Mg–Fe Layered Double Hydroxides/Polyacrylonitrile Nanofibers for Solar-Light Induced Peroxymonosulfate Elimination of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Mg–Fe/PAN Nanofibers
2.2. Photo-PMSTesting
3. Results and Discussion
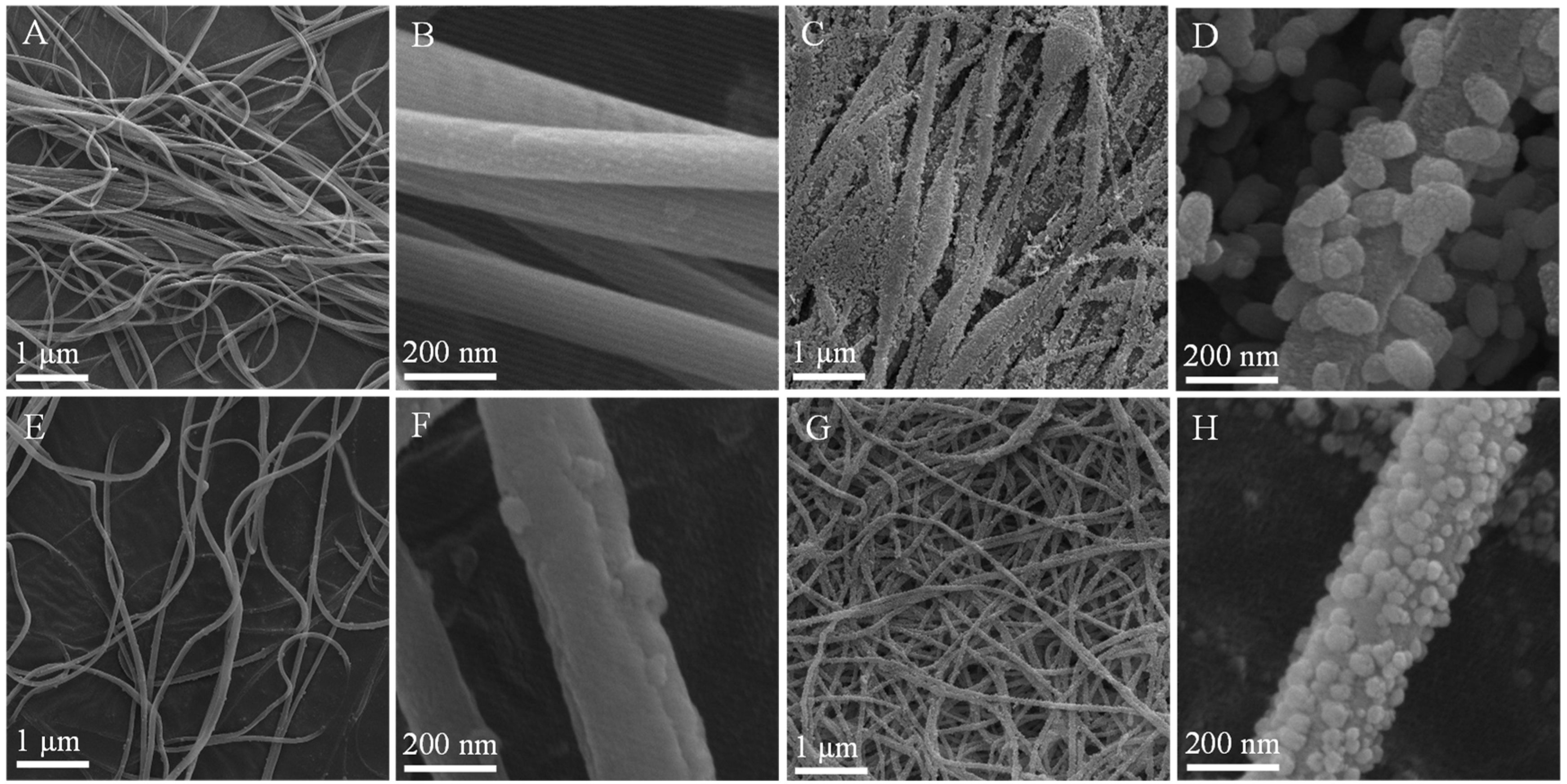
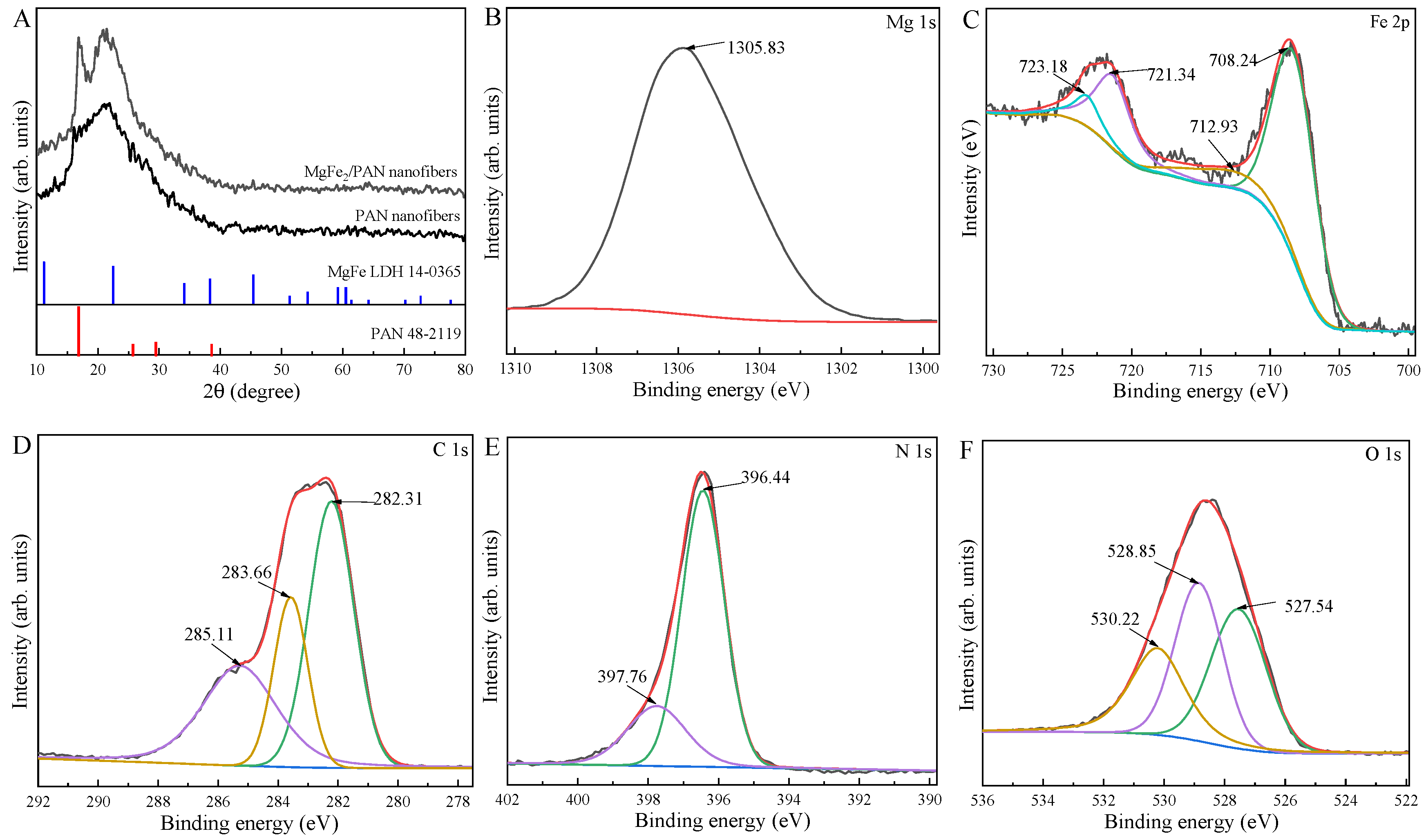
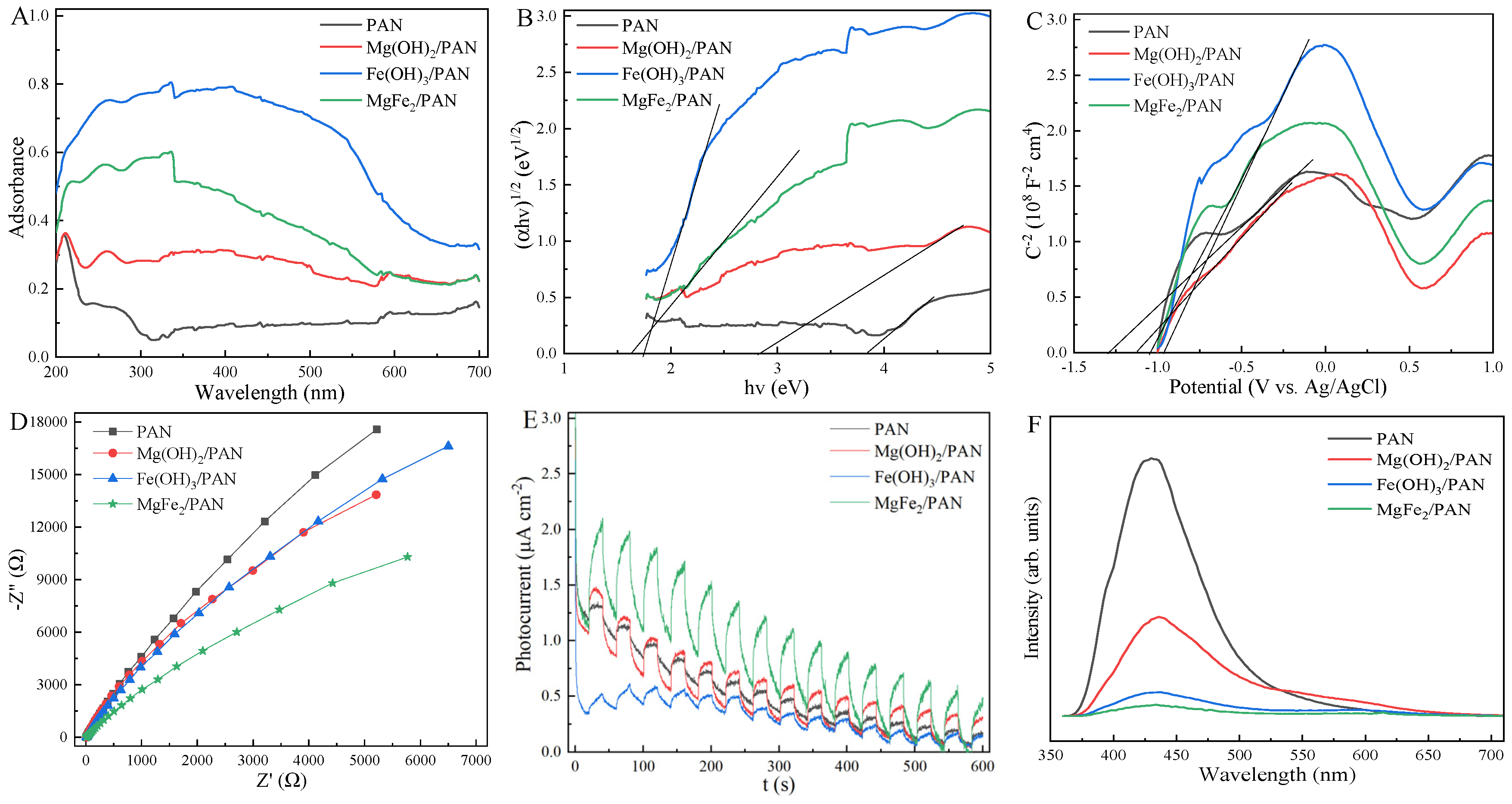
3.1. Characterization
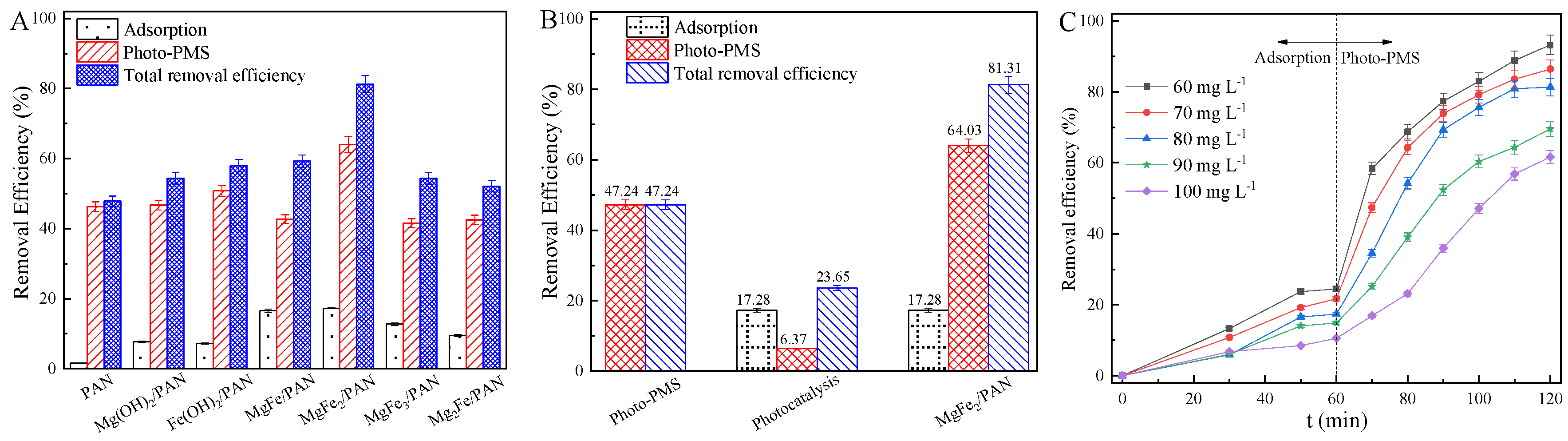
3.2. Photo-PMS Performance
3.3. Photo-PMS Durability
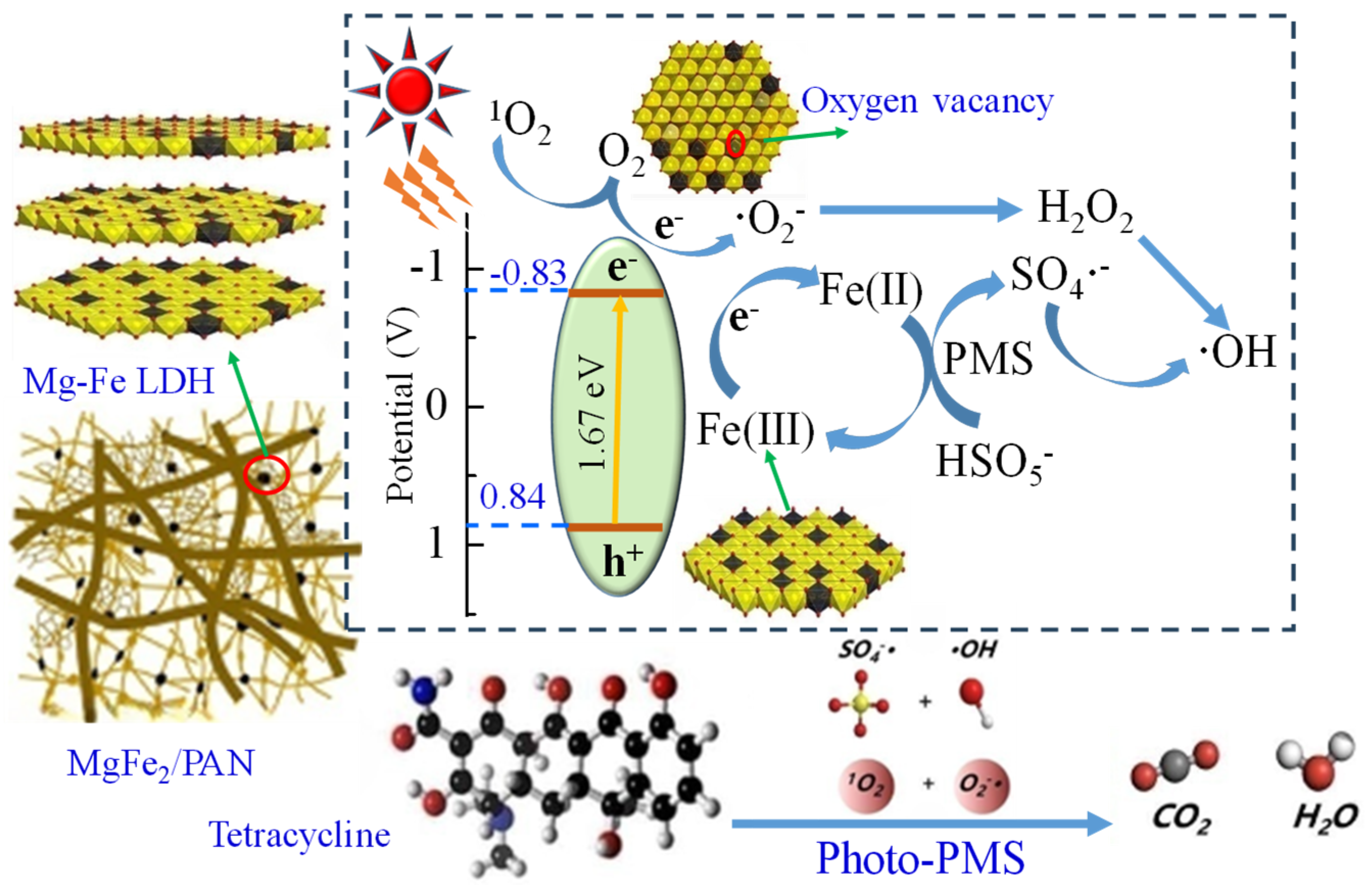
3.4. Photo-PMS Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswal, B.K.; Balasubramanian, R. Adsorptive removal of sulfonamides, tetracyclines and quinolones from wastewater and water using carbon-based materials: Recent developments and future directions. J. Clean. Prod. 2022, 349, 131421. [Google Scholar] [CrossRef]
- Sugitha, S.K.J.; Venkatesan, R.; Latha, R.G.; Vetcher, A.A.; Al-Asbahi, B.A.; Kim, S.C. A study on the antibacterial, antispas-modic, antipyretic, and anti-Inflammatory activity of ZnO nanoparticles using leaf extract from Jasminum sambac (L. Aiton). Molecules 2024, 29, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakash, P.; Venkatesan, R.; Muthu, S.E.; Hatshan, M.R.; Vetcher, A.A.; Kim, S.-C.; Kim, I. Effect of different etching times on the structural, morphological, electrical, and antimicrobial properties of mesoporous silicon. Heliyon 2023, 9, e23105. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual exist-ence in the environment. Environ. Poll. 2017, 221, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, Y.; Zhao, C.; Zhang, Z.; Zhou, Z. Tetracycline antibiotics: Potential anticancer drugs. Eur. J. Pharmacol. 2023, 956, 175949. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Sawunyama, L.; Ravele, M.P.; Rasheed-Adeleke, A.A.; Seheri, N.H.; Onwudiwe, D.C.; Mhlanga, S.D. Synthesis approaches to ceramic membranes, their composites, and application in the removal of tetracycline from water. Environ. Adv. 2023, 12, 100371. [Google Scholar] [CrossRef]
