scholar] [CrossRef]
- Karlson, B.; Godhe, A.; Cusack, C.; Bresnan, E. Introduction to methods for quantitative phytoplankton analysis. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; UNESCO: London, UK, 2010; p. 5. [Google Scholar]
- Dong, Y. Research and Development of Algae in the Nine Plateau Lakes in Yunnan. Environ. Sci. Guide 2014, 33, 1–8. [Google Scholar]
- Zhang, Y.; Zhang, H.; Liu, Q.; Duan, L.; Zhou, Q. Total nitrogen and community turnover determine phosphorus use efficiency of phytoplankton along nutrient gradients in plateau lakes. Environ. Sci. 2023, 124, 699–711. [Google Scholar] [CrossRef]
- Hou, P.; Chang, F.; Duan, L.; Zhang, Y.; Zhang, H. Seasonal Variation and Spatial Heterogeneity of Water Quality Parameters in Lake Chenghai in Southwestern China. Water 2022, 14, 1640. [Google Scholar] [CrossRef]
- Wang, J.H.; Yang, C.; Dao, G.H.; Du, J.S.; Han, Y.P.; Wu, G.X.; Hu, H.Y. Meteorological factors and water quality changes of Plateau Lake Dianchi in China (1990–2015) and their joint influences on cyanobacterial blooms. Sci. Total Environ. 2019, 665, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Roesler, C.S.; Perry, M.J. In situ phytoplankton absorption, fluorescence emission, and particulate backscattering spectra determined from reflectance. Geophys. Res. Ocean. 1995, 100, 13279–13294. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D. Fluorometric determination of chlorophyll. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Yang, J.; Wang, T.; Ma, W.P. The research of methods of measuring chlorophyll an in water. Environ. Monit. China 2011, 27, 24–27. [Google Scholar]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Xu, W.; Duan, L.; Wen, X.; Li, H.; Li, D.; Zhang, Y.; Zhang, H. Effects of seasonal variation on water quality parameters and eutrophication in Lake Yangzong. Water 2022, 14, 2732. [Google Scholar] [CrossRef]
- Desortová, B. Relationship between chlorophyll-α concentration and phytoplankton biomass in several reservoirs in Czechoslovakia. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1981, 66, 153–169. [Google Scholar] [CrossRef]
- Hunter, B.L.; Laws, E.A. ATP and chlorophyll a as estimators of phytoplankton carbon biomass. Limnol. Oceanogr. 1981, 26, 944–956. [Google Scholar] [CrossRef]
- Barçante, B.; Nascimento, N.O.; Silva, T.F.; Reis, L.A.; Giani, A. Cyanobacteria dynamics and phytoplankton species richness as a measure of waterbody recovery: Response to phosphorus removal treatment in a tropical eutrophic reservoir. Ecol. Indic. 2020, 117, 106702. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.J.; Cheng, S.P.; Chai, P.H.; Liang, W.; Wu, Z.B. The spatial and temporal distribution of chlorophyll-a and its correlation with environmental factors in the Nanfeihe River. Acta Hydrobiol. Sin. 2014, 38, 342–350. [Google Scholar]
- Zhang, G.G. Spatial-temporal distribution of chlorophyll-a and its correlation with environment factors in Dongting Lake. Environ. Monit. China 2016, 32, 84–90. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algal species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Zhong, W. Application of Fluorescence Technique in Early Warning Monitor to Cyanobacteria Bloom-forming in Taihu Lake. Environ. Monit. China 2009, 25, 23–26. [Google Scholar]
- Righetti, D.; Vogt, M.; Gruber, N.; Psomas, A.; Zimmermann, N.E. Global pattern of phytoplankton diversity driven by temperature and environmental variability. Sci. Adv. 2019, 5, eaau6253. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.G.; Cheng, S.P. Phytoplankton community structure and water quality assessment in an ecological restoration area of Baiyangdian Lake, China. Environ. Sci. Technol. 2021, 18, 1529–1536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).