- Filho, F.G.N.; Filho, E.C.S.; Osajima, J.A.; de Melo Alves, A.P.; Fonseca, M.G. Adsorption of tetracycline using chi-tosan–alginate–bentonite composites. Appl. Clay Sci. 2023, 239, 106952. [Google Scholar] [CrossRef]
- Varadharajan, V.; Senthilkumar, D.S.; Senthilkumar, K.; Sundramurthy, V.P.; Manikandan, R.; Senthilarasan, H.; Ganesan, H.; Kesavamoorthy, I.; Ramasamy, A. Process modeling and toxicological evaluation of adsorption of tetracycline onto the magnet-ized cotton dust biochar. J. Water Process Eng. 2022, 49, 103046. [Google Scholar] [CrossRef]
- Wang, J.; Yi, X.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W.; et al. Eliminating tetracy-cline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: Batch and continuous experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Aryee, A.A.; Ma, Y.; Wang, J.; Han, R.; Qu, L. A magnetic biomass/MOF composite as a functional material for the oxidative removal of tetracycline: Degradation mechanism and toxicity study. J. Environ. Chem. Eng. 2023, 11, 110663. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Chen, J.; Yan, S.; Xie, S. Multi-omic profiling of a novel activated sludge strain Sphingobacterium sp. WM1 reveals the mechanism of tetracycline biodegradation and its merits of potential application. Water Res. 2023, 243, 120397. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Zeng, Z.; Wang, Y.; Han, M. Efficient removal of tetracycline in water by a Fe3O4-mediated persulfate acti-vation and biodegradation coupled system: Performance, validation, and mechanism. J. Water Process Eng. 2023, 53, 103718. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Gao, D.; Wang, X.; Suo, N.; Yu, Y.; Zhang, S. Electrocatalysis degradation of tetracycline in a three-dimensional aeration electrocatalysis reactor (3D-AER) with a flotation-tailings particle electrode (FPE): Physicochemical properties, influencing factors and the degradation mechanism. J. Hazard. Mater. 2020, 407, 124361. [Google Scholar] [CrossRef]
- Shi, C.; Yu, S.; Wang, L.; Zhang, X.; Lin, X.; Li, C. Degradation of tetracycline/oxytetracycline by electrospun aligned polyac-rylonitrile-based carbon nanofibers as anodic electrocatalysis microfiltration membrane. J. Environ. Chem. Eng. 2021, 9, 106540. [Google Scholar] [CrossRef]
- Swedha, M.; Balasurya, S.; Syed, A.; Das, A.; Khan, S.S. Continuous photocatalysis via Z-scheme based nanocatalyst system for environmental remediation of pharmaceutically active compound: Modification, reaction site, defect engineering and challenges on the nanocatalyst. J. Mol. Liq. 2022, 353, 118745. [Google Scholar] [CrossRef]
- Haleem, A.; Pan, J.-M.; Shah, A.; Hussain, H.; He, W.-D. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Amaly, N.; EL-Moghazy, A.Y.; Nitin, N.; Sun, G.; Pandey, P.K. Design, preparation, and application of novel multilayer met-al-polyphenol composite on macroporous framework melamine foam for effective filtration removal of tetracycline in fluidic systems. Sep. Purif. Technol. 2023, 321, 124238. [Google Scholar] [CrossRef]
- Xiong, H.-Q.; Bao, H.-R.; Long, F.; Du, Y.-Y.; Qu, J.-Z.; Luan, Z.-X.; Sun, X.-L. From lab to nature: Overcoming challenges in applying in-situ photocatalysis to water bodies. J. Environ. Chem. Eng. 2024, 12, 112656. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Qiu, L.; Yang, W.; Yu, Y.; Li, J.; Liu, Y. Z-scheme NiFe LDH/Bi4O5I2 heterojunction for photo-Fenton oxida-tion of tetracycline. J. Alloys Compd. 2023, 944, 169124. [Google Scholar] [CrossRef]
- Fazli, A.; Brigante, M.; Khataee, A.; Mailhot, G. Fe2.5Co0.3Zn0.2O4/CuCr-LDH as a visible-light-responsive photocatalyst for the degradation of caffeine, bisphenol A, and simazine in pure water and real wastewater under photo-Fenton-like degrada-tion process. Chemosphere 2022, 291, 132920. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, Q.; Liang, M.; Sun, W. Sb(III) and Sb(V) removal from water by a hydroxyl-intercalated, mechanochemically synthesized Mg-Fe-LDH. Appl. Clay Sci. 2020, 196, 105766. [Google Scholar] [CrossRef]
- Hudcová, B.; Fein, J.B.; Tsang, D.C.W.; Komárek, M. Mg-Fe LDH-coated biochars for metal(loid) removal: Surface complex-ation modeling and structural change investigations. Chem. Eng. J. 2022, 432, 134360. [Google Scholar] [CrossRef]
- Ji, Y.; Song, Z.; Xu, Y.; Zhang, Y. Cu-Fe LDHs/Bi2WO6 composite for superior photo-Fenton Rhodamine B removal through combination of photogenerated electrons and multivalent bimetal redox for accelerating Fe3+/Fe2+ cycles. J. Alloys Compd. 2022, 925, 166655. [Google Scholar] [CrossRef]
- Dong, Z.; Du, X.; Zhu, X.; Huang, E.; An, Y. Regulate the behaviors of photo-generated carriers in CuFe-LDH/TiO2 based on positive bias voltage to enhance the capability of photo-Fenton on nitrobenzene degradation. Appl. Surf. Sci. 2022, 602, 154312. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Liu, Z.; Lai, C.; Yang, X.; Shi, X.; Liu, S.; Zhang, M.; Fu, Y.; Zhou, X.; et al. Degradation of tetracycline by FeNi-LDH/Ti3C2 photo-Fenton system in water: From performance to mechanism. Chemosphere 2022, 294, 133736. [Google Scholar] [CrossRef] [PubMed]
- Costa-Serge, N.d.M.; Gonçalves, R.G.L.; Ramirez-Ubillus, M.A.; Li, C.; Hammer, P.; Chiron, S.; Nogueira, F.P. Effect of the interlamellar anion on CuMgFe-LDH in solar photo-Fenton and Fenton-like degradation of the anticancer drug 5-fluorouracil. Appl. Catal. B Environ. 2022, 315, 121537. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Tang, L.; Liang, Q.; He, Q.; Wu, T.; Pan, Y.; Cheng, M.; Liu, Y.; Tan, X.; et al. Construction of Bi2WO6/CoAl-LDHs S-scheme heterojunction with efficient photo-Fenton-like catalytic performance: Experimental and theoretical studies. Chemosphere 2022, 291, 133001. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y.; Li, J.; Zhang, L.; Xie, B.; Ni, Z.; Xia, S. The performance and mechanism of persulfate activation boosted MoO2@LDHs Z-scheme heterojunction for efficient photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2023, 11, 110257. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, Y.; Xu, L.; Mao, Y.; Wu, D. Enhanced abiotic integrated polyphenol-Maillard humification by Mg/Fe layered double hydroxide (LDH): Role of Fe(III)-polyphenol complexation. Chem. Eng. J. 2021, 425, 130521. [Google Scholar] [CrossRef]
- Gonçalves, R.G.L.; Mendes, H.M.; Bastos, S.L.; D’Agostino, L.C.; Tronto, J.; Pulcinelli, S.H.; Santilli, C.V.; Neto, J.L. Fenton-like degradation of methylene blue using Mg/Fe and MnMg/Fe layered double hydroxides as reusable catalysts. Appl. Clay Sci. 2020, 187, 105477. [Google Scholar] [CrossRef]
- Pelalak, R.; Hassani, A.; Heidari, Z.; Zhou, M. State-of-the-art recent applications of layered double hydroxides (LDHs) ma-terial in Fenton-based oxidation processes for water and wastewater treatment. Chem. Eng. J. 2023, 474, 145511. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Khan, N. LDH-based nanomaterials for photocatalytic applications: A comprehensive review on the role of bi/trivalent cations, anions, morphology, defect engineering, memory effect, and heterojunction formation. J. Energy Chem. 2023, 84, 242–276. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A comprehensive review on adsorption, photocatalytic and chemical degrada-tion of dyes and nitro-compounds over different kinds of porous and composite materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Long, K.; Sun, X.; Yuan, H.; Li, W. Activities in photocatalytic hydrogen evolution of In2O3/In2S3 heterostructure and In2O3/In2S3@PAN nanofibers. Ceram. Int. 2023, 49, 24093–24099. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, G.; Bai, J.; He, R.; Li, C. Enhanced photocatalytic activities of CdS-BiOCl/PAN composites towards photocata-lytic hydrogen evolution. Mater. Res. Bull. 2019, 117, 9–17. [Google Scholar] [CrossRef]
- Mao, Y.; Lin, L.; Chen, Y.; Yang, M.; Zhang, L.; Dai, X.; He, Q.; Jiang, Y.; Chen, H.; Liao, J.; et al. Preparation of site-specific Z-scheme g-C3N4/PAN/PANI@LaFeO3 cable nanofiber membranes by coaxial electrospinning: Enhancing filtration and photocatalysis performance. Chemosphere 2023, 328, 138553. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, Z.; Yu, S.; Yang, B.; Yin, Y.; Zan, L.; Myung, N.V. Piezo-photocatalytic flexible PAN/TiO2 composite nanofibers for environmental remediation. Sci. Total Environ. 2022, 824, 153790. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, K.; Zhu, X.; Yuan, H.; Wang, C. Photocatalytic activities of Bi2O2CO3/g-C3N4@PAN nanofibers in hydrogen production. Appl. Surf. Sci. 2022, 599, 154013. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Qian, F.; Cao, M.; Cheng, Y.; Li, J.; Tian, M.; Li, W.; Wang, L. The design of novel swash plate photocatalytic reactor with PAN/BiInOCl membrane photocatalyst for excellent RhB degradation. J. Alloys Compd. 2023, 968, 171894. [Google Scholar] [CrossRef]
- Teng, P.; Zhu, J.; Li, Z.; Li, K.; Copner, N.; Gao, S.; Zhao, E.; Zhu, X.; Liu, Z.; Tian, F.; et al. Flexible PAN-Bi2O2CO3–BiOI heterojunction nanofiber and the photocatalytic degradation property. Opt. Mater. 2022, 134, 112935. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Korobeinyk, A.V.; Yeleuov, M.; Azat, S.; Bakbolat, B.; Umirzakov, A.; Mansurov, Z. Bio-waste-derived few-layered graphene/SrTiO3/PAN as efficient photocatalytic system for water splitting. Appl. Surf. Sci. 2021, 549, 149176. [Google Scholar] [CrossRef]
- Chen, G.; Wong, N.H.; Sunarso, J.; Wang, Y.; Liu, Z.; Chen, D.; Wang, D.; Dai, G. Flexible Bi2MoO6/S-C3N4/PAN heterojunction nanofibers made from electrospinning and solvothermal route for boosting visible-light photocatalytic performance. Appl. Surf. Sci. 2023, 612, 155893. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Duan, X.; Sarmah, A.K.; Zhao, X. Remediation of environmentally persistent organic pollutants (POPs) by persulfates oxidation system (PS): A review. Sci. Total Environ. 2023, 863, 160818. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Mohamadi, L.; Kazemzadeh, S.; Zafar, M.N.; Rahdar, A.; Khaksefidi, R. Synthesis and characterization of poly(styrene-block-acrylic acid) diblock copolymer modified magnetite nanocomposite for efficient removal of penicillin G. Compos. Part B Eng. 2019, 182, 107643. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2019, 107, 100591. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.R.; Scudiero, L.; McEwen, J.S.; Garcia-Perez, M. Deconvoluting the XPS spectra for ni-trogen-doped chars: An analysis from first principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced photocatalytic activities of RhB degradation and H2 evolution from in situ for-mation of the electrostatic heterostructure MoS2/NiFe LDH nanocomposite through the Z-scheme mechanism via p–n het-erojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Lian, Z.; Wu, T.; Zhang, X.; Cai, S.; Xiong, Y.; Yang, R. Synergistic degradation of tetracycline from Mo2C/MoOx films medi-ated peroxymonosulfate activation and visible-light triggered photocatalysis. Chem. Eng. J. 2023, 469, 143774. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, C.; Hu, Q.; Ding, L. 0D/3D NiCo2O4/defected UiO-66 catalysts for enhanced degradation of tetracy-cline in peroxymonosulfate/simulated sunlight systems: Degradation mechanisms and pathways. Chemosphere 2022, 299, 134322. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhang, R.; Ren, X.; Guo, W. Vacancy-rich structure inducing efficient persulfate activation for tetracycline degradation over Ni-Fe layered double hydroxide nanosheets. Sep. Purif. Technol. 2022, 289, 120663. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Pan, S.; Zhang, B.; Ding, J.; Rong, S.; et al. Effective mineralization and detoxification of tetracycline hydrochloride enabled by oxygen vacancies in g-C3N4/LDH composites. Sep. Purif. Technol. 2023, 305, 122554. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Li, Y.; Dong, W.; Wu, S.; Duan, Q. Porphyrin-TiO2 encapsulated ZIF-8/PAN-derived porous carbon nanofiber ternary photocatalyst with enhanced photodegradation efficiency through synergistic effect. Vacuum 2023, 218, 112673. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, G.; Yu, S.; Liu, Z.; Yin, J.; Xue, M.; Sun, Q.; Shen, F.; Li, X.; Yin, Z.; et al. Novel MIL-88B(Fe)/ZnTi-LDH high-low junctions for adsorption and photodegradation of tetracycline: Characteristics, performance, and mechanisms. Chem. Eng. J. 2023, 473, 145198. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Wang, T.; Liu, X.; Xu, T.; Wei, M.; Yang, L.; Li, C. Improved photocatalytic activity of Ni2P/NiCo-LDH composites via a Co–P bond charge transfer channel to degrade tetracycline under visible light. J. Alloys Compd. 2021, 852, 156963. [Google Scholar] [CrossRef]
- Devi, S.K.; Thirumal, V.; Balamurugan, A.; Avula, B.; Pongiya, U.D.; Kim, J.; Surya, C. Photocatalytic degradation of tetracycline over ternary NiCoMn-LDH under visible light illumination. ACS Mater. Lett. 2023, 350, 134910. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Deng, Q.; Zhang, X.; Xiong, L.; Tang, Z.; Li, P.; Yin, N.; Sun, A.; Chen, D.; et al. In-situ construction of 3D marigold-like CoAl-LDH/Ti3C2 heterosystem collaborating with 2D/2D interface for efficient photodegradation of multiple antibiotics. Appl. Surf. Sci. 2021, 569, 151084. [Google Scholar] [CrossRef]
- Shi, H.; Yang, X.; Zuo, Y.; Yang, H.; Zhang, R.; Zhang, Y.; Fan, Y.; Du, X.; Jiang, L. Construction of Fe3O4/FeP binary composite catalyst for degradation of tetracycline in wastewater. Int. J. Electrochem. Soc. 2021, 16, 210314. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, C.; Qin, L.; Fu, Y.; He, J.; Huang, D.; Li, B.; Zhang, M.; Liu, S.; Li, L.; et al. ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn-O-Fe structure for TC degradation. Chem. Eng. J. 2020, 392, 124851. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Ye, B.; Luo, M.; Zheng, X. Mg–Fe Layered Double Hydroxides/Polyacrylonitrile Nanofibers for Solar-Light Induced Peroxymonosulfate Elimination of Tetracycline Hydrochloride. Water 2024, 16, 1345. https://doi.org/10.3390/w16101345
Peng H, Ye B, Luo M, Zheng X. Mg–Fe Layered Double Hydroxides/Polyacrylonitrile Nanofibers for Solar-Light Induced Peroxymonosulfate Elimination of Tetracycline Hydrochloride. Water. 2024; 16(10):1345. https://doi.org/10.3390/w16101345
Chicago/Turabian StylePeng, Hao, Beilei Ye, Meiying Luo, and Xiaogang Zheng. 2024. "Mg–Fe Layered Double Hydroxides/Polyacrylonitrile Nanofibers for Solar-Light Induced Peroxymonosulfate Elimination of Tetracycline Hydrochloride" Water 16, no. 10: 1345. https://doi.org/10.3390/w16101345
APA StylePeng, H., Ye, B., Luo, M., & Zheng, X. (2024). Mg–Fe Layered Double Hydroxides/Polyacrylonitrile Nanofibers for Solar-Light Induced Peroxymonosulfate Elimination of Tetracycline Hydrochloride. Water, 16(10), 1345. https://doi.org/10.3390/w16101345